Unlocking Mangiferin: A Therapeutic Candidate Revolutionizing Liver Disease Therapy
Abstract
1. Introduction
2. Method
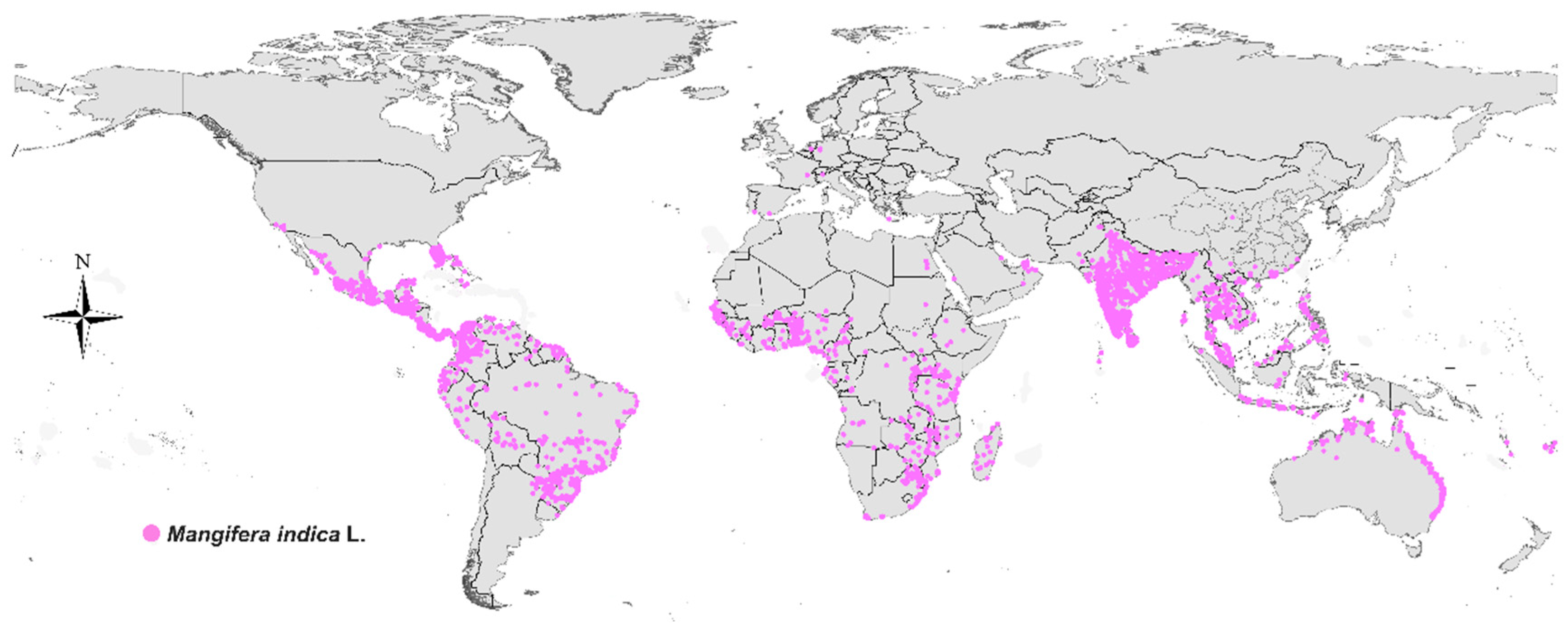
3. Source and Phytochemical Properties of MF
| No. | Scientific Name | Common Name | Source Parts in Plants |
|---|---|---|---|
| 1 | Mangifera indica | Mango | Fruits, leaves, bark |
| 2 | Mangifera persiciformis | Peach-shaped Mango | Fruits, leaves, bark |
| 3 | Mangifera sylvatica | Forest Mango | Fruits, leaves, bark |
| 4 | Hiptage benghalensis | Hiptage | Leaves; Flowers |
| 5 | Mammea americana | Mammee Apple | Branch; Leaves |
| 6 | Carica papaya | Papaya | Leaves |
| 7 | Citrus limon | Lemon | Fruit peel |
| 8 | Terminalia chebula | Chebulae Fructus | Fruit pulp |
| 9 | Cydonia oblonga | quince | Fruits; Seeds |
| 10 | Prunus amygdalus | European plum | Fruits |
| 11 | Garcinia mangostana | Mangosteen | Fruit peel |
| 12 | Coffea arabica | Arabian coffee | Leaves |
| 13 | Phaleria capitata | - | Seeds |
| 14 | Phaleria macrocarpa | - | Seeds |
| 15 | Aspalathus linearis | Rooibos | Leaves |
| 16 | Gypsophila pacifica | - | Roots; Leaves |
| 17 | Curio radicans | - | Roots; Stem |
| 18 | Penthorum chinense | all-grass of Chinese Penthorum | Leaves; Fruits |
| 19 | Swertia macrosperma | - | Whole herb |
| 20 | Swertia mussotii | Herba Swertiae Mussotii | Whole herb |
| 21 | Swertia punicea | - | Whole herb |
| 22 | Swertia kingii | - | Whole herb |
| 23 | Swertia franchetiana | - | Whole herb |
| 24 | Gentianella turkestanorum | - | Leaves |
| 25 | Gentiana algida | Alpine Gentian | Leaves; Flowers; Whole herb |
| 26 | Gentiana rhodantha | - | Leaves; Flowers; Whole herb |
| 27 | Hedysarum alpinum | - | Rhizome; Leaves |
| 28 | Hedysarum flavescens | - | Rhizome; Leaves |
| 29 | Astragalus membranaceus | Astragali Radix | Rhizome; Leaves |
| 30 | Anemarrhena asphodeloides | Anemarrhenae Rhizoma | Rhizome |
| 31 | Aquilaria sinensis | Chinese Eaglewood | Resin-containing heartwood |
| 32 | Aquilaria crassna | Eaglewood | Resin-containing heartwood |
| 33 | Sesamum indicum | sesame | Seeds |
| 34 | Dobinea delavayi | - | Rhizome |
| 35 | Reynoutria japonica | Giant Knotweed Rhizome | Rhizome; Leaves |
| 36 | Polygala tenuifolia | Thinleaf Milkwort Root-bark | Roots; Stem; Leaves |
4. Extraction and Separation Methods of MF
5. Synthesis of MF
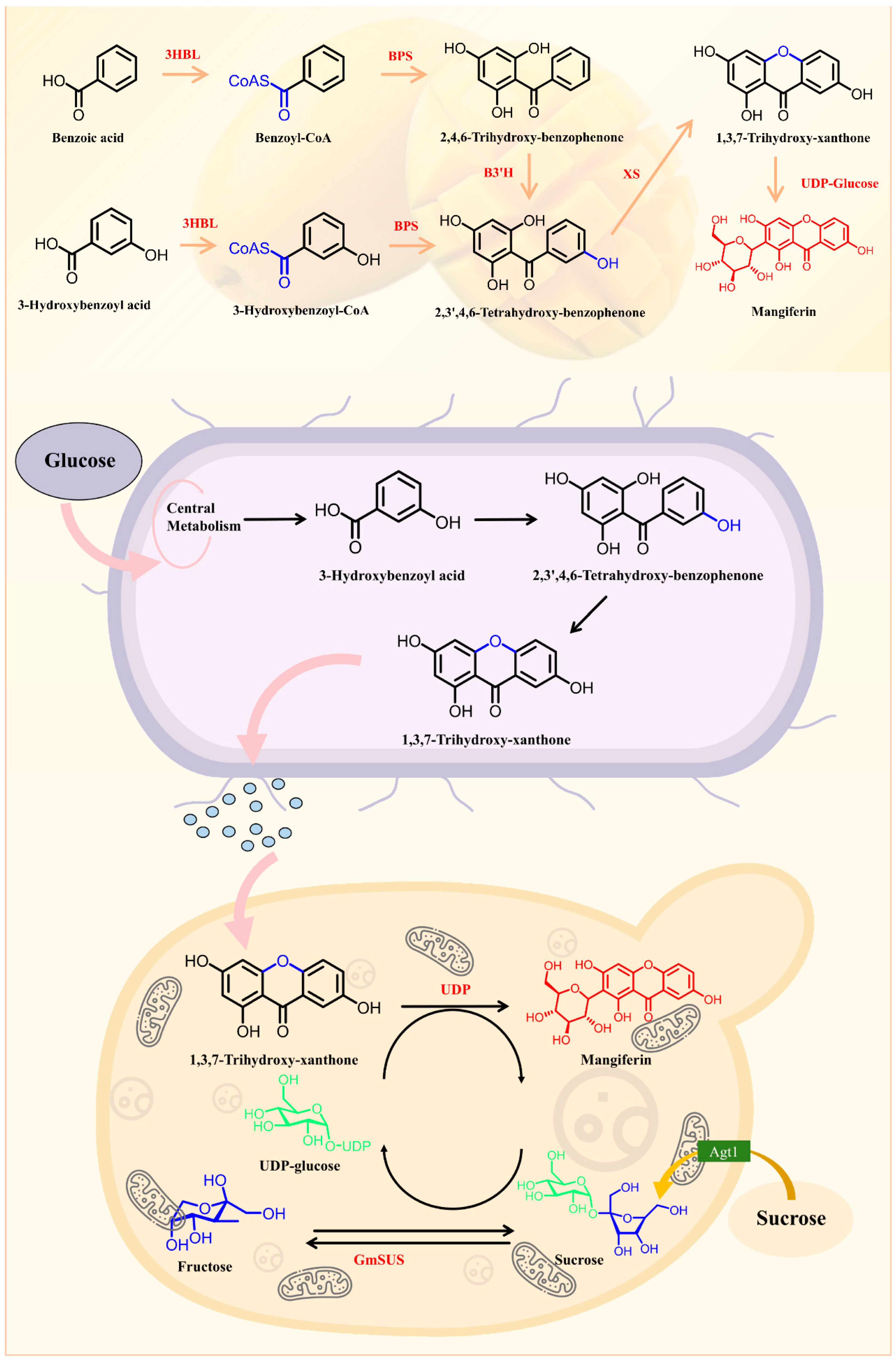
5.1. Biosynthesis
5.2. Chemosynthesis
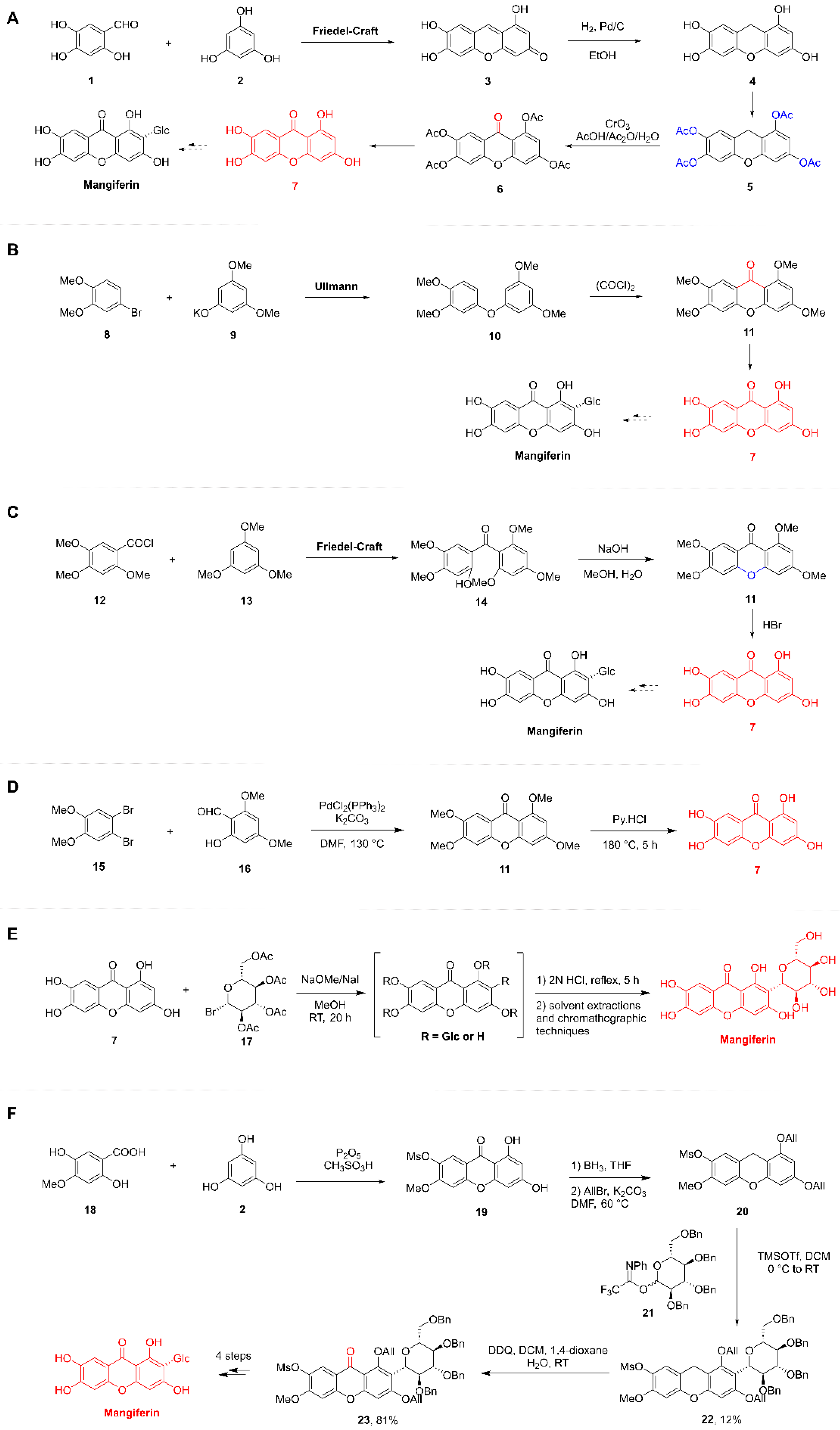
5.2.1. Total Synthesis of MF
Synthesis of the Xanthone Moiety
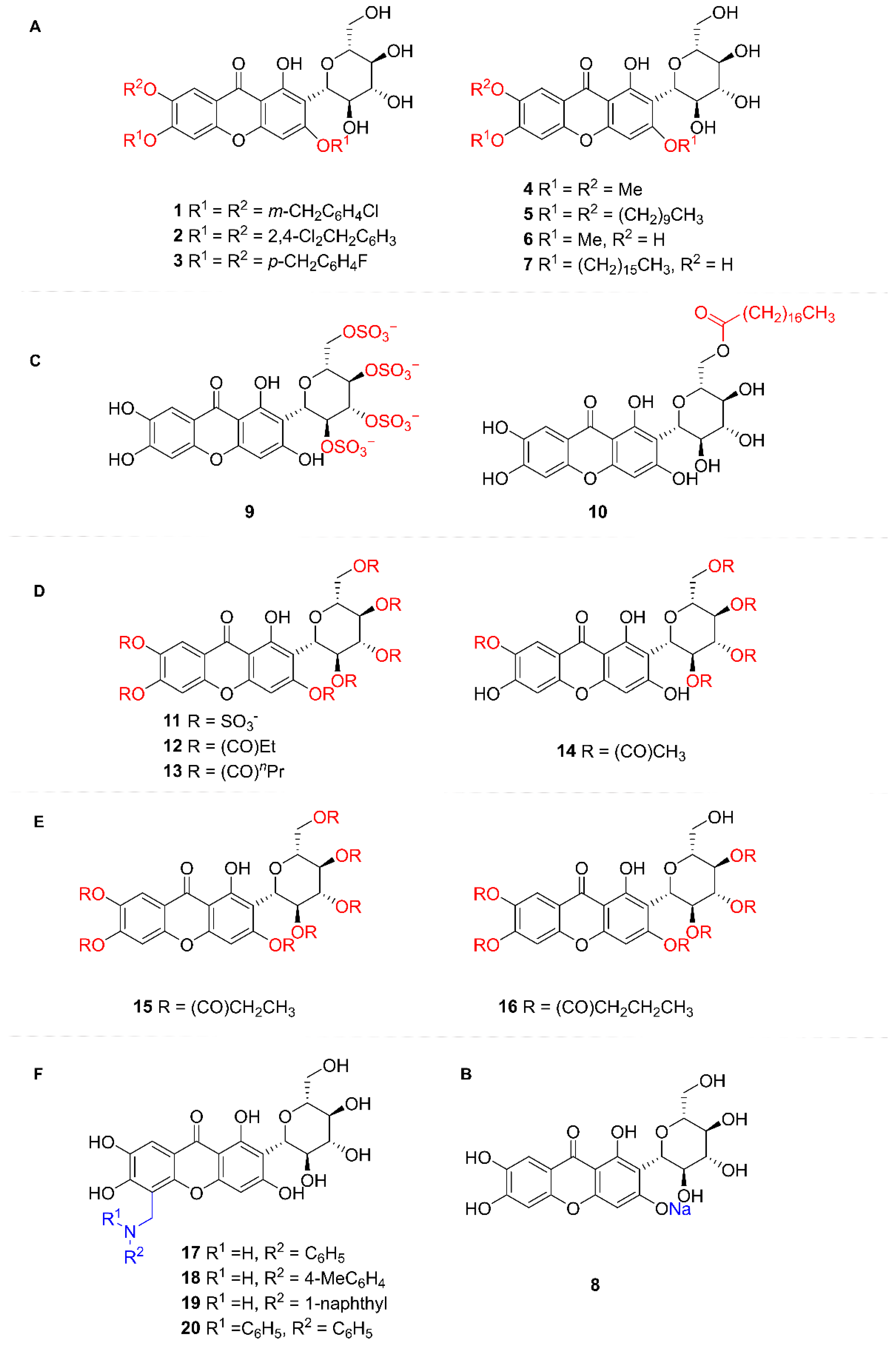
5.2.2. Structural Modification of MF
- (1)
- Hydroxy groups on the xanthone nucleus (Benzyl, alkyl, and other groups)
- (2)
- Sodium phenolate salts on the xanthone nucleus
- (3)
- Hydroxy groups of glucose (Sulfonic group, acyl group)
- (4)
- Hydroxy groups of MF (sulfonic acid and acyl group)
- (5)
- Oxygenated xanthone core’s C-8 position
6. Pharmacokinetics of MF
7. Safety of MF
8. Mechanism of MF in the Treatment of Liver Disease
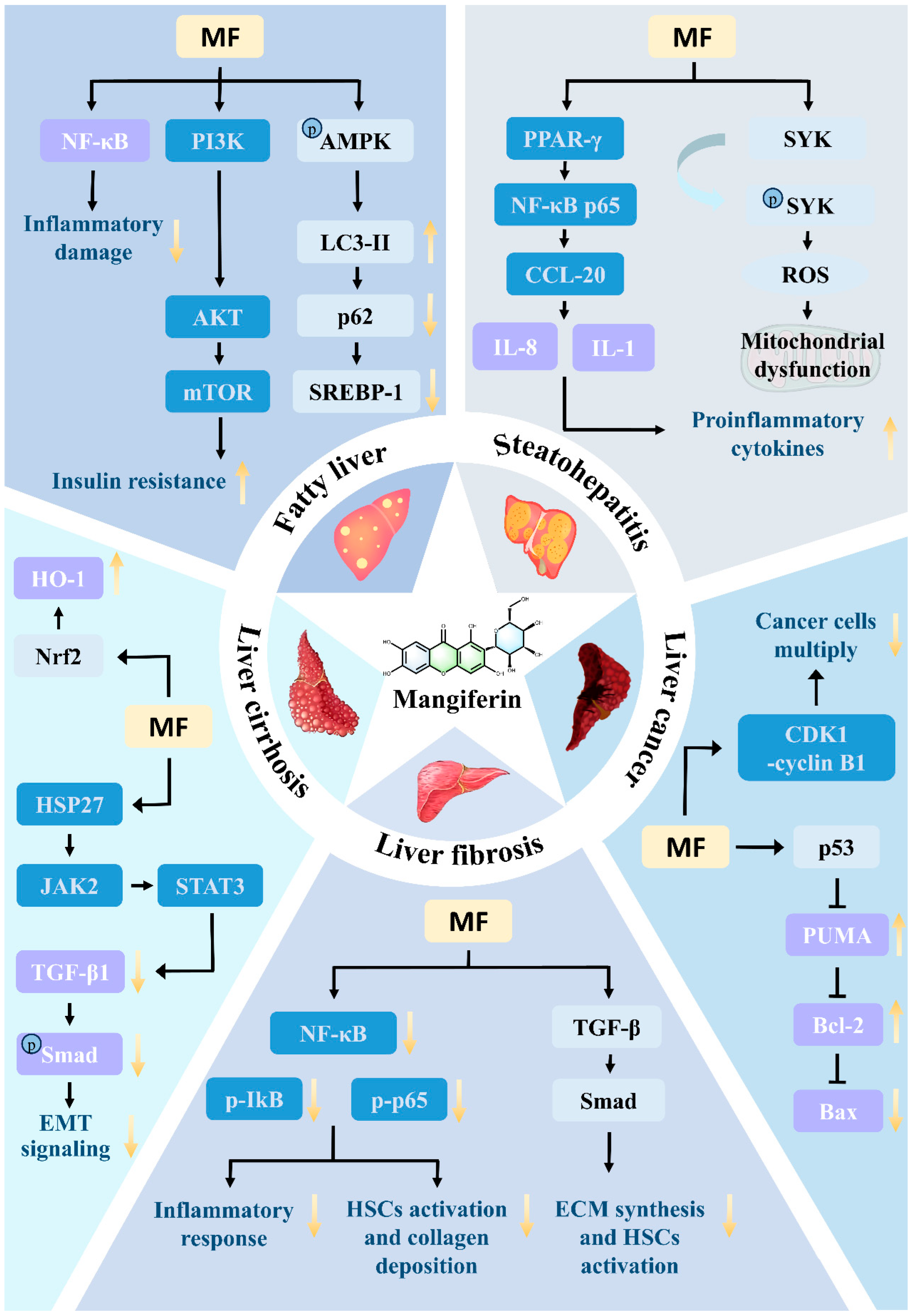
8.1. Acute Liver Injury
8.2. Fatty Liver
8.3. Steatohepatitis
8.4. Liver Fibrosis
8.5. Liver Cirrhosis
8.6. Liver Cancer
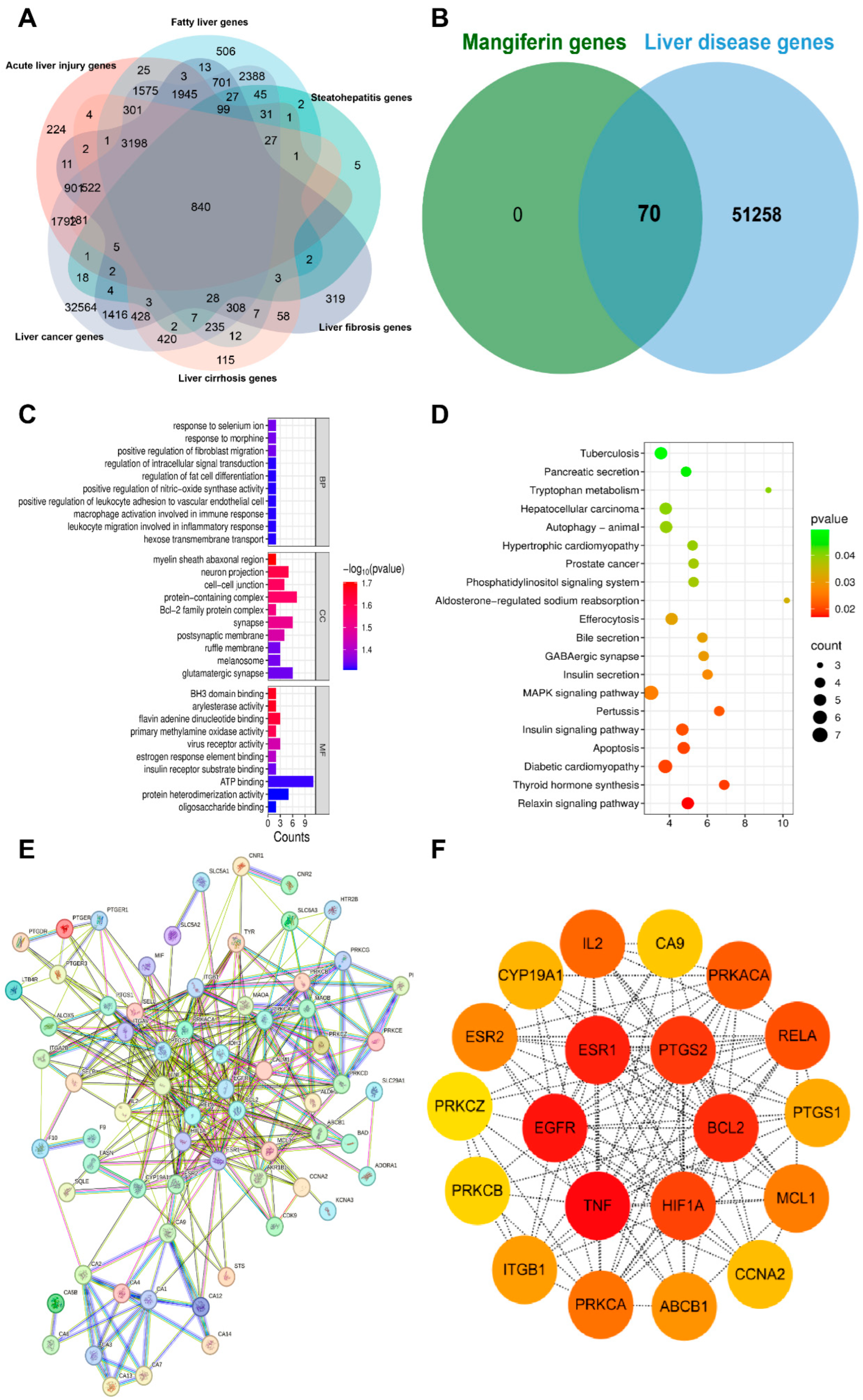
9. Network Pharmacology Analysis of MF
9.1. Collection Targets of Liver Disease Genes
9.2. GO and KEGG Pathway Enrichment Analysis
9.3. Protein–Protein Interaction (PPI) Network Construction
9.4. Molecular Docking Analysis
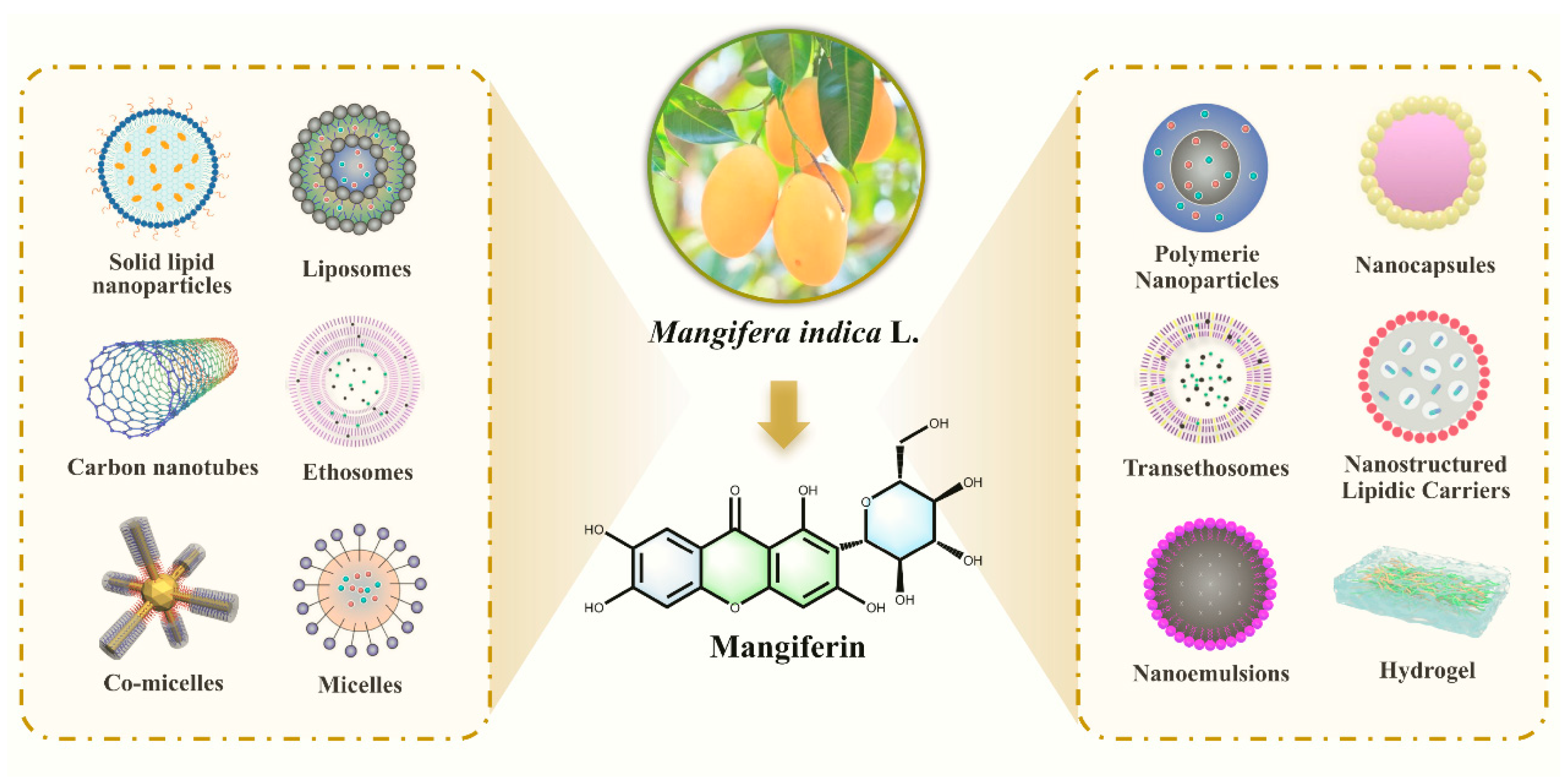
10. Study on the Bioavailability Enhancement Strategy of MF and Its Targeted Therapeutic Mechanism
10.1. New Dosage Form Development: Multimodal Carrier Systems Reshape Drug Delivery Characteristics
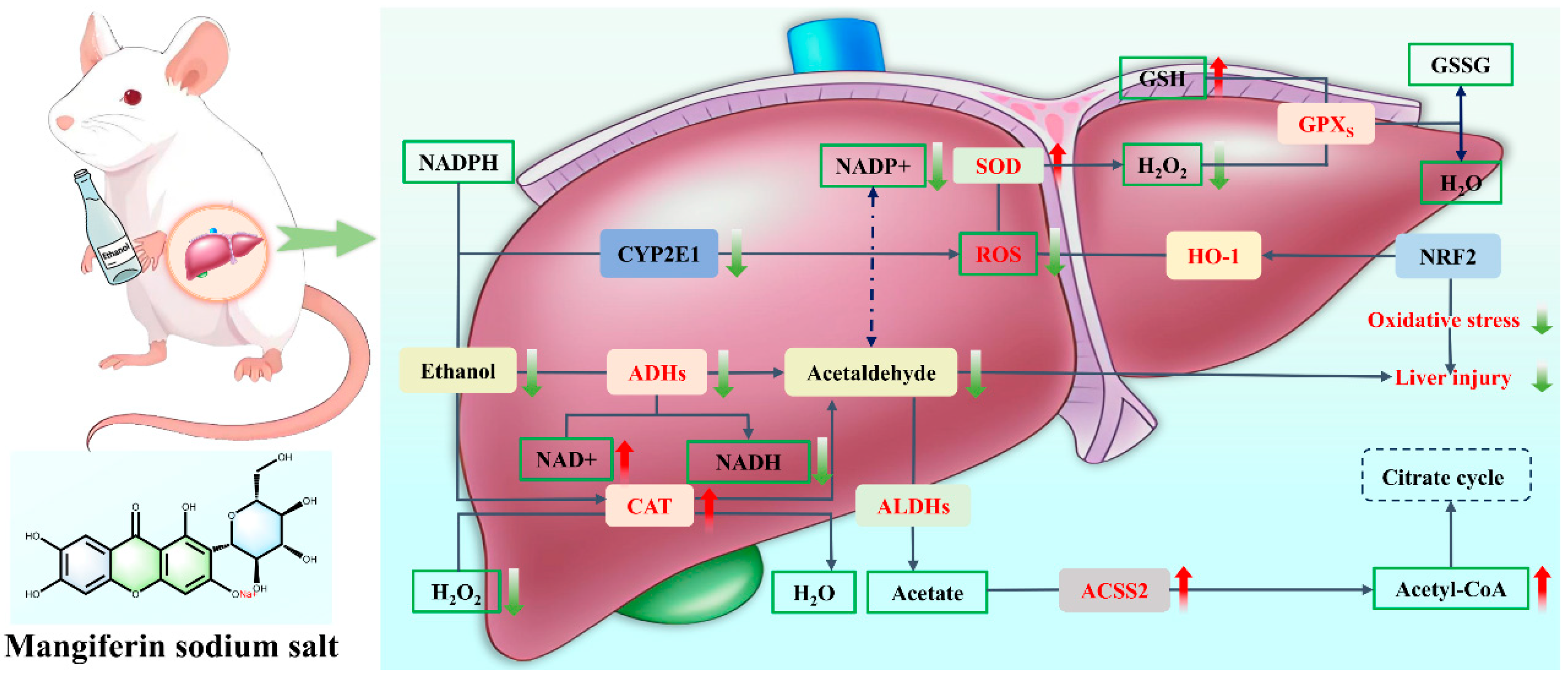
10.2. Structural Modification: Target Potentiation and Hepatic Injury Therapeutic Mechanism of MF Sodium Salt
10.3. Biotransformation: Bacterial Colony Intervention and Metabolic Pathway Modulation to Enhance Drug Stability
11. Future Perspectives
12. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MF | Mangiferin |
| ALD | Alcoholic liver disease |
| MAFLD | Metabolism-associated fatty liver disease |
| Nrf2 | nuclear factor E2-related factor 2 |
| CCl4 | carbon tetrachloride |
| HO-1 | heme oxygenase-1 |
| PPI | Protein–protein interaction |
| API | active pharmaceutical ingredients |
| NDV | Newcastle disease virus |
References
