Mapping Research Trends in Frailty and Nutrition: A Combined Bibliometric and Structured Review (2000–2024)
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion Criteria and Exclusion Criteria
2.2.1. Inclusion Criteria
- (1)
- Study design: employed a well-defined observational or interventional design. Priority was given to randomized controlled trials (RCTs), prospective cohort studies, and large-scale cross-sectional studies in that order.
- (2)
- Participants: community-dwelling or institutionalized adults with a mean age of ≥50 years, who were classified as pre-frail or frail according to any validated criteria.
- (3)
- Exposure/intervention: for observational studies, a clearly defined nutritional exposure (e.g., intake levels of specific nutrients, food groups, or dietary patterns) was required. For interventional studies, a clearly defined nutritional intervention (e.g., supplementation) with an appropriate control or comparison group was required.
- (4)
- Outcome measures: required to report results from at least one validated frailty assessment instrument. The accepted instruments included, but were not limited to: Fried Frailty Phenotype (FP), Frailty Index (FI), Tilburg Frailty Indicator (TFI), Clinical Frailty Scale (CFS), Objective measures (grip strength, SPPB), Electronic Frailty Index, FRAIL scale, Groningen Frailty Indicator, Edmonton Frailty Scale, Frailty Risk Score, PRISMA-7, Hospital Frailty Risk Score, etc.
- (5)
- Publication status: peer-reviewed original research articles or reviews published in English between 1 January 2000 and 31 December 2024.
2.2.2. Exclusion Criteria
- (1)
- Methodological concerns: lack of a control or comparison group, insufficient sample size (e.g., n < 100 for cross-sectional studies; n < 30 per group for interventional trials), or inadequate statistical adjustment for key confounders (as a minimum, age and sex; ideally also including total energy intake, comorbidities, and socioeconomic status).
- (2)
- Population characteristics: individuals with terminal illnesses (e.g., metastatic cancer, end-stage renal disease), major organ failure (NYHA Class IV heart failure, severe COPD), or those in acute, unstable hospitalization settings.
- (3)
- Intervention issues: multi-component interventions (e.g., combined exercise, cognitive training, and nutrition) where the effects of the nutritional component could not be isolated for analysis.
- (4)
- Publication type: abstracts, conference proceedings, editorials, letters, case reports, and non-English publications.
2.3. Data Analysis
2.3.1. Bibliometric Software and Specific Parameters
3. Results
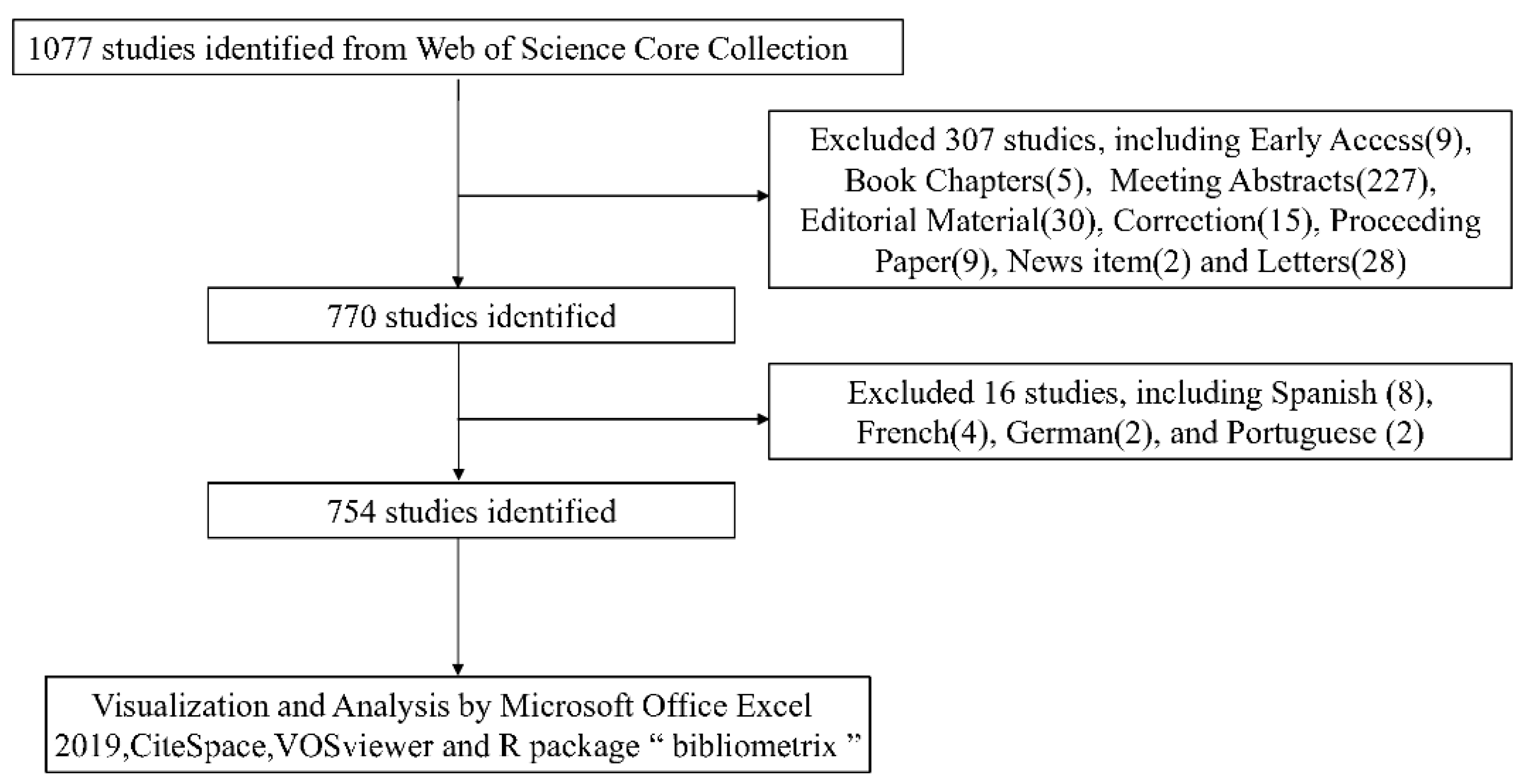
3.1. The Literature Search and Selection
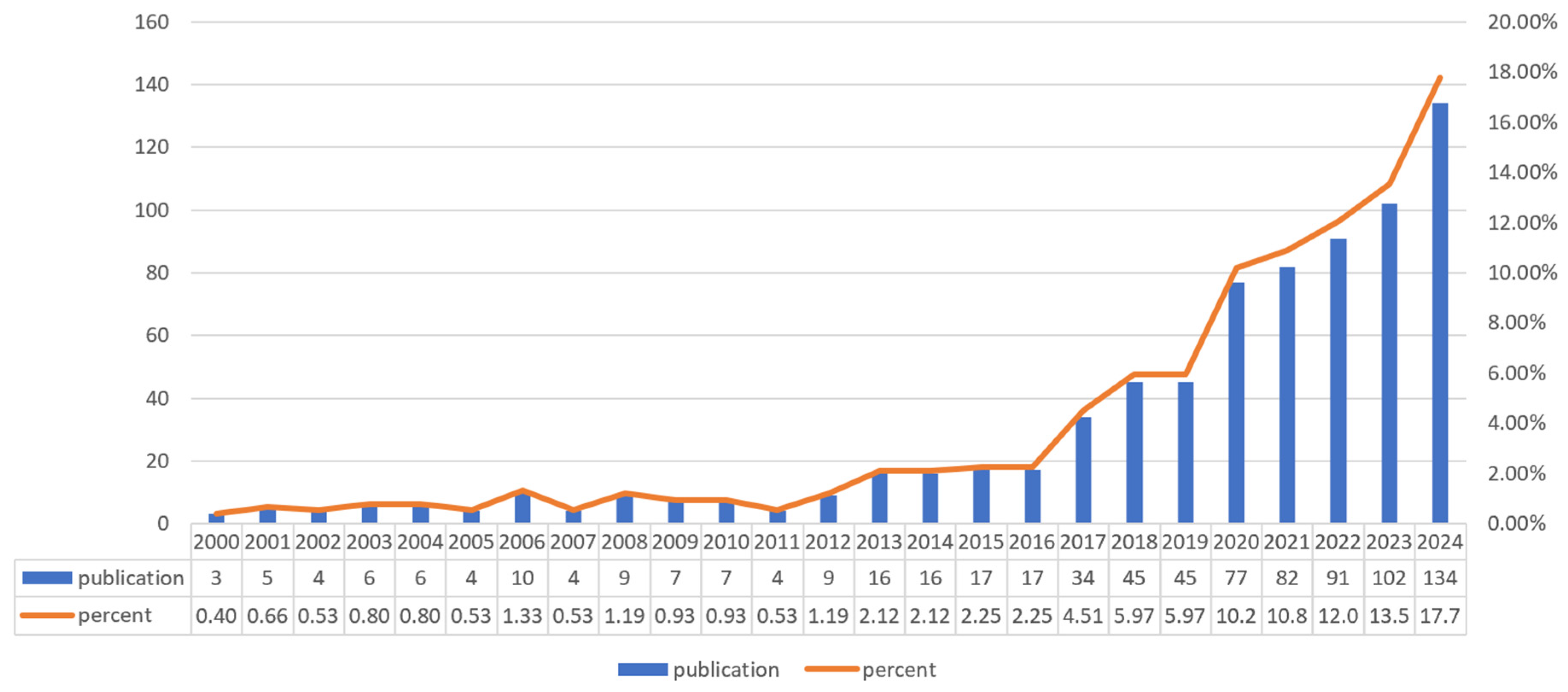
3.2. Analysis of Publication
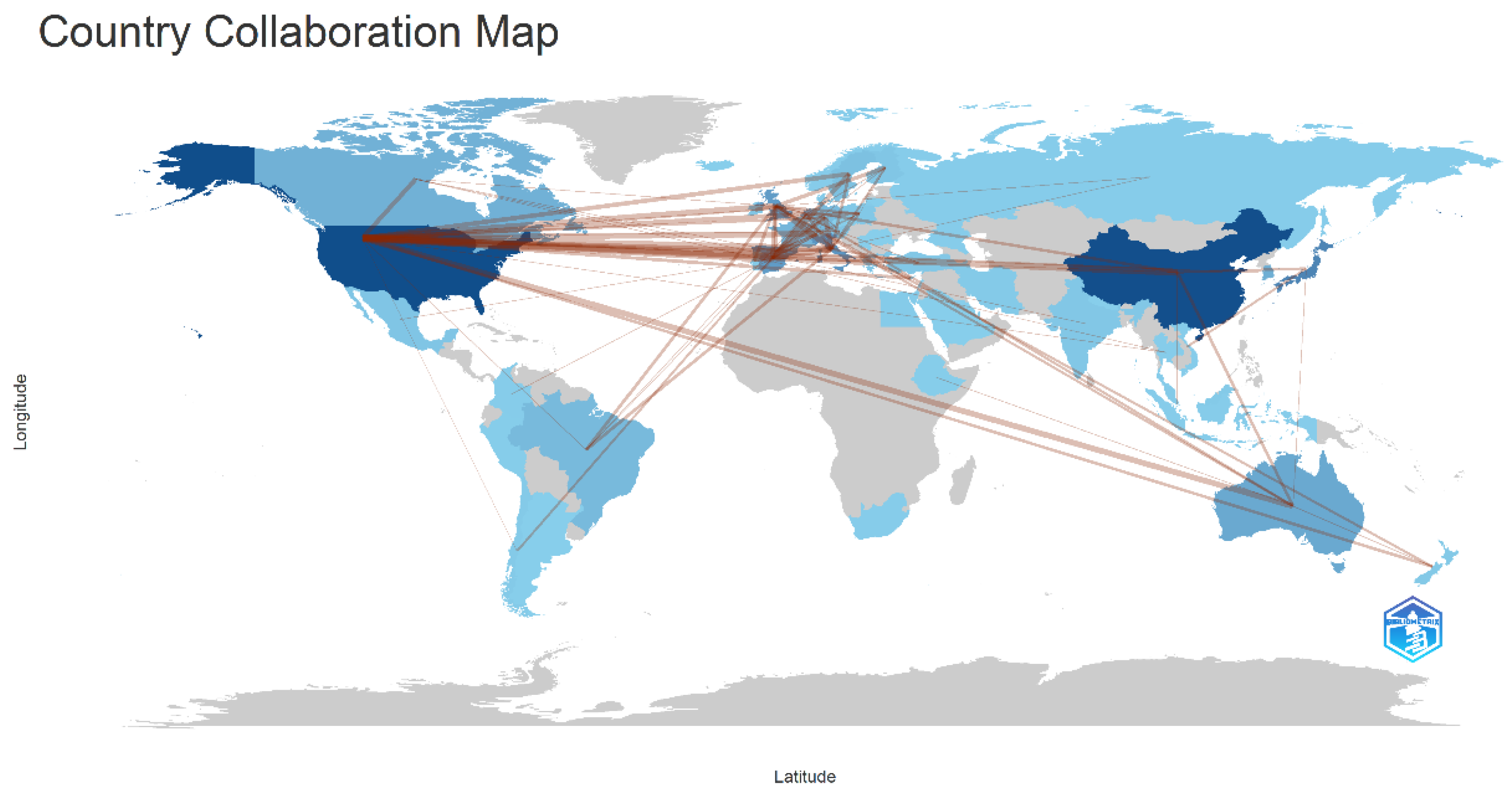
3.3. Country and Institution Analysis
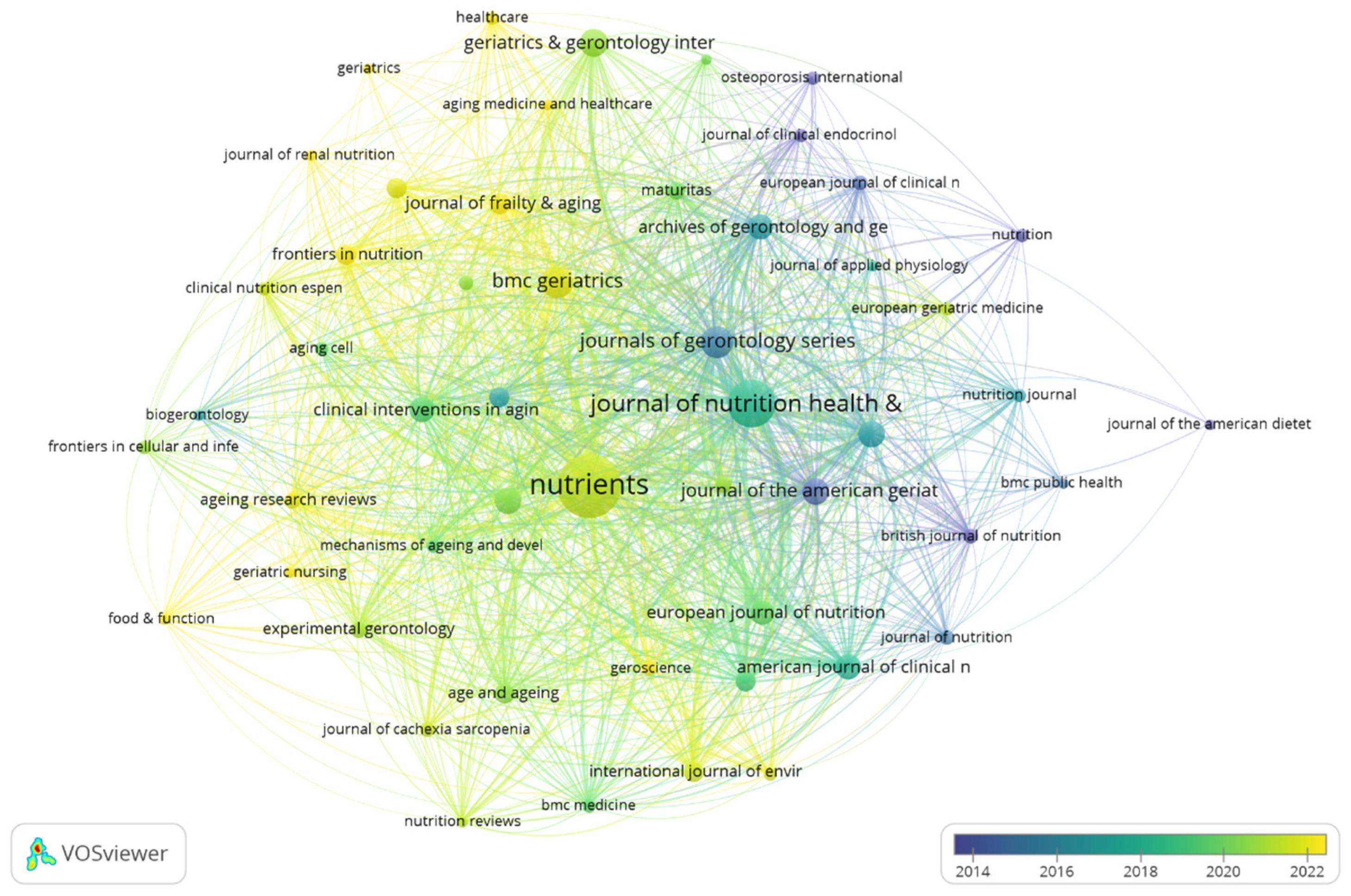
3.4. Journals and Co-Cited Journals
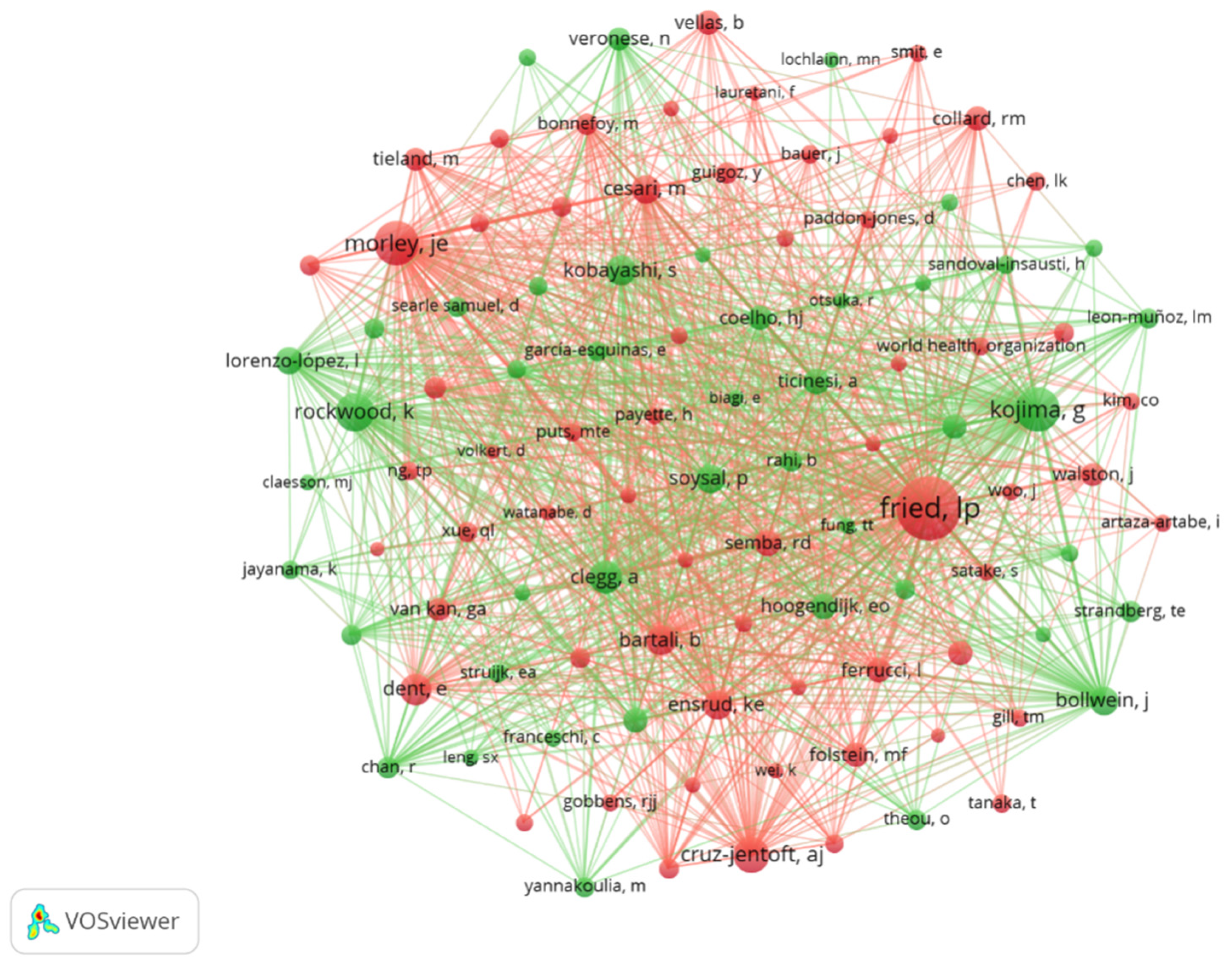
3.5. Authors and Co-Cited Authors
3.6. Co-Cited References
3.7. References with Citation Bursts
3.8. Hotspots and Frontiers
3.9. Key Findings of Nutrition and Frailty
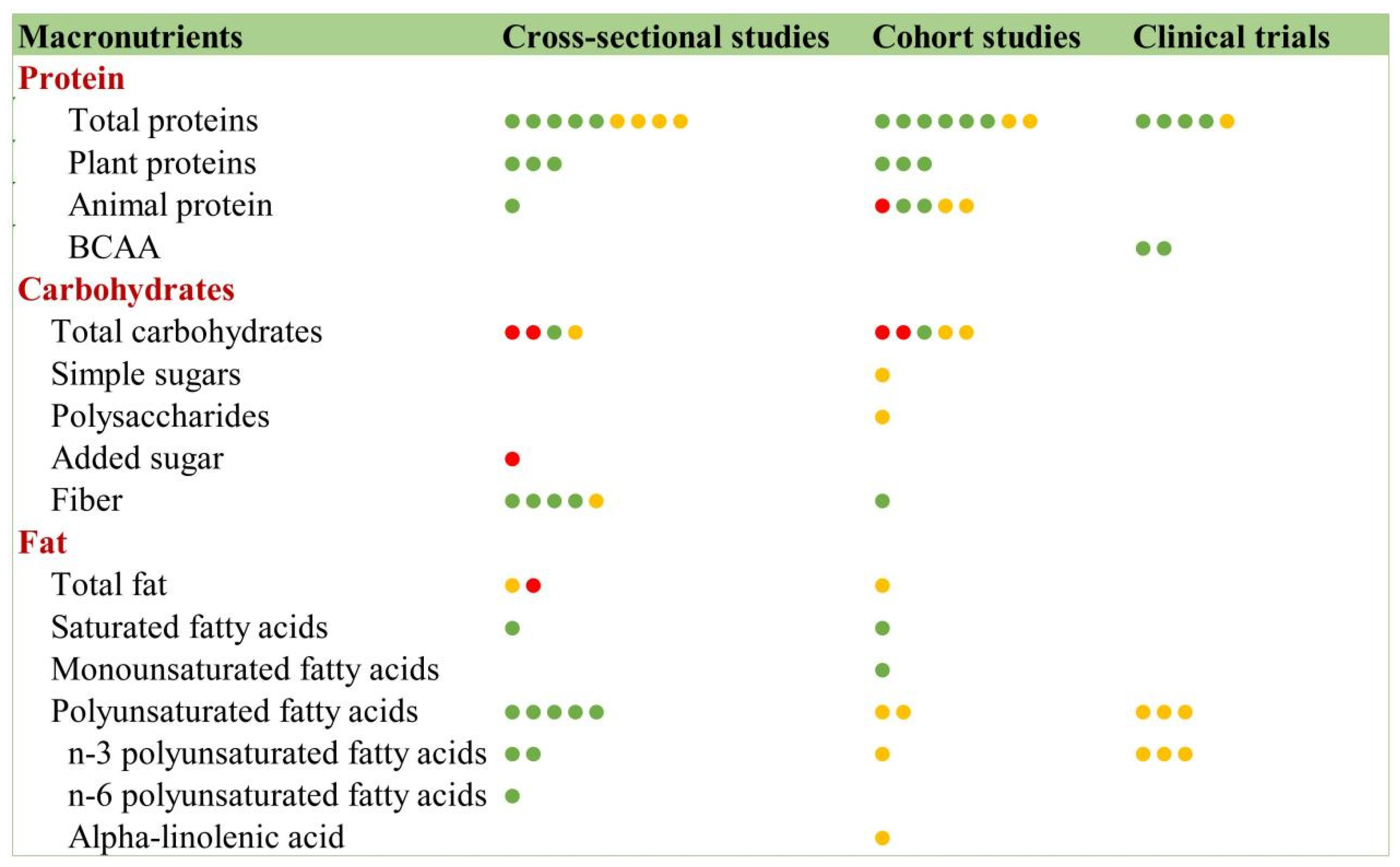
3.9.1. Macronutrients and Frailty
Protein
Carbohydrates
Fats
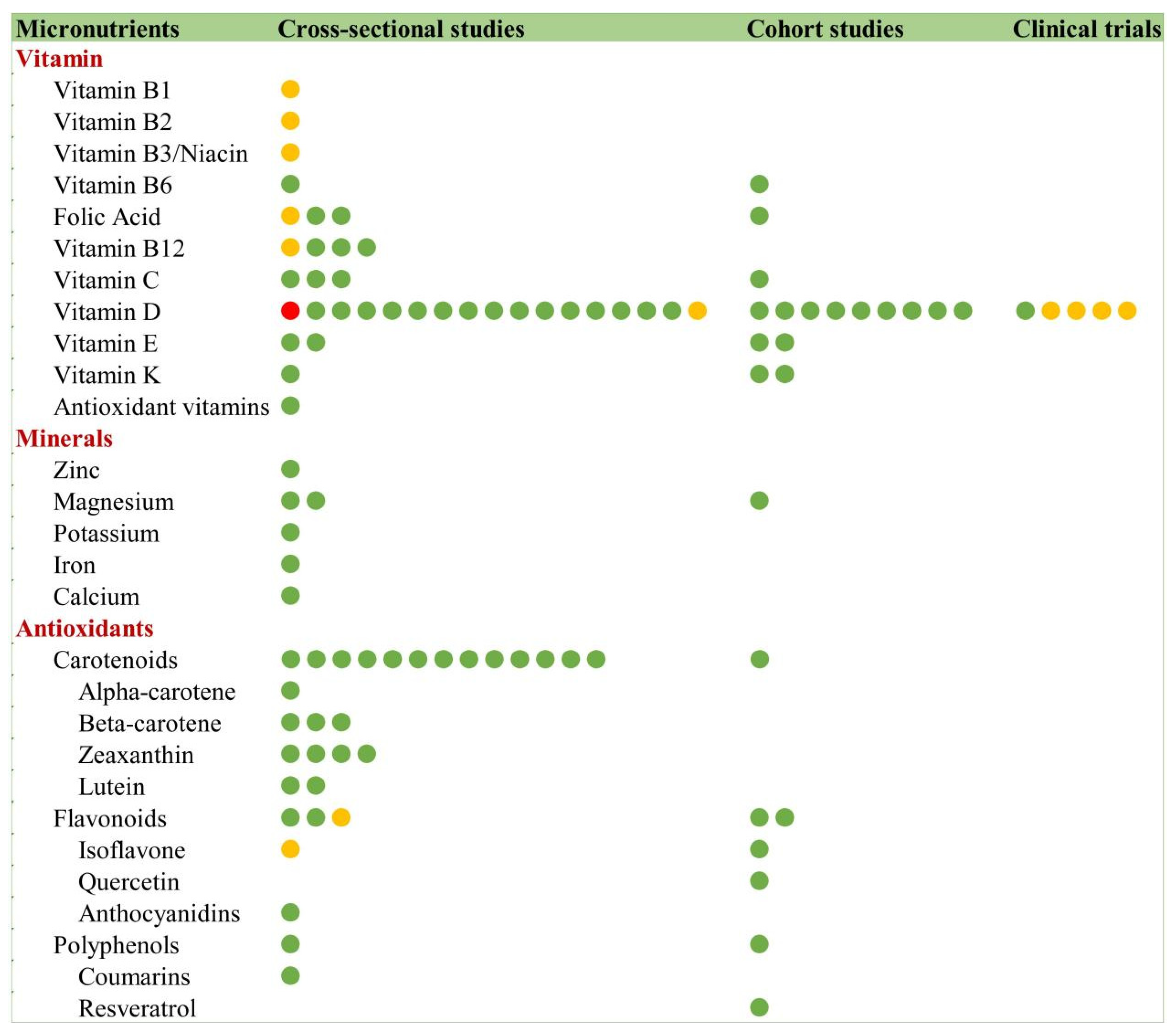
3.9.2. Micronutrients and Frailty
Vitamins
Minerals
Antioxidants
- Carotenoids
- Flavonoids and Polyphenols
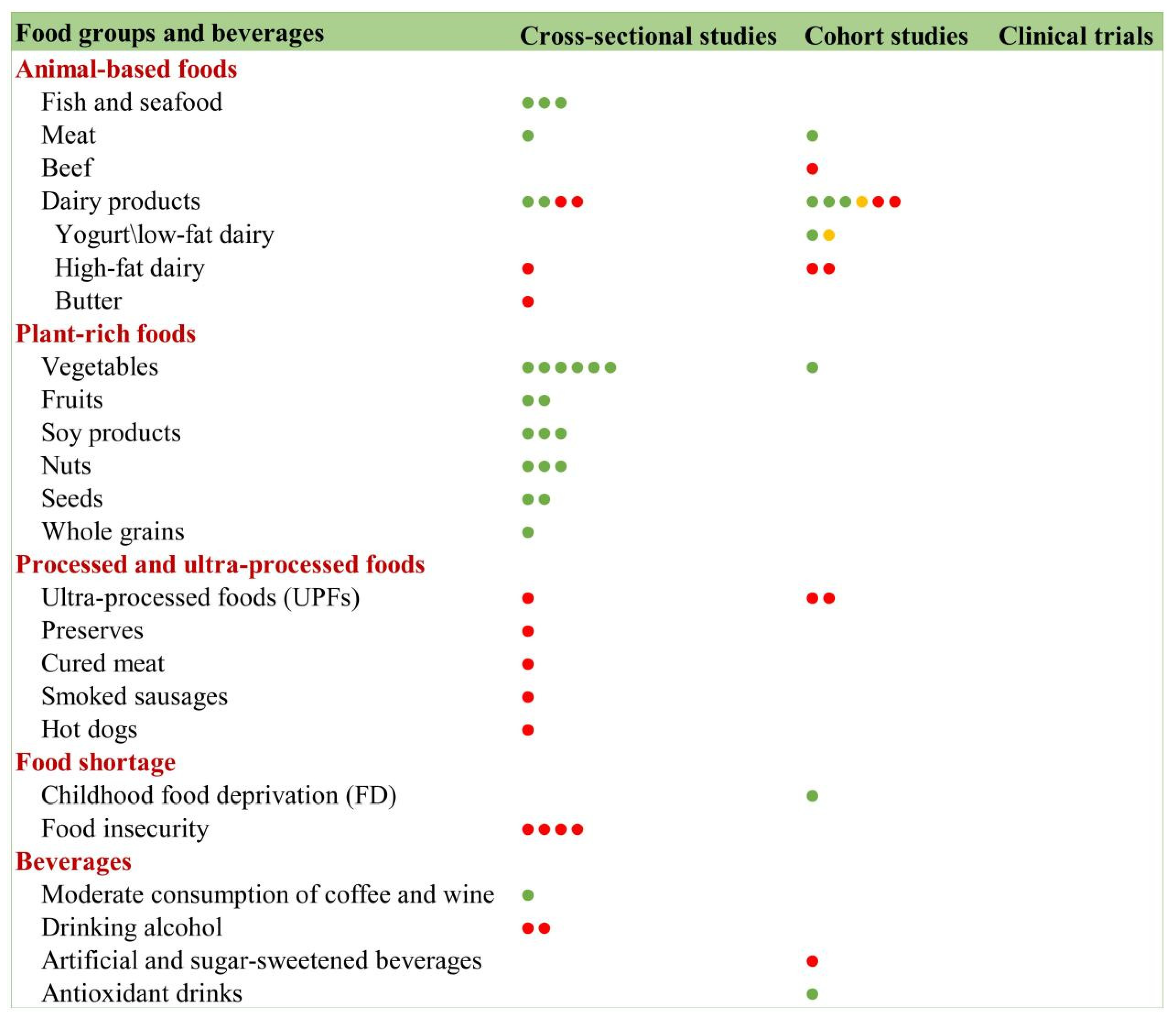
3.9.3. Food Groups and Frailty
Animal-Based Foods
Plant-Based Foods
Ultra-Processed Foods (UPFs)
Food Insecurity
Beverages
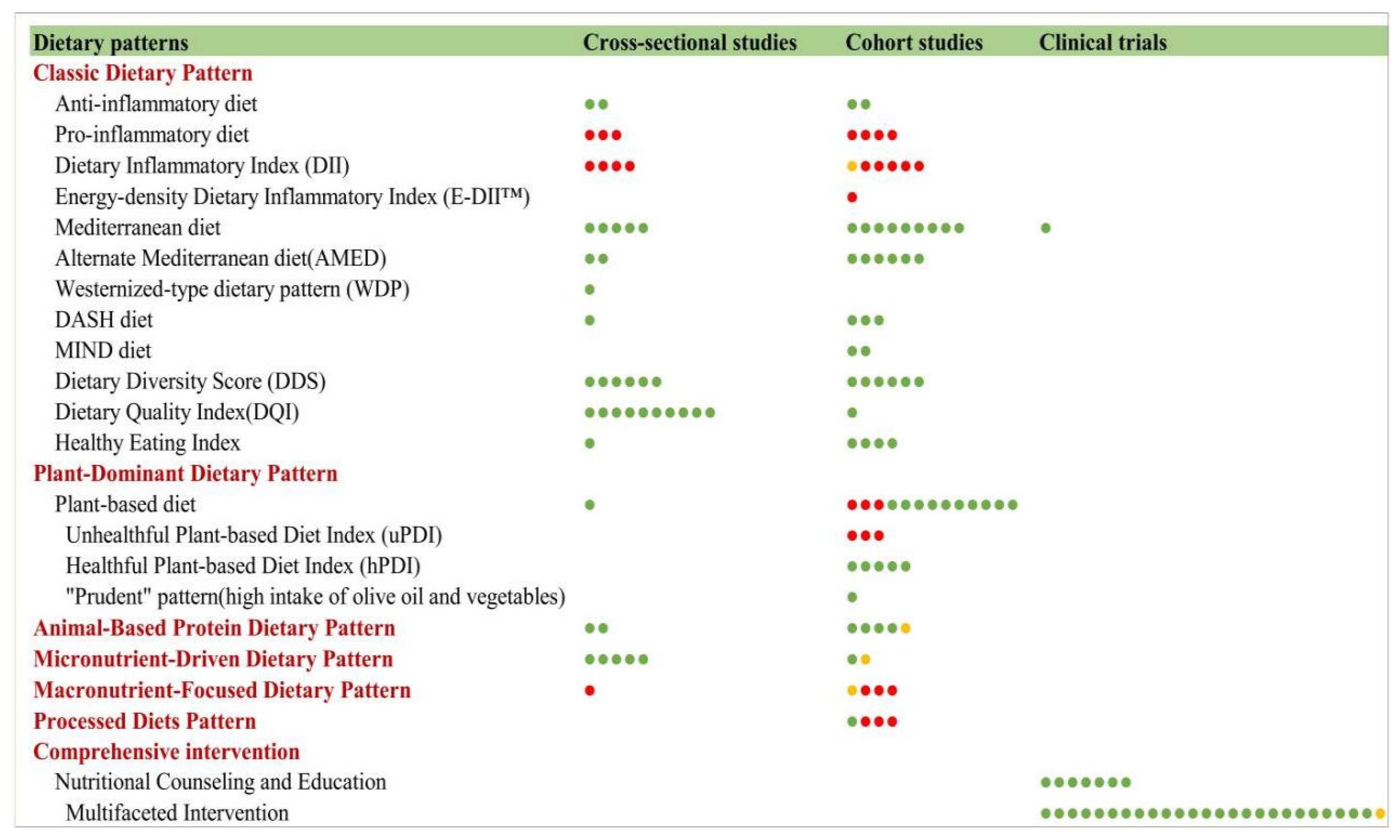
3.9.4. Dietary Patterns and Frailty
Classic Dietary Patterns
Data-Driven Posteriori Dietary Patterns
Comprehensive Interventions and Frailty
4. Discussion
4.1. Current Status of Publications
4.2. Country and Institution Analysis
4.3. Journals and Co-Cited Journals
4.4. Authors and Co-Cited Authors
4.5. Co-Cited References
4.6. Citation Bursts
4.7. Hotspots and Frontiers
4.8. Key Findings of Nutrition and Frailty
4.9. Strengths
4.10. Limitations
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Vermeulen, J.; Neyens, J.C.; van Rossum, E.; Spreeuwenberg, M.D.; de Witte, L.P. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: A systematic review. BMC Geriatr. 2011, 11, 33. [Google Scholar] [CrossRef]
