Effect of Oral and Topical Sodium Bicarbonate on Functional Recovery and Soccer-Specific Performance After Exercise-Induced Muscle Damage: A Randomized, Double-Blind, Placebo-Controlled Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
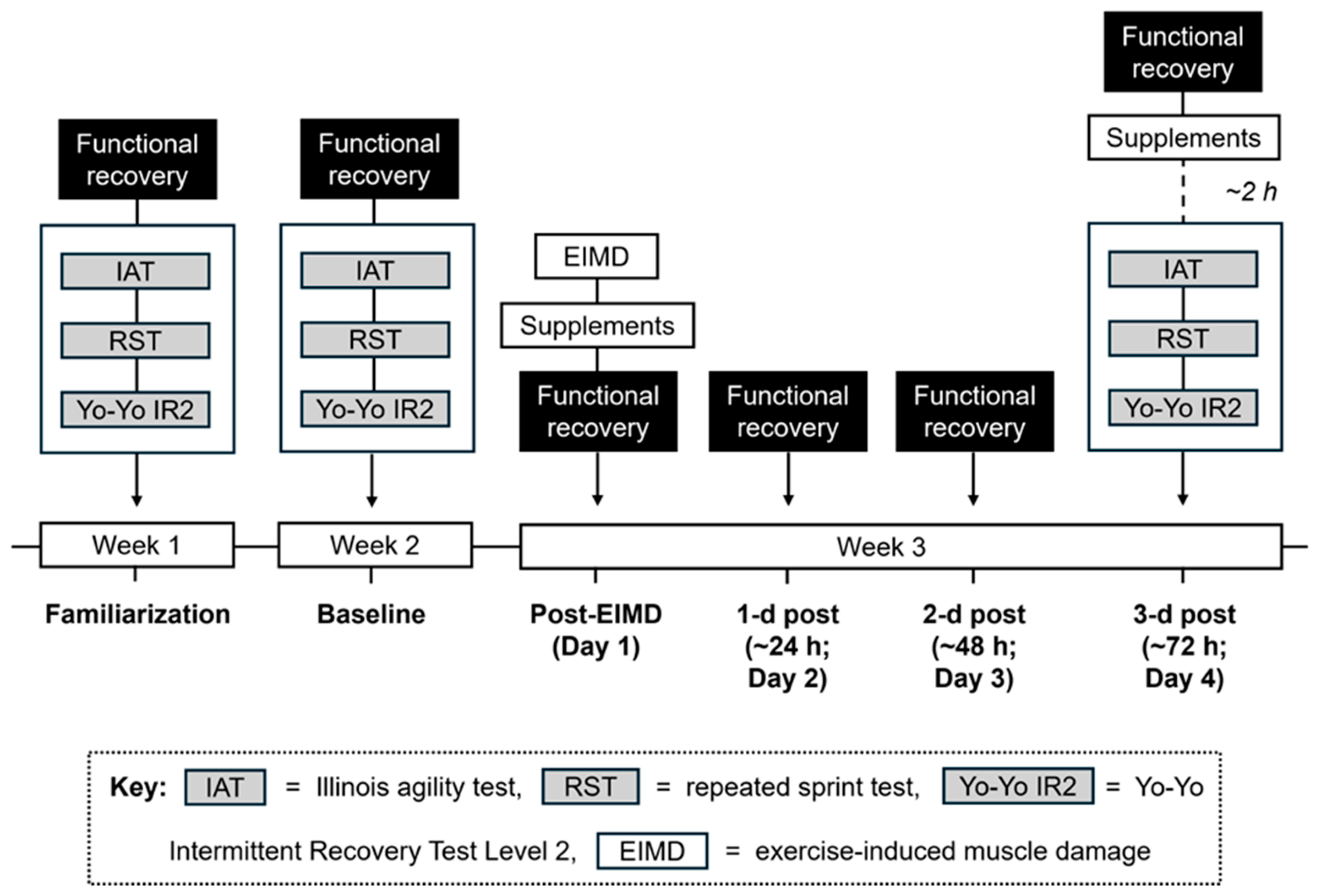
2.2. Study Design
2.3. Procedures, Familiarization and Baseline Measures
2.4. Exercise-Induced Muscle Damage Task
2.5. Nutritional Supplements
2.6. Experimental Testing Week (Day 1–4)
2.7. Statistical Analysis
3. Results
3.1. Baseline Measures and Test–Retest Reproducibility
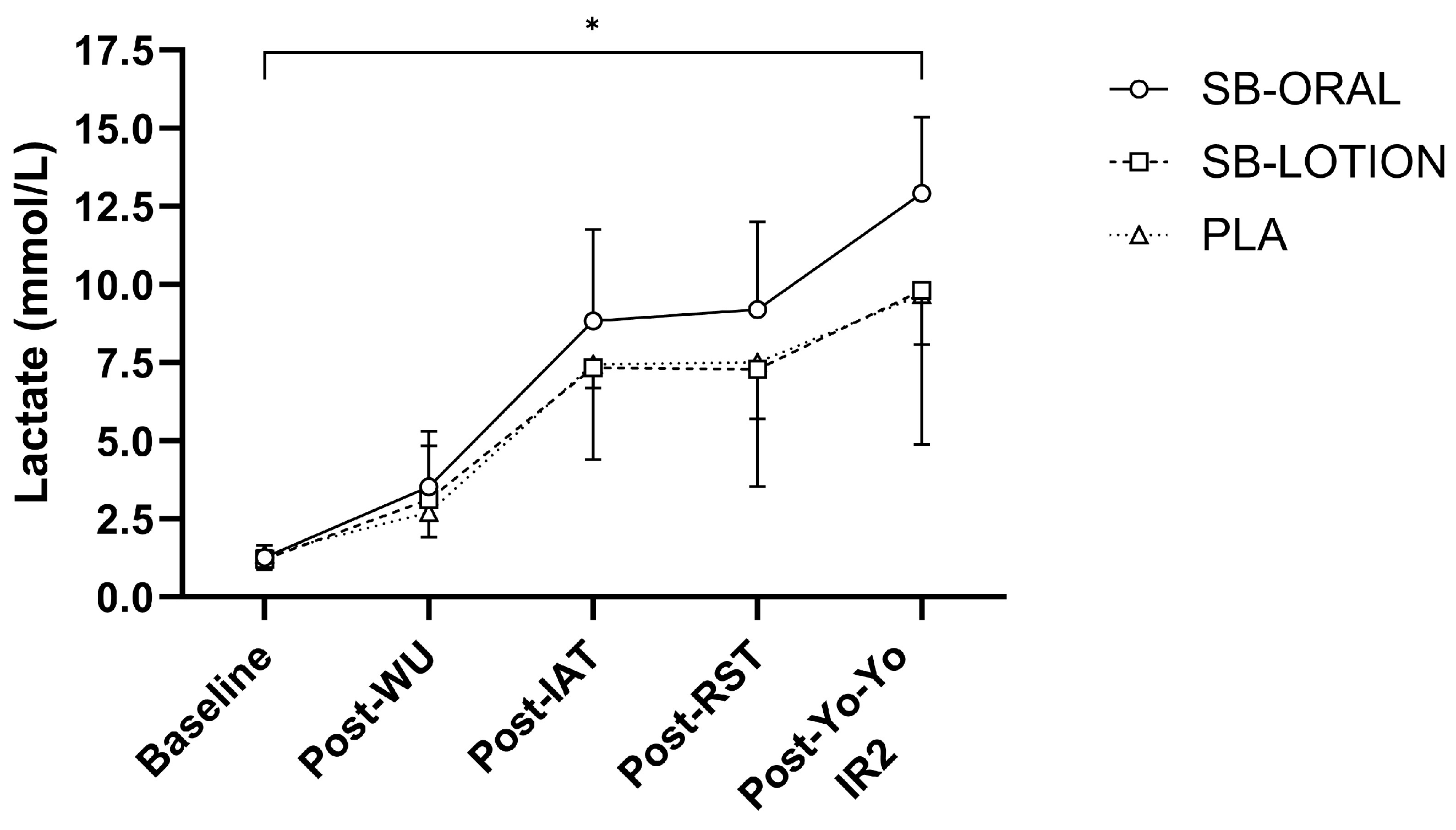
3.2. Repeated Sprint Muscle Damage Protocol
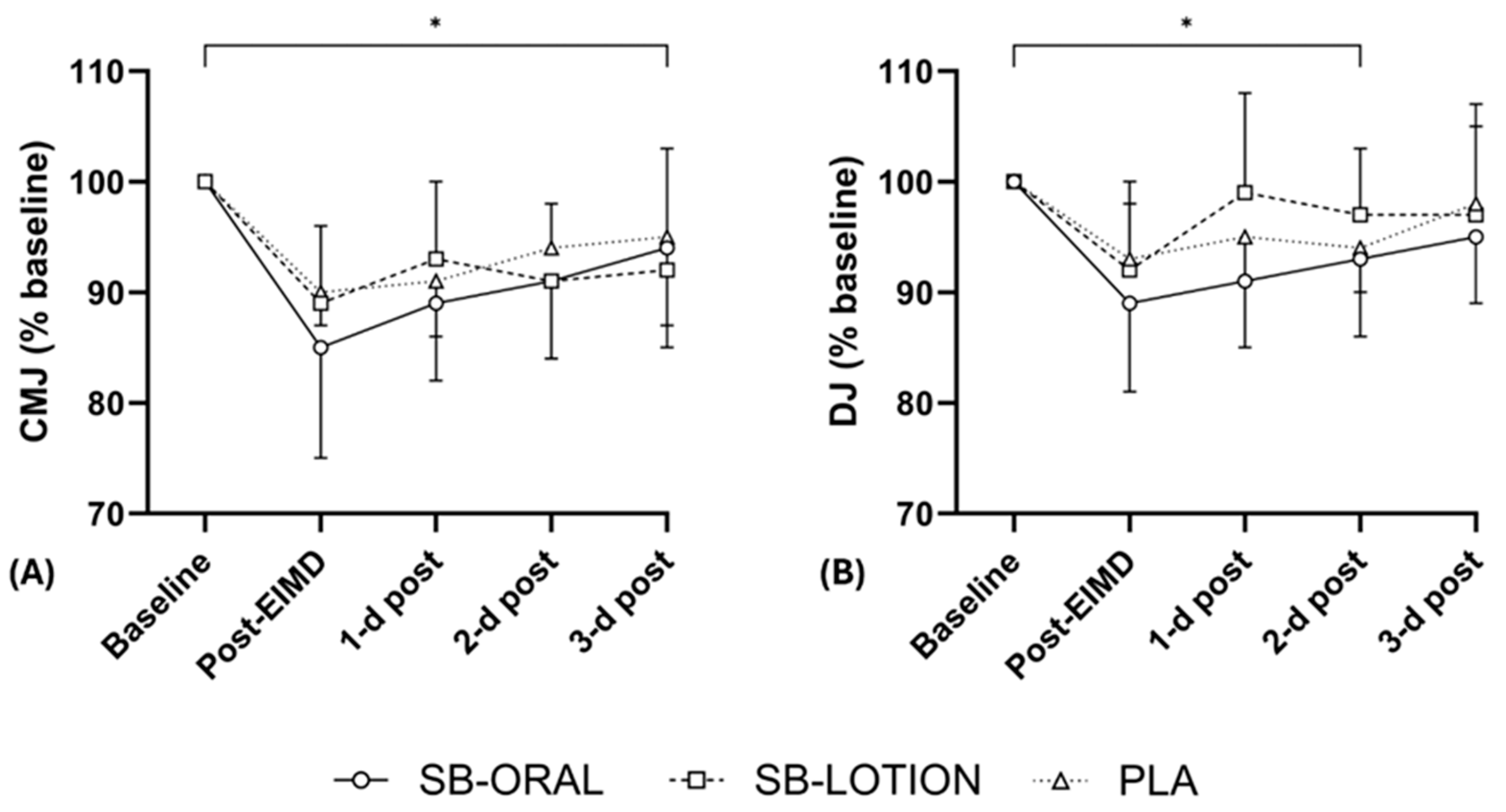
3.3. Vertical Jump Height
3.4. Maximal Voluntary Isometric Contraction
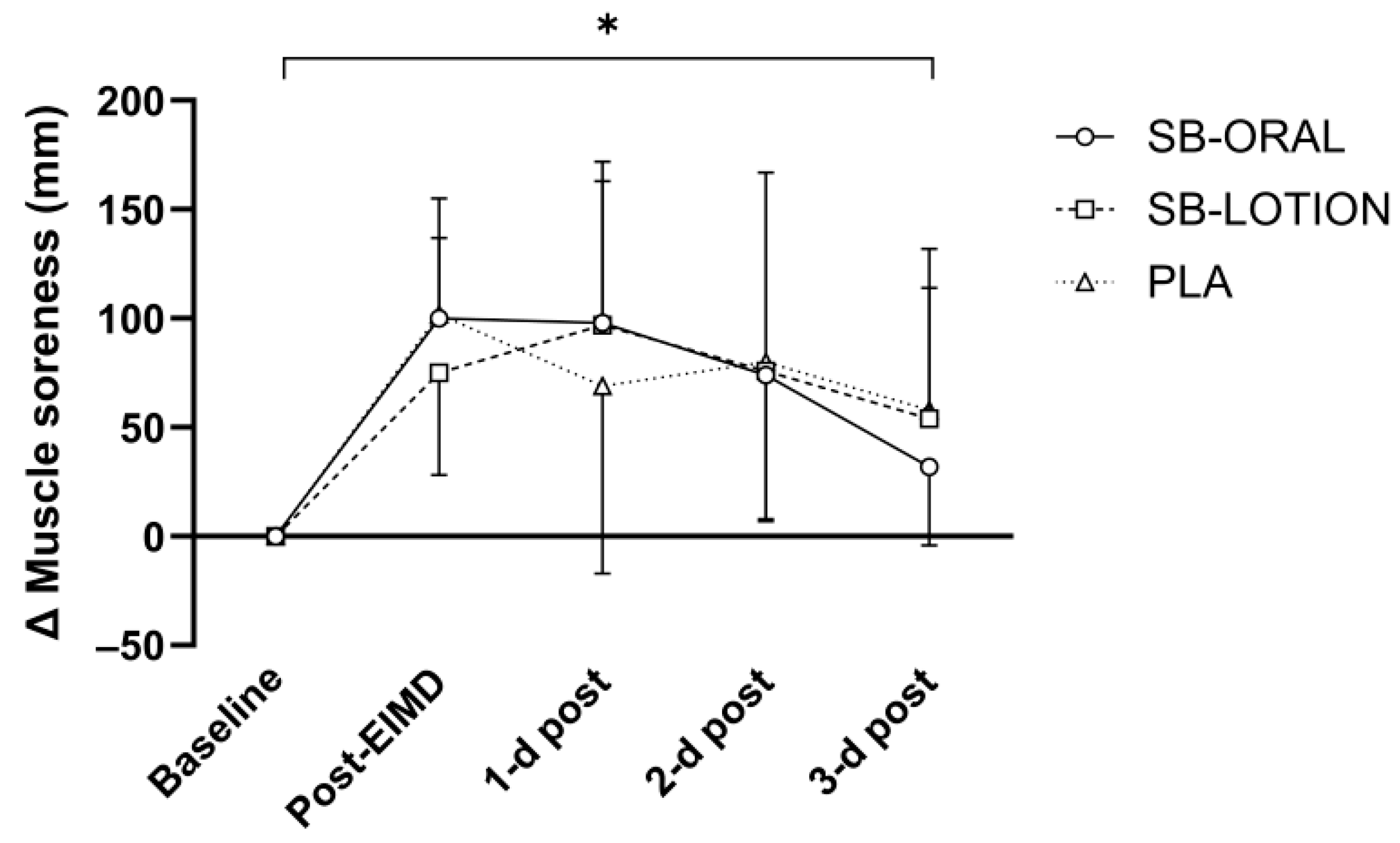
3.5. Muscle Soreness and Wellbeing Score
3.6. Soccer-Specific Performance
3.7. Acid-Base Balance and Lactate
3.8. Gastrointestinal Distress, Cooling Sensations, and Blinding
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BM | Body mass |
| CMJ | Countermovement jump |
| DJ | Drop jump |
| EIMD | Exercise-induced muscle damage |
| GI | Gastrointestinal |
| H+ | Hydrogen ion |
| HCO3− | Bicarbonate |
| HSP72 | Heat shock protein-72 |
| IAT | Illinois Agility Test |
| ICC | Intraclass correlation coefficient |
| MVIC | Maximal voluntary isometric contraction |
| PGC-1α | Peroxisome proliferator-activated receptor-γ coactivator 1-alpha |
| SB | Sodium bicarbonate |
| TB | Testing battery |
| TEM | Typical error of measurement |
| TRPM8 | Transient receptor potential melastatin-eight |
| Yo-Yo IR2 | Yo-Yo Intermittent Recovery Test Level 2 |
References
- Abbott, W.; Hansell, E.J.; Brett, A.; Škarabot, J.; James, L.J.; Clifford, T. Curcumin Attenuates Delayed-Onset Muscle Soreness and Muscle Function Deficits Following a Soccer Match in Male Professional Soccer Players. Int. J. Sports Physiol. Perform. 2023, 18, 347–353. [Google Scholar] [CrossRef]
- Bell, P.G.; Stevenson, E.; Davison, G.W.; Howatson, G. The Effects of Montmorency Tart Cherry Concentrate Supplementation on Recovery Following Prolonged, Intermittent Exercise. Nutrients 2016, 8, 441. [Google Scholar] [CrossRef]
- Daab, W.; Bouzid, M.A.; Lajri, M.; Bouchiba, M.; Saafi, M.A.; Rebai, H. Chronic Beetroot Juice Supplementation Accelerates Recovery Kinetics Following Simulated Match Play in Soccer Players. J. Am. Coll. Nutr. 2021, 40, 61–69. [Google Scholar] [CrossRef]
