Lactiplantibacillus plantarum WLPL04 from Human Breast Milk Attenuates Hyperuricemia via Coordinated Purine Salvage Pathway, Renal Transporter Regulation, and Gut Microbiota Remodeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of L. plantarum WLPL04 Under Purine Substrate
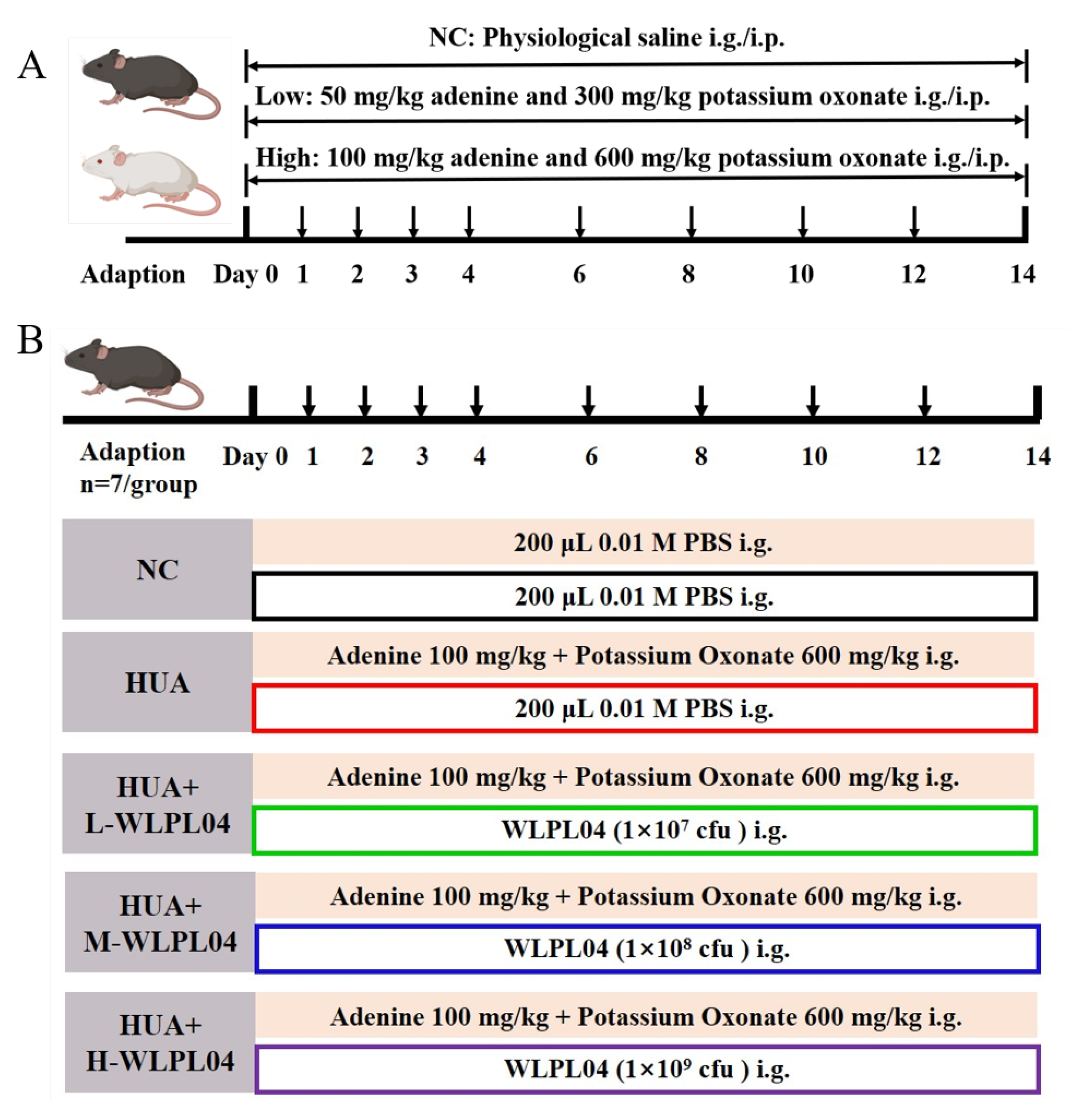
2.2. Hyperuricemia Modeling Design
2.3. L. plantarum WLPL04 Intervention in Hyperuricemia Model
2.4. Preparation of Specimens
2.5. Urine Collection
2.6. Serum Biochemical Assay
2.7. Histopathological Analysis
2.8. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
2.9. 16 S Ribosomal RNA Gene Sequencing and Data Analysis
2.10. RAW264.7 Cells Co-Culture with WLPL04
2.11. Statistical Analysis
3. Results
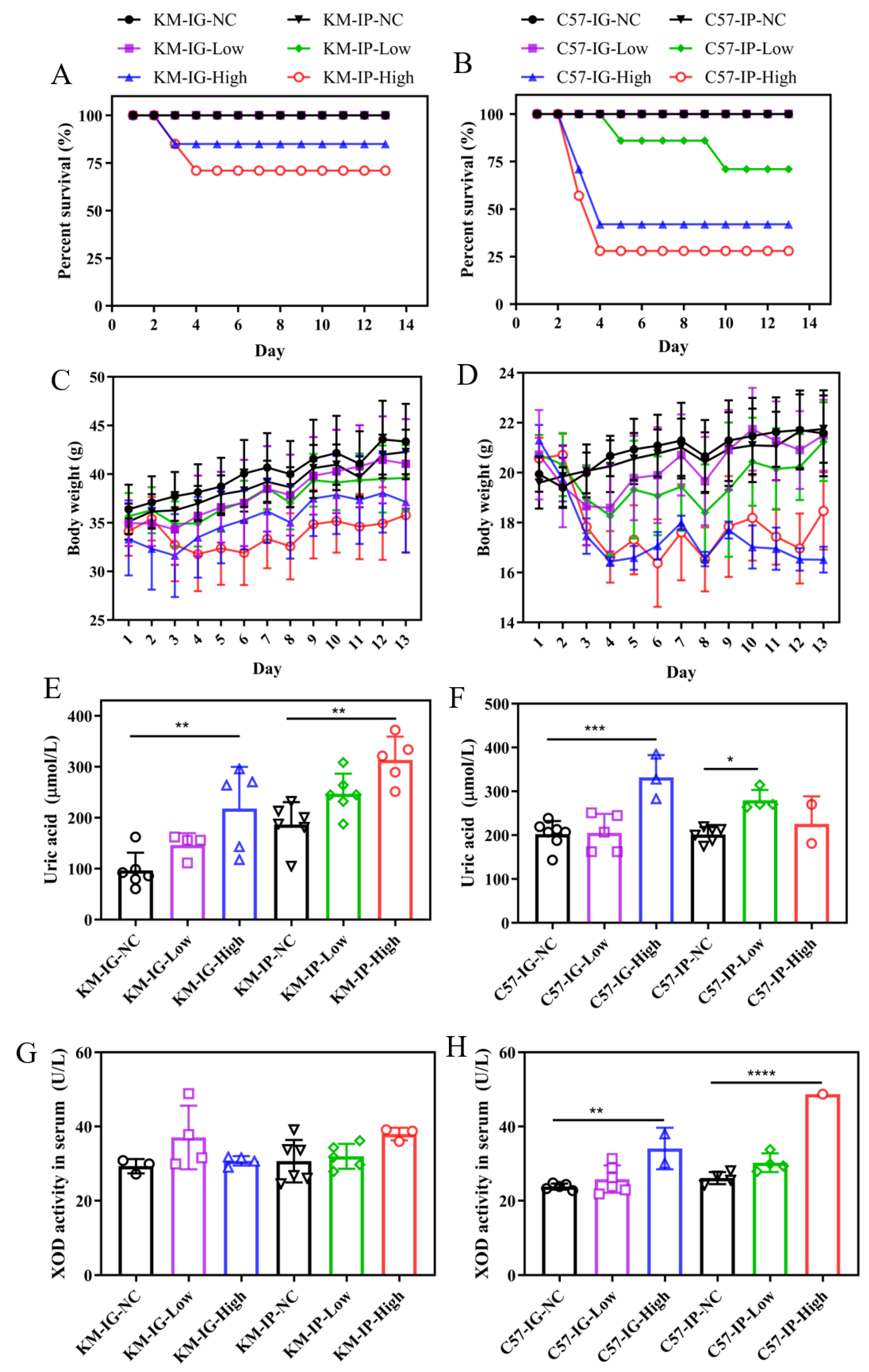
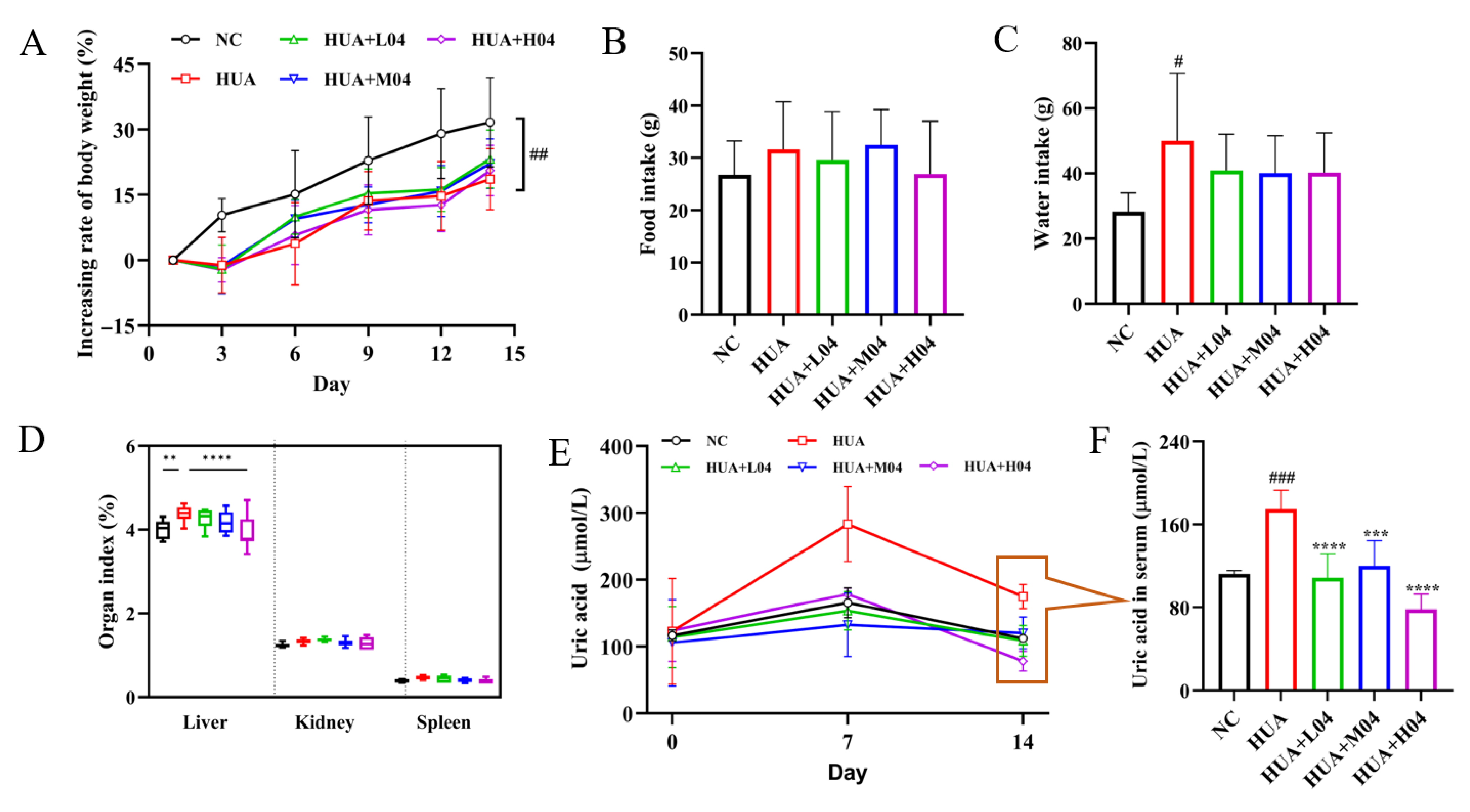
3.1. Effects of Different Models on Survival, Body Weight, and UA Metabolism on Mice
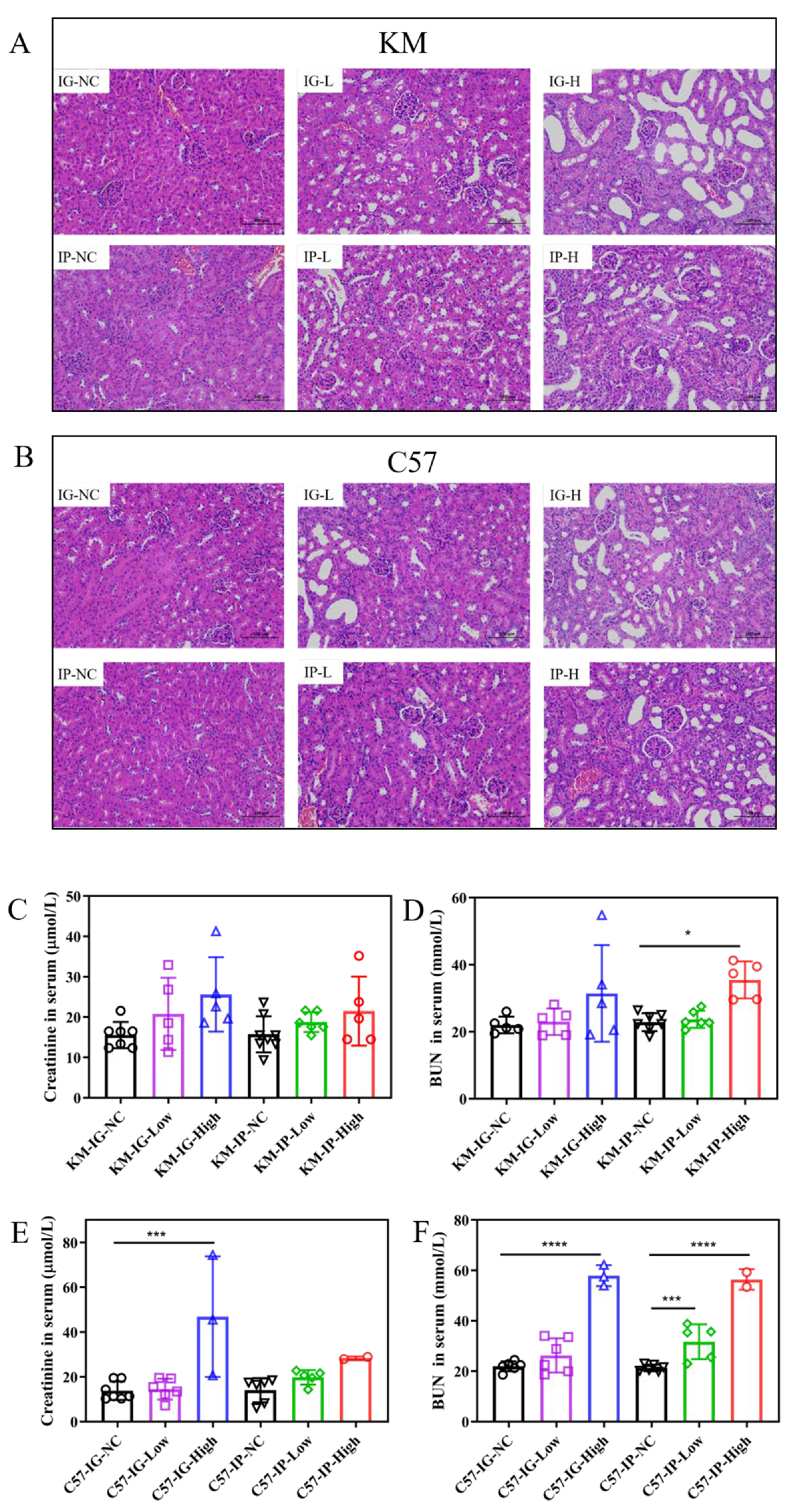
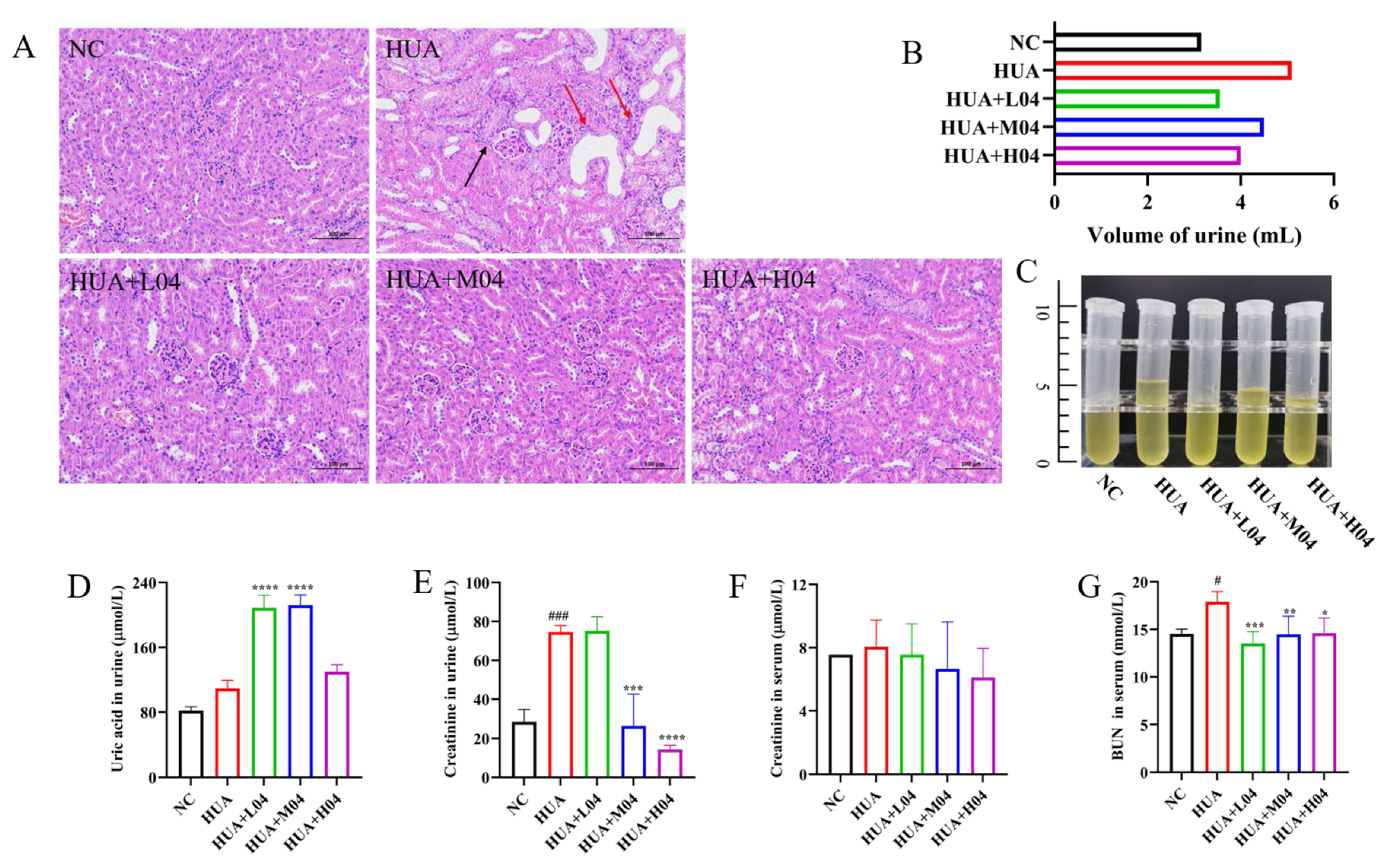
3.2. Renal Function Implications of Hyperuricemia Models
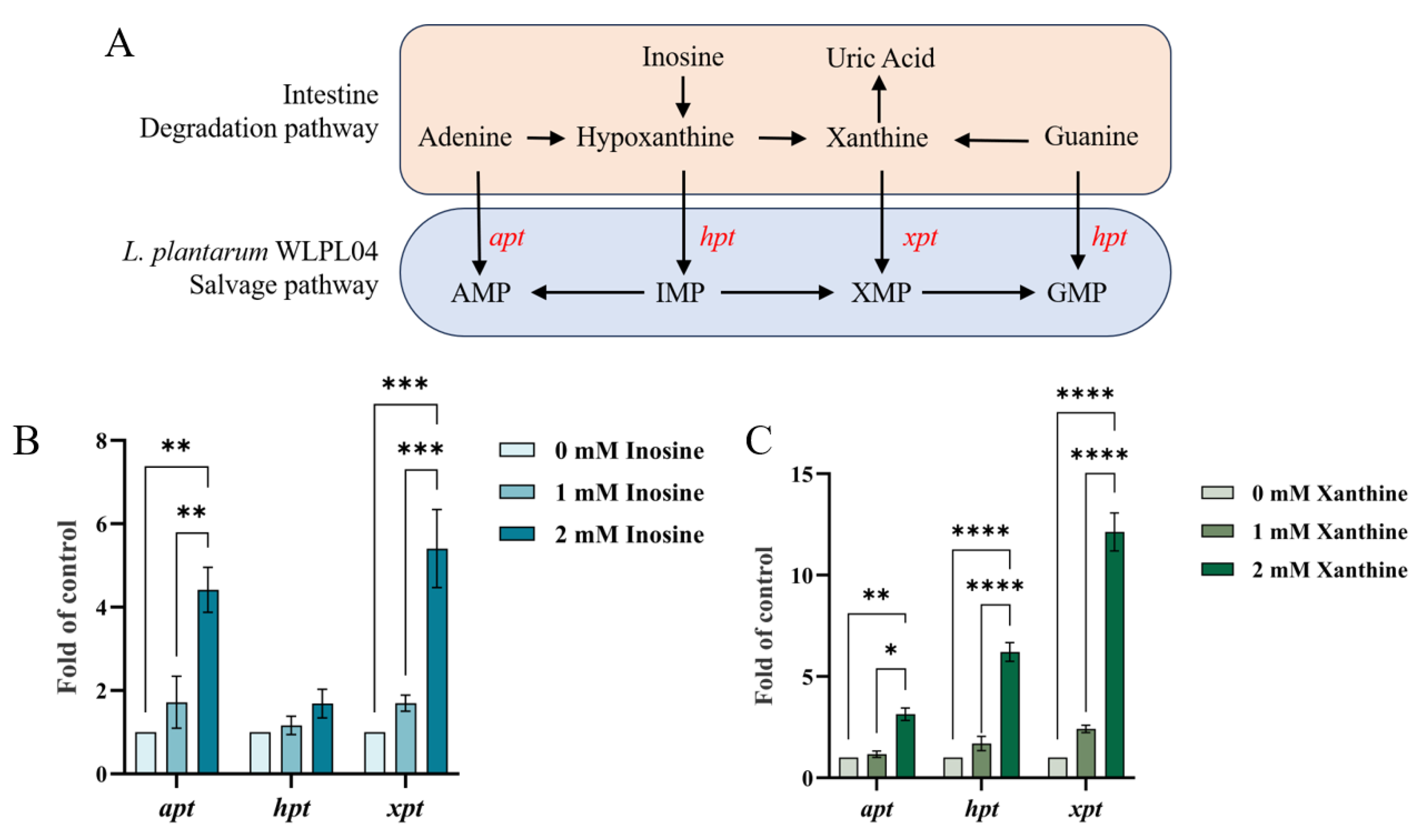
3.3. Expression of Purine Salvage Pathway Genes in L. plantarum WLPL04
3.4. Effect of L. plantarum WLPL04 on Uric Acid and Physiological Parameters
3.5. Renal Protective Effect of L. plantarum WLPL04 in Hyperuricemia
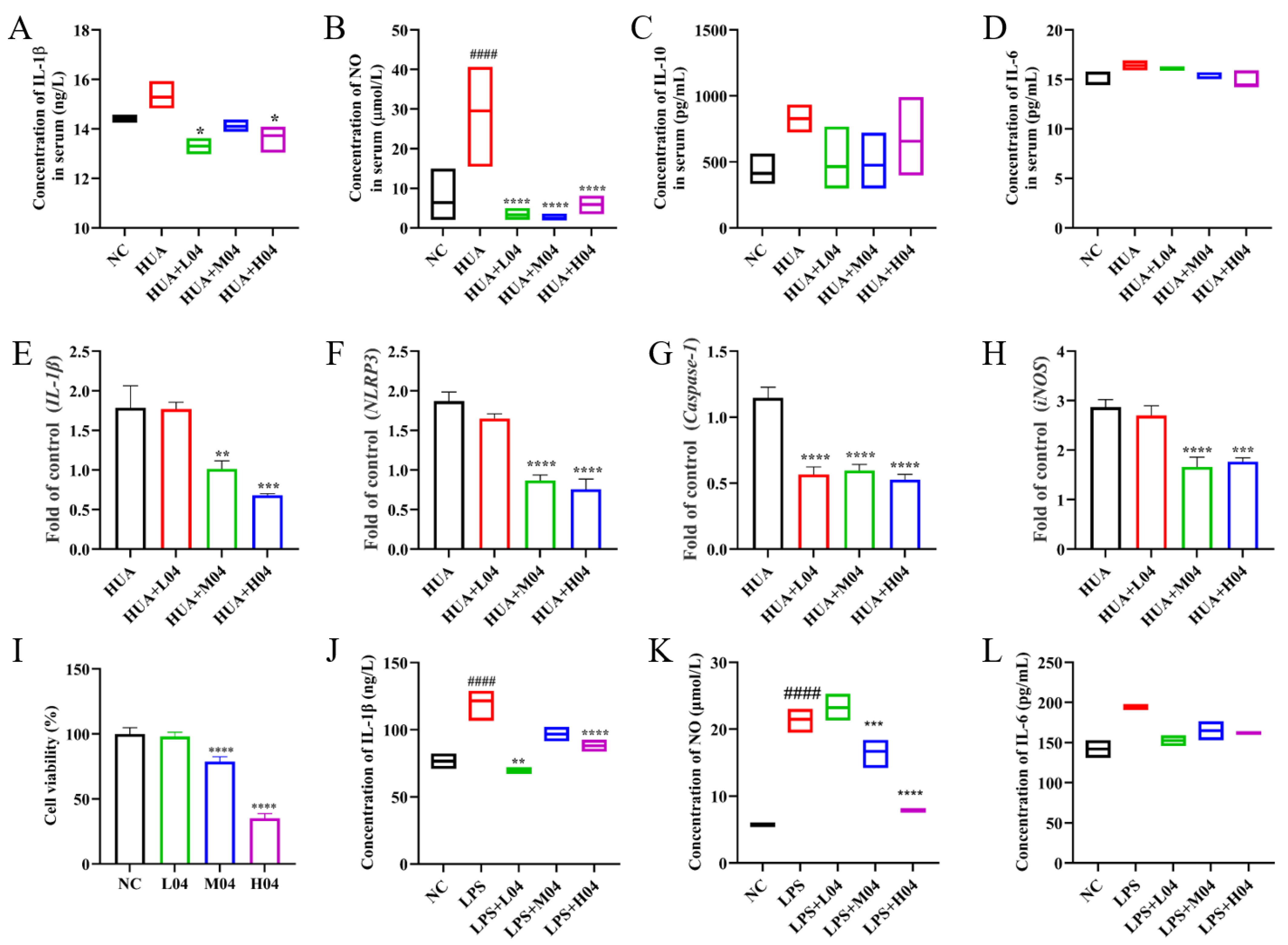
3.6. Effects of L. plantarum WLPL04 on Inflammation
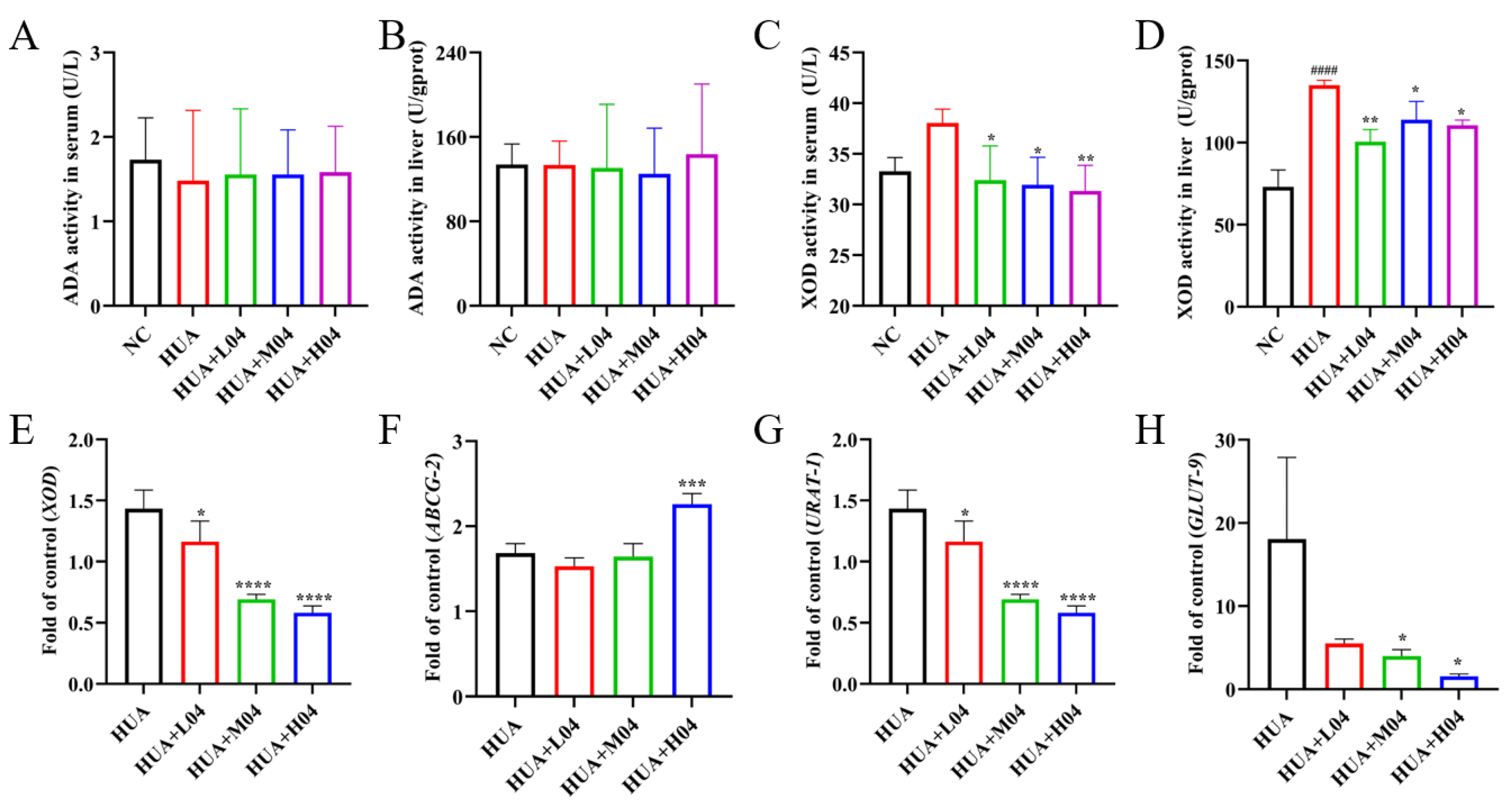
3.7. Regulation of Uric Acid Metabolism by L. plantarum WLPL04
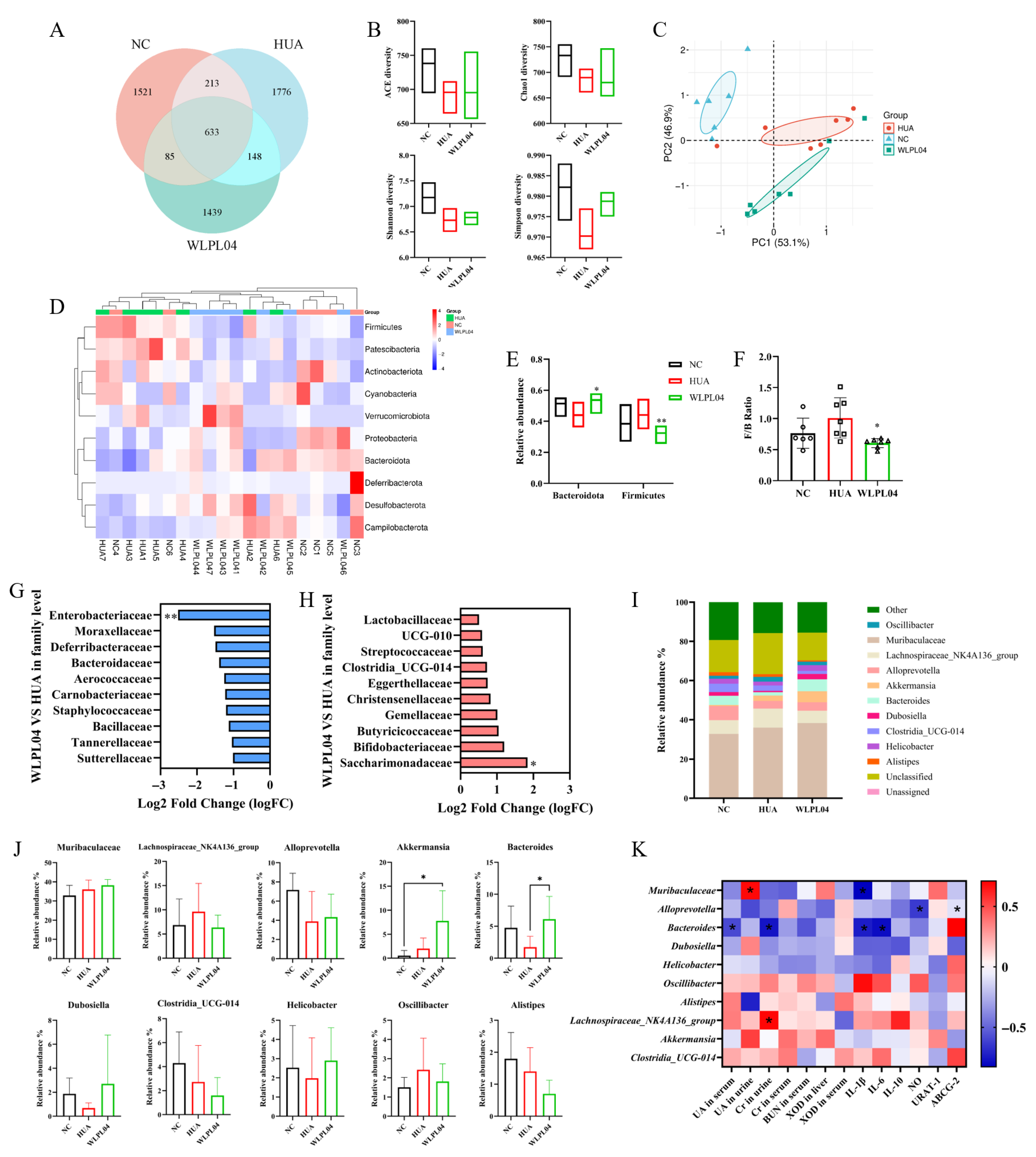
3.8. L. plantarum WLPL04 Modulates Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, L.; Zong, Y.; Li, H.; Wang, Q.; Xie, L.; Yang, B.; Pang, Y.; Zhang, C.; Zhong, Z.; Gao, J. Hyperuricemia and its related diseases: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 212. [Google Scholar] [CrossRef]
