Neurodevelopmental Changes in the Guinea Pig Brain Caused by Time-Limited Complete Vitamin C Deprivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Environment
2.2. Experimental Protocol
2.3. Fixation and Histology Procedure
2.4. Determination of Hydroxyproline Concentration
2.5. Isolation of RNA
2.6. Reverse Transcription Reaction
2.7. Relative Quantification of mRNA in Real-Time PCR
2.8. Statistical Analysis
3. Results
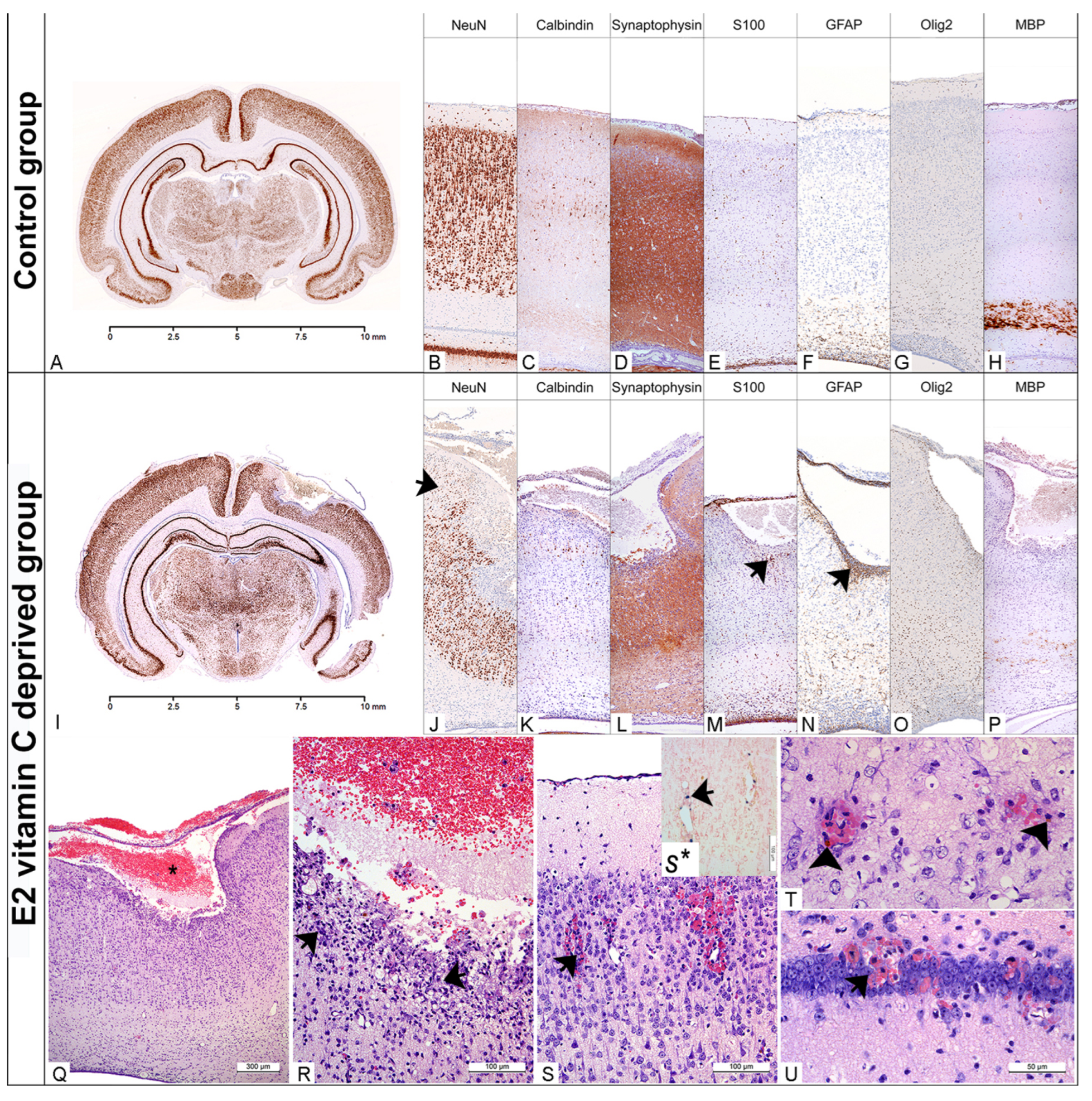
3.1. Morphological Analysis of Cerebellar and Cerebral Tissue
3.1.1. Macroscopic Cerebellar and Cerebral Analysis
3.1.2. Microscopic Cerebral Analysis
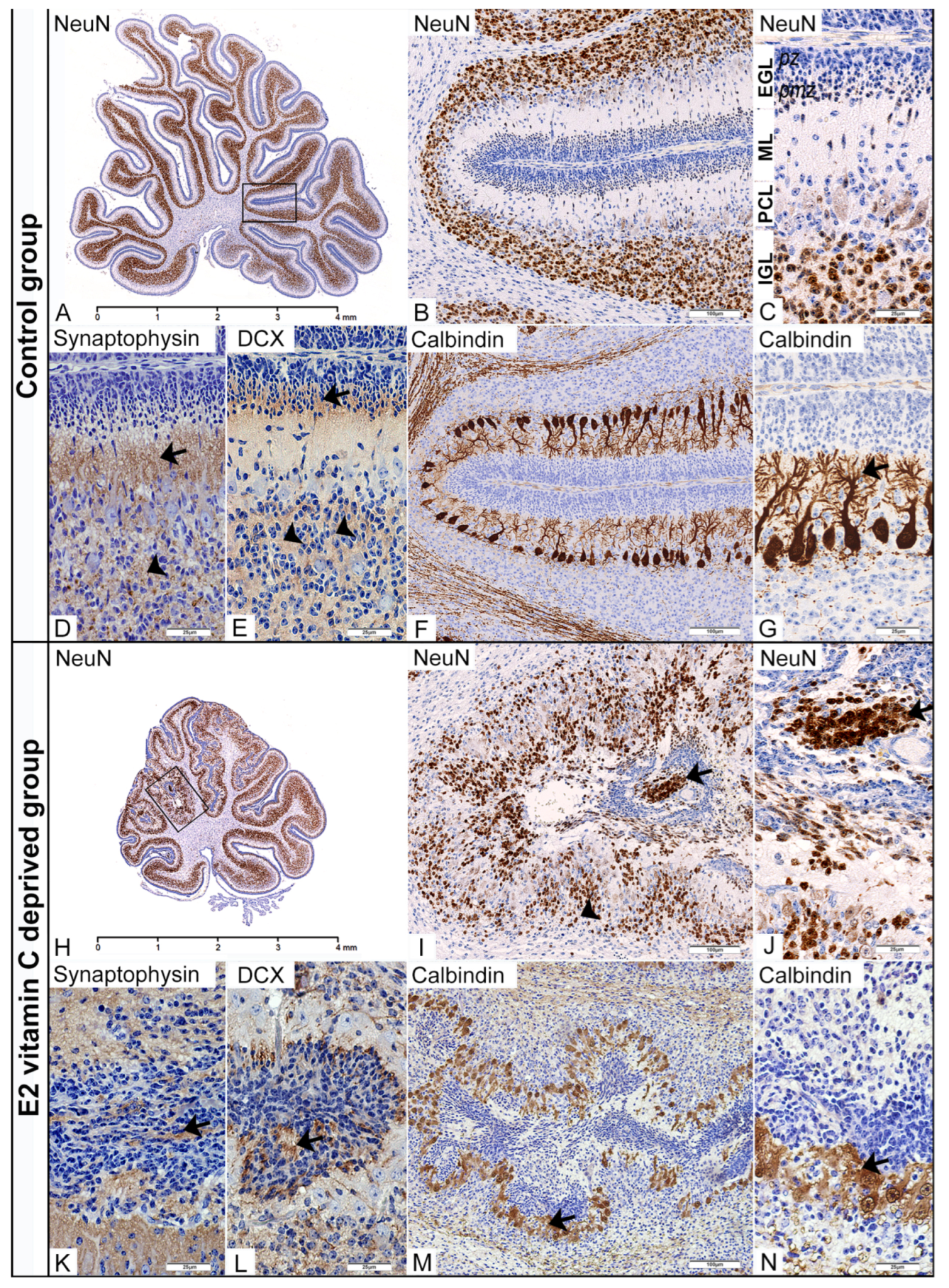
3.1.3. Microscopic Cerebellar Analysis
- Specificity of normal cortical neurogenesis in cerebellum of the control group of foetuses
- Specificity of aberrant cortical neurogenesis in cerebellum of vitamin C-deprived foetuses
- Specificity of Cytoarchitectonic Disturbance of Bergman Glia Cells and the Process of Gliogenesis
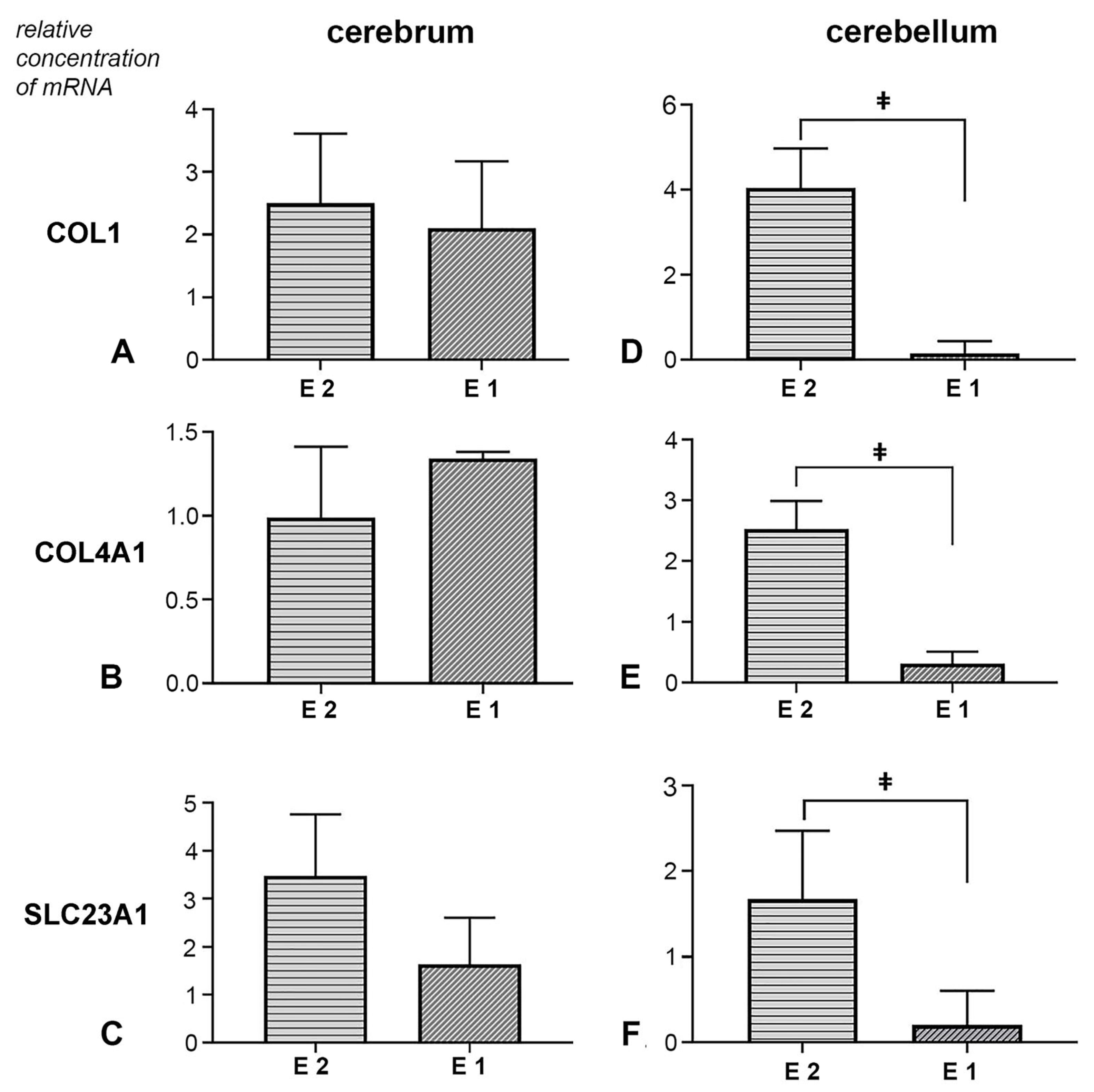
3.2. Biochemical Analysis of the Amount of Hydroxyproline in Cerebellar and Cerebral Tissue Samples
3.3. Molecular Analysis of COL1, COL4A1 and SLC23A1 Gene Expression in Cerebral and Cerebellar Tissue Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GULO | L-Gulonolactone oxidase |
| PMB | pial basal membrane |
| EGL | external granular layer |
| ML | molecular layer |
| IGL | internal granular layer |
| PCL | Purkinje cell layer |
References
- Sato, P.; Udenfriend, S. Scurvy-prone animals, including man, monkey, and guinea pig, do not express the gene for gulonolactone oxidase. Arch. Biochem. Biophys. 1978, 187, 158–162. [Google Scholar] [CrossRef]
- Drouin, G.; Godin, J.R.; Pagé, B. The genetics of vitamin C loss in vertebrates. Curr. Genom. 2011, 12, 371–378. [Google Scholar] [CrossRef]
- Nishikimi, M.; Kawai, T.; Yagi, K. Guinea pigs possess a highly mutated gene for L-gulono-gamma-lactone oxidase, the key enzyme for L-ascorbic acid biosynthesis, missing in this species. J. Biol. Chem. 1992, 267, 21967–21972. [Google Scholar] [CrossRef]
- Grant, M.E.; Prockop, D.J. The biosynthesis of collagen—(Third of Three Parts). N. Engl. J. Med. 1972, 286, 291–300. [Google Scholar] [CrossRef]
- Grosso, G.; Bei, R.; Mistretta, A.; Marventano, S.; Calabrese, G.; Masuelli, L.; Giganti, M.G.; Modesti, A.; Galvano, F.; Gazzolo, D. Effects of vitamin C on health: A review of evidence. Front. Biosci. 2013, 18, 1017–1029. [Google Scholar] [CrossRef]
