Temporal Patterns of Eating and Diet Composition of Night Shift Workers Are Influenced More by Shift Type than by Chronotype
Abstract
1. Introduction
2. Materials and Methods
2.1. Work Diary
2.2. Sleep Diary
2.3. Food Diary
2.4. Chronotype Questionnaire
2.5. Statistical Analysis
3. Results
| MS | 1stNS | SNS | 1stDONS | DO | |
|---|---|---|---|---|---|
| n | 43 | 93 | 97 | 97 | 108 |
| Female | 14 (33%) | 52 (56%) | 56 (58%) | 56 (58%) | 61 (56%) |
| Age (years) | 45.0 ± 9.8 | 47.2 ± 10.2 | 47.5 ± 10.0 | 47.4 ± 9.7 | 46.0 ± 10.3 |
| BMI (kg/m2) | 33.2 ± 4.0 | 33.7 ± 5.1 | 33.9 ± 5.4 | 33.8 ± 5.8 | 34.1 ± 5.9 |
| Work | |||||
| Work start time (hh:mm) | 08:29 ± 00:13 a | 19:55 ± 00:12 b | 20:00 ± 00:12 b | - | - |
| Work end time (hh:mm) | 17:38 ± 00:11 a | 06:49 ± 00:09 b | 06:58 ± 00:08 b | - | - |
| Work duration (hh:mm) | 09:21 ± 00:15 a | 10:52 ± 00:12 b | 10:57 ± 00:11 b | - | - |
| Sleep | |||||
| Wake up time (hh:mm) * | 06:00 ± 00:19 a | 08:51 ± 00:16 b | 13:57 ± 00:14 c | 12:56 ± 00:16 d | 08:04 ± 00:13 e |
| Sleep onset (hh:mm) # | 23:26 ± 00:19 a | 08:00 ± 00:16 b | 08:53 ± 00:13 c | 23:48 ± 00:16 a | 23:35 ± 00:12 a |
| Wake window duration (hh:mm) ^ | 17:21 ± 00:18 a | 23:14 ± 00:17 b | 18:49 ± 00:13 c | 10:56 ± 00:16 d | 15:46 ± 00:12 e |
3.1. Day Type
3.2. Chronotype
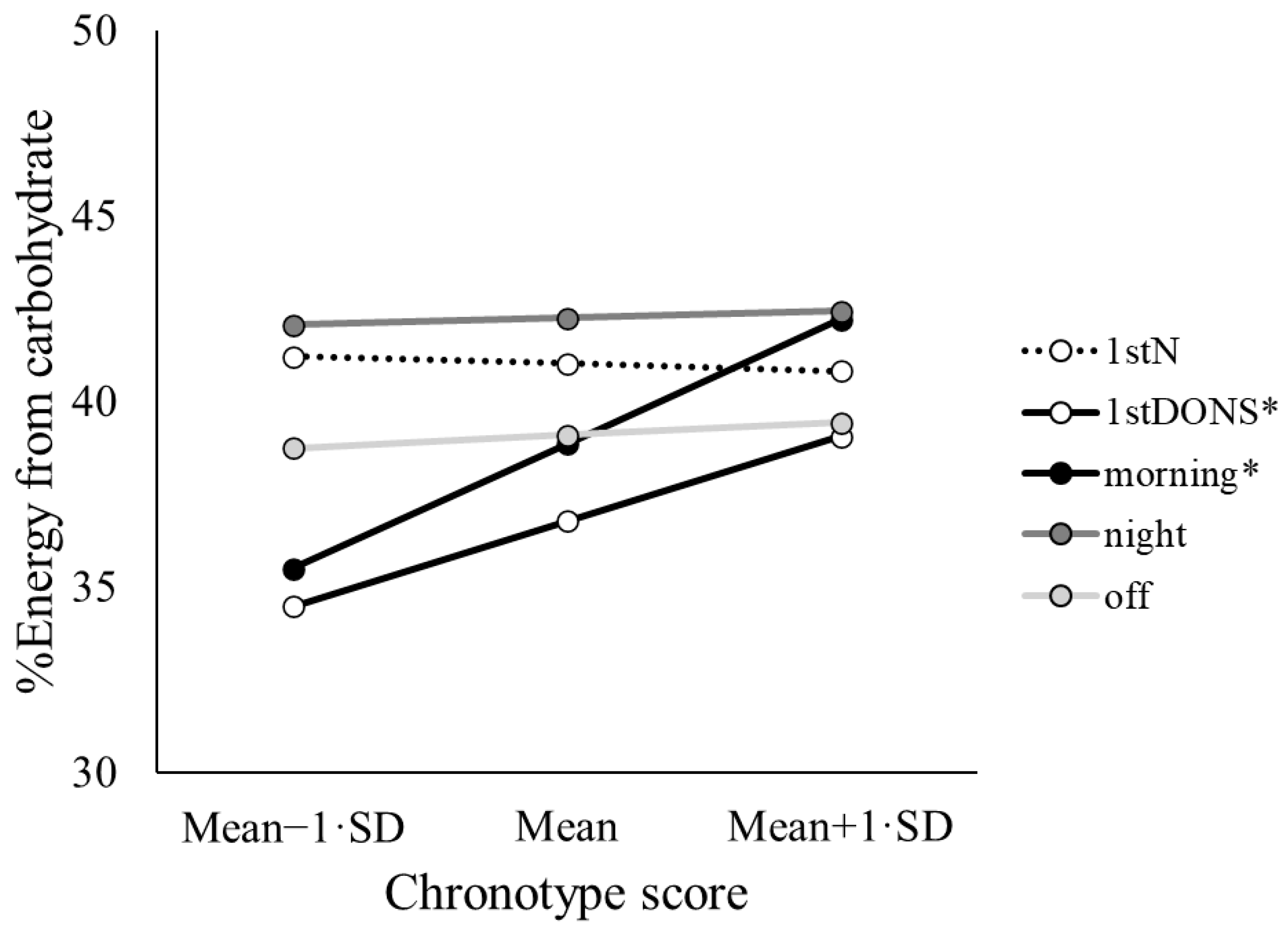
3.3. Day Type by Chronotype
4. Discussion
4.1. Night-Time Energy Loading, Implications, and Considerations
4.2. Nutrient Intake in the Context of Shift Type
4.3. Considering Shift Worker Chronotype
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnston, J.D. Physiological responses to food intake throughout the day. Nutr. Res. Rev. 2014, 27, 107–118. [Google Scholar] [CrossRef]
- Shaw, E.; Dorrian, J.; Coates, A.M.; Leung, G.K.W.; Davis, R.; Rosbotham, E.; Warnock, R.; Huggins, C.E.; Bonham, M.P. Temporal pattern of eating in night shift workers. Chronobiol. Int. 2019, 36, 1613–1625. [Google Scholar] [CrossRef]
- de Assis, M.A.; Kupek, E.; Nahas, M.V.; Bellisle, F. Food intake and circadian rhythms in shift workers with a high workload. Appetite 2003, 40, 175–183. [Google Scholar] [CrossRef]