- Higa, S.; Yoshida, M.; Shima, D.; Ii, Y.; Kitazaki, S.; Yamamoto, Y.; Fujimoto, Y. A Retrospective, Cross-Sectional Study on the Prevalence of Hyperuricemia Using a Japanese Healthcare Database. Arch. Rheumatol. 2020, 35, 41–51. [Google Scholar] [CrossRef]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011, 63, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Han, C.; Wu, D.; Xia, X.; Gu, J.; Guan, H.; Shan, Z.; Teng, W. Prevalence of Hyperuricemia and Gout in Mainland China from 2000 to 2014: A Systematic Review and Meta-Analysis. Biomed Res. Int. 2015, 2015, 762820. [Google Scholar] [CrossRef]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Molecular biological and clinical understanding of the pathophysiology and treatments of hyperuricemia and its association with metabolic syndrome, cardiovascular diseases and chronic kidney disease. Int. J. Mol. Sci. 2021, 22, 9221. [Google Scholar] [CrossRef]
- Cicero, A.F.; Fogacci, F.; Cincione, R.I.; Tocci, G.; Borghi, C. Clinical effects of xanthine oxidase inhibitors in hyperuricemic patients. Med. Princ. Pract. 2021, 30, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Roman, Y.M. The role of uric acid in human health: Insights from the uricase gene. J. Pers. Med. 2023, 13, 1409. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef]
- Yin, H.; Liu, N.; Chen, J. The role of the intestine in the development of hyperuricemia. Front. Immunol. 2022, 13, 845684. [Google Scholar] [CrossRef]
- Xu, X.; Li, C.; Zhou, P.; Jiang, T. Uric acid transporters hiding in the intestine. Pharm. Biol. 2016, 54, 3151–3155. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, M.; Zhao, J.; Chen, W.; Wang, G. The human gut microbiota and uric acid metabolism: Genes, metabolites, and diet. Crit. Rev. Food Sci. Nutr. 2025, 20, 1–21. [Google Scholar] [CrossRef]
