Selected Lipidome Components and Their Association with Perinatal Depression
Abstract
1. Introduction
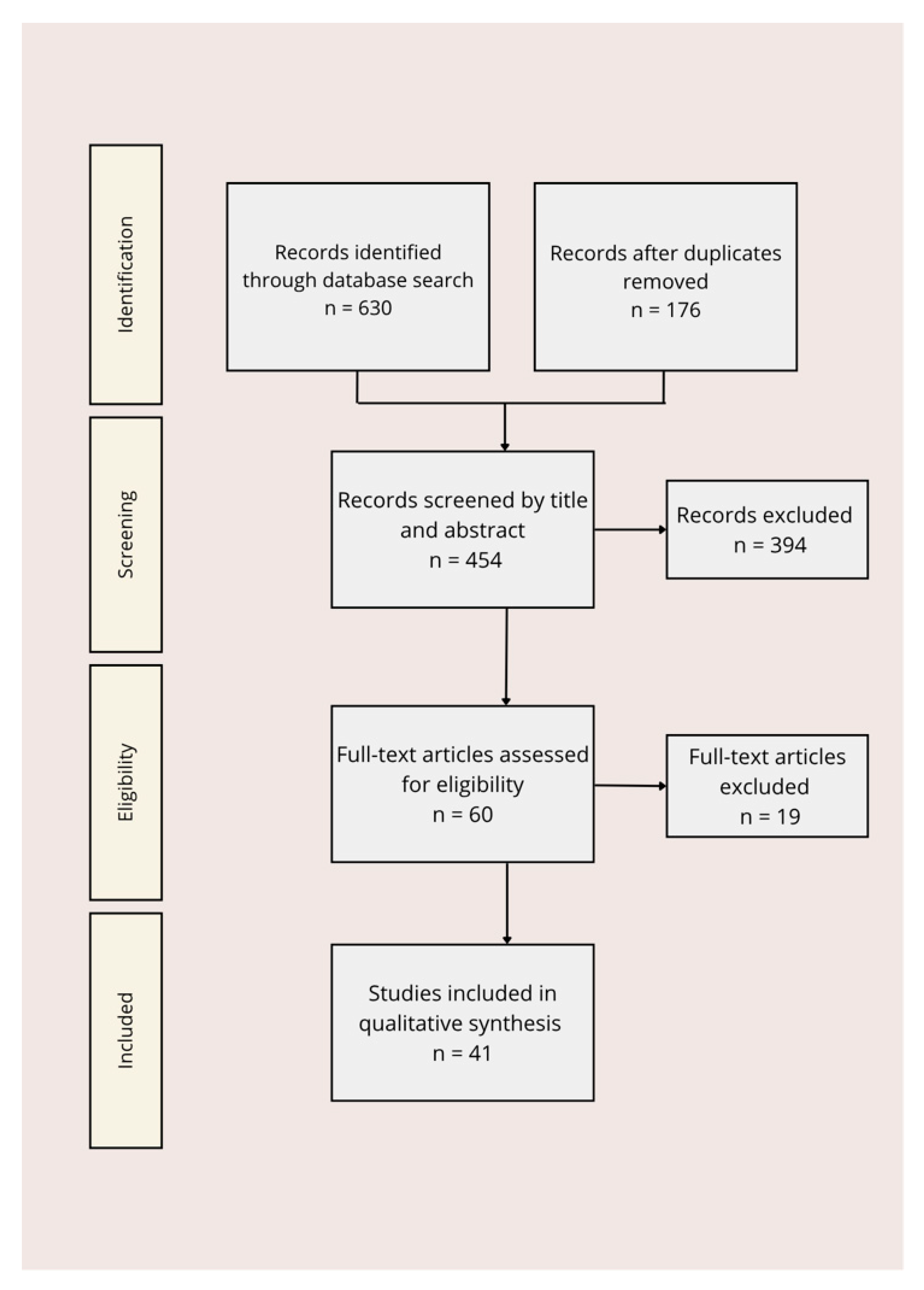
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Extraction
2.4. Data Synthesis
3. Results and Discussion
3.1. Changes in Lipid Metabolism During Pregnancy
3.2. Fatty Acids
3.2.1. Inflammation, Perinatal Depression and Their Connection to Fatty Acids
3.2.2. Omega-3 Supplementation as a Potential Intervention for Perinatal Depression
3.3. Lecithin and Choline
Choline and Depression
4. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Author (Year) | Country/Setting | Population | Study Design | Main Lipid Component(s) | Key Findings |
|---|---|---|---|---|---|
| Rees et al. (2009) [31] | Australia | Pregnant women | Case–control | Omega-3 PUFAs (EPA, DHA) | Low omega-3 PUFA levels strongly associated with antenatal depression. |
| Hoge et al. (2019) [37] | Belgium | Pregnant women | Prospective cohort | Omega-6/Omega-3 ratio | Imbalance (↑ omega-6/omega-3) predicted postpartum depression. |
| Hamazaki et al. (2019) [38] | Japan (JECS cohort) | Nationwide cohort of pregnant women | Longitudinal | Fish intake, omega-3 PUFAs | High dietary omega-3/fish intake associated with reduced postpartum depression risk. |
| Mocking et al. (2020) [39] | Multicenter | Perinatal women | RCT | Omega-3 supplementation | No preventive effect on perinatal depression, modest therapeutic effect in postpartum depression. |
| Liao et al. (2019) [62] | International (meta-analysis) | Patients with depression | Meta-analysis | Omega-3 PUFAs (EPA ≥ 60%) | Higher EPA proportion showed stronger antidepressant effect: evidence variable. |
| Hsu et al. (2018) [61] | Taiwan | Pregnant and postpartum women | Clinical trial | EPA- and DHA-rich oils | EPA-rich oils reduced depressive symptoms during and after pregnancy: DHA had preventive effects. |
| Ilavská et al. (2024) [29] | Slovakia | Children/adolescents with depression | RTC | Omega-3 and omega-6 fatty acids | Omega-3 modulated kynurenine/tryptophan ratio: omega-6 increased kynurenine production. |
| Carabelli et al. (2020) [59] | Brazil (animal study) | Rodent depression model | Experimental | Fish oil (EPA/DHA) | Fish oil ↓ IDO activity ↑ hippocampus serotonin levels. |
| Borsini et al. (2017) [58] | UK (cell model) | Human hippocampus progenitors | In vitro | Omega-3 fatty acids | Omega-3 counteracted IL-1β-induced reduction in neurogenesis. |
References
- Al-abri, K.; Edge, D.; Armitage, C.J. Prevalence and correlates of perinatal depression. Soc. Psychiatry Psychiatr. Epidemiol. 2023, 58, 1581–1590. [Google Scholar] [CrossRef]
- Tebeka, S.; Le Strat, Y.; De Premorel Higgons, A.; Benachi, A.; Dommergues, M.; Kayem, G.; Lepercq, J.; Luton, D.; Mandelbrot, L.; Ville, Y.; et al. Prevalence and incidence of postpartum depression and environmental factors: The IGEDEPP cohort. J. Psychiatr. Res. 2021, 138, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Roddy Mitchell, A.; Gordon, H.; Lindquist, A.; Walker, S.P.; Homer, C.S.E.; Middleton, A.; Cluver, C.A.; Tong, S.; Hastie, R. Prevalence of perinatal depression in low- and middle-income countries. JAMA Psychiatry 2023, 80, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, S.; Wang, G. Prevalence and Risk Factors of Postpartum Depression in Women: A Systematic Review and Meta-analysis. J. Clin. Nurs. 2021, 31, 2665–2677. [Google Scholar] [CrossRef]
- Cox, E.Q.; Sowa, N.A.; Meltzer-Brody, S.E.; Gaynes, B.N. The Perinatal Depression Treatment Cascade. J. Clin. Psychiatry 2016, 77, 1189–1200. [Google Scholar] [CrossRef]
