Bioactive Peptides from Quinoa (Chenopodium quinoa Willd.) as Modulators of the Gut Microbiome: A Scoping Review of Preclinical Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Methodological Framework
2.2. Research Question and PCC Framework
- Population (P): Preclinical experimental models, including in vitro studies (e.g., fecal fermentation, bacterial cultures, simulated digestion systems) and in vivo animal models.
- Concept (C): Modulation of the gut microbiome through the administration of bioactive peptides or protein hydrolysates derived from quinoa (Chenopodium quinoa Willd.).
- Context (C): Preclinical studies investigating the effects of quinoa peptides on the gut ecosystem, including the production of SCFAs, inhibition of pathogens, and promotion of beneficial bacteria.
2.3. Eligibility Criteria
2.3.1. Inclusion Criteria
- Study Type: Preclinical investigations, both in vitro and in vivo. Studies using whole quinoa or quinoa flour were also included if the original paper’s discussion attributed the observed microbiome-modulating effects, at least in part, to the fermentation of its protein content and the subsequent in situ generation of bioactive peptides.
- Intervention: Bioactive peptides obtained from quinoa proteins through enzymatic hydrolysis, fermentation, or simulated digestion.
- Outcomes of Interest: Studies reporting at least one of the following outcomes: changes in microbiota composition, modulation of bacterial growth, or production of metabolites such as SCFAs.
- Period and Language: Articles published between January 2000 and July 2025, in English, Spanish, or Portuguese.
2.3.2. Exclusion Criteria
- Publication Format: Conference abstracts, letters to the editor, or preprint articles.
- Non-Specific Intervention: Studies using crude quinoa extracts without peptide characterization.
- Focus on Non-Peptidic Compounds: Studies exclusively addressing saponins, polyphenols, or isolated polysaccharides.
- Concomitant Interventions: Studies in which the simultaneous application of other substances precluded discerning the specific effect of peptides.
2.4. Information Sources and Search Strategy
2.5. Study Selection and Data Extraction
2.6. Data Synthesis and Visualization
3. Results
3.1. Study Selection and General Characteristics of the Evidence
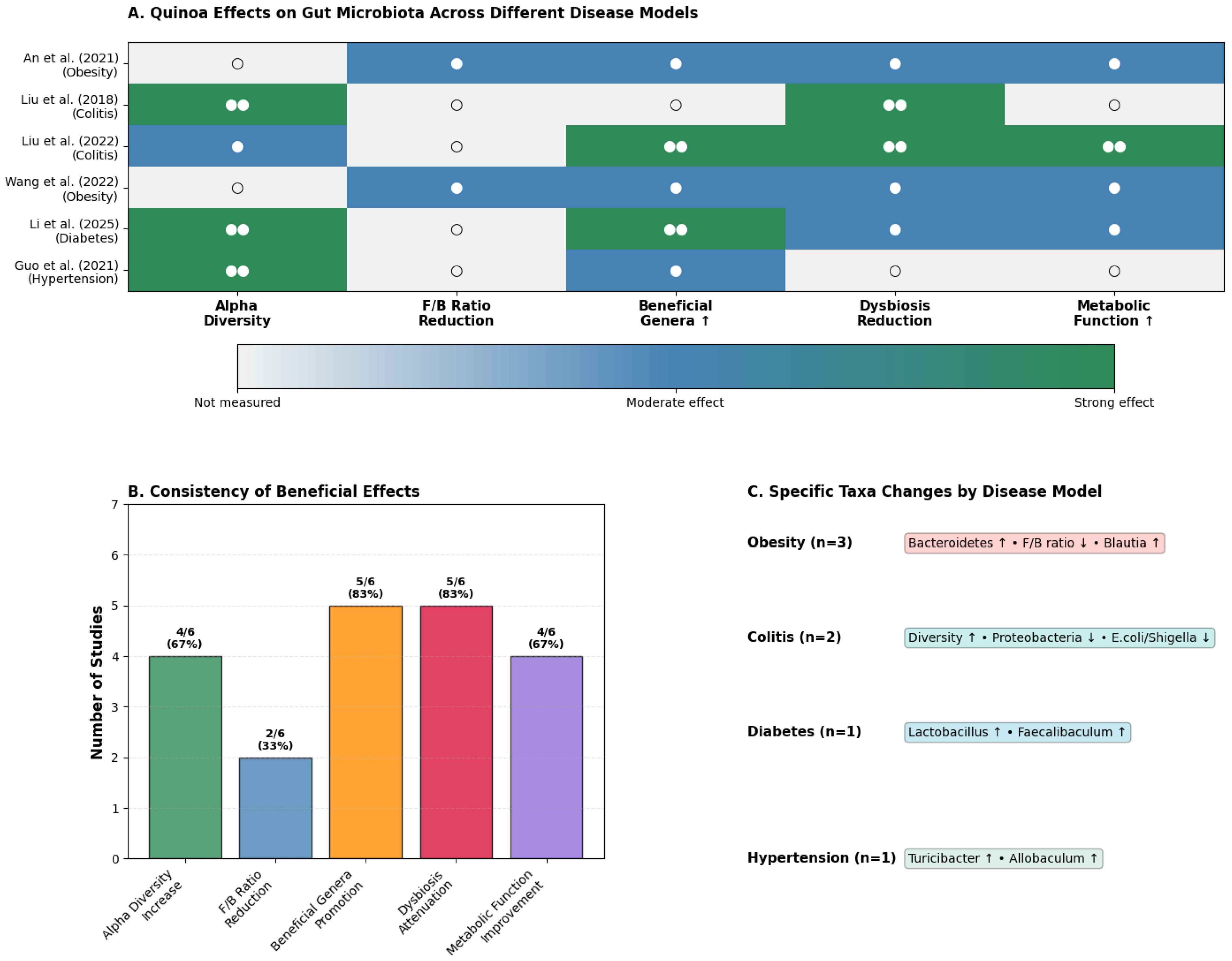
3.2. Gut Microbiota Modulation: Context-Dependent Therapeutic Signatures
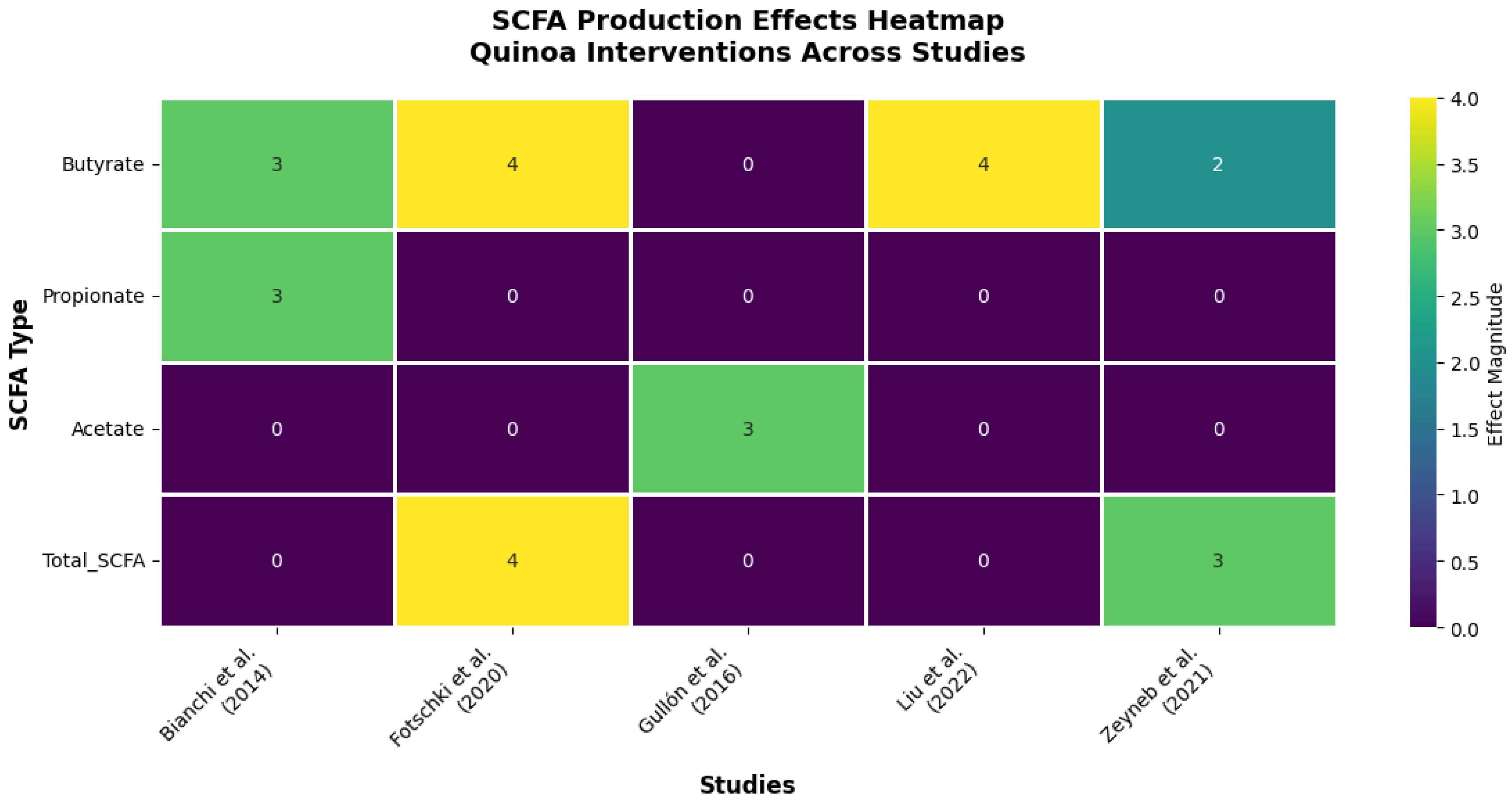
3.3. Metabolic Microbiome Activation: The Butyrate-Centric Response
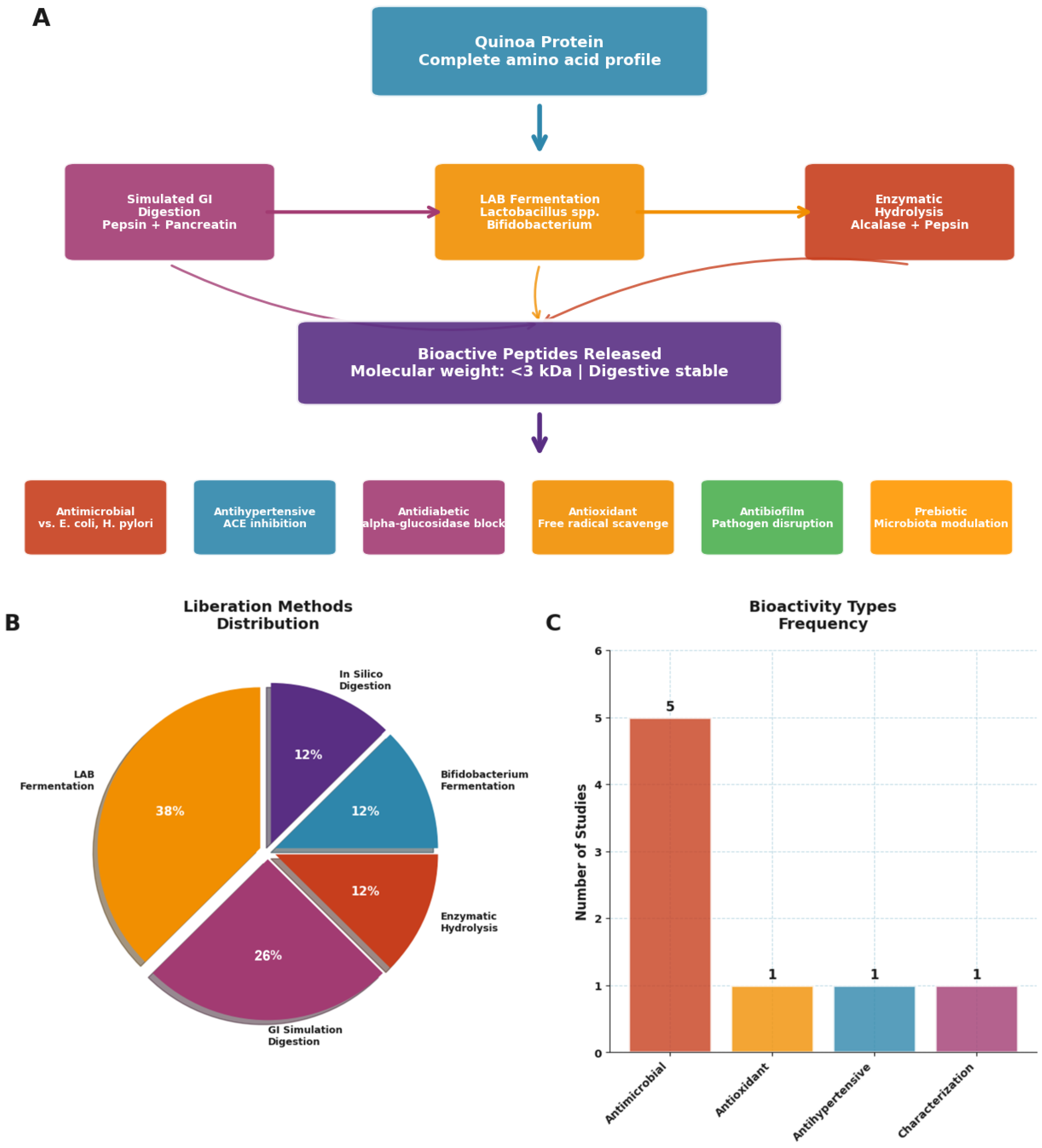
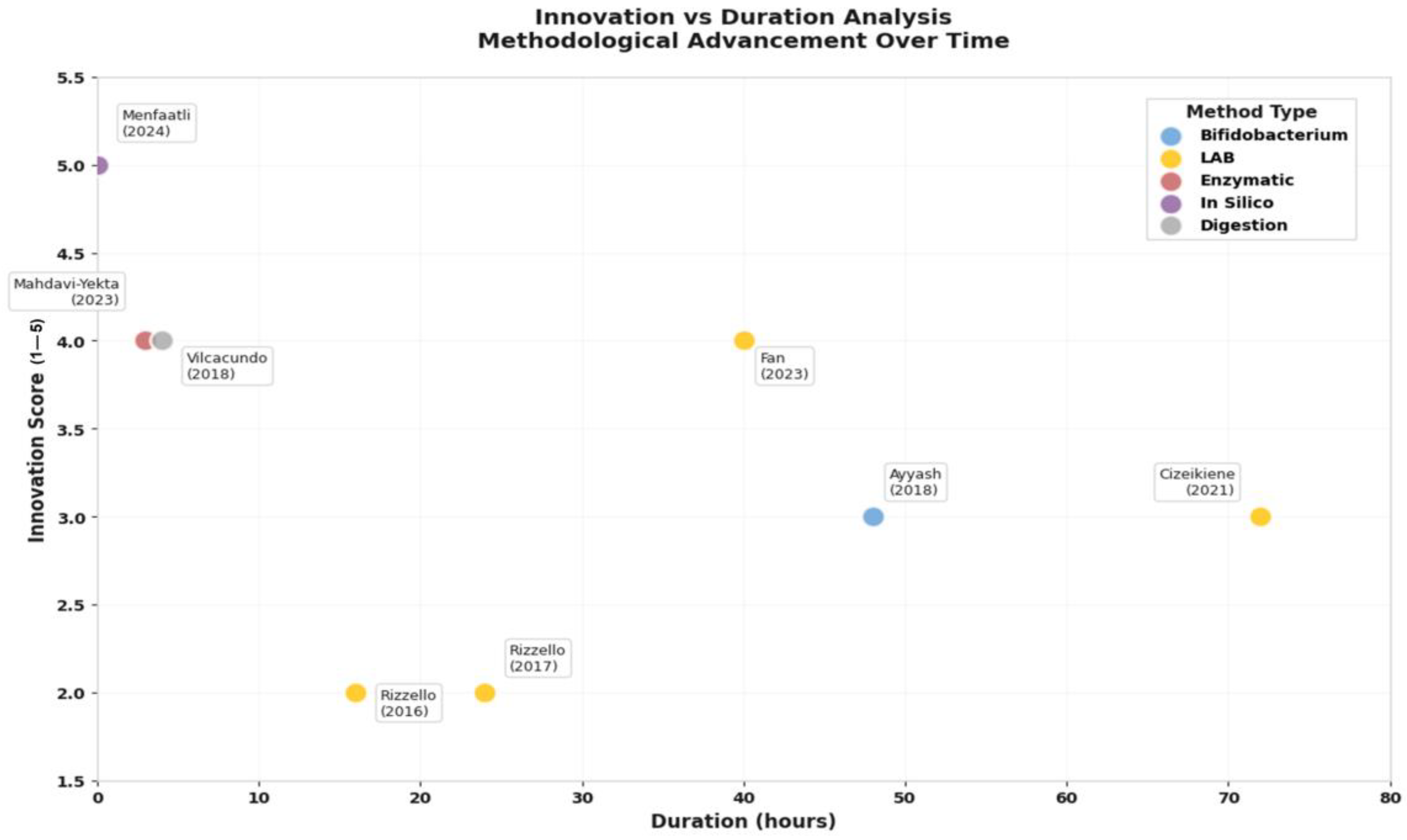
3.4. Bioactive Peptide Liberation: Innovation Meets Functionality
4. Discussion
4.1. Principal Findings and Mechanistic Insights
4.2. Disease-Specific Modulation Patterns and Mechanisms of Action
4.3. Bioactive Peptide Generation and Antimicrobial Properties
4.4. SCFA Production and Metabolic Implications
4.5. Comparative Analysis with Other Plant-Derived Peptides
4.6. Clinical Translation Challenges and Opportunities
4.7. Implications for Functional Food Development
4.8. Future Perspectives and Research Directions
4.9. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOM | Azoxymethane |