- Tong, Y.; Wei, Y.; Ju, Y.; Li, P.; Zhang, Y.; Li, L.; Gao, L.; Liu, S.; Liu, D.; Hu, Y. Anaerobic purinolytic enzymes enable dietary purine clearance by engineered gut bacteria. Cell Chem. Biol. 2023, 30, 1104–1114. e1107. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, K.; Kerby, R.L.; Zhang, Q.; Pradhan, M.; Mehrabian, M.; Lusis, A.J.; Bergström, G.; Bäckhed, F.; Rey, F.E. Gut bacterial metabolism contributes to host global purine homeostasis. Cell Host Microbe 2023, 31, 1038–1053.e10. [Google Scholar] [CrossRef] [PubMed]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Zhong, H.; Chen, F.; Regenstein, J.; Hu, X.; Cai, L.; Feng, F. The gut microbiota as a target to control hyperuricemia pathogenesis: Potential mechanisms and therapeutic strategies. Crit. Rev. Food Sci. Nutr. 2022, 62, 3979–3989. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, X.; Li, J.; Li, Z.; Hu, Q.; Li, L.; Hao, X.; Song, M.; Li, C. Using probiotics for type 2 diabetes mellitus intervention: Advances, questions, and potential. Crit. Rev. Food Sci. Nutr. 2020, 60, 670–683. [Google Scholar] [CrossRef]
- James, A.; Ke, H.; Yao, T.; Wang, Y. The role of probiotics in purine metabolism, hyperuricemia and gout: Mechanisms and interventions. Food Rev. Int. 2023, 39, 261–277. [Google Scholar] [CrossRef]
- Zhong, F.; Feng, X.; Cao, J.; Li, M.; Tian, J.; Wang, J.; Wang, X.; Luo, X. Novel Potential Probiotics from Chinese Baijiu Fermentation Grains: Dual Action of Lactiplantibacillus plantarum LTJ1/LTJ48 in Uric Acid Reduction and Gut Microbiota Restoration for Hyperuricemia Therapy in Mice. Nutrients 2025, 17, 2097. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, X.-D.; Li, J.-Z.; Mo, Q.-Y.; Wang, X.; Zhao, Y.; Zhang, Y.-M.; Luo, H.-T.; Xia, D.-Y.; Ma, W.-Q.; et al. Host-derived Lactobacillus plantarum alleviates hyperuricemia by improving gut microbial community and hydrolase-mediated degradation of purine nucleosides. eLife 2024, 13, e100068. [Google Scholar] [CrossRef]
