Abstract
Background: Depression is a significant complication of the peripartum period that can result in profound long-term detrimental implications for the affected woman, her child, and her family. It is possible that micronutrient imbalances could contribute to the development of perinatal depression through their roles in neurotransmitter synthesis and neuroendocrine and neuroimmune pathways. Micronutrient imbalances are more likely during the perinatal period due to the additional physiological demands on the body during this time. The objective of this systematic review was to review and summarise the existing evidence regarding the association between micronutrient levels and perinatal depression. Methods: MEDLINE, EMBASE, PsycINFO, CINAHL, Scopus, and Web of Science were searched for studies examining blood levels of micronutrients and assessment of depression within the peripartum period using validated rating tools. Results: A total of 58 studies met the eligibility criteria and were included in this review. Of these, 31 studies reported a significant inverse association between perinatal depression and at least one of the following: vitamin D, iron status, vitamin B12, folate, or zinc. Vitamin D was the most frequently investigated nutrient, examined in 28 of the 58 articles. The remaining 27 did not demonstrate a significant association. Conclusion: This review found that vitamin D deficiency has the greatest evidence of an association with perinatal depression. The evidence for other micronutrients is mixed, inconclusive, or limited. Further research is required to determine the significance of these micronutrients in the development of perinatal depression.
1. Introduction
Perinatal depression is a significant complication of pregnancy and the postpartum period. One study estimated the world-wide prevalence of perinatal depression to be 11.98% (95% CI 11.4–12.5) [1]. Perinatal (or peripartum) depression refers to depression arising during pregnancy (antenatal period) or after childbirth, up to 12 months postpartum [2]. Peripartum depression can have profound long-term detrimental implications for the affected woman, her child, and her family. The UK-based confidential enquiry into maternal deaths study found suicide to be the leading cause of maternal death during the postpartum period [3]. Depression during pregnancy is associated with reduced prenatal care, increased maternal substance use, and higher rates of premature delivery and small-for-gestational-age (SGA) infants [4]. Postpartum depression (PPD) is associated with problems with maternal behaviour and interpersonal relations, including difficulty with infant care and other responsibilities, and difficulty bonding with the child and marital relationship issues [5]. Importantly, perinatal depression may have lasting effects on the child’s development [6].
The current literature indicates that several micronutrients may be implicated in the development of perinatal depression. This is plausible considering that a number of micronutrients play important roles in brain neurochemistry. Decreased brain iron stores, for example, impair the activity of iron-dependent enzymes that are necessary for the synthesis of serotonin, dopamine, and noradrenaline [7]. Vitamin B12 and folate are linked to the effective functioning of the folate cycle, which is necessary for the regeneration of tetrahydrobiopterin, a co-factor that has a crucial role in the synthesis of neurotransmitters [8]. Zinc has a key role in neurotransmitter actions, including serotonin, the functioning of GABA and NMDA receptors, and the activation of neurotrophic factors [9]. Particularly, during the perinatal period, women are more vulnerable to developing micronutrient imbalances due to the increased physiological needs of the body [10]. Thus, our hypothesis is that micronutrient imbalances contribute to the development of perinatal depression. Current Australian guidelines informing clinical practice for perinatal mental illness do not provide any recommendations regarding pathology testing for perinatal depression [11,12,13,14]. Understanding the impact of micronutrient deficiencies during the perinatal period may be able to help inform current clinical practice with regard to the testing and management of these micronutrient imbalances to help in the prevention or treatment of perinatal depression.
2. Methods
2.1. Search Strategy
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. The protocol is registered in PROSPERO (CRD42024620320) and is accessible at https://www.crd.york.ac.uk/PROSPERO/view/CRD42024620320 (accessed on 30 October 2025). A comprehensive search of the electronic databases (Ovid databases, MEDLINE, EMBASE, PsycINFO, CINAHL, Scopus, and Web of Science) was undertaken from the time of inception on 14 June 2024.
Two main searches were performed as described below and then combined with the word “AND” to produce the search for each search engine. Each of the following terms was searched as keywords on their own and as subject headings when possible:
- “Postpartum depression” OR “Postnatal depression” OR “Antepartum depression” OR “Antenatal depression: OR “Peripartum depression” OR “Perinatal depression”
- “Micronutrient*” OR “Biological marker*” OR “Biomarker*” OR “Trace element*” OR “Trace metal*” OR “Zinc” OR “Copper” OR “Selenium” OR “Magnesium” OR “Iron” OR “Vitamin*” OR “Folate”.
All search results were imported into Covidence systematic review software for screening and selection of studies. The titles and abstracts were independently screened by two reviewers to identify any eligible studies. Full-text articles were then retrieved and evaluated by both reviewers using the predefined inclusion and exclusion criteria to confirm eligibility.
Any discrepancies in the assessment of study eligibility were resolved through a discussion involving an independent third reviewer. The full study selection process, which includes the number of articles screened, excluded, and included, is depicted in Figure 1.
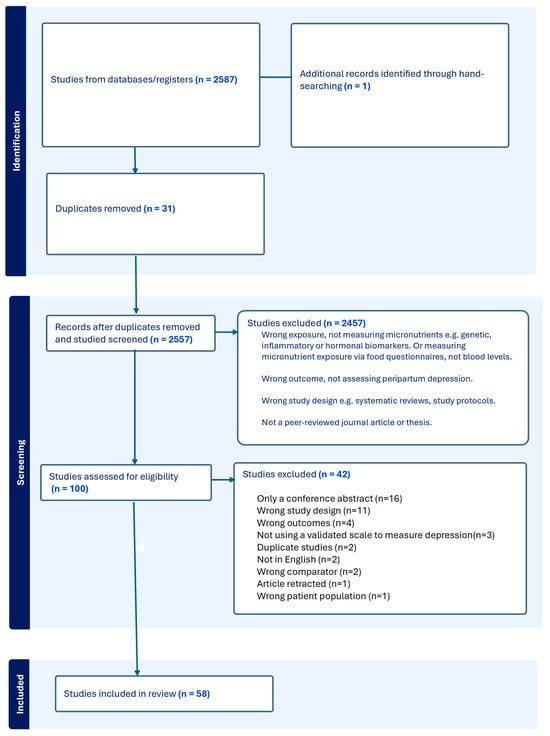
Figure 1.
PRISMA diagram depicting the selection of studies for this review.
2.2. Eligibility Criteria
The inclusion criteria for the studies included in this review are outlined below:
- -
- Peer-reviewed journal articles or dissertations written in English.
- -
- Population (P): Women of any age who were pregnant or within 12 months postpartum.
- -
- Intervention/Exposure (I): Assessment of blood micronutrient levels, where micronutrients are defined as vitamins and minerals essential for human life [15]. Measurements in terms of serum levels, plasma levels, whole blood levels, or blood cell levels of the micronutrient were all considered acceptable.
- -
- Comparator (C): The comparator groups include women with differing micronutrient levels, women without micronutrient deficiency, or no comparator group (as applicable to the study design).
- -
- Outcome (O): Presence and/or severity of perinatal depression, determined either by a validated rating scale or by a clinical diagnosis according to recognised classification systems, such as the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM) or the World Health Organization’s International Classification of Diseases (ICD) [16,17].
2.3. Quality Assessment
The quality of observational studies was assessed by a single reviewer using the Newcastle–Ottawa Scale (NOS) [18]. We acknowledge that independent dual assessment would have strengthened the rigour of this study, and this represents a limitation of the review [18]. Randomised controlled trials (RCTs) were assessed using the Joanna Briggs Institute (JBI) checklist [19]. The NOS evaluates a number of domains, including selection, comparability of groups, and ascertainment of outcome. Studies with scores of 0–3 are considered low quality, scores of 4–6 are considered moderate quality, and scores of 7–9 are considered high quality [20]. The JBI checklist is a descriptive measure of quality that explores domains of randomisation, concealment of allocation, blinding of allocation, and whether treatment groups were managed the same way outside of the intervention [19].
2.4. Statistical Analysis
A structured narrative synthesis was conducted to summarise and compare the findings across all included studies. Data was extracted from each article with regard to author and date of publication; conflicts of interest from authors; funding sources; study design; number of participants; the population from which participants were retrieved, including country and clinic setting; the micronutrients studied and the method that was undertaken; the timing of micronutrients tested in terms of the perinatal period, the depression rating tool used, and the cut-off; the timing of depression assessment in relation to the perinatal period; the statistical methods used to deduce associations between micronutrient levels and depression scores; and the measurement of confounding factors as well as the statistical analysis used to account for these. Descriptive statistics reported in the original publications, including medians, ranges, percentages of events, and confidence intervals, where available, were extracted and presented to outline the variability in clinical outcomes across studies and subgroups. Summary tables and figures were constructed to display study characteristics, intervention details, and reported outcomes in a clear and consistent format. Results were organised according to the micronutrient examined.
3. Results
3.1. Study Selection and Description of Studies
The search through six databases (PsycINFO, Web of Science, MEDLINE, Scopus, EMBASE, and CINHAHL) yielded 2587 records, including one dissertation. Hand-searching produced another relevant journal article. After 31 duplicates were excluded at this stage, 2556 records were screened by title and abstract. A total of 100 studies were selected for full-text evaluation, of which 58 met the eligibility criteria. Please see Figure 1, which depicts the selection of studies for this review in accordance with PRISMA guidelines.
