Gestational Diabetes Mellitus Subtypes Derived by Clustering Analysis Show Heterogeneity in Glucometabolic Parameters Already at Early Pregnancy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Laboratory Methods and Calculations
2.3. Classification of GDM Subgroups and Related Web Application
2.4. Statistical Analysis
2.5. Sample Size Justification
3. Results
3.1. Characteristics of the Study Cohort
3.2. Metabolic Assessments
3.3. Glucose-Lowering Medication and Pregnancy Outcomes
3.4. Comparison with a Classification Based on Isolated and Combined Fasting and Post-Prandial Hyperglycemia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| BMI | body mass index |
| BMIPG | pregestational body mass index |
| CL | clusters |
| DIORAL | oral disposition index |
| FCP | fasting C-peptide |
| FFQ | food frequency questionnaire |
| FI | fasting insulin |
| FPG | fasting plasma glucose |
| GDM | gestational diabetes mellitus |
| GWAS | genome-wide association study |
| HbA1c | glycated hemoglobin A1c |
| IGIF | insulinogenic index at fasting |
| IQR | interquartile range |
| LGA | large for gestational age |
| NGT | normal glucose tolerant |
| OGTT | oral glucose tolerance test |
| OR | odds ratios |
| QUICKI | quantitative insulin sensitivity check index |
| TyGIS | triglyceride-glucose insulin sensitivity index |
| T2D | type 2 diabetes |
References
- Powe, C.E.; Allard, C.; Battista, M.-C.; Doyon, M.; Bouchard, L.; Ecker, J.L.; Perron, P.; Florez, J.C.; Thadhani, R.; Hivert, M.-F. Heterogeneous Contribution of Insulin Sensitivity and Secretion Defects to Gestational Diabetes Mellitus. Diabetes Care 2016, 39, 1052–1055. [Google Scholar] [CrossRef]
- Powe, C.E.; Hivert, M.-F.; Udler, M.S. Defining Heterogeneity Among Women with Gestational Diabetes Mellitus. Diabetes 2020, 69, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Skyler, J.S. Non-insulin-dependent diabetes mellitus: A clinical strategy. Diabetes Care 1984, 7 (Suppl. 1), 118–129. [Google Scholar] [PubMed]
- Mahajan, A.; Taliun, D.; Thurner, M.; Robertson, N.R.; Torres, J.M.; Rayner, N.W.; Payne, A.J.; Steinthorsdottir, V.; Scott, R.A.; Grarup, N.; et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 2018, 50, 1505–1513. [Google Scholar] [CrossRef]
- Salvatori, B.; Wegener, S.; Kotzaeridi, G.; Herding, A.; Eppel, F.; Dressler-Steinbach, I.; Henrich, W.; Piersanti, A.; Morettini, M.; Tura, A.; et al. Identification and validation of gestational diabetes subgroups by data-driven cluster analysis. Diabetologia 2024, 67, 1552–1566. [Google Scholar] [CrossRef]
- HAPO Study Cooperative Research Group; Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; et al. Hyperglycemia and Adverse Pregnancy Outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef]
- Lowe, W.L.; Scholtens, D.M.; Lowe, L.P.; Kuang, A.; Nodzenski, M.; Talbot, O.; Catalano, P.M.; Linder, B.; Brickman, W.J.; Clayton, P.; et al. Association of Gestational Diabetes with Maternal Disorders of Glucose Metabolism and Childhood Adiposity. JAMA 2018, 320, 1005–1016. [Google Scholar] [CrossRef]
- Farrar, D.; Simmonds, M.; Bryant, M.; Sheldon, T.A.; Tuffnell, D.; Golder, S.; Dunne, F.; Lawlor, D.A. Hyperglycaemia and risk of adverse perinatal outcomes: Systematic review and meta-analysis. BMJ 2016, 354, i4694. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef]
- Catalano, P.M.; McIntyre, H.D.; Cruickshank, J.K.; McCance, D.R.; Dyer, A.R.; Metzger, B.E.; Lowe, L.P.; Trimble, E.R.; Coustan, D.R.; Hadden, D.R.; et al. The Hyperglycemia and Adverse Pregnancy Outcome Study. Diabetes Care 2012, 35, 780–786. [Google Scholar] [CrossRef]