- Bandeen-Roche, K.; Seplaki, C.L.; Huang, J.; Buta, B.; Kalyani, R.R.; Varadhan, R.; Xue, Q.L.; Walston, J.D.; Kasper, J.D. Frailty in Older Adults: A Nationally Representative Profile in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1427–1434. [Google Scholar] [CrossRef]
- Walston, J.; Hadley, E.C.; Ferrucci, L.; Guralnik, J.M.; Newman, A.B.; Studenski, S.A.; Ershler, W.B.; Harris, T.; Fried, L.P. Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J. Am. Geriatr. Soc. 2006, 54, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Lohman, M.C.; Mezuk, B.; Dumenci, L. Depression and frailty: Concurrent risks for adverse health outcomes. Aging Ment. Health 2017, 21, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, E.Y.; Waite, L.J. Social disconnectedness, perceived isolation, and health among older adults. J. Health Soc. Behav. 2009, 50, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Cacioppo, J.T.; Hawkley, L.C.; Thisted, R.A. Perceived social isolation makes me sad: 5-year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago Health, Aging, and Social Relations Study. Psychol. Aging 2010, 25, 453–463. [Google Scholar] [CrossRef]
- McLachlan, K.J.J.; Gale, C.R. The effects of psychological distress and its interaction with socioeconomic position on risk of developing four chronic diseases. J. Psychosom. Res. 2018, 109, 79–85. [Google Scholar] [CrossRef]
- Ringer, T.; Hazzan, A.A.; Agarwal, A.; Mutsaers, A.; Papaioannou, A. Relationship between family caregiver burden and physical frailty in older adults without dementia: A systematic review. Syst. Rev. 2017, 6, 55. [Google Scholar] [CrossRef]
- Tana, C.; Lauretani, F.; Ticinesi, A.; Gionti, L.; Nouvenne, A.; Prati, B.; Meschi, T.; Maggio, M. Impact of Nutritional Status on Caregiver Burden of Elderly Outpatients. A Cross-Sectional Study. Nutrients 2019, 11, 281. [Google Scholar] [CrossRef]
- Kawada, T. Social Isolation, Loneliness, and Frailty in Older Adults: A Risk Assessment. J. Am. Med. Dir. Assoc. 2023, 24, 1222. [Google Scholar] [CrossRef]
- Liu, W.; Qin, R.; Qiu, Y.; Luan, T.; Qiu, B.; Yan, K.; Chen, Z.; Miao, B.; Liu, Y. Multidimensional frailty as a predictor of mortality among older adults: A systematic review and meta-analysis. BMC Geriatr. 2024, 24, 793. [Google Scholar] [CrossRef]
- Wang, S.K.; Wang, Q.J.; Wang, P.; Li, X.Y.; Cui, P.; Wang, D.F.; Chen, X.L.; Kong, C.; Lu, S.B. The impact of frailty on clinical outcomes of older patients undergoing enhanced recovery after lumbar fusion surgery: A prospective cohort study. Int. J. Surg. 2024, 110, 4785–4795. [Google Scholar] [CrossRef]
- Chitalu, P.; Tsui, A.; Searle, S.D.; Davis, D. Life-space, frailty, and health-related quality of life. BMC Geriatr. 2022, 22, 646. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 2021, 50, 96–104. [Google Scholar] [CrossRef]
- He, B.; Ma, Y.; Wang, C.; Jiang, M.; Geng, C.; Chang, X.; Ma, B.; Han, L. Prevalence and Risk Factors for Frailty among Community-Dwelling Older People in China: A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2019, 23, 442–450. [Google Scholar] [CrossRef]
- Sinclair, D.R.; Maharani, A.; Chandola, T.; Bower, P.; Hanratty, B.; Nazroo, J.; O’Neill, T.W.; Tampubolon, G.; Todd, C.; Wittenberg, R.; et al. Frailty among Older Adults and Its Distribution in England. J. Frailty Aging 2022, 11, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Iliffe, S.; Taniguchi, Y.; Shimada, H.; Rakugi, H.; Walters, K. Prevalence of frailty in Japan: A systematic review and meta-analysis. J. Epidemiol. 2017, 27, 347–353. [Google Scholar] [CrossRef]
- Satake, S.; Shimada, H.; Yamada, M.; Kim, H.; Yoshida, H.; Gondo, Y.; Matsubayashi, K.; Matsushita, E.; Kuzuya, M.; Kozaki, K.; et al. Prevalence of frailty among community-dwellers and outpatients in Japan as defined by the Japanese version of the Cardiovascular Health Study criteria. Geriatr. Gerontol. Int. 2017, 17, 2629–2634. [Google Scholar] [CrossRef]
- Ofori-Asenso, R.; Chin, K.L.; Mazidi, M.; Zomer, E.; Ilomaki, J.; Zullo, A.R.; Gasevic, D.; Ademi, Z.; Korhonen, M.J.; LoGiudice, D.; et al. Global Incidence of Frailty and Prefrailty Among Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e198398. [Google Scholar] [CrossRef]