3.2. Main Findings
Studies were grouped according to the type of micronutrient biomarker studied. The most studied micronutrient was vitamin D, with 28 studies examining its relationship with either prenatal or postnatal depression [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46]. Regarding the other micronutrients, 13 articles studied iron [29,44,47,48,49,50,51,52,53,54], 12 articles studied folate [55,56,57,58,59,60,61,62,63,64], 10 articles studied vitamin B12 [59,60,62,63,64,65,66,67,68], 5 articles studied zinc [10,69,70,71], 3 studied magnesium [42,71,72], 3 studied copper [42,72,73], 2 studied selenium [72,74], 2 studied vitamin C [61,75], 1 studied calcium [42], 2 studied vitamin E [45,61], 1 studied vitamin A [45,61], 1 studied beta-carotene [61], 1 studied vitamin B2 [45], and 1 studied manganese [72]. A number of studies investigated more than one micronutrient [29,44,45,59,60,61,62,63,64,72]. Sample sizes varied from 31 participants (Ohsuga et al.) to 16,528 participants (Jani et al.) [40,53].
The Edinburgh Postnatal Depression Scale (EPDS) was the most common rating tool used in the studies to assess perinatal depression. In Australia, it is the recommended screening tool for depression in the perinatal period [11]. The EPDS is a 10-item validated self-rating scale developed by Cox et al. in 1987 [76] to screen for perinatal depression [16]. Depressive symptoms in most studies were assessed using the Edinburgh Postnatal Depression Scale (EPDS), with cut-off scores for probable depression varying between ≥10 and ≥13, which may influence reported prevalence and effect estimates. Other rating tools used to assess depression included the following: the Center for Epidemiologic Studies Depression Scale (CES-D); Beck Depression Inventory (BDI); Kessler Psychological Distress Scale (K-10); Depression Anxiety and Stress Scale (DASS-21); Patient Health Questionnaire (PHQ-9); Mini International Neuropsychiatric Interview (MINI); and Structured Clinical Interview for DSM (SCID). Table 1 summarises the findings of the review articles below.

Table 1.
Summary of findings for vitamin D and perinatal depression.
3.2.1. Vitamin D
Of the 28 studies examining vitamin D, 25 studies were observational in design and 3 were randomised controlled trials (RCTs). Six out of the eight studies examining depression scores in the antenatal period found a significant inverse relationship between vitamin D levels and depression scores in pregnancy [25,26,27,30,40,44]. The two largest studies in this review, Jani et al. (n = 16,528) and Brandenbarg et al. (n = 4101), supported this association. Jani et al., for example, found women with a high perinatal depression risk had increased odds of being vitamin D deficient (adjusted OR 1.321, 95% CI 1.105–1.579) [40]. Brandenbarg et al. demonstrated a significant inverse correlation between vitamin D status and antenatal depression scores (Spearman p = −0.188, p < 0.001) [25].
Nine studies exclusively examined the relationship between vitamin D and depression scores in the postnatal period [21,22,33,35,41,42,45,46,77]. Five studies demonstrated a significant inverse relationship between vitamin D levels and depression scores [21,22,33,46,77].
Eight studies that investigated the relationship between vitamin D levels and both antenatal and postnatal depression yielded mixed findings [23,29,31,32,34,37,38,41]. Lamb et al., for example, found a significant inverse relationship between vitamin D and EPDS scores antenatally and postnatally. Their study demonstrated that women with elevated depressive symptoms (i.e., EPDS score ≥ 10) had significantly lower vitamin D levels (t = −2.09, p = 0.039) [32].
However, Nassr et al. and Noshiro et al. did not find any significant association between vitamin D levels and antenatal or postnatal depression [34,41]. These null findings may reflect that studies with smaller sample sizes were not sufficiently powered to detect an association. Results from a study by King et al. suggested that women with vitamin D deficiency (defined as serum levels ≤ 20 ng/mL) had an odds ratio (OR) of 2.40 (95% CI 0.92–6.27) for elevated depression scores. However, this finding was not statistically significant (p = 0.07) [31].
While Accortt et al., 2021 did not find vitamin D to be significantly associated with perinatal depression risk, they found that a lower vitamin D metabolite ratio (VMR) was associated with postpartum depression (OR of 1.43 (95% CI 1.10–1.87, p = 0.007) [23]. Wang et al. found a significant relationship between vitamin D and postnatal depression, but not antenatal depression [37]. The authors found that for every 5 ng/mL increase in serum 25(OH)D, the EPDS score for ‘depressed mood’ decreased by 0.1 points [37].
In contrast, Williams et al. and Evanchuk et al. both found a significant relationship between vitamin D levels and antenatal depression scores, but not postnatal depression scores [29,38]. Williams et al. found that vitamin D at 12–20 weeks was a significant predictor of the BDI score at 12–20 weeks (p < 0.05) and at 34–36 weeks of gestation (p < 0.05) [38]. For every one unit increase in vitamin D in early pregnancy, there was an approximate 0.14 unit decrease in the antenatal BDI score (95% CI −0.26 to −0.017) [38]. Evanchuk et al. found that higher vitamin D levels were associated with lower EPDS scores in the third trimester (p = 0.001) but did not find any association between vitamin D levels and postpartum EPDS scores [29].
Three RCTs examined the effect of vitamin D supplementation on PPD [24,28,36]. None of these studies found a relationship between serum vitamin D levels and peripartum depression scores at baseline. Please see a summary of results in Table 1.
3.2.2. Iron
A total of 13 studies assessed the association between iron status and iron-related biomarkers and perinatal depression. Of these, 12 were observational studies and 1 was an RCT. Five studies examined depression in the antenatal period, five examined postnatal depression, and two examined both time periods.
Only two studies found a significant association between deficiency of iron (or iron-related biomarkers) and antenatal depression. Dama et al. found the adjusted odds ratio for those who were iron-deficient in pregnancy developing depression was 2.51 (95% CI 1.14–5.52) [51]. The other study supporting a significant association was by Basutkar et al. (2022) [49]. This paper found a significantly higher mean EPDS score in the iron-deficiency anaemia (IDA) group (12.78 +/− 3.40) compared with the non-IDA group (8.82 +/− 3.12), 95% CI 2.94–4.87, p < 0.005 [49]. Ferritin concentration was one of the predictors of EPDS scores (correlation coefficient r = −0.50, p < 0.001) [49]. Serum iron was also negatively correlated with EPDS with a correlation coefficient, r = −0.38, p < 0.001 [49]. Pregnant individuals with iron deficiency were 12 times more likely to develop depression compared to those without iron deficiency [49]. The remaining three studies, by Hasdemir et al., Bodnar et al. and Basutkar et al. (2021), did not find any significant association between depression scores and iron deficiency [44,52,61].
Only one study (Albacar et al.) found a significant relationship between iron studies and depression scores in the postpartum period [47]. Ferritin concentrations were significantly lower in the group with postpartum depression (PPD) compared to the non-PPD group (15.4 +/− 12.7 mcg/L vs. 21.6 +/− 13.5 mcg/L, p = 0.002) [47]. Albacar et al. was the only study that measured inflammation as a covariate through CRP and found that ferritin persisted as a marker of postpartum depression even after CRP was taken into consideration through multivariate logistic regression analysis [47]. Both Ohsuga et al. and Evanchuk et al. examined the relationship between iron studies and depression scores during the antenatal and postnatal periods. Evanchuk et al. identified a significant relationship between low iron levels in mid-pregnancy and EPDS scores in the third trimester [29]. However, there was no relationship between serum ferritin levels and postpartum EPDS scores [29]. Ohsuga et al. found no significant difference in the median EPDS scores between individuals with non-anaemic iron deficiency (NAID) and those without [53]. However, within the NAID group, EPDS scores increased significantly from mid-pregnancy to one month postpartum [53]. Paoletti et al., in a randomised control trial, found no significant association between haematological or iron values at any timepoint and peripartum depression scores [54]. Please find a summary of these results in Table 2.

Table 2.
Summary of findings for iron studies and perinatal depression.
3.2.3. Folate
Twelve studies examined the relationship between blood folate levels and perinatal depressive symptoms. Five of these studied the antenatal period, four studied the postnatal period, and three studied both time periods. Only two articles found a statistically significant relationship between folate levels and depressive symptoms. Chong et al. found a significant association between lower folate concentrations in those with probable antenatal depression compared to those without (mean +/− SSD: [27.3 +/− 113.8 vs. 40.4 +/− 336.5 nmol/L]; p = 0.011) [64]. However, they did not find any association between folate and postnatal depression [64]. The second study by Abou-Saleh et al. found lower folate levels in those with postpartum depression (p < 0.01) [60]. This study considered the effect of vitamin B12 on this association through stepwise multiple regression analysis; however, other covariate factors, such as inflammatory markers and supplementation, were not included. Three studies measured red cell folate, which is a more accurate reflection of folate status than serum folate [78], plasma folate, or dietary folate [78]. These three studies found no association between red blood cell folate levels and depression scores [56,62,68]. Please find a summary of these results in Table 3.

Table 3.
Summary of findings for folate and perinatal depression.
