The Effect of Maternal Folic Acid Supplementation on Neurodevelopmental Disorders in Offspring: An Umbrella Review of Systematic Reviews and Meta-Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Registration and Protocol
2.2. Eligibility Criteria for the Studies
2.2.1. Outcomes
2.2.2. Inclusion Criteria
2.2.3. Exclusion Criteria
2.3. Information Sources and Search Strategies
2.4. Studies Selection and Extraction of Data
2.5. Quality Assessment
2.6. Credibility of the Evidence
2.7. Statistical Analysis
3. Results
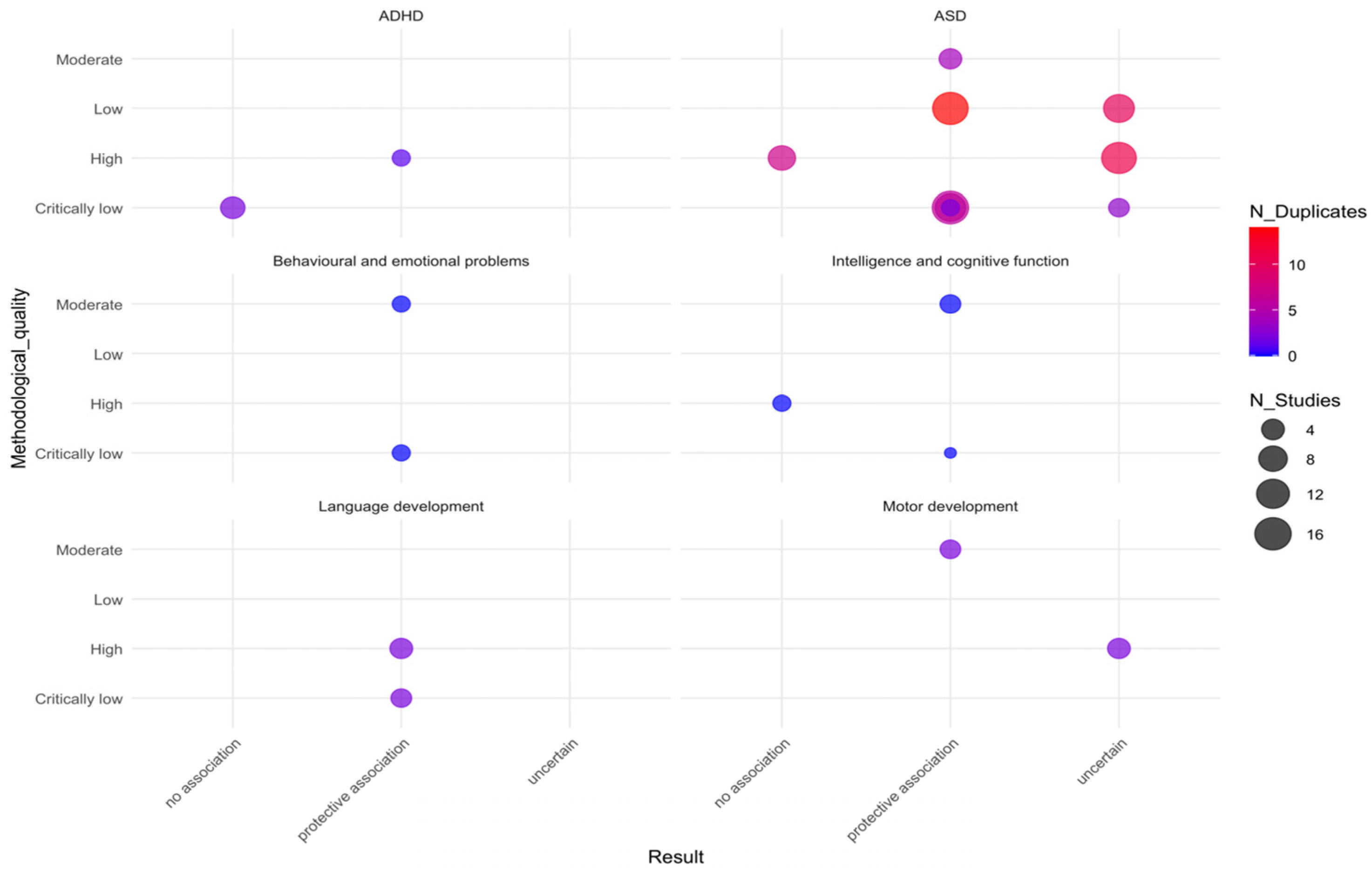
3.1. AMSTAR-2 Assessment of Included Systematic Reviews
3.2. GRADE Assessment of Interventions
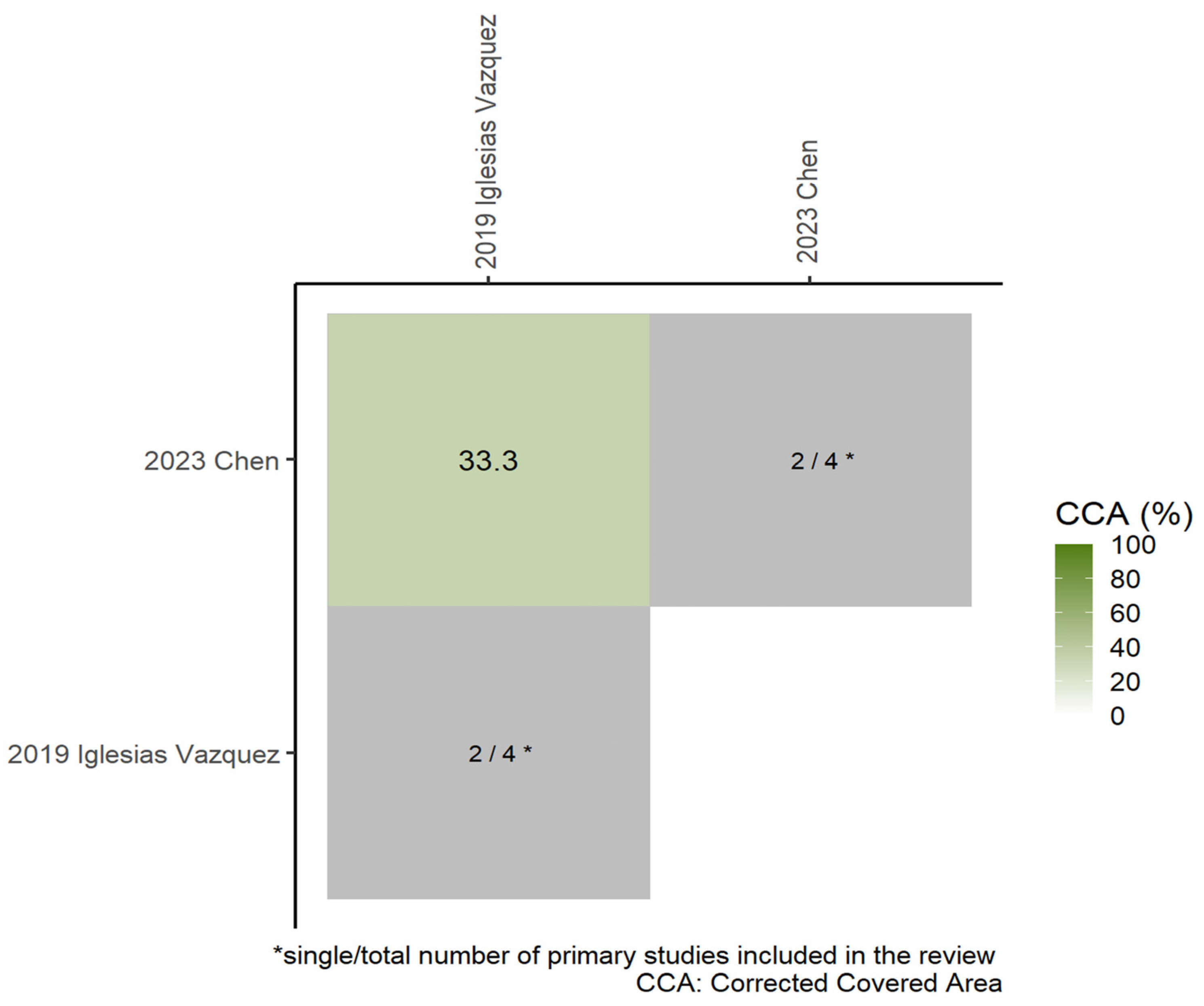
3.3. Meta-Analysis
| Author (year) | Exposure | Exposure Comparison | Assessment Criteria/Tool | Study Design (Number of Studies) | No. of Participants (Cases) | Databases Searched | Locations | Statistical Model/Effect Size Indicator (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|
| Dose | Window | ||||||||
| ASD | |||||||||
| Wang et al. [11] | NA | Before and/or during pregnancy | No folic acid supplementation | DSM-IV, CARS, ICD-10, ADI-R, ADOS, etc. | All: 12 Cohort studies: 4 RR = 0.903 (0.786–0.998) Case–control studies: 6 RR = 0.435 (0.264–0.717) | 631,905 (4514) | PubMed, Web of Knowledge, and Wanfang Database (as recent as March 2017) | Asian, European, American | Random effects model/RR = 0.771 (0.641–0.928) |
| Guo et al. [14] | NA | Periconception period, early pregnancy, mid-pregnancy, pre-pregnancy | No folic acid supplementation | DSM-IV, DSM-IV-TR, ICD-9/10, ADI-R, ADOS, ADOS-G | All: 8 Prospective cohort studies: 4 Registry-based cohort study: 1 Case–cohort study: 1 Case–control studies: 2 | 840,776 (7127) | PubMed, EMBASE, PsycINFO, Scopus, Web of Science, and Cochrane Library (up to 7 June 2018) | Denmark, Norway, Sweden, the United States, and Israel | Random effects model/OR = 0.91 (0.73–1.13) and HR = 0.66 (0.38–1.17) |
| Yu et al. [29] | NA | Before pregnancy and early pregnancy | No folic acid supplementation | Diagnostic reference standards with internationally recognized or expert consensus are available | All: 10 Cohort studies: 7 Case–control studies: 3 | 1,230,294 (4459) | PubMed, Embase, Scopus, Cochrane Library, EBSCO, CNKI, Wanfang Data, VIP, and CBD | China, Norway, Denmark, the United States of America, The Netherlands, Oman | Random effects model/OR = 0.798 (0.669–0.952) |
| Liu et al. [10] | No detail doses; detail doses: ≥400 µg/d, ≥500 µg/d, ≥800 µg/d | Before pregnancy (12 weeks before the start of pregnancy); early pregnancy (start of pregnancy-12 weeks after); during pregnancy (no specific pregnancy and folic acid exposure period occurring at the period from 270 days before childbirth up to the date of delivery); before pregnancy to early pregnancy | No folic acid supplementation | DSM-IV, ICD-8/9/10, ADOS, ADI-R, SCQ | All: 10 Cohort studies: 6 Case–control studies: 4 | NA (9795) | PubMed, EMBASE, and Cochrane Library (Until 31 January 2020) | European countries, the United States of America, Israel, and China | Random effects model/OR during the prenatal period = 0.570 (0.460–0.720) |
| Jia et al. [30] | NA | Pre-pregnancy and pregnancy, early pregnancy, 4 weeks before pregnancy to 8 weeks after pregnancy | No folic acid supplementation | DSM-V/IV, ICD-10, CARS | All: 17 Cohort studies: 8 Case–control studies: 9 | 887,053 (10,812) | PubMed, Embase, Web of Science, The Cochrane Library, Scopus, CNKI, WanFang Data, VIP, and CBM (31 December 2020) | China, Israel, Norway, Sweden, the Netherlands, Oman, the United States, Denmark | Random effects model/OR = 0.890 (0.770–1.030) |