- Popovich, D.; McAlhany, A.; Adewumi, A.O.; Barnes, M.M. Scurvy: Forgotten but definitely not gone. J. Pediatr. Health Care 2009, 23, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Velandia, B.; Centor, R.M.; McConnell, V.; Shah, M. Scurvy is still present in developed countries. J. Gen. Intern. Med. 2008, 23, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.J.; Constable, B.J.; Kodicek, E. Studies in vivo on the biosynthesis of collagen and elastin in ascorbic acid-deficient guinea pigs. Biochem. J. 1969, 113, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Gore, I.; Wada, M.; Goodman, M.L. Capillary hemorrhage in ascorbic-acid-deficient guinea pigs. Ultrastructural basis. Arch. Pathol. 1968, 85, 493–502. [Google Scholar]
- Sievers, J.; von Knebel, D.C.; Pehlemann, F.W.; Berry, M. Meningeal cells influence cerebellar development over a critical period. Anat. Embryol. 1986, 175, 91–100. [Google Scholar] [CrossRef]
- Halfter, W.; Dong, S.; Yip, Y.-P.; Willem, M.; Mayer, U. A critical function of the pial basement membrane in cortical histogenesis. J. Neurosci. 2002, 22, 6029–6040. [Google Scholar] [CrossRef]
- Nguyen, H.; Ostendorf, A.P.; Satz, J.S.; Westra, S.; Ross-Barta, S.E.; Campbell, K.P.; Moore, S.A. Glial scaffold required for cerebellar granule cell migration is dependent on dystroglycan function as a receptor for basement membrane proteins. Acta Neuropathol. Commun. 2013, 1, 58. [Google Scholar] [CrossRef] [PubMed]
- Pöschl, E.; Schlötzer-Schrehardt, U.; Brachvogel, B.; Saito, K.; Ninomiya, Y.; Mayer, U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 2004, 131, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa-Tomikawa, N.; Ogawa, J.; Douet, V.; Xu, Z.; Kamikubo, Y.; Sakurai, T.; Kohsaka, S.; Chiba, H.; Hattori, N.; Yamada, Y.; et al. Laminin α1 is essential for mouse cerebellar development. Matrix Biol. 2012, 31, 17–28. [Google Scholar] [CrossRef]
- Li, J.; Yu, M.; Feng, G.; Hu, H.; Li, X. Breaches of the pial basement membrane are associated with defective dentate gyrus development in mouse models of congenital muscular dystrophies. Neurosci. Lett. 2011, 505, 19–24. [Google Scholar] [CrossRef]
- Graus-Porta, D.; Blaess, S.; Senften, M.; Littlewood-Evans, A.; Damsky, C.; Huang, Z.; Orban, P.; Klein, R.; Schittny, J.C.; Müller, U. β1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron 2001, 31, 367–379. [Google Scholar] [CrossRef]