3.2.4. Vitamin B12
There were 10 articles in total that examined the link between vitamin B12 and perinatal depression. Five articles examined the postnatal period only [57,59,60,66,67], three examined the antenatal period only [43,62,63], and two articles examined both time periods [64,65]. Three articles found a significant relationship between vitamin B12 levels and depressive symptoms in the postpartum period [60,66,67] and one in the antenatal period (Peppard et al.) [63]. Abou-Saleh et al. was the only study that showed a positive relationship between B12 levels and depressive symptoms; the other studies showed a negative relationship [60]. It is important to note that Abou Saleh et al.’s study had a number of limitations, including a relatively small sample size, a short follow-up period, and limited confounding factors considered, which may have resulted in an overestimation of the association [60]. Four of the ten studies into vitamin B12 examined the link between homocysteine levels and depression scores [59,62,65,67]. Only Aishwarya et al. found a significant association between homocysteine levels with postpartum depression. A positive association was found at both 24–28 h postpartum (p = 0.001) as well as six weeks postpartum (p = 0.001) [59]. Please find a summary of these results in Table 4.

Table 4.
Summary of findings for vitamin B12 and perinatal depression.
3.2.5. Zinc
All five articles studying serum zinc measured depressive symptoms in the postpartum period, and two studies also assessed the antenatal period. Four articles found an inverse association between zinc and depressive symptoms. Roomruangwong et al. found a significant inverse relationship between serum zinc and depressive symptoms both antenatally and postnatally using various assessment tools [10]. This was supported by Kavitha et al. [70], who found a strong inverse relationship (r = −0.24, p < 0.05) between zinc and postpartum depression. Wojcik et al. also found a negative association between serum zinc and EPDS scores in the postpartum period [71]. Indriasari et al., however, found the strength of the relationship between zinc and postpartum depression to be weak [79]. Only the Kurniati et al. study found no correlation [69]. Please find a summary of these results in Table 5.

Table 5.
Summary of results for zinc and perinatal depression.
3.2.6. Copper
There were three studies that examined blood copper levels and perinatal depression [42,72,73]. In a retrospective cohort study of 902 patient records, of which 78 were women with postpartum depression, Crayton and Walsh found that women with a history of postpartum depression had significantly elevated serum copper levels (131 mcg/dL +/− 39 mcg/dL) compared to women with a history of depression but without postpartum depression (111 +/− 25 mcg/dL, p < 0.001) and to non-depressed controls (106 +/− 20 mcg/dL, p < 0.001) [73]. Bahramy et al. found that mean serum copper measured between 26- and 32-week gestation was significantly higher in women with depression compared to those without (p = 0.048) [42]. In contrast, Rokoff et al. found no association between erythrocyte copper concentrations and elevated EPDS at any timepoint [72]. Please find a summary of these results in Table 6.

Table 6.
Summary of results for copper and perinatal depression.
3.2.7. Other Micronutrients
No associations were found between peripartum depression and selenium [72,74], magnesium [42,71,72], B-carotene [61], vitamin C [47,75], vitamin A [61], and vitamin E [45,47]. Lin et al., however, did find plasma riboflavin level to be associated with decreased risk of postpartum depression (OR 0.747, 95% CI 0.566–0.987, p = 0.040) [45]. Please find a summary of these results in Table 7.

Table 7.
Summary of findings of other micronutrients and perinatal depression.
4. Methodological Quality
Only one study included in this review was found to be of low quality. Of the 54 observational studies, 8 studies were of moderate quality, and the remaining 45 studies were of high quality. The NOS scores and micronutrients studied in each of the observational studies are presented in Table 8. Table 9 presents the qualitative JBI quality assessment for the RCTs included in the review.

Table 8.
NOS assessments and micronutrients studied.

Table 9.
Quality assessment for RCTs as per JBI critical appraisal tool.
In general, most studies provided a reliable exposure measurement with clear descriptions of the method by which micronutrients were tested from the blood samples drawn. Due to the eligibility criteria, all studies used a standardised rating tool for assessment of depression symptoms, and only one article used a diagnosis based on a validated diagnostic standard, i.e., DSM-IV [73]. In a number of studies, it was not clear what cut-off score the authors used to define probable perinatal depression [57,68,69].
Most studies clearly described the source of the study populations. Sample sizes were, in general, of moderate to large size, with 43 out of 58 studies having sample sizes greater than 100. However, only 15 studies published a power calculation regarding their sample size [24,32,42,46,49,50,54,56,59,61,66,70,75]. Most of these had a power of at least 80% or over, with a significance level of 0.05 to detect a moderate effect size. Several studies fell short of the required number of participants needed to reach a power of 80% to detect a moderate effect size [30,72,75].
The majority of studies took into consideration multiple confounding factors and used appropriate statistical methods to account for their effects. These included regression-based models such as linear regression models, multiple logistic regression, and multivariate general linear model analysis, as well as stratified models. Three papers provided no clear description of any confounding factors [41,68,71]. The remaining 11 studies either took into account only a few confounding factors, were not clear on how the confounding factors were adjusted for statistically, or could have used more sophisticated statistical methods to adjust for these [28,42,53,54,57,59,60,69,70,73,74,75,79].
Multiple confounding factors were found to have a statistically significant association with depression scores. A number of articles supported an association between younger age and depression scores [25,37,64,70,72]. Repeatedly, an association was found between obesity and depression scores [25,27,40,43,55,63]. A number of studies suggested that lower education levels in the patients were positively associated with depression scores [25,33,55,64]. This contrasted with other studies, which reported that higher education levels were associated with depression scores [72]. A number of studies from India suggested that the middle socioeconomic group was actually associated with higher rates of perinatal depression [46,59,67]. The reason for this is unclear, but it may be due to greater access for the middle—upper class to healthcare, including psychiatric services. Studies were consistent in finding a positive association between unemployment and depression scores [46,47]. Ethnicity featured as an important confounding factor in some studies, with a recurrent finding of higher depression scores in Hispanic and African American patients compared to Caucasian patients [23,33,72]. Marital factors associated with higher depression scores included marital dissatisfaction [46,67,77], divorce or separation [27,33], polygamy [43], and consanguinity [49]. Studies from India suggested that dissatisfaction with the gender of the child was associated with depression scores [46,67]. A history of depression or mental illness was also found to have a strong relationship with depression scores [27,56,77]. For example, Blunden et al. found a history of a mental health condition had a relative risk of 1.80 (95% CI 1.50–2.15) p < 0.001 with postpartum depression [56]. However, only eleven articles mentioned a past history of mental illness as a confounding factor. There were many other confounding factors that associated positively with depression scores. Some of these included smoking [37], passive smoke exposure [43,64], drinking alcohol [37], formula feeding [33], pregnancy complications [59,62], mode of delivery [67], stressful life events [43,77], and unplanned pregnancy [67]. Breastfeeding [56] and a healthy diet [43] appeared to be protective factors against depression.
5. Discussion
This systematic review summarises the evidence of associations between a broad number of micronutrients and perinatal depression. The evidence was strongest for vitamin D, with 19 high-quality studies linking low vitamin D levels to perinatal depression. There was also evidence for an inverse association between iron studies, vitamin B12, and zinc levels and perinatal depression. There was less evidence for an association between folate levels and perinatal depression. There was little evidence for a relationship between other micronutrients and perinatal depression. Conversely, high serum copper levels were associated with depression.
Most of the studies included in this review were of high quality. Of the 58 studies included in this review, 31 studies supported a significant association between micronutrient level and antenatal or postnatal depression, and 27 studies found no association. Most of the studies that found a significant association suggested an inverse relationship between the micronutrient level and perinatal depression, i.e., that a micronutrient deficiency was associated with perinatal depression.
Copper is the only exception to this trend, with higher levels being associated with perinatal depression in two studies, albeit one of these studies was of poor quality [42,73]. Both serum copper and ceruloplasmin increase under the influence of inflammatory conditions. This was not considered as a covariate in the articles included [80]. Serum copper rises in pregnancy, likely as a result of rising oestrogen, and falls significantly in the postpartum reaching pre-pregnancy levels after six weeks [81]. Thus, the timing of copper testing in relation to the timing in the perinatal period is likely to affect copper levels also. Current literature suggests that an elevated cellular copper can lead to neuronal injury and induce oxidative stress and pro-inflammatory responses, potentially contributing to the development of depression [82]. Ni et al.’s systematic review of 21 observational studies supports this, with a finding that blood copper levels were higher in patients with depression [83]. This finding was also supported by Hulsbosch et al.’s study of 2036 pregnant women, which found that the copper-to-zinc ratio (Cu:Zn ratio) was independently associated with a persistently high negative affect, as measured by the Tilbury Distress Scale negative affect subscale (adjusted OR 1.52, 95% CI 1.13–2.04) [84].
In this review, the micronutrient with the greatest evidence base for its association with perinatal depression was vitamin D. All except one of the articles studying vitamin D were of high quality. Vitamin D studies accounted for 28 out of the 58 studies in this review. Of these studies, 19 supported a significant inverse association between vitamin D and perinatal depression. Eight studies found no association at all. The variation in findings may be for a number of reasons. The null findings from these studies may reflect studies with smaller sample sizes that were not sufficiently powered to detect an association. Secondly, it may be difficult to observe an association in populations with a high prevalence of vitamin D deficiency, as both groups of women have high rates of deficiency [34]. Sunlight exposure and seasonality are important confounding factors affecting vitamin D levels. However, only four studies attempted to measure sunlight exposure as a confounding factor [21,36,44,46], and only ten studies measured seasonality [21,26,27,28,30,32,35,37,39,41,77]. The existence of 11 systematic reviews on vitamin D and perinatal depression is notable, as no other micronutrient has been similarly reviewed [85,86,87,88,89,90,91,92,93,94,95]. In general, these reviews supported an inverse relationship between vitamin D and perinatal depression scores.
