Effects of Donor Human Milk and Formula Supplementation on Bone Metabolism and Clinical Outcomes in Preterm Infants Receiving Mother’s Own Milk
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Data Collection
2.3. Statistical Analysis
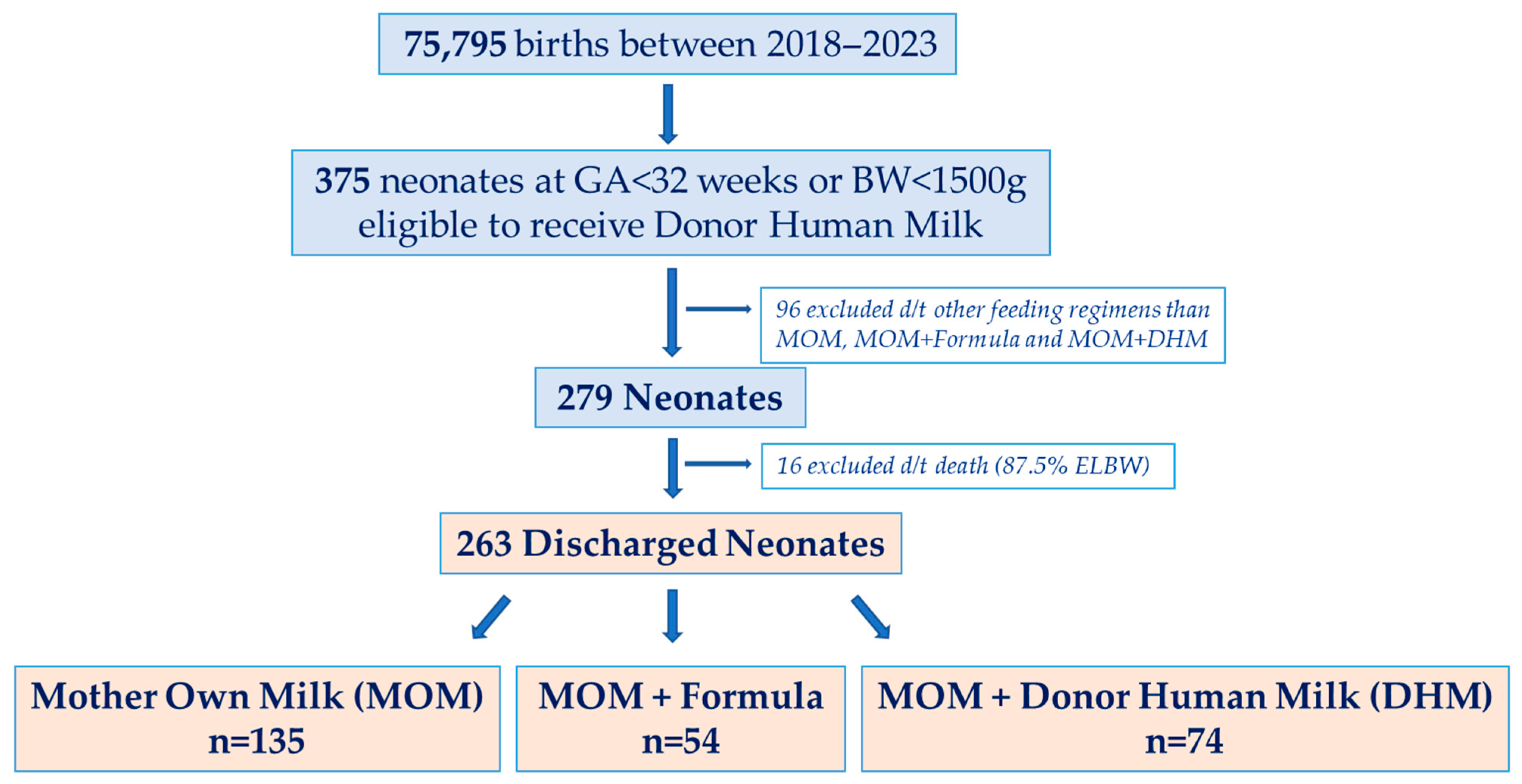
3. Results
3.1. Study Population
3.2. Demographic and Feeding Characteristics
3.3. Clinical Outcomes
3.4. Predictors of Growth and Clinical Outcomes
3.5. Bone Metabolism Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Section on Breastfeeding; Eidelman, A.I.; Schanler, R.J.; Johnston, M.; Landers, S.; Noble, L.; Szucs, K.; Viehmann, L. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A. Human milk for the premature infant. Pediatr. Clin. N. Am. 2013, 60, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Lapidaire, W.; Lucas, A.; Clayden, J.D.; Clark, C.; Fewtrell, M.S. Human milk feeding and cognitive outcome in preterm infants: The role of infection and NEC reduction. Pediatr. Res. 2022, 91, 1207–1214. [Google Scholar] [CrossRef]
- Moro, G.E.; Arslanoglu, S. Editorial: Human Milk in the Feeding of Preterm Infants: Established and Debated Aspects. Front. Pediatr. 2020, 8, 378. [Google Scholar] [CrossRef]
- Embleton, N.D.M.; Jennifer Moltu, S.; Lapillonne, A.; van den Akker, C.H.; Carnielli, V.; Fusch, C.; Gerasimidis, K.; van Goudoever, J.B.; Haiden, N.M.; Iacobelli, S.; et al. Enteral Nutrition in Preterm Infants (2022): A Position Paper from the ESPGHAN Committee on Nutrition and Invited Experts. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 248–268. [Google Scholar] [CrossRef]
- Arslanoglu, S.; Boquien, C.-Y.; King, C.; Lamireau, D.; Tonetto, P.; Barnett, D.; Bertino, E.; Gaya, A.; Gebauer, C.; Grovslien, A.; et al. Fortification of Human Milk for Preterm Infants: Update and Recommendations of the European Milk Bank Association (EMBA) Working Group on Human Milk Fortification. Front. Pediatr. 2019, 7, 76. [Google Scholar] [CrossRef]
- Kavurt, S.; Demirel, N.; Yücel, H.; Unal, S.; Yıldız, Y.T.; Bas, A.Y. Evaluation of radiologic evidence of metabolic bone disease in very low birth weight infants at fourth week of life. J. Perinatol. 2021, 41, 2668–2673. [Google Scholar] [CrossRef]
- Yang, J.; Tang, Q.; Zhou, P. Narrative review of methodological advances in human milk fortification: For better preterm infant growth. Front. Pediatr. 2024, 12, 1466528. [Google Scholar] [CrossRef]
- Silveira, R.C.; Procianoy, R.S. Effects of Early Nutrition on Premature Infants. Nutrients 2025, 17, 1648. [Google Scholar] [CrossRef]