| Vazque et al. [15] | NA | Periconception and early pregnancy | No folic acid supplementation | ADI-R, ADOS, SRS, DSM-V/IV, ICD-9/10 | About: 9 Cohort studies: 6 Case–control studies: 3 | 739,226 (6396) | PubMed, Scopus, and The Cochrane Library (until June 2018) | The United States, Norway, Denmark, Sweden, and Israel | Random effects model/OR = 0.580 (0.460–0.750) |
| Friel et al. [31] | NA | Periconception period, early pregnancy, and pregnancy | Low or no supplement intake | ICD-9/10, DSM, etc. | About: 10 Cohort studies: 4 Case–control studies: 3 Nested case–control studies: 2 Population-based cohort and sibling case–control: 1 | 904,947 (8159) | MEDLINE (OVID), EMBASE (OVID), PsycINFO (EBSCO), Web of Science core collection, Open gray, and BioRix (until 8 June 2020) | Sweden, the United States, Israel, China, Denmark, Norway | Random effects model/RR = 0.740 (0.530–1.040) |
| Li et al. [32] | NA | Periconception period, early pregnancy, and pregnancy | No folic acid supplementation and/or normal diet | Population-based patient registries, medical records, ASD diagnosis or screening, and service registries | About: 6 Cohort studies: 6 | 528,810 (NA) | PubMed and Embase (through March 2019) | The United States, Israel, Denmark, Sweden, and Norway | Random effects model/RR = 0.640 (0.460–0.900) |
| Chen et al. [12] | NA | Before pregnancy, early pregnancy, pregnancy | No use, the lowest dose, below the recommended dose, or the shortest period | CABS, DSM-IV, ICD-10/9, ADI-R, ADOS, ABC, etc. | About: 16 Cohort studies: 8 Case–control studies: 8 | 360,282 (NA) | MEDLINE, Web of Science, Cochrane Library, Scopus, EMBASE, and PsychInfo (until 24 September 2021) | China, Sweden, Israel, Norway, the United States, Denmark, Saudi Arabia | Case–control studies: random effects model/OR = 0.670 (0.430–1.050) Cohort studies: random effects model/OR = 0.690 (0.510–0.930) |
| ADHD | |||||||||
| Chen et al. [12] | NA | Before and/or during pregnancy | No use, the lowest dose, below the recommended dose, or the shortest period | SDQ Preschool Version, CPT-II Omission errors, DSM-IV, etc. | About: 6 Cohort studies: 6 | 35,402 (NA) | MEDLINE, Web of Science, Cochrane Library, Scopus, EMBASE, and PsychInfo (until 24 September 2021) | New Zealand, Spain, Japan, Denmark | Fixed effect model/OR = 0.860 (0.780–0.950) |
| Motor Development | |||||||||
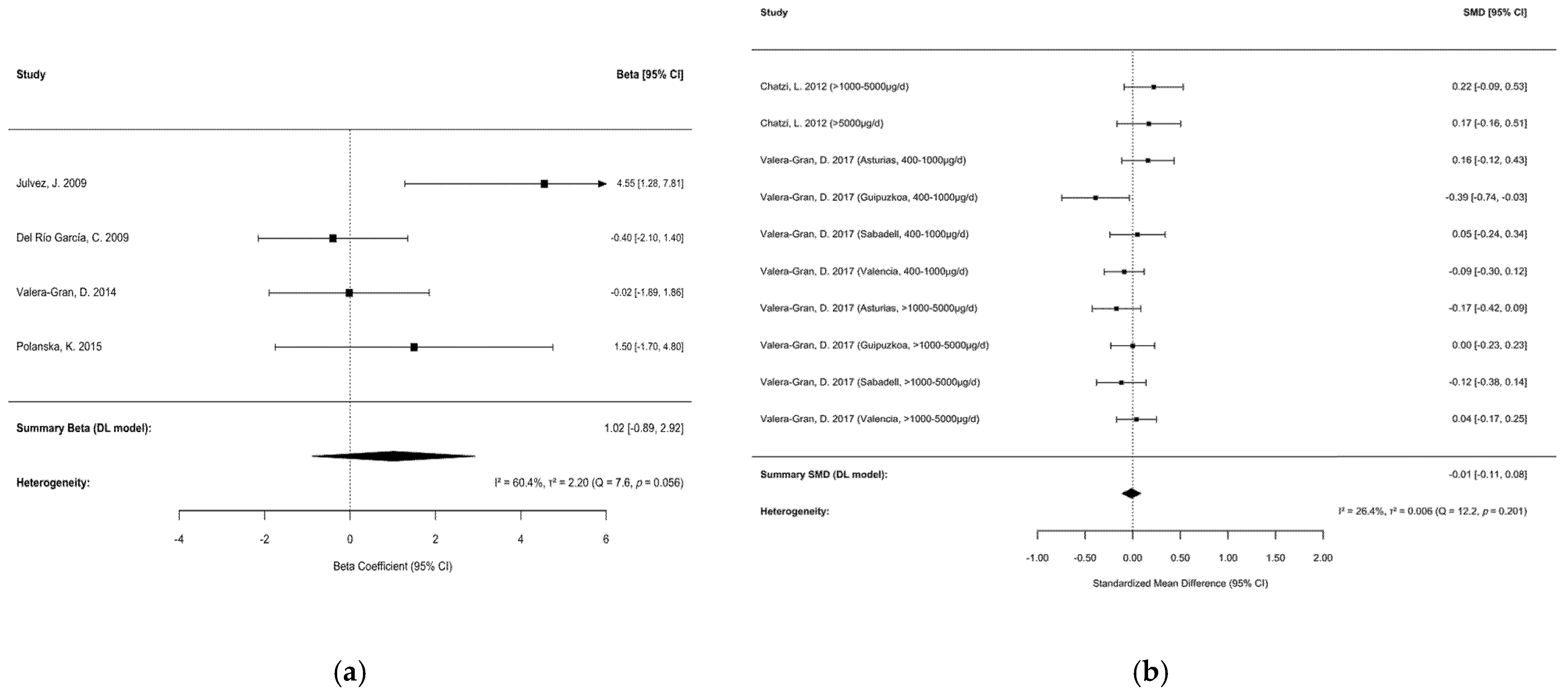
| Chen et al. [12] | NA | Before and/or during pregnancy | No use, the lowest dose, below the recommended dose, or the shortest period | BSID-II, MCSA, Bayley test, the psychomotor scale (Ps) of BSID-I | About: 4 Cohort studies: 8 | 3424 (NA) | MEDLINE, Web of Science, Cochrane Library, Scopus, EMBASE, and PsychInfo (until 24 September 2021) | Spain, Poland, Mexico | Random effects model/Beta = 1.02 (−0.890–2.920) |
| Vazque et al. [15] | 400–1000 μg/daily, 1000–5000 μg/daily, 5000 μg/daily | Periconception and early pregnancy | Less than 400 μg/d | Bayley, McCarthy | About: 4 Cohort studies: 4 | 8804 (NA) | PubMed, Scopus, and The Cochrane Library (until June 2018) | Greece, Spain, Poland | Random effects model/SMD = −0.020 (−0.080–0.040) |
| Intellectual or cognitive development | |||||||||