The strong evidence base for vitamin D may stem from the ease, reliability, and low cost of testing vitamin D serum levels, making it relatively easy to research. Vitamin D is unique in that it is also a neuro-steroid [96,97]. Various biological mechanisms have been hypothesised in the literature to explain the association between low vitamin D levels and depression. These include the role of vitamin D in the synthesis of neurotransmitters such as acetylcholine, dopamine, serotonin, and gamma aminobutyric acid; its antioxidant and anti-inflammatory effects in the central nervous system; its regulation of neuronal levels of calcium; and its role in enhancing nerve growth factors, such as BDNF [98,99,100,101,102]. Furthermore, vitamin D acts as a transcriptional regulator for many genes and also has roles in neuronal function and plasticity [98].
The second largest number of articles were regarding iron and its association with perinatal depression. Five out of twelve studies on iron suggested a significant inverse relationship with perinatal depression, i.e., iron deficiency was associated with higher depression scores. This relationship with postnatal depression is biologically plausible as iron is an essential co-factor in the synthesis of neurotransmitters such as serotonin, melatonin, norepinephrine, and dopamine, as well as myelin synthesis in the central nervous system [103]. Iron also plays key roles in the functioning of the hippocampus as well as the pre-frontal cortex, which are important brain networks involved in the pathophysiology of major depressive disorder [103]. In animal models, iron deficiency during the perinatal period in mice was associated with impaired maternal care postpartum as well as impaired neurodevelopment in the infant mice [103]. However, one explanation for the mixed outcome from these high-quality studies may be due to the fact that iron status in the body is measured through multiple biomarkers (serum ferritin, serum iron, and transferrin saturation). This complicates the interpretation of the relationship of iron with depression scores and make direct associations more difficult to establish. Moreover, ferritin is an acute-phase reactant that can mask iron deficiency when it rises with inflammation [104]. Only one study in this review considered inflammation as a covariate [47]. Studies also varied in whether they focused on anaemic or non-anaemic patients, which may represent an important confounding factor influencing the observed relationships with depression outcomes.
Folate and vitamin B12 play a central role in the DNA methylation process, which is crucial to the normal development and function of the central nervous system [105]. There is substantial evidence to support that inappropriate methylation patterns can contribute to a range of neuropsychiatric issues such as depression, autism, and schizophrenia [105]. Deficiencies in these micronutrients can result in elevated homocysteine levels, which have been associated with depression [8,106] and also cognitive impairment [107,108]. Deficiencies in vitamin B12 and folate can become overt during pregnancy and lactation, when demands of the growing foetus and delayed repletion impose significant nutritional demand [7,109]. In particular, it is well known that deficiencies of folate in pregnancy can result in foetal neural tube defects [109].
This review found limited evidence for the association between vitamin B12 and perinatal depression despite these studies being of high quality. Three articles suggested an inverse relationship, and one study (Abou-Saleh et al.) suggested a positive relationship with depression scores [60]. It is important to note that the Abou-Saleh et al. study had a number of limitations, as described previously.
Similarly, regarding folate, while the majority of studies examining this micronutrient were of high quality, only two of the twelve studies found a significant association between blood folate levels and depression scores [60,64]. For folate, the absence of a significant association in some studies may reflect an incomplete adjustment for other one-carbon metabolites, such as vitamin B12 and homocysteine, which are biochemically interdependent and may confound the relationship between folate status and perinatal depression.
There were five studies that studied blood zinc levels and perinatal depression, of which three demonstrated a strong inverse relationship. However, the quality of these studies was poorer when compared with the studies of the other micronutrients. Kurniati et al. did not provide clear cut-off scores to define depression, and it was unclear at what timepoint postpartum participants were recruited [79]. Wojcik et al. did not clearly identify any confounding factors and did not directly statistically quantify the nature and strength of the association between serum zinc and depression scores [71]. Only Roomruangwon et al. appeared to consider the diurnal variation of zinc and measured fasting morning zinc levels [10]. Roomruangwong et al. was also the only study that considered CRP as a covariate, which is important considering that zinc levels decrease in inflammatory states [110]. Roomruangwong et al. found there was a significant inverse relationship between zinc and CRP in the prenatal period. Both lower zinc and higher CRP were associated with prenatal and postnatal depression [10].
With regard to the other micronutrients measured, the evidence base was very limited. In general, no significant association was found between these micronutrient levels and perinatal depression, except for a positive association between riboflavin and depression scores [45]. Further research is required to support an association between any of these micronutrients and perinatal depression.
This systematic review found a significant link between low vitamin D levels and perinatal depression. This review provides evidence that antenatally screening routinely for vitamin D enables supplementation and potentially reduces the risk of peripartum depression. Vitamin D supplementation in perinatal women with a deficiency is likely to have multiple health benefits for the mother and baby. Severe vitamin D deficiency can lead to delayed early infant motor development as well as growth retardation, rickets, osteomalacia, and hypocalcaemia in children, and it has a critical role in bone mineralisation [111,112]. There is also emerging evidence that vitamin D deficiency may be related to neurodevelopmental disorders [113,114,115] as well as allergic disorders in children [116,117,118] because it has important roles in brain development and functioning [96,119] and in the regulation of the immune system [120]. In adult women, it is well known that vitamin D deficiency is associated with the development of osteoporosis [121]. However, there is also emerging evidence for the association between low vitamin D and a number of other conditions, including autoimmune conditions, cancers, and cardiovascular disease [120].
The risks of developing vitamin D deficiency may be higher in postpartum women who may struggle to find the time to obtain sufficient sunlight exposure, and even higher in women with postpartum depression who often struggle to leave the home. Women of culturally diverse backgrounds are also likely to be at a higher risk of vitamin D deficiency due to decreased skin absorption for those with darker skin pigmentation and also cultural practices related to veiling [122]. Therefore, routine antenatal screening for vitamin D deficiency and appropriate supplementation offers an opportunity not only to potentially prevent perinatal depression but also to promote broader maternal and infant health.
In Australia, vitamin D levels are not routinely tested antenatally. The current Royal Australian and New Zealand guidelines for Obstetrics and Gynaecology state, with regard to vitamin D testing, not to “test Vitamin D levels in pregnancy as part of routine pregnancy screening, regardless of maternal risk factors” [123]. Medicare currently will not subsidise routine testing for vitamin D in the perinatal period [124]. Thus, it is highly likely that vitamin D deficiency in pregnancy may be underdiagnosed in the Australian perinatal population, and a valuable opportunity to reduce the risk of perinatal depression through vitamin D supplementation is being overlooked.
Strengths, Limitations, and Future Directions
This systematic review has a number of strengths in that it involved a comprehensive search of the literature that spanned multiple databases and involved forward citation-tracking of articles. It aimed to include a detailed assessment of the studies’ methodological quality. However, there were several limitations of this review. There was significant variability across the studies with regard to the micronutrients measured, the instruments used to assess depressive symptoms, and the cut-offs used to define probable depression. For example, there were differences in the EPDS thresholds applied across studies (e.g., ≥10 vs. ≥13), which likely contributed to the variability in reported associations between micronutrient levels and perinatal depression. Variability in findings across studies may reflect differences in dietary patterns, baseline nutritional status, and genetic polymorphisms such as MTHFR that influence folate and B12 metabolism. Another explanation for this variability is that articles studied women from various geographical locations, ethnicities, and cultures. Confounding factors such as seasonality, inflammation, body mass index, socioeconomic status, and comorbidities may also partly explain the inconsistent results. In addition, methodological variability, including differences in assay techniques, diagnostic tools for depression, and definitions of micronutrient deficiency, likely contributes to heterogeneity. Variation in the timing of micronutrient assessment across studies—such as measurement in early versus late pregnancy or postpartum—may also contribute to inconsistent findings. Micronutrient levels fluctuate throughout pregnancy and after delivery due to physiological changes, haemodilution, dietary intake, and supplement use, which may affect their observed relationship with depressive symptoms. Micronutrient deficiencies may be more difficult to detect in well-nourished individuals from higher socioeconomic populations compared with lower socioeconomic areas, which may have a greater prevalence of malnutrition [61]. As a result of this variability, a meta-analysis or formal pooled analysis of these results was not possible.
Due to the variability of association measures across the papers, a meta-analysis could not be provided. It is possible that publication bias may be present, as studies finding an association may be more likely to be published than those not finding an association. The scope of this review was limited to measures of micronutrient levels in blood. However, the accuracy, availability, and cost of blood sampling micronutrient levels vary. For some micronutrients, assessment of dietary patterns or urinary micronutrient concentrations may be more accurate than blood testing.