- De Curtis, M.; Rigo, J. The nutrition of preterm infants. Early Hum. Dev. 2012, 88 (Suppl. S1), S5–S7. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, M.; Rumbold, A.R.; Miller, J.; Chong, Y.F.; Collins, C.T. A Systematic Review and Meta-Analysis of Human Milk Feeding and Short-Term Growth in Preterm and Very Low Birth Weight Infants. Nutrients 2021, 13, 2089. [Google Scholar] [CrossRef]
- Gates, A.; Hair, A.B.; Salas, A.A.; Thompson, A.B.; Stansfield, B.K. Nutrient Composition of Donor Human Milk and Comparisons to Preterm Human Milk. J. Nutr. 2023, 153, 2622–2630. [Google Scholar] [CrossRef]
- Parker, L.A.; Koernere, R.; Fordham, K.; Bubshait, H.; Eugene, A.; Gefre, A.; Bendixen, M. Mother’s Own Milk Versus Donor Human Milk: What’s the Difference? Crit. Care Nurs. Clin. N. Am. 2024, 36, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.S.; Harrison, C.M. Formula feeding results in better growth and weight gain compared to donor breast milk in preterm and low birthweight infants, with a greater risk in necrotising enterocolitis. Arch. Dis. Child.-Educ. Pract. 2020, 105, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Muts, J.; van Keulen, B.J.; van Goudoever, J.B.; van den Akker, C.H. Formula protein versus human milk protein and the effects on growth in preterm born infants. Curr. Opin. Clin. Nutr. Metab. Care 2025, 28, 33–38. [Google Scholar] [CrossRef]
- Quigley, M.; Embleton, N.D.; Meader, N.; McGuire, W. Donor human milk for preventing necrotising enterocolitis in very preterm or very low-birthweight infants. Cochrane Database Syst. Rev. 2024, CD002971. [Google Scholar] [CrossRef] [PubMed]
- Neu, J. Necrotizing enterocolitis. World Rev. Nutr. Diet. 2014, 110, 253–263. [Google Scholar] [CrossRef]
- Faienza, M.F.; D’AMato, E.; Natale, M.P.; Grano, M.; Chiarito, M.; Brunetti, G.; D’AMato, G. Metabolic Bone Disease of Prematurity: Diagnosis and Management. Front. Pediatr. 2019, 7, 143. [Google Scholar] [CrossRef]
- Faerk, J.; Petersen, S.; Peitersen, B.; Michaelsen, K.F. Diet and bone mineral content at term in premature infants. Pediatr. Res. 2000, 47, 148–156. [Google Scholar] [CrossRef]
- Mimouni, F.B.; Mandel, D.; Lubetzky, R.; Senterre, T. Calcium, phosphorus, magnesium and vitamin D requirements of the preterm infant. World Rev. Nutr. Diet. 2014, 110, 140–151. [Google Scholar] [CrossRef]
- Czech-Kowalska, J. Mineral and nutritional requirements of preterm infant. Semin. Fetal Neonatal Med. 2020, 25, 101071. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.T.; Chatzixiros, E.; Grummer-Strawn, L.; Engmann, C.; Israel-Ballard, K.; Mansen, K.; O’COnnor, D.; Unger, S.; Herson, M.; Weaver, G.; et al. Developing global guidance on human milk banking. Bull. World Health Organ. 2021, 99, 892–900. [Google Scholar] [CrossRef]