| Chen et al. [12] | NA | Before and/or during pregnancy | No use, the lowest dose, below the recommended dose, or shortest period | Bayley-III, MCSA, BSID, etc. | About: 5 Cohort studies: 5 | 4910 (NA) | MEDLINE, Web of Science, Cochrane Library, Scopus, EMBASE, and PsychInfo (until 24 September 2021) | Greece, Spain, Poland, China | Random effects model/Beta = 1.30 (−1.610–4.210) |
| Behavior development | |||||||||
| Chen et al. [12] | NA | Before and/or during pregnancy | No use | SDQ, CBCL, etc. | About: 3 Cohort studies: 3 | 36,275 (NA) | MEDLINE, Web of Science, Cochrane Library, Scopus, EMBASE and PsychInfo (until 24 September 2021) | New Zealand, The Netherlands, Denmark | Fixed effect model/OR = 0.750 (0.630–0.910) |
| Language development | |||||||||
| Chen et al. [12] | NA | Before and/or during pregnancy | No use, the lowest dose, below the recommended dose, or the shortest period | Bayley test, CDIs, MCSA, etc. | About: 4 Cohort studies: 4 | 8408 (NA) | MEDLINE, Web of Science, Cochrane Library, Scopus, EMBASE, and PsychInfo (until 24 September 2021) | New Zealand, Poland, Spain | Random effects model/Beta = 0.780 (−1.170–2.720) |
| Mental Development | |||||||||
| Vazque et al. [15] | 400–1000 μg/d, 1000–5000 μg/d, 5000 μg/d | Periconception, early pregnancy, and pregnancy | Less than 400 μg/d | Bayley, Popper–Szondi functional development test, Brunet–Lézine Scales, etc. | About: 7 RCT: 2 Cohort studies: 5 | 11,302 (NA) | PubMed, Scopus, and The Cochrane Library (until June 2018) | Hungary, Germany, Spain, Greece, Poland | Random effects model/SMD = −0.060 (−0.110–0.000) |
3.3.1. ASD
- 1.
- Folic acid supplementation dosage: preference was given to groups receiving doses closest to the recommended 400 μg/d for neural tube defect prevention [37];
- 2.
- Timing of exposure: data reflecting prenatal exposure were prioritized, as this period is most critical for neurodevelopment related to ASD [38];
- 3.
- Follow-up duration: outcomes with the longest follow-up (e.g., 5 years) were selected to enhance diagnostic stability.
Subgroup Analyses
Sensitivity Analysis
3.3.2. Motor Development
3.3.3. ADHD, Mental Development, Language, Behavior, Intellectual or Cognitive Development
3.4. Systematic Review
4. Discussion
4.1. Main Finding
4.2. Discussion on ASD
4.3. Discussion on ADHD
4.4. Discussion on Motor Development
4.5. Discussion on Intellectual or Cognitive Development
4.6. Discussion on Behavioral and Emotional Problems
4.7. Discussion on Language Development
4.8. Discussion on Mental Development and Neurodevelopmental Disorders
4.9. Strength
4.10. Limitations
4.11. Implications of Findings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NDDs | Neurodevelopmental disorders |
| SRs | Systematic reviews |
| MAs | Meta-analyses |
| ASD | Autism spectrum disorder |
| ADHD | Attention-deficit/hyperactivity disorder |
| DSM-5 | The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition |
| ICD-10/11 | The 10th/11th revisions of the International Classification of Diseases |
| IDD | Intellectual Developmental Disorder |
| TDs | Tic disorders |
| NTDs | Neural tube defects |
| PROSPERO | The International Prospective Register of Systematic Reviews |
| CL | Critically low |
| GRADE | Grading of Recommendations, Assessment, Development, and Evaluations |
| RCTs | Randomized controlled trials |
| DL | DerSimonian and Laird |
| CIs | Confidence intervals |
| CCA | Corrected covered area |
| AD | Autism disorder |
| OR | Odds ratio |
| CI | Confidence interval |
References
- World Health Organization. Mental Disorders. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-disorders (accessed on 8 June 2025).