- Kosmadopoulos, A.; Kervezee, L.; Boudreau, P.; Gonzales-Aste, F.; Vujovic, N.; Scheer, F.; Boivin, D.B. Effects of shift work on the eating behavior of police officers on patrol. Nutrients 2020, 12, 999. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J.; Yang, J.N.; Garcia, J.I.; Myers, S.; Bozzi, I.; Wang, W.; Buxton, O.M.; Shea, S.A.; Scheer, F.A. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci. USA 2015, 112, E2225–E2234. [Google Scholar] [CrossRef]
- Gooley, J.J.; Chua, E.C. Diurnal regulation of lipid metabolism and applications of circadian lipidomics. J. Genet. Genom. 2014, 41, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Feng, W.; Wang, F.; Li, P.; Li, Z.; Li, M.; Tse, G.; Vlaanderen, J.; Vermeulen, R.; Tse, L.A. Meta-analysis on shift work and risks of specific obesity types. Obes. Rev. 2018, 19, 28–40. [Google Scholar] [CrossRef]
- Shan, Z.; Li, Y.; Zong, G.; Guo, Y.; Li, J.; Manson, J.E.; Hu, F.B.; Willett, W.C.; Schernhammer, E.S.; Bhupathiraju, S.N. Rotating night shift work and adherence to unhealthy lifestyle in predicting risk of type 2 diabetes: Results from two large US cohorts of female nurses. BMJ 2018, 363, k4641. [Google Scholar] [CrossRef]
- Torquati, L.; Mielke, G.I.; Brown, W.J.; Kolbe-Alexander, T. Shift work and the risk of cardiovascular disease. A systematic review and meta-analysis including dose-response relationship. Scand. J. Work. Environ. Health 2018, 44, 229–238. [Google Scholar] [CrossRef]
- Almoosawi, S.; Vingeliene, S.; Gachon, F.; Voortman, T.; Palla, L.; Johnston, J.D.; Van Dam, R.M.; Darimont, C.; Karagounis, L.G. Chronotype: Implications for epidemiologic studies on chrono-nutrition and cardiometabolic health. Adv. Nutr. 2019, 10, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Shibata, S. Chronobiology and nutrition. Neuroscience 2013, 253, 78–88. [Google Scholar] [CrossRef]
- Phoi, Y.Y.; Rogers, M.; Bonham, M.P.; Dorrian, J.; Coates, A.M. A scoping review of chronotype and temporal patterns of eating of adults: Tools used, findings, and future directions. Nutr. Res. Rev. 2021, 35, 112–135. [Google Scholar] [CrossRef] [PubMed]
- Phoi, Y.Y.; Bonham, M.P.; Rogers, M.; Dorrian, J.; Coates, A.M. Content validation of a chrononutrition questionnaire for the general and shift work populations: A Delphi study. Nutrients 2021, 13, 4087. [Google Scholar] [CrossRef]