- Dennis, C.L.; Singla, D.R.; Brown, H.K.; Savel, K.; Clark, C.T.; Grigoriadis, S.; Vigod, S.N. Postpartum Depression: A Clinical Review of Impact and Current Treatment Solutions. Drugs 2024, 84, 645–659. [Google Scholar] [CrossRef]
- Dimcea, D.A.-M.; Petca, R.C.; Dumitrașcu, M.C.; Șandru, F.; Mehedințu, C.; Petca, A. Postpartum Depression: Etiology, Treatment, and Consequences for Maternal Care. Diagnostics 2024, 14, 865. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Z.; Deng, Y.; Zhang, L.; He, C.; Wu, Y.; Xu, X. Risk factors for the development of postpartum depression in individuals who screened positive for antenatal depression. BMC Psychiatry 2023, 23, 557. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.; Obst, S.; Teague, S.J.; Rossen, L.; Spry, E.A.; Macdonald, J.A.; Sunderland, M.; Olsson, C.A.; Youssef, G.; Hutchinson, D. Association between maternal perinatal depression and anxiety and child and adolescent development. JAMA Pediatr. 2020, 174, 1082. [Google Scholar] [CrossRef]
- Jahan, N.; Went, T.R.; Sultan, W.; Sapkota, A.; Khurshid, H.; Qureshi, I.A.; Alfonso, M. Untreated Depression During Pregnancy and Its Effect on Pregnancy Outcomes: A Systematic Review. Cureus 2021, 13, e17251. [Google Scholar] [CrossRef]
- Luciano, M.; Di Vincenzo, M.; Brandi, C.; Tretola, L.; Toricco, R.; Perris, F.; Volpicelli, A.; Torella, M.; La Verde, M.; Fiorillo, A.; et al. Does antenatal depression predict post-partum depression and obstetric complications? Results from a longitudinal, long-term, real-world study. Front. Psychiatry 2022, 13, 1082762. [Google Scholar] [CrossRef]
- Li, S.; Yang, Z.; Yao, M.; Shen, Y.; Zhu, H.; Jiang, Y.; Ji, Y.; Yin, J. Exploration for biomarkers of postpartum depression based on metabolomics: A systematic review. J. Affect. Disord. 2022, 317, 298–306. [Google Scholar] [CrossRef]
- Yang, R.; Lin, Z.; Cai, Y.; Chen, N.; Zhou, Y.; Zhang, J.; Hong, G. Assessing the risk of prenatal depressive symptoms in Chinese women: An integrated evaluation of serum metabolome, multivitamin supplement intake, and clinical blood indicators. Front. Psychiatry 2024, 14, 1234461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; He, L.; Li, Z.; Ding, R.; Han, X.; Chen, B.; Cao, G.; Ye, J.-H.; Li, T.; Fu, R. Bridging Neurobiological Insights and Clinical Biomarkers in Postpartum Depression: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 8835. [Google Scholar] [CrossRef] [PubMed]
- Padilha, M.; Ferreira, A.L.L.; Normando, P.; Schincaglia, R.M.; Freire, S.R.; Keller, V.N.; Figueiredo, A.C.C.; Yin, X.; Brennan, L.; Kac, G. Maternal serum amino acids and hydroxylated sphingomyelins at pregnancy are associated with anxiety symptoms during pregnancy and throughout the first year after delivery. J. Affect. Disord. 2024, 351, 579–587. [Google Scholar] [CrossRef]
- Yu, Z.; Matsukawa, N.; Saigusa, D.; Motoike, I.N.; Ono, C.; Okamura, Y.; Onuma, T.; Takahashi, Y.; Sakai, M.; Kudo, H.; et al. Plasma metabolic disturbances during pregnancy and postpartum in women with depression. iScience 2022, 25, 105666. [Google Scholar] [CrossRef]
- Müller, C.P.; Reichel, M.; Mühle, C.; Rhein, C.; Gulbins, E.; Kornhuber, J. Brain membrane lipids in major depression and anxiety disorders. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 1052–1065. [Google Scholar] [CrossRef]
- Cermenati, G.; Mitro, N.; Audano, M.; Melcangi, R.C.; Crestani, M.; De Fabiani, E.; Caruso, D. Lipids in the nervous system: From biochemistry and molecular biology to patho-physiology. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 51–60. [Google Scholar] [CrossRef]