- Dollberg, S.; Haklai, Z.; Mimouni, F.B.; Gorfein, I.; Gordon, E.-S. Birth weight standards in the live-born population in Israel. Isr. Med. Assoc. J. 2005, 7, 311–314. [Google Scholar]
- Zhang, H.; Jia, Q.; Piao, M.; Chang, Y.; Zhang, J.; Tong, X.; Han, T. Screening of Serum Alkaline Phosphatase and Phosphate Helps Early Detection of Metabolic Bone Disease in Extremely Low Birth Weight Infants. Front. Pediatr. 2021, 9, 642158. [Google Scholar] [CrossRef] [PubMed]
- Chacham, S.; Pasi, R.; Chegondi, M.; Ahmad, N.; Mohanty, S.B. Metabolic Bone Disease in Premature Neonates: An Unmet Challenge. J. Clin. Res. Pediatr. Endocrinol. 2020, 12, 332–339. [Google Scholar] [CrossRef]
- Manuck, T.A.; Rice, M.M.; Bailit, J.L.; Grobman, W.A.; Reddy, U.M.; Wapner, R.J.; Thorp, J.M.; Caritis, S.N.; Prasad, M.; Tita, A.T.; et al. Preterm neonatal morbidity and mortality by gestational age: A contemporary cohort. Am. J. Obstet. Gynecol. 2016, 215, e1–e103. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.M.; Grandi, S.M.; Pullenayegum, E.; McDonald, S.D.; Beltempo, M.; Premji, S.S.; Pole, J.D.; Bacchini, F.; Shah, P.S.; Pechlivanoglou, P. Short-Term and Long-Term Mortality Risk After Preterm Birth. JAMA Netw. Open 2024, 7, e2445871. [Google Scholar] [CrossRef]
- Morkuniene, R.; Levuliene, R.; Gegzna, V.; Jakimaviciene, E.M.; Tutkuviene, J. Surviving prematurity: Retrospective longitudinal study of multisystem consequences in preterm-born individuals from infancy to adolescence. BMC Pediatr. 2025, 25, 46. [Google Scholar] [CrossRef]
- Körnmann, M.N.; Christmann, V.; Gradussen, C.J.W.; Rodwell, L.; Gotthardt, M.; Van Goudoever, J.B.; Van Heijst, A.F.J. Growth and Bone Mineralization of Very Preterm Infants at Term Corrected Age in Relation to Different Nutritional Intakes in the Early Postnatal Period. Nutrients 2017, 9, 1318. [Google Scholar] [CrossRef]
- Underwood, M.A. The fifty billion dollar question: Does formula cause necrotizing enterocolitis? J. Perinatol. 2025, 45, 565–571. [Google Scholar] [CrossRef]
- Report of Necrotizing Enterocolitis (NEC) in Preterm Infants Working Group of the National Advisory Council of Child Health and Human Development (NACHHD). Available online: https://dpcpsi.nih.gov/oepr/infoquality/report-necrotizing-enterocolitis-nec-preterm-infants-working-group-national (accessed on 9 July 2025).
