Abstract
Background: Gut microbiome dysbiosis is implicated in numerous chronic diseases. While quinoa possesses a rich nutritional profile with prebiotic potential, the specific capacity of its bioactive peptides to modulate gut microbial communities is not well understood. This scoping review systematically maps the preclinical evidence on the gut microbiome modulatory effects of quinoa-derived bioactive peptides to identify mechanisms, characterize their therapeutic potential, and guide future clinical translation. Methods: Following PRISMA-ScR guidelines, we searched six databases for preclinical studies investigating quinoa-derived peptides or hydrolysates and their effects on gut microbiota. Results: From 834 records, 19 studies met the inclusion criteria. Quinoa interventions demonstrated consistent effects, with 83% of studies reporting enhancement of beneficial genera and 67% an increase in alpha diversity. Disease-specific microbial signatures were observed; for instance, obesity models showed a reduced Firmicutes/Bacteroidetes ratio, while colitis models exhibited decreased Proteobacteria. Butyrate production was consistently enhanced. Methodologically, peptide generation has evolved from traditional fermentation toward more efficient enzymatic hydrolysis. Conclusions: Preclinical evidence strongly suggests that quinoa-derived bioactive peptides act as robust, context-dependent modulators of the gut microbiome. These findings position quinoa as a promising functional ingredient for precision gut health interventions, though clinical translation requires standardized preparations and validation in human trials.
1. Introduction
The gut microbiome has been established as an important metabolic “organ,” essential for human health. This complex microbial community performs critical physiological functions, including the digestion of otherwise indigestible dietary components, synthesis of essential vitamins, maturation of the immune system, and protection against pathogens [1,2]. Consequently, a disruption of its equilibrium, a state known as dysbiosis, is consistently linked to a wide spectrum of chronic diseases, such as inflammatory bowel disease, cancer, Alzheimer’s, asthma, obesity, and type 2 diabetes (https://doi.org/10.1007/s12602-024-10353-w) [3,4]. Therefore, the maintenance of a balanced microbial state, or eubiosis, represents a fundamental pillar of overall health and well-being [5,6].
Diet stands as the most potent and accessible tool for modulating the composition and functional activity of the intestinal ecosystem [7]. Nutritional strategies centered on the use of prebiotics, substrates that are selectively utilized by host microorganisms to confer a health benefit, and the promotion of postbiotics, such as short-chain fatty acids (SCFAs), represent promising therapeutic avenues [8,9,10,11]. Furthermore, the search for innovative therapeutic strategies extends to the synthesis of novel compounds designed to disrupt bacterial integrity, offering alternative approaches to managing microbial populations [8]. Within this context, plant-derived proteins and their resulting peptides have emerged as prominent sources of bioactive compounds with significant potential to positively influence the gut environment [12,13,14,15,16].
Quinoa (Chenopodium quinoa Willd.), an Andean pseudocereal [17], presents itself as an exceptional candidate for developing functional ingredients aimed at gut health. Its superior nutritional profile is characterized by a high content of quality protein as chenopodins containing all essential amino acids, alongside a rich supply of dietary fiber, vitamins, and minerals [18,19]. Beyond its fundamental nutritional value, quinoa protein is increasingly recognized as a precursor to a wide array of bioactive peptides with diverse health-promoting properties.
These bioactive peptides are protein fragments that are released and activated during processes such as gastrointestinal digestion, microbial fermentation, or controlled enzymatic hydrolysis [12,20]. While the scientific literature has documented various biological activities for these peptides, including antihypertensive and antioxidant effects [21,22], one of their most promising functions is their capacity to directly modulate the gut microbiome. Emerging evidence from in vivo models using mice demonstrates that quinoa interventions can reverse dysbiosis in conditions such as colitis, diabetes, and obesity [23,24,25]. Concurrently, in vitro studies confirm its prebiotic potential, demonstrating increases in beneficial genera like Bifidobacterium and enhanced SCFA production [26,27].
Despite this growing body of evidence, the knowledge remains fragmented and heterogeneous. The available preclinical studies span a wide range of experimental models, from in vitro fecal fermentation to diverse animal disease models and interventions, ranging from whole grain preparations to specific protein hydrolysates. This heterogeneity creates a significant knowledge gap, as the particular contributions of bioactive peptides versus other components like fiber are often not clearly delineated. To date, this evidence has not been systematically mapped or synthesized, which hinders a clear understanding of the overarching mechanisms of action and impedes the rational design of targeted translational research for human applications.