- Labelle-Dumais, C.; Dilworth, D.J.; Harrington, E.P.; de Leau, M.; Lyons, D.; Kabaeva, Z.; Manzini, M.C.; Dobyns, W.B.; Walsh, C.A.; Michele, D.E.; et al. COL4A1 mutations cause ocular dysgenesis, neuronal localization defects, and myopathy in mice and Walker-Warburg syndrome in humans. PLoS Genet. 2011, 7, e1002062. [Google Scholar] [CrossRef]
- Coker, S.J.; Berry, M.J.; Vissers, M.C.M.; Dyson, R.M. Maternal Vitamin C Intake during Pregnancy Influences Long-Term Offspring Growth with Timing- and Sex-Specific Effects in Guinea Pigs. Nutrients 2024, 16, 369. [Google Scholar] [CrossRef] [PubMed]
- Coker, S.J.; Dyson, R.M.; Smith-Díaz, C.C.; Vissers, M.C.M.; Berry, M.J. Effects of Low Vitamin C Intake on Fertility Parameters and Pregnancy Outcomes in Guinea Pigs. Nutrients 2023, 15, 4107. [Google Scholar] [CrossRef]
- Schjoldager, J.G.; Paidi, M.D.; Lindblad, M.M.; Birck, M.M.; Kjærgaard, A.B.; Dantzer, V.; Lykkesfeldt, J.; Tveden-Nyborg, P. Maternal vitamin C deficiency during pregnancy results in transient fetal and placental growth retardation in guinea pigs. Eur. J. Nutr. 2015, 54, 667–676. [Google Scholar] [CrossRef]
- Hansen, S.N.; Jørgensen, J.M.B.; Nyengaard, J.R.; Lykkesfeldt, J.; Tveden-Nyborg, P. Early Life Vitamin C Deficiency Does Not Alter Morphology of Hippocampal CA1 Pyramidal Neurons or Markers of Synaptic Plasticity in a Guinea Pig Model. Nutrients 2018, 10, 749. [Google Scholar] [CrossRef]
- Tveden-Nyborg, P.; Johansen, L.K.; Raida, Z.; Villumsen, C.K.; Larsen, J.O.; Lykkesfeldt, J. Vitamin C deficiency in early postnatal life impairs spatial memory and re-duces the number of hippocampal neurons in guinea pigs. Am. J. Clin. Nutr. 2009, 90, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Paidi, M.D.; Schjoldager, J.G.; Lykkesfeldt, J.; Tveden–Nyborg, P. Prenatal vitamin C deficiency results in differential levels of oxidative stress during late gestation in foetal guinea pig brains. Redox. Biol. 2014, 2, 361–367. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Perez, T.G.; Poulsen, H.E.; Christen, S. Vitamin C deficiency in weanling guinea pigs: Differential expression of oxidative stress and DNA repair in liver and brain. Br. J. Nutr. 2007, 98, 1116–1119. [Google Scholar] [CrossRef]