- Newham, D.J. The Consequences of Eccentric Contractions and Their Relationship to Delayed Onset Muscle Pain. Eur. J. Appl. Physiol. 1988, 57, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Bangsbo, J.; Iaia, F.M.; Krustrup, P. Metabolic Response and Fatigue in Soccer. Int. J. Sports Physiol. Perform. 2007, 2, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Krustrup, P.; Mohr, M.; Steensberg, A.; Bencke, J.; Kjaer, M.; Bangsbo, J. Muscle and Blood Metabolites during a Soccer Game: Implications for Sprint Performance. Med. Sci. Sports Exerc. 2006, 38, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Ispirlidis, I.; Fatouros, I.G.; Jamurtas, A.Z.; Nikolaidis, M.G.; Michailidis, I.; Douroudos, I.; Margonis, K.; Chatzinikolaou, A.; Kalistratos, E.; Katrabasas, I.; et al. Time-Course of Changes in Inflammatory and Performance Responses Following a Soccer Game. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2008, 18, 423–431. [Google Scholar] [CrossRef]
- Souglis, A.; Bogdanis, G.C.; Chryssanthopoulos, C.; Apostolidis, N.; Geladas, N.D. Time Course of Oxidative Stress, Inflammation, and Muscle Damage Markers for 5 Days After a Soccer Match: Effects of Sex and Playing Position. J. Strength Cond. Res. 2018, 32, 2045. [Google Scholar] [CrossRef]
- Dupont, G.; Nedelec, M.; McCall, A.; McCormack, D.; Berthoin, S.; Wisløff, U. Effect of 2 Soccer Matches in a Week on Physical Performance and Injury Rate. Am. J. Sports Med. 2010, 38, 1752–1758. [Google Scholar] [CrossRef]
- Hunt, J.E.A.; Coelho, M.O.C.; Buxton, S.; Butcher, R.; Foran, D.; Rowland, D.; Gurton, W.; Macrae, H.; Jones, L.; Gapper, K.S.; et al. Consumption of New Zealand Blackcurrant Extract Improves Recovery from Exercise-Induced Muscle Damage in Non-Resistance Trained Men and Women: A Double-Blind Randomised Trial. Nutrients 2021, 13, 2875. [Google Scholar] [CrossRef]
- Gurton, W.H.; King, D.G.; Ranchordas, M.K.; Siegler, J.C.; Gough, L.A. Enhancing Exercise Performance and Recovery through Sodium Bicarbonate Supplementation: Introducing the Ingestion Recovery Framework. Eur. J. Appl. Physiol. 2024, 124, 3175–3190. [Google Scholar] [CrossRef]