| BAPs | Bioactive Antimicrobial Peptides |
| DSS | Dextran Sulfate Sodium |
| F/B ratio | Firmicutes/Bacteroidetes Ratio |
| GI | Gastrointestinal |
| HFD | High-Fat Diet |
| JBI | Joanna Briggs Institute |
| LAB | Lactic Acid Bacteria |
| PCC | Population, Concept, and Context |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews |
| QBSDF | Quinoa Bran Soluble Dietary Fiber |
| QHP | Quinoa Protein Hydrolysate |
| QP | Quinoa Protein |
| SCFAs | Short-Chain Fatty Acids |
| SHR | Spontaneously Hypertensive Rats |
| SHIME® | Simulator of the Human Intestinal Microbial Ecosystem |
| UAE | United Arab Emirates |
| USA | United States of America |
References
- Khalil, M.; Di Ciaula, A.; Mahdi, L.; Jaber, N.; Di Palo, D.M.; Graziani, A.; Baffy, G.; Portincasa, P. Unraveling the Role of the Human Gut Microbiome in Health and Diseases. Microorganisms 2024, 12, 2333. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Prabhakar, M.R.; Mohanty, A.; Meena, S.S. Influence of Gut Microbiome on the Human Physiology. Syst. Microbiol. Biomanuf. 2022, 2, 217–231. [Google Scholar] [CrossRef]
- Protasiewicz-Timofticiuc, D.-C.; Bădescu, D.; Moța, M.; Ștefan, A.-G.; Mitrea, A.; Clenciu, D.; Efrem, I.C.; Roșu, M.M.; Vladu, B.E.; Gheonea, T.C.; et al. Back to Roots: Dysbiosis, Obesity, Metabolic Syndrome, Type 2 Diabetes Mellitus, and Obstructive Sleep Apnea—Is There an Objective Connection? A Narrative Review. Nutrients 2024, 16, 4057. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing A Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Q.; Yang, Y.; Zhong, W.; He, F.; Li, J. The Mycobiome as Integral Part of the Gut Microbiome: Crucial Role of Symbiotic Fungi in Health and Disease. Gut Microbes 2024, 16, 2440111. [Google Scholar] [CrossRef]
- Martinez Guevara, D.; Vidal Cañas, S.; Palacios, I.; Gómez, A.; Estrada, M.; Gallego, J.; Liscano, Y. Effectiveness of Probiotics, Prebiotics, and Synbiotics in Managing Insulin Resistance and Hormonal Imbalance in Women with Polycystic Ovary Syndrome (PCOS): A Systematic Review of Randomized Clinical Trials. Nutrients 2024, 16, 3916. [Google Scholar] [CrossRef]
- Turroni, S.; Benítez-Páez, A. Editorial: Remodeling Composition and Function of Microbiome by Dietary Strategies-Functional Foods Perspective. Front. Nutr. 2021, 8, 811102. [Google Scholar] [CrossRef]
- Aragón-Muriel, A.; Liscano-Martínez, Y.; Rufino-Felipe, E.; Morales-Morales, D.; Oñate-Garzón, J.; Polo-Cerón, D. Synthesis, Biological Evaluation and Model Membrane Studies on Metal Complexes Containing Aromatic N,O-Chelate Ligands. Heliyon 2020, 6, e04126. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Ashaolu, J.O.; Adeyeye, S.A.O. Fermentation of Prebiotics by Human Colonic Microbiota in Vitro and Short-chain Fatty Acids Production: A Critical Review. J. Appl. Microbiol. 2021, 130, 677–687. [Google Scholar] [CrossRef]
- Bedu-Ferrari, C.; Biscarrat, P.; Langella, P.; Cherbuy, C. Prebiotics and the Human Gut Microbiota: From Breakdown Mechanisms to the Impact on Metabolic Health. Nutrients 2022, 14, 2096. [Google Scholar] [CrossRef] [PubMed]