- Zhao, C.; Pu, Z.; Gao, J.; Liu, C.; Xing, J.; Lang, W.; Chen, J.; Yuan, C.; Zhou, C. “Multiomics” Analyses Combined with Systems Pharmacology Reveal the Renoprotection of Mangiferin Monosodium Salt in Rats with Diabetic Nephropathy: Focus on Improvements in Renal Ferroptosis, Renal Inflammation, and Podocyte Insulin Resistance. J. Agric. Food. Chem. 2023, 71, 358–381. [Google Scholar] [CrossRef]
- Gendy, A.M.; El-Gazar, A.A.; Ragab, G.M.; Al-Mokaddem, A.K.; El-Haddad, A.E.; Selim, H.M.R.M.; Yousef, E.M.; Hamed, N.O.; Ibrahim, S.S.A. Possible Implication of Nrf2, PPAR-γ and MAPKs Signaling in the Protective Role of Mangiferin against Renal Ischemia/Reperfusion in Rats. Pharmaceuticals 2022, 16, 6. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, W.; Deng, J.; Du, Z.; Zeng, X.; Zhou, B.; Hao, E. Mangiferin alleviates renal inflammatory injury in spontaneously hypertensive rats by inhibiting MCP-1/CCR2 signaling pathway. Chin. Herb. Med. 2023, 15, 556–563. [Google Scholar] [CrossRef]
- Chen, L.; Li, S.; Zhu, J.; You, A.; Huang, X.; Yi, X.; Xue, M. Mangiferin prevents myocardial infarction-induced apoptosis and heart failure in mice by activating the Sirt1/FoxO3a pathway. J. Cell. Mol. Med. 2021, 25, 2944–2955. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Chen, C.Y.; Wang, Y.F.; Guo, Z.Y.; Zhang, Y. Mangiferin: A natural neuroprotective polyphenol with anti-inflammatory and anti-oxidant properties for depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2025, 139, 111401. [Google Scholar] [CrossRef]
- Tan, H.; Liang, D.; Lu, N.; Zhang, J.; Zhang, S.; Tan, G. Mangiferin attenuates lipopolysaccharide-induced neuronal injuries in primary cultured hippocampal neurons. Aging 2024, 16, 8645–8656. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Zhang, M.; Wang, P.; Dai, Z.; Li, X.; Li, D.; Jing, L.; Qi, C.; Fan, H.; Qin, M.; et al. Transcriptome analysis reveals the anti-Parkinson’s activity of Mangiferin in zebrafish. Biomed. Pharmacother. 2024, 179, 117387. [Google Scholar] [CrossRef]
- Deng, X.; Lin, B.; Wang, F.; Xu, P.; Wang, N. Mangiferin attenuates osteoporosis by inhibiting osteoblastic ferroptosis through Keap1/Nrf2/SLC7A11/GPX4 pathway. Phytomedicine 2024, 124, 155282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, M.; Li, Y.; Guo, Y.; Yu, Y.; Zhou, Z.; Lu, H.; Yao, S.; Wu, C.; Zhang, X.; et al. Mangiferin ameliorates polycystic ovary syndrome in rats by modulating insulin resistance, gut microbiota, and ovarian cell apoptosis. Front. Pharmacol. 2024, 15, 1457467. [Google Scholar] [CrossRef]
- Wang, M.; Liang, Y.; Chen, K.; Wang, M.; Long, X.; Liu, H.; Sun, Y.; He, B. The management of diabetes mellitus by mangiferin: Advances and prospects. Nanoscale 2022, 14, 2119–2135. [Google Scholar] [CrossRef]
- Kalligeros, M.; Henry, L.; Younossi, Z.M. Metabolic dysfunction-associated steatotic liver disease and its link to cancer. Metabolism 2024, 160, 156004. [Google Scholar] [CrossRef]
- Mak, L.Y.; Liu, K.; Chirapongsathorn, S.; Yew, K.C.; Tamaki, N.; Rajaram, R.B.; Panlilio, M.T.; Lui, R.; Lee, H.W.; Lai, J.C.; et al. Liver diseases and hepatocellular carcinoma in the Asia-Pacific region: Burden, trends, challenges and future directions. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 834–851. [Google Scholar] [CrossRef]
- Oliveira, B.G.; Costa, H.B.; Ventura, J.A.; Kondratyuk, T.P.; Barroso, M.E.S.; Correia, R.M.; Pimentel, E.F.; Pinto, F.E.; Endringer, D.C.; Romão, W. Chemical profile of mango (Mangifera indica L.) using electrospray ionisation mass spectrometry (ESI-MS). Food Chem. 2016, 204, 37–45. [Google Scholar] [CrossRef]
- Alañón, M.E.; Pimentel-Moral, S.; Arráez-Román, D.; Segura-Carretero, A. HPLC-DAD-Q-ToF-MS profiling of phenolic compounds from mango (Mangifera indica L.) seed kernel of different cultivars and maturation stages as a preliminary approach to determine functional and nutraceutical value. Food Chem. 2021, 337, 127764. [Google Scholar] [CrossRef] [PubMed]
- Heo, N.Y.; Kim, H. Epidemiology and updated management for autoimmune liver disease. Clin. Mol. Hepatol. 2023, 29, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Newman, K.L.; Vélez, C.; Paul, S.; Radix, A.E.; Streed, C.G.; Targownik, L.E. Research Considerations in Digestive and Liver Disease in Transgender and Gender-Diverse Populations. Clin. Gastroenterol. Hepatol. 2023, 21, 2443–2449. [Google Scholar] [CrossRef] [PubMed]