- Čapo, I.; Hinić, N.; Lalošević, D.; Vučković, N.; Stilinović, N.; Marković, J.; Sekulić, S. Vitamin C Depletion in Prenatal Guinea Pigs as a Model of Lissencephaly Type II. Vet. Pathol. 2015, 52, 1263–1271. [Google Scholar] [CrossRef]
- Mary, T.H.; Leonard, E. Some Embryological Aspects of Vitamin C-Deficiency in the Guinea Pig (Cavia Cobaya). Trans. Kans. Acad. Sci. 1951, 54, 42–57. [Google Scholar] [CrossRef][Green Version]
- van der Veer, B.K.; Custers, C.; Brangers, W.; Cornelis, R.; Tsaniras, S.C.; Ridder, K.; Thienpont, B.; Cheng, H.; Chen, Q.; Kraushaar, D.; et al. Maternal Vitamin C Deficiency and Genetic Risk Factors Contribute to Congenital Defects through Dysregulation of DNA Methylation. bioRxiv 2025. [Google Scholar] [CrossRef]
- Harrison, F.E.; Dawes, S.M.; Meredith, M.E.; Babaev, V.R.; Li, L.; May, J.M. Low vitamin C and increased oxidative stress and cell death in mice that lack the sodium-dependent vitamin C transporter SVCT2. Free Radic. Biol. Med. 2010, 49, 821–829. [Google Scholar] [CrossRef]
- Gould, D.B.E.; Phalan, F.C.E.; Breedveld, G.J.E.; van Mil, S.E.E.; Smith, R.S.E.; Schimenti, J.C.E.; Aguglia, U.E.; van der Knaap, M.S.E.; Heutink, P.E.; John, S.W. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science 2005, 308, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.; Sonnberger, M.; Dunzinger, A.; Voglmayr, E.; Aichholzer, M.; Kleiser, R.; Strasser, P. Imaging Brain Diseases: A Neuroradiology, Nuclear Medicine, Neurosurgery, Neuropathology and Molecular Biology-based Approach, 1st ed.; Springer: Berlin, Germany, 2020; p. 484. [Google Scholar]
- Putbrese, B.; Kennedy, A. Findings and differential diagnosis of fetal intracranial haemorrhage and fetal ischaemic brain injury: What is the role of fetal MRI? Br. J. Radiol. 2017, 90, 20160253. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodian, F.; Peterkofsky, B. Vitamin C deficiency in guinea pigs differentially affects the expression of type IV colagen, laminin, and elastin in blood vessels. J. Nutr. 1999, 129, 83–91. [Google Scholar] [CrossRef][Green Version]
- Willem, M.; Miosge, N.; Halfter, W.; Smyth, N.; Jannetti, I.; Burghart, E.; Timpl, R.; Mayer, U. Specific ablation of the nidogen-binding site in the laminin gamma1 chain interferes with kidney and lung development. Development 2002, 129, 2711–2722. [Google Scholar] [CrossRef]
- Larsell, O. The morphogenesis and adult pattern of the lobules and fissures of the cerebellum of the white rat. J. Comp. Neurol. 1952, 97, 281–356. [Google Scholar] [CrossRef] [PubMed]