- Rivas-Ruiz, F.; Machón, M.; Contreras-Fernández, E.; Vrotsou, K.; Padilla-Ruiz, M.; Díez Ruiz, A.I.; de Mesa Berenguer, Y.; Vergara, I. Prevalence of frailty among community-dwelling elderly persons in Spain and factors associated with it. Eur. J. Gen. Pract. 2019, 25, 190–196. [Google Scholar] [CrossRef]
- Cechinel, C.; Lenardt, M.H.; Rodrigues, J.A.M.; Binotto, M.A.; Aristides, M.M.; Kraus, R. Frailty and delirium in hospitalized older adults: A systematic review with meta-analysis. Rev. Lat. Am. Enferm. 2022, 30, e3687. [Google Scholar] [CrossRef]
- Gómez Jiménez, E.; Avendaño Céspedes, A.; Cortés Zamora, E.B.; García Molina, R.; Abizanda, P. Frailty prevalence in hospitalized older adults. A systematic review. Rev. Esp. Salud Publica 2021, 95, e202110158. [Google Scholar]
- Turner, G.; Clegg, A. Best practice guidelines for the management of frailty: A British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age Ageing 2014, 43, 744–747. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Woodhouse, L.; Rodríguez-Mañas, L.; Fried, L.P.; Woo, J.; Aprahamian, I.; Sanford, A.; Lundy, J.; et al. Physical Frailty: ICFSR International Clinical Practice Guidelines for Identification and Management. J. Nutr. Health Aging 2019, 23, 771–787. [Google Scholar] [CrossRef]
- Dent, E.; Lien, C.; Lim, W.S.; Wong, W.C.; Wong, C.H.; Ng, T.P.; Woo, J.; Dong, B.; de la Vega, S.; Hua Poi, P.J.; et al. The Asia-Pacific Clinical Practice Guidelines for the Management of Frailty. J. Am. Med. Dir. Assoc. 2017, 18, 564–575. [Google Scholar] [CrossRef]
- Cooper, I.D. Bibliometrics basics. J. Med. Libr. Assoc. 2015, 103, 217–218. [Google Scholar] [CrossRef]
- Othman, Z.; Abdul Halim, A.S.; Azman, K.F.; Ahmad, A.H.; Zakaria, R.; Sirajudeen, K.N.S.; Wijaya, A.; Ahmi, A. Profiling the Research Landscape on Cognitive Aging: A Bibliometric Analysis and Network Visualization. Front. Aging Neurosci. 2022, 14, 876159. [Google Scholar] [CrossRef]
- Liu, T.; Song, F.; Su, D.; Tian, X. Bibliometric analysis of research trends in relationship between sarcopenia and surgery. Front. Surg. 2022, 9, 1056732. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; He, K.; Fang, D.; Qiu, X.; Xiao, W.; Lou, S.; Yong, R. Trends in Nutrition Research for Sarcopenia: A Bibliometric Analysis. Nutrients 2022, 14, 4262. [Google Scholar] [CrossRef]
- Tomaszewski, R. Visibility, impact, and applications of bibliometric software tools through citation analysis. Scientometrics 2023, 128, 4007–4028. [Google Scholar] [CrossRef]
- Nazari, M. Systematic Approaches to a Successful Literature Review. Online Inf. Rev. 2014, 38, 321–326. [Google Scholar]
- Kang, L.; Gao, Y.; Liu, X.; Liang, Y.; Chen, Y.; Liang, Y.; Zhang, L.; Chen, W.; Pang, H.; Peng, L.N. Effects of whey protein nutritional supplement on muscle function among community-dwelling frail older people: A multicenter study in China. Arch. Gerontol. Geriatr. 2019, 83, 7–12. [Google Scholar] [CrossRef]
- Kim, C.O.; Lee, K.R. Preventive effect of protein-energy supplementation on the functional decline of frail older adults with low socioeconomic status: A community-based randomized controlled study. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 309–316. [Google Scholar] [CrossRef]
- Park, Y.; Choi, J.E.; Hwang, H.S. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2018, 108, 1026–1033. [Google Scholar] [CrossRef]
- Abe, S.; Ezaki, O.; Suzuki, M. Medium-Chain Triglycerides in Combination with Leucine and Vitamin D Increase Muscle Strength and Function in Frail Elderly Adults in a Randomized Controlled Trial. J. Nutr. 2016, 146, 1017–1026. [Google Scholar] [CrossRef]
- Tanaka, T.; Kafyra, M.; Jin, Y.; Chia, C.W.; Dedoussis, G.V.; Talegawkar, S.A.; Ferrucci, L. Quality Specific Associations of Carbohydrate Consumption and Frailty Index. Nutrients 2022, 14, 5072. [Google Scholar] [CrossRef]
- Orkaby, A.R.; Dushkes, R.; Ward, R.; Djousse, L.; Buring, J.E.; Lee, I.M.; Cook, N.R.; LeBoff, M.S.; Okereke, O.I.; Copeland, T.; et al. Effect of Vitamin D3 and Omega-3 Fatty Acid Supplementation on Risk of Frailty: An Ancillary Study of a Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2231206. [Google Scholar] [CrossRef]
