Dietary Copper on the Onset of Puberty in Rats: Possible Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Experimental Design
2.2. Detection of Estrous Stage and Sampling & Preservation of Blood/Tissues
2.3. Copper Content Testing
2.4. Determination of Reproduction-Related Hormone Levels in the HPOA Axis
2.5. Total RNA Extraction and Real Time Polymerase Chain ReactionPCR
2.6. Transcriptomic Analysis
2.7. Western Blot Analysis
2.8. Isolation, Culture, and Identification of Hypothalamic Mixed Neurons
2.9. Screening for the Optimal Copper Concentration and Concentrations of PKC Activators and Inhibitors for Hypothalamic Mixed Neurons
2.10. Statistical Analyses
3. Results
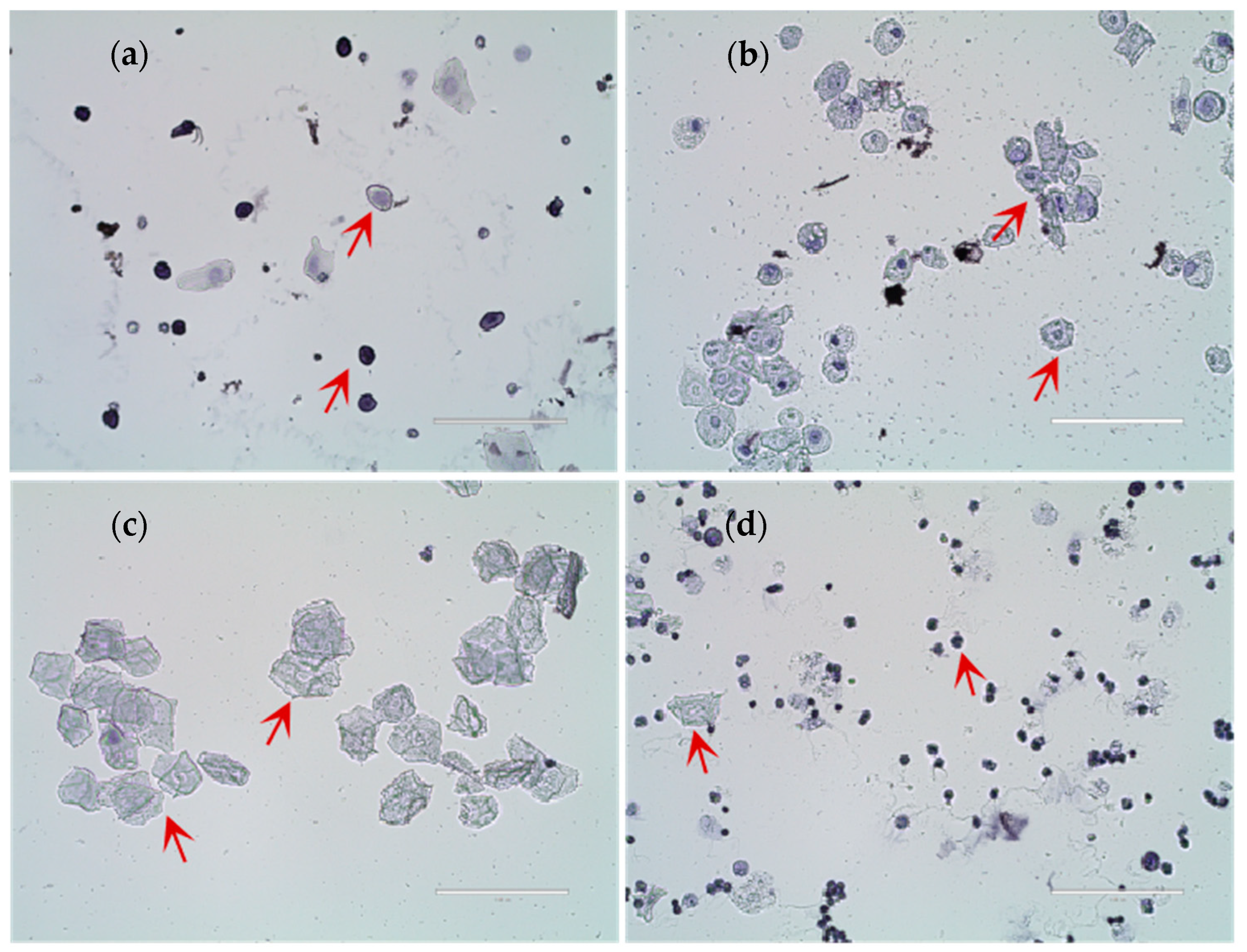
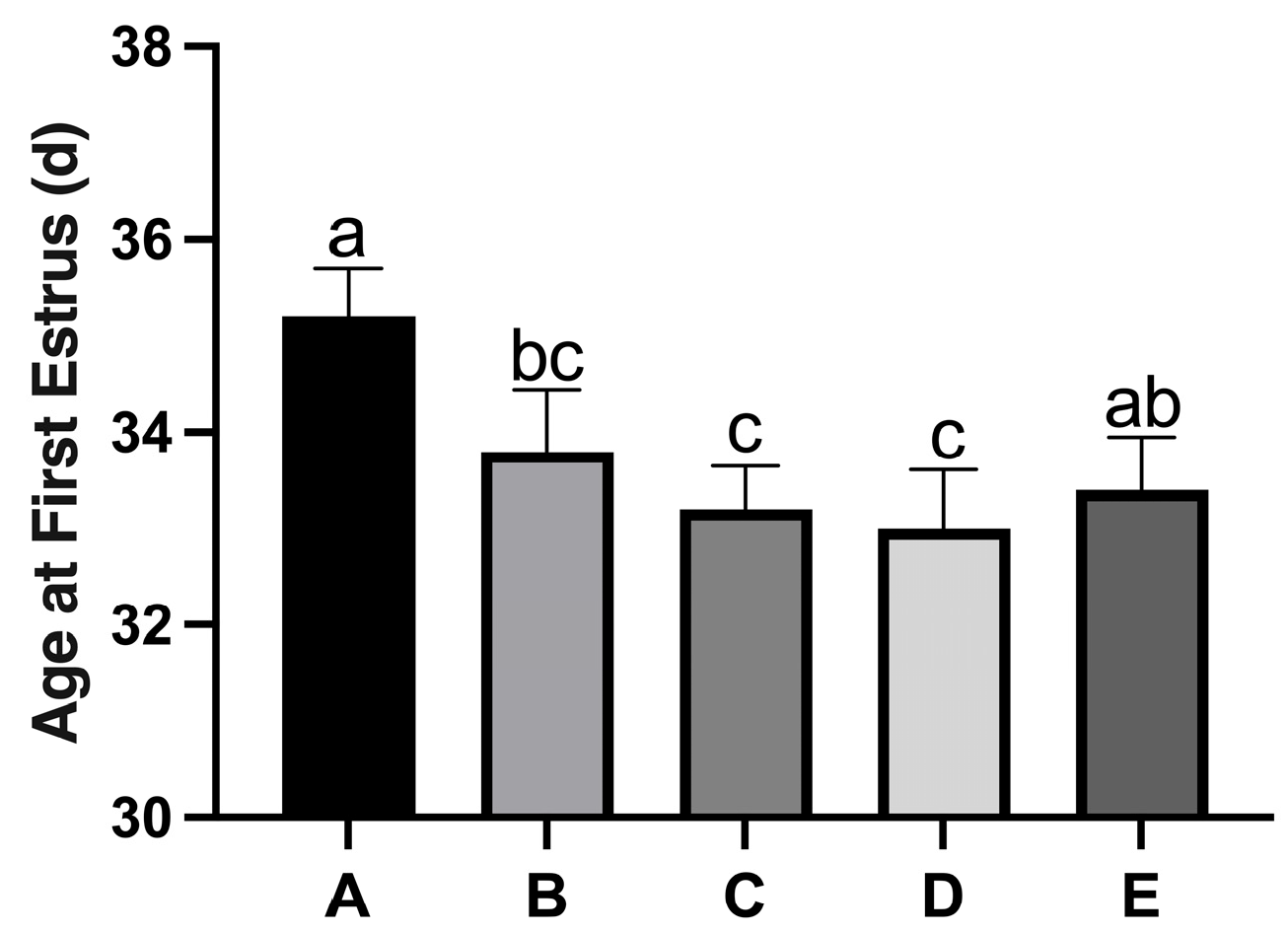
3.1. Identification of Rat Estrus and Effects of Copper on Puberty Age
3.2. Detection of Copper Content in the Hypothalamus and Serum of Rats
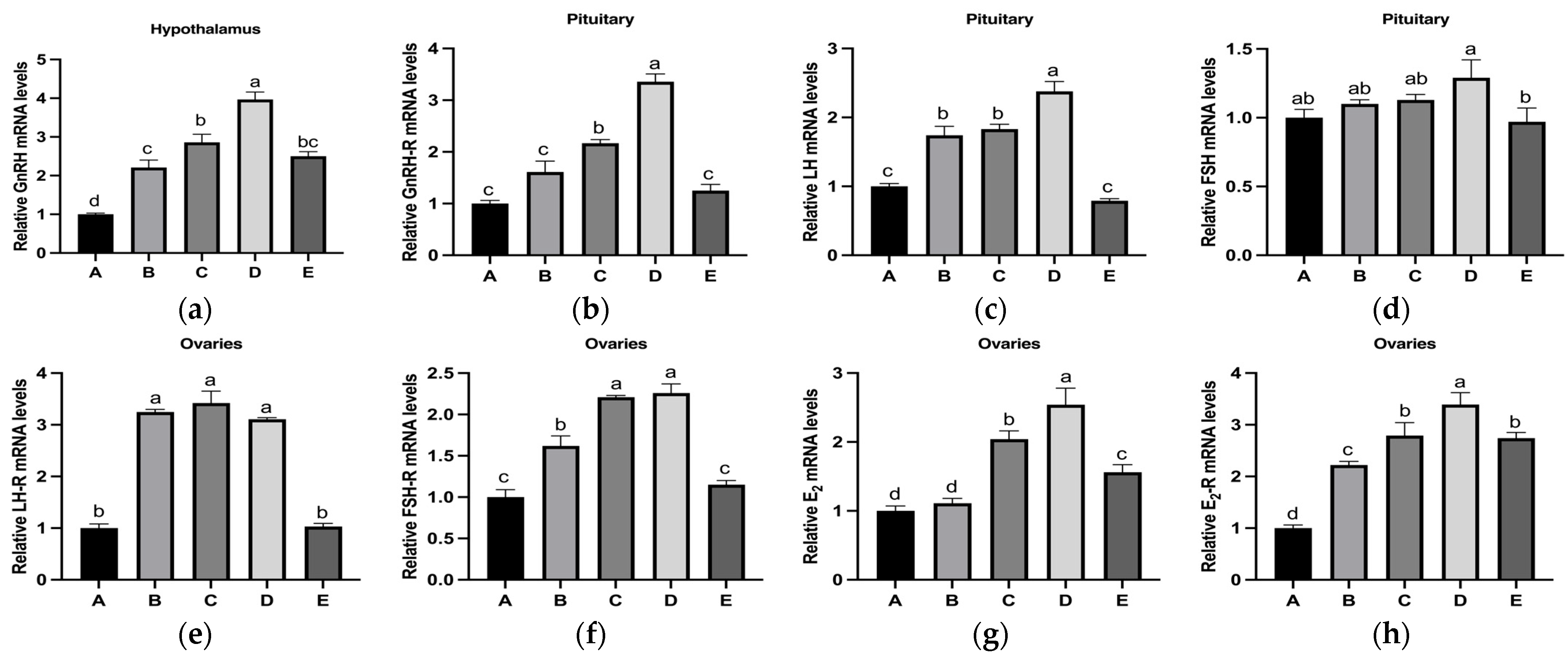
3.3. Effects of Copper on the Secretion of Reproduction-Related Hormones in the HPOA Axis During Puberty Initiation in Rats
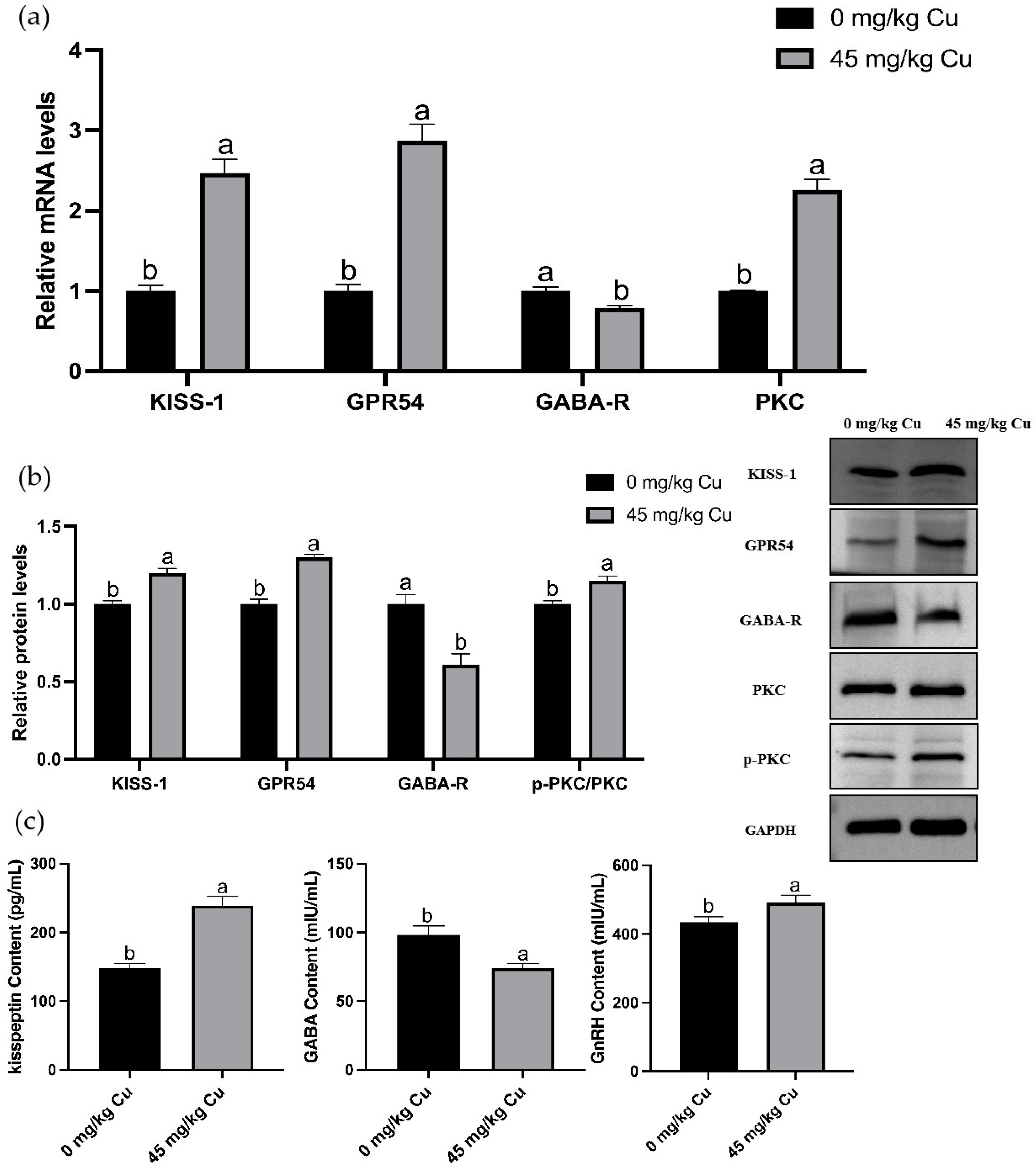
3.4. Effects of Copper on the Relative mRNA Expression Levels of Reproduction-Related Hormones in the HPOA Axis During Puberty Initiation in Rats
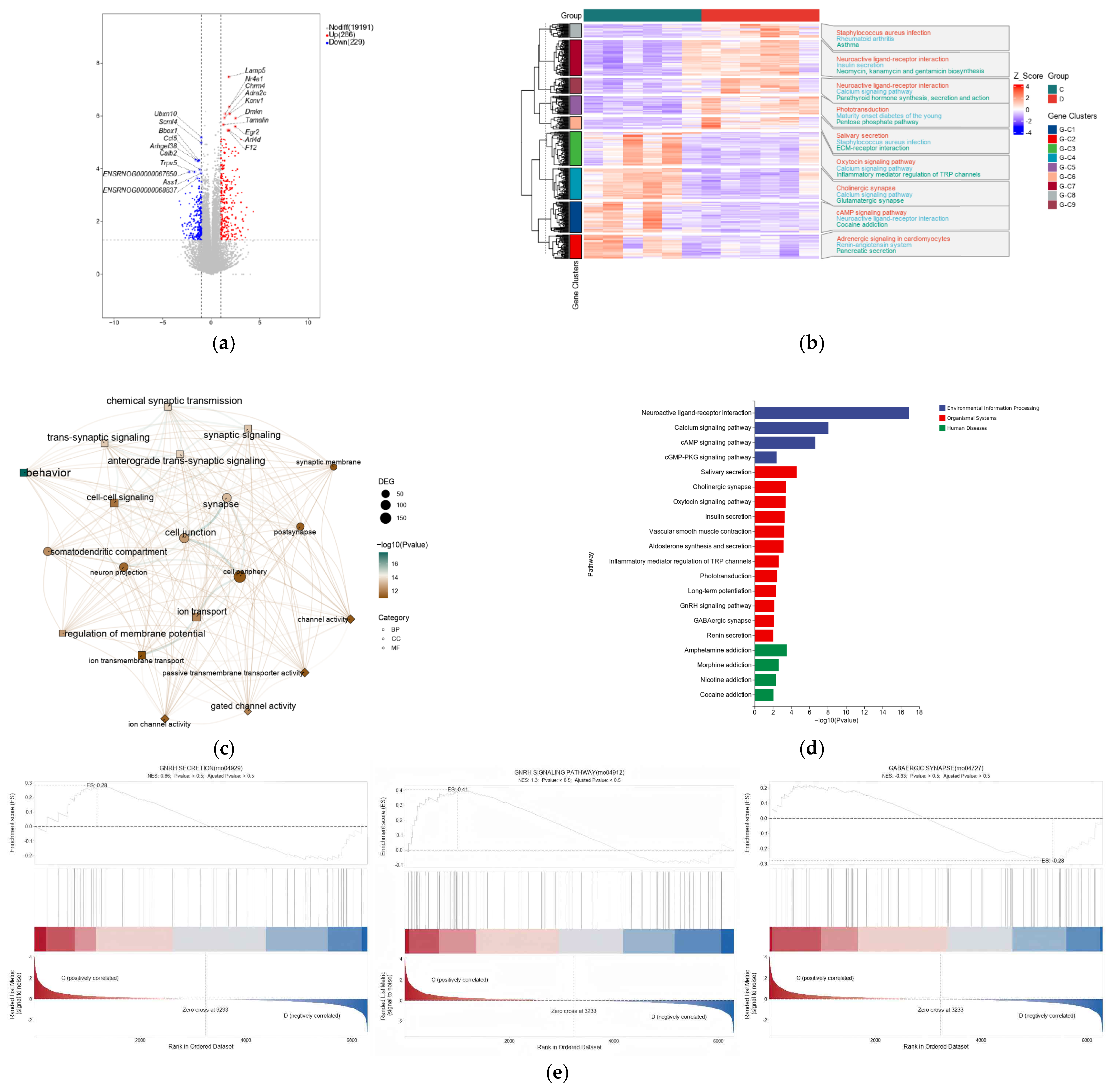
3.5. Transcriptome Analysis and Validation
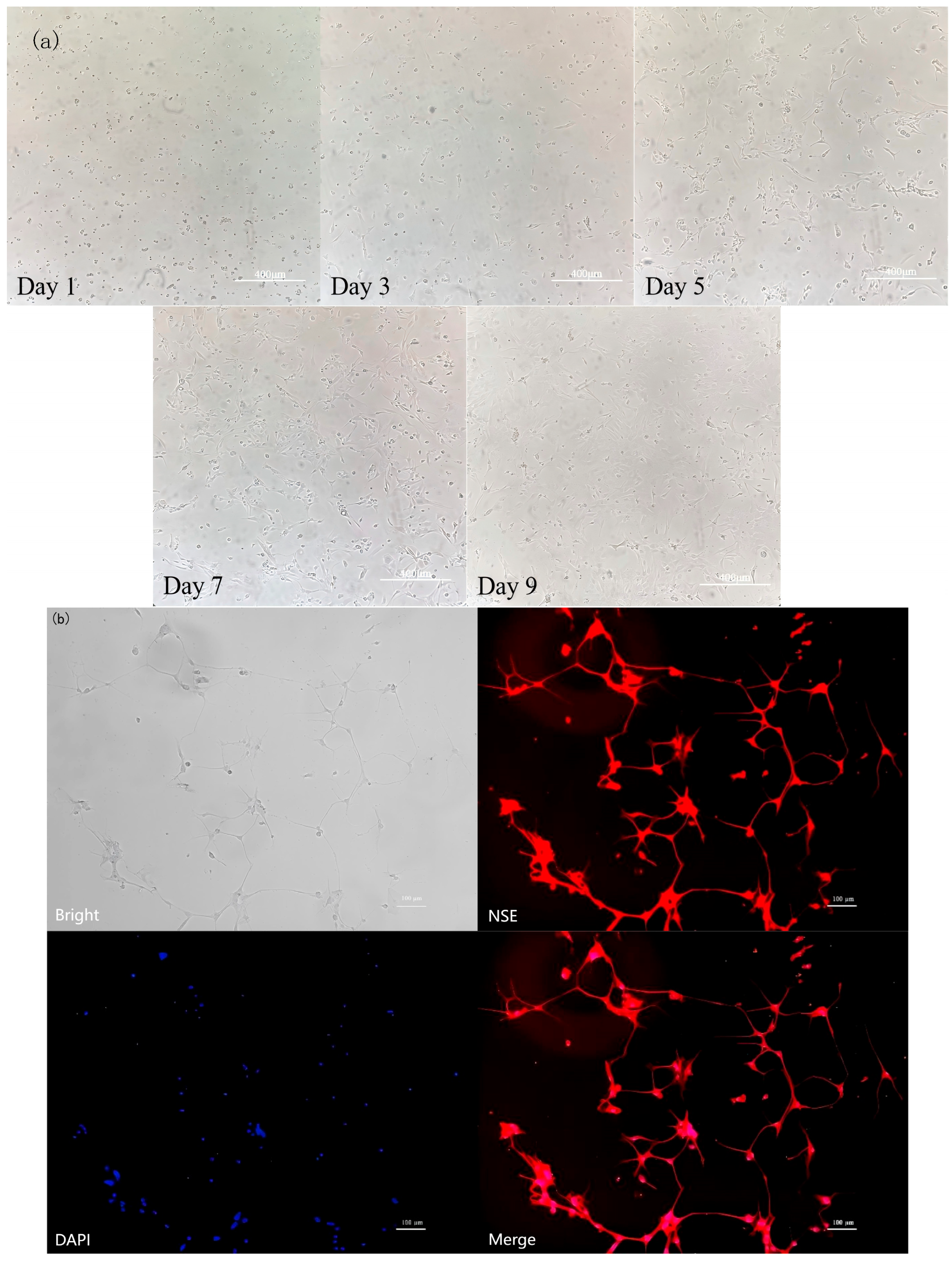
3.6. Identification of Rat Hypothalamic Neuronal Cells
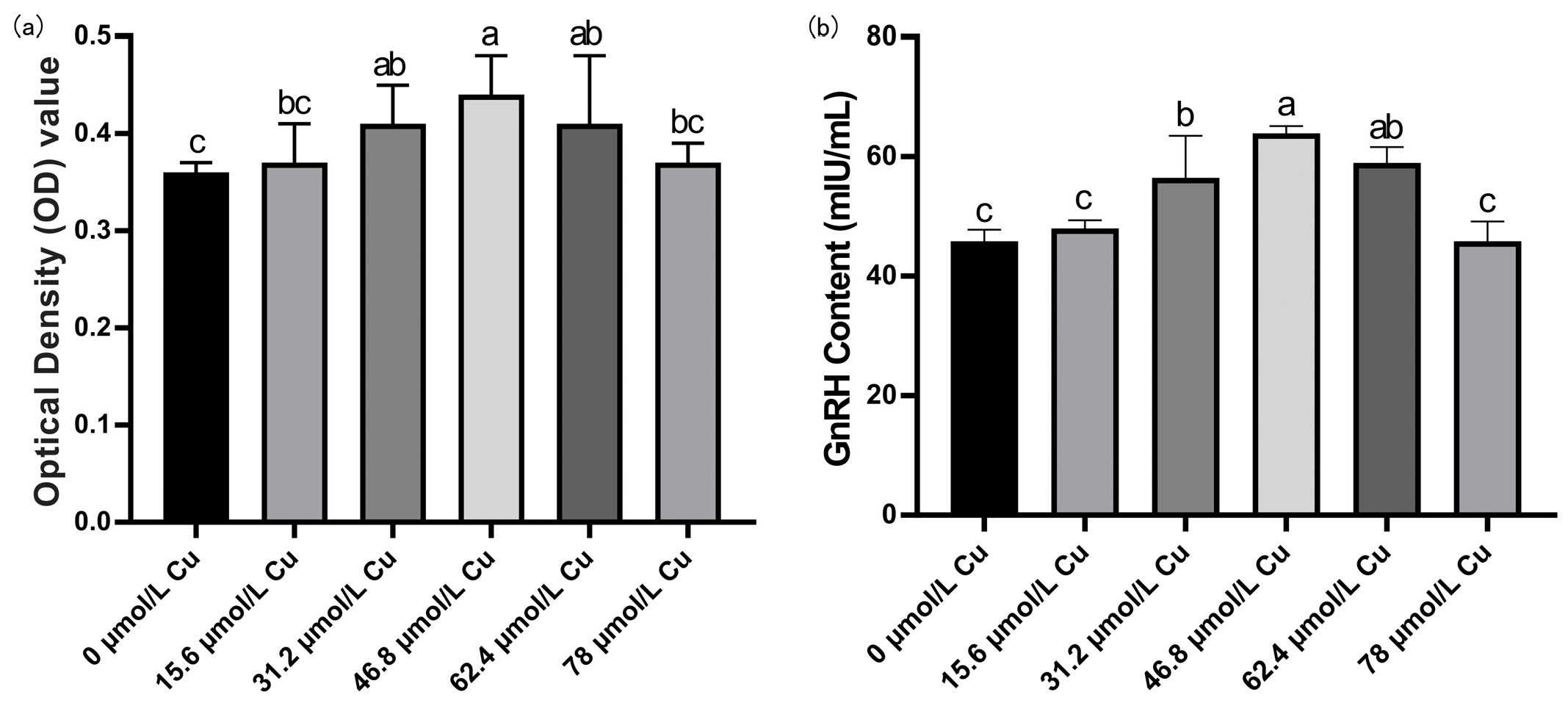
3.7. Screening of Optimal Copper Concentration
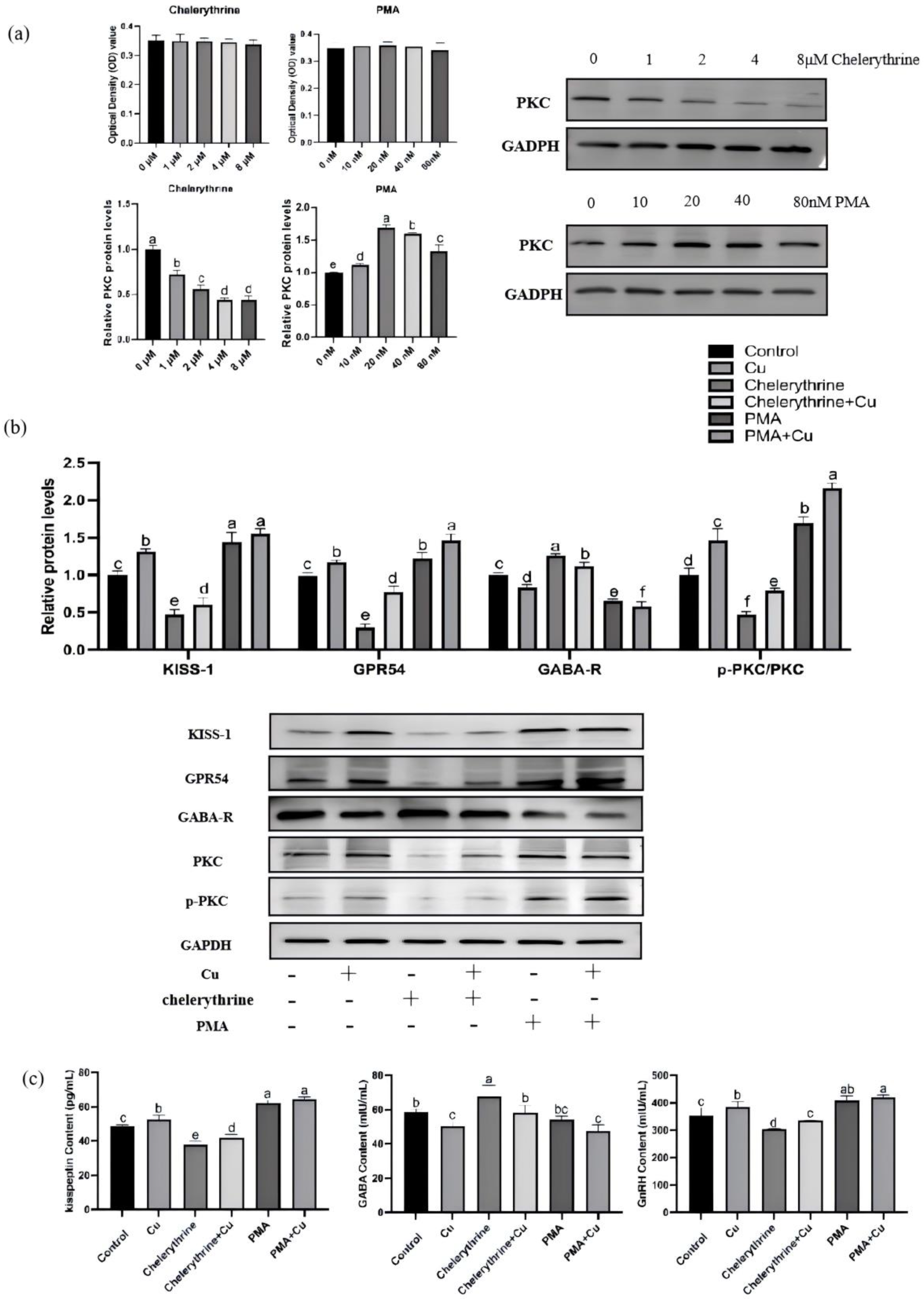
3.8. Effects of PMA and Chelerythrine Treatments on Copper-Regulated GABA and GnRH Pathways in Hypothalamic Mixed Neurons
4. Discussion
4.1. Dose-Dependent Regulation of Puberty by Copper
4.2. Activation of the HPOA Axis by Copper
4.3. Transcriptomic Insights: Involvement of GnRH and GABA Signaling Pathways
4.4. PKC as a Critical Convergence Point in Copper-Mediated Puberty Regulation
4.5. In Vitro Validation of the PKC-Centered Regulatory Pathway
4.6. Study Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gohil, A.; Eugster, E.A. Delayed and Precocious Puberty–Genetic Underpinnings and Treatments. Endocrinol. Metab. Clin. N. Am. 2020, 49, 741. [Google Scholar] [CrossRef]
- Mohanraj, S.; Prasad, H.K. Delayed Puberty. Indian J. Pediatr. 2023, 90, 590–597. [Google Scholar] [CrossRef]
- Wang, Y.; Gou, H.; Guo, J. Risk Factors for Precocious Puberty: A Systematic Review and Meta-Analysis. Psychoneuroendocrinology 2025, 176, 107427. [Google Scholar] [CrossRef]
- Ducharme, J.R.; Collu, R. Pubertal Development: Normal, Precocious and Delayed. Clin. Endocrinol. Metab. 1982, 11, 57–87. [Google Scholar] [CrossRef]
- Naulé, L.; Maione, L.; Kaiser, U.B. Puberty, a Sensitive Window of Hypothalamic Development and Plasticity. Endocrinology 2021, 162, bqaa209. [Google Scholar] [CrossRef]