- Bishop, D.; Edge, J.; Davis, C.; Goodman, C. Induced Metabolic Alkalosis Affects Muscle Metabolism and Repeated-Sprint Ability. Med. Sci. Sports Exerc. 2004, 36, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Gurton, W.H.; Greally, J.; Chudzikiewicz, K.; Gough, L.A.; Lynn, A.; Ranchordas, M.K. Beneficial Effects of Oral and Topical Sodium Bicarbonate during a Battery of Team Sport-Specific Exercise Tests in Recreationally Trained Male Athletes. J. Int. Soc. Sports Nutr. 2023, 20, 2216678. [Google Scholar] [CrossRef] [PubMed]
- Gurton, W.H.; Gough, L.A.; Siegler, J.C.; Lynn, A.; Ranchordas, M.K. Oral but Not Topical Sodium Bicarbonate Improves Repeated Sprint Performance During Simulated Soccer Match Play Exercise in Collegiate Athletes. Int. J. Sport Nutr. Exerc. Metab. 2024, 34, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Krustrup, P.; Ermidis, G.; Mohr, M. Sodium Bicarbonate Intake Improves High-Intensity Intermittent Exercise Performance in Trained Young Men. J. Int. Soc. Sports Nutr. 2015, 12, 25. [Google Scholar] [CrossRef]
- Gough, L.A.; Brown, D.; Deb, S.K.; Sparks, S.A.; McNaughton, L.R. The Influence of Alkalosis on Repeated High-Intensity Exercise Performance and Acid–Base Balance Recovery in Acute Moderate Hypoxic Conditions. Eur. J. Appl. Physiol. 2018, 118, 2489–2498. [Google Scholar] [CrossRef]
- Gurton, W.; Macrae, H.; Gough, L.; King, D.G. Effects of Post-Exercise Sodium Bicarbonate Ingestion on Acid-Base Balance Recovery and Time-to-Exhaustion Running Performance: A Randomised Crossover Trial in Recreational Athletes. Appl. Physiol. Nutr. Metab. 2021, 46, 1111–1118. [Google Scholar] [CrossRef]
- Tee, J.C.; Bosch, A.N.; Lambert, M.I. Metabolic Consequences of Exercise-Induced Muscle Damage. Sports Med. 2007, 37, 827–836. [Google Scholar] [CrossRef]
- Peart, D.J.; McNaughton, L.R.; Midgley, A.W.; Taylor, L.; Towlson, C.; Madden, L.A.; Vince, R.V. Pre-Exercise Alkalosis Attenuates the Heat Shock Protein 72 Response to a Single-Bout of Anaerobic Exercise. J. Sci. Med. Sport 2011, 14, 435–440. [Google Scholar] [CrossRef]
- Peart, D.J.; Kirk, R.J.; Hillman, A.R.; Madden, L.A.; Siegler, J.C.; Vince, R.V. The Physiological Stress Response to High-Intensity Sprint Exercise Following the Ingestion of Sodium Bicarbonate. Eur. J. Appl. Physiol. 2013, 113, 127–134. [Google Scholar] [CrossRef]
- Peart, D.J.; Kirk, R.J.; Madden, L.A.; Siegler, J.C.; Vince, R.V. The Influence of Exogenous Carbohydrate Provision and Pre-Exercise Alkalosis on the Heat Shock Protein Response to Prolonged Interval Cycling. Amino Acids 2013, 44, 903–910. [Google Scholar] [CrossRef]