- Paternostro, R.; Sieghart, W.; Trauner, M.; Pinter, M. Cancer and hepatic steatosis. ESMO 2021, 6, 100185. [Google Scholar] [CrossRef]
- Cao, G.; Jing, W.; Liu, J.; Liu, M. Countdown on hepatitis B elimination by 2030: The global burden of liver disease related to hepatitis B and association with socioeconomic status. Hepatol. Int. 2022, 16, 1282–1296. [Google Scholar] [CrossRef]
- Saha, S.; Sadhukhan, P.; Sil, P.C. Mangiferin: A xanthonoid with multipotent anti-inflammatory potential. Biofactors 2016, 42, 459–474. [Google Scholar] [CrossRef]
- Akkewar, A.S.; Mishra, K.A.; Sethi, K.K. Mangiferin: A natural bioactive immunomodulating glucosylxanthone with potential against cancer and rheumatoid arthritis. J. Biochem. Mol. Toxic. 2024, 38, e23765. [Google Scholar] [CrossRef]
- Zivković, J.; Kumar, K.A.; Rushendran, R.; Ilango, K.; Fahmy, N.M.; El-Nashar, H.A.S.; El-Shazly, M.; Ezzat, S.M.; Melgar-Lalanne, G.; Romero-Montero, A.; et al. Pharmacological properties of mangiferin: Bioavailability, mechanisms of action and clinical perspectives. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 763–781. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.Y.; Wang, S.J.; Li, Y.B. Research progress on pharmacological effects of mangiferin. Strait Pharm. J. 2019, 31, 31–33. [Google Scholar]
- Sun, C.Y.; Xie, G.Y.; Qin, M.J. Research progress on distribution and pharmacological activities of mangiferin in plant kingdom. Chin. Wild Plant Resour. 2017, 36, 39–45+49. [Google Scholar]
- Fan, X.C.; Jiao, G.Y.; Zhang, F.; Han, J.; Chen, W.S. Research progress on pharmacodynamic effects of mangiferin. Acad. J. Nav. Med. Univ. 2024, 45, 219–226. [Google Scholar]
- Zhang, L.; Huang, C.; Fan, S. Mangiferin and organ fibrosis: A mini review. Biofactors 2021, 47, 59–68. [Google Scholar] [CrossRef]
- Mittal, S.; Iqubal, M.K.; Iqbal, B.; Gupta, M.M.; Ali, J.; Baboota, S. A pervasive scientific overview on mangiferin in the prevention and treatment of various diseases with preclinical and clinical updates. J. Complementary Integr. Med. 2020, 18, 9–21. [Google Scholar] [CrossRef]
- Li, L.; Dong, Y.; Liu, X.; Wang, M. Mangiferin for the Management of Liver Diseases: A Review. Foods 2023, 12, 2469. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Das, T.; Gopalakrishnan, A.V.; Saha, S.C.; Ghorai, M.; Nandy, S.; Kumar, M.; Radha; Ghosh, A.; Mukerjee, N.; et al. Mangiferin: The miraculous xanthone with diverse pharmacological properties. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.Q.; Zhen, H.S.; Xiong, W.N.; Ping, J.J.; Qiong, D.M. Determination of mangiferin in mango peel by RP-HPLC. Chin. Tradit. Herb. Drugs 2006, 37, 1262–1263. [Google Scholar] [CrossRef]
- Huang, H.B.; Li, X.J.; Liang, Q.Y. Determination of mangiferin in mango leaves by RP-HPLC. China J. Chin. Mater. Med. 2003, 28, 49–51. [Google Scholar]
- Zhu, F.R.; Wang, S.X.; Mao, D.Y.; Ao, W.; Qing, L.W. Optimization of ultrasound-assisted extraction process of mangiferin from mango kernel. Sci. Technol. Food Ind. 2024, 45, 161–167. [Google Scholar]
- Liu, C.K.; Yang, L.; He, L.F. Determination of total flavonoids and mangiferin content in mango pulp. Hubei Agric. Sci. 2020, 59, 138–140+144. [Google Scholar]
- Chen, M.M.; Yang, C.Y.; Wan, Y.Y.; Chen, X.; Guo, M.R.; Du, M.X. Determination of mangiferin in Anemarrhena asphodeloides by HPLC. Shandong Chem. Ind. 2022, 51, 115–119+122. [Google Scholar]
- Wu, J.Y.; Zhang, W.G.; Lang, Y.F.; Huang, Z.C.; Yang, W.L.; Yang, J.; Chen, H.F. Chemical composition analysis of mango kernel based on UPLC-Q-TOF-MS/MS. Nat. Prod. Res. Dev. 2023, 35, 949–965. [Google Scholar]
- Zou, D.F.; Gao, Y.; Zhang, K.F. Determination of mangiferin content in leaves of different mango varieties. J. Anhui Agric. Sci. 2010, 38, 2947–2948. [Google Scholar]
- Deng, J.G.; Feng, X.; Wang, Q.; Tan, J.P.; Ye, Y. Comparative study on mangiferin content in mango leaves from different origins and varieties. Chin. Tradit. Pat. Med. 2006, 28, 1755–1756. [Google Scholar] [CrossRef]
- Yang, K.S.; Zhao, H.Y.; Gu, C.Z.; Li, Z.H.; Lu, J.; Cao, J.X. Determination of mangiferin in different mango varieties from Yunnan by HPLC. Sci. Technol. Food Ind. 2014, 35, 61–63+67. [Google Scholar]