- Fotschki, B.; Juśkiewicz, J.; Jurgoński, A.; Amarowicz, R.; Opyd, P.; Bez, J.; Muranyi, I.; Lykke Petersen, I.; Laparra Llopis, M. Protein-Rich Flours from Quinoa and Buckwheat Favourably Affect the Growth Parameters, Intestinal Microbial Activity and Plasma Lipid Profile of Rats. Nutrients 2020, 12, 2781. [Google Scholar] [CrossRef]
- Fan, X.; Ma, X.; Maimaitiyiming, R.; Aihaiti, A.; Yang, J.; Li, X.; Wang, X.; Pang, G.; Liu, X.; Qiu, C.; et al. Study on the Preparation Process of Quinoa Anti-Hypertensive Peptide and Its Stability. Front. Nutr. 2023, 9, 1119042. [Google Scholar] [CrossRef]
- Ocampo-Ibáñez, I.D.; Liscano, Y.; Rivera-Sánchez, S.P.; Oñate-Garzón, J.; Lugo-Guevara, A.D.; Flórez-Elvira, L.J.; Lesmes, M.C. A Novel Cecropin D-Derived Short Cationic Antimicrobial Peptide Exhibits Antibacterial Activity Against Wild-Type and Multidrug-Resistant Strains of Klebsiella pneumoniae and Pseudomonas aeruginosa. Evol. Bioinform. 2020, 16, 1176934320936266. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Sanchez, S.P.; Ocampo-Ibáñez, I.D.; Liscano, Y.; Martínez, N.; Muñoz, I.; Manrique-Moreno, M.; Martinez-Martinez, L.; Oñate-Garzon, J. Integrating In Vitro and In Silico Analysis of a Cationic Antimicrobial Peptide Interaction with Model Membranes of Colistin-Resistant Pseudomonas Aeruginosa Strains. Pharmaceutics 2022, 14, 1248. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.P.; Bangar, S.P.; Albaloosh, M.; Ajayi, F.F.; Mudgil, P.; Maqsood, S. Plant-Derived Proteins as a Sustainable Source of Bioactive Peptides: Recent Research Updates on Emerging Production Methods, Bioactivities, and Potential Application. Crit. Rev. Food Sci. Nutr. 2022, 63, 9539–9560. [Google Scholar] [CrossRef]
- Trejos, M.; Aristizabal, Y.; Aragón-Muriel, A.; Oñate-Garzón, J.; Liscano, Y. Characterization and Classification In Silico of Peptides with Dual Activity (Antimicrobial and Wound Healing). Int. J. Mol. Sci. 2023, 24, 13091. [Google Scholar] [CrossRef]
- Caicedo Cerón, N.; Oñate-Garzon, J.F.; Liscano, Y. Evaluation of the Anti-Aging Activity of Peptides Obtained by Enzymatic Hydrolysis with Actinidin from Colombian Quinoa Seed Proteins. Rev. Prod. Nat. 2025, 6, 129–132. [Google Scholar]
- Hernández-Ledesma, B. Quinoa (Chenopodium quinoa Willd.) as Source of Bioactive Compounds: A Review. Bioact. Compd. Health Dis. 2019, 2, 27. [Google Scholar] [CrossRef]
- Ng, C.Y.; Wang, M. The Functional Ingredients of Quinoa (Chenopodium quinoa) and Physiological Effects of Consuming Quinoa: A Review. Food Front. 2021, 2, 329–356. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Miralles, B.; Carrillo, W.; Hernández-Ledesma, B. In Vitro Chemopreventive Properties of Peptides Released from Quinoa (Chenopodium quinoa Willd.) Protein under Simulated Gastrointestinal Digestion. Food Res. Int. 2018, 105, 403–411. [Google Scholar] [CrossRef]
- Ayyash, M.; Johnson, S.K.; Liu, S.-Q.; Al-Mheiri, A.; Abushelaibi, A. Cytotoxicity, Antihypertensive, Antidiabetic and Antioxidant Activities of Solid-State Fermented Lupin, Quinoa and Wheat by Bifidobacterium Species: In-Vitro Investigations. LWT 2018, 95, 295–302. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Lorusso, A.; Russo, V.; Pinto, D.; Marzani, B.; Gobbetti, M. Improving the Antioxidant Properties of Quinoa Flour through Fermentation with Selected Autochthonous Lactic Acid Bacteria. Int. J. Food Microbiol. 2017, 241, 252–261. [Google Scholar] [CrossRef]