- Harris, J.C. New classification for neurodevelopmental disorders in DSM-5. Curr. Opin. Psychiatry 2014, 27, 95–97. [Google Scholar] [CrossRef]
- Maw, K.J.; Beattie, G.; Burns, E.J. Cognitive strengths in neurodevelopmental disorders, conditions and differences: A critical review. Neuropsychologia 2024, 197, 108850. [Google Scholar] [CrossRef]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F., 3rd; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of Nutrition for Development-Folate Review. J. Nutr. 2015, 145, 1636s–1680s. [Google Scholar] [CrossRef]
- MRC Vitamin Study Research Group. Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet 1991, 338, 131–137. [Google Scholar] [CrossRef]
- Li, Z.; Berry, R.J.; Li, S. Preventing neural tube defects with periconceptional folic acid supplementation: A population-based intervention program in the China. Zhonghua Yi Xue Za Zhi 2000, 80, 493–498. [Google Scholar]
- Kancherla, V.; Black, R.E. Historical perspective on folic acid and challenges in estimating global prevalence of neural tube defects. Ann. N. Y. Acad. Sci. 2018, 1414, 20–30. [Google Scholar] [CrossRef]
- Greenblatt, J.M.; Huffman, L.C.; Reiss, A.L. Folic acid in neurodevelopment and child psychiatry. Prog. Neuropsychopharmacol. Biol. Psychiatry 1994, 18, 647–660. [Google Scholar] [CrossRef]
- Cordero, A.M.; Crider, K.S.; Rogers, L.M.; Cannon, M.J.; Berry, R.J. Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects: World Health Organization guidelines. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 421–423. [Google Scholar] [PubMed]
- Liu, X.; Zou, M.; Sun, C.; Wu, L.; Chen, W.-X. Prenatal Folic Acid Supplements and Offspring’s Autism Spectrum Disorder: A Meta-analysis and Meta-regression. J. Autism Dev. Disord. 2022, 52, 522–539. [Google Scholar] [CrossRef]
- Wang, M.; Li, K.; Zhao, D.; Li, L. The association between maternal use of folic acid supplements during pregnancy and risk of autism spectrum disorders in children: A meta-analysis. Mol. Autism 2017, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qin, L.; Gao, R.; Jin, X.; Cheng, K.; Zhang, S.; Hu, X.; Xu, W.; Wang, H. Neurodevelopmental effects of maternal folic acid supplementation: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 3771–3787. [Google Scholar] [CrossRef]
- Gao, Y.; Sheng, C.; Xie, R.-H.; Sun, W.; Asztalos, E.; Moddemann, D.; Zwaigenbaum, L.; Walker, M.; Wen, S.W. New Perspective on Impact of Folic Acid Supplementation during Pregnancy on Neurodevelopment/Autism in the Offspring Children—A Systematic Review. PLoS ONE 2016, 11, e0165626. [Google Scholar] [CrossRef]
- Guo, B.-Q.; Li, H.-B.; Zhai, D.-S.; Ding, S.-B. Association of maternal prenatal folic acid intake with subsequent risk of autism spectrum disorder in children: A systematic review and meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 94, 109650. [Google Scholar] [CrossRef]
- Iglesias Vazquez, L.; Canals, J.; Arija, V. Review and meta-analysis found that prenatal folic acid was associated with a 58% reduction in autism but had no effect on mental and motor development. Acta Paediatr. 2019, 108, 600–610. [Google Scholar] [CrossRef]
- Vasconcelos, C.; Perry, I.S.; Gottfried, C.; Riesgo, R.; Castro, K. Folic acid and autism: Updated evidences. Nutr. Neurosci. 2024, 28, 273–307. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Castro, K.; Klein, L.d.S.; Baronio, D.; Gottfried, C.; Riesgo, R.; Perry, I.S. Folic acid and autism: What do we know? Nutr. Neurosci. 2016, 19, 310–317. [Google Scholar] [CrossRef]
- Veena, S.R.; Gale, C.R.; Krishnaveni, G.V.; Kehoe, S.H.; Srinivasan, K.; Fall, C.H. Association between maternal nutritional status in pregnancy and offspring cognitive function during childhood and adolescence; a systematic review. BMC Pregnancy Childbirth 2016, 16, 220. [Google Scholar] [CrossRef] [PubMed]
- Scofield, J.E.; Buchanan, E.M.; Kostic, B. A meta-analysis of the survival-processing advantage in memory. Psychon. Bull. Rev. 2018, 25, 997–1012. [Google Scholar] [CrossRef]
- Luo, M.-L.; Tan, H.-Z.; Zhou, Q.; Wang, S.-Y.; Cai, C.; Guo, Y.-W.; Shen, L. Realizing the Meta-Analysis of Single Rate in R Software. J. Evid.-Based Med. 2013, 13, 181–184+188. (In Chinese) [Google Scholar]
- Bougioukas, K.I.; Diakonidis, T.; Mavromanoli, A.C.; Haidich, A.B. ccaR: A package for assessing primary study overlap across systematic reviews in overviews. Res. Synth. Methods 2023, 14, 443–454. [Google Scholar] [CrossRef]
- Pieper, D.; Antoine, S.L.; Mathes, T.; Neugebauer, E.A.; Eikermann, M. Systematic review finds overlapping reviews were not mentioned in every other overview. J. Clin. Epidemiol. 2014, 67, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Z.; Chen, B.; Li, J.; Yuan, X.; Li, J.; Wang, W.; Dai, T.; Chen, H.; Wang, Y.; et al. Dietary sugar consumption and health: Umbrella review. BMJ 2023, 381, e071609. [Google Scholar] [CrossRef]
- Yu, X.-F.; Li, M.; Zheng, Y. Association between maternal folate supplementation during pregnancy and the risk of autism spectrum disorder in the offspring: A Meta analysis. Zhongguo Dang Dai Er Ke Za Zhi Chin. J. Contemp. Pediatr. 2017, 19, 286–291. [Google Scholar]
- Jia, R.; Jin, S. Association between folic acid supplementation during pregnancy and the risk of autism spectrum disorder in the offspring: A meta-analysis. Chin. J. Evid.-Based Med. 2021, 21, 1141–1147. [Google Scholar] [CrossRef]
- Friel, C.; Leyland, A.H.; Anderson, J.J.; Havdahl, A.; Borge, T.; Shimonovich, M.; Dundas, R. Prenatal Vitamins and the Risk of Offspring Autism Spectrum Disorder: Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2558. [Google Scholar] [CrossRef]
- Li, M.; Francis, E.; Hinkle, S.N.; Ajjarapu, A.S.; Zhang, C. Preconception and Prenatal Nutrition and Neurodevelopmental Disorders: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 1628. [Google Scholar] [CrossRef]
- Li, W.; Li, Z.; Li, S.; Wang, X.; Wilson, J.X.; Huang, G. Periconceptional Folic Acid Supplementation Benefit to Development of Early Sensory-Motor Function through Increase DNA Methylation in Rat Offspring. Nutrients 2018, 10, 292. [Google Scholar] [CrossRef]
- Steenweg-de Graaff, J.; Ghassabian, A.; Jaddoe, V.W.; Tiemeier, H.; Roza, S.J. Folate concentrations during pregnancy and autistic traits in the offspring. The Generation R Study. Eur. J. Public Health 2015, 25, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Al-Farsi, Y.M.; Waly, M.I.; Deth, R.C.; Al-Sharbati, M.M.; Al-Shafaee, M.; Al-Farsi, O.; Al-Khaduri, M.M.; Gupta, I.; Ali, A.; Al-Khalili, M.; et al. Low folate and vitamin B12 nourishment is common in Omani children with newly diagnosed autism. Nutrition 2013, 29, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.J.; Kogan, V.; Shelton, J.F.; Delwiche, L.; Hansen, R.L.; Ozonoff, S.; Ma, C.C.; McCanlies, E.C.; Bennett, D.H.; Hertz-Picciotto, I.; et al. Combined Prenatal Pesticide Exposure and Folic Acid Intake in Relation to Autism Spectrum Disorder. Environ. Health Perspect. 2017, 125, 097007. [Google Scholar] [CrossRef] [PubMed]