- Deng, H.M.; Chen, Y.J.; Lin, Z.H.; Zheng, L.W.; Sun, Z.F.; Guo, W.F. Separation, purification and identification of mangiferin. Agric. Prod. Process. 2018, 17, 1–4+9. [Google Scholar] [CrossRef]
- Ma, Y.L.; Li, X.N.; Qian, D.M.; Tan, J.L. Extraction of mangiferin and its antioxidant activity. Shandong Chem. Ind. 2020, 49, 24–26. [Google Scholar]
- Castro-Muñoz, R.; Cabezas, R.; Plata-Gryl, M. Mangiferin: A comprehensive review on its extraction, purification and uses in food systems. Adv. Colloid Interface Sci. 2024, 329, 103188. [Google Scholar] [CrossRef]
- Mei, S.; Perumal, M.; Battino, M.; Kitts, D.D.; Xiao, J.; Ma, H.; Chen, X. Mangiferin: A review of dietary sources, absorption, metabolism, bioavailability, and safety. Crit. Rev. Food Sci. 2023, 63, 3046–3064. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Patel, S. Mangiferin: A phytochemical with panacea potential. Biomed. Pharmacother. 2017, 96, 1562–1564. [Google Scholar] [CrossRef]
- Ma, X.H.; Wei, Y.L.; Li, G.Z. Research progress of mango food products. Agric. Prod. Process. 2024, 23, 108–111. [Google Scholar] [CrossRef]
- López-Cárdenas, F.G. Advances in Mangiferin: Biosynthetic Pathways, Bioavailability and Bioactivity. In Handbook of Dietary Flavonoids; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–37. [Google Scholar]
- Guan, L.; Yang, C.Q.; Li, R.J.; Zhao, H.R.; Li, W.Z. Research progress on mangiferin. Contemp. Chem. Ind. 2021, 50, 478–481+486. [Google Scholar]
- Ehianeta, T.S.; Laval, S.; Yu, B. Bio- and chemical syntheses of mangiferin and congeners. Biofactors 2016, 42, 445–458. [Google Scholar] [CrossRef]
- Quillinan, A.J.; Scheinmann, F. Studies in the xanthone series. Part XII. A general synthesis of polyoxygenated xanthones from benzophenone precursors. J. Chem. Soc. Perkin Trans. I 1973, 1, 1329–1337. [Google Scholar] [CrossRef]
- Hu, L.; Hu, H.; Wu, W.; Chai, X.; Luo, J.; Wu, Q. Discovery of novel xanthone derivatives as xanthine oxidase inhibitors. Bioorgan. Med. Chem. Lett. 2011, 21, 4013–4015. [Google Scholar] [CrossRef]
- Qin, Q.X.; Yang, J.; Yang, B. A novel and efficient synthesis of norathyriol using Pd(II) as a catalyst. Res. Chem. Intermed. 2014, 40, 1633–1636. [Google Scholar] [CrossRef]
- Wu, Z.; Wei, G.; Lian, G.; Yu, B. Synthesis of mangiferin, isomangiferin, and homomangiferin. J. Org. Chem. 2010, 7, 5725–5728. [Google Scholar] [CrossRef]
- Liao, H.L. Isolation and Structure Modification of Mangiferin from Anemarrhena asphodeloides Bge. Master’s Thesis, Naval Medical University, Shanghai, China, 2005. [Google Scholar]
- Yang, X.L.; Liu, X.; Lan, C.; Nong, J.C.Z.; Pan, L.L.; Jiao, W. Effects of mangiferin on proliferation, apoptosis and cycle of nasopharyngeal carcinoma CNE2 cells. Shandong Med. J. 2009, 49, 23–24. [Google Scholar]
- Huang, X.O.; Deng, J.G.; Chen, Z. Protective effect of mangiferin dropping pills on chronic liver injury in rats. Front. Pharm. Sci. 2009, 12, 701–704. [Google Scholar]
- Deng, J.G.; Yang, K.; Yan, L.; Guo, L.C.; Tang, H.Q. Effect of mangiferin on T lymphocyte proliferation in immunosuppressed mice. Pharmacol. Clin. Chin. Mater. Med. 2007, 23, 64–65. [Google Scholar]
- Pu, Z.J. Improving Effect of Mangiferin Sodium Salt on Alcoholic Hepatitis and Regulatory Mechanism of Metabolomics. Master’s Thesis, Hebei University, Baoding, China, 2023. [Google Scholar] [CrossRef]
- Tang, T. Inhibitory Study of Mangiferin Derivatives on 3t3-l1adipocytes. Master’s Thesis, Jiangsu University, Zhenjiang, China, 2024. [Google Scholar] [CrossRef]
- Correia-da-Silva, M.; Sousa, E.; Duarte, B.; Marques, F.; Cunha-Ribeiro, L.M.; Pinto, M.M. Polysulfated xanthones: Multipathway development of a new generation of dual anticoagulant/antiplatelet agents. J. Med. Chem. 2011, 15, 5373–5384. [Google Scholar] [CrossRef]
- Li, X.J. Chemical Synthesis and Pharmacological Activity Study of Mangiferin Esterified Derivatives. Ph.D. Thesis, Guangxi Medical University, Nanning, China, 2012. [Google Scholar] [CrossRef]
- Singh, S.K.; Sinha, S.K.; Prasad, S.K.; Kumar, R.; Bithu, B.S.; Kumar, S.S.; Singh, P. Synthesis and evaluation of novel analogues of mangiferin as potent antipyretic. Aaian Pac. J. Trop. Med. 2011, 11, 866–869. [Google Scholar] [CrossRef]
- Liu, H.; Tang, K.Y.; Sun, Z.; Jian, L.; Li, Z.; Wu, B.; Huang, C. Structure elucidation of in vivo and in vitro metabolites of mangiferin. J. Pharm. Biomed. Anal. 2011, 55, 1075–1082. [Google Scholar] [CrossRef]