- Li, L.; Hu, X.; Sun, X.; Wei, X.; Zhang, H.; Wang, P. Quinoa Alleviates the Diabetic Symptoms of Db/Db Mice by Upregulating Insulin Signaling and Modulating Gut Microbiota Composition. J. Funct. Foods 2025, 128, 106813. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Mai, P.; Hao, Y.; Wang, Z.; Wang, J. Quinoa Bran Soluble Dietary Fiber Ameliorates Dextran Sodium Sulfate Induced Ulcerative Colitis in BALB/c Mice by Maintaining Intestinal Barrier Function and Modulating Gut Microbiota. Int. J. Biol. Macromol. 2022, 216, 75–85. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Tao, S.-Y.; Wu, Y.-X.; An, T.; Lv, B.-H.; Liu, J.-X.; Liu, Y.-T.; Jiang, G.-J. Quinoa Reduces High-Fat Diet-Induced Obesity in Mice via Potential Microbiota-Gut-Brain-Liver Interaction Mechanisms. Microbiol. Spectr. 2022, 10, e00329-22. [Google Scholar] [CrossRef]
- Gullón, B.; Gullón, P.; Tavaria, F.K.; Yáñez, R. Assessment of the Prebiotic Effect of Quinoa and Amaranth in the Human Intestinal Ecosystem. Food Funct. 2016, 7, 3782–3788. [Google Scholar] [CrossRef]
- Zeyneb, H.; Pei, H.; Cao, X.; Wang, Y.; Win, Y.; Gong, L. In Vitro Study of the Effect of Quinoa and Quinoa Polysaccharides on Human Gut Microbiota. Food Sci. Nutr. 2021, 9, 5735–5745. [Google Scholar] [CrossRef]
- Santos, W.M.D.; Secoli, S.R.; Püschel, V.A.D.A. The Joanna Briggs Institute Approach for Systematic Reviews. Rev. Lat.-Am. Enferm. 2018, 26, e3074. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, M.; Zhao, B.; Yang, F. Isolation of Antioxidant Peptides from Yak Casein Hydrolysate. RSC Adv. 2020, 10, 19844–19851. [Google Scholar] [CrossRef]
- Bianchi, F.; Rossi, E.A.; Sakamoto, I.K.; Adorno, M.A.T.; Van De Wiele, T.; Sivieri, K. Beneficial Effects of Fermented Vegetal Beverages on Human Gastrointestinal Microbial Ecosystem in a Simulator. Food Res. Int. 2014, 64, 43–52. [Google Scholar] [CrossRef]
- Cizeikiene, D.; Gaide, I.; Basinskiene, L. Effect of Lactic Acid Fermentation on Quinoa Characteristics and Quality of Quinoa-Wheat Composite Bread. Foods 2021, 10, 171. [Google Scholar] [CrossRef]
- Fan, X.; Guo, H.; Teng, C.; Yang, X.; Qin, P.; Richel, A.; Zhang, L.; Blecker, C.; Ren, G. Supplementation of Quinoa Peptides Alleviates Colorectal Cancer and Restores Gut Microbiota in AOM/DSS-Treated Mice. Food Chem. 2023, 408, 135196. [Google Scholar] [CrossRef]
- Mahdavi-Yekta, M.; Reihani, S.F.S.; Mohammadi, M. Antimicrobial Activity of Quinoa Protein Hydrolysate against Streptococcus pyogenes and Escherichia coli. J. Food Qual. 2023, 2023, 9993241. [Google Scholar] [CrossRef]
- Menfaatli, E.; Kavruk, M.; Gundeger, E. In Silico Evaluation of Antimicrobial Potential of Amaranth, Chia and Quinoa Peptides Released During the Simulated Gastric Digestion and Their Effects on Helicobacter Pylori. Trends Pept. Protein Sci. 2024, 9, 1–8. [Google Scholar]
- Rizzello, C.G.; Lorusso, A.; Montemurro, M.; Gobbetti, M. Use of Sourdough Made with Quinoa (Chenopodium quinoa) Flour and Autochthonous Selected Lactic Acid Bacteria for Enhancing the Nutritional, Textural and Sensory Features of White Bread. Food Microbiol. 2016, 56, 1–13. [Google Scholar] [CrossRef]