- Wexler, D.J.; Powe, C.E.; Barbour, L.A.; Buchanan, T.; Coustan, D.R.; Corcoy, R.; Damm, P.; Dunne, F.; Feig, D.S.; Ferrara, A.; et al. Research Gaps in Gestational Diabetes Mellitus. Obstet. Gynecol. 2018, 132, 496–505. [Google Scholar] [CrossRef]
- Linder, T.; Eder, A.; Monod, C.; Rosicky, I.; Eppel, D.; Redling, K.; Geissler, F.; Huhn, E.A.; Hösli, I.; Göbl, C.S. Impact of Prepregnancy Overweight and Obesity on Treatment Modality and Pregnancy Outcome in Women with Gestational Diabetes Mellitus. Front. Endocrinol. 2022, 13, 799625. [Google Scholar] [CrossRef]
- Langer, O.; Yogev, Y.; Xenakis, E.M.; Brustman, L. Overweight and obese in gestational diabetes: The impact on pregnancy outcome. Am. J. Obstet. Gynecol. 2005, 192, 1768–1776. [Google Scholar] [CrossRef]
- Black, M.H.; Sacks, D.A.; Xiang, A.H.; Lawrence, J.M. The Relative Contribution of Prepregnancy Overweight and Obesity, Gestational Weight Gain, and IADPSG-Defined Gestational Diabetes Mellitus to Fetal Overgrowth. Diabetes Care 2013, 36, 56–62. [Google Scholar] [CrossRef]
- Mecacci, F.; Lisi, F.; Vannuccini, S.; Ottanelli, S.; Rambaldi, M.P.; Serena, C.; Simeone, S.; Petraglia, F. Different Gestational Diabetes Phenotypes: Which Insulin Regimen Fits Better? Front. Endocrinol. 2021, 12, 630903. [Google Scholar] [CrossRef]
- Chatzakis, C.; Eleftheriades, A.; Demertzidou, E.; Dinas, K.; Vlahos, N.; Sotiriadis, A.; Eleftheriades, M. Pregnancy outcomes in the different phenotypes of gestational diabetes mellitus based on the oral glucose tolerance test. A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2023, 204, 110913. [Google Scholar] [CrossRef] [PubMed]
- Kotzaeridi, G.; Blätter, J.; Eppel, D.; Rosicky, I.; Linder, T.; Geissler, F.; Huhn, E.A.; Hösli, I.; Tura, A.; Göbl, C.S. Characteristics of gestational diabetes subtypes classified by oral glucose tolerance test values. Eur. J. Clin. Investig. 2021, 51, e13628. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wang, Q.; Chen, S.; Liu, Y.; Li, C.; Kang, R.; Wang, J.; Wei, T.; Wang, Q.; Li, X.; et al. Heterogeneity of Gestational Diabetes and Risk for Adverse Pregnancy Outcome: A Cohort Study. J. Clin. Endocrinol. Metab. 2025, 110, e2264–e2272. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadou, K.I.; Paschou, S.A.; Markozannes, G.; Vasileiou, V.; Kanouta, F.; Mitropoulou, M.; Antsaklis, P.; Theodora, M.; Psaltopoulou, T.; Daskalakis, G.; et al. Gestational diabetes mellitus subtypes according to oral glucose tolerance test and pregnancy outcomes. Endocrine 2025, 90, 95–103. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, W.; Meng, X.; Zhao, W.; Pan, J.; Tang, J.; Huang, Y.; Tao, M.; Liu, F. Heterogeneity of insulin resistance and beta cell dysfunction in gestational diabetes mellitus: A prospective cohort study of perinatal outcomes. J. Transl. Med. 2018, 16, 289. [Google Scholar] [CrossRef]
- Koenigbauer, J.T.; Fangmann, L.; Rostin, P.; Balke, S.; Weid, P.; Henrich, W.; Weichert, A.; Christian, G. Advanced maternal age (AMA) and 75 g oGTT glucose levels are pedictors for insulin therapy in women with gestational diabetes (GDM). J. Perinat. Med. 2023, 51, 1154–1162. [Google Scholar] [CrossRef]
- Kotzaeridi, G.; Blätter, J.; Eppel, D.; Rosicky, I.; Mittlböck, M.; Yerlikaya-Schatten, G.; Schatten, C.; Husslein, P.; Eppel, W.; Huhn, E.A.; et al. Performance of early risk assessment tools to predict the later development of gestational diabetes. Eur. J. Clin. Investig. 2021, 51, e13630. [Google Scholar] [CrossRef]
- Haftenberger, M.; Heuer, T.; Heidemann, C.; Kube, F.; Krems, C.; Mensink, G.B. Relative validation of a food frequency questionnaire for national health and nutrition monitoring. Nutr. J. 2010, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. 2013. Available online: https://www.who.int/publications/i/item/WHO-NMH-MND-13.2 (accessed on 30 May 2024).