- Mangold, U.; Sievers, J.; Berry, M. 6-Hydroxydopamine induced ectopia of external granule cells in the subarachnoid space covering the cerebellum. II. Differentiation of granule cells: A scanning and transmission electron microscopic study. J. Comp. Neurol. 1984, 227, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Sievers, J.; Mangold, U.; Berry, M. 6-OHDA-induced ectopia of external granule cells in the subarachnoid space covering the cerebellum. Genesis and topography. Cell Tissue Res. 1983, 230, 309–336. [Google Scholar] [CrossRef] [PubMed]
- Adle-Biassette, H.; Golden, J.A.; Harding, B. Developmental and perinatal brain diseases. Handb. Clin. Neurol. 2017, 145, 51–78. [Google Scholar] [CrossRef]
- Holzfeind, P.J.; Grewal, P.K.; Reitsamer, H.A.; Kechvar, J.; Lassmann, H.; Hoeger, H.; Hewitt, J.E.; Bittner, R.E. Skeletal, cardiac and tongue muscle pathology, defective retinal transmission, and neuronal migration defects in the Largemyd mouse defines a natural model for glycosylation-deficient muscle—Eye—Brain disorders. Hum. Mol. Genet. 2002, 11, 2673–2687. [Google Scholar] [CrossRef]
- Devisme, L.; Bouchet, C.; Gonzalès, M.; Alanio, E.; Bazin, A.; Bessières, B.; Bigi, N.; Blanchet, P.; Bonneau, D.; Bonnières, M.; et al. Cobblestone lissencephaly: Neuropathological subtypes and correlations with genes of dystroglycanopathies. Brain 2012, 135, 469–482. [Google Scholar] [CrossRef]
- Hu, H.; Yang, Y.; Eade, A.; Xiong, Y.; Qi, Y. Breaches of the pial basement membrane and disappearance of the glia limitans during development underlie the cortical lamination defect in the mouse model of muscle-eye-brain disease. J. Comp. Neurol. 2007, 501, 168–183. [Google Scholar] [CrossRef]
- Pogledic, I.; Mankad, K.; Severino, M.; Lerman-Sagie, T.; Jakab, A.; Hadi, E.; Jansen, A.C.; Bahi-Buisson, N.; Di Donato, N.; Oegema, R.; et al. Prenatal assessment of brain malformations on neuroimaging: An expert panel review. Brain 2024, 147, 3982–4002. [Google Scholar] [CrossRef]
- Colgrave, M.L.; Allingham, P.G.; Tyrrell, K.; Jones, A. Multiple Reaction Monitoring for the Accurate Quantification of Amino Acids: Using Hydroxyproline to Estimate Collagen Content. In Amino Acid Analysis: Methods in Molecular Biology; Alterman, M., Ed.; Humana: New York, NY, USA, 2019; Volume 2030, pp. 33–45. [Google Scholar] [CrossRef]