- Kaprara, A.; Huhtaniemi, I.T. The Hypothalamus-Pituitary-Gonad Axis: Tales of Mice and Men. Metabolism 2018, 86, 3–17. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, T.S.; Augusto, K.V.Z.; Han, Y.; Sartori, M.M.P.; Denadai, J.C.; Santos, C.T.; Sobral, N.C.; Roça, R.O.; Sartori, J.R. High Levels of Copper and Zinc Supplementation in Broiler Diets on Growth Performance, Carcase Traits and Apparent Ileal Mineral Absorption. Br. Poult. Sci. 2021, 62, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garrido, M.A.; Tena-Sempere, M. Metabolic Control of Puberty: Roles of Leptin and Kisspeptins. Horm. Behav. 2013, 64, 187–194. [Google Scholar] [CrossRef]
- Faienza, M.F.; Urbano, F.; Moscogiuri, L.A.; Chiarito, M.; De Santis, S.; Giordano, P.J. Genetic, Epigenetic and Enviromental Influencing Factors on the Regulation of Precocious and Delayed Puberty. Front. Endocrinol. 2022, 13, 1019468. [Google Scholar] [CrossRef] [PubMed]
- Campisi, S.C.; Humayun, K.N.; Rizvi, A.; Lou, W.; Söder, O.; Bhutta, Z.A. Later Puberty Onset Among Chronically Undernourished Adolescents Living in a Karachi Slum, Pakistan. Acta Paediatr. 2020, 109, 1019–1025. [Google Scholar] [CrossRef]
- Iwasa, T.; Matsuzaki, T.; Murakami, M.; Fujisawa, S.; Kinouchi, R.; Gereltsetseg, G.; Kuwahara, A.; Yasui, T.; Irahara, M. Effects of Intrauterine Undernutrition on Hypothalamic Kiss1 Expression and the Timing of Puberty in Female Rats. J. Physiol. 2010, 588, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.-O.; Kröncke, K.-D.; Buchczyk, D.P.; Sies, H. Role of Copper, Zinc, Selenium and Tellurium in the Cellular Defense Against Oxidative and Nitrosative Stress. J. Nutr. 2003, 133, 1448S–1451S. [Google Scholar] [CrossRef]
- Sinquin, G.; Morfin, R.F.; Charles, J.-F.; Floch, H. Testosterone Metabolism by Homogenates of Human Prostates with Benign Hyperplasia: Effects of Zinc, Cadmium and Other Bivalent Cations. J. Steroid Biochem. 1984, 20, 773–780. [Google Scholar] [CrossRef]
- Peacey, L.; Elphick, M.R.; Jones, C.E. Roles of Copper in Neurokinin B and Gonadotropin-Releasing Hormone Structure and Function and the Endocrinology of Reproduction. Gen. Comp. Endocrinol. 2020, 287, 113342. [Google Scholar] [CrossRef]
- Wang, J.; Zou, Y.; Guan, R.; Tan, S.; Su, L.; Zhao, Z.; Cao, Z.; Jiang, K.; Wang, T.; Zheng, G.J. Copper Supplementation Alleviates Hypoxia-Induced Ferroptosis and Oxidative Stress in Neuronal cells. Int. J. Mol. Med. 2024, 54, 117. [Google Scholar] [CrossRef]
- Vega, P.J.; Llanillo, L.H. Wilson’s Disease: Forms of Presentation in Childhood. Gastroenterol. Hepatol. 2006, 29, 560–567. [Google Scholar]
- Kaler, S.G. Inborn Errors of Copper Metabolism. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 113, pp. 1745–1754. [Google Scholar] [PubMed]
- de Bie, P.; Muller, P.; Wijmenga, C.; Klomp, L.W.J. Molecular Pathogenesis of Wilson and Menkes Disease: Correlation of Mutations with Molecular Defects and Disease Phenotypes. J. Med. Genet. 2007, 44, 673–688. [Google Scholar] [CrossRef]
- Cicolari, S.; Dacrema, M.; Tsetegho Sokeng, A.J.; Xiao, J.; Atchan Nwakiban, A.P.; Di Giovanni, C.; Santarcangelo, C.; Magni, P.; Daglia, M. Hydromethanolic Extracts from Adansonia digitata L. Edible Parts Positively Modulate Pathophysiological Mechanisms Related to the Metabolic Syndrome. Molecules 2020, 25, 2858. [Google Scholar] [CrossRef]