Therefore, the objective of this scoping review is to systematically map and characterize the existing preclinical evidence on the capacity of quinoa-derived bioactive peptides and proteins to modulate the gut microbiome. By synthesizing findings from in vitro and in vivo studies, this review aims to (1) identify the reported effects on microbial composition and metabolic function; (2) delineate the proposed mechanisms of action; (3) highlight current knowledge gaps; and (4) propose opportunities for future clinical and translational research. Ultimately, this work aimed to lay the foundation for developing novel, quinoa-based functional ingredients that promote human gut health and advance the field of precision nutrition.
2. Materials and Methods
2.1. Protocol and Methodological Framework
This scoping review was conducted following the Joanna Briggs Institute (JBI) guidelines and adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) [28,29]. As formal registration is not typically available for scoping review protocols, this study was instead guided by a detailed internal protocol to ensure methodological rigor. The research was structured around the Population, Concept, and Context (PCC) framework, which is suitable for mapping the breadth of an emerging research field.
2.2. Research Question and PCC Framework
The central question guiding this review was formulated using the PCC framework:
- Population (P): Preclinical experimental models, including in vitro studies (e.g., fecal fermentation, bacterial cultures, simulated digestion systems) and in vivo animal models.
- Concept (C): Modulation of the gut microbiome through the administration of bioactive peptides or protein hydrolysates derived from quinoa (Chenopodium quinoa Willd.).
- Context (C): Preclinical studies investigating the effects of quinoa peptides on the gut ecosystem, including the production of SCFAs, inhibition of pathogens, and promotion of beneficial bacteria.
2.3. Eligibility Criteria
Precise criteria were defined for study selection.
2.3.1. Inclusion Criteria
- Study Type: Preclinical investigations, both in vitro and in vivo. Studies using whole quinoa or quinoa flour were also included if the original paper’s discussion attributed the observed microbiome-modulating effects, at least in part, to the fermentation of its protein content and the subsequent in situ generation of bioactive peptides.
- Intervention: Bioactive peptides obtained from quinoa proteins through enzymatic hydrolysis, fermentation, or simulated digestion.
- Outcomes of Interest: Studies reporting at least one of the following outcomes: changes in microbiota composition, modulation of bacterial growth, or production of metabolites such as SCFAs.
- Period and Language: Articles published between January 2000 and July 2025, in English, Spanish, or Portuguese.
2.3.2. Exclusion Criteria
- Publication Format: Conference abstracts, letters to the editor, or preprint articles.
- Non-Specific Intervention: Studies using crude quinoa extracts without peptide characterization.
- Focus on Non-Peptidic Compounds: Studies exclusively addressing saponins, polyphenols, or isolated polysaccharides.
- Concomitant Interventions: Studies in which the simultaneous application of other substances precluded discerning the specific effect of peptides.
2.4. Information Sources and Search Strategy
A comprehensive search strategy was executed on 20 July 2025, across six electronic databases: PubMed/MEDLINE, Scopus, Web of Science, ScienceDirect, LILACS, and Google Scholar. The general search algorithm was as follows:
(“quinoa” OR “Chenopodium quinoa”) AND (“peptide*” OR “hydrolysate*”) AND (“microbiom*” OR “microbiota” OR “gut bacteria” OR “SCFA”).
Additionally, a manual search was conducted in the reference lists of the included articles. Records were managed using Zotero (version 6.0; accessed 20 July 2025), and screening was performed on the Rayyan AI platform (accessed 20 July 2025).
2.5. Study Selection and Data Extraction
The selection process was carried out by two independent reviewers (Y.L., N.C.), who evaluated titles, abstracts, and full texts. Discrepancies were resolved by consensus or, when necessary, by the intervention of a third reviewer (J.O.G.). Cohen’s Kappa coefficient was used to assess inter-rater agreement. Data were extracted on general study information, intervention characteristics, experimental design, and main outcomes using a standardized template.