- Bates, C.J.; Tsuchiya, H. Comparison of vitamin C deficiency with food restriction on collagen cross-link ratios in bone, urine and skin of weanling guinea-pigs. Br. J. Nutr. 2003, 89, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Bates, C.J. Ascorbic acid deficiency in guinea pigs: Contrasting effects of tissue ascorbic acid depletion and of associated inanition on status indices related to collagen and vitamin D. Br. J. Nutr. 1994, 72, 745–752. [Google Scholar] [CrossRef]
- Nováková, V.; Hrubá, F.; Mrhová, O.; Ginter, E.; Masek, J. The effect of vitamin C deficiency on the aorta of the guinea pig. Atherosclerosis 1982, 43, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Rivers, J.M.; Krook, L.; Cormier, A. Biochemical and histological study of guinea pig fetal and uterine tissue in ascorbic acid deficiency. J. Nutr. 1970, 100, 217–227. [Google Scholar] [CrossRef]
- Parsons, K.K.; Maeda, N.; Yamauchi, M.; Banes, A.J.; Koller, B.H. Ascorbic acid-independent synthesis of collagen in mice. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1131–E1139. [Google Scholar] [CrossRef]
- Qiu, B.; Wei, F.; Sun, X.; Wang, X.; Duan, B.; Shi, C.; Zhang, J.; Zhang, J.; Qiu, W.; Mu, W. Measurement of hydroxyproline in collagen with three different methods. Mol. Med. Rep. 2014, 10, 1157–1163. [Google Scholar] [CrossRef]
- Barnes, M.J.; Constable, B.J.; Morton, L.F.; Kodicek, E. Studies in vivo on the biosynthesis of collagen and elastin in ascorbic acid-deficient guinea pigs. Evidence for the formation and degradation of a partially hydroxylated collagen. Biochem. J. 1970, 119, 575–585. [Google Scholar] [CrossRef]
- Quaglino, D.; Fornieri, C.; Botti, B.; Davidson, J.M.; Pasquali-Ronchetti, I. Opposing effects of ascorbate on collagen and elastin deposition in the neonatal rat aorta. Eur. J. Cell Biol. 1991, 54, 18–26. [Google Scholar]
- Wang, Y.; Mackenzie, B.; Tsukaguchi, H.; Weremowicz, S.; Morton, C.C.; Hediger, M.A. Human vitamin C (L-ascorbic acid) transporter SVCT1. Biochem. Biophys. Res. Commun. 2000, 267, 488–494. [Google Scholar] [CrossRef]
- Welch, R.W.; Bergsten, P.; Butler, J.D.; Levine, M. Ascorbic acid accumulation and transport in human fibroblasts. Biochem. J. 1993, 294, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Hasselholt, S.; Tveden-Nyborg, P.; Lykkesfeldt, J. Distribution of vitamin C is tissue specific with early saturation of the brain and adrenal glands following differential oral dose regimens in guinea pigs. Br. J. Nutr. 2015, 113, 1539–1549. [Google Scholar] [CrossRef]