6. Conclusions
This systematic review found a significant association between low vitamin D levels and perinatal depression. Currently, the Royal Australian and New Zealand College for Obstetricians and Gynaecologists (RANZCOG) guidelines do not recommend the routine screening of vitamin D levels antenatally [123]. This review provides evidence for routinely screening for vitamin D antenatally to enable supplementation and potentially reduce the risk of perinatal depression. There is mixed and conflicting evidence regarding other micronutrients, including iron, folate, vitamin B12, zinc, and copper, and thus further research is needed. Variability in findings across studies likely reflects differences in dietary patterns, baseline nutritional status, genetic polymorphisms, and variability in the timing of measuring the micronutrient. Differences in the cut-off thresholds applied to depression assessment tools (such as the EPDS) are likely to contribute to variability in the associations between micronutrients and perinatal depression. Confounding factors such as seasonality, inflammation, body mass index, socioeconomic status, and comorbidities may partly explain the inconsistent results. Future research needs to be adequately powered, use appropriate statistical methods to account for multiple confounding factors, and provide clear cut-off scores for defining cases of perinatal depression. Such research may help clarify the role of micronutrients in both the prevention and adjunctive treatment of perinatal depression. In addition, future studies could incorporate precision-nutrition approaches to account for individual variability in nutrient metabolism, genetic polymorphisms, and dietary intake. In addition, Mendelian randomization studies may help clarify whether observed associations between micronutrient status and perinatal depression reflect causal relationships or residual confounding. Future research into this area may help inform the role of micronutrients in the prevention of perinatal depression and as adjunctive treatments for perinatal depression.
Author Contributions
Conceptualisation N.I. and J.V.; methodology: N.I. and J.V.; software, Covidence; validation A.S. and J.S., review and editing: A.S. and J.S., supervision J.V. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Woody, C.A.; Ferrari, A.J.; Siskind, D.J.; Whiteford, H.A.; Harris, M.G. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J. Affect. Disord. 2017, 219, 86–92. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetrics and Gynecology. Treatment and Management of Mental Health Conditions During Pregnancy and Postpartum: ACOG Clinical Practice Guidelines number 5. Obs. Gynecol. 2023, 141, 1262–1288. [Google Scholar] [CrossRef]
- Oates, M. Perinatal psychiatry disroders: A leading cause of maternal morbidity and mortality. Br. Med. Bull. 2003, 67, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Davalos, D.B.; Yaden, C.A.; Tregallos, H.C. Untreated prenatal maternal depression and the potential risks to the offspring: A review. Arch. Women’s Ment. Health 2012, 15, 1–14. [Google Scholar] [CrossRef]
- Paulson, J.F.; Bazelmore, S.D. Prenatal and postpartum depression in fathers and its association with maternal depression: A meta-analysis. JAMA Psychiatry 2010, 303, 1961–1969. [Google Scholar]
- Wickramaratne, P.; Gameroff, M.J.; Pilowsky, D.J.; Hughes, C.W.; Garber, J.; Malloy, E.; King, C.; Cerda, G.; Sood, A.B.; Alpert, J.E.; et al. Children of depressed mothers 1 year after remission of maternal depression: Findings from the STAR*D-child study. Am. J. Psychiatry 2011, 168, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Wisner, K.L. Nutrition and depression: Implications for improving mental health among childbearing-aged women. Biol. Psychiatry 2005, 58, 679–685. [Google Scholar] [CrossRef]
- Bottiglieri, T. Homocysteine and folate metabolism in depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 1103–1112. [Google Scholar] [CrossRef]
- Barrondo, S.; Salles, J. Allosteric modulation of 5HT1A receptors in zinc: Binding studies. Neuropharmacology 2009, 56, 455–462. [Google Scholar] [CrossRef]
- Roomruangwong, C.; Kanchanatawan, B.; Sirivichayakul, S.; Mahieu, B.; Nowak, G.; Maes, M. Lower Serum Zinc and Higher CRP Strongly Predict Prenatal Depression and Physio-somatic Symptoms, Which All Together Predict Postnatal Depressive Symptoms. Mol. Neurobiol. 2017, 54, 1500–1512. [Google Scholar] [CrossRef]
- Centre of Perinatal Excellence (COPE). Mental Health Care in the Perinatal Period: Australian Clinical Practice Guideline; Department of Health and Aged Care: Phillip, Australia, 2023; Available online: https://www.cope.org.au/uploads/images/Health-professionals/COPE_2023_Perinatal_Mental_Health_Practice_Guideline.pdf (accessed on 30 October 2025).
- RANZCOG. Distress, Depression and Anxiety During Pregnancy and Following Birth. 2017. Available online: https://ranzcog.edu.au/wp-content/uploads/Depression-Anxiety-During-Pregnancy.pdf (accessed on 30 October 2025).
- Malhi, G.S.; Bell, E.; Bassett, D.; Boyce, P.; Bryant, R.; Hazell, P.; Hopwood, M.; Lyndon, B.; Mulder, R.; Porter, R.; et al. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust. New Zealand J. Psychiatry 2021, 55, 7–117. [Google Scholar] [CrossRef]
- RANZCP. Perinatal mental health services. RANZCP. 2021. Available online: https://www.ranzcp.org/clinical-guidelines-publications/clinical-guidelines-publications-library/perinatal-mental-health-services (accessed on 30 October 2025).
- World Health Organisation. Micronutrients. Available online: https://www.who.int/health-topics/micronutrients#tab=tab_1 (accessed on 30 October 2025).
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders Fifth Edition Text Revision DSM-5-TR; American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- World Health Organisation. International Classification of Diseases for Mortality and Morbidity Statistics (11th Revision); World Health Organisation: Geneva, Switzerland, 2019. [Google Scholar]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 30 October 2025).
- Barker, T.H.; Stone, J.C.; Sears, K.; Klugar, M.; Tufanaru, C.; Leonardi-Bee, J.; Aromataris, E.; Munn, Z. The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid. Synth. 2023, 21, 494–506. [Google Scholar] [CrossRef]
- Trujillo, J.; Vieira, M.C.; Lepsch, J.; Rebelo, F.; Poston, L.; Pasupathy, D.; Kac, G. A systematic review of the associations between maternal nutritional biomarkers and depression and/or anxiety during pregnancy and postpartum. J. Affect. Disord. 2018, 232, 185–203. [Google Scholar] [CrossRef]
- Abedi, P.; Bovayri, M.; Fakhri, A.; Jahanfar, S. The Relationship Between Vitamin D and Postpartum Depression in Reproductive-Aged Iranian Women. J. Med. Life 2018, 11, 286–292. [Google Scholar] [CrossRef]
- Accortt, E.; Schetter, C.; Peters, R.; Cassidy-Bushrow, A. Lower prenatal vitamin D status and postpartum depressive symptomatology in African American women: Preliminary evidence for moderation by inflammatory cytokines. Arch. Women’s Ment. Health 2016, 19, 373–383. [Google Scholar] [CrossRef]
- Accortt, E.E.; Arora, C.; Mirocha, J.; Jackman, S.; Liang, R.; Karumanchi, S.A.; Berg, A.H.; Hobel, C.J. Low prenatal vitamin D metabolite ratio and subsequent postpartum depression risk. J. Women’s Health 2021, 30, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Amani, R.; Jafarirad, S.; Cheraghian, B.; Sayyah, M.; Hemmati, A.A. The effect of vitamin D and calcium supplementation on inflammatory biomarkers, estradiol levels and severity of symptoms in women with postpartum depression: A randomized double-blind clinical trial. Nutr. Neurosci. 2022, 25, 22–32. [Google Scholar] [CrossRef]
- Brandenbarg, J.; Vrijkotte, T.G.M.; Goedhart, G.; Van Eijsden, M. Maternal early-pregnancy vitamin d status is associated with maternal depressive symptoms in the amsterdam born children and their development cohort. Psychosom. Med. 2012, 74, 751–757. [Google Scholar] [CrossRef]