- De-Regil, L.M.; Fernández-Gaxiola, A.C.; Dowswell, T.; Peña-Rosas, J.P. Effects and safety of periconceptional folate supplementation for preventing birth defects. Cochrane Database Syst. Rev. 2010, 12, Cd007950. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, C.; Kuang, M.; Lin, L.; Xu, G.; Pan, N.; Weng, X.; Jing, J.; Shi, L.; Yi, Q.; et al. Examining associations of folic acid supplements administered to mothers during pre-conceptional and prenatal periods with autism spectrum disorders in their offspring: Insights from a multi-center study in China. Front. Public Health 2024, 12, 1321046. [Google Scholar] [CrossRef]
- Surén, P.; Roth, C.; Bresnahan, M.; Haugen, M.; Hornig, M.; Hirtz, D.; Lie, K.K.; Lipkin, W.I.; Magnus, P.; Reichborn-Kjennerud, T.; et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. Jama 2013, 309, 570–577. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, L.-X.; Xu, W.-J.; Chen, H.; Zhong, J.-Q.; Zeng, C.-X. Research on the prevalence and the risk factors of autism spectrum disorder from 1.5 to 3 years old in Zhuhai city. Chin. J. Child Health Care 2014, 22, 649–651. (In Chinese) [Google Scholar] [CrossRef]
- Li, X.; Ling, Z.-Y.; Wang, J.-M.; Yang, S.-P.; Qin, Y.-Y.; Xie, S.-N.; Yang, S.-B.; Zhang, J. Periconceptional Risk Factors for Childhood Autism:A 1:1 Matched Case-control Study. Acta Med. Univ. Sci. Technol. Huazhong 2015, 44, 357–361. (In Chinese) [Google Scholar]
- Su, Y.-Y. Effect of Environmental Risk Factor on Children with Autistic disorder and Mental Retardation. Master’s Thesis, Tianjin Medical University, Tianjin, China, 2012. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, H.; Liu, L.; Sun, D.L.; Yin, X.N.; Chen, Z.D.; Wu, C.A.; Chen, W.Q. Interaction between passive smoking and folic acid supplement during pregnancy on autism spectrum disorder behaviors in children aged 3 years. Zhonghua Liu Xing Bing Xue Za Zhi 2016, 37, 940–944. [Google Scholar] [CrossRef]
- Nilsen, R.M.; Surén, P.; Gunnes, N.; Alsaker, E.R.; Bresnahan, M.; Hirtz, D.; Hornig, M.; Lie, K.K.; Lipkin, W.I.; Reichborn-Kjennerud, T.; et al. Analysis of self-selection bias in a population-based cohort study of autism spectrum disorders. Paediatr. Perinat. Epidemiol. 2013, 27, 553–563. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Tancredi, D.J.; Ozonoff, S.; Hansen, R.L.; Hartiala, J.; Allayee, H.; Schmidt, L.C.; Tassone, F.; Hertz-Picciotto, I. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am. J. Clin. Nutr. 2012, 96, 80–89. [Google Scholar] [CrossRef]
- Virk, J.; Liew, Z.; Olsen, J.; Nohr, E.A.; Catov, J.M.; Ritz, B. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism 2016, 20, 710–718. [Google Scholar] [CrossRef]
- Sun, Y.; Shao, T.; Yao, Y.; Tao, H.; Ni, L.; Yan, S.; Gu, C.; Cao, H.; Huang, K.; Tao, F. Pregnancy-related anxiety and subthreshold autism trait in preschool children based a birth cohort study. Zhonghua Yu Fang Yi Xue Za Zhi 2016, 50, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Desoto, M.; Robert, H. Synthetic folic acid supplementation during pregnancy may increase the risk of developing autism. J. Pediatr. Biochem. 2016, 02, 251–261. [Google Scholar] [CrossRef]
- DeVilbiss, E.A.; Magnusson, C.; Gardner, R.M.; Rai, D.; Newschaffer, C.J.; Lyall, K.; Dalman, C.; Lee, B.K. Antenatal nutritional supplementation and autism spectrum disorders in the Stockholm youth cohort: Population based cohort study. BMJ 2017, 359, j4273. [Google Scholar] [CrossRef]
- Levine, S.Z.; Kodesh, A.; Viktorin, A.; Smith, L.; Uher, R.; Reichenberg, A.; Sandin, S. Association of Maternal Use of Folic Acid and Multivitamin Supplements in the Periods Before and During Pregnancy With the Risk of Autism Spectrum Disorder in Offspring. JAMA Psychiatry 2018, 75, 176–184. [Google Scholar] [CrossRef]
- Strøm, M.; Granström, C.; Lyall, K.; Ascherio, A.; Olsen, S.F. Research Letter: Folic acid supplementation and intake of folate in pregnancy in relation to offspring risk of autism spectrum disorder. Psychol. Med. 2018, 48, 1048–1054. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Iosif, A.M.; Guerrero Angel, E.; Ozonoff, S. Association of Maternal Prenatal Vitamin Use With Risk for Autism Spectrum Disorder Recurrence in Young Siblings. JAMA Psychiatry 2019, 76, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Shen, Y.D.; Li, Y.J.; Xun, G.L.; Liu, H.; Wu, R.R.; Xia, K.; Zhao, J.P.; Ou, J.J. Maternal dietary patterns, supplements intake and autism spectrum disorders: A preliminary case-control study. Medicine 2018, 97, e13902. [Google Scholar] [CrossRef]
- Tan, M.; Yang, T.; Zhu, J.; Li, Q.; Lai, X.; Li, Y.; Tang, T.; Chen, J.; Li, T. Maternal folic acid and micronutrient supplementation is associated with vitamin levels and symptoms in children with autism spectrum disorders. Reprod. Toxicol. 2020, 91, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Sharman Moser, S.; Davidovitch, M.; Rotem, R.S.; Chodick, G.; Shalev, V.; Koren, G. High dose folic acid during pregnancy and the risk of autism; The birth order bias: A nested case-control study. Reprod. Toxicol. 2019, 89, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Froehlich, T.; Kalkbrenner, A.; Pfeiffer, C.M.; Fazili, Z.; Yolton, K.; Lanphear, B.P. Brief report: Are autistic-behaviors in children related to prenatal vitamin use and maternal whole blood folate concentrations? J. Autism Dev. Disord. 2014, 44, 2602–2607. [Google Scholar] [CrossRef]
- Goodrich, A.J.; Volk, H.E.; Tancredi, D.J.; McConnell, R.; Lurmann, F.W.; Hansen, R.L.; Schmidt, R.J. Joint effects of prenatal air pollutant exposure and maternal folic acid supplementation on risk of autism spectrum disorder. Autism Res. 2018, 11, 69–80. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Hansen, R.L.; Hartiala, J.; Allayee, H.; Schmidt, L.C.; Tancredi, D.J.; Tassone, F.; Hertz-Picciotto, I. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology 2011, 22, 476–485. [Google Scholar] [CrossRef]
- Al-maqati, T.N.; Al-Otaibi, N.M.; Al-Merbati, L.S.; Al-Dossary, D.M. Prenatal factors influencing the risk of autism spectrum disorder. Adv. Neurodev. Disord. 2021, 5, 71–76. [Google Scholar] [CrossRef]
- Chatzi, L.; Papadopoulou, E.; Koutra, K.; Roumeliotaki, T.; Georgiou, V.; Stratakis, N.; Lebentakou, V.; Karachaliou, M.; Vassilaki, M.; Kogevinas, M. Effect of high doses of folic acid supplementation in early pregnancy on child neurodevelopment at 18 months of age: The mother-child cohort ‘Rhea’ study in Crete, Greece. Public Health Nutr. 2012, 15, 1728–1736. [Google Scholar] [CrossRef]