- Peart, D.J.; Kirk, R.J.; Madden, L.A.; Vince, R.V. Implications of a Pre-Exercise Alkalosis-Mediated Attenuation of HSP72 on Its Response to a Subsequent Bout of Exercise. Amino Acids 2016, 48, 499–504. [Google Scholar] [CrossRef]
- Percival, M.E.; Martin, B.J.; Gillen, J.B.; Skelly, L.E.; MacInnis, M.J.; Green, A.E.; Tarnopolsky, M.A.; Gibala, M.J. Sodium Bicarbonate Ingestion Augments the Increase in PGC-1α mRNA Expression during Recovery from Intense Interval Exercise in Human Skeletal Muscle. J. Appl. Physiol. 2015, 119, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S. PGC-1α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid. Med. Cell. Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef] [PubMed]
- Battazza, R.A.; Kalytczak, M.M.; Leite, C.D.F.C.; Rica, R.L.; Lamolha, M.A.; Junior, A.H.L.; Maia, A.F.; Bergamin, M.; Baker, J.S.; Politti, F.; et al. Effect of Sodium Bicarbonate Supplementation on Muscle Performance and Muscle Damage: A Double Blind, Randomized Crossover Study. J. Diet. Suppl. 2023, 20, 689–705. [Google Scholar] [CrossRef] [PubMed]
- Gillis, D.J.; Vellante, A.; Gallo, J.A.; D’Amico, A.P. Influence of Menthol on Recovery from Exercise-Induced Muscle Damage. J. Strength Cond. Res. 2020, 34, 451. [Google Scholar] [CrossRef]
- Hyldahl, R.D.; Keadle, J.; Rouzier, P.A.; Pearl, D.; Clarkson, P.M. Effects of Ibuprofen Topical Gel on Muscle Soreness. Med. Sci. Sports Exerc. 2010, 42, 614–621. [Google Scholar] [CrossRef]
- Johar, P.; Grover, V.; Topp, R.; Behm, D.G. A Comparison of Topical Menthol to Ice on Pain, Evoked Tetanic and Voluntary Force during Delayed Onset Muscle Soreness. Int. J. Sports Phys. Ther. 2012, 7, 314–322. [Google Scholar]
- Argoff, C.E. Topical Analgesics in the Management of Acute and Chronic Pain. Mayo Clin. Proc. 2013, 88, 195–205. [Google Scholar] [CrossRef]
- McKay, A.K.A.; Peeling, P.; Binnie, M.J.; Goods, P.S.R.; Sim, M.; Cross, R.; Siegler, J. Topical Sodium Bicarbonate: No Improvement in Blood Buffering Capacity or Exercise Performance. Int. J. Sports Physiol. Perform. 2020, 15, 1005–1011. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.M.; Needham, K.W.; Kaiser, B.W.; Wilkins, B.W.; Minson, C.T.; Halliwill, J.R. Transcutaneous Delivery of Sodium Bicarbonate Increases Intramuscular pH. Front. Physiol. 2023, 14, 1142567. [Google Scholar] [CrossRef] [PubMed]