- Li, M.; Yang, D.; Mei, L.; Yuan, L.; Xie, A.; Yuan, J. Screening and characterization of purine nucleoside degrading lactic acid bacteria isolated from Chinese sauerkraut and evaluation of the serum uric acid lowering effect in hyperuricemic rats. PLoS ONE 2014, 9, e105577. [Google Scholar] [CrossRef]
- Hsieh, M.-W.; Chen, H.-Y.; Tsai, C.-C. Screening and evaluation of purine-nucleoside-degrading lactic acid bacteria isolated from winemaking byproducts in vitro and their uric acid-lowering effects in vivo. Fermentation 2021, 7, 74. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, X.; Zhang, L.; Meng, F.; Zhou, L.; Pang, X.; Lu, Z.; Lu, Y. Lacticaseibacillus rhamnosus Fmb14 prevents purine induced hyperuricemia and alleviate renal fibrosis through gut-kidney axis. Pharmacol. Res. 2022, 182, 106350. [Google Scholar] [CrossRef] [PubMed]
- Nei, S.; Matsusaki, T.; Kawakubo, H.; Ogawa, K.; Nishiyama, K.; Tsend-Ayush, C.; Nakano, T.; Takeshita, M.; Shinyama, T.; Yamasaki, M. Lactiplantibacillus plantarum 06CC2 Enhanced the Expression of Intestinal Uric Acid Excretion Transporter in Mice. Nutrients 2024, 16, 3042. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wen, X.; Lessing, D.J.; Chu, W. Uric Acid-Degrading Lacticaseibacillus paracasei CPU202306 Ameliorates Hyperuricemia by Regulating Uric Acid Metabolism and Intestinal Microecology in Mice. In Probiotics and Antimicrobial Proteins; Springer: Berlin/Heidelberg, Germany, 2025; pp. 1–25. [Google Scholar]
- Wang, Q.; Liang, J.; Zou, Q.; Wang, W.; Yan, G.; Guo, R.; Yuan, T.; Wang, Y.; Liu, X.; Liu, Z. Tryptophan metabolism-regulating probiotics alleviate hyperuricemia by protecting the gut barrier integrity and enhancing colonic uric acid excretion. J. Agric. Food Chem. 2024, 72, 26746–26761. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Werlinger, P.; Suh, J.-W.; Cheng, J. Potential probiotic Lacticaseibacillus paracasei MJM60396 prevents hyperuricemia in a multiple way by absorbing purine, suppressing xanthine oxidase and regulating urate excretion in mice. Microorganisms 2022, 10, 851. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, F.; Wan, C.; Xiong, Y.; Shah, N.P.; Wei, H.; Tao, X. Evaluation of probiotic properties of Lactobacillus plantarum WLPL04 isolated from human breast milk. J. Dairy Sci. 2016, 99, 1736–1746. [Google Scholar] [CrossRef]
- Allgoewer, A.; Mayer, B. Sample Size Estimation for Pilot Animal Experiments by Using a Markov Chain Monte Carlo Approach. Altern. Lab. Anim. 2017, 45, 83–90. [Google Scholar] [CrossRef]
- Liu, Y.-r.; Wang, J.-q.; Li, J. Role of NLRP3 in the pathogenesis and treatment of gout arthritis. Front. Immunol. 2023, 14, 1137822. [Google Scholar] [CrossRef]
- Bi, C.; Zhang, L.; Liu, J.; Chen, L. Lactobacillus paracasei 259 alleviates hyperuricemia in rats by decreasing uric acid and modulating the gut microbiota. Front. Nutr. 2024, 11, 1450284. [Google Scholar] [CrossRef]
- Tseng, W.-T.; Kong, X.-R.; Han, Y.-T.; Lin, W.-Y.; Yin, D.; Du, L.; Xie, J.; Chang, T.-H. Lacticaseibacillus paracasei LT12—A Probiotic Strain That Reduces Hyperuricemia via Inhibiting XO Activity and Regulating Renal Uric Acid Transportation Protein. Fermentation 2025, 11, 96. [Google Scholar] [CrossRef]
- Yang, Q.; Su, S.; Luo, N.; Cao, G. Adenine-induced animal model of chronic kidney disease: Current applications and future perspectives. Ren. Fail. 2024, 46, 2336128. [Google Scholar] [CrossRef]
- Liu, J.; Hong, S.; Yang, J.; Zhang, X.; Wang, Y.; Wang, H.; Peng, J.; Hong, L. Targeting purine metabolism in ovarian cancer. J. Ovarian Res. 2022, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Takemura, H.; Choi, J.-H.; Fushimi, K.; Narikawa, R.; Wu, J.; Kondo, M.; Nelson, D.C.; Suzuki, T.; Ouchi, H.; Inai, M.; et al. Role of hypoxanthine-guanine phosphoribosyltransferase in the metabolism of fairy chemicals in rice. Org. Biomol. Chem. 2023, 21, 2556–2561. [Google Scholar] [CrossRef]