- Valera-Gran, D.; Navarrete-Muñoz, E.M.; Garcia de la Hera, M.; Fernández-Somoano, A.; Tardón, A.; Ibarluzea, J.; Balluerka, N.; Murcia, M.; González-Safont, L.; Romaguera, D.; et al. Effect of maternal high dosages of folic acid supplements on neurocognitive development in children at 4–5 y of age: The prospective birth cohort Infancia y Medio Ambiente (INMA) study. Am. J. Clin. Nutr. 2017, 106, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Julvez, J.; Fortuny, J.; Mendez, M.; Torrent, M.; Ribas-Fitó, N.; Sunyer, J. Maternal use of folic acid supplements during pregnancy and four-year-old neurodevelopment in a population-based birth cohort. Paediatr. Perinat. Epidemiol. 2009, 23, 199–206. [Google Scholar] [CrossRef]
- del Río Garcia, C.; Torres-Sánchez, L.; Chen, J.; Schnaas, L.; Hernández, C.; Osorio, E.; Portillo, M.G.; López-Carrillo, L. Maternal MTHFR 677C>T genotype and dietary intake of folate and vitamin B(12): Their impact on child neurodevelopment. Nutr. Neurosci. 2009, 12, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Valera-Gran, D.; García de la Hera, M.; Navarrete-Muñoz, E.M.; Fernandez-Somoano, A.; Tardón, A.; Julvez, J.; Forns, J.; Lertxundi, N.; Ibarluzea, J.M.; Murcia, M.; et al. Folic acid supplements during pregnancy and child psychomotor development after the first year of life. JAMA Pediatr. 2014, 168, e142611. [Google Scholar] [CrossRef] [PubMed]
- Polańska, K.; Muszyński, P.; Sobala, W.; Dziewirska, E.; Merecz-Kot, D.; Hanke, W. Maternal lifestyle during pregnancy and child psychomotor development—Polish Mother and Child Cohort study. Early Hum. Dev. 2015, 91, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Campoy, C.; Escolano-Margarit, M.V.; Ramos, R.; Parrilla-Roure, M.; Csábi, G.; Beyer, J.; Ramirez-Tortosa, M.C.; Molloy, A.M.; Decsi, T.; Koletzko, B.V. Effects of prenatal fish-oil and 5-methyltetrahydrofolate supplementation on cognitive development of children at 6.5 y of age. Am. J. Clin. Nutr. 2011, 94, 1880s–1888s. [Google Scholar] [CrossRef]
- Czeizel, A.E.; Dobó, M. Postnatal somatic and mental development after periconceptional multivitamin supplementation. Arch. Dis. Child. 1994, 70, 229–233. [Google Scholar] [CrossRef]
- Dobó, M.; Czeizel, A.E. Long-term somatic and mental development of children after periconceptional multivitamin supplementation. Eur. J. Pediatr. 1998, 157, 719–723. [Google Scholar] [CrossRef]
- Sampaio, A.C.; Matos, F.F.d.N.; Lopes, L.d.L.; Marques, I.M.M.; Tavares, R.M.; Fernandes, M.V.d.M.; Teixeira, M.R.V.d.S.; Brito, A.B.d.; Feitosa, A.C.; Guedes, T.O.; et al. Association of the Maternal Folic Acid Supplementation with the Autism Spectrum Disorder: A Systematic Review. Rev. Bras. De Ginecol. E Obs./RBGO Gynecol. Obstet. 2021, 43, 775–781. [Google Scholar] [CrossRef]
- Hoxha, B.; Hoxha, M.; Domi, E.; Gervasoni, J.; Persichilli, S.; Malaj, V.; Zappacosta, B. Folic Acid and Autism: A Systematic Review of the Current State of Knowledge. Cells 2021, 10, 1976. [Google Scholar] [CrossRef]
- DeVilbiss, E.A.; Gardner, R.M.; Newschaffer, C.J.; Lee, B.K. Maternal folate status as a risk factor for autism spectrum disorders: A review of existing evidence. Br. J. Nutr. 2015, 114, 663–672. [Google Scholar] [CrossRef]
- Zhong, C.; Tessing, J.; Lee, B.K.; Lyall, K. Maternal Dietary Factors and the Risk of Autism Spectrum Disorders: A Systematic Review of Existing Evidence. Autism Res. Off. J. Int. Soc. Autism Res. 2020, 13, 1634–1658. [Google Scholar] [CrossRef]
- Viswanathan, M.; Urrutia, R.P.; Hudson, K.N.; Middleton, J.C.; Kahwati, L.C. Folic Acid Supplementation to Prevent Neural Tube Defects: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2023, 330, 460–466. [Google Scholar] [CrossRef]
- Cheng, J.; Eskenazi, B.; Widjaja, F.; Cordero, J.F.; Hendren, R.L. Improving autism perinatal risk factors: A systematic review. Med. Hypotheses 2019, 127, 26–33. [Google Scholar] [CrossRef]
- Chmielewska, A.; Dziechciarz, P.; Gieruszczak-Białek, D.; Horvath, A.; Pieścik-Lech, M.; Ruszczyński, M.; Skórka, A.; Szajewska, H. Effects of prenatal and/or postnatal supplementation with iron, PUFA or folic acid on neurodevelopment: Update. Br. J. Nutr. 2019, 122, S10–S15. [Google Scholar] [CrossRef]
- Jalali Chimeh, F.; Aghaie, E.; Ghavi, S.; Fatahnia, R. Investigation of the Effects of Maternal Nutrition during Pregnancy on Cognitive Functions of Toddlers: A Systematic Review. Int. J. Prev. Med. 2024, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Freedman, R.; Hunter, S.K.; Hoffman, M.C. Prenatal Primary Prevention of Mental Illness by Micronutrient Supplements in Pregnancy. Am. J. Psychiatry 2018, 175, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Chamova, R.; Toneva, A.; Brajkova, R.; Pancheva, R. Impact of Maternal Nutrition on Child Neurodevelopment: Insights from Recent Studies. Biomed. Rev. 2023, 34, 109–119. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Z.; Lin, Y.; Xie, H. Risk of Excess Maternal Folic Acid Supplementation in Offspring. Nutrients 2024, 16, 755. [Google Scholar] [CrossRef]
- Reynolds, E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006, 5, 949–960. [Google Scholar] [CrossRef]
- Virdi, S.; Jadavji, N.M. The Impact of Maternal Folates on Brain Development and Function after Birth. Metabolites 2022, 12, 876. [Google Scholar] [CrossRef]
- Bhate, V.K.; Joshi, S.M.; Ladkat, R.S.; Deshmukh, U.S.; Lubree, H.G.; Katre, P.A.; Bhat, D.S.; Rush, E.C.; Yajnik, C.S. Vitamin B12 and folate during pregnancy and offspring motor, mental and social development at 2 years of age. J. Dev. Orig. Health Dis. 2012, 3, 123–130. [Google Scholar] [CrossRef]
- McNulty, H.; Rollins, M.; Cassidy, T.; Caffrey, A.; Marshall, B.; Dornan, J.; McLaughlin, M.; McNulty, B.A.; Ward, M.; Strain, J.J.; et al. Effect of continued folic acid supplementation beyond the first trimester of pregnancy on cognitive performance in the child: A follow-up study from a randomized controlled trial (FASSTT Offspring Trial). BMC Med. 2019, 17, 196. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, A.; McNulty, H.; Rollins, M.; Prasad, G.; Gaur, P.; Talcott, J.B.; Witton, C.; Cassidy, T.; Marshall, B.; Dornan, J.; et al. Effects of maternal folic acid supplementation during the second and third trimesters of pregnancy on neurocognitive development in the child: An 11-year follow-up from a randomised controlled trial. BMC Med. 2021, 19, 73. [Google Scholar] [CrossRef] [PubMed]
- Skórka, A.; Gieruszczak-Bialek, D.; Piescik, M.; Szajewska, H. Effects of prenatal and/or postnatal (maternal and/or child) folic acid supplementation on the mental performance of children. Crit. Rev. Food Sci. Nutr. 2012, 52, 959–964. [Google Scholar] [CrossRef] [PubMed]