- Noratto, G.D.; Murphy, K.; Chew, B.P. Quinoa Intake Reduces Plasma and Liver Cholesterol, Lessens Obesity-Associated Inflammation, and Helps to Prevent Hepatic Steatosis in Obese Db/Db Mouse. Food Chem. 2019, 287, 107–114. [Google Scholar] [CrossRef]
- Guo, H.; Hao, Y.; Fan, X.; Richel, A.; Everaert, N.; Yang, X.; Ren, G. Administration with Quinoa Protein Reduces the Blood Pressure in Spontaneously Hypertensive Rats and Modifies the Fecal Microbiota. Nutrients 2021, 13, 2446. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Qiu, B.; Fan, S.; Ding, H.; Liu, Z. Quinoa Whole Grain Diet Compromises the Changes of Gut Microbiota and Colonic Colitis Induced by Dextran Sulfate Sodium in C57BL/6 Mice. Sci. Rep. 2018, 8, 14916. [Google Scholar] [CrossRef]
- An, T.; Liu, J.-X.; Yang, X.; Lv, B.; Wu, Y.; Jiang, G. Supplementation of Quinoa Regulates Glycolipid Metabolism and Endoplasmic Reticulum Stress in the High-Fat Diet-Induced Female Obese Mice. Nutr. Metab. 2021, 18, 95. [Google Scholar] [CrossRef]
- Carías Domínguez, A.M.; De Jesús Rosa Salazar, D.; Stefanolo, J.P.; Cruz Serrano, M.C.; Casas, I.C.; Zuluaga Peña, J.R. Intestinal Dysbiosis: Exploring Definition, Associated Symptoms, and Perspectives for a Comprehensive Understanding—A Scoping Review. Probiotics Antimicrob. Proteins 2025, 17, 440–449. [Google Scholar] [CrossRef]
- Hemalatha, P.; Bomzan, D.P.; Rao, B.V.S.; Sreerama, Y.N. Distribution of Phenolic Antioxidants in Whole and Milled Fractions of Quinoa and Their Inhibitory Effects on α-Amylase and α-Glucosidase Activities. Food Chem. 2016, 199, 330–338. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Feng, M.; Zhou, X.; Lu, Y.; Gao, L.; Yu, C.; Jiang, X.; Zhao, J. Thyroid-Stimulating Hormone Decreases HMG-CoA Reductase Phosphorylation via AMP-Activated Protein Kinase in the Liver. J. Lipid Res. 2015, 56, 963–971. [Google Scholar] [CrossRef]
- Fukatsu, A.; Tsuzukibashi, O.; Suzuk, H.; Asaka, K.; Ono, Y.; Fuchigami, M.; Kobayashi, T.; Uchibori, S.; Takahashi, Y.; Komine, C.; et al. One-Step Multiplex PCR for Simultaneous Detection and Identification of Eight Medically Important Candida Species. Open J. Stomatol. 2021, 11, 14–24. [Google Scholar] [CrossRef]
- Fathurrohmah, N.N.; Murti, S.T.C.; Suryani, C.L. Effect of Zn–Chlorophyll Complexes Formation on the Color Stability of Pandan (Pandanus amaryllifolius) Leaf Extract. Food Sci. Technol. 2023, 11, 161–167. [Google Scholar] [CrossRef]
- Tang, Y.; Tsao, R. Phytochemicals in Quinoa and Amaranth Grains and Their Antioxidant, Anti-inflammatory, and Potential Health Beneficial Effects: A Review. Mol. Nutr. Food Res 2017, 61, 1600767. [Google Scholar] [CrossRef]
- Rocchetti, G.; Chiodelli, G.; Giuberti, G.; Masoero, F.; Trevisan, M.; Lucini, L. Evaluation of Phenolic Profile and Antioxidant Capacity in Gluten-Free Flours. Food Chem. 2017, 228, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, T.; Abeyrathne, E.D.N.S.; Ahn, D.U. Effect of Bioactive Peptides on Gut Microbiota and Their Relations to Human Health. Foods 2024, 13, 1853. [Google Scholar] [CrossRef]