- Vetter, C. Circadian disruption: What do we actually mean? Eur. J. Neurosci. 2020, 51, 531–550. [Google Scholar] [CrossRef] [PubMed]
- Mazri, F.H.; Manaf, Z.A.; Shahar, S.; Mat Ludin, A.F. The association between chronotype and dietary pattern among adults: A scoping review. Int. J. Environ. Res. Public Health 2019, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, C.; Münch, M.; Kruger, R. Chronotype differences in body composition, dietary intake and eating behavior outcomes: A scoping systematic review. Adv. Nutr. 2022, 13, 2357–2405. [Google Scholar] [CrossRef]
- Merikanto, I.; Lahti, T.; Puolijoki, H.; Vanhala, M.; Peltonen, M.; Laatikainen, T.; Vartiainen, E.; Salomaa, V.; Kronholm, E.; Partonen, T. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiol. Int. 2013, 30, 470–477. [Google Scholar] [CrossRef]
- Juda, M.; Vetter, C.; Roenneberg, T. Chronotype modulates sleep duration, sleep quality, and social jet lag in shift-workers. J. Biol. Rhythm. 2013, 28, 141–151. [Google Scholar] [CrossRef]
- van de Ven, H.A.; van der Klink, J.J.; Vetter, C.; Roenneberg, T.; Gordijn, M.; Koolhaas, W.; de Looze, M.P.; Brouwer, S.; Bültmann, U. Sleep and need for recovery in shift workers: Do chronotype and age matter? Ergonomics 2016, 59, 310–324. [Google Scholar] [CrossRef]
- Dickerman, B.A.; Markt, S.C.; Koskenvuo, M.; Hublin, C.; Pukkala, E.; Mucci, L.A.; Kaprio, J. Sleep disruption, chronotype, shift work, and prostate cancer risk and mortality: A 30-year prospective cohort study of Finnish twins. Cancer Causes Control 2016, 27, 1361–1370. [Google Scholar] [CrossRef]
- Hansen, J.; Lassen, C.F. Nested case-control study of night shift work and breast cancer risk among women in the Danish military. Occup. Environ. Med. 2012, 69, 551–556. [Google Scholar] [CrossRef]
- Vetter, C.; Devore, E.E.; Ramin, C.A.; Speizer, F.E.; Willett, W.C.; Schernhammer, E.S. Mismatch of sleep and work timing and risk of type 2 diabetes. Diabetes Care 2015, 38, 1707–1713. [Google Scholar] [CrossRef]
- Vetter, C.; Fischer, D.; Matera, J.L.; Roenneberg, T. Aligning work and circadian time in shift workers improves sleep and reduces circadian disruption. Curr. Biol. 2015, 25, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Kervezee, L.; Gonzales-Aste, F.; Boudreau, P.; Boivin, D.B. The relationship between chronotype and sleep behavior during rotating shift work: A field study. Sleep 2021, 44, zsaa225. [Google Scholar] [CrossRef]