- Zhang, P.; Zhong, J.; Liu, X.; Sun, W. The association between dynamic changes in vitamin D and frailty alterations: A prospective analysis of UK Biobank participants. J. Cachexia Sarcopenia Muscle 2024, 15, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tang, X.Y.; Yang, J.; Feng, Z.Q.; Yuan, Y.; Jiang, Y.; Hu, G.M.; Dong, J.C. Cognitive frailty in relation to vitamin B12 and 25-hydroxyvitamin D in an elderly population: A cross-sectional study from NHANES. Front. Nutr. 2024, 11, 1430722. [Google Scholar] [CrossRef]
- Pramyothin, P.; Techasurungkul, S.; Lin, J.; Wang, H.; Shah, A.; Ross, P.D.; Puapong, R.; Wasnich, R.D. Vitamin D status and falls, frailty, and fractures among postmenopausal Japanese women living in Hawaii. Osteoporos. Int. 2009, 20, 1955–1962. [Google Scholar] [CrossRef]
- Cai, Y.; Wanigatunga, A.A.; Mitchell, C.M.; Urbanek, J.K.; Miller, E.R., 3rd; Juraschek, S.P.; Michos, E.D.; Kalyani, R.R.; Roth, D.L.; Appel, L.J.; et al. The effects of vitamin D supplementation on frailty in older adults at risk for falls. BMC Geriatr. 2022, 22, 312. [Google Scholar] [CrossRef]
- Kotlarczyk, M.P.; Perera, S.; Ferchak, M.A.; Nace, D.A.; Resnick, N.M.; Greenspan, S.L. Vitamin D deficiency is associated with functional decline and falls in frail elderly women despite supplementation. Osteoporos. Int. 2017, 28, 1347–1353. [Google Scholar] [CrossRef]
- Imaoka, M.; Higuchi, Y.; Todo, E.; Kitagwa, T.; Ueda, T. Low-frequency Exercise and Vitamin D Supplementation Reduce Falls Among Institutionalized Frail Elderly. Int. J. Gerontol. 2016, 10, 202–206. [Google Scholar] [CrossRef]
- Balboa-Castillo, T.; Struijk, E.A.; Lopez-Garcia, E.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillon, P. Low vitamin intake is associated with risk of frailty in older adults. Age Ageing 2018, 47, 872–879. [Google Scholar] [CrossRef]
- Matteini, A.M.; Walston, J.D.; Fallin, M.D.; Bandeen-Roche, K.; Kao, W.H.L.; Semba, R.D.; Allen, R.H.; Guralnik, J.; Fried, L.P.; Stabler, S.P. Markers of B-vitamin deficiency and frailty in older women. J. Nutr. Health Aging 2008, 12, 303–308. [Google Scholar] [CrossRef]
- Soh, Y.; Won, C.W. Association between frailty and vitamin B12 in the older Korean population. Medicine 2020, 99, e22327. [Google Scholar] [CrossRef]
- Dhonukshe-Rutten, R.A.; Lips, M.; de Jong, N.; Chin, A.P.M.J.; Hiddink, G.J.; van Dusseldorp, M.; De Groot, L.C.; van Staveren, W.A. Vitamin B-12 status is associated with bone mineral content and bone mineral density in frail elderly women but not in men. J. Nutr. 2003, 133, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Suh, H.S.; Lee, E.E. Association between dietary supplements and frailty: A cross-sectional study using national survey data in South Korea. Int. J. Food Sci. Nutr. 2024, 75, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, K.; Kusunoki, H.; Tsuji, S.; Wada, Y.; Nagai, K.; Itoh, M.; Sano, K.; Amano, M.; Maeda, H.; Hasegawa, Y.; et al. The Relationship between Dietary Habits and Frailty in Rural Japanese Community-Dwelling Older Adults: Cross-Sectional Observation Study Using a Brief Self-Administered Dietary History Questionnaire. Nutrients 2018, 10, 1982. [Google Scholar] [CrossRef]
- Luong, R.; Ribeiro, R.V.; Rangan, A.; Naganathan, V.; Blyth, F.; Waite, L.M.; Handelsman, D.J.; Cumming, R.G.; Le Couteur, D.G.; Hirani, V. Changes in Dietary Total and Nonheme Iron Intake Is Associated with Incident Frailty in Older Men: The Concord Health and Aging in Men Project. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1853–1865. [Google Scholar] [CrossRef]
- De Nucci, S.; Zupo, R.; Donghia, R.; Castellana, F.; Lofù, D.; Aresta, S.; Guerra, V.; Bortone, I.; Lampignano, L.; De Pergola, G.; et al. Dietary profiling of physical frailty in older age phenotypes using a machine learning approach: The Salus in Apulia Study. Eur. J. Nutr. 2023, 62, 1217–1229. [Google Scholar] [CrossRef]
- Kaimoto, K.; Yamashita, M.; Suzuki, T.; Makizako, H.; Koriyama, C.; Kubozono, T.; Takenaka, T.; Ohishi, M.; Kanouchi, H.; The Tarumizu Study Diet, G. Association of Protein and Magnesium Intake with Prevalence of Prefrailty and Frailty in Community-Dwelling Older Japanese Women. J. Nutr. Sci. Vitaminol. 2021, 67, 39–47. [Google Scholar] [CrossRef]
- Liang, M.; Ren, X.; Zhang, Q.; Ruan, Z.; Jin, M.; Xu, Y.; Chen, X.; Qiu, Z. The Association Between Dietary Magnesium Intake and Frailty in Patients with Chronic Obstructive Pulmonary Disease: National Health and Nutrition Examination Survey. Int. J. Chron. Obs. Pulmon Dis. 2024, 19, 2651–2660. [Google Scholar] [CrossRef]