- Wang, T.; Wu, L.; Chen, Q.; Chen, K.; Tan, F.; Liu, J.; Liu, X.; Han, H. Copper Deposition in Wilson’s Disease Causes Male Fertility Decline by Impairing Reproductive Hormone Release Through Inducing Apoptosis and Inhibiting ERK Signal in Hypothalamic-Pituitary of Mice. Front. Endocrinol. 2022, 13, 961748. [Google Scholar] [CrossRef] [PubMed]
- Tobet, S.A.; Schwarting, G.A. Minireview: Recent Progress in Gonadotropin-Releasing Hormone Neuronal Migration. Endocrinology 2006, 147, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Tsukamura, H.; Yamada, S.; Homma, T.; Inamoto, Y.; Uenoyama, Y.; Ohkura, S.; Inoue, N.; Maeda, K.-I. Metastin/Kisspeptin-GPR54 System Regulating Reproductive Functions in Mammals. Biol. Reprod. 2008, 78, 280. [Google Scholar] [CrossRef]
- Oakley, A.E.; Clifton, D.K.; Steiner, R.A. Kisspeptin Signaling in the Brain. Endocr. Rev. 2009, 30, 713–743. [Google Scholar] [CrossRef]
- Castellano, J.; Navarro, V.; Fernandez-Fernandez, R.; Nogueiras, R.; Tovar, S.; Roa, J.; Vazquez, M.; Vigo, E.; Casanueva, F.; Aguilar, E.; et al. Changes in Hypothalamic KiSS-1 System and Restoration of Pubertal Activation of the Reproductive Axis by Kisspeptin in Undernutrition. Endocrinology 2005, 146, 3917–3925. [Google Scholar] [CrossRef]
- Rønnekleiv, O.K.; Qiu, J.; Kelly, M.J. Hypothalamic Kisspeptin Neurons and the Control of Homeostasis. Endocrinology 2022, 163, bqab253. [Google Scholar] [CrossRef]
- Maffucci, J.A.; Gore, A.C. Hypothalamic Neural Systems Controlling the Female Reproductive Life Cycle: Gonadotropin-Releasing Hormone, Glutamate, and GABA. Int. Rev. Cell Mol. Biol. 2009, 274, 69–127. [Google Scholar]
- Taylor-Burds, C.; Cheng, P.; Wray, S. Chloride accumulators NKCC1 and AE2 in mouse GnRH neurons: Implications for GABAA mediated excitation. PLoS ONE 2015, 10, e0131076. [Google Scholar] [CrossRef] [PubMed]
- McGee, T.P.; Houston, C.M.; Brickley, S.G. Copper Block of Extrasynaptic GABAA Receptors in the Mature Cerebellum and Striatum. J. Neurosci. 2013, 33, 13431–13435. [Google Scholar] [CrossRef]
- Constantin, S.; Wray, S. Gonadotropin-Releasing Hormone-1 Neuronal Activity is Independent of Hyperpolarization-Activated Cyclic Nucleotide-Modulated Channels but Is Sensitive to Protein Kinase a-Dependent Phosphorylation. Endocrinology 2008, 149, 3500–3511. [Google Scholar] [CrossRef]
- Davis, C.D.; Johnson, W.T. Protein Kinase C Isozyme Protein and mRNA. BioFactors 2001, 15, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, J.; Zhou, B.; Li, P.; Hu, X.; Zhu, Z.; Tan, Y.; Chang, C.; Lü, J.; Song, B. Anomalous Behavior of Membrane Fluidity Caused by Copper-Copper Bond Coupled Phospholipids. Sci. Rep. 2018, 8, 14093. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Wang, Z.; Li, M.; Du, T.; Jia, S.; Yang, W.; Yang, L. Regulatory Effects of Copper on Ghrelin Secretion in Rat Fundic Glands. J. Anim. Physiol. Anim. Nutr. 2025, 109, 521–532. [Google Scholar] [CrossRef]