| Author (year) | Exposure | Exposure Comparison | Assessment Criteria/Tool | Study Design (Number of Studies) | No. of Participants (Cases) | Databases Searched | Locations | Main Findings | |
|---|---|---|---|---|---|---|---|---|---|
| Dose | Window | ||||||||
| ASD | |||||||||
| Sampaio et al. [69] | NA | Preconception period and at the beginning of pregnancy | Not applicable or insufficient | NA | All: 17 | NA | BIREME virtual bank, Virtual Health Library, the Medical Literature Analysis and Retrieval System Online (between February 2018 and February 2020) | The Netherlands, the United States, Denmark, China, Colombia, Norway, Palestine | Maternal FA supplementation in the pre-conception period and beginning of pregnancy as a protective effect in relation to ASD. |
| Hoxha et al. [70] | NA | Before and/or during pregnancy | No folic acid supplementation | PDD-NOS, MABC, etc. | All: 10 Cohort studies: 9 Case–control cohort study: 1 | 1,083,144 (NA) | PubMed, Scopus, Medline, and Embase (until 31 May 2021) | Denmark, Israel, Norway, Sweden, the United States, the Netherlands, Nepal | In some of them, the maternal FA supplementation results in a reduced ASD risk; other studies do not confirm these positive results, finding an enhanced risk following the supplementation; furthermore, some authors did not obtain any association between folate intake and ASD risk, or not satisfactory conclusions about the utility of folate supplementation. |
| Gao et al. [13] | NA | Before and/or during pregnancy | No folic acid supplementation | ADI-R, ADOS, etc. | About: 4 Cohort studies: 2 Case–control studies: 2 | 90,613 (NA) | Medline, EMBASE (until 31 December 2014) | Norway, the United States, and Spain | Folic acid supplementation in pregnancy may protect against impaired neurodevelopment, including ASDs in children. |
| Castro et al. [22] | NA | Perinatal period and/or pregnancy | No folic acid supplementation | ADI-R, ADOS | About: 2 Cohort study: 1 Case–control study: 1 | 86,013 (829) | MEDLIN (from January 2003 to July 2013) | Norway, the United States | Periconceptional folic acid may reduce ASD risk. |
| DeVilbiss et al. [71] | NA | pre- and peri-conceptional periods | No folic acid supplementation | SRS, ADI-R, etc. | All: 10 Cohort studies: 9 Case–control study: 1 | 138,100 (NA) | PubMed (until 15 April 2015) | Norway, the United States, The Netherlands, Spain, India, Nepal, and Hungary | Observational studies: Maternal FA intake during the periconceptional period and early pregnancy has a protective effect on the risk of ASD in children. RCT: Maternal folic acid supplementation may have a positive effect on autism in offspring, or it may be unrelated. |
| Zhong et al. [72] | NA | Perinatal period and/or pregnancy | With lower/lowest levels as the referent group | DSM-IV, ADOS, MSEL, ADI-R, SCQ, SRS | About: 15 Cohort studies: 9 Case–control studies: 4 nested case–control studies: 2 | 661,282 (NA) | PubMed (until July 2020) | Norway, the United States, Israel, the Netherlands, Denmark, Sweden, China | Higher or moderate intake of prenatal FA was associated with reductions in odds of ASD, though results have not been uniform, and there is a need to clarify differences in findings based on biomarkers versus reported intake. |
| Viswanathan et al. [73] | NA | Perinatal period and/or pregnancy | No folic acid supplementation | ICD-10, DSM-IV, etc. | About: 7 Cohort studies: 6 Case–control study: 1 | 761,125 (NA) | PubMed, Cochrane Library, Embase, and trial registries (from 1 July 2015, through 2 July 2021, with surveillance through 10 February 2023) | Israel, Denmark, Sweden, Norway | There is no association between folic acid supplementation before or during pregnancy and the risk of autism in offspring. |
| Vasconcelos et al. [16] | NA | Perinatal period and/or pregnancy | No folic acid supplementation | DSM-5, ICD-9/10 | About: 14 Case–control studies: 13 Cross-sectional studies: 4 Both: 3 | 69,484 (5085) | PubMed (MEDLINE), EBSCO, and CINAHL databases (from 2013 to 2024) | The United States, Sweden, Israel, China | An association between the risk of ASD and maternal folic acid intake was not established by most studies included in this review. Further investigation is needed to define a causal relationship between maternal folic acid intake and the risk of autism. |
| Cheng et al. [74] | NA | During the perinatal period or pregnancy | No folic acid supplementation | ICD, ADI-R, ADOS | About: 3 Prospective Cohort study: 1 Case–control studies: 2 | 2700 (NA) | MEDLINE (between 1 January 2005 and 1 July 2018) | The United States | There may be an optimal level of folate during pregnancy for reducing the offspring ASD risk. |
| Chmielewska et al. [75] | NA | Periconceptional period | No folic acid supplementation | ADI-R, ADOS, expert diagnosis | About: 2 Cohort study: 1 case–control study: 1 | 86,013 (NA) | Cochrane Library (2009–May 2014) | The United States, Norway | Maternal folic acid intake during pregnancy is associated with a lower risk of ASD in offspring. |
| ADHD | |||||||||
| Li et al. [32] | NA | Periconception period, early pregnancy | No folic acid supplementation and/or normal diet | ICD-10, SDQ, patient registries, ADHD Rating Scale-IV | All: 5 Cohort studies: 5 | 43,063 (NA) | PubMed and Embase (through March 2019) | New Zealand, Japan, Denmark, Spain, United Kingdom | No convincing evidence supports an association between folate intake from food or supplements and ADHD risk. |
| Gao et al. [13] | NA | Periconception period, early pregnancy | No folic acid supplementation | NA | About: 1 Cohort study: 1 | 393 (NA) | Medline, EMBASE (until 31 December 2014) | Norway, the United States, Spain | Omission errors were lower in those children whose mothers took dietary supplementation with folic acid during pregnancy. |
| Sargoor et al. [23] | NA | Periconception period, early pregnancy | No folic acid supplementation | By two psychologists and teachers, CPT-II | About: 2 Cohort studies: 2 | 813 (NA) | Medline, PubMed, and the Cochrane Library (from January 1960 to October2014) | Spain, the United Kingdom | Folic acid supplementation during pregnancy may have beneficial effects on symptoms of inattention and hyperactivity/impulsivity. |
| Chmielewska et al. [75] | NA | Periconception period, early pregnancy | No folic acid supplementation | SDQ | About: 1 prospective cohort study: 1 | 100 (NA) | MEDLINE, the Cochrane Library (2009–May 2014) | The United Kingdom | Higher total folate intake from food and supplements during early pregnancy is associated with higher rates of attention deficit hyperactivity disorder in children. |
| Motor Development | |||||||||