- Basak, S.; Mallick, R.; Duttaroy, A.K. Maternal Docosahexaenoic Acid Status during Pregnancy and Its Impact on Infant Neurodevelopment. Nutrients 2020, 12, 3615. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, F.; Edwards, M.J.; Mühle, C.; Carpinteiro, A.; Wilson, G.; Wilker, B.; Soddemann, M.; Keitsch, S.; Scherbaum, N.; Müller, B.W.; et al. Ceramide levels in blood plasma correlate with major depressive disorder severity and its neutralization abrogates depressive behavior in mice. J. Biol. Chem. 2022, 298, 102185. [Google Scholar] [CrossRef]
- Bharti, V.; Bhardwaj, A.; Hood, K.; Elias, D.A.; Metcalfe, A.W.S.; Kim, J.S. A systematic review and meta-analysis of lipid metabolomic signatures of Major Depressive Disorder. J. Psychiatr. Res. 2021, 139, 197–205. [Google Scholar] [CrossRef]
- Mulder, J.W.C.M.; Kusters, D.M.; Roeters van Lennep, J.E.; Hutten, B.A. Lipid metabolism during pregnancy: Consequences for mother and child. Curr. Opin. Lipidol. 2024, 35, 133–140. [Google Scholar] [CrossRef]
- Armistead, B.; Johnson, E.; VanderKamp, R.; Kula-Eversole, E.; Kadam, L.; Drewlo, S.; Kohan-Ghadr, H.-R. Placental regulation of energy homeostasis during human pregnancy. Endocrinology 2020, 161, bqaa076. [Google Scholar] [CrossRef]
- Preda, A.; Preda, S.D.; Mota, M.; Iliescu, D.G.; Zorila, L.G.; Comanescu, A.C.; Mitrea, A.; Clenciu, D.; Mota, E.; Vladu, I.M. Dyslipidemia in pregnancy: A systematic review of molecular alterations and clinical implications. Biomedicines 2024, 12, 2252. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Qiu, H.; Wang, J.; Liu, X.; Chen, S.; Li, B. Correlation between lipid metabolism levels and pregnancy outcomes. Front. Med. 2025, 12, 1530525. [Google Scholar] [CrossRef] [PubMed]
- Duttaroy, A.K.; Basak, S. Maternal fatty acid metabolism in pregnancy and its consequences in the feto-placental development. Front. Physiol. 2022, 12, 787848. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Asch, R.H.; Lindquist, D.M.; Krikorian, R. Role of polyunsaturated fatty acids in human brain structure and function across the lifespan: An update on neuroimaging findings. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 23–34. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Belury, M.A.; Porter, K.; Beversdorf, D.Q.; Lemeshow, S.; Glaser, R. Depressive symptoms, omega-6:omega-3 fatty acids, and inflammation in older adults. Psychosom. Med. 2007, 69, 217–224. [Google Scholar] [CrossRef]
- Ilavská, L.; Morvová, M.; Paduchová, Z.; Muchová, J.; Garaiova, I.; Ďuračková, Z.; Šikurová, L.; Trebatická, J. The kynurenine and serotonin pathway, neopterin and biopterin in depressed children and adolescents: An impact of omega-3 fatty acids, and association with markers related to depressive disorder. A randomized, blinded, prospective study. Front. Psychiatry 2024, 15, 1347178. [Google Scholar] [CrossRef]
- Stachowicz, K. The role of polyunsaturated fatty acids in neuronal signaling in depression and cognitive processes. Arch. Biochem. Biophys. 2023, 737, 109555. [Google Scholar] [CrossRef]
- Rees, A.M.; Austin, M.P.; Owen, C.; Parker, G. Omega-3 deficiency associated with perinatal depression: Case control study. Psychiatry Res. 2009, 166, 254–259. [Google Scholar] [CrossRef]
- Mischoulon, D.; Dunlop, B.W.; Kinkead, B.; Schettler, P.J.; Lamon-Fava, S.; Rakofsky, J.J.; Nierenberg, A.A.; Clain, A.J.; Crowe, T.M.; Wong, A.; et al. Omega-3 fatty acids for major depressive disorder with high inflammation. J. Clin. Psychiatry 2022, 83, 21m14074. [Google Scholar] [CrossRef]
- Zhang, M.M.; Zou, Y.; Li, S.M.; Wang, L.; Sun, Y.H.; Shi, L.; Lu, L.; Bao, Y.-P.; Li, S.-X. The efficacy and safety of omega-3 fatty acids on depressive symptoms in perinatal women: A meta-analysis of randomized placebo-controlled trials. Transl. Psychiatry 2020, 10, 193. [Google Scholar] [CrossRef]