- Cassidy-Bushrow, A.E.; Peters, R.M.; Johnson, D.A.; Li, J.; Rao, D.S. Vitamin D nutritional status and antenatal depressive symptoms in African American women. J. Women’s Health 2012, 21, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Cunha Figueiredo, A.C.; Trujillo, J.; Freitas-Vilela, A.A.; Franco-Sena, A.B.; Rebelo, F.; Cunha, G.M.; de Castro, M.B.T.; Farnum, A.; Mokhtar, R.R.; Holick, M.F.; et al. Association between plasma concentrations of vitamin D metabolites and depressive symptoms throughout pregnancy in a prospective cohort of Brazilian women. J. Psychiatr. Res. 2017, 95, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dabbaghmanesh, M.H.; Vaziri, F.; Najib, F.; Nasiri, S.; Pourahmad, S. The effect of vitamin D consumption during pregnancy on maternal thyroid function and depression: A randomized, placebo-controlled, clinical trial. Jundishapur J. Nat. Pharm. Prod. 2019, 14, e65328. [Google Scholar] [CrossRef]
- Evanchuk, J.L.; Kozyrskyj, A.; Vaghef-Mehrabani, E.; Lamers, Y.; Giesbrecht, G.F.; Letourneau, N.; Aghajafari, F.; Dewey, D.; Leung, B.; Bell, R.C.; et al. Maternal Iron and Vitamin D Status during the Second Trimester Is Associated with Third Trimester Depression Symptoms among Pregnant Participants in the APrON Cohort. J. Nutr. 2024, 154, 174–184. [Google Scholar] [CrossRef]
- Huang, J.Y.; Arnold, D.; Qiu, C.-f.; Miller, R.S.; Williams, M.A.; Enquobahrie, D.A. Association of serum vitamin D with symptoms of depression and anxiety in early pregnancy. J. Women’s Health 2014, 23, 588–595. [Google Scholar] [CrossRef]
- King, C.E.; Wilkerson, A.; Newman, R.; Wagner, C.L.; Guille, C. Sleep, Anxiety, and Vitamin D Status and Risk for Peripartum Depression. Reprod. Sci. 2022, 29, 1851–1858. [Google Scholar] [CrossRef]
- Lamb, A.R.; Lutenbacher, M.; Wallston, K.A.; Pepkowitz, S.H.; Holmquist, B.; Hobel, C.J. Vitamin D deficiency and depressive symptoms in the perinatal period. Arch. Women’s Ment. Health 2018, 21, 745–755. [Google Scholar] [CrossRef]
- Murphy, P.K.; Mueller, M.; Hulsey, T.C.; Ebeling, M.D.; Wagner, C.L. An exploratory study of postpartum depression and vitamin D. J. Am. Psychiatr. Nurses Assoc. 2010, 16, 170–177. [Google Scholar] [CrossRef]
- Nassr, O.A.; Mohammed, M.M.; Showman, H.A. Relationship between inflammatory biomarkers, vitamin D levels, and depressive symptoms in late pregnancy and during the postpartum period: A prospective, observational study. Middle East Curr. Psychiatry 2022, 29, 78. [Google Scholar] [CrossRef]
- Uslu Yuvaci, H.; Yazar, H.; Kose, E.; Coban, B.N.; Aslan, M.M.; Yazici, E.; Akdemir, N.; Cevrioglu, A.S. Evaluation of the relationship between the level of vitamin D in maternal blood and breast milk and postpartum depression. J. Clin. Obstet. Gynecol. 2020, 30, 58–64. [Google Scholar] [CrossRef]
- Vaziri, F.; Nasiri, S.; Tavana, Z.; Dabbaghmanesh, M.H.; Sharif, F.; Jafari, P. A randomized controlled trial of vitamin D supplementation on perinatal depression: In Iranian pregnant mothers. BMC Pregnancy Childbirth 2016, 16, 239. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, W.; Zhao, A.; Szeto, I.M.-Y.; Lan, H.; Zhang, J.; Li, P.; Ren, Z.; Mao, S.; Jiang, H.; et al. Perinatal depression and serum vitamin D status: A cross-sectional study in urban China. J. Affect. Disord. 2023, 322, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Romero, V.C.; Clinton, C.M.; Vazquez, D.M.; Marcus, S.M.; Chilimigras, J.L.; Hamilton, S.E.; Allbaugh, L.J.; Vahratian, A.M.; Schrader, R.M.; et al. Vitamin D levels and perinatal depressive symptoms in women at risk: A secondary analysis of the mothers, omega-3, and mental health study. BMC Pregnancy Childbirth 2016, 16, 203. [Google Scholar] [CrossRef]
- Woo, J.G. What Is the Relationship Between Vitamin D Status, Pregnancy Symptoms, and Quality of Life? Ph.D. Thesis, Loyola University Chicago, Chicago, IL, USA, 2017. [Google Scholar]
- Jani, R.; Knight-Agarwal, C.R.; Bloom, M.; Takito, M.Y. The association between pre-pregnancy body mass index, perinatal depression and maternal vitamin D status: Findings from an australian cohort study. Int. J. Women’s Health 2020, 12, 213–219. [Google Scholar] [CrossRef]
- Noshiro, K.; Umazume, T.; Inubashiri, M.; Tamura, M.; Hosaka, M.; Watari, H. Association between Edinburgh Postnatal Depression Scale and Serum Levels of Ketone Bodies and Vitamin D, Thyroid Function, and Iron Metabolism. Nutrients 2023, 15, 768. [Google Scholar] [CrossRef] [PubMed]
- Bahramy, P.; Mohammad-Alizadeh-Charandabi, S.; Ramezani-Nardin, F.; Mirghafourvand, M. Serum Levels of Vitamin D, Calcium, Magnesium, and Copper, and their Relations with Mental Health and Sexual Function in Pregnant Iranian Adolescents. Biol. Trace Elem. Res. 2020, 198, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Al-Sabah, R.; Al-Taiar, A.; Ziyab, A.H.; Akhtar, S.; Hammoud, M.S. Antenatal Depression and its Associated Factors: Findings from Kuwait Birth Cohort Study. J. Epidemiol. Glob. Health 2024, 14, 847–859. [Google Scholar] [CrossRef]
- Basutkar, R.S.; Eipe, T.; Divya, P.; Sivasankaran, P. Correlation of antenatal depression among the iron and s-25(OH)D deficient pregnant women: An observational study in a south Indian population. Int. J. Pharm. Res. 2021, 13, 3414–3420. [Google Scholar]
- Lin, Y.-H.; Chen, C.-M.; Su, H.-M.; Mu, S.-C.; Chang, M.-L.; Chu, P.-Y.; Li, S.-C. Association between Postpartum Nutritional Status and Postpartum Depression Symptoms. Nutrients 2019, 11, 1204. [Google Scholar] [CrossRef]
- Pillai, R.R.; Premkumar, N.R.; Kattimani, S.; Sagili, H.; Wilson, A.B.; Sharon, L.; Rajendiran, S. Reduced Maternal Serum Total, Free and Bioavailable Vitamin D Levels and its Association with the Risk for Postpartum Depressive Symptoms. Arch. Med. Res. 2021, 52, 84–92. [Google Scholar] [CrossRef]
- Albacar, G.; Sans, T.; Martin-Santos, R.; Garcia-Esteve, L.; Guillamat, R.; Sanjuan, J.; Canellas, F.; Gratacos, M.; Cavalle, P.; Arija, V.; et al. An association between plasma ferritin concentrations measured 48h after delivery and postpartum depression. J. Affect. Disord. 2011, 131, 136–142. [Google Scholar] [CrossRef]
- Armony-Sivan, R.; Shao, J.; Li, M.; Zhao, G.; Zhao, Z.; Xu, G.; Zhou, M.; Zhan, J.; Bian, Y.; Ji, C.; et al. No relationship between maternal iron status and postpartum depression in two samples in China. J. Pregnancy 2012, 2012, 521431. [Google Scholar] [CrossRef] [PubMed]
- Basutkar, R.S.; Sudarsan, P.; Vinod, C.E.; Varghese, R.; Perumal, D.; Sivasankaran, P. Association between iron-deficiency anemia and antenatal depression in a semi-urban population of South India: A cross-sectional study. Int. J. Acad. Med. 2022, 8, 137–144. [Google Scholar] [CrossRef]
- Chandrasekaran, N.; De Souza, L.R.; Urquia, M.L.; Young, B.; McLeod, A.; Windrim, R.; Berger, H. Is anemia an independent risk factor for postpartum depression in women who have a cesarean section?—A prospective observational study. BMC Pregnancy Childbirth 2018, 18, 400. [Google Scholar] [CrossRef]
- Dama, M.; Van Lieshout, R.J.; Mattina, G.; Steiner, M. Iron Deficiency and Risk of Maternal Depression in Pregnancy: An Observational Study. J. Obstet. Gynaecol. Can. 2018, 40, 698–703. [Google Scholar] [CrossRef]
- Hasdemir, O.K.; Bilgic, D.; Altun, I.K. Relationship Between Gestasyonel Depression and Iron Deficiency Anemia In Pregnancy: A Case-Control Study. Int. J. Caring Sci. 2022, 15, 1545–1555. [Google Scholar]
- Ohsuga, T.; Egawa, M.; Kii, M.; Ikeda, Y.; Ueda, A.; Chigusa, Y.; Mogami, H.; Mandai, M. Association between non-anemic iron deficiency in early pregnancy and perinatal mental health: A retrospective pilot study. J. Obstet. Gynaecol. Res. 2022, 48, 2730–2737. [Google Scholar] [CrossRef]
- Paoletti, A.M.; Orru, M.M.; Marotto, M.F.; Pilloni, M.; Zedda, P.; Fais, M.F.; Piras, B.; Piano, C.; Pala, S.; Lello, S.; et al. Observational study on the efficacy of the supplementation with a preparation with several minerals and vitamins in improving mood and behaviour of healthy puerperal women. Gynecol. Endocrinol. 2013, 29, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Avalos, L.A.; Nance, N.; Caan, B.; Sujan, A.C.; Uriu-Adams, J.Y.; Li, D.-K.; Quesenberry, C.P.; Hedderson, M.M. Association of serum folate levels during pregnancy and prenatal depression. J. Matern.-Fetal Neonatal Med. 2023, 36, 2145878. [Google Scholar] [CrossRef]