| Gao et al. [13] | NA | Before and/or during pregnancy | No folic acid supplementation | MABC, BSID, etc. | About: 4 RCT: 1 Cohort studies: 3 | 10,083 (NA) | Medline, EMBASE (until 31 December 2014) | Nepal, Spain | Folic acid supplementation in pregnancy may improve motor function. |
| Sargoor et al. [23] | NA | Before and/or during pregnancy | No folic acid supplementation | DDST, Questionnaire, etc. | About: 4 Prospective observational study: 1 Population-based longitudinal study: 1 Cohort studies: 2 | 46,244 (NA) | Medline/PubMed and the Cochrane Library (from January 1960 to October 2014) | The United Kingdom, Spain, Norway, and the United States | Half of the studies showed no association between folic acid supplementation and motor development, while half demonstrated a protective effect. |
| Chmielewska et al. [75] | NA | Periconceptional period | No folic acid supplementation | Denver development scale | About: 1 Population-based longitudinal study: 1 | 6774 (NA) | MEDLINE, the Cochrane Library (2009–May 2014) | The United States | Folic acid use was associated with improved gross motor development. |
| Intellectual or cognitive development | |||||||||
| Gao et al. [13] | NA | Before and/or during pregnancy | No folic acid supplementation | WISC-III, K-ABC, etc. | All: 3Cohort studies: 3 | 1900 (NA) | Medline, EMBASE (until 31 December 2014) | Nepal, India | Folic acid supplementation in pregnancy may improve intellectual and cognitive function. |
| Chimeh et al. [76] | NA | Perinatal period and/or pregnancy | No folic acid supplementation | BSID-III, Cognitive, linguistic, motor, socio-emotional, and adaptive behavioral subscales, MDI | About: 2 Prospective cohort study: 1 Two-way randomized trial:1 | 908 (NA) | Scopus, SID, Google Scholar, PubMed, and Science Direct (until March 2022) | Greece, China | Taking folic acid supplements can improve cognitive function in young children. |
| Sargoor et al. [23] | NA | Early pregnancy and mid-pregnancy | No folic acid supplementation | WRAML2, PPVT-III | About: 2 Prospective observational study: 1 Cohort study: 1 | 2105 (NA) | Medline/PubMed and the Cochrane Library (from January 1960 to October2014) | The United States | No association of folate intake with cognitive function. |
| Behavioral and emotional problems | |||||||||
| Gao et al. [13] | NA | Before and/or during pregnancy | No folic acid supplementation | CBCL 11/2–5 | About: 2 Cohort studies: 2 | 7504 (NA) | Medline, EMBASE (until 31 December 2014) | Netherlands | Folic acid supplementation in pregnancy may improve behavioral and emotional problems. |
| Freedman et al. [77] | NA | Perinatal period and/or pregnancy | No folic acid supplementation | ANT, CBCL | About: 3 Prospective, randomized study: 1 Prospective observational studies: 2 | 3521 (NA) | EDLINE (from 1990 through 2017) | Europe, Netherlands | Decreased emotional problems (child). |
| Chmielewska et al. [75] | NA | Early pregnancy | No folic acid supplementation | Child Behavior Checklist | About: 1 Cohort study: 1 | 4214 (NA) | Cochrane Library (2009–May 2014) | The Netherlands | Children of mothers who did not use folic acid supplements during early pregnancy had a higher risk of developing problems. |
| Language development | |||||||||
| Gao et al. [13] | NA | Before and/or during pregnancy | No folic acid supplementation | Language grammar rating scale | About: 1 Cohort study: 1 | 38,954 (NA) | Medline, EMBASE (until 31 December 2014) | Norway | Folic acid supplementation in pregnancy may improve language function. |
| Sargoor et al. [23] | NA | Before and/or during pregnancy | No folic acid supplementation | PPVT-III, DDST, Questionnaire | About: 4 Prospective observational studies: 2 Cohort studies: 2 | 40,680 (NA) | Medline/PubMed and the Cochrane Library (from January 1960 to October 2014) | The United States, Norway, the United Kingdom, Spain | Most opinions support the potential benefits of folic acid for language function. |
| Chmielewska et al. [75] | NA | Before and/or during pregnancy | No folic acid supplementation | PPVT-III, Bayley | About: 3 prospective observational study: 1 cohort studies: 2 | 40,717 (NA) | MEDLINE, the Cochrane Library (2009–May 2014) | The United States, Norway, Greece | Early pregnancy use of folic acid supplements is associated with a reduced risk of language development delays in offspring. |
| Mental development | |||||||||
| Gao et al. [13] | NA | Before and/or during pregnancy | No folic acid supplementation | Bayley test | About: 2 RCT: 1 Cohort study: 1 | 878 (NA) | Medline, EMBASE (until 31 December 2014) | Hungary, Mexico | Folic acid supplementation in pregnancy may improve mental development in children. |
| Sargoor et al. [23] | Deficient daily folate intake (≥400 μg/d) | First trimester | Deficient daily folate intake (<400 μg/d) | Bayley test | About: 1 (Prospective birth cohort) | 253 (NA) | Medline/PubMed and the Cochrane Library (from January 1960 to October 2014) | Mexico | Dietary intake of folate (<400 mg/day) reduced the mental development index only among children of mothers who were carriers of the TT genotype. |
| Neurodevelopment | |||||||||
| Chamova et al. [78] | 400 μg/d | Users who used it for 1–3 months, 3–6 months, and >6 months during pregnancy | Did not use folic acid supplements during pregnancy | Standard neuropsychological examination table | About: 1 Birth Cohort study: 1 | 1186 (NA) | PubMed, Scopus, Mendeley (2018–2023) | China | Maternal FA supplementation during pregnancy favors neurodevelopment in the offspring at 1 month old. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Hu, Y.; Hou, L.; Wu, X.; Chen, X.; Yan, R.; Dong, J.; Wu, J. The Effect of Maternal Folic Acid Supplementation on Neurodevelopmental Disorders in Offspring: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients 2025, 17, 3443. https://doi.org/10.3390/nu17213443
Yu M, Hu Y, Hou L, Wu X, Chen X, Yan R, Dong J, Wu J. The Effect of Maternal Folic Acid Supplementation on Neurodevelopmental Disorders in Offspring: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients. 2025; 17(21):3443. https://doi.org/10.3390/nu17213443
Chicago/Turabian StyleYu, Miao, Yiming Hu, Lei Hou, Xiaomin Wu, Xiangxin Chen, Ruohan Yan, Jie Dong, and Jing Wu. 2025. "The Effect of Maternal Folic Acid Supplementation on Neurodevelopmental Disorders in Offspring: An Umbrella Review of Systematic Reviews and Meta-Analyses" Nutrients 17, no. 21: 3443. https://doi.org/10.3390/nu17213443
APA StyleYu, M., Hu, Y., Hou, L., Wu, X., Chen, X., Yan, R., Dong, J., & Wu, J. (2025). The Effect of Maternal Folic Acid Supplementation on Neurodevelopmental Disorders in Offspring: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients, 17(21), 3443. https://doi.org/10.3390/nu17213443