- Martinat, M.; Rossitto, M.; Di Miceli, M.; Layé, S. Perinatal dietary polyunsaturated fatty acids in brain development, role in neurodevelopmental disorders. Nutrients 2021, 13, 1185. [Google Scholar] [CrossRef]
- Koletzko, B.; Cetin, I.; Brenna, J.T. Dietary fat intakes for pregnant and lactating women. Br. J. Nutr. 2007, 98, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, T.H.; Williams, S.M. Low Omega-3 intake is associated with high rates of depression and preterm birth on the country level. Sci. Rep. 2020, 10, 19749. [Google Scholar] [CrossRef] [PubMed]
- Hoge, A.; Tabar, V.; Donneau, A.F.; Dardenne, N.; Degée, S.; Timmermans, M.; Nisolle, M.; Guillaume, M.; Castronovo, V. Imbalance between omega-6 and omega-3 polyunsaturated fatty acids in early pregnancy is predictive of postpartum depression in a Belgian cohort. Nutrients 2019, 11, 876. [Google Scholar] [CrossRef]
- Hamazaki, K.; Matsumura, K.; Tsuchida, A.; Kasamatsu, H.; Tanaka, T.; Ito, M.; Inadera, H. Dietary intake of fish and n-3 polyunsaturated fatty acids and risk of postpartum depression: A nationwide longitudinal study—The Japan Environment and Children’s Study (JECS). Psychol. Med. 2019, 50, 2416–2424. [Google Scholar] [CrossRef]
- Mocking, R.J.T.; Steijn, K.; Roos, C.; Assies, J.; Bergink, V.; Ruhé, H.G.; Schene, A.H. Omega-3 fatty acid supplementation for perinatal depression. J. Clin. Psychiatry 2020, 81, 19r13106. [Google Scholar] [CrossRef] [PubMed]
- Silva-Fernandes, A.; Conde, A.; Marques, M.; Caparros-Gonzalez, R.A.; Fransson, E.; Mesquita, A.R.; Figueiredo, B.; Skalkidou, A. Inflammatory biomarkers and perinatal depression: A systematic review. PLoS ONE 2024, 19, e0280612. [Google Scholar] [CrossRef]
- Sha, Q.; Madaj, Z.; Keaton, S.; Escobar Galvis, M.L.; Smart, L.; Krzyzanowski, S.; Fazleabas, A.T.; Leach, R.; Postolache, T.T.; Achtyes, E.D.; et al. Cytokines and tryptophan metabolites can predict depressive symptoms in pregnancy. Transl. Psychiatry 2022, 12, 35. [Google Scholar] [CrossRef]
- Zhu, J.; Jin, J.; Tang, J. Inflammatory pathophysiological mechanisms implicated in postpartum depression. Front. Pharmacol. 2022, 13, 955672. [Google Scholar] [CrossRef]
- Brown, S.J.; Huang, X.F.; Newell, K.A. The kynurenine pathway in major depression: What we know and where to next. Neurosci. Biobehav. Rev. 2021, 127, 917–927. [Google Scholar] [CrossRef]
- Lashgari, N.A.; Roudsari, N.M.; Shayan, M.; Niazi Shahraki, F.; Hosseini, Y.; Momtaz, S.; Abdolghaffari, A.H. IDO/Kynurenine; novel insight for treatment of inflammatory diseases. Cytokine 2023, 166, 156206. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Miller, B.J.; Stefanek, M.E.; Miller, A.H. Inflammation-induced activation of the indoleamine 2,3-dioxygenase pathway: Relevance to cancer-related fatigue. Cancer 2015, 121, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Giuliani, F. The role of inflammation in depression and fatigue. Front. Immunol. 2019, 10, 1696. [Google Scholar] [CrossRef]
- Fujigaki, H.; Yamamoto, Y.; Saito, K. L-Tryptophan-kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases: Focus on cell type differences. Neuropharmacology 2017, 112, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Dziurkowska, E.; Wesolowski, M. Cortisol as a biomarker of mental disorder severity. J. Clin. Med. 2021, 10, 5204. [Google Scholar] [CrossRef]
- Sordrager, F. Relationship Between Cortisol and Tryptophan 2,3-Dioxygenase in Health and Depression. Thesis, University of Groningen, Groningen, The Netherlands, 2014. Available online: https://umcg.studenttheses.ub.rug.nl/2526/ (accessed on 10 February 2025).