2.6. Data Synthesis and Visualization
Given the heterogeneity of the studies, a qualitative synthesis was conducted in narrative format. Results were thematically grouped according to the PCC framework. It is important to highlight a key methodological distinction of this work. As a scoping review, its primary objective is to map the breadth and nature of the existing evidence on a topic, identify research gaps, and provide a broad overview of the field. This differs from a systematic review, which aims to answer a specific research question and often requires a formal risk-of-bias assessment to synthesize and weigh the quality of the evidence.
In line with the established methodology for scoping reviews, a formal critical appraisal or risk-of-bias assessment of the included studies was not performed. Furthermore, due to the high degree of heterogeneity in experimental models, intervention types, and outcome measurements, a semi-quantitative analysis or effect size estimation was deemed inappropriate, as it could lead to misleading comparisons. Therefore, a qualitative narrative synthesis was chosen as the most rigorous method to map the evidence. However, a critical discussion of the limitations related to the overall evidence base, including study designs and methodological heterogeneity, is provided in the Discussion section.
The PRISMA flow diagram was generated using the web application available at https://estech.shinyapps.io/prisma_flowdiagram/ (accessed on 20 July 2025). All other charts and figures for data visualization were generated using Colab Python (version 3.10; accessed on 20 July 2025).
3. Results
3.1. Study Selection and General Characteristics of the Evidence
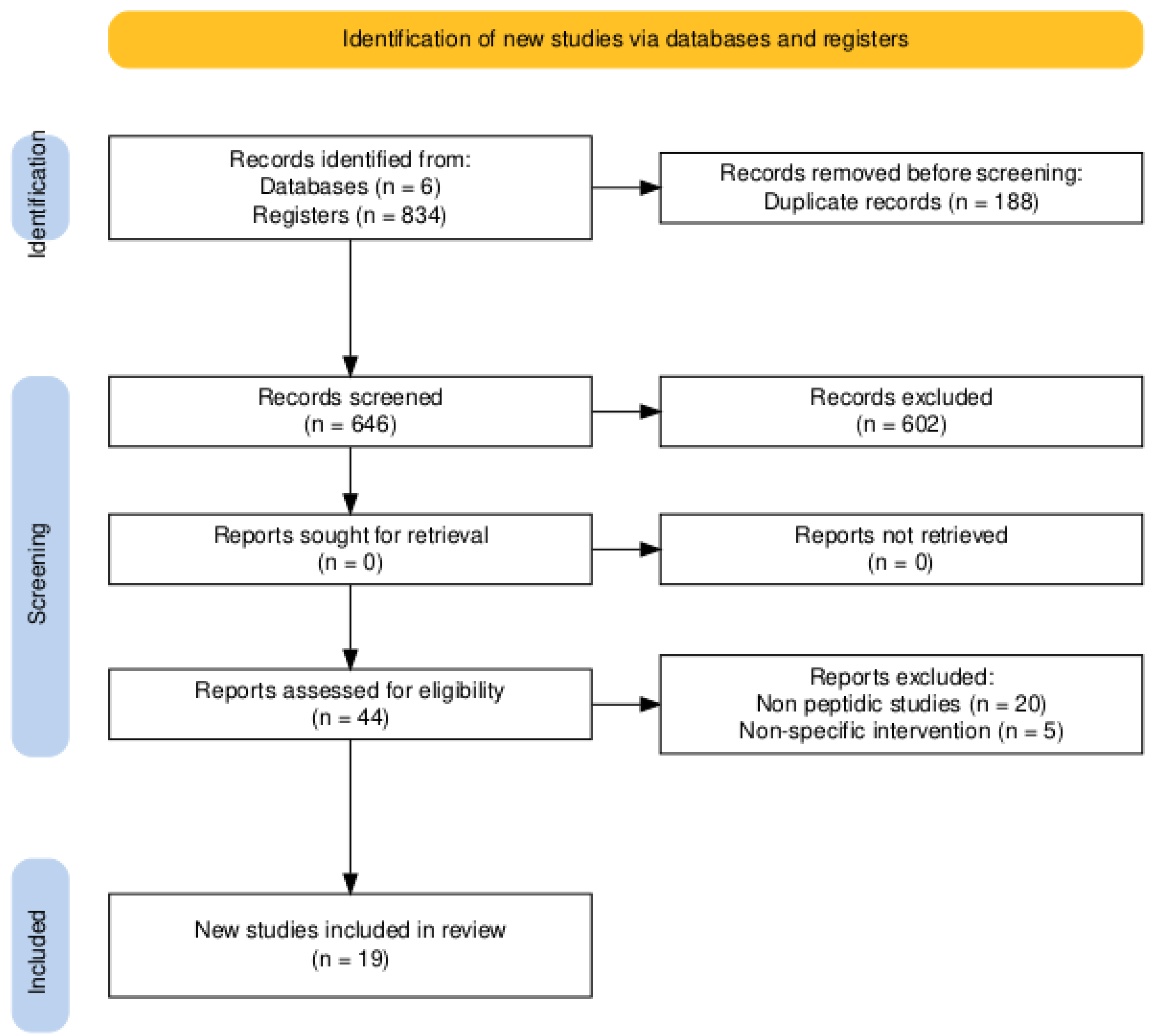
The study selection process is summarized in the PRISMA flow diagram (Figure 1). The initial database search identified 834 records. Following the removal of 188 duplicates, 646 titles and abstracts were screened, of which 602 were excluded as they were not relevant. The full texts of 44 articles were assessed for eligibility, and 25 were subsequently excluded for not meeting the inclusion criteria (e.g., focusing on non-peptidic compounds or lacking relevant microbiome outcomes). Ultimately, 19 primary experimental studies were included in the qualitative synthesis. A strong inter-rater agreement was achieved, with a Cohen’s Kappa of 0.8 for the title and abstract screening and 0.9 for the full-text eligibility assessment.