- Villar, J.; Ismail, L.C.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative Insulin Sensitivity Check Index: A Simple, Accurate Method for Assessing Insulin Sensitivity in Humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef]
- Salvatori, B.; Linder, T.; Eppel, D.; Morettini, M.; Burattini, L.; Göbl, C.; Tura, A. TyGIS: Improved triglyceride-glucose index for the assessment of insulin sensitivity during pregnancy. Cardiovasc. Diabetol. 2022, 21, 215. [Google Scholar] [CrossRef]
- Tura, A.; Kautzky-Willer, A.; Pacini, G. Insulinogenic indices from insulin and C-peptide: Comparison of beta-cell function from OGTT and IVGTT. Diabetes Res. Clin. Pract. 2006, 72, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Retnakaran, R.; Qi, Y.; Goran, M.I.; Hamilton, J.K. Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet. Med. 2009, 26, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Ahren, B.; Pacini, G. Importance of quantifying insulin secretion in relation to insulin sensitivity to accurately assess beta cell function in clinical studies. Eur. J. Endocrinol. 2004, 150, 97–104. [Google Scholar] [CrossRef]
- Salvatori, B. Compute Subgroup for Patients with Gestational Diabetes Mellitus (GDM). 2024. Available online: https://clugdm.shinyapps.io/clugdm/ (accessed on 31 October 2024).
- Konietschke, F.; Placzek, M.; Schaarschmidt, F.; Hothorn, L.A. nparcomp: An R Software Package for Nonparametric Multiple Comparisons and Simultaneous Confidence Intervals. J. Stat. Softw. 2015, 64, 1–17. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Immanuel, J.; Simmons, D.; Harreiter, J.; Desoye, G.; Corcoy, R.; Adelantado, J.M.; Devlieger, R.; Lapolla, A.; Dalfra, M.G.; Bertolotto, A.; et al. Metabolic phenotypes of early gestational diabetes mellitus and their association with adverse pregnancy outcomes. Diabet. Med. 2021, 38, e14413. [Google Scholar] [CrossRef]
- Benhalima, K.; Van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Maes, T.; Dufraimont, E.; De Block, C.; et al. Characteristics and pregnancy outcomes across gestational diabetes mellitus subtypes based on insulin resistance. Diabetologia 2019, 62, 2118–2128. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Storm, P.; Käräjämäki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.B.; Aly, D.M.; Almgren, P.; et al. Novel subgroups of adult-onset diabetes and their association with outcomes: A data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef]