- Reiter, A.M.; Roach, G.D.; Sargent, C. No effect of chronotype on hunger or snack consumption during a night shift with acute sleep deprivation. Nutrients 2022, 14, 1324. [Google Scholar] [CrossRef]
- Yoshizaki, T.; Komatsu, T.; Tada, Y.; Hida, A.; Kawano, Y.; Togo, F. Association of habitual dietary intake with morningness-eveningness and rotating shift work in Japanese female nurses. Chronobiol. Int. 2018, 35, 392–404. [Google Scholar] [CrossRef]
- Yoshizaki, T.; Kawano, Y.; Noguchi, O.; Onishi, J.; Teramoto, R.; Sunami, A.; Yokoyama, Y.; Tada, Y.; Hida, A.; Togo, F. Association of eating behaviours with diurnal preference and rotating shift work in Japanese female nurses: A cross-sectional study. BMJ Open 2016, 6, e011987. [Google Scholar] [CrossRef]
- Faulkner, R.; Rangel, T.; Penders, R.A.; Saul, T.; Bindler, R.; Miller, L.; Wilson, M. Differences in nutritional profile by chronotype among 12-h day shift and night shift nurses. Chronobiol. Int. 2024, 41, 17–28. [Google Scholar] [CrossRef]
- Rogers, M.; Coates, A.; Huggins, C.E.; Dorrian, J.; Clark, A.B.; Davis, C.; Leung, G.K.; Davis, R.; Phoi, Y.Y.; Kellow, N.J.; et al. Study protocol for the Shifting Weight using Intermittent Fasting in night shift workers (SWIFt) study: A three-arm randomised controlled trial comparing three weight loss strategies in night shift workers with obesity. BMJ Open 2022, 12, e060520. [Google Scholar] [CrossRef] [PubMed]
- Foodworks.online. Foodworks 10 Professional, version 10.0; Xyris Pty Ltd.: Brisbane, Australia, 2019. [Google Scholar]
- Smith, C.S.; Reilly, C.; Midkiff, K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J. Appl. Psychol. 1989, 74, 728–738. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand; The Australian Government Department of Health and Ageing: Canberra, Australia, 2006. [Google Scholar]
- Clark, A.B.; Coates, A.M.; Davidson, Z.E.; Bonham, M.P. Dietary patterns under the influence of rotational shift work schedules: A systematic review and meta-analysis. Adv. Nutr. 2023, 14, 295–316. [Google Scholar] [CrossRef]