- Blunden, C.H.; Inskip, H.M.; Robinson, S.M.; Cooper, C.; Godfrey, K.M.; Kendrick, T.R. Postpartum depressive symptoms: The B-vitamin link. Ment. Health Fam. Med. 2012, 9, 5–13. [Google Scholar]
- Morris, E.; Slomp, C.; Hippman, C.; Inglis, A.; Carrion, P.; Batallones, R.; Andrighetti, H.; Austin, J. A Matched Cohort Study of Postpartum Placentophagy in Women With a History of Mood Disorders: No Evidence for Impact on Mood, Energy, Vitamin B12 Levels, or Lactation. J. Obstet. Gynaecol. Can. 2019, 41, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- van Lee, L.; Quah, P.L.; Saw, S.M.; Yap, F.K.P.; Godfrey, K.M.; Chong, Y.S.; Meaney, M.J.; Chen, H.; Chong, M.F.-F. Maternal choline status during pregnancy, but not that of betaine, is related to antenatal mental well-being: The growing up in Singapore toward healthy outcomes cohort. Depress. Anxiety 2017, 34, 877–887. [Google Scholar] [CrossRef]
- Aishwarya, S.; Rajendiren, S.; Kattimani, S.; Dhiman, P.; Haritha, S.; AnanthaNarayanan, P.H. Homocysteine and serotonin: Association with postpartum depression. Asian J. Psychiatry 2013, 6, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Abou-Saleh, M.T.; Ghubash, R.; Karim, L.; Krymski, M.; Anderson, D.N. The role of pterins and related factors in the biology of early postpartum depression. Eur. Neuropsychopharmacol. 1999, 9, 295–300. [Google Scholar] [PubMed]
- Bodnar, L.M.; Wisner, K.L.; Luther, J.F.; Powers, R.W.; Evans, R.W.; Gallaher, M.J.; Newby, P.; Bodnar, L.M.; Wisner, K.L.; Luther, J.F.; et al. An exploratory factor analysis of nutritional biomarkers associated with major depression in pregnancy. Public Health Nutr. 2012, 15, 1078–1086. [Google Scholar] [CrossRef]
- Lukose, A.; Ramthal, A.; Thomas, T.; Bosch, R.; Kurpad, A.V.; Duggan, C.; Srinivasan, K. Nutritional factors associated with antenatal depressive symptoms in the early stage of pregnancy among Urban South Indian women. Matern. Child Health J. 2014, 18, 161–170. [Google Scholar] [CrossRef]
- Peppard, L.; Oh, K.M.; Gallo, S.; Milligan, R. Risk of depression in pregnant women with low-normal serum Vitamin B12. Res. Nurs. Health 2019, 42, 264–272. [Google Scholar] [CrossRef]
- Chong, M.F.F.; Wong, J.X.Y.; Colega, M.; Chen, L.-W.; van Dam, R.M.; Tan, C.S.; Lim, A.L.; Cai, S.; Broekman, B.F.P.; Lee, Y.S.; et al. Relationships of maternal folate and vitamin B12 status during pregnancy with perinatal depression: The GUSTO study. J. Psychiatr. Res. 2014, 55, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Batalha, M.A.; Costa, P.N.D.; Ferreira, A.L.L.; Freitas-Costa, N.C.; Figueiredo, A.C.C.; Shahab-Ferdows, S.; Hampel, D.; Allen, L.H.; Perez-Escamilla, R.; Kac, G. Maternal Mental Health in Late Pregnancy and Longitudinal Changes in Postpartum Serum Vitamin B-12, Homocysteine, and Milk B-12 Concentration Among Brazilian Women. Front. Nutr. 2022, 9, 923569. [Google Scholar] [CrossRef]
- Cruz-Rodriguez, J.; Canals-sans, J.; Hernandez-Martinez, C.; Arija, V. Association between of vitamin b12 status during pregnancy and probable postpartum depression: The eclipses study. J. Reprod. Infant Psychol. 2024, 43, 1111–1125. [Google Scholar] [CrossRef]
- Dhiman, P.; Pillai, R.R.; Wilson, A.B.; Premkumar, N.; Bharadwaj, B.; Ranjan, V.P.; Rajendiran, S. Cross-sectional association between vitamin B12 status and probable postpartum depression in Indian women. BMC Pregnancy Childbirth 2021, 21, 146. [Google Scholar] [CrossRef]
- Morris, E.; Hippman, C.; Albert, A.; Slomp, C.; Inglis, A.; Carrion, P.; Batallones, R.; Andrighetti, H.; Ross, C.; Dyer, R.; et al. A prospective study to explore the relationship between MTHFR C677T genotype, physiological folate levels, and postpartum psychopathology in at-risk women. PLoS ONE 2020, 15, e0243936. [Google Scholar] [CrossRef]
- Kurniati, Y.; Sinrang, W.; Syamsuddin, S. Postpartum blues syndrome: Serum zinc and psychosocial factors. Enferm. Clin. 2020, 30, 18–21. [Google Scholar]
- Kavitha, M.M.; Dharambhat, S.; Mutalik, N.; Chandrashekaraya, S.H.; Kashinakunti, S.V. Correlation of serum zinc levels with post-partum depression—A case-control study in North Karnataka. J. Clin. Diagn. Res. 2021, 15, 1–3. [Google Scholar]
- Wojcik, J.; Dudek, D.; Schlegel-Zawadzka, M.; Grabowska, M.; Marcinek, A.; Florek, E.; Piekoszewski, W.; Nowak, R.J.; Opoka, W.; Nowak, G. Antepartum/postpartum depressive symptoms and serum zinc and magnesium levels. Pharmacol. Rep. 2006, 58, 571–576. [Google Scholar]
- Rokoff, L.B.; Cardenas, A.; Lin, P.-I.D.; Rifas-Shiman, S.L.; Wright, R.O.; Bosquet Enlow, M.; Coull, B.A.; Oken, E.; Korrick, S.A. Early pregnancy essential and non-essential metal mixtures and maternal antepartum and postpartum depressive symptoms. Neurotoxicology 2023, 94, 206–216. [Google Scholar] [CrossRef]
- Crayton, J.W.; Walsh, W.J. Elevated serum copper levels in women with a history of post-partum depression. J. Trace Elem. Med. Biol. 2007, 21, 17–21. [Google Scholar] [CrossRef]
- Jin, Y.; Coad, J.; Pond, R.; Kim, N.; Brough, L. Selenium intake and status of postpartum women and postnatal depression during the first year after childbirth in New Zealand—Mother and Infant Nutrition Investigation (MINI) study. J. Trace Elem. Med. Biol. 2020, 61, 126503. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Bradley, H.A.; Vlasiuk, E.; Pierard, H.; Beddow, J.; Rucklidge, J.J. Inflammation and Vitamin C in Women with Prenatal Depression and Anxiety: Effect of Multinutrient Supplementation. Antioxidants 2023, 12, 941. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.L.; Holden, J.M.; Sagovsky, K. Detection of postnatal depression: Development. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef]
- Fu, C.W.; Liu, J.T.; Tu, W.J.; Yang, J.Q.; Cao, Y. Association between serum 25-hydroxyvitamin D levels measured 24 hours after delivery and postpartum depression. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 1688–1694. [Google Scholar]
- British Committee For Standards in Haematology. Guidelines on the investigation and diagnosis of cobalamin and folate deficiencies. Clin. Lab. Haematol. 1994, 16, 101–115. [Google Scholar]
- Indriasari, R.; Kurniati, Y.; Syam, A.; Syamsuddin, S.; Mansur, M.A.; Amir, S. Relationship between serum zinc concentration with post-partum depression among women in coastal area of Indonesia. Pak. J. Nutr. 2019, 18, 747–752. [Google Scholar] [CrossRef]
- Flynn, A. Estrogen Modulation of Blood Copper and Other Essential Metal Concentrations. In Inflammatory Diseases and Copper: The Metabolic and Therapeutic Roles of Copper and Other Essential Metalloelements in Humans; Sorenson, J.R.J., Ed.; Humana Press: Totowa, NJ, USA, 1982; pp. 17–30. [Google Scholar]
- Vir, S.C.; Love, A.H.G.; Thompson, W. Serum and hair concentrations of copper during pregnancy. Am. J. Clin. Nutr. 1981, 34, 2382–2388. [Google Scholar] [CrossRef]
- Chen, J.; Song, W.; Zhang, W. The emerging role of copper in depression. Front. Neurosci. 2023, 17, 01–08. [Google Scholar] [CrossRef]
- Ni, M.; You, Y.; Chen, J.; Zhang, L. Copper in depressive disorder: A systematic review and meta-analysis of observational studies. Psychiatry Res. 2018, 267, 506–515. [Google Scholar] [CrossRef]
- Hulsbosch, L.P.; Boekhorst, M.; Gigase, F.A.J.; Broeren, M.A.C.; Krabbe, J.G.; Maret, W.; Pop, V.J.M. The first trimester plasma copper-zinc ratio is independently related to pregnancy-specific psychological distress symptoms throughout pregnancy. Nutrition 2023, 109, 111938. [Google Scholar] [CrossRef]
- Tiderencel, K.A.; Zelig, R.; Parker, A. The Relationship Between Vitamin D and Postpartum Depression: A Review of Current Literature. Top. Clin. Nutr. 2019, 34, 301–314. [Google Scholar] [CrossRef]
- Aghajafari, F.; Letourneau, N.; Mahinpey, N.; Cosic, N.; Giesbrecht, G. Vitamin D Deficiency and Antenatal and Postpartum Depression: A Systematic Review. Nutrients 2018, 10, 478. [Google Scholar] [CrossRef]
- Wang, J.; Liu, N.; Sun, W.; Chen, D.; Zhao, J.; Zhang, W. Association between vitamin D deficiency and antepartum and postpartum depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gynecol. Obstet. 2018, 298, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Fallah, M.; Askari, G.; Asemi, Z. Is Vitamin D Status Associated with Depression, Anxiety and Sleep Quality in Pregnancy: A Systematic Review. Adv. Biomed. Res. 2020, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Liu, S.; Chen, D. Poor vitamin D status and the risk of maternal depression: A dose-response meta-analysis of observational studies. Public Health Nutr. 2021, 24, 2161–2170. [Google Scholar] [CrossRef]