- Eppel, D.; Feichtinger, M.; Lindner, T.; Kotzaeridi, G.; Rosicky, I.; Yerlikaya-Schatten, G.; Eppel, W.; Husslein, P.; Tura, A.; Göbl, C.S. Association between maternal triglycerides and disturbed glucose metabolism in pregnancy. Acta Diabetol. 2021, 58, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Linder, T.; Eppel, D.; Kotzaeridi, G.; Yerlikaya-Schatten, G.; Rosicky, I.; Morettini, M.; Tura, A.; Göbl, C.S. Glucometabolic Alterations in Pregnant Women with Overweight or Obesity but without Gestational Diabetes Mellitus: An Observational Study. Obes. Facts 2024, 17, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Takele, W.W.; Vesco, K.K.; Josefson, J.; Redman, L.M.; Hannah, W.; Bonham, M.P.; Chen, M.; Chivers, S.C.; Fawcett, A.J.; Grieger, J.A.; et al. Effective interventions in preventing gestational diabetes mellitus: A systematic review and meta-analysis. Commun. Med. 2024, 4, 75. [Google Scholar] [CrossRef]
- Hayes, M.G.; Urbanek, M.; Hivert, M.-F.; Armstrong, L.L.; Morrison, J.; Guo, C.; Lowe, L.P.; Scheftner, D.A.; Pluzhnikov, A.; Levine, D.M.; et al. Identification of HKDC1 and BACE2 as Genes Influencing Glycemic Traits During Pregnancy Through Genome-Wide Association Studies. Diabetes 2013, 62, 3282–3291. [Google Scholar] [CrossRef]
- Lee, K.; Kuang, A.; Bain, J.R.; Hayes, M.G.; Muehlbauer, M.J.; Ilkayeva, O.R.; Newgard, C.B.; Powe, C.E.; Hivert, M.-F.; Scholtens, D.M.; et al. Metabolomic and genetic architecture of gestational diabetes subtypes. Diabetologia 2024, 67, 895–907. [Google Scholar] [CrossRef]
- Elliott, A.; Walters, R.K.; Pirinen, M.; Kurki, M.; Junna, N.; Goldstein, J.I.; Reeve, M.P.; Lemmelä, S.M.; Turley, P.; Lahtela, E.; et al. Distinct and shared genetic architectures of gestational diabetes mellitus and type 2 diabetes. Nat. Genet. 2024, 56, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Udler, M.S.; Hsu, S.; Allard, C.; Kuang, A.; Manning, A.K.; Perron, P.; Bouchard, L.; Lowe, W.L.; Scholtens, D.; et al. Genetic Loci and Physiologic Pathways Involved in Gestational Diabetes Mellitus Implicated Through Clustering. Diabetes 2021, 70, 268–281. [Google Scholar] [CrossRef]