- Veronese, N.; Stubbs, B.; Maggi, S.; Notarnicola, M.; Barbagallo, M.; Firth, J.; Dominguez, L.J.; Caruso, M.G. Dietary Magnesium and Incident Frailty in Older People at Risk for Knee Osteoarthritis: An Eight-Year Longitudinal Study. Nutrients 2017, 9, 1253. [Google Scholar] [CrossRef]
- Michelon, E.; Blaum, C.; Semba, R.D.; Xue, Q.L.; Ricks, M.O.; Fried, L.P. Vitamin and carotenoid status in older women: Associations with the frailty syndrome. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 600–607. [Google Scholar] [CrossRef]
- Henning, T.; Kochlik, B.; Ara, I.; González-Gross, M.; Fiorillo, E.; Marongiu, M.; Cucca, F.; Rodriguez-Artalejo, F.; Carnicero Carreño, J.A.; Rodriguez-Mañas, L.; et al. Patterns of Dietary Blood Markers Are Related to Frailty Status in the FRAILOMIC Validation Phase. Nutrients 2023, 15, 1142. [Google Scholar] [CrossRef]
- Semba, R.D.; Bartali, B.; Zhou, J.; Blaum, C.; Ko, C.W.; Fried, L.P. Low serum micronutrient concentrations predict frailty among older women living in the community. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 594–599. [Google Scholar] [CrossRef]
- Rietman, M.L.; Spijkerman, A.M.W.; Wong, A.; van Steeg, H.; Bürkle, A.; Moreno-Villanueva, M.; Sindlinger, T.; Franceschi, C.; Grubeck-Loebenstein, B.; Bernhardt, J.; et al. Antioxidants linked with physical, cognitive and psychological frailty: Analysis of candidate biomarkers and markers derived from the MARK-AGE study. Mech. Ageing Dev. 2019, 177, 135–143. [Google Scholar] [CrossRef]
- Rabassa, M.; Zamora-Ros, R.; Urpi-Sarda, M.; Bandinelli, S.; Ferrucci, L.; Andres-Lacueva, C.; Cherubini, A. Association of habitual dietary resveratrol exposure with the development of frailty in older age: The Invecchiare in Chianti study. Am. J. Clin. Nutr. 2015, 102, 1534–1542. [Google Scholar] [CrossRef]
- Oei, S.; Millar, C.L.; Nguyen Lily, T.N.; Mukamal, K.J.; Kiel, D.P.; Lipsitz, L.A.; Hannan, M.T.; Sahni, S. Higher intake of dietary flavonols, specifically dietary quercetin, is associated with lower odds of frailty onset over 12 years of follow-up among adults in the Framingham Heart Study. Am. J. Clin. Nutr. 2023, 118, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, K.; Kin, S.K.; Yamada, S.; Akishita, M.; Ogawa, S. Sex-related differences in the association between frailty and dietary consumption in Japanese older people: A cross-sectional study. BMC Geriatr. 2019, 19, 211. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.L.; Coppinger, T.; Lacey, S.; Walton, J.; Arsenic, T.; McCarthy, A.L. Associations between Food Group Intake and Physical Frailty in Irish Community-Dwelling Older Adults. Nutr. Metab. Insights 2021, 14, 11786388211006447. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Yamada, Y.; Nanri, H.; Nozawa, Y.; Itoi, A.; Yoshimura, E.; Watanabe, Y.; Yoshida, T.; Yokoyama, K.; Goto, C.; et al. Association between the Frequency of Protein-Rich Food Intakes and Kihon-Checklist Frailty Indices in Older Japanese Adults: The Kyoto-Kameoka Study. Nutrients 2018, 10, 84. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.T.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.C.; Rao, Z.Y.; Du, L.; Zhao, R.; Yi, M.S.; et al. Fish consumption and multiple health outcomes: Umbrella review. Trends Food Sci. Technol. 2020, 99, 273–283. [Google Scholar] [CrossRef]
- Otsuka, R.; Zhang, S.; Tange, C.; Nishita, Y.; Tomida, M.; Kinoshita, K.; Kato, Y.; Ando, F.; Shimokata, H.; Arai, H. Association of Dietary Intake with the Transitions of Frailty among Japanese Community-Dwelling Older Adults. J. Frality Aging 2022, 11, 26–32. [Google Scholar] [CrossRef]
- Fung, T.; Rossato, S.L.; Chen, Z.L.; Khandpur, N.; Rodriguez-Artalejo, F.; Willett, W.C.; Struijk, E.A.; Lopez-Garcia, E. Ultraprocessed foods, unprocessed or minimally processed foods, and risk of frailty in a cohort of United States females. Am. J. Clin. Nutr. 2024, 120, 232–239. [Google Scholar] [CrossRef]
- Muhammad, T.; Saravanakumar, P.; Sharma, A.; Srivastava, S.; Irshad, C. Association of food insecurity with physical frailty among older adults: Study based on LASI, 2017–2018. Arch. Gerontol. Geriatr. 2022, 103, 104762. [Google Scholar] [CrossRef]
- Pérez-Zepeda, M.U.; Castrejón-Pérez, R.C.; Wynne-Bannister, E.; García-Peña, C. Frailty and food insecurity in older adults. Public Health Nutr. 2016, 19, 2844–2849. [Google Scholar] [CrossRef]