- Fang, P. Data on the Association between Vitamin D and Perinatal Depression. In Proceedings of the 2021 International Conference on Public Health and Data Science, Chengdu, China, 9–11 July 2021; pp. 206–212. [Google Scholar]
- Chai, Y. Recent Advance in Understanding Vitamin D in Postpartum Depression. Int. J. Biomed. Sci. 2022, 18, 60–65. [Google Scholar] [CrossRef]
- Ribamar, A.; Almeida, B.; Soares, A.; Peniche, B.; Jesus, P.; Cruz, S.P.d.; Ramalho, A. Relationship between vitamin D deficiency and both gestational and postpartum depression. Nutr. Hosp. 2020, 37, 1238–1245. [Google Scholar] [CrossRef]
- Gould, J.F.; Gibson, R.A.; Green, T.J.; Makrides, M. A Systematic Review of Vitamin D during Pregnancy and Postnatally and Symptoms of Depression in the Antenatal and Postpartum Period from Randomized Controlled Trials and Observational Studies. Nutrients 2022, 14, 2300. [Google Scholar] [CrossRef]
- Mahmood, I.; Owens, C.T.; Hoover, R.M. Association Between Vitamin D Levels During Pregnancy and Postpartum Depression. J. Pharm. Technol. 2015, 31, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Jafarirad, S.; Amani, R. Postpartum depression and vitamin D: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Feron, F.; Cui, X.; Kesby, J.P.; Harms, L.H.; Ko, P.; McGrath, J.J.; Burne, T.H.J. Developmental vitamin D causes abnormal brain development. Psychoneuroendocrinology 2009, 34, S247–S257. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (IOM). Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Musazadeh, V.; Keramati, M.; Ghalichi, F.; Kavyani, Z.; Ghoreishi, Z.; Alras, K.A.; Albadawi, N.; Salem, A.; Albadawi, M.I.; Salem, R.; et al. Vitamin D protects against depression: Evidence from an umbrella meta-analysis on interventional and observational meta-analyses. Pharmacol. Res. 2023, 187, 106605. [Google Scholar] [CrossRef]
- Kouba, B.R.; Camargo, A.; Gil-Mohapel, J.; Rodrigues, A.L.S. Molecular Basis Underlying the Therapeutic Potential of Vitamin D for the Treatment of Depression and Anxiety. Int. J. Mol. Sci. 2022, 23, 7077. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Q.; Wang, L.; Zhang, C.; Li, Y.; Zhang, Y. Effects of 25-hydroxyvitamin d3 and oral calcium bolus on lactation performance, ca homeostasis, and health of multiparous dairy cows. Animals 2021, 11, 1576. [Google Scholar] [CrossRef]
- Moretti, R.; Morelli, M.E.; Caruso, P. Vitamin D in neurological diseases: A rationale for a pathogenic impact. Int. J. Mol. Sci. 2018, 19, 2245. [Google Scholar] [CrossRef]
- Berridge, M.J. Vitamin D and Depression: Cellular and Regulatory Mechanisms. Pharmacol. Rev. 2017, 69, 80–92. [Google Scholar] [CrossRef]
- Duarte-Silva, E.; Maes, M.; Peixoto, C.A. Iron metabolism dysfunction in neuropsychiatric disorders: Implications for therapeutic intervention. Behav. Brain Res. 2025, 479, 115343. [Google Scholar] [CrossRef]
- Thachil, J. The beneficial effect of acute phase increase in serum ferritin. Eur. J. Intern. Med. 2016, 35, e16–e17. [Google Scholar] [CrossRef]
- Stevens, A.J.; Rucklidge, J.J.; Kennedy, M.A. Epigenetics, nutrition and mental health. Is there a relationship? Nutr. Neurosci. 2018, 21, 602–613. [Google Scholar] [CrossRef]
- Bjelland, I.; Tell, G.S.; Vollset, S.E.; Refsum, H.; Ueland, P.M. Folate, vitamin B12, Homocysteine and the MTHFR 677C-> T polymorphism in anxiety and depression. The Hordaland Homocysteine Study. Arch. Gen. Psychiatry 2003, 60, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmio, L. B vitamins and one carbon metabolism: Implications in human health and disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J. Nutr. Health Aging 2002, 6, 39–42. [Google Scholar]
- Kominiarek, M.A.; Rajan, P. Nutrition Recommendations in Pregnancy and Lactation. Med. Clin. N. Am. 2016, 100, 1199–1215. [Google Scholar] [CrossRef]
- McDonald, C.M.; Suchdev, P.S.; Krebs, N.F.; Hess, S.Y.; Wessells, K.R.; Ismaily, S.; Rahman, S.; Wieringa, F.T.; Williams, A.M.; Brown, K.H.; et al. Adjusting plasma or serum zinc concentrations for inflammation: Biomarkers reflecting inflammation and nutritional determinants of anemia (BRINDA) project. Am. J. Clin. Nutr. 2020, 111, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.A.; Zapalowski, C.; Kappy, M.S. Disorders of calcium, phosphate, parathyroid hormone and vitamin D. In Principles and Practice of Pediatric Endocrinology; Kappy, M.S., Allen, D.B., Geffner, M.D., Eds.; Charles C Thomas Co.: Springfield, IL, USA, 2005. [Google Scholar]
- Zhang, H.; Liu, S.; Si, Y.; Zhang, S.; Tian, Y.; Liu, Y.; Li, H.; Zhu, Z. Natural sunlight plus vitamin D supplementation ameliorate delayed early motor development in newborn infants from maternal perinatal depression. J. Affect. Disord. 2019, 257, 241–249. [Google Scholar] [CrossRef]
- Ma, S.-S.; Zhu, D.-M.; Yin, W.-J.; Hao, J.-H.; Huang, K.; Tao, F.-B.; Tao, R.-X.; Zhu, P. The role of neonatal vitamin D in the association of prenatal depression with toddlers ADHD symptoms: A birth cohort study. J. Affect. Disord. 2021, 281, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Tahir, H.; Munir, N.; Iqbal, S.S.; Bacha, U.; Amir, S.; Umar, H.; Riaz, M.; Tahir, I.M.; Shah, S.M.A.; Shafiq, A.; et al. Maternal vitamin D status and attention deficit hyperactivity disorder (ADHD), an under diagnosed risk factor; A review. Eur. J. Inflamm. 2023, 21, 1–15. [Google Scholar] [CrossRef]
- Lisi, G.; Ribolsi, M.; Siracusano, A.; Niolu, C. Maternal vitamin D and its role in determining fetal origins of mental health. Curr. Pharm. Des. 2020, 26, 2497–2509. [Google Scholar] [CrossRef]
- Fu, H.; Li, Y.; Huang, H.; Wang, D. Serum vitamin D level and efficacy of vitamin D supplementation in children with atopic dermatitis: A systematic review and meta-analysis. Comput. Math. Methods Med. 2022, 2022, 9407888. [Google Scholar] [CrossRef]
- Wang, Q.; Ying, Q.; Zhu, W.; Chen, J. Vitamin D and asthma occurrence in children: A systematic review and meta-analysis. J. Pediatr. Nurs. 2022, 62, e60–e68. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Jin, Y.; Luo, J.; Jiang, F.; Zhang, L.; Wang, X.; Zhang, H.; Ma, S.; Li, Y. Comparison of the vitamin D level between children with and without cow’s milk protein allergy: A systematic review with meta-analysis. Front. Pediatr. 2025, 13, 1649825. [Google Scholar] [CrossRef] [PubMed]
- Sabir, M.S.; Haussler, M.R.; Mallick, S.; Kaneko, I.; Lucas, D.A.; Haussler, C.A.; Whitfield, G.K.; Jurutka, P.W. Optimal vitamin D spurs serotonin: 1,25-dihydroxyvitamin D represses serotonin reuptake transport (SERT) and degradation (MAO-A) gene expression in cultured rat serotonergic neuronal cell lines. Genes Nutr. 2018, 13, 19. [Google Scholar] [CrossRef]
- Bouillon, R. Vitamin D and Extraskeletal Health. UpToDate. 2025. Available online: https://www.uptodate.com/contents/vitamin-d-and-extraskeletal-health (accessed on 30 October 2025).
- Rosen, H.N. Calcium and Vitamin D Supplement in Osteoporosis. Up to Date. 2023. Available online: https://www.uptodate.com/contents/calcium-and-vitamin-d-supplementation-in-osteoporosis (accessed on 30 October 2025).
- Munns, C.F.; Simm, P.J.; Rodda, C.P.; Garnett, S.P.; Zacharin, M.R.; Ward, L.M.; Geddes, J.; Cherian, S.; Zurynski, Y.; Cowell, C.T. Incidence of vitamin D deficiency rickets among Australian children: An Australian Paediatric Surveillance Unit study. Med. J. Aust. 2012, 196, 466–468. [Google Scholar] [CrossRef]
- Royal Australian and New Zealand College of Obstetrics and Gynaecology (RANZCOG). Routine Antenatal Assessment in the Absence of Pregnancy Complications 2022. Available online: https://ranzcog.edu.au/wp-content/uploads/Routine-Antenatal-Assessment.pdf (accessed on 30 October 2025).
- Australian Government Department of Health and Ageing. MBS Online Medicare Benefits Schedule: Department of Health, Disability and Ageing. 2025. Available online: https://www9.health.gov.au/mbs (accessed on 30 October 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).