- Hebert, C. NTP Technical Report on the Toxicity Studies of Cupric Sulfate (CAS No. 7758-99-8) Administered in Drinking Water and Feed to F344/N Rats and B6C3F1 Mice. Toxic. Rep. Ser. 1993, 29, 1-D3. [Google Scholar]
- Sakhaee, E.; Emadi, L.; Abshenas, J.; Kheirandish, R.; Azari, O.; Amiri, E. Evaluation of Epididymal Sperm Quality Following Experimentally Induced Copper Poisoning in Male Rats. Andrologia 2012, 44, 110–116. [Google Scholar] [CrossRef]
- Radke, S.L.; Ensley, S.M.; Hansen, S.L. Inductively Coupled Plasma Mass Spectrometry Determination of Hepatic Copper, Manganese, Selenium, and Zinc Concentrations in Relation to Sample Amount and Storage Duration. J. Vet. Diagn. Investig. 2020, 32, 103–107. [Google Scholar] [CrossRef]
- Kawano, T.; Inokuchi, J.; Eto, M.; Murata, M.; Kang, J.-H. Activators and Inhibitors of Protein Kinase C (PKC): Their Applications in Clinical Trials. Pharmaceutics 2021, 13, 1748. [Google Scholar] [CrossRef] [PubMed]
- McGivern, R.F.; Yellon, S.M. Delayed Onset of Puberty and Subtle Alterations in GnRH Neuronal Morphology in Female Rats Exposed Prenatally to Ethanol. Alcohol 1992, 9, 335–340. [Google Scholar] [CrossRef]
- Kim, H.S.; Shin, J.-H.; Moon, H.J.; Kim, T.S.; Kang, I.H.; Seok, J.-H.; Kim, I.Y.; Park, K.L.; Han, S.Y. Evaluation of the 20-Day Pubertal Female Assay in Sprague-Dawley Rats Treated with DES, Tamoxifen, Testosterone, and Flutamide. Toxicol. Sci. 2002, 67, 52–62. [Google Scholar] [CrossRef][Green Version]
- Al-Othman, A.A.; Rosenstein, F.; Lei, K.Y. Pool Size and Concentration of Plasma Cholesterol Are Increased and Tissue Copper Levels Are Reduced During Early Stages of Copper Deficiency in Rats. J. Nutr. 1994, 124, 628–635. [Google Scholar] [CrossRef]
- Ghayour-Mobarhan, M.; Taylor, A.; New, S.; Lamb, D.; Ferns, G. Determinants of Serum Copper, Zinc and Selenium in Healthy Subjects. Ann. Clin. Biochem. 2005, 42, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, A.K.; Mogulkoc, R.; Belviranli, M. Serum Levels of Calcium, Selenium, Magnesium, Phosphorus, Chromium, Copper and Iron—Their Relation to Zinc in Rats with Induced Hypothyroidism. Acta Clin. Croat 2013, 52, 151–156. [Google Scholar]
- Merriam, G.R.; Nunnelley, L.L.; Trish, J.W.; Naftolin, F. Sex-Related and Cyclic Variation of Trace Elements in Rat Hypothalamus and Pituitary. Brain Res. 1979, 171, 503–510. [Google Scholar] [CrossRef]
- Abel, M.H.; Wootton, A.N.; Wilkins, V.; Huhtaniemi, I.; Knight, P.G.; Charlton, H.M. The Effect of a Null Mutation in the Follicle-Stimulating Hormone Receptor Gene on Mouse Reproduction. Endocrinology 2000, 141, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Schvartz, I.; Hazum, E. Copper Induces Luteinizing Hormone Release and Desensitization of Pituitary Gonadotropes. Biochem. Biophys. Res. Commun. 1986, 136, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Clayton, R.N. Role of GnRH in the Maturation of Pituitary Gonadotroph Function. Reproduction 1985, 75, 307–315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Millar, R.P. GnRHs and GnRH Receptors. Anim. Reprod. Sci. 2005, 88, 5–28. [Google Scholar] [CrossRef]