- Mazurkiewicz-Pisarek, A.; Baran, J.; Ciach, T. Antimicrobial Peptides: Challenging Journey to the Pharmaceutical, Biomedical, and Cosmeceutical Use. Int. J. Mol. Sci. 2023, 24, 9031. [Google Scholar] [CrossRef]
- Parvez, A.K.; Jubyda, F.T.; Ayaz, M.; Sarker, A.; Haque, N.; Khan, M.S.; Mou, T.J.; Rahman, M.A.; Huq, M.A. Microbial- and Plant-Derived Bioactive Peptides and Their Applications against Foodborne Pathogens: Current Status and Future Prospects. Int. J. Microbiol. 2024, 2024, 9978033. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.S.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.P.; Stahl, B.; Harsselaar, J.V.; et al. Short Chain Fatty Acids in Human Gut and Metabolic Health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Hays, K.E.; Pfaffinger, J.M.; Ryznar, R. The Interplay between Gut Microbiota, Short-Chain Fatty Acids, and Implications for Host Health and Disease. Gut Microbes 2024, 16, 2393270. [Google Scholar] [CrossRef]
- Rekha, K.; Venkidasamy, B.; Samynathan, R.; Nagella, P.; Rebezov, M.B.; Khayrullin, M.; Ponomarev, E.E.; Bouyahya, A.; Sarkar, T.; Shariati, M.A.; et al. Short-Chain Fatty Acid: An Updated Review on Signaling, Metabolism, and Therapeutic Effects. Crit. Rev. Food Sci. Nutr. 2022, 64, 2461–2489. [Google Scholar] [CrossRef]
- Ren, G.; Teng, C.; Fan, X.; Guo, S.; Zhao, G.; Zhang, L.; Liang, Z.; Qin, P. Nutrient Composition, Functional Activity and Industrial Applications of Quinoa (Chenopodium quinoa Willd.). Food Chem. 2023, 410, 135290. [Google Scholar] [CrossRef] [PubMed]
- Lagoumintzis, G.; Patrinos, G.P. Triangulating Nutrigenomics, Metabolomics and Microbiomics toward Personalized Nutrition and Healthy Living. Hum. Genom. 2023, 17, 109. [Google Scholar] [CrossRef] [PubMed]
- Singar, S.; Nagpal, R.; Arjmandi, B.H.; Akhavan, N.S. Personalized Nutrition: Tailoring Dietary Recommendations through Genetic Insights. Nutrients 2024, 16, 2673. [Google Scholar] [CrossRef] [PubMed]
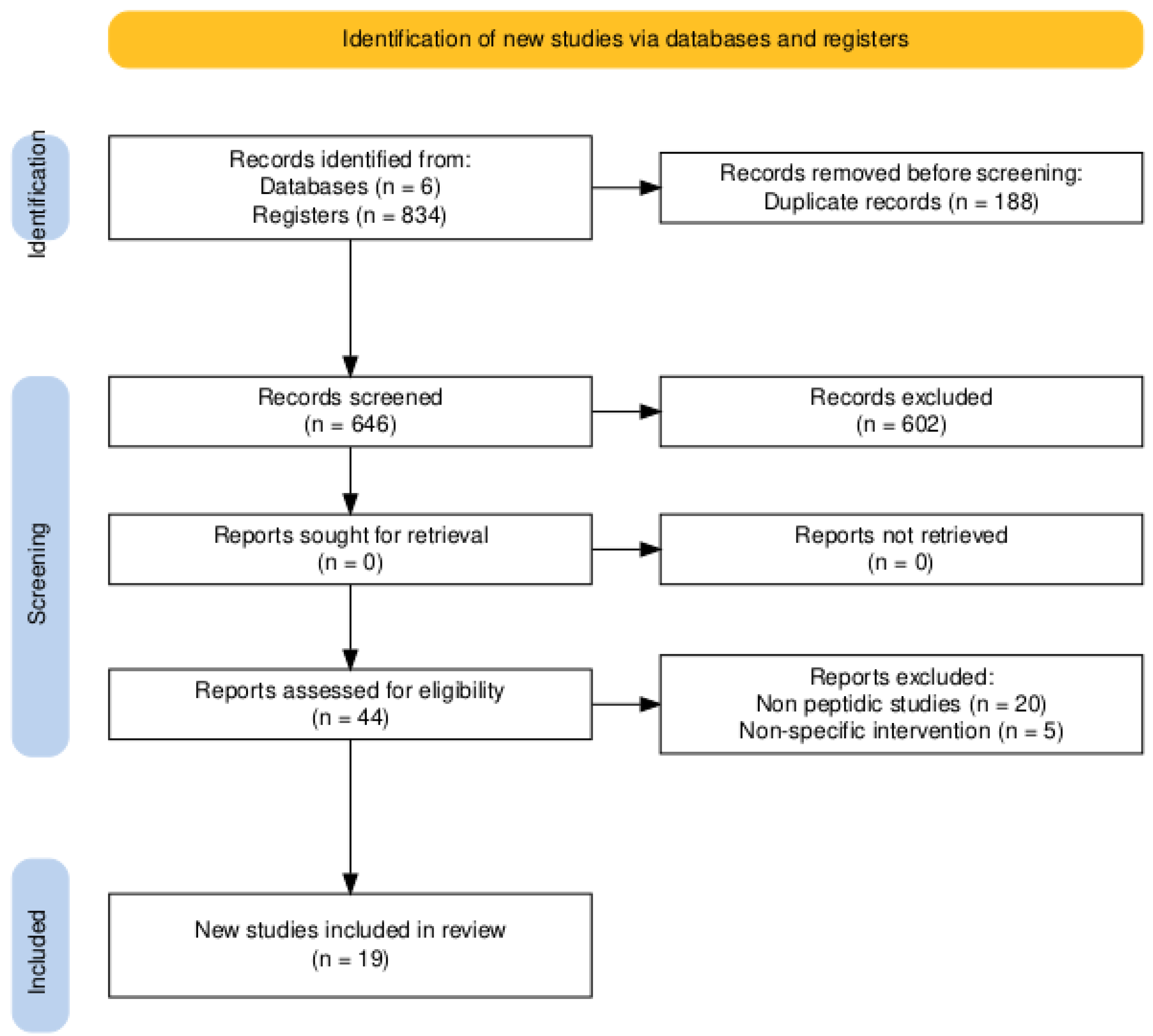
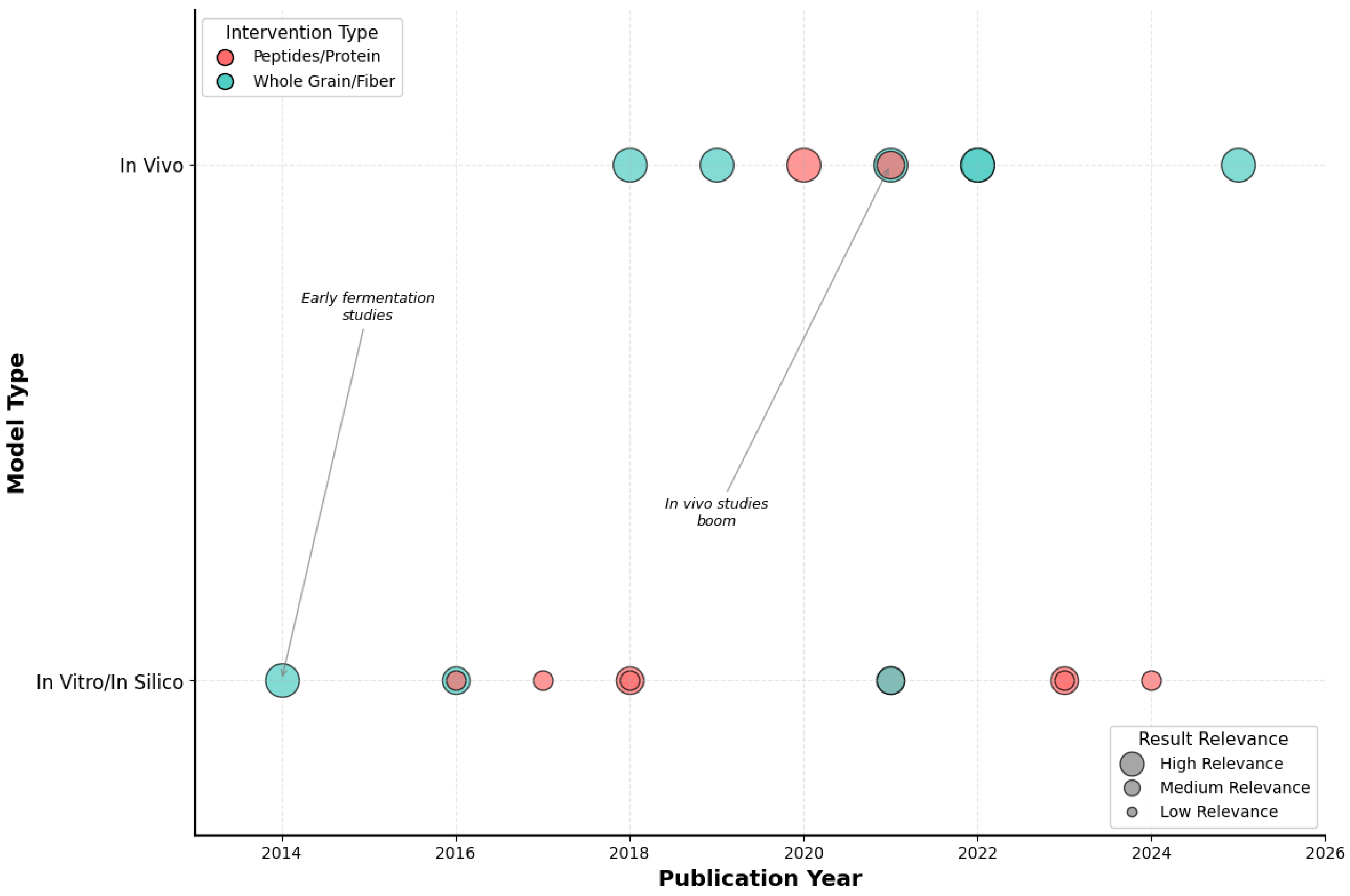
| Reference (Author, Year, Country) | Main Objective | Experimental Model | Main Intervention |
|---|---|---|---|
| Ayyash et al. (2018), UAE [21] | To investigate bioactivity generation through quinoa fermentation. | In vitro (Solid-state fermentation). | Quinoa flour fermented with Bifidobacterium. |
| Bianchi et al. (2014), Brazil [31] | To evaluate the effect of a quinoa beverage in a human GI ecosystem simulator. | In vitro (SHIME® simulator). | Fermented soy–quinoa (30%) beverage. |
| Cizeikiene et al. (2021), Lithuania [32] | To evaluate the effect of lactic fermentation on quinoa characteristics. | In vitro (LAB fermentation). | Quinoa flour fermented with Lactobacillus. |
| Fan et al. (2023), China [33] | To study the preparation and stability of antihypertensive quinoa peptides. | In vitro (Fermentation). | Quinoa flour fermented with L. paracasei. |
| Gullón et al. (2016), Spain/Portugal [26] | To evaluate the prebiotic effect of quinoa. | In vitro (Human fecal fermentation). | Cooked quinoa flour (post-digestion). |
| Mahdavi-Yekta et al. (2023), Iran [34] | To evaluate the antimicrobial activity of quinoa protein hydrolysate. | In vitro (Agar diffusion assay). | Quinoa protein hydrolysate (QHP). |