- Zhou, L.; Xiong, J.Y.; Chai, Y.Q.; Huang, L.; Tang, Z.Y.; Zhang, X.F.; Liu, B.; Zhang, J.T. Possible antidepressant mechanisms of omega-3 polyunsaturated fatty acids acting on the central nervous system. Front. Psychiatry 2022, 13, 933704. [Google Scholar] [CrossRef]
- Okuda, S.; Nishiyama, N.; Saito, H.; Katsuki, H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J. Neurochem. 2002, 70, 299–307. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative stress in depression: The link with the stress response, neuroinflammation, serotonin, neurogenesis and synaptic plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef]
- Klein, C.; Patte-Mensah, C.; Taleb, O.; Bourguignon, J.J.; Schmitt, M.; Bihel, F.; Maitre, M.; Mensah-Nyagan, A.G. The neuroprotector kynurenic acid increases neuronal cell survival through neprilysin induction. Neuropharmacology 2013, 70, 254–260. [Google Scholar] [CrossRef]
- Badawy, A.A.-B.; Dawood, S.; Bano, S. Kynurenine pathway of tryptophan metabolism in pathophysiology and therapy of major depressive disorder. World J. Psychiatry 2023, 13, 141–148. [Google Scholar] [CrossRef]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2022, 28, 3243–3256. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Bao, C.H.; Wu, Y.; Liang, S.H.; Wang, D.; Wu, L.Y.; Huang, Y.; Liu, H.R.; Wu, H.G. Tryptophan-kynurenine metabolism: A link between the gut and brain for depression in inflammatory bowel disease. J. Neuroinflamm. 2021, 18, 135. [Google Scholar] [CrossRef]
- Irum, N.; Afzal, T.; Faraz, M.H.; Aslam, Z.; Rasheed, F. The role of gut microbiota in depression: An analysis of the gut-brain axis. Front. Behav. Neurosci. 2023, 17, 1185522. [Google Scholar] [CrossRef] [PubMed]
- Borsini, A.; Alboni, S.; Horowitz, M.A.; Tojo, L.M.; Cannazza, G.; Su, K.P.; Pariante, C.M.; Zunszain, P.A. Rescue of IL-1β-induced reduction of human neurogenesis by omega-3 fatty acids and antidepressants. Brain Behav. Immun. 2017, 65, 230–238. [Google Scholar] [CrossRef]
- Carabelli, B.; Delattre, A.M.; Waltrick, A.P.F.; Araújo, G.; Suchecki, D.; Machado, R.B.; de Souza, L.E.R.; Zanata, S.M.; Zanoveli, J.M.; Ferraz, A.C. Fish-oil supplementation decreases Indoleamine-2,3-Dioxygenase expression and increases hippocampal serotonin levels in the LPS depression model. Behav. Brain Res. 2020, 390, 112675. [Google Scholar] [CrossRef]
- Wang, C.C.; Yang, C.J.; Wu, L.H.; Lin, H.C.; Wen, Z.H.; Lee, C.H. Eicosapentaenoic acid reduces indoleamine 2,3-dioxygenase 1 expression in tumor cells. Int. J. Med. Sci. 2018, 15, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.C.; Tung, C.Y.; Chen, H.E. Omega-3 polyunsaturated fatty acid supplementation in prevention and treatment of maternal depression: Putative mechanism and recommendation. J. Affect. Disord. 2018, 238, 47–61. [Google Scholar] [CrossRef]
- Liao, Y.; Xie, B.; Zhang, H.; He, Q.; Guo, L.; Subramaniapillai, M.; Fan, B.; Lu, C.; McIntyre, R.S. Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl. Psychiatry 2019, 9, 190. [Google Scholar] [CrossRef]
- Li, S.; Li, R.; Hu, X.; Zhang, Y.; Wang, D.; Gao, Y.; Wang, J.; Wang, Q.; Song, C.; Huang, S.; et al. Omega-3 supplementation improves depressive symptoms, cognitive function and niacin skin flushing response in adolescent depression: A randomized controlled clinical trial. J. Affect. Disord. 2024, 345, 394–403. [Google Scholar] [CrossRef]
- Serefko, A.; Jach, M.E.; Pietraszuk, M.; Świąder, M.; Świąder, K.; Szopa, A. Omega-3 polyunsaturated fatty acids in depression. Int. J. Mol. Sci. 2024, 25, 8675. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, S.; Manohar, K.; Shariff, A.; Kinattingal, N.; Wani, S.U.D.; Alshehri, S.; Imam, M.T.; Shakeel, F.; Krishna, K.L. Omega-3 fatty acids supplementation in the treatment of depression: An observational study. J. Pers. Med. 2023, 13, 224. [Google Scholar] [CrossRef]
- Onaolapo, M.C.; Alabi, O.D.; Akano, O.P.; Olateju, B.S.; Okeleji, L.O.; Adeyemi, W.J.; Ajayi, A.F. Lecithin and cardiovascular health: A comprehensive review. Egypt. Heart J. 2024, 76, 92. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of lecithin and other phosphoglycerides as used in cosmetics. Int. J. Toxicol. 2020, 39 (Suppl. S2), 5S–25S. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, E.; Obeid, R. Choline, neurological development and brain function: A systematic review focusing on the first 1000 days. Nutrients 2020, 12, 1731. [Google Scholar] [CrossRef]
- Sam, C.; Bordoni, B. Physiology, Acetylcholine. In StatPearls [Internet]; [Updated 10 April 2023]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557825/ (accessed on 15 February 2025).