- Ye, C.; Aihemaitijiang, S.; Wang, R.; Halimulati, M.; Zhang, Z. Associations between Early-Life Food Deprivation and Risk of Frailty of Middle-Age and Elderly People: Evidence from the China Health and Retirement Longitudinal Study. Nutrients 2021, 13, 3066. [Google Scholar] [CrossRef] [PubMed]
- Jayanama, K.; Theou, O.; Blodgett, J.M.; Cahill, L.; Rockwood, K. Frailty, nutrition-related parameters, and mortality across the adult age spectrum. BMC Med. 2018, 16, 188. [Google Scholar] [CrossRef] [PubMed]
- Shiau, M.H.; Lee, M.C.; Lin, F.L.; Hurng, B.S.; Yeh, C.J. Cross-Sectional, Short-, Medium-, and Long-Term Effects of Dietary Pattern on Frailty in Taiwan. Int. J. Environ. Res. Public Health 2021, 18, 9717. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Daou, T.; Abi Kharma, J.; Daccache, A.; Bassil, M.; Naja, F.; Rahi, B. Association between Lebanese Mediterranean Diet and Frailty in Community-Dwelling Lebanese Older Adults-A Preliminary Study. Nutrients 2022, 14, 3084. [Google Scholar] [CrossRef]
- León-Muñoz, L.M.; García-Esquinas, E.; López-García, E.; Banegas, J.R.; Rodríguez-Artalejo, F. Major dietary patterns and risk of frailty in older adults: A prospective cohort study. BMC Med. 2015, 13, 11. [Google Scholar] [CrossRef]
- Huang, C.H.; Martins, B.A.; Okada, K.; Matsushita, E.; Uno, C.; Satake, S.; Kuzuya, M. A 3-year prospective cohort study of dietary patterns and frailty risk among community-dwelling older adults. Clin. Nutr. 2021, 40, 229–236. [Google Scholar] [CrossRef]
- Vohra, V.; Leland, E.M.; Schlosser, R.J.; Kamath, V.; Rowan, N.R. Association of Frailty Status and Dietary Patterns in a Nationally Representative Sample of United States Adults with Olfactory Dysfunction. Nutrients 2022, 14, 1238. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Q.; Guo, L.; Xue, Y.; Dang, Y.; Liu, W.; Yin, T.; Zhang, Y.; Zhao, Y. Associations between Low-Carbohydrate Diets and Low-Fat Diets with Frailty in Community-Dwelling Aging Chinese Adults. Nutrients 2023, 15, 3084. [Google Scholar] [CrossRef] [PubMed]
- Pilleron, S.; Ajana, S.; Jutand, M.A.; Helmer, C.; Dartigues, J.F.; Samieri, C.; Féart, C. Dietary Patterns and 12-Year Risk of Frailty: Results From the Three-City Bordeaux Study. J. Am. Med. Dir. Assoc. 2017, 18, 169–175. [Google Scholar] [CrossRef]
- Hickson, M.; Child, J.; Collinson, A. Impact of a dietitian in general practice: Care of the frail and malnourished. J. Hum. Nutr. Diet. 2022, 35, 145–153. [Google Scholar] [CrossRef]
- Wu, S.Y.; Hsu, L.L.; Hsu, C.C.; Hsieh, T.J.; Su, S.C.; Peng, Y.W.; Guo, T.M.; Kang, Y.W.; Pan, W.H. Dietary education with customised dishware and food supplements can reduce frailty and improve mental well-being in elderly people: A single-blind randomized controlled study. Asia Pac. J. Clin. Nutr. 2018, 27, 1018–1030. [Google Scholar] [CrossRef]
- Casals, C.; Avila-Cabeza-de-Vaca, L.; González-Mariscal, A.; Marín-Galindo, A.; Costilla, M.; Ponce-Gonzalez, J.G.; Vázquez-Sánchez, M.A.; Corral-Pérez, J. Effects of an educational intervention on frailty status, physical function, physical activity, sleep patterns, and nutritional status of older adults with frailty or pre-frailty: The FRAGSALUD study. Front. Public Health 2023, 11, 1267666. [Google Scholar] [CrossRef]
- Wang, E.L.; Keller, H.; Mourtzakis, M.; Rodrigues, I.B.; Steinke, A.; Ashe, M.C.; Thabane, L.; Brien, S.; Funnell, L.; Cheung, A.M.; et al. MoveStrong at home: A feasibility study of a model for remote delivery of functional strength and balance training combined with nutrition education for older pre-frail and frail adults. Appl. Physiol. Nutr. Metab. 2022, 47, 1172–1186. [Google Scholar] [CrossRef]
- Haider, S.; Grabovac, I.; Winzer, E.; Kapan, A.; Schindler, K.E.; Lackinger, C.; Titze, S.; Dorner, T.E. Change in inflammatory parameters in prefrail and frail persons obtaining physical training and nutritional support provided by lay volunteers: A randomized controlled trial. PLoS ONE 2017, 12, e0185879. [Google Scholar] [CrossRef]
- Lammes, E.; Rydwik, E.; Akner, G. Effects of nutritional intervention and physical training on energy intake, resting metabolic rate and body composition in frail elderly. A randomised, controlled pilot study. J. Nutr. Health Aging 2012, 16, 162–167. [Google Scholar] [CrossRef]