- Ruddick-Collins, L.C.; Morgan, P.J.; Johnstone, A.M. Mealtime: A circadian disruptor and determinant of energy balance? J. Neuroendocrinol. 2020, 32, e12886. [Google Scholar] [CrossRef]
- Manoogian, E.N.C.; Zadourian, A.; Lo, H.C.; Gutierrez, N.R.; Shoghi, A.; Rosander, A.; Pazargadi, A.; Ormiston, C.K.; Wang, X.; Sui, J.; et al. Feasibility of time-restricted eating and impacts on cardiometabolic health in 24-h shift workers: The Healthy Heroes randomized control trial. Cell Metab. 2022, 34, 1442–1456.e1447. [Google Scholar] [CrossRef]
- Leung, G.K.W.; Davis, R.; Huggins, C.E.; Ware, R.S.; Bonham, M.P. Does rearranging meal times at night improve cardiovascular risk factors? An Australian pilot randomised trial in night shift workers. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1890–1902. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.S.; Silva, C.M.; Silva, A.T.F.; Santos, L.L.D.; de Paiva Maia, Y.C.; Pedrazzoli, M.; Wright, K.P., Jr.; Crispim, C.A. Influence of fasting during the night shift on next day eating behavior, hunger, and glucose and insulin levels: A randomized, three-condition, crossover trial. Eur. J. Nutr. 2023, 62, 1281–1293. [Google Scholar] [CrossRef]
- Gupta, C.C.; Coates, A.M.; Dorrian, J.; Banks, S. The factors influencing the eating behaviour of shiftworkers: What, when, where and why. Ind. Health 2019, 57, 419–453. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.C.; Centofanti, S.; Dorrian, J.; Coates, A.; Stepien, J.M.; Kennaway, D.; Wittert, G.; Heilbronn, L.; Catcheside, P.; Noakes, M.; et al. Altering meal timing to improve cognitive performance during simulated nightshifts. Chronobiol. Int. 2019, 36, 1691–1713. [Google Scholar] [CrossRef]
- Gupta, C.C.; Centofanti, S.; Dorrian, J.; Coates, A.M.; Stepien, J.M.; Kennaway, D.; Wittert, G.; Heilbronn, L.; Catcheside, P.; Noakes, M.; et al. Subjective hunger, gastric upset, and sleepiness in response to altered meal siming during simulated shiftwork. Nutrients 2019, 11, 1352. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics. Food and Nutrients. 2023. Available online: https://www.abs.gov.au/statistics/health/food-and-nutrition/food-and-nutrients/latest-release (accessed on 10 November 2025).