- Jaiswal, A.; Dewani, D.; Reddy, L.S.; Patel, A. Choline supplementation in pregnancy: Current evidence and implications. Cureus. 2023, 15, e48538. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Dietary reference values for choline. EFSA J. 2016, 14, 4484. [Google Scholar] [CrossRef]
- Office of Dietary Supplements (ODS). Choline. Office of Dietary Supplements (ODS). [cited b.d.]. Available online: https://ods.od.nih.gov/factsheets/Choline-HealthProfessional/ (accessed on 1 March 2025).
- Obeid, R.; Schön, C.; Derbyshire, E.; Jiang, X.; Mellott, T.J.; Blusztajn, J.K.; Zeisel, S.H. A narrative review on maternal choline intake and liver function of the fetus and the infant; implications for research, policy, and practice. Nutrients 2024, 16, 260. [Google Scholar] [CrossRef]
- Taesuwan, S.; McDougall, M.Q.; Malysheva, O.V.; Bender, E.; Nevins, J.E.H.; Devapatla, S.; Vidavalur, R.; Caudill, M.A.; Klatt, K.C. Choline metabolome response to prenatal choline supplementation across pregnancy: A randomized controlled trial. FASEB J. 2021, 35, e22063. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Kounatidis, D.; Psallida, S.; Panagopoulos, F.; Stratigou, T.; Geladari, E.; Karampela, I.; Tsilingiris, D. The interplay between dietary choline and cardiometabolic disorders: A review of current evidence. Curr. Nutr. Rep. 2024, 13, 152–165. [Google Scholar] [CrossRef]
- Walsh, J.; Palandra, J.; Goihberg, E.; Deng, S.; Hurst, S.; Neubert, H. Analysis of β-nerve growth factor and its precursor during human pregnancy by immunoaffinity-liquid chromatography tandem mass spectrometry. Sci. Rep. 2023, 13, 9180. [Google Scholar] [CrossRef]
- Numakawa, T.; Kajihara, R. The role of brain-derived neurotrophic factor as an essential mediator in neuronal functions and the therapeutic potential of its mimetics for neuroprotection in neurologic and psychiatric disorders. Molecules 2025, 30, 848. [Google Scholar] [CrossRef]
- Christian, L.M.; Mitchell, A.M.; Gillespie, S.L.; Palettas, M. Serum brain-derived neurotrophic factor (BDNF) across pregnancy and postpartum: Associations with race, depressive symptoms, and low birth weight. Psychoneuroendocrinology 2016, 74, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yi, H.; Li, T.C.; Wang, Y.; Wang, H.; Chen, X. Role of vascular endothelial growth factor (VEGF) in human embryo implantation: Clinical implications. Biomolecules 2021, 11, 253. [Google Scholar] [CrossRef]
- Sferruzzi-Perri, A.N. Regulating needs: Exploring the role of insulin-like growth factor-2 signaling in materno-fetal resource allocation. Placenta 2018, 64, S16–S22. [Google Scholar] [CrossRef]
- Zuk, E.; Nikrandt, G.; Chmurzynska, A. Dietary choline intake in European and non-European populations: Current status and future trends—A narrative review. Nutr. J. 2024, 23, 68. [Google Scholar] [CrossRef] [PubMed]
- Alhajj, M.J.; Montero, N.; Yarce, C.J.; Salamanca, C.H. Lecithins from vegetable, land, and marine animal sources and their potential applications for cosmetic, food, and pharmaceutical sectors. Cosmetics 2020, 7, 87. [Google Scholar] [CrossRef]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Gundert-Remy, U.; Husøy, T.; et al. Safety of use of oat lecithin as a food additive. EFSA J. 2020, 18, 5969. [Google Scholar] [CrossRef]
- Kansakar, U.; Trimarco, V.; Mone, P.; Varzideh, F.; Lombardi, A.; Santulli, G. Choline supplements: An update. Front. Endocrinol. 2023, 14, 1148166. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Tomassoni, D.; Nittari, G.; Traini, E.; Amenta, F. Effects of choline containing phospholipids on the neurovascular unit: A review. Front. Cell. Neurosci. 2022, 16, 988759. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C.; Gant, N. The biochemistry of choline. In Magnetic Resonance Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2014; pp. 104–110. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Salem, M.A.; Mahdy, N.M.E.; Mahfouz, M.M. Lecithin. In Antioxidants Effects in Health; Elsevier: Amsterdam, The Netherlands, 2022; pp. 375–386. [Google Scholar] [CrossRef]