- Durkalec-Michalski, K.; Nowaczyk, P.M.; Kamińska, J.; Saunders, B.; Łoniewski, I.; Czubaszek, D.; Steffl, M.; Podgórski, T. The Interplay between Bicarbonate Kinetics and Gastrointestinal Upset on Ergogenic Potential after Sodium Bicarbonate Intake: A Randomized Double-Blind Placebo-Controlled Trial. Sci. Rep. 2023, 13, 7081. [Google Scholar] [CrossRef] [PubMed]
- Ranchordas, M.K.; Dawson, J.T.; Russell, M. Practical Nutritional Recovery Strategies for Elite Soccer Players When Limited Time Separates Repeated Matches. J. Int. Soc. Sports Nutr. 2017, 14, 35. [Google Scholar] [CrossRef]
- Clifford, T.; Bell, O.; West, D.J.; Howatson, G.; Stevenson, E.J. The Effects of Beetroot Juice Supplementation on Indices of Muscle Damage Following Eccentric Exercise. Eur. J. Appl. Physiol. 2016, 116, 353–362. [Google Scholar] [CrossRef]
- Gathercole, R.J.; Sporer, B.C.; Stellingwerff, T.; Sleivert, G.G. Comparison of the Capacity of Different Jump and Sprint Field Tests to Detect Neuromuscular Fatigue. J. Strength Cond. Res. 2015, 29, 2522. [Google Scholar] [CrossRef]
- McKay, A.K.A.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining Training and Performance Caliber: A Participant Classification Framework. Int. J. Sports Physiol. Perform. 2022, 17, 317–331. [Google Scholar] [CrossRef]
- Durkalec–Michalski, K.; Zawieja, E.E.; Zawieja, B.E.; Michałowska, P.; Podgórski, T. The Gender Dependent Influence of Sodium Bicarbonate Supplementation on Anaerobic Power and Specific Performance in Female and Male Wrestlers. Sci. Rep. 2020, 10, 1878. [Google Scholar] [CrossRef]
- Reilly, T. Human Circadian Rhythms and Exercise. Crit. Rev. Biomed. Eng. 1990, 18, 165–180. [Google Scholar]
- Maughan, R.J.; Merson, S.J.; Broad, N.P.; Shirreffs, S.M. Fluid and Electrolyte Intake and Loss in Elite Soccer Players during Training. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 333–346. [Google Scholar] [CrossRef]
- Bangsbo, J.; Iaia, F.M.; Krustrup, P. The Yo-Yo Intermittent Recovery Test. Sports Med. 2008, 38, 37–51. [Google Scholar] [CrossRef]
- Hooper, S.L.; Mackinnon, L.T. Monitoring Overtraining in Athletes. Recommendations. Sports Med. 1995, 20, 321–327. [Google Scholar] [CrossRef]
- McLean, B.D.; Coutts, A.J.; Kelly, V.; McGuigan, M.R.; Cormack, S.J. Neuromuscular, Endocrine, and Perceptual Fatigue Responses during Different Length between-Match Microcycles in Professional Rugby League Players. Int. J. Sports Physiol. Perform. 2010, 5, 367–383. [Google Scholar] [CrossRef]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; Van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of Tart Cherry Juice on Indices of Recovery Following Marathon Running: Cherry Juice Supplementation and Marathon Running. Scand. J. Med. Sci. Sports 2010, 20, 843–852. [Google Scholar] [CrossRef]