- Søgaard, D.; Lindblad, M.M.; Paidi, M.D.; Hasselholt, S.; Lykkesfeldt, J.; Tveden-Nyborg, P. In vivo vitamin C deficiency in guinea pigs increases ascorbate transporters in liver but not kidney and brain. Nutr. Res. 2014, 34, 639–645. [Google Scholar] [CrossRef]
- Clark, A.G.; Rohrbaugh, A.L.; Otterness, I.; Kraus, V.B. The effects of ascorbic acid on cartilage metabolism in guinea pig articular cartilage explants. Matrix Biol. 2002, 21, 175–184. [Google Scholar] [CrossRef]
- Paidi, M.D.; Schjoldager, J.G.; Lykkesfeldt, J.; Tveden-Nyborg, P. Chronic vitamin C deficiency promotes redox imbalance in the brain but does not alter sodium-dependent vitamin C transporter 2 expression. Nutrients 2014, 6, 1809–1822. [Google Scholar] [CrossRef] [PubMed]
- Alberts, A.; Moldoveanu, E.-T.; Niculescu, A.-G.; Grumezescu, A.M. Vitamin C: A Comprehensive Review of Its Role in Health, Disease Prevention, and Therapeutic Potential. Molecules 2025, 30, 748. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Yao, F.; Xi, G.; Yang, J.; Zhang, Z.; Yang, Q.; Tian, J.; An, L. Vitamin C Rescues In vitro Embryonic Development by Correcting Impaired Active DNA Demethylation. Front. Cell Dev. Biol. 2021, 19, 784244. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Falcone, T.; Attaran, M.; Goldberg, J.M.; Agarwal, A.; Sharma, R.K. Vitamin C and vitamin E supplementation reduce oxidative stress-induced embryo toxicity and improve the blastocyst development rate. Fertil. Steril. 2002, 78, 1272–1277. [Google Scholar] [CrossRef]



| Antibody Name | Clone | Source | Company | Catalogue No. | Dilution |
|---|---|---|---|---|---|
| Anti-NeuN | EPR12763 | Rabbit monoclonal | Abcam (Cambridge, UK) | ab177487 | 1:3000 |
| Anti-DCX | polyclonal | Rabbit polyclonal | Abcam (Cambridge, UK) | ab18723 | 1:500 |
| Anti-Nestin | EPR1301(2) | Rabbit monoclonal | Abcam (Cambridge, UK) | ab176571 | 1:250 |
| Anti-S100 | EP1576Y | Rabbit monoclonal | Abcam (Cambridge, UK) | ab52642 | 1:1000 |
| Anti-MBP | 12 | Rat monoclonal | Abcam (Cambridge, UK) | ab7349 | 1:100 |
| Anti-Synaptophysin | SYP02 | Mouse monoclonal | Thermo Scientific (Waltham, MA, USA) | MS-1150-S0 | 1:40 |
| Anti-GFAP | polyclonal | Rabbit polyclonal | Thermo Scientific (Waltham, MA, USA) | RB-087-A0 | 1:200 |