- Zhou, H.; Song, S.; Lan, X.; Li, Y.; Yuan, X.; Yang, J.; Li, M.; Cao, T.; Zhang, J. Comprehensive profiling of mangiferin metabolites in vivo and in vitro based on the “drug metabolite clusters” analytical strategy. ACS Omega 2023, 8, 9934–9946. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.G.; Zhao, Y.; Yang, S.Y.; Fang, L.H.; Song, J.K.; Lv, Y.; Du, G.H. Pharmacokinetics of three kinds of mangiferin polymorphs in rats. Her. Med. 2019, 38, 208–212. [Google Scholar]
- Fomenko, E.V.; Chi, Y. Mangiferin modulation of metabolism and metabolic syndrome. Biofactors 2016, 42, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Gao, Y.; Xu, Z.; Lian, S.; Ma, Y.; Guo, X.; Hu, P.; Li, Z.; Huang, C. Pharmacokinetics of mangiferin and its metabolite-Norathyriol, Part 1: Systemic evaluation of hepatic first-pass effect in vitro and in vivo. Biofactors 2016, 42, 533–544. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, Y.W.; Zhao, D.; Xu, Y.Y.; Xu, M.J.; Hai, X.D. Hypoglycemic activity and in vivo pharmacokinetics of mangiferin. J. Chongqing Med. Univ. 2022, 47, 387–392. [Google Scholar]
- Li, J.; Liu, M.; Yu, H.; Wang, W.; Han, L.; Chen, Q.; Ruan, J.; Wen, S.; Zhang, Y.; Wang, T. Mangiferin Improves Hepatic Lipid Metabolism Mainly Through Its Metabolite-Norathyriol by Modulating SIRT-1/AMPK/SREBP-1c Signaling. Front. Pharmacol. 2018, 9, 201. [Google Scholar] [CrossRef]
- Huang, H.X.; Tan, Z.Y.; Deng, J.G.; Liang, Q.Y.; Nong, Y.M.; Song, N.M. In vitro metabolic transformation of mangiferin by human intestinal flora. China J. Chin. Mater. Med. 2011, 36, 443–445. [Google Scholar]
- Sarwar, A.R.; Iqbal, F.M.; Jamil, M.A.; Abbas, K. Nanocrystals of Mangiferin Using Design Expert: Preparation, Characterization, and Pharmacokinetic Evaluation. Molecules 2023, 28, 5918. [Google Scholar] [CrossRef]
- Sun, X.G.; Zhang, J.; Pang, X.; Liang, Q.Y.; Nong, Y.M.; Song, N.M. Research progress on metabolic pathways of natural flavonoid glycosides. Chin. Tradit. Herb. Drugs 2020, 51, 3078–3089. [Google Scholar]
- Li, M.; Wu, C.; Guo, H.; Chu, C.; Hu, M.; Zhou, C. Mangiferin improves hepatic damage-associated molecular patterns, lipid metabolic disorder and mitochondrial dysfunction in alcohol hepatitis rats. Food Funct. 2019, 10, 3514–3534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, C.; Yin, L.; Huang, C.; Fan, S. Mangiferin relieves CCl4-induced liver fibrosis in mice. Sci. Rep. 2023, 13, 4172. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhen, Y.; Chen, X.; Cao, L.; Song, J.; Liu, X.; Wang, M. Mangiferin Protects DNase 2 Abundance via Nrf2 Activation to Prevent Cytosolic mtDNA Accumulation during Liver Injury. Mol. Nutr. Food Res. 2023, 67, e2200885. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhang, X.Y.; Ge, X.Q.; Liu, M.X. Mangiferin prevents hepatocyte epithelial-mesenchymal transition in liver fibrosis via targeting HSP27-mediated JAK2/STAT3 and TGF-β1/Smad pathway. Phytother. Res. 2022, 36, 4167–4182. [Google Scholar] [CrossRef]
- Yang, S.; Kuang, G.; Zhang, L.; Wu, S.; Zhao, Z.; Wang, B.; Yin, X.; Gong, X.; Wan, J. Mangiferin Attenuates LPS/D-GalN-Induced Acute Liver Injury by Promoting HO-1 in Kupffer Cells. Front. Immunol. 2020, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, S.; Chen, W.; Yu, J.; Fang, P.; Zhou, G.; Li, J.; Jin, L.; Chen, Y.; Chen, P.; et al. Mangiferin alleviates endoplasmic reticulum stress in acute liver injury by regulating the miR-20a/miR-101a-Nrf2 axis. Biochem. J. 2020, 168, 365–374. [Google Scholar] [CrossRef]
- Pan, C.W.; Pan, Z.Z.; Hu, J.J.; Chen, W.L.; Zhou, G.Y.; Lin, W.; Jin, L.X.; Xu, C.L. Mangiferin alleviates lipopolysaccharide and D-galactosamine-induced acute liver injury by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Eur. J. Pharmacol. 2016, 770, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Li, L.; Li, G.; Song, J.; Liu, B.; Liu, X.; Wang, M. Mangiferin protects against alcoholic liver injury via suppression of inflammation-induced adipose hyperlipolysis. Food Funct. 2020, 11, 8837–8851. [Google Scholar] [CrossRef] [PubMed]
- Mi, K.; Huang, R. Mechanism of mangiferin on liver fibrosis in rats based on TGF-β/Smad signaling pathway. Sichuan Med. J. 2020, 41, 142–146. [Google Scholar]