- Higley, M.J.; Picciotto, M.R. Neuromodulation by acetylcholine: Examples from schizophrenia and depression. Curr. Opin. Neurobiol. 2014, 29, 88–95. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Obayemi, A.; Wigestrand, M.B.; Fote, G.M.; Calarco, C.A.; Li, A.M.; Picciotto, M.R. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc. Natl. Acad. Sci. USA 2013, 110, 3573–3578. [Google Scholar] [CrossRef]
- Janowsky, D.S.; El-Yousef, K.M.; Davis, J.M. Acetylcholine and depression. Psychosom. Med. 1974, 36, 248–257. [Google Scholar] [CrossRef]
- Riley, C.A.; Renshaw, P.F. Brain choline in major depression: A review of the literature. Psychiatry Res. Neuroimaging 2018, 271, 142–153. [Google Scholar] [CrossRef]
- Dulawa, S.C.; Janowsky, D.S. Cholinergic regulation of mood: From basic and clinical studies to emerging therapeutics. Mol. Psychiatry 2018, 24, 694–709. [Google Scholar] [CrossRef]
- Hong, C.H.; Woo, J.I.; Suh, Y.H.; Park, C.-W.; Lee, C.K. Effect of ketamine on the acetylcholine concentration of various regions of rat brain. Seoul. Med. J. 1987, 28, 347–351. [Google Scholar]
- van der Spek, A.; Stewart, I.D.; Kühnel, B.; Pietzner, M.; Alshehri, T.; Gauß, F.; Hysi, P.G.; MahmoudianDehkordi, S.; Heinken, A.; Luik, A.I.; et al. Circulating metabolites modulated by diet are associated with depression. Mol. Psychiatry 2023, 28, 3874–3887. [Google Scholar] [CrossRef] [PubMed]
- van Lee, L.; Quah, P.L.; Saw, S.M.; Yap, F.K.P.; Godfrey, K.M.; Chong, Y.S.; Meaney, M.J.; Chen, H.; Chong, M.F.-F. Maternal choline status during pregnancy, but not that of betaine, is related to antenatal mental well-being: The growing up in Singapore toward healthy outcomes cohort. Depress. Anxiety 2017, 34, 877–887. [Google Scholar] [CrossRef]
- Mudimela, S.; Vishwanath, N.K.; Pillai, A.; Morales, R.; Marrelli, S.P.; Barichello, T.; Giridharan, V.V. Clinical significance and potential role of trimethylamine N-oxide in neurological and neuropsychiatric disorders. Drug Discov. Today 2022, 27, 103334. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Chen, X.; Zhang, Y.; Zhang, H.; Xie, P. Gut microbiota and its metabolites in depression: From pathogenesis to treatment. EBioMedicine 2023, 90, 104527. [Google Scholar] [CrossRef] [PubMed]
- Meinitzer, S.; Baranyi, A.; Holasek, S.; Schnedl, W.J.; Zelzer, S.; Mangge, H.; Herrmann, M.; Meinitzer, A.; Enko, D. Sex-specific associations of trimethylamine-N-oxide and zonulin with signs of depression in carbohydrate malabsorbers and nonmalabsorbers. Dis. Markers 2020, 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, Y.; Wang, H.; Wang, X. Trimethylamine-N-oxide enhances post-stroke depression progression via ROS-p38/MAPK signaling. Hum. Exp. Toxicol. 2024, 43. [Google Scholar] [CrossRef] [PubMed]
- Von Lewinski, D.; Enko, D.; Rotenhaeusler, H.P.; Amouzadeh-Ghadikolai, O.; Harpf, H.; Harpf, L.; Traninger, H.; Obermayer-Pietsch, B.; Von Lewinski, F.; Schweinzer, M.; et al. TMAO (trimethylamine N-oxide) as a potential biomarker of individual severe stress perception in posttraumatic stress disorder (PTSD)-vulnerable patients after acute myocardial infarction. Eur. Heart J. 2021, 42 (Suppl. S1), ehab724.1265. [Google Scholar] [CrossRef]


| Fatty Acid | Type | Symbol | Physiological Role | Brain Presence/Function | Association with Perinatal Depression |
|---|---|---|---|---|---|
| α-Linolenic Acid (ALA) | Omega-3 PUFA | 18:3n-3 | Precursor of EPA and DHA | Crosses BBB but quickly oxidized; low proportion in brain lipids | Deficiency limits DHA synthesis, indirectly increasing depression risk |
| Eicosapentaenoic Acid (EPA) | Omega-3 PUFA | 20:5n-3 | Anti-inflammatory, supports neurotransmission | Crosses BBB; limited accumulation | Higher EPA/DHA ratio (≥1.5) associated with improved mood; deficiency linked to depressive symptoms |
| Docosahexaenoic Acid (DHA) | Omega-3 PUFA | 22:6n-3 | Essential for neuronal membranes, synaptic function, neurogenesis | Major n-3 PUFA in grey matter; accumulates in fetal brain | Low maternal DHA linked to increased risk of antenatal and postpartum depression |
| Docosapentaenoic Acid (DPA) | Omega-3 PUFA | 22:5n-3 | Intermediate between EPA and DHA | Crosses BBB but rapidly oxidized | Limited evidence; may support DHA-related neuroprotection |