- D’Amico, A.P.; Gillis, J. Influence of Foam Rolling on Recovery from Exercise-Induced Muscle Damage. J. Strength Cond. Res. 2019, 33, 2443. [Google Scholar] [CrossRef]
- Gurton, W.H.; Matta, G.G.; Gough, L.A.; Hurst, P. Efficacy of Sodium Bicarbonate Ingestion Strategies for Protecting Blinding. Eur. J. Appl. Physiol. 2022, 122, 2555–2563. [Google Scholar] [CrossRef] [PubMed]
- Gurton, W.H.; Dabin, L.; Marshall, S. No Effect of Individualized Sodium Bicarbonate Supplementation on 200-m or 400-m Freestyle-Swimming Time-Trial Performance in Well-Trained Athletes. Int. J. Sports Physiol. Perform. 2025, 20, 224–231. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; ISBN 978-0-203-77158-7. [Google Scholar]
- Lakens, D. Calculating and Reporting Effect Sizes to Facilitate Cumulative Science: A Practical Primer for t-Tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Delfour-Peyrethon, R.; Lambert, K.; Granata, C.; Hobbs, T.; Hanon, C.; Bishop, D.J. The Effect of Pre-Exercise Alkalosis on Lactate/pH Regulation and Mitochondrial Respiration Following Sprint-Interval Exercise in Humans. Front. Physiol. 2023, 14, 1073407. [Google Scholar] [CrossRef]
- Schweiker, M.; Huebner, G.M.; Kingma, B.R.M.; Kramer, R.; Pallubinsky, H. Drivers of Diversity in Human Thermal Perception—A Review for Holistic Comfort Models. Temp. Multidiscip. Biomed. J. 2018, 5, 308–342. [Google Scholar] [CrossRef]
- Simoneau, J.A.; Bouchard, C. Human Variation in Skeletal Muscle Fiber-Type Proportion and Enzyme Activities. Am. J. Physiol.-Endocrinol. Metab. 1989, 257, E567–E572. [Google Scholar] [CrossRef] [PubMed]
- Macutkiewicz, D.; Sunderland, C. Sodium Bicarbonate Supplementation Does Not Improve Elite Women’s Team Sport Running or Field Hockey Skill Performance. Physiol. Rep. 2018, 6, e13818. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.L.; McLay-Cooke, R.T.; Brown, R.C.; Gray, A.R.; Fairbairn, K.A. Increased Blood pH but Not Performance with Sodium Bicarbonate Supplementation in Elite Rugby Union Players. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.; Slater, G.; Gore, C.; Dawson, B.; Burke, L. Effect of Sodium Bicarbonate on [HCO3−], pH, and Gastrointestinal Symptoms. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 189–194. [Google Scholar] [CrossRef]