- Boini, S.; Bourgkard, E.; Ferrières, J.; Esquirol, Y. What do we know about the effect of night-shift work on cardiovascular risk factors? An umbrella review. Front. Public Health 2022, 10, 1034195. [Google Scholar] [CrossRef]
- Marot, L.P.; Rosa, D.E.; Lopes, T.; Moreno, C.R.C.; Crispim, C.A. Eating duration throughout a rotating shift schedule: A case study. J. Am. Coll. Nutr. 2021, 40, 624–631. [Google Scholar] [CrossRef]
- Stutz, B.; Krueger, B.; Goletzke, J.; Jankovic, N.; Alexy, U.; Herder, C.; Dierkes, J.; Berg-Beckhoff, G.; Jakobsmeyer, R.; Reinsberger, C.; et al. Glycemic response to meals with a high glycemic index differs between morning and evening: A randomized cross-over controlled trial among students with early or late chronotype. Eur. J. Nutr. 2024, 63, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Mazri, F.H.; Manaf, Z.A.; Shahar, S.; Mat Ludin, A.F.; Karim, N.A.; Hazwari, N.D.D.; Kek, Q.W.; Abdul Basir, S.M.; Arifin, A. Do temporal eating patterns differ in healthy versus unhealthy overweight/obese individuals? Nutrients 2021, 13, 4121. [Google Scholar] [CrossRef]
- Juda, M.; Vetter, C.; Roenneberg, T. The Munich ChronoType Questionnaire for Shift-workers (MCTQShift). J. Biol. Rhythms 2013, 28, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Leech, R.M.; Worsley, A.; Timperio, A.; McNaughton, S.A. Characterizing eating patterns: A comparison of eating occasion definitions. Am. J. Clin. Nutr. 2015, 102, 1229–1237. [Google Scholar] [CrossRef] [PubMed]

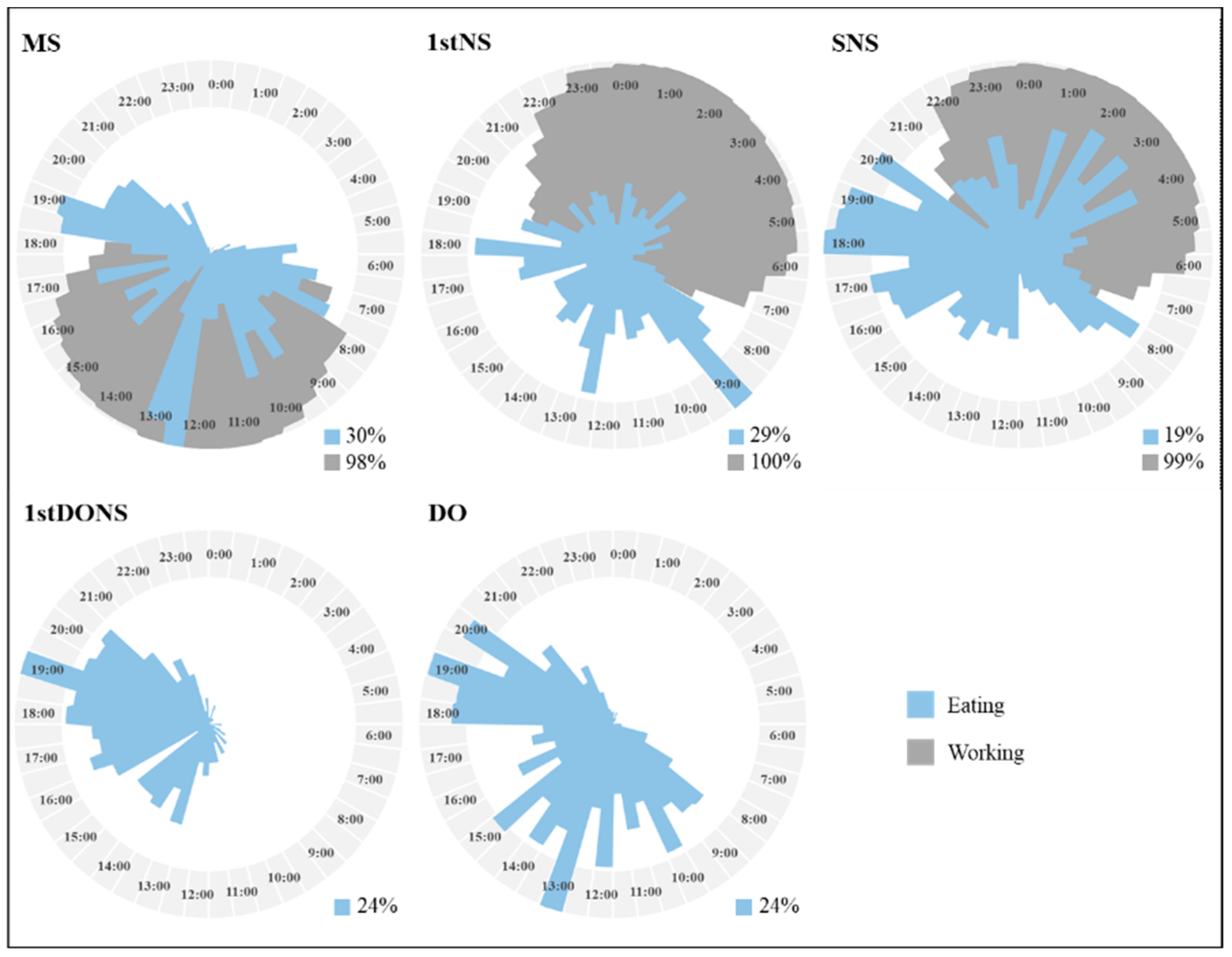
| Day Type | Chronotype | Day Type × Chronotype | ||||
|---|---|---|---|---|---|---|
| F/Wald χ2(df) | p | F/Wald χ2(df) | p | F/Wald χ2(df) | p | |
| Temporal patterns of eating | ||||||
| FEO | 11.03(4,730) | <0.001 | 13.22(1,130) | <0.001 | 1.94(4,729) | 0.102 |
| LEO | 49.03(4,686) | <0.001 | 6.86(1,123) | 0.010 | 0.96(4,687) | 0.428 |
| DEW | 10.41(4,700) | <0.001 | 0.03(1,134) | 0.863 | 1.54(4,696) | 0.188 |
| LarEO | 2.80(4,708) | 0.025 | 3.99(1,129) | 0.048 | 0.692(4,703) | 0.597 |
| Meal frequency # | 29.54(4) | <0.001 | 3.17(1) | 0.075 | 7.30(4) | 0.121 |
| Snack frequency # | 8.65(4) | 0.070 | 2.17(1) | 0.141 | 5.06(4) | 0.281 |
| Total eating frequency # | 15.94(4) | 0.003 | 0.33(1) | 0.568 | 7.02(4) | 0.135 |