- Shi, C.; Li, Y.; You, Z.; Tian, Y.; Zhu, X.; Xu, H.; Yang, M.; Zhang, Y.; Dong, R.; Quan, H.; et al. Mangiferin Ameliorates CCl4-Triggered Acute Liver Injury by Inhibiting Inflammatory Response and Oxidative Stress: Involving the Nrf2-ARE Pathway. J. Inflamm. Res. 2024, 17, 7081–7097. [Google Scholar] [CrossRef]
- Chu, C.; Zhao, Y.Y.; Zhou, C.Y. Protective Effect of Mangiferin on Alcoholic Hepatitis in Rats. Nat. Prod. Res. Dev. 2018, 30, 753–760. [Google Scholar]
- Chen, X.; Xia, X.D.; Tong, D.Y.; Che, D.B. Experimental Study on Mangiferin in Improving the Liver Function in Model Mice with Liver Injury. China Pharm. 2019, 28, 17–20. [Google Scholar]
- Chu, C. Experimental Study on Proteotive effects of Mangiferin on Alcoholic Liver Disease. Master’s Thesis, Hebei University, Baoding, China, 2018. [Google Scholar]
- Chowdhury, A.; Lu, J.; Zhang, R.; Nabila, J.; Gao, H.; Wan, Z.; Adelusi-Temitope, I.; Yin, X.; Sun, Y. Mangiferin ameliorates acetaminophen-induced hepatotoxicity through APAP-Cys and JNK modulation. Biomed. Pharmacother. 2019, 117, 109097. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, Y.Y.; Wang, L.; Teng, T.; Zhou, M.; Wang, S.G.; Tian, Y.Z.; Du, L.; Yin, X.X.; Sun, Y. Mangiferin ameliorates fatty liver via modulation of autophagy and inflammation in high-fat-diet induced mice. Biomed. Pharmacother. 2017, 96, 328–335. [Google Scholar] [CrossRef]
- Li, L.S. Mangiferin Protects Against Liver Injury Through Suppressing Cytosolic mtDNA Accumulation. Ph.D. Thesis, Hebei Normal University, Baoding, China, 2024. [Google Scholar]
- Xu, J.; Liu, X.J.; Lv, Q.Z.; Yu, H.; Cheng, W.G.; Ji, M.L. Protective effect of mangiferin on acute liver failure in mice. J. Xinxiang Med. Univ. 2020, 37, 327–331. [Google Scholar]
- Morozkina, S.N.; Nhung-Vu, T.H.; Generalova, Y.E.; Snetkov, P.P.; Uspenskaya, M.V. Mangiferin as New Potential Anti-Cancer Agent and Mangiferin-Integrated Polymer Systems-A Novel Research Direction. Biomolecules 2021, 11, 79. [Google Scholar] [CrossRef]
- Du, Z.C.; Deng, J.G.; Huang, H.X.; Liu, X.J.; Chen, L.; Li, H.W. Effect of Mango Leaf Extract on Acute Alcoholic Liver Injury in Mice. Chin. J. Exp. Tradit. Med. Formulae 2013, 19, 250–253. [Google Scholar]
- Toledo, R.C.L.; Brito, L.F.; Caetano, M.M.M.; Nakajima, V.M.; Da-Silva, B.P.; Soares, F.E.F.; Martino, H.S.D.; De-Queiroz, J.H. Acute treatment with Mangifera indica L. leaf extract attenuates liver inflammation in rats fed a cafeteria diet. Food Funct. 2019, 10, 4861–4867. [Google Scholar] [CrossRef]
- Yi, H.H. Preparation and Pharmacokinetics of mPEG-PLGA-Mangiferin Nanoparticles. Master’s Thesis, Northwest University, Xi’an, China, 2018. [Google Scholar]
- Han, D.D.; Chen, C.J.; Zhang, C.; Zhang, Y.; Tang, X. Determination of mangiferin in rat plasma by liquid–liquid extraction with UPLC-MS/MS. J. Pharm. Biomed. Anal. 2010, 51, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Kammalla, A.K.; Ramasamy, M.K.; Inampudi, J.; Dubey, G.P.; Agrawal, A.; Kaliappan, I. Comparative Pharmacokinetic Study of Mangiferin After Oral Administration of Pure Mangiferin and US Patented Polyherbal Formulation to Rats. AAPS J. 2015, 16, 250–258. [Google Scholar] [CrossRef]
- Li, X.Y.; Ye, X.Q.; Si, J.R.; Si, N.Q. Biopharmaceutics Classification System and Formulation Strategies. J. Jilin Med. Univ. 2016, 37, 60–63. [Google Scholar]
- Barakat, S.; Nasr, M.; Ahmed, R.F.; Badawy, S.; Mortada, N. Recent formulation advances of mangiferin. Rev. Bras. Farmacogn. 2022, 32, 871–882. [Google Scholar] [CrossRef]
- Baghel, M.; Baghel, I.; Kumari, P.; Bharkatiya, M.; Joshi, G.; Sakure, K.; Badwaik, H. Nano-delivery Systems and Therapeutic Applications of Phytodrug Mangiferin. Appl. Biochem. Biotechnol. 2024, 196, 7429–7463. [Google Scholar] [CrossRef]
- Mu, X.H. Preparation of Mangiferin PLGA Porous Microspheres by Pickering Emulsion Template Method. Master’s Thesis, Guangxi University of Chinese Medicine, Nanning, China, 2017. [Google Scholar]
- Huang, H.X.; Liang, Q.Y.; Deng, J.G.; Nong, Y.M.; Wei, W.; Chen, W.W. Effect of different pharmaceutical excipients on Mangiferin Absorption and Transportation in Caco-2 Cell Model. J. Guangxi Univ. Chin. Med. 2014, 17, 60–62. [Google Scholar]
- Alkholifi, F.K.; Alam, A.; Foudah, A.I.; Yusufoglu, H.S. Phospholipid-Based Topical Nano-Hydrogel of Mangiferin: Enhanced Topical Delivery and Improved Dermatokinetics. Gels 2023, 9, 178. [Google Scholar] [CrossRef]