- FDA, CDC, NIH: Evidence Points to Lack of Human Milk, Not Specialty Formulas, as Increasing Risk of NEC. Available online: https://publications.aap.org/aapnews/news/30429/FDA-CDC-NIH-Evidence-points-to-lack-of-human-milk (accessed on 9 July 2025).
| Characteristics | MOM N = 135 | MOM + Formula N = 54 | MOM + DHM N = 74 | p-Value |
|---|---|---|---|---|
| Mode of delivery | 0.188 | |||
| Spontaneous vaginal delivery | 40 (29.6) | 19 (35.2) | 30 (40.5) | |
| Emergency Cesarean section | 94 (69.6) | 33 (61.1) | 42 (56.8) | |
| Elective Cesarean section | 1 (0.7) | 2 (3.7) | 2 (2.7) | |
| Infant of diabetic mothers | 10 (7.4) | 8 (14.8) | 11 (14.9) | 0.157 |
| Celestone treatment | 99 (73.3) | 42 (77.8) | 61 (82.4) | 0.323 |
| Intrauterine growth restriction (IUGR) | 24 (17.8) | 14 (25.9) | 8 (11) | 0.090 |
| Gestational age (GA) (week) | 28 [27–30] | 30 [29–31] | 30 [28–31] | <0.001 |
| Birth weight (BW) (g) | 1070 [825–1320] | 1450 [1157.5–1612.5] | 1362.5 [1073.8–1486.3] | <0.001 |
| Small for Gestational Age | 27 (20) | 15 (27.8) | 12 (16.2) | 0.272 |
| Gender (Female) | 75 (55.6) | 37 (68.5) | 43 (58.1) | 0.258 |
| Day of initiating enteral feeding (day) | 2 [1–2] | 2 [1–2] | 2 [1–2] | 0.058 |
| Day at reaching BW | 10 [7–13.3] | 9 [6–11] | 9 [7–12] | 0.260 |
| Duration of parenteral nutrition (day) | 15 [10–29] | 9 [4.5–10.5] | 9 [6–13] | <0.001 |
| Day of reaching full enteral feeding (day) | 15 [11–28] | 10 [6–12] | 9 [6.5–13] | <0.001 |
| Length of hospital stay (day) | 68 [45–95] | 39 [31–46.3] | 43 [37–74.5] | <0.001 |
| Feeding Intolerance | 41 (30.4) | 9 (16.7) | 14 (18.9) | 0.062 |
| Surfactant use | 74 (54.8) | 17 (31.5) | 26 (35.1) | 0.002 |
| Necrotizing Enterocolitis | 25 (18.5) | 3 (5.6) | 9 (12.2) | 0.059 |
| Respiratory Distress Syndrome | 126 (93.3) | 38 (70.4) | 55 (74.3) | <0.001 |
| Bronchopulmonary Dysplasia | 87 (64.4) | 8 (14.8) | 26 (35.1) | <0.001 |
| Blood Stream Infection | 27 (20) | 3 (5.6) | 6 (8.1) | 0.009 |
| Retinopathy of Prematurity | 15 (11.1) | 2 (3.7) | 8 (10.8) | 0.264 |
| Hypoglycemia | 27 (20) | 10 (18.5) | 16 (21.6) | 0.909 |
| Percentage of MOM (%) | 100 | 79.5 * [64.4–90] | 90 * [79–93] | 0.013 * |
| Characteristics | MOM N = 135 | MOM + Formula N = 54 | MOM + DHM N = 74 | p-Value |
|---|---|---|---|---|
| Alkaline Phosphatase (ALKP, IU/L) first measurement | 189 [156–235] | 191 [162–254] | 216.5 [170.8–276.8] | 0.056 |
| ALKP (IU/L) on day 7 | 261 * [216.5–337] | 277 [205–351] | 303 * [256–378] | 0.024 * |
| ALKP > 500 IU/L on day 7 | 11 (8.3) | 2 (3.8) | 3 (4.2) | 0.482 |
| ALKP (IU/L) on day 14 | 397 * [300.8–506] | 343 * [269.5–413] | 350.5 [262.3–457.0] | 0.005 * |