- Tzoupis, H.; Nteli, A.; Androutsou, M.-E.; Tselios, T. Gonadotropin-Releasing Hormone and GnRH Receptor: Structure, Function and Drug Development. Curr. Med. Chem. 2020, 27, 6136–6158. [Google Scholar] [CrossRef]
- Michaluk, A.; Kochman, K. Involvement of Copper in Female Reproduction. Reprod. Biol. 2007, 7, 193–205. [Google Scholar]
- Tran, K.K.; Jayawardena, B.M.; Elphick, M.R.; Jones, C.E. A Gonadotropin-Releasing Hormone Type Neuropeptide with a High Affinity Binding Site for Copper (II) and Nickel (II). Metallomics 2019, 11, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Colledge, W.H. Kisspeptins and GnRH Neuronal Signalling. Trends Endocrinol. Metab. 2009, 20, 115–121. [Google Scholar] [CrossRef]
- Weaver, A.; Kelly, J.; Kind, K.; Gatford, K.; Kennaway, D.; Herde, P.; Van Wettere, W.H.E.J. Oocyte Maturation and Embryo Survival in Nulliparous Female Pigs (gilts) Is Improved by Feeding a Lupin-Based High-Fibre Diet. Reprod. Fertil. Dev. 2013, 25, 1216–1223. [Google Scholar] [CrossRef]
- Han, S.-K.; Gottsch, M.L.; Lee, K.J.; Popa, S.M.; Smith, J.T.; Jakawich, S.K.; Clifton, D.K.; Steiner, R.A.; Herbison, A.E. Activation of Gonadotropin-Releasing Hormone Neurons by Kisspeptin as a Neuroendocrine Switch for the Onset of Puberty. J. Neurosci. 2005, 25, 11349–11356. [Google Scholar] [CrossRef] [PubMed]
- Messager, S.; Chatzidaki, E.E.; Ma, D.; Hendrick, A.G.; Zahn, D.; Dixon, J.; Thresher, R.R.; Malinge, I.; Lomet, D.; Carlton, M.B.L.; et al. Kisspeptin Directly Stimulates Gonadotropin-Releasing Hormone Release via G Protein-Coupled Receptor 54. Proc. Natl. Acad. Sci. USA 2005, 102, 1761–1766. [Google Scholar] [CrossRef]
- Pielecka-Fortuna, J.; Chu, Z.; Moenter, S.M. Kisspeptin Acts Directly and Indirectly to Increase Gonadotropin-Releasing Hormone Neuron Activity and Its Effects Are Modulated by Estradiol. Endocrinology 2008, 149, 1979–1986. [Google Scholar] [CrossRef]
- Quaynor, S.; Hu, L.; Leung, P.K.; Feng, H.; Mores, N.; Krsmanovic, L.Z.; Catt, K.J. Expression of a Functional G Protein-Coupled Receptor 54-Kisspeptin Autoregulatory System in Hypothalamic Gonadotropin-Releasing Hormone Neurons. Mol. Endocrinol. 2007, 21, 3062–3070. [Google Scholar] [CrossRef]
- Gottsch, M.L.; Cunningham, M.J.; Smith, J.T.; Popa, S.M.; Acohido, B.V.; Crowley, W.F.; Seminara, S.; Clifton, D.K.; Steiner, R.A. A Role for Kisspeptins in the Regulation of Gonadotropin Secretion in the Mouse. Endocrinology 2004, 145, 4073–4077. [Google Scholar] [CrossRef]
- Irwig, M.S.; Fraley, G.S.; Smith, J.T.; Acohido, B.V.; Popa, S.M.; Cunningham, M.J.; Gottsch, M.L.; Clifton, D.K.; Steiner, R.A. Kisspeptin Activation of Gonadotropin Releasing Hormone Neurons and Regulation of KiSS-1 mRNA in the Male Rat. Neuroendocrinology 2005, 80, 264–272. [Google Scholar] [CrossRef]