- Lv, D.X.; Yu, R.; Qu, Z.G.; Fan, M.S. Preparation and in vivopharmacokinetics of mangiferin solid lipid nanoparticles. Chin. Trad. Pat. Med. 2020, 42, 2835–2839. [Google Scholar]
- Xuan, X.Y.; Wang, Y.J.; Tian, H.; Pi, J.X.; Sun, T.Z.; Zhang, W.L. Study on Prescription of Self-microemulsifying Drug Delivery System of Mangiferin Phospholipid Complex. J. Chin. Med. Mater. 2012, 35, 1508–1511. [Google Scholar]
- Zheng, Y.; Wang, Y.J. Physicochemical property and percutaneous permeability in vitro of mangiferin liposomes. Drugs Clin. 2014, 29, 147–150. [Google Scholar]
- Xuan, X.Y.; Wang, Y.J.; Zhang, W.L.; Pi, J.X.; Zheng, Y.; Gao, X. Preparation of self-micro emulsifying drug delivery systems of mangiferin and its pharmacokinetic study in rats. Drug Eval. Res. 2013, 36, 166–170. [Google Scholar]
- Liu, M.; Liu, Y.; Ge, Y.; Zhong, Z.; Wang, Z.; Wu, T.; Zhao, X.; Zu, Y. Solubility, Antioxidation, and Oral Bioavailability Improvement of Mangiferin Microparticles Prepared Using the Supercritical Antisolvent Method. Pharmaceutics 2020, 12, 90. [Google Scholar] [CrossRef]
- Telange, D.R.; Sohail, N.K.; Hemke, A.T.; Kharkar, P.S.; Pethe, A.M. Phospholipid complex-loaded self-assembled phytosomal soft nanoparticles: Evidence of enhanced solubility, dissolution rate, ex vivo permeability, oral bioavailability, and antioxidant potential of mangiferin. Drug. Deliv. Transl. Res. 2021, 11, 1056–1083. [Google Scholar] [CrossRef]
- Yang, L.P.; Wang, H.J.; Li, W.H.; Wang, F.Y.; Fang, X.D. Formulation optimization of mangiferin-amino-modified mesoporous silica nanoparticles and oral pharmacokinetics evaluation. Chin. Tradit. Herb. Drugs 2024, 55, 2542–2552. [Google Scholar]
- Li, Z.J.; Pu, Z.J.; Gao, Y.W.; Zhou, M.; Zhang, Z.; Xiao, P.F.; Chen, J.T.; Zhou, C.Y. Integrated serum and liver metabolomics decipher the hepatoprotective mechanisms of mangiferin sodium salt through modulating alcohol metabolism on alcoholic liver disease. Food Biosci. 2024, 61, 104631. [Google Scholar] [CrossRef]
- Xiong, W.N.; Yue, G.H.; Ye, M.L.; Liang, J.Q.; Wang, Z.P.; Tang, Z.Y.; Wu, Y.Q. Preparation and characterization of mangiferin-polyvinylpyrrolidone solid dispersons and its in vitro metabolism by human intestinal microflora. Chin. J. Hosp. Pharm. 2017, 37, 1028–1032. [Google Scholar]
- Yang, X.; Yang, C.; Zhang, S.; Geng, H.; Zhu, A.X.; Bernards, R.; Qin, W.; Fan, J.; Wang, C.; Gao, Q. Precision treatment in advanced hepatocellular carcinoma. Cancer Cell 2024, 42, 180–197. [Google Scholar] [CrossRef]
- Terrault, N.A.; Francoz, C.; Berenguer, M.; Charlton, M.; Heimbach, J. Liver Transplantation 2023: Status Report, Current and Future Challenges. Clin. Gastroenterol. Hepatol. 2023, 21, 2150–2166. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Piedvache, C.; Chiche, L.; Adam, J.P.; Salamé, E.; Bucur, P.; Cherqui, D.; Scatton, O.; Granger, V.; Ducreux, M.; et al. Collaborative TransMet group. Liver transplantation plus chemotherapy versus chemotherapy alone in patients with permanently unresectable colorectal liver metastases (TransMet): Results from a multicentre, open-label, prospective, randomised controlled trial. Lancet 2024, 404, 1107–1118. [Google Scholar]
- Mallapaty, S. First pig-to-human liver transplant recipient ‘doing very well’. Nature 2024, 630, 18. [Google Scholar] [CrossRef]
- Zhong, L.; Gan, L.; Wang, B.; Wu, T.; Yao, F.; Gong, W.; Peng, H.; Deng, Z.; Xiao, G.; Liu, X.; et al. Hyperacute rejection-engineered oncolytic virus for interventional clinical trial in refractory cancer patients. Cell 2025, 188, 1119–1136. [Google Scholar] [CrossRef]
- Sidik, S. How to trick the immune system into attacking tumours. Nature, 2025; online ahead of print. [Google Scholar] [CrossRef]
- Irshad, N.; Naeem, H.; Shahbaz, M.; Imran, M.; Mujtaba, A.; Hussain, M.; Al-Abdulmonem, W.; Alsagaby, S.A.; Yehuala, T.F.; Abdelgawad, M.A.; et al. Mangiferin: An effective agent against human malignancies. Food. Sci. Nutr. 2024, 12, 7137–7157. [Google Scholar] [CrossRef]
- Iqbal, H.; Inam-Ur-Raheem, M.; Munir, S.; Rabail, R.; Kafeel, S.; Shahid, A.; Mousavi Khaneghah, A.; Aadil, R.M. Therapeutic potential of mangiferin in cancer: Unveiling regulatory pathways, mechanisms of action, and bioavailability enhancements-An updated review. Food. Sci. Nutr. 2023, 12, 1413–1429. [Google Scholar] [CrossRef]