- Payette, H.; Boutier, V.; Coulombe, C.; Gray-Donald, K. Benefits of nutritional supplementation in free-living, frail, undernourished elderly people: A prospective randomized community trial. J. Am. Diet. Assoc. 2002, 102, 1088–1095. [Google Scholar] [CrossRef]
- Han, C.Y.; Sharma, Y.; Yaxley, A.; Baldwin, C.; Woodman, R.; Miller, M. Individualized Hospital to Home, Exercise-Nutrition Self-Managed Intervention for Pre-Frail and Frail Hospitalized Older Adults: The INDEPENDENCE Randomized Controlled Pilot Trial. Clin. Interv. Aging 2023, 18, 809–825. [Google Scholar] [CrossRef]
- Jaroch, A.; Kozakiewicz, M.; Kowalkowska, A.; Glówczewska-Siedlecka, E.; Kedziora-Kornatowska, K. Comprehensive nutritional assessment of frail older adults and a tailored protein-enhanced diet as a way to improve the nutritional status. Nutr. Food Sci. 2021, 51, 1163–1173. [Google Scholar] [CrossRef]
- Gagesch, M.; Wieczorek, M.; Vellas, B.; Kressig, R.W.; Rizzoli, R.; Kanis, J.; Willett, W.C.; Egli, A.; Lang, W.; Orav, E.J.; et al. Effects of Vitamin D, Omega-3 Fatty Acids and a Home Exercise Program on Prevention of Pre-Frailty in Older Adults: The DO-HEALTH Randomized Clinical Trial. J. Frality Aging 2023, 12, 71–77. [Google Scholar] [CrossRef]
- Tieland, M.; Dirks, M.L.; van der Zwaluw, N.; Verdijk, L.B.; van de Rest, O.; de Groot, L.; van Loon, L.J.C. Protein Supplementation Increases Muscle Mass Gain During Prolonged Resistance-Type Exercise Training in Frail Elderly People: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Med. Dir. Assoc. 2012, 13, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Merchant, R.A.; Chan, Y.H.; Anbarasan, D.; Seetharaman, S.; Au, L.Y.; Nachammai, V.; Lai, A.L.X.; Ho, V.D.; Wong, B.L.L.; Pang, E.N.C.; et al. Impact of exercise and leucine-enriched protein supplementation on physical function, body composition, and inflammation in pre-frail older adults: A quasi-experimental study. Front. Med. 2023, 10, 1204198. [Google Scholar] [CrossRef] [PubMed]
- Bonnefoy, M.; Cornu, C.; Normand, S.; Boutitie, F.; Bugnard, F.; Rahmani, A.; Lacour, J.R.; Laville, M. The effects of exercise and protein-energy supplements on body composition and muscle function in frail elderly individuals: A long-term controlled randomised study. Br. J. Nutr. 2003, 89, 731–738. [Google Scholar] [CrossRef]
- Park, W.; Lee, J.S.; Hong, K.S.; Park, H.Y.; Park, S.; Kim, N.; Park, J. Protein-Added Healthy Lunch-Boxes Combined with Exercise for Improving Physical Fitness and Vascular Function in Pre-Frail Older Women: A Community-Based Randomized Controlled Trial. Clin. Interv. Aging 2023, 18, 13–27. [Google Scholar] [CrossRef]
- Amasene, M.; Cadenas-Sanchez, C.; Echeverria, I.; Sanz, B.; Alonso, C.; Tobalina, I.; Irazusta, J.; Labayen, I.; Besga, A. Effects of Resistance Training Intervention along with Leucine-Enriched Whey Protein Supplementation on Sarcopenia and Frailty in Post-Hospitalized Older Adults: Preliminary Findings of a Randomized Controlled Trial. J. Clin. Med. 2022, 11, 97. [Google Scholar] [CrossRef]
- Karelis, A.D.; Messier, V.; Suppère, C.; Briand, P.; Rabasa-Lhoret, R. Effect of cysteine-rich whey protein (ImmunocalA®) supplementation in combination with resistance training on muscle strength and lean body mass in non-frail elderly subjects: A randomized, double-blind controlled study. J. Nutr. Health Aging 2015, 19, 531–536. [Google Scholar] [CrossRef]
- Nakazeko, T.; Shobako, N.; Shioya, N.; Iwama, Y.; Hirano, Y.; Fujii, S.; Nakamura, F.; Honda, K. Frailty-Preventing Effect of an Intervention Program Using a Novel Complete Nutritional “COMB-FP Meal”: A Pilot Randomized Control Trial. Nutrients 2023, 15, 4317. [Google Scholar] [CrossRef]






| Rank | Country | Counts | Institution | Counts |
|---|---|---|---|---|
| 1 | USA (America) | 157 (20.8%) | Harvard T.H. Chan School of Public Health | 22 (2.9%) |
| 2 | China (Asia) | 124 (16.4%) | Harvard Medical School | 19 (2.5%) |
| 3 | Japan (Asia) | 87 (11.5%) | National Institute on Aging | 15 (1.9%) |
| 4 | Italy (Europe) | 67 (8.9%) | Universidad Autónoma de Madrid | 14 (1.8%) |
| 5 | Spain (Europe) | 59 (7.8%) | The University of Sydney | 14 (1.8%) |
| 6 | England (Europe) | 54 (7.2%) | Columbia University | 13 (1.7%) |