- Smith, J.T.; Acohido, B.V.; Clifton, D.K.; Steiner, R.A. KiSS-1 Neurones Are Direct Targets for Leptin in the ob/ob Mouse. J. Neuroendocrinol. 2006, 18, 298–303. [Google Scholar] [CrossRef]
- Tomikawa, J.; Homma, T.; Tajima, S.; Shibata, T.; Inamoto, Y.; Takase, K.; Inoue, N.; Ohkura, S.; Uenoyama, Y.; Maeda, K.-I.; et al. Molecular Characterization and Estrogen Regulation of Hypothalamic KISS1 Gene in the Pig. Biol. Reprod. 2010, 82, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Harlow, K.; Griesgraber, M.J.; Seman, A.D.; Shuping, S.L.; Sommer, J.R.; Griffith, E.H.; Hileman, S.M.; Nestor, C.C. The Impact of Undernutrition on KNDy (Kisspeptin/Neurokinin B/Dynorphin) Neurons in Female Lambs. J. Neuroendocrinol. 2022, 34, e13135. [Google Scholar] [CrossRef] [PubMed]
- Hoogeveen, R.C.; Reaves, S.K.; Reid, P.M.; Reid, B.L.; Lei, K.Y. Copper Deficiency Shifts Energy Substrate Utilization from Carbohydrate to Fat and Reduces Fat Mass in Rats. J. Nutr. 1994, 124, 1660–1666. [Google Scholar] [CrossRef]
- Tolbert, M.E.; Kamalu, J.A.; Draper, G.D. Effects of Cadmium, Zinc, Copper and Manganese on Hepatic Parenchymal Cell Gluconeogenesis. J. Environ. Sci. Health Part B 1981, 16, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Fadlalla, I.; Barri, M. A Possible Association Between Dietary Intake of Copper, Zinc and Phosphate and Delayed Puberty in Heifers in Sudan. Trop. Anim. Health Prod. 2002, 34, 75–80. [Google Scholar] [CrossRef]
- Binesh, A.; Venkatachalam, K. Copper in Human Health and Disease: A Comprehensive Review. J. Biochem. Mol. Toxicol. 2024, 38, e70052. [Google Scholar] [CrossRef]
- Bhat, R.; Axtell, R.; Mitra, A.; Miranda, M.; Lock, C.; Tsien, R.W.; Steinman, L. Inhibitory Role for GABA in Autoimmune Inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 2580–2585. [Google Scholar] [CrossRef]
- Catavero, C.; Bao, H.; Song, J. Neural Mechanisms Underlying GABAergic Regulation of Adult Hippocampal Neurogenesis. Cell Tissue Res. 2018, 371, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Ligon, B.; Yang, J.; Morin, S.; Ruberti, M.; Steer, M.L. Regulation of Pancreatic Islet Cell Survival and Replication by γ-Aminobutyric Acid. Diabetologia 2007, 50, 764–773. [Google Scholar] [CrossRef]
- Wu, L.-X.; Xu, Y.-C.; Pantopoulos, K.; Tan, X.-Y.; Wei, X.-L.; Zheng, H.; Luo, Z. Glycophagy Mediated Glucose-Induced Changes of Hepatic Glycogen Metabolism via OGT1-AKT1-FOXO1Ser238 Pathway. J. Nutr. Biochem. 2023, 117, 109337. [Google Scholar] [CrossRef]
- Herbison, A.E.; Moenter, S.M. Depolarising and Hyperpolarising Actions of GABAA Receptor Activation on Gonadotrophin-Releasing Hormone Neurones: Towards an Emerging Consensus. J. Neuroendocrinol. 2011, 23, 557–569. [Google Scholar] [CrossRef]
- Hozumi, A.; Matsunobu, S.; Mita, K.; Treen, N.; Sugihara, T.; Horie, T.; Sakuma, T.; Yamamoto, T.; Shiraishi, A.; Hamada, M.; et al. GABA-Induced GnRH Release Triggers Chordate Metamorphosis. Curr. Biol. 2020, 30, 1555–1561.e4. [Google Scholar] [CrossRef] [PubMed]