| Menfaatli et al. (2024), Turkey [35] | To evaluate in silico the antimicrobial potential of quinoa peptides. | In silico (Simulated digestion). | Predicted quinoa peptides. |
| Rizzello et al. (2016), Italy [36] | To evaluate the use of quinoa sourdough to improve bread. | In vitro (Fermentation). | Quinoa flour sourdough. |
| Rizzello et al. (2017), Italy [22] | To enhance quinoa antioxidant properties through fermentation. | In vitro (Fermentation). | Quinoa flour fermented with L. plantarum. |
| Vilcacundo et al. (2018), Ecuador [20] | To evaluate the properties of quinoa peptides. | In vitro (Simulated GI digestion). | Digested quinoa protein. |
| Zeyneb et al. (2021), China [27] | To study the effect of quinoa on human microbiota. | In vitro (Human fecal fermentation). | Raw/cooked quinoa (post-digestion). |
| Reference (Author, Year, Country) | Main Objective | Experimental Model | Main Intervention | Dose (% of Diet, w/w) | Time of Administration |
|---|---|---|---|---|---|
| Fotschki et al. (2020), Poland [11] | To evaluate the effect of quinoa flours on intestinal microbial activity. | In vivo (Wistar rats). | Quinoa protein-rich flour. | 28% daily | 2 weeks |
| Guo et al. (2021), China [38] | To evaluate the effect of quinoa protein on blood pressure and microbiota. | In vivo (SHR hypertensive rats). | Quinoa protein (QP). | 100 mg/kg, 200 mg/kg, and 400 mg/kg | 2 weeks |
| Li et al. (2025), China [23] | To evaluate the effect of quinoa on diabetes and microbiota. | In vivo (db/db diabetic mice). | Quinoa. | 60% and 100% | 9 weeks |
| Liu et al. (2018), China [39] | To investigate the effect of quinoa on colitis and intestinal dysbiosis. | In vivo (DSS-induced colitis mice). | Whole quinoa grain. | 907 g/kg | 10 days |
| Liu et al. (2022), China [24] | To study the effect of quinoa fiber on colitis and microbiota. | In vivo (DSS-induced colitis mice). | Quinoa bran soluble dietary fiber (QBSDF). | 1.5 g/kg | 2 weeks |
| Noratto et al. (2019), USA [37] | To investigate the effect of quinoa on cholesterol metabolism. | In vivo (db/db diabetic mice). | Whole quinoa grain. | 125 g/kg | 8 weeks |
| Wang et al. (2022), China [25] | To investigate the mechanisms of quinoa in obesity. | In vivo (HFD mice). | Whole quinoa grain. | 2 g/day | 6 weeks |
| Fan et al. (2023), China [33] | To study the effect of quinoa on colorectal cancer. | In vivo (C57BL/6 mice) | Digested quinoa protein. | 100 and 400 mg/kg/day | 5 days |
| An et al. (2021), China [40] | To evaluate the effect of quinoa on metabolism and dysbiosis in obese mice. | In vivo (HFD mice). | Whole quinoa grain (with/without saponins). | 2 g/day | 12 weeks |
| Reference (Author, Year) | Animal Model | Intervention | Effect on Alpha Diversity | Key Modulated Taxa |
|---|---|---|---|---|
| An et al. (2021) [40] | HFD mice | Whole grain | Not reported | ↑ Bacteroidetes, ↑ Actinobacteria; ↓ F/B ratio. |
| Guo et al. (2021) [38] | Hypertensive rats | Quinoa protein | Increased | ↑ Turicibacter, ↑ Allobaculum. |
| Li et al. (2025) [23] | Diabetic mice | Whole grain | Increased | ↑ Lactobacillus, ↑ Faecalibaculum; ↓ Helicobacter. |