| NGT | CL1 | CL2 | CL3 | |
|---|---|---|---|---|
| (n = 893) | (n = 15) | (n = 70) | (n = 110) | |
| Age (years) | 31.4 ± 5.8 | 35.9 ± 5.3 * | 31.4 ± 5.2† | 33.2 ± 5.6 * |
| Parity (≥1) | 541 (60.6) | 11 (73.3) | 51 (72.9) | 72 (65.5) |
| GDM in previous pregnancy | 52 (5.8) | 7 (46.7) * | 14 (20.0) * | 30 (27.3) * |
| Ethnicity (non-Caucasian) | 184 (20.6) | 5 (33.3) | 20 (28.6) | 32 (29.1) |
| BMI, before pregnancy (kg/m2) | 24.3 ± 5.2 | 34.0 ± 5.8 * | 28.3 ± 5.4 *† | 25.1 ± 4.5 †§ |
| BMI, early pregnancy (kg/m2) | 24.8± 5.1 | 34.3 ± 5.5 * | 29.1 ± 5.5 *† | 25.8 ± 4.5 †§ |
| Family history of diabetes (1st grade) | 214 (23.9) | 10 (66.7) * | 16 (22.9) † | 46 (41.8) *§ |
| Family history of diabetes (1st & 2nd grade) | 386 (43.2) | 12 (80.0) * | 33 (47.1) | 74 (67.3) *§ |
| Multiple pregnancy | 107 (12.0) | 0 (0.0) | 8 (11.4) | 10 (9.1) |
| Triglycerides, early pregnancy (mg/dl) | 114 ± 44 | 158 ± 38 * | 126 ± 45 | 139 ± 53 * |
| Total-cholesterol, early pregnancy (mg/dl) | 188 ± 35.0 | 185 ± 28 | 185 ± 35 | 194 ± 37 |
| LDL-cholesterol, early pregnancy (mg/dl) | 94.1 ± 27.9 | 92.7 ± 26.1 | 96.8 ± 29.2 | 97.2 ± 28.0 |
| HDL-cholesterol, early pregnancy (mg/dl) | 71.1 ± 16.1 | 60.1 ± 12.0 * | 63.6 ± 12.7 * | 69.2 ± 16.2 |
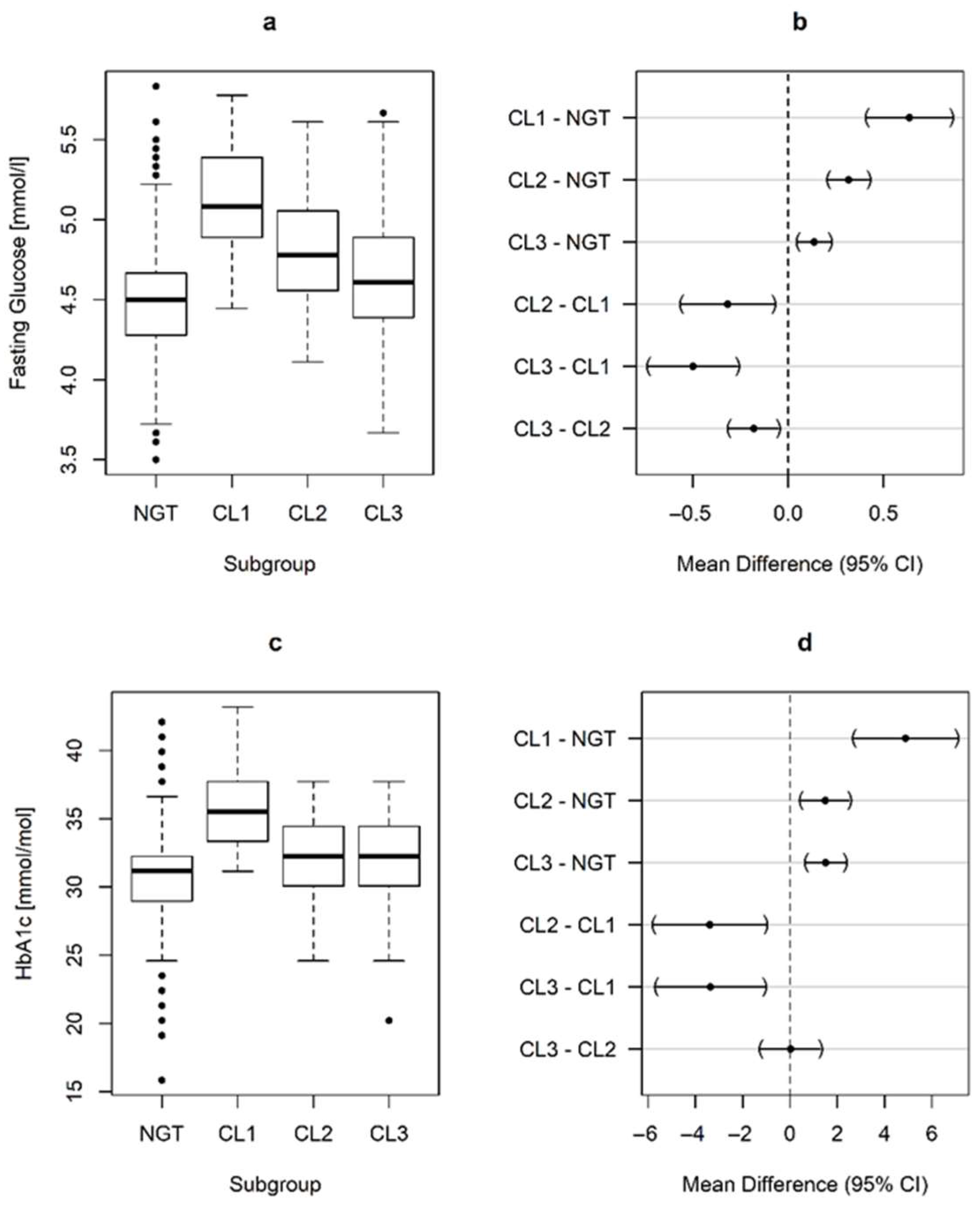
| FPG, early pregnancy (mmol/L) | 4.48 ± 0.32 | 5.12 ± 0.44 * | 4.80 ± 0.31 *† | 4.62 ± 0.38 *†§ |
| HbA1c, early pregnancy (%) | 4.95 ± 0.29 | 5.40 ± 0.29 * | 5.09 ± 0.25 *† | 5.09 ± 0.30 *† |
| HbA1c, early pregnancy (mmol/mol) | 30.6 ± 3.2 | 35.5 ± 3.1 * | 32.1 ± 2.8 *† | 32.2 ± 3.2 *† |
| OGTT-G 0′ (mmol/L) | 4.37 ± 0.37 | 5.84 ± 0.46 * | 5.35 ± 0.31 *† | 4.76 ± 0.50 *†§ |
| OGTT-G 60′ (mmol/L) | 6.90 ± 1.49 | 12.02 ± 1.52 * | 8.25 ± 1.42 *† | 10.54 ± 1.08 *†§ |
| OGTT-G 120′ (mmol/L) | 5.64 ± 1.12 | 8.69 ± 1.33 * | 5.97 ± 0.98 † | 8.29 ± 1.49 *§ |
| Fasting insulin, early pregnancy (µU/mL) | 7.5 (5.3–10.7) | 15.7 (14.3–27.5) * | 11.6 (7.3–16.9) *† | 9.8 (6.6–12.8) *† |