- Wang, H.; Mei, L.; Deng, Y.; Liu, Y.; Wei, X.; Liu, M.; Zhou, J.; Ma, H.; Zheng, P.; Yuan, J. Lactobacillus brevis DM9218 ameliorates fructose-induced hyperuricemia through inosine degradation and manipulation of intestinal dysbiosis. Nutrition 2019, 62, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, X.; Guo, Z.; Gao, R.; Ni, Z.; Cui, H.; Zong, M.; Van Bockstaele, F.; Lou, W. Lactiplantibacillus plantarum enables blood urate control in mice through degradation of nucleosides in gastrointestinal tract. Microbiome 2023, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Moon, J.S.; Kim, J.E.; Jang, Y.-J.; Choi, H.S.; Oh, I. Evaluation of purine-nucleoside degrading ability and in vivo uric acid lowering of Streptococcus thermophilus IDCC 2201, a novel antiuricemia strain. PLoS ONE 2024, 19, e0293378. [Google Scholar] [CrossRef]
- Ty, M.C.; Zuniga, M.; Götz, A.; Kayal, S.; Sahu, P.K.; Mohanty, A.; Mohanty, S.; Wassmer, S.C.; Rodriguez, A. Malaria inflammation by xanthine oxidase-produced reactive oxygen species. EMBO Mol. Med. 2019, 11, e9903. [Google Scholar] [CrossRef]
- Kim, S.-K. The mechanism of the NLRP3 inflammasome activation and pathogenic implication in the pathogenesis of gout. J. Rheum. Dis. 2022, 29, 140–153. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Guo, W.; Mao, B.; Tang, X.; Zhang, Q.; Zhao, J.; Zhang, H.; Chen, W.; Cui, S. Improvement of inflammatory bowel disease by lactic acid bacteria-derived metabolites: A review. Crit. Rev. Food Sci. Nutr. 2025, 65, 1261–1278. [Google Scholar] [CrossRef] [PubMed]
- Kropp, C.; Le Corf, K.; Relizani, K.; Tambosco, K.; Martinez, C.; Chain, F.; Rawadi, G.; Langella, P.; Claus, S.P.; Martin, R. The Keystone commensal bacterium Christensenella minuta DSM 22607 displays anti-inflammatory properties both in vitro and in vivo. Sci. Rep. 2021, 11, 11494. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Garrett, S.; Hong, W.; Zhang, J. Staphylococcus aureus Infections and Human Intestinal Microbiota. Pathogens 2024, 13, 276. [Google Scholar] [CrossRef]