| Anti Calbindin D-28k | CB38 | Rabbit monoclonal | Swant (Tägerwilen, Switzerland) | - | 1:5000 |
| Anti-Olig2 | polyclonal | Rabbit polyclonal | IBL (Fujioka, Japan) | 18953 | 1:100 |
| Anti-TPPP | 6C10 | Mouse monoclonal | Flow Labs UK (London, UK) | - | 1:2000 |
| Gene | Primers | Primer Length |
|---|---|---|
| COL1 | F: 5′-ATGTCTAGGGTCTAGACATGTTCA-3′ | 24 bp |
| R: 5′-CCTTGCCGTTGTCGCAGACG-3′ | 20 bp | |
| COL4A1 | F: 5′-TATCTCTGGGGACAACATCCG-3′ | 21 bp |
| R: 5′-CATCTCGCTTCTCTCTATGGTG-3′ | 22 bp | |
| SLC23A1 | F: 5′-TACCTGACATGCTTCAGTGG-3′ | 20 bp |
| R: 5′-CGGCTGCCCACCTTGGTAAT-3′ | 20 bp | |
| GAPDH | F: 5′-GCGCCGAGTATGTAGTGGAA-3′ | 20 bp |
| R: 5′-TGATTCACGCCCATCACGAA-3′ | 20 bp |
| Intra-Axial Haemorrhage (Intraparenchymal) | Extra-Axial Haemorrhage (Subarachnoid) | |||||
|---|---|---|---|---|---|---|
| Group No. of Foetuses | Cerebral Cortex | Hippocampus | Thalamus and Hypothalamus | Cerebellum | ||
| Control | 20 | 0 | 0 | 0 | 0 | 0 |
| E1 | 21 | 7 1 | 6 4 | 7 7 | 2 10 | 0 |
| E2 | 19 | 19 2,3 | 19 5,6 | 19 8,9 | 19 11,12 | 11 13,14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čapo, I.; Andrijević, I.; Čapo, N.; Popović, M.; Milenković, I.; Ratajac, R.; Vranješ, D.; Milutinović, D.; Simin, D.; Sekulić, S. Neurodevelopmental Changes in the Guinea Pig Brain Caused by Time-Limited Complete Vitamin C Deprivation. Nutrients 2025, 17, 3484. https://doi.org/10.3390/nu17213484
Čapo I, Andrijević I, Čapo N, Popović M, Milenković I, Ratajac R, Vranješ D, Milutinović D, Simin D, Sekulić S. Neurodevelopmental Changes in the Guinea Pig Brain Caused by Time-Limited Complete Vitamin C Deprivation. Nutrients. 2025; 17(21):3484. https://doi.org/10.3390/nu17213484
Chicago/Turabian StyleČapo, Ivan, Ilija Andrijević, Nataša Čapo, Milan Popović, Ivan Milenković, Radomir Ratajac, Dejan Vranješ, Dragana Milutinović, Dragana Simin, and Slobodan Sekulić. 2025. "Neurodevelopmental Changes in the Guinea Pig Brain Caused by Time-Limited Complete Vitamin C Deprivation" Nutrients 17, no. 21: 3484. https://doi.org/10.3390/nu17213484
APA StyleČapo, I., Andrijević, I., Čapo, N., Popović, M., Milenković, I., Ratajac, R., Vranješ, D., Milutinović, D., Simin, D., & Sekulić, S. (2025). Neurodevelopmental Changes in the Guinea Pig Brain Caused by Time-Limited Complete Vitamin C Deprivation. Nutrients, 17(21), 3484. https://doi.org/10.3390/nu17213484