| 7 | Australia (Oceania) | 44 (5.8%) | Wageningen University and Research | 13 (1.7%) |
| 8 | Netherlands (Europe) | 42 (5.6%) | The Johns Hopkins University | 12 (1.6%) |
| 9 | South Korea (Asia) | 37 (4.9%) | National Health Research Institutes | 12 (1.6%) |
| 10 | Canada (America) | 34 (4.5%) | Ciberesp Ciber Epidemiol and Publ Hlth | 11 (1.5%) |
| Rank | Journal | Counts | IF | Q |
|---|---|---|---|---|
| 1 | Nutrients | 89 (11.8%) | 5.0 | Q1 |
| 2 | The Journal of Nutrition, Health and Aging | 52 (6.9%) | 4.0 | Q1 |
| 3 | BMC Geriatrics | 23 (3.1%) | 3.8 | Q2 |
| 4 | Journals of Gerontology Series A: Biological Sciences and Medical Sciences | 22 (2.9%) | 3.8 | Q1 |
| 5 | Geriatrics and Gerontology International | 17 (2.3%) | 2.5 | Q2 |
| 6 | Aging Clinical and Experimental Research | 16 (2.1%) | 3.4 | Q2 |
| 7 | Journal of the American Geriatrics Society | 16 (2.1%) | 4.3 | Q1 |
| 8 | Journal of the American Medical Directors Association | 16 (2.1%) | 3.8 | Q1 |
| 9 | Archives of Gerontology and Geriatrics | 15 (1.9%) | 3.8 | Q2 |
| 10 | The American Journal of Clinical Nutrition | 14 (1.8%) | 6.5 | Q1 |
| 11 | Clinical Interventions in Aging | 14 (1.8%) | 3.7 | Q3 |
| 12 | European Journal of Nutrition | 13 (1.7%) | 4.3 | Q1 |
| 13 | The Journal of Frailty and Aging | 11 (1.5%) | 3.3 | Q2 |
| 14 | Age and aging | 9 (1.2%) | 7.1 | Q1 |
| 15 | BMJ Open | 9 (1.2%) | 2.3 | Q2 |
| Rank | Journal | Co-Citation | IF | Q |
|---|---|---|---|---|
| 1 | Journals of Gerontology Series A: Biological Sciences and Medical Sciences | 2058 | 4.3 | Q1 |
| 2 | Journal of the American Geriatrics Society | 1309 | 4.5 | Q1 |
| 3 | Nutrients | 1216 | 5.0 | Q1 |
| 4 | The Journal of Nutrition, Health and Aging | 1152 | 4.3 | Q1 |
| 5 | Journal of the American Medical Directors Association | 1127 | 3.8 | Q1 |
| 6 | The American Journal of Clinical Nutrition | 1006 | 6.5 | Q1 |
| 7 | Clinical Nutrition | 667 | 7.4 | Q1 |
| 8 | Age and aging | 629 | 7.1 | Q1 |
| 9 | BMC Geriatrics | 556 | 3.8 | Q1 |
| 10 | The Lancet | 525 | 88.5 | Q1 |
| 11 | PLoS ONE | 422 | 2.6 | Q1 |
| 12 | Aging Research Reviews | 414 | 12.5 | Q1 |
| 13 | The Journal of Nutrition | 407 | 3.7 | Q2 |
| 14 | British Journal of Nutrition | 323 | 3.0 | Q2 |
| 15 | Archives of Gerontology and Geriatrics | 313 | 3.8 | Q2 |
| Rank | Authors | Country | Institution | Counts | Co-Cited Authors | Citations |
|---|---|---|---|---|---|---|
| 1 | Rodriguez–Artalejo, Fernando | Spain | Universidad Autónoma de Madrid | 19 | Fried, Lp | 535 |
| 2 | Lopez–Garcia, Esther | Spain | Universidad Autónoma de Madrid | 14 | Morley, Je | 248 |
| 3 | Ferrucci, Luigi | USA | National Institute on Aging (NIA) | 10 | Kojima, G | 245 |
| 4 | Cesari, Matteo | Italy | University of Milan | 9 | Rockwood, K | 192 |
| 5 | Veronese, Nicola | Italy | University of Padua | 8 | Cruz–Jentoft, Aj | 166 |
| 6 | Guallar–Castillon, Pilar | Spain | Universidad Autónoma de Madrid | 7 | Clegg, A | 138 |
| 7 | Struijk, Ellen A. | Netherlands | Wageningen University | 7 | Dent, E | 125 |
| 8 | Feart, Catherine | France | University of Bordeaux | 7 | Kobayashi, S | 116 |
| 9 | Ara, Ignacio | Spain | University of Castilla–La Mancha | 6 | Bartali, B | 113 |
| 10 | Fung, Teresa T. | USA | Simmons University | 6 | Cesari, M | 109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Zhang, H.; Tian, J.; Tu, Y.; Fan, R.; Zhu, W.; Zhang, Z. Mapping Research Trends in Frailty and Nutrition: A Combined Bibliometric and Structured Review (2000–2024). Nutrients 2025, 17, 3541. https://doi.org/10.3390/nu17223541
Han Y, Zhang H, Tian J, Tu Y, Fan R, Zhu W, Zhang Z. Mapping Research Trends in Frailty and Nutrition: A Combined Bibliometric and Structured Review (2000–2024). Nutrients. 2025; 17(22):3541. https://doi.org/10.3390/nu17223541
Chicago/Turabian StyleHan, Yaxin, Haohao Zhang, Jiajing Tian, Yahui Tu, Rui Fan, Wenli Zhu, and Zhaofeng Zhang. 2025. "Mapping Research Trends in Frailty and Nutrition: A Combined Bibliometric and Structured Review (2000–2024)" Nutrients 17, no. 22: 3541. https://doi.org/10.3390/nu17223541
APA StyleHan, Y., Zhang, H., Tian, J., Tu, Y., Fan, R., Zhu, W., & Zhang, Z. (2025). Mapping Research Trends in Frailty and Nutrition: A Combined Bibliometric and Structured Review (2000–2024). Nutrients, 17(22), 3541. https://doi.org/10.3390/nu17223541