| Fasting C-Peptide, early pregnancy (ng/mL) | 1.5 (1.2–1.9) | 2.8 (2.3–3.5) * | 1.9 (1.6–2.5) *† | 1.8 (1.4–2.3) *† |
| QUICKI, early pregnancy (dimensionless) × 102 | 36.2 ± 3.4 | 30.9 ± 1.8 * | 33.9 ± 3.1 *† | 34.9 ± 3.2 *† |
| TyGIS, early pregnancy (mg kg−1 min−1) | 6.6 (5.6–7.4) | 2.8 (1.6–4.0) * | 5.3 (4.0–6.4) *† | 5.8 (4.8–6.8) *† |
| IGIF, early pregnancy (ng/mg) | 2.04 ± 0.75 | 3.19 ± 0.72 * | 2.45 ± 0.82 *† | 2.28 ± 0.73 *† |
| DIORAL, early pregnancy (ng mg−1 (µU/mL)−1) × 102 | 24.7 (20.6–30.8) | 17.4 (13.2–19.0) * | 21.1 (17.3–25.7) *† | 22.8 (19.4–27.3) † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotzaeridi, G.; Salvatori, B.; Piersanti, A.; Heinzl, F.; Zarotti, S.; Kiss, H.; Wegener, S.; Dressler-Steinbach, I.; Henrich, W.; Morettini, M.; et al. Gestational Diabetes Mellitus Subtypes Derived by Clustering Analysis Show Heterogeneity in Glucometabolic Parameters Already at Early Pregnancy. Nutrients 2025, 17, 3252. https://doi.org/10.3390/nu17203252
Kotzaeridi G, Salvatori B, Piersanti A, Heinzl F, Zarotti S, Kiss H, Wegener S, Dressler-Steinbach I, Henrich W, Morettini M, et al. Gestational Diabetes Mellitus Subtypes Derived by Clustering Analysis Show Heterogeneity in Glucometabolic Parameters Already at Early Pregnancy. Nutrients. 2025; 17(20):3252. https://doi.org/10.3390/nu17203252
Chicago/Turabian StyleKotzaeridi, Grammata, Benedetta Salvatori, Agnese Piersanti, Florian Heinzl, Sophie Zarotti, Herbert Kiss, Silke Wegener, Iris Dressler-Steinbach, Wolfgang Henrich, Micaela Morettini, and et al. 2025. "Gestational Diabetes Mellitus Subtypes Derived by Clustering Analysis Show Heterogeneity in Glucometabolic Parameters Already at Early Pregnancy" Nutrients 17, no. 20: 3252. https://doi.org/10.3390/nu17203252
APA StyleKotzaeridi, G., Salvatori, B., Piersanti, A., Heinzl, F., Zarotti, S., Kiss, H., Wegener, S., Dressler-Steinbach, I., Henrich, W., Morettini, M., Tura, A., & Göbl, C. S. (2025). Gestational Diabetes Mellitus Subtypes Derived by Clustering Analysis Show Heterogeneity in Glucometabolic Parameters Already at Early Pregnancy. Nutrients, 17(20), 3252. https://doi.org/10.3390/nu17203252