- Ji, H.W.; Wang, C.R.; Yuan, X.W.; Wang, J.; Wang, L.; Cao, Q.L.; Li, Y.H.; Xu, Y.N.; Kim, N.H. Mangiferin improves early porcine embryonic development by reducing oxidative stress. Reprod. Domest. Anim. 2024, 59, e14565. [Google Scholar] [CrossRef]
- Rao, J.; Qiu, P.; Zhang, Y.; Wang, X. Gut microbiota trigger host liver immune responses that affect drug-metabolising enzymes. Front. Immunol. 2024, 15, 1511229. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Trauner, M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022, 34, 1700–1718. [Google Scholar] [CrossRef]
- Imparato, G.; Urciuolo, F.; Netti, P.A. Organ on Chip Technology to Model Cancer Growth and Metastasis. Bioengineering 2022, 9, 28. [Google Scholar] [CrossRef]
- Monteduro, A.G.; Rizzato, S.; Caragnano, G.; Trapani, A.; Giannelli, G.; Maruccio, G. Organs-on-chips technologies—A guide from disease models to opportunities for drug development. Biosens. Bioelectron. 2023, 231, 115271. [Google Scholar] [CrossRef]
- Alonso-Roman, R.; Mosig, A.S.; Figge, M.T.; Papenfort, K.; Eggeling, C.; Schacher, F.H.; Hube, B.; Gresnigt, M.S. Organ-on-chip models for infectious disease research. Nat. Microbiol. 2024, 9, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Lucchetti, M.; Aina, K.O.; Grandmougin, L.; Jäger, C.; Pérez Escriva, P.; Letellier, E.; Mosig, A.S.; Wilmes, P. An Organ-on-Chip Platform for Simulating Drug Metabolism Along the Gut-Liver Axis. Adv. Healthc. Mater. 2024, 13, e2303943. [Google Scholar] [CrossRef] [PubMed]

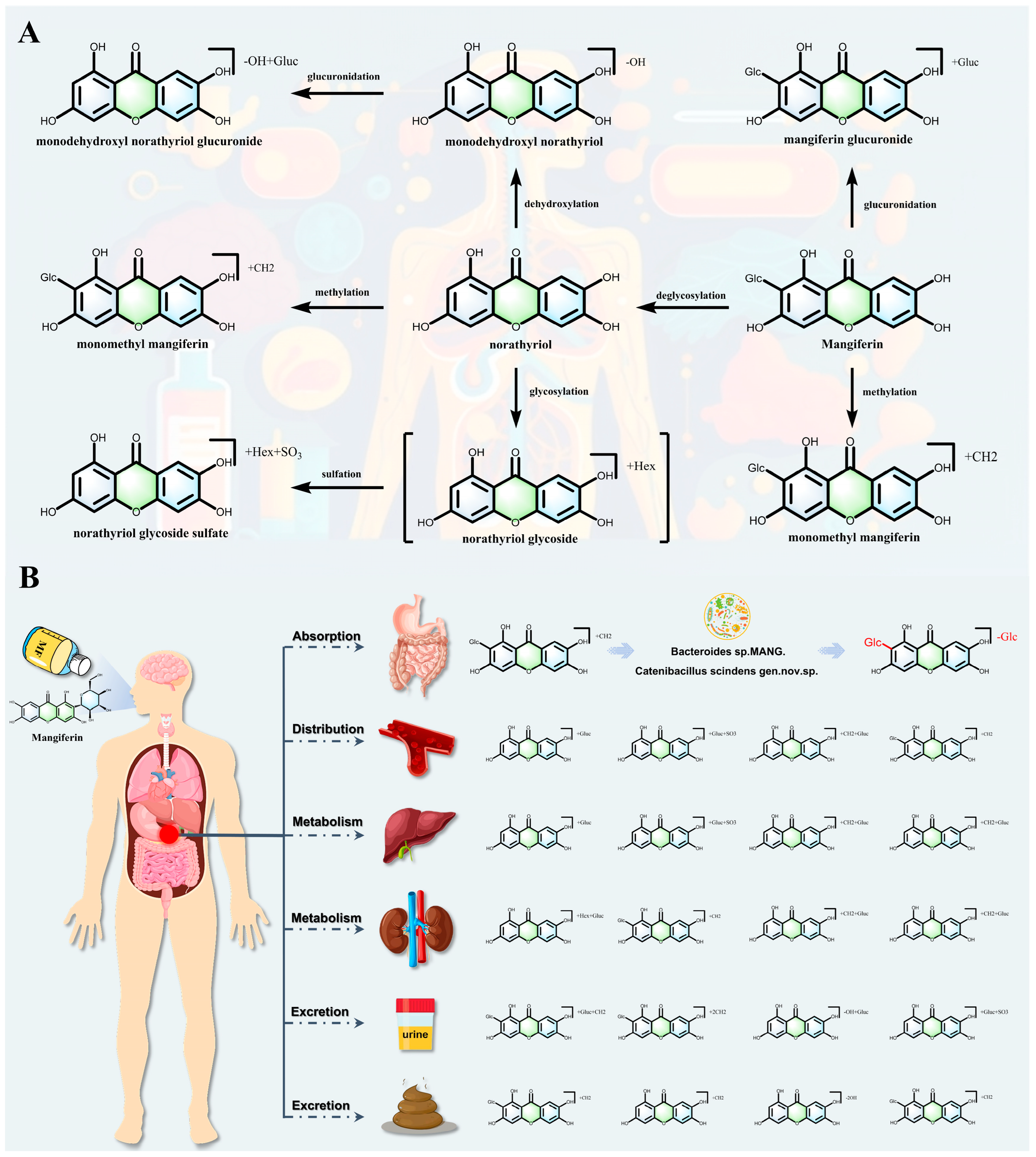
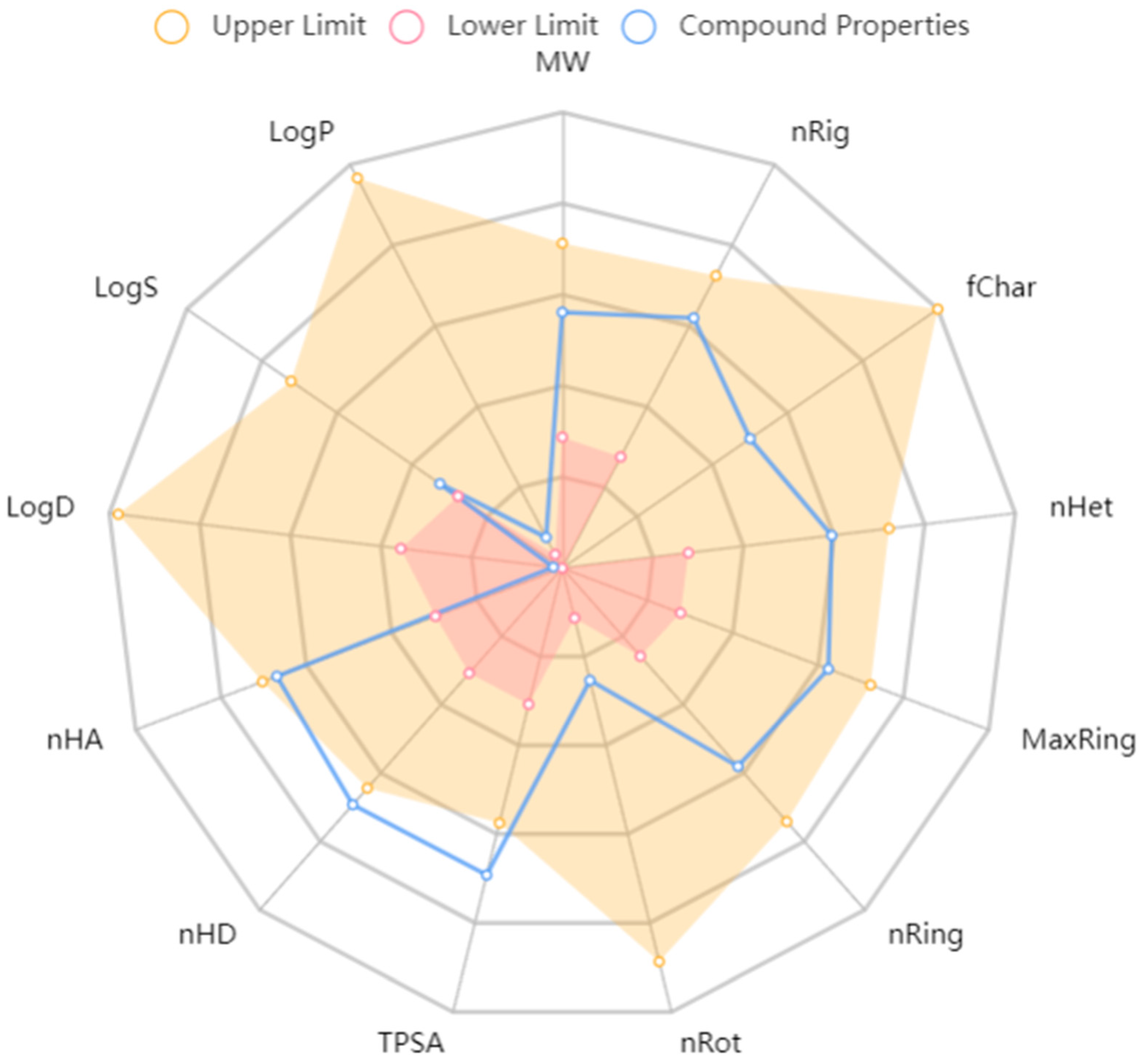
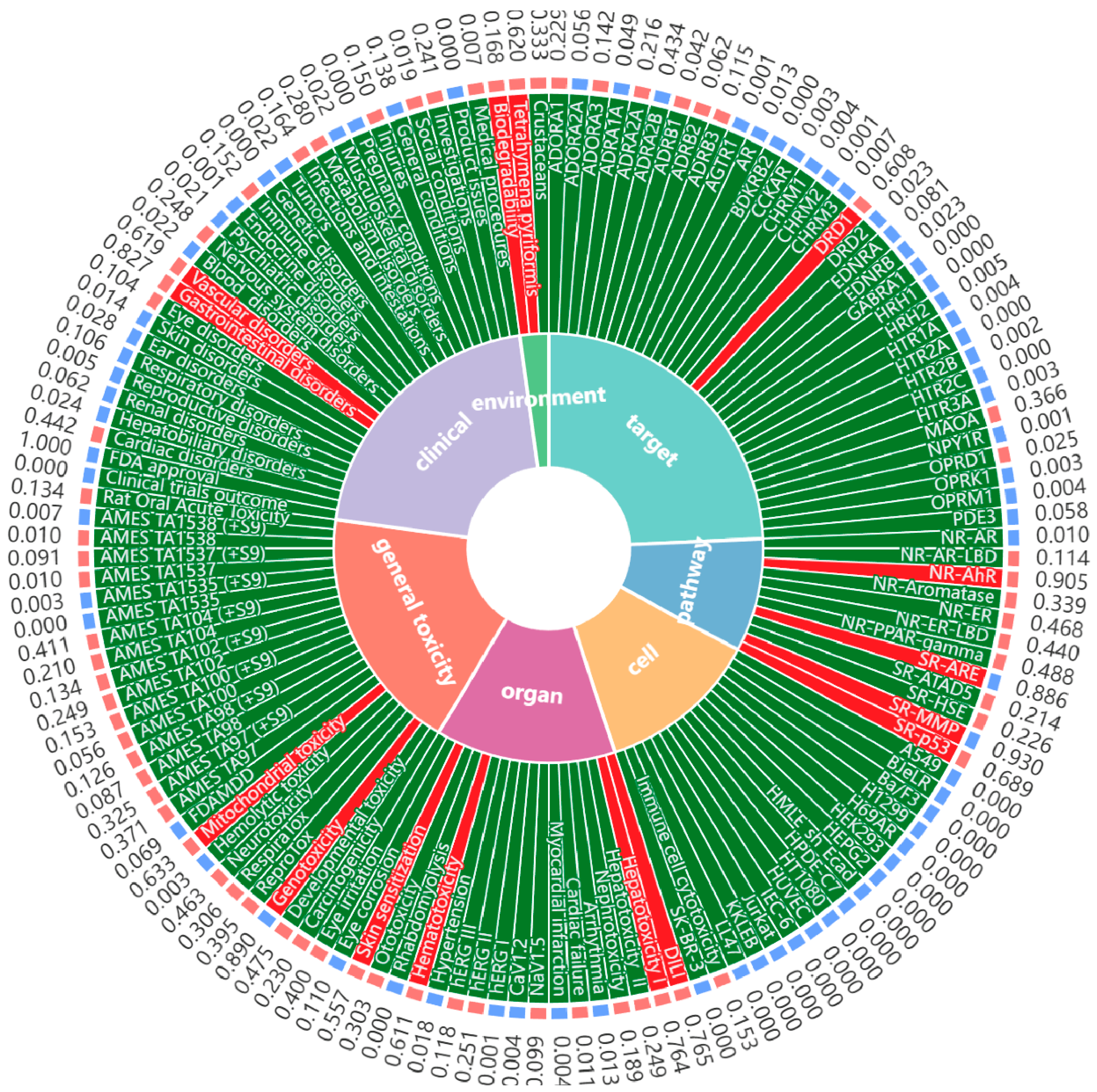
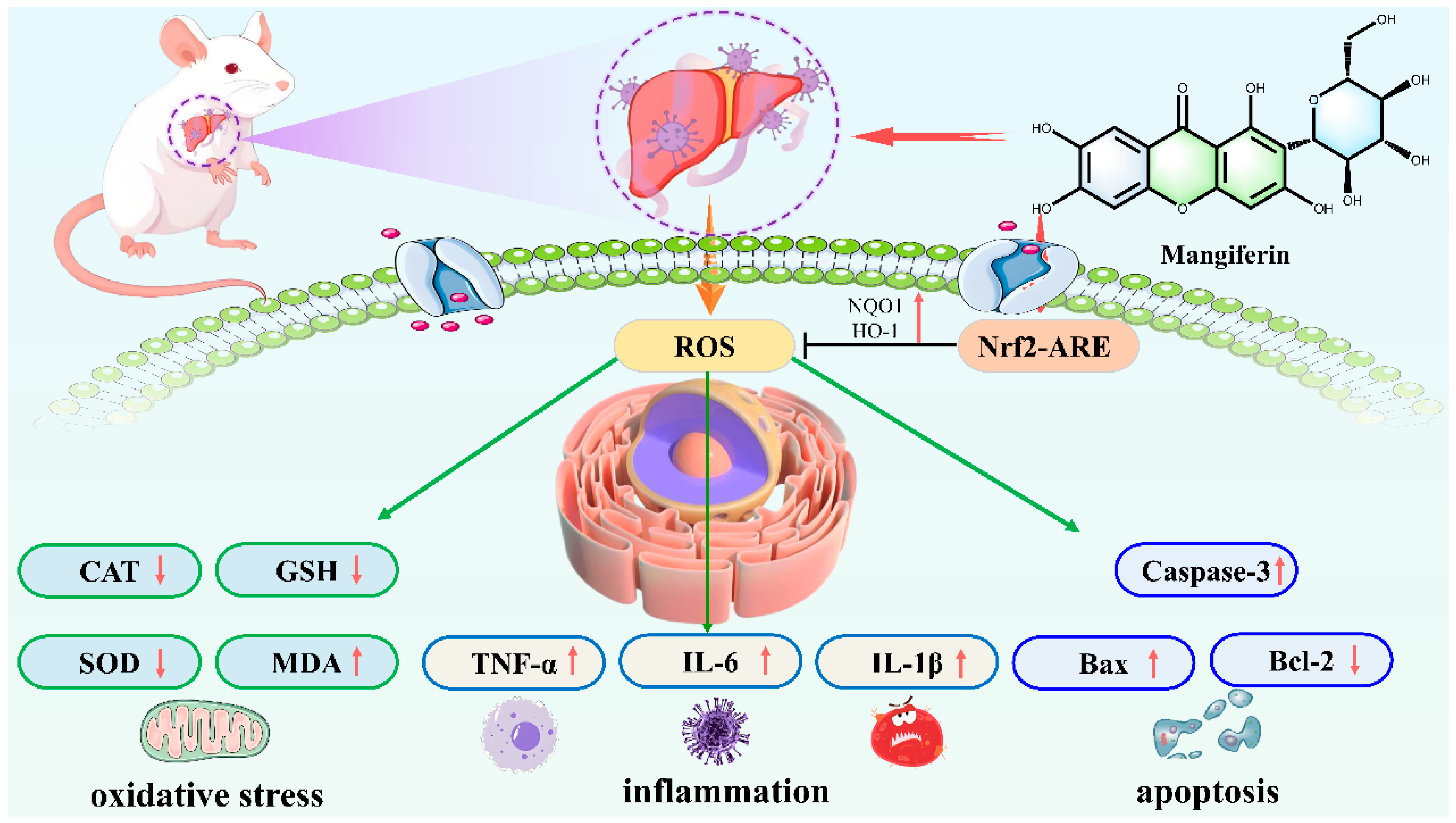


| Liver Disease | Experimental Model | Molding Method | Dose | Core Mechanisms of Action and Targets | References |
|---|---|---|---|---|---|
| Acute liver injury | mice model | Combined treatment with lipopolysaccharide and D-galactosamine | 20–100 mg/kg | ↓TLR4, ↓NF-κB, ↓NLRP3, ↓TNF-α, ↓IL-1β, ↓caspase-1, ↑Nrf2, ↑HO-1, ↓ROS, ↓MDA | [74,76] |
| mice model | Acetaminophen | no standards | ↓APAP-Cys, ↓p-JNK, ↓ROS, ↑AMPK, ↑Nrf2, ↑HO-1 | [83] | |
| mice model | CCl4 | no standards | ↑Nrf2, ↑NQO1, ↑HO-1, ↓p-p65 | [80] | |
| Fatty liver | mice model | High-fat diet feeding | 10–50 mg/kg | ↑AMPKα, ↓mTOR, ↓p-p70S6K, ↓LC3-II, ↓p62, ↓NF-κB, ↓TNF-α, ↓p-JNK, ↑PI3K, ↑p-AKT, ↓TG, ↓TC, ↓SREBP-1 | [82] |
| Steatohepatitis | rat model | Alcohol gavage combined with a high-fat diet | 20–80 mg/kg | ↓NF-κB, ↓p65, ↓IL-6, ↓TNF-α, ↓SREBP-1c | [70,77] |
| Liver fibrosis | rat model | CCl4 subcutaneous injection | 10–50 mg/kg | ↓α-SMA, ↓COL1, ↓p-p65, ↓p-IkB α | [71,72,78] |
| Liver cirrhosis | mice model | CCl4 subcutaneous (2 mL/kg,10% olive oil solution) twice weekly for 12 weeks | 50–100 mg/kg | ↓EMT, ↓α-SMA, ↑E-cadherin, ↓p-JAK2, ↓p-STAT3, ↓TGF-β1 | [71,73] |
| mice model (BDL) | Bile duct ligation surgery with mancozeb intervention started 7 days postoperatively | 100 mg/kg | ↑DNase 2, ↓mtDNA, ↓TLR9, ↓MyD88, ↓NF-κB, ↑Nrf2, ↓ROS | [72] | |
| Rat model (alcohol) | Alcohol gavage (50% ethanol, 5 g/kg/d) in combination with a high-fat diet for 24 weeks | 80–100 mg/kg | ↑PPARα, ↑PGC-1α, ↓HMGB1, ↓HSP90, ↓NLRP3 | [70,78] | |
| Liver cancer | rat model | Diethylnitrosamine induced | 30–60 mg/kg | ↑Bax, ↓Bcl-2, ↑Caspase-3, ↓NF-κB | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Su, S.; Wu, J.; Yang, X.; Zhang, Q.; Shen, X.; Zhao, L.; Wang, T.; Feng, N.; Su, J.; et al. Unlocking Mangiferin: A Therapeutic Candidate Revolutionizing Liver Disease Therapy. Nutrients 2025, 17, 3401. https://doi.org/10.3390/nu17213401
Xie J, Su S, Wu J, Yang X, Zhang Q, Shen X, Zhao L, Wang T, Feng N, Su J, et al. Unlocking Mangiferin: A Therapeutic Candidate Revolutionizing Liver Disease Therapy. Nutrients. 2025; 17(21):3401. https://doi.org/10.3390/nu17213401
Chicago/Turabian StyleXie, Jihang, Sijing Su, Jianfa Wu, Xing Yang, Qian Zhang, Xiaojiang Shen, Linlin Zhao, Ting Wang, Nana Feng, Jinsong Su, and et al. 2025. "Unlocking Mangiferin: A Therapeutic Candidate Revolutionizing Liver Disease Therapy" Nutrients 17, no. 21: 3401. https://doi.org/10.3390/nu17213401
APA StyleXie, J., Su, S., Wu, J., Yang, X., Zhang, Q., Shen, X., Zhao, L., Wang, T., Feng, N., Su, J., & Zhang, Y. (2025). Unlocking Mangiferin: A Therapeutic Candidate Revolutionizing Liver Disease Therapy. Nutrients, 17(21), 3401. https://doi.org/10.3390/nu17213401