- Piet, R.; De Croft, S.; Liu, X.; Herbison, A.E. Electrical Properties of Kisspeptin Neurons and Their Regulation of GnRH Neurons. Front. Neuroendocrinol. 2015, 36, 15–27. [Google Scholar] [CrossRef]
- Piet, R.; Kalil, B.; McLennan, T.; Porteous, R.; Czieselsky, K.; Herbison, A.E. Dominant Neuropeptide Cotransmission in Kisspeptin-GABA Regulation of GnRH Neuron Firing Driving Ovulation. J. Neurosci. 2018, 38, 6310–6322. [Google Scholar] [CrossRef]
- Plant, T.M. The Role of KiSS-1 in the Regulation of Puberty in Higher Primates. Eur. J. Endocrinol. 2006, 155, S11–S16. [Google Scholar] [CrossRef]
- Bown, A.W.; Shelp, B.J. The Metabolism and Functions of [Gamma]-Aminobutyric acid. Plant Physiol. 1997, 115, 1. [Google Scholar] [CrossRef]
- Nakamura, U.; Nohmi, T.; Sagane, R.; Hai, J.; Ohbayashi, K.; Miyazaki, M.; Yamatsu, A.; Kim, M.; Iwasaki, Y. Dietary Gamma-Aminobutyric Acid (GABA) Induces Satiation by Enhancing the Postprandial Activation of Vagal Afferent Nerves. Nutrients 2022, 14, 2492. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Sobolev, A.P.; Nabavi, S.F.; Sureda, A.; Moghaddam, A.H.; Khanjani, S.; Xiao, J.; Shirooie, S.; Tsetegho Sokeng, A.J.; Baldi, A.; et al. Post-Stroke Depression: Antidepressive Effects of a Chemically Characterized Maqui Berry Extract in a Mouse Model (Aristotelia Chilensis (Molina) Stuntz). Food Chem. Toxicol. 2019, 131, 110641. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, J.; Liu, L.; Zhu, X.; Wang, X.; Liu, Z.; Wang, Z.; Yang, L.; Liu, G. Effect of High Dietary Copper on Somatostatin and Growth Hormone-Releasing Hormone Levels in the Hypothalami of Growing Pigs. Biol. Trace Elem. Res. 2011, 143, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Baumann, C.; Viveiros, M.M. Lack of Protein Kinase C-Delta (PKCδ) Disrupts Fertilization and Embryonic Development. Mol. Reprod. Dev. 2015, 82, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Mondadori, R.; Neves, J.; Goncalves, P. Protein Kinase C (PKC) Role in Bovine Oocyte Maturation and Early Embryo Development. Anim. Reprod. Sci. 2008, 107, 20–29. [Google Scholar] [CrossRef]
- Dyck, J.R.; Kudo, N.; Barr, A.J.; Davies, S.P.; Hardie, D.G.; Lopaschuk, G.D. Phosphorylation Control of Cardiac Acetyl-CoA Carboxylase by cAMP-Dependent Protein Kinase and 5′-AMP Activated Protein Kinase. Eur. J. Biochem. 1999, 262, 184–190. [Google Scholar] [CrossRef]
- Klausen, C.; Severson, D.L.; Chang, J.P.; Habibi, H.R. Role of PKC in the Regulation of Gonadotropin Subunit mRNA Levels: Interaction with Two Native Forms of Gonadotropin-Releasing Hormone. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2005, 289, R1634–R1643. [Google Scholar] [CrossRef][Green Version]
- Naor, Z.; Harris, D.; Shacham, S. Mechanism of GnRH Receptor Signaling: Combinatorial Cross-Talk of Ca2+ and Protein Kinase C. Front. Neuroendocrinol. 1998, 19, 1–19. [Google Scholar] [CrossRef]