- Durkalec-Michalski, K.; Nowaczyk, P.M.; Adrian, J.; Kamińska, J.; Podgórski, T. The Influence of Progressive-Chronic and Acute Sodium Bicarbonate Supplementation on Anaerobic Power and Specific Performance in Team Sports: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Nutr. Metab. 2020, 17, 38. [Google Scholar] [CrossRef]
- Gough, L.A.; Sparks, S.A. The Effects of a Carbohydrate Hydrogel System for the Delivery of Bicarbonate Mini-Tablets on Acid–Base Buffering and Gastrointestinal Symptoms in Resting Well-Trained Male Cyclists. Sports Med.-Open 2024, 10, 17. [Google Scholar] [CrossRef]
- Gurton, W.H.; Matta, G.G.; Gough, L.A.; Ranchordas, M.K.; King, D.G.; Hurst, P. Sodium Bicarbonate and Time-to-Exhaustion Cycling Performance: A Retrospective Analysis Exploring the Mediating Role of Expectation. Sports Med.-Open 2023, 9, 65. [Google Scholar] [CrossRef]

| Variable | SB-ORAL | SB-LOTION | PLA | p Value |
|---|---|---|---|---|
| Sex (M/F) | 5 M, 3 F | 5 M, 3 F | 6 M, 2 F | - |
| Body mass (kg) | 76.8 ± 12.5 | 73.6 ± 7.0 | 81.4 ± 11.9 | 0.359 |
| Stature (cm) | 176.9 ± 11.1 | 176.1 ± 8.8 | 181.3 ± 8.3 | 0.505 |
| Age (y) | 21.1 ± 2.2 | 23.2 ± 2.6 | 22.0 ± 4.0 | 0.395 |
| Variable | SB-ORAL | SB-LOTION | PLA | p Value |
|---|---|---|---|---|
| Soreness (mm) | 49 ± 36 | 36 ± 21 | 36 ± 31 | 0.644 |
| CMJ height (cm) | 33.8 ± 6.9 | 34.1 ± 8.0 | 33.7 ± 5.2 | 0.993 |
| DJ height (cm) | 31.2 ± 4.6 | 32.6 ± 6.1 | 32.6 ± 5.9 | 0.843 |
| MVIC (nm) | 196 ± 74 | 183 ± 49 | 212 ± 51 | 0.634 |
| IAT (s) | 17.05 ± 0.92 | 17.18 ± 1.13 | 17.13 ± 1.02 | 0.967 |
| 8 × 25 m sprints (s) | 4.04 ± 0.35 | 4.04 ± 0.26 | 4.04 ± 0.16 | 0.999 |
| Yo-Yo IR2 (m) | 425 ± 254 | 435 ± 220 | 425 ± 181 | 0.995 |
| Variable | SB-ORAL | SB-LOTION | PLA | p Value |
|---|---|---|---|---|
| 40 × 15 m sprints | ||||
| Average (s) | 2.80 ± 0.18 | 2.81 ± 0.24 | 2.85 ± 0.12 | 0.872 |
| Fastest (s) | 2.65 ± 0.18 | 2.68 ± 0.19 | 2.70 ± 0.15 | 0.885 |
| Total (s) | 112.07 ± 7.43 | 112.73 ± 9.44 | 114.11 ± 4.72 | 0.857 |
| Decrement (%) | 5.6 ± 1.8 | 5.1 ± 2.2 | 5.9 ± 3.2 | 0.764 |
| Variable | Pre-EIMD | Post-EIMD | 1 d Post | 2 d Post | 3 d Post | Pre- TB | Post-TB |
|---|---|---|---|---|---|---|---|
| pH (au) | |||||||
| SB-ORAL | 7.41 ± 0.02 | 7.38 ± 0.05 | 7.41 ± 0.01 * | 7.41 ± 0.02 | 7.40 ± 0.02 | 7.46 ± 0.03 ** | 7.32 ± 0.05 * |