- Baldelli, V.; Scaldaferri, F.; Putignani, L.; Del Chierico, F. The Role of Enterobacteriaceae in Gut Microbiota Dysbiosis in Inflammatory Bowel Diseases. Microorganisms 2021, 9, 697. [Google Scholar] [CrossRef]
- Pan, L.; Feng, R.; Hu, J.; Yu, H.; Tong, Q.; Yang, X.; Song, J.; Xu, H.; Ye, M.; Zhang, Z.; et al. Bacteroides fragilis-derived succinic acid promotes the degradation of uric acid by inhibiting hepatic AMPD2: Insight into how plant-based berberine ameliorates hyperuricemia. Acta Pharm. Sin. B 2025, 15, 5244–5260. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, J.; Cao, Q.; Zhu, S.; Chen, W.; Luo, H.; Zhao, Y.; Bernard, L.A.; Wang, X.; Tu, Q.; et al. Gut-kidney axis modulation by viable and inactivated Akkermansia muciniphila mitigates avian hyperuricemia through microbial-metabolic crosstalk. mSystems 2025, 10, e00773-25. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Duan, J.; Wang, D.; Liu, J.; Chen, X.; Qin, X.Y.; Yu, W. Akkermansia muciniphila Alleviates Persistent Inflammation, Immunosuppression, and Catabolism Syndrome in Mice. Metabolites 2023, 13, 194. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, X.; Li, Y.; Guo, W.; Li, M. Jiedu-Yizhi Formula Alleviates Neuroinflammation in AD Rats by Modulating the Gut Microbiota. Evid.-Based Complement. Altern. Med. 2022, 2022, 4023006. [Google Scholar] [CrossRef]
- Yuan, S.; Jia, W.; Liu, X.; Liu, R.; Cao, M.; Wu, Y.; Li, Y.; Xu, W.; Xiao, C.; Hong, Z.; et al. Therapeutic effect of fecal microbiota transplantation on hyperuricemia mice by improving gut microbiota. Front. Microbiol. 2025, 16, 1599107. [Google Scholar] [CrossRef]
| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| apt | TGGTTTTGCACCTGCTCGTA | TAGCACATTTTGTCCCGGCT |
| xpt | GGCGGTTCAAGGGATGTTTG | CTAAACGGACACCCCGATCA |
| hpt | CGTACTCAAGGGTGCCGTTT | ACATCACGACCCGTCACATC |