- Lau, C.G.; Takayasu, Y.; Rodenas-Ruano, A.; Paternain, A.V.; Lerma, J.; Bennett, M.V.; Zukin, R.S. SNAP-25 Is a Target of Protein Kinase C Phosphorylation Critical to NMDA Receptor Trafficking. J. Neurosci. 2010, 30, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Matti, U.; Nehring, R.B.; Binz, T.; Rettig, J.; Neher, E.; Sørensen, J.B. Protein Kinase C-Dependent Phosphorylation of Synaptosome-Associated Protein of 25 kDa at Ser187 Potentiates Vesicle Recruitment. J. Neurosci. 2002, 22, 9278–9286. [Google Scholar] [CrossRef] [PubMed]
- Bright, D.P.; Smart, T.G. Protein Kinase C Regulates Tonic GABAA Receptor-Mediated Inhibition in the Hippocampus and Thalamus. Eur. J. Neurosci. 2013, 38, 3408–3423. [Google Scholar] [CrossRef]
- Lien, C.-F.; Chen, S.-J.; Tsai, M.-C.; Lin, C.-S. Potential Role of Protein Kinase C in the Pathophysiology of Diabetes-Associated Atherosclerosis. Front. Pharmacol. 2021, 12, 716332. [Google Scholar] [CrossRef] [PubMed]
- Mahato, B.; Home, P.; Rajendran, G.; Paul, A.; Saha, B.; Ganguly, A.; Ray, S.; Roy, N.; Swerdlow, R.H.; Paul, S. Regulation of Mitochondrial Function and Cellular Energy Metabolism by Protein Kinase C-λ/ι: A Novel Mode of Balancing Pluripotency. Stem Cells 2014, 32, 2880–2892. [Google Scholar] [CrossRef]
- Mehta, K.D. Emerging Role of Protein Kinase C in Energy Homeostasis: A Brief Overview. World J. Diabetes 2014, 5, 385. [Google Scholar] [CrossRef]
- Gabryel-Skrodzka, M.; Nowak, M.; Grajewski, J.; Jastrząb, R. Biocoordination Reactions in Copper (II) Ions and Phosphocholine Systems Including Pyrimidine Nucleosides and Nucleotides. Sci. Rep. 2023, 13, 10787. [Google Scholar] [CrossRef]
| Items | A | B | C | D | E |
|---|---|---|---|---|---|
| Hypothalamus (mg/kg) | 2.77 ± 0.07 | 2.91 ± 0.29 | 2.92 ± 0.30 | 3.10 ± 0.31 | 3.15 ± 0.33 |
| Serum (mg/L) | 1.20 ± 0.11 | 1.43 ± 0.15 | 1.59 ± 0.05 | 1.64 ± 0.23 | 1.74 ± 0.23 |
| Items | A | B | C | D | E |
|---|---|---|---|---|---|
| GnRH (mIU/mL) | 63.23 ± 2.90 d | 68.48 ± 3.41 b,c | 70.69 ± 6.06 a,b | 74.71 ± 3.87 a | 65.08 ± 2.63 c,d |
| FSH (mIU/mL) | 9.24 ± 0.13 b | 9.43 ± 0.22 b | 10.05 ± 0.41 a | 9.43 ± 0.21 b | 9.17 ± 0.28 b |
| LH (mIU/mL) | 30.73 ± 3.31 c | 35.41 ± 1.11 b | 39.24 ± 2.86 a | 40.05 ± 1.23 a | 24.8 ± 1.14 d |
| E2 (pmol/mL) | 45.69 ± 2.60 b | 45.27 ± 3.95 b | 49.14 ± 2.78 a | 49.56 ± 1.55 a | 45.41 ± 1.20 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, R.; Wang, Z.; Li, C.; Li, M.; Yang, W.; Yang, L. Dietary Copper on the Onset of Puberty in Rats: Possible Mechanism. Nutrients 2025, 17, 3534. https://doi.org/10.3390/nu17223534
Sun R, Wang Z, Li C, Li M, Yang W, Yang L. Dietary Copper on the Onset of Puberty in Rats: Possible Mechanism. Nutrients. 2025; 17(22):3534. https://doi.org/10.3390/nu17223534
Chicago/Turabian StyleSun, Rui, Zhongshen Wang, Cheng Li, Meng Li, Wenyan Yang, and Lianyu Yang. 2025. "Dietary Copper on the Onset of Puberty in Rats: Possible Mechanism" Nutrients 17, no. 22: 3534. https://doi.org/10.3390/nu17223534
APA StyleSun, R., Wang, Z., Li, C., Li, M., Yang, W., & Yang, L. (2025). Dietary Copper on the Onset of Puberty in Rats: Possible Mechanism. Nutrients, 17(22), 3534. https://doi.org/10.3390/nu17223534