| SB-LOTION | 7.40 ± 0.03 | 7.39 ± 0.02 | 7.39 ± 0.02 | 7.40 ± 0.03 | 7.39 ± 0.03 | 7.40 ± 0.01 | 7.31 ± 0.03 * |
| PLA | 7.41 ± 0.02 | 7.37 ± 0.04 | 7.39 ± 0.01 | 7.40 ± 0.02 | 7.40 ± 0.02 | 7.41 ± 0.02 | 7.25 ± 0.07 |
| HCO3− (mmol·L−1) | |||||||
| SB-ORAL | 24.1 ± 1.3 | 20.4 ± 3.6 | 24.5 ± 1.8 | 23.6 ± 1.6 | 23.8 ± 2.2 | 28.7 ± 2.7 ** | 16.9 ± 2.4 |
| SB-LOTION | 24.2 ± 1.9 | 20.9 ± 2.1 | 24.1 ± 1.0 | 24.4 ± 2.2 | 23.9 ± 1.4 | 25.5 ± 1.9 | 17.3 ± 2.6 * |
| PLA | 24.3 ± 1.3 | 20.5 ± 2.0 | 24.0 ± 1.7 | 24.0 ± 1.7 | 23.7 ± 1.1 | 23.8 ± 1.8 | 14.0 ± 2.7 |
| Variable | Post- EIMD | Post- Supp-1 | Pre- Supp-4 | Post- Supp-4 | Pre-TB | Post-TB |
|---|---|---|---|---|---|---|
| GI (mm) | ||||||
| SB-ORAL | - | - | 23 ± 25 | 24 ± 23 | 20 ± 17 | 29 ± 28 |
| SB-LOTION | - | - | 11 ± 14 | 19 ± 33 | 14 ± 24 | 22 ± 25 |
| PLA | - | - | 19 ± 17 | 21 ± 17 | 22 ± 11 | 25 ± 23 |
| Cooling (au) | ||||||
| SB-ORAL | 1 ± 1 | −2 ± 2 | 0 ± 1 | −2 ± 2 | 0 ± 1 | 2 ± 2 |
| SB-LOTION | 1 ± 1 | −2 ± 2 | 0 ± 2 | −2 ± 3 | −1 ± 2 | 2 ± 2 |
| PLA | 1 ± 1 | −2 ± 2 | 0 ± 1 | −2 ± 1 | −1 ± 2 | 3 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurton, W.H.; Gough, L.A.; Lynn, A.; Ranchordas, M.K. Effect of Oral and Topical Sodium Bicarbonate on Functional Recovery and Soccer-Specific Performance After Exercise-Induced Muscle Damage: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2025, 17, 3383. https://doi.org/10.3390/nu17213383
Gurton WH, Gough LA, Lynn A, Ranchordas MK. Effect of Oral and Topical Sodium Bicarbonate on Functional Recovery and Soccer-Specific Performance After Exercise-Induced Muscle Damage: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2025; 17(21):3383. https://doi.org/10.3390/nu17213383
Chicago/Turabian StyleGurton, William H., Lewis A. Gough, Anthony Lynn, and Mayur K. Ranchordas. 2025. "Effect of Oral and Topical Sodium Bicarbonate on Functional Recovery and Soccer-Specific Performance After Exercise-Induced Muscle Damage: A Randomized, Double-Blind, Placebo-Controlled Study" Nutrients 17, no. 21: 3383. https://doi.org/10.3390/nu17213383
APA StyleGurton, W. H., Gough, L. A., Lynn, A., & Ranchordas, M. K. (2025). Effect of Oral and Topical Sodium Bicarbonate on Functional Recovery and Soccer-Specific Performance After Exercise-Induced Muscle Damage: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients, 17(21), 3383. https://doi.org/10.3390/nu17213383