| Liu et al. (2018) [39] | Colitis mice | Whole grain | Increased | ↓ Proteobacteria, ↓ Escherichia/Shigella. |
| Liu et al. (2022) [24] | Colitis mice | Bran fiber | Increased | ↑ Lachnospiraceae (butyrate producer). |
| Wang et al. (2022) [25] | HFD mice | Whole grain | Not reported | ↑ Blautia; ↓ F/B ratio. |
| Reference (Author, Year) | Experimental Model | Effect on SCFAs | Other Functional Results |
|---|---|---|---|
| Bianchi et al. (2014) [31] | In vitro (SHIME®) | ↑ Butyrate, ↑ Propionate | ↓ Ammonia. |
| Fotschki et al. (2020) [11] | In vivo (Rats) | ↑ Total SCFAs, ↑ Butyrate | ↓ Cecal pH; ↑ Microbial enzymatic activity. |
| Gullón et al. (2016) [26] | In vitro (Fecal) | ↑ Acetate | Not measured. |
| Liu et al. (2022) [24] | In vivo (Mice) | ↑ Butyrate | ↑ Intestinal barrier integrity. |
| Zeyneb et al. (2021) [27] | In vitro (Fecal) | ↑ Total SCFAs | ↓ pH. |
| Reference (Author, Year) | Peptide Production Method | Demonstrated Bioactivity | Target Bacteria | Duration (Hours) |
|---|---|---|---|---|
| Ayyash et al. (2018) [21] | Fermentation with Bifidobacterium. | Release of peptides with antihypertensive activity. | Not applicable. | 48 |
| Cizeikiene et al. (2021) [32] | Fermentation with LAB. | Antimicrobial activity. | E. coli, S. aureus. | 72 |
| Fan et al. (2023) [33] | Fermentation with L. paracasei. | Antimicrobial activity, digestion stability. | E. coli, S. aureus. | 40 |
| Mahdavi-Yekta et al. (2023) [34] | Enzymatic hydrolysis (pepsin, alcalase). | Antimicrobial activity. | E. coli. | 2.5–3.5 |
| Menfaatli et al. (2024) [35] | In silico digestion (pepsin). | Predicted antimicrobial and antibiofilm activity. | H. pylori. | Not applicable. |
| Rizzello et al. (2016) [36] | Fermentation with LAB (sourdough). | Increased proteolysis. | Not applicable. | 16 |
| Rizzello et al. (2017) [22] | Fermentation with LAB. | Release of peptides with antioxidant activity. | Not applicable. | 24 |
| Vilcacundo et al. (2018) [20] | Simulated GI digestion (pepsin–pancreatin). | Identification of released peptides (<3 kDa). | Not applicable. | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caicedo, N.; Liscano, Y.; Oñate-Garzón, J. Bioactive Peptides from Quinoa (Chenopodium quinoa Willd.) as Modulators of the Gut Microbiome: A Scoping Review of Preclinical Evidence. Nutrients 2025, 17, 3215. https://doi.org/10.3390/nu17203215
Caicedo N, Liscano Y, Oñate-Garzón J. Bioactive Peptides from Quinoa (Chenopodium quinoa Willd.) as Modulators of the Gut Microbiome: A Scoping Review of Preclinical Evidence. Nutrients. 2025; 17(20):3215. https://doi.org/10.3390/nu17203215
Chicago/Turabian StyleCaicedo, Nicolás, Yamil Liscano, and Jose Oñate-Garzón. 2025. "Bioactive Peptides from Quinoa (Chenopodium quinoa Willd.) as Modulators of the Gut Microbiome: A Scoping Review of Preclinical Evidence" Nutrients 17, no. 20: 3215. https://doi.org/10.3390/nu17203215
APA StyleCaicedo, N., Liscano, Y., & Oñate-Garzón, J. (2025). Bioactive Peptides from Quinoa (Chenopodium quinoa Willd.) as Modulators of the Gut Microbiome: A Scoping Review of Preclinical Evidence. Nutrients, 17(20), 3215. https://doi.org/10.3390/nu17203215