| Diet composition | ||||||
| Energy (kJ) | 2.88(4,726) | 0.022 | 0.12(1,128) | 0.728 | 1.13(4,727) | 0.342 |
| Protein (%E) | 2.19(4,732) | 0.069 | 1.28(1,132) | 0.259 | 2.09(4,731) | 0.081 |
| Fat (%E) | 1.63(4,733) | 0.164 | 7.18(1,134) | 0.008 | 1.19(4,732) | 0.313 |
| Sat fat (%E) | 0.5(4,727) | 0.736 | 5.91(1,132) | 0.016 | 0.46(4,727) | 0.762 |
| Carbohydrate (%E) | 4.16(4,730) | 0.002 | 3.42(1,134) | 0.067 | 2.76(4,731) | 0.027 |
| Fibre (g) | 4.75(4,724) | <0.001 | 0.19(1,128) | 0.666 | 2.29(4,725) | 0.058 |
| Alcohol (yes/no) | 1.71(4) | 0.790 | 0.05(1) | 0.832 | 1.64(4) | 0.802 |
| MS a | 1stNS b | SNS c | 1stDONS d | DO e | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EMM | SE | EMM | SE | EMM | SE | EMM | SE | EMM | SE | |
| Temporal patterns of eating | ||||||||||
| FEO (hh:mm) | 7:37 a | 0:22 | 10:32 be | 00:18 | 14:33 cd | 00:14 | 14:23 cd | 00:17 | 09:57 be | 00:13 |
| LEO (hh:mm) | 20:40 ade | 00:27 | 03:21 b | 00:21 | 04:54 c | 00:18 | 20:34 ade | 00:21 | 20:09 ade | 00:17 |
| DEW (hh:mm) | 12:56 ac | 00:31 | 16:15 b | 00:25 | 13:42 ac | 00:20 | 06:08 d | 00:24 | 10:24 e | 00:18 |
| LarEO (hh:mm) | 16:13 ae | 00:33 | 17:42 bd | 00:27 | 20:09 c | 00:20 | 18:05 bd | 00:26 | 16:35 ae | 00:18 |
| Meal frequency | 2.8 ab | 0.1 | 2.9 ab | 0.1 | 2.2 c | 0.1 | 1.7 d | 0.1 | 2.6 e | 0.0 |
| Snack frequency | 2.4 | 0.3 | 2.1 | 0.2 | 2.2 | 0.1 | 1.5 | 0.1 | 1.9 | 0.1 |
| Total eating frequency | 5.2 ab | 0.3 | 5.1 ab | 0.2 | 4.4 ce | 0.1 | 3.2 d | 0.2 | 4.4 ce | 0.1 |
| Diet composition | ||||||||||
| Energy (kJ) | 9152.7 abe,ac | 446.1 | 9596.6 abe | 362.2 | 8744.0 ac | 308.6 | 7492.3 d | 351.7 | 9599.2 abe | 287.1 |
| Protein (%E) | 20.7 | 0.8 | 20.3 | 0.6 | 20.2 | 0.5 | 18.9 | 0.6 | 18.5 | 0.5 |
| Fat (%E) | 35.5 | 1.1 | 35.5 | 0.9 | 34.5 | 0.7 | 37.7 | 0.9 | 36.0 | 0.7 |
| Sat fat (%E) | 14.4 | 0.6 | 13.8 | 0.5 | 13.4 | 0.4 | 14.1 | 0.5 | 14.1 | 0.4 |
| Carbohydrate (%E) | 38.8 ab,ad,ae | 1.3 | 41.0 ab,bc,be | 1.0 | 42.2 bc | 0.9 | 36.8 ad | 1.0 | 39.1 ae,be | 0.8 |
| Fibre (g) | 20.2 ace | 1.3 | 24.9 b | 1.1 | 20.7 ace | 0.9 | 16.2 d | 1.0 | 21.2 ace | 0.8 |
| Alcohol (% yes) ^ | 20% | 8.3% | 6.9% | 27.9% | 33.3% | |||||
| Mean − 1·SD | Mean | Mean + 1·SD | ||||