| ALKP > 500 IU/L on day 14 | 35 (26.9) | 3 (5.7) | 7 (9.7) | <0.001 |
| ALKP (IU/L) on day 28 | 354.5 [285–473.8] | 386.5 [279.8–457.0] | 349 [289.5–420.0] | 0.609 |
| ALKP > 500 IU/L on day 28 | 23 (18) | 8 (17.4) | 7 (10.1) | 0.331 |
| Phosphorus (mg/dL) first measurement | 5.8 [4.9–6.6] | 5.8 [4.9–6.5] | 5.5 [4.8–6.4] | 0.796 |
| Phosphorus (mg/dL) on day 7 | 5.7 [4.8–6.6] | 6 [5.4–6.9] | 5.8 [5.0–6.7] | 0.145 |
| Phosphorus < 4.8 mg/dL on day 7 | 32 (24.2) | 5 (10) | 13 (18.8) | 0.096 |
| Phosphorus (mg/dL) on day 14 | 6.1 ± 1.1 (3.0–8.4) | 6.8 ± 1 (4.9–8.9) | 6.6 ± 1.1 (3.3–8.8) | <0.001 |
| Phosphorus < 4.8 mg/dL on day 14 | 12 (9.7) | 0 (0) | 4 (6.1) | 0.046 |
| Phosphorus (mg/dL) on day 28 | 6.4 ± 0.8 (4.2–8.0) | 6.6 ± 0.6 (5.5–8.1) | 6.6 ± 0.8 (4.4–8.1) | 0.218 |
| Phosphorus < 4.8 mg/dL on day 28 | 5 (4.0) | 0 | 1 (1.5) | 0.341 |
| Calcium (mg/dL) first measurement | 8.7 ± 0.8 (6.9–10.4) | 9.0 ± 0.8 (7.8–11.1) | 9.1 ± 0.9 (6.7–11.6) | <0.001 |
| Calcium (mg/dL) on day 7 | 9.9 [9.3–10.3] | 10.3 [9.8–10.7] | 10.3 [9.8–10.7] | <0.001 |
| Calcium < 7 mg/dL on day 7 | 0 | 0 | 0 | NA |
| Calcium (mg/dL) on day 14 | 10.0 [9.7–10.5] | 10.4 [10.1–10.7] | 10.4 [10.0–10.8] | <0.001 |
| Calcium < 7 mg/dL on day 14 | 0 | 0 | 1 (1.5) | 0.483 |
| Calcium (mg/dL) on day 28 | 10.2 * [9.8–10.5] | 10.2 [9.9–10.5] | 10.4 * [10.0–10.8] | 0.033 * |
| Calcium < 7 mg/dL on day 28 | 0 | 0 | 0 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herzlich, J.; Frumer, B.; Mandel, D.; Morag, S.; Halperin, A.; Mangel, L. Effects of Donor Human Milk and Formula Supplementation on Bone Metabolism and Clinical Outcomes in Preterm Infants Receiving Mother’s Own Milk. Nutrients 2025, 17, 3263. https://doi.org/10.3390/nu17203263
Herzlich J, Frumer B, Mandel D, Morag S, Halperin A, Mangel L. Effects of Donor Human Milk and Formula Supplementation on Bone Metabolism and Clinical Outcomes in Preterm Infants Receiving Mother’s Own Milk. Nutrients. 2025; 17(20):3263. https://doi.org/10.3390/nu17203263
Chicago/Turabian StyleHerzlich, Jacky, Bar Frumer, Dror Mandel, Sharon Morag, Ariel Halperin, and Laurence Mangel. 2025. "Effects of Donor Human Milk and Formula Supplementation on Bone Metabolism and Clinical Outcomes in Preterm Infants Receiving Mother’s Own Milk" Nutrients 17, no. 20: 3263. https://doi.org/10.3390/nu17203263
APA StyleHerzlich, J., Frumer, B., Mandel, D., Morag, S., Halperin, A., & Mangel, L. (2025). Effects of Donor Human Milk and Formula Supplementation on Bone Metabolism and Clinical Outcomes in Preterm Infants Receiving Mother’s Own Milk. Nutrients, 17(20), 3263. https://doi.org/10.3390/nu17203263