| Linoleic Acid (LA) | Omega-6 PUFA | 18:2n-6 | Precursor of arachidonic acid (AA) | Supports cell membrane integrity | Imbalance (excess omega-6) increases inflammation and depression risk |
| Arachidonic Acid (AA) | Omega-6 PUFA | 20:4n-6 | Inflammatory mediator, signal transduction | Major n-6 PUFA in brain grey matter | High AA/DHA ratio associated with impaired synaptic function and depressive symptoms |
| First Author (Year) | Ref. | Country/Setting | Population | Study Design | Key Findings |
|---|---|---|---|---|---|
| Mineur (2013) | [89] | USA | Mice | Preclinical in vivo | Higher acetylocholine levels in the hippocampus are linked to more anxiety- and depression-like behaviour; this effect is reversible with fluoxetine |
| Janowsky (1974) | [90] | USA | Adults | Clinical challenge experiment | Central cholinergic stymulation provoked depressive symptoms |
| Riley (2018) | [91] | USA | - | Narrative review | Most neuroimaging studies show elevated choline levels in major depressive disorder (MDD) |
| Dulawa (2018) | [92] | USA | - | Narrative review | Increased cholinergic activity linked to low mood; AchE inhibitors often worsen depressive symptoms |
| Hong (1987) | [93] | South Korea | Rats | Preclinical in vivo | Ketamine has the capacity to alter acetocholine levels |
| van der Spek (2023) | [94] | Multi-Country European Cohorts | Adults (n = 13,596) | Multi-cohort observational study | Altered diet-related metabolites in depression with elevated levels of lecithine and reduced SCFAs concentration |
| van Lee (2017) | [95] | Singapore (Gusto Cohort) | Pregnant women (n = 949) | Prospective cohort | Higher maternal choline linked to more antenatal depressive/anxiety symptoms |
| Mudimela (2022) | [96] | International | - | Narrative review | TMAO promotes neuroinflammation via oxidative stress and micoglial activation which can lead to psychiatric disoreders |
| Liu (2023) | [97] | International | - | Narrative review | Altered TMAO, SCFAs, kynurenine pathway activation linked to gut dysbosis with neuroinflammation and depression |
| Meinitzer (2020) | [98] | Austria | Adults (n = 251) | Cross-sectional observational | Increased TMAO linked to depressive symptoms; zonulin associations were sex-specific |
| Hu (2024) | [99] | China | Rodent model; cellular assays | Preclinical in vivo | TMAO expression aggreviated post-stroke depression, increased brain blood barrier (BBB) permeability and decreased neutrophic signalling |
| Von Lewinski (2021) | [100] | Austria | Myocardial infarction (MI) patients (n = 52) | Observational cohort | Higher TMAO after myocardial infarction linked to severe perceived stress; potential stress biomarker |
| Aspect | Type of Evidence | Strengh of Evidence | Main Limitations |
|---|---|---|---|
| Omega-3 PUFAS and Perinatal Depression | Randomized controlled trials, cohort studies | Moderate to strong |
|
| Lecithin and Choline in Perinatal Mood Regulation | Observational and animal studies | Weak to moderate |
|
| Inflammatory and Kynurenine Pathways | Mechanistic and clinical studies | Moderate |
|
| Supplementation Efficacy (EPA vs. DHA Ratio) | Meta-analyses, clinical trials | Moderate |
|
| Overall Lipidome–Mood Associations | Integrative reviews, metabolomic studies | Moderate |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ładno, D.; Nowak, B.; Palka, A.; Strzelecki, D.; Gawlik-Kotelnicka, O. Selected Lipidome Components and Their Association with Perinatal Depression. Nutrients 2025, 17, 3590. https://doi.org/10.3390/nu17223590
Ładno D, Nowak B, Palka A, Strzelecki D, Gawlik-Kotelnicka O. Selected Lipidome Components and Their Association with Perinatal Depression. Nutrients. 2025; 17(22):3590. https://doi.org/10.3390/nu17223590
Chicago/Turabian StyleŁadno, Dominika, Beata Nowak, Aleksandra Palka, Dominik Strzelecki, and Oliwia Gawlik-Kotelnicka. 2025. "Selected Lipidome Components and Their Association with Perinatal Depression" Nutrients 17, no. 22: 3590. https://doi.org/10.3390/nu17223590
APA StyleŁadno, D., Nowak, B., Palka, A., Strzelecki, D., & Gawlik-Kotelnicka, O. (2025). Selected Lipidome Components and Their Association with Perinatal Depression. Nutrients, 17(22), 3590. https://doi.org/10.3390/nu17223590