| 16s | AGAGTTTGATCCTGGCTCAG | CTACGGCTACCTTGTTACGA |
| iNOS | GCGAAAGGTCATGGCTTCAC | TCTTCCAAGGTGCTTGCCTT |
| IL-1β | GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGTCCGTCAACT |
| NLRP-3 | CCTGGGGGACTTTGGAATCAG | GATCCTGACAACACGCGGA |
| Caspase-1 | ATCTTTCTCCGAGGGTTGG | AAGTCTTGTGCTCTGGGCAG |
| XOD | ATGACGAGGACAACGGTAGAT | TCATACTTGGAGATCATCACGGT |
| ABCG2 | AGAATAGCATTAAGGCCAGGTTT | AGAATAGCATTAAGGCCAGGTTT |
| GLUT9 | TGGACTCAATGCGATCTGG | AGAGAAGATAGCAGCCAGTGTTT |
| URAT1 | CCGCTTCCGACAACCTCA | CTTCTGCGCCCAAACCTATC |
| β-actin | GCTCCTCCTGAGCGCAAGTA | CAGCTCAGTAACAGTCCGCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, M.; Hu, Y.; Zhang, Z.; Qiu, L.; Tao, X.; Wei, H. Lactiplantibacillus plantarum WLPL04 from Human Breast Milk Attenuates Hyperuricemia via Coordinated Purine Salvage Pathway, Renal Transporter Regulation, and Gut Microbiota Remodeling. Nutrients 2025, 17, 3447. https://doi.org/10.3390/nu17213447
Wei M, Hu Y, Zhang Z, Qiu L, Tao X, Wei H. Lactiplantibacillus plantarum WLPL04 from Human Breast Milk Attenuates Hyperuricemia via Coordinated Purine Salvage Pathway, Renal Transporter Regulation, and Gut Microbiota Remodeling. Nutrients. 2025; 17(21):3447. https://doi.org/10.3390/nu17213447
Chicago/Turabian StyleWei, Min, Yingsheng Hu, Zhihong Zhang, Liang Qiu, Xueying Tao, and Hua Wei. 2025. "Lactiplantibacillus plantarum WLPL04 from Human Breast Milk Attenuates Hyperuricemia via Coordinated Purine Salvage Pathway, Renal Transporter Regulation, and Gut Microbiota Remodeling" Nutrients 17, no. 21: 3447. https://doi.org/10.3390/nu17213447
APA StyleWei, M., Hu, Y., Zhang, Z., Qiu, L., Tao, X., & Wei, H. (2025). Lactiplantibacillus plantarum WLPL04 from Human Breast Milk Attenuates Hyperuricemia via Coordinated Purine Salvage Pathway, Renal Transporter Regulation, and Gut Microbiota Remodeling. Nutrients, 17(21), 3447. https://doi.org/10.3390/nu17213447