|---|---|---|---|---|---|---|
| EMM | SE | EMM | SE | EMM | SE | |
| Temporal patterns of eating | ||||||
| FEO (hh:mm) | 12:04 | 00:15 | 11:24 | 00:10 | 10:45 | 00:14 |
| LEO (hh:mm) | 14:33 | 00:20 | 13:55 | 00:14 | 13:17 | 00:19 |
| DEW (hh:mm) | 11:51 | 00:19 | 11:53 | 00:13 | 11:56 | 00:18 |
| LarEO (hh:mm) | 18:11 | 00:19 | 17:45 | 00:13 | 17:18 | 00:18 |
| Meal frequency | 2.3 | 0.1 | 2.4 | 0.0 | 2.5 | 0.0 |
| Snack frequency | 2.2 | 0.1 | 2.0 | 0.1 | 1.9 | 0.1 |
| Total eating frequency | 4.5 | 0.2 | 4.4 | 0.1 | 4.4 | 0.2 |
| Diet composition | ||||||
| Energy (kJ) | 9002.6 | 348.8 | 8917.0 | 237.1 | 8831.3 | 333.8 |
| Protein (%E) | 19.3 | 0.6 | 19.7 | 0.4 | 20.2 | 0.5 |
| Fat (%E) | 37.3 | 0.8 | 35.8 | 0.5 | 34.4 | 0.7 |
| Sat fat (%E) | 14.8 | 0.5 | 14.0 | 0.3 | 13.1 | 0.5 |
| Carbohydrate (%E) | 38.4 | 0.9 | 39.6 | 0.6 | 40.8 | 0.9 |
| Fibre (g) | 20.3 | 1.0 | 20.6 | 0.7 | 20.9 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phoi, Y.Y.; Dorrian, J.; Rogers, M.; Leung, G.K.W.; Davis, R.; Clark, A.B.; Davis, C.; Bonham, M.P.; Coates, A.M. Temporal Patterns of Eating and Diet Composition of Night Shift Workers Are Influenced More by Shift Type than by Chronotype. Nutrients 2025, 17, 3561. https://doi.org/10.3390/nu17223561
Phoi YY, Dorrian J, Rogers M, Leung GKW, Davis R, Clark AB, Davis C, Bonham MP, Coates AM. Temporal Patterns of Eating and Diet Composition of Night Shift Workers Are Influenced More by Shift Type than by Chronotype. Nutrients. 2025; 17(22):3561. https://doi.org/10.3390/nu17223561
Chicago/Turabian StylePhoi, Yan Yin, Jillian Dorrian, Michelle Rogers, Gloria K. W. Leung, Rochelle Davis, Angela B. Clark, Corinne Davis, Maxine P. Bonham, and Alison M. Coates. 2025. "Temporal Patterns of Eating and Diet Composition of Night Shift Workers Are Influenced More by Shift Type than by Chronotype" Nutrients 17, no. 22: 3561. https://doi.org/10.3390/nu17223561
APA StylePhoi, Y. Y., Dorrian, J., Rogers, M., Leung, G. K. W., Davis, R., Clark, A. B., Davis, C., Bonham, M. P., & Coates, A. M. (2025). Temporal Patterns of Eating and Diet Composition of Night Shift Workers Are Influenced More by Shift Type than by Chronotype. Nutrients, 17(22), 3561. https://doi.org/10.3390/nu17223561