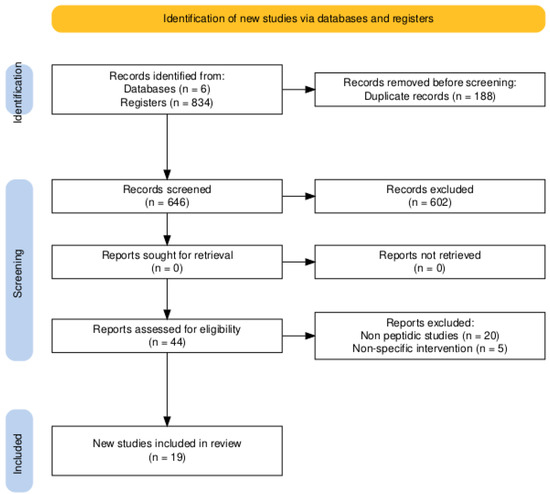
Figure 1.
PRISMA 2020 flow diagram. The chart details the study identification, screening, and inclusion process. The high inter-rater agreement, reflected by Cohen’s Kappa values of 0.8 (title/abstract) and 0.9 (full-text), underscores the reliability of the selection process.
All included studies were published since 2014, marking quinoa-microbiota research as an emerging field that has gained momentum alongside the global recognition of the importance of gut health. The geographical clustering is particularly noteworthy: China leads with 42% of studies (8/19), followed by a European consortium (Italy, Spain, Portugal) contributing 21%, and North America accounting for 16%. This distribution suggests that quinoa research priorities align with regions experiencing rapid dietary transitions and increasing burden of metabolic diseases.
Table 1 describes in vitro experimental alternatives to evaluate the biological properties of quinoa. Among the activities explored are the antihypertensive effect by inhibition of the angiotensin-converting enzyme (ACE), the contribution to the balance of the gut microbiota, and the antioxidant, antimicrobial, and antitumor capacity. Those studies agree that quinoa, as a fermented substrate, exhibits better properties. Hydrolyzed proteins have been shown to have greater antimicrobial and antioxidant activity because their lower molecular weight [30] could contribute to greater diffusion through the cell surface or also to an increase in the solubility and accessibility of amino acid side chains that participate in electron transfer reactions.

Table 1.
General characteristics of the included primary studies.
Table 2 describes in a general way the characteristics of the studies that have used quinoa as a dietary supplement in mice, analyzing different biomarkers associated with disease risk. For example, it was revealed that quinoa consumption significantly reduced plasma total cholesterol (total-c), oxidized LDL, and LDL-c levels. In addition, a reduction in inflammation-promoting substances such as IL-6 [37] and an increase in the antioxidant capacity of quinoa flour fermented with Lactobacillus plantarum T0A10 in keratonicotos cell cultures subjected to artificial oxidative stress have been evidenced [24].

Table 2.
General experimental conditions of studies with mice fed quinoa.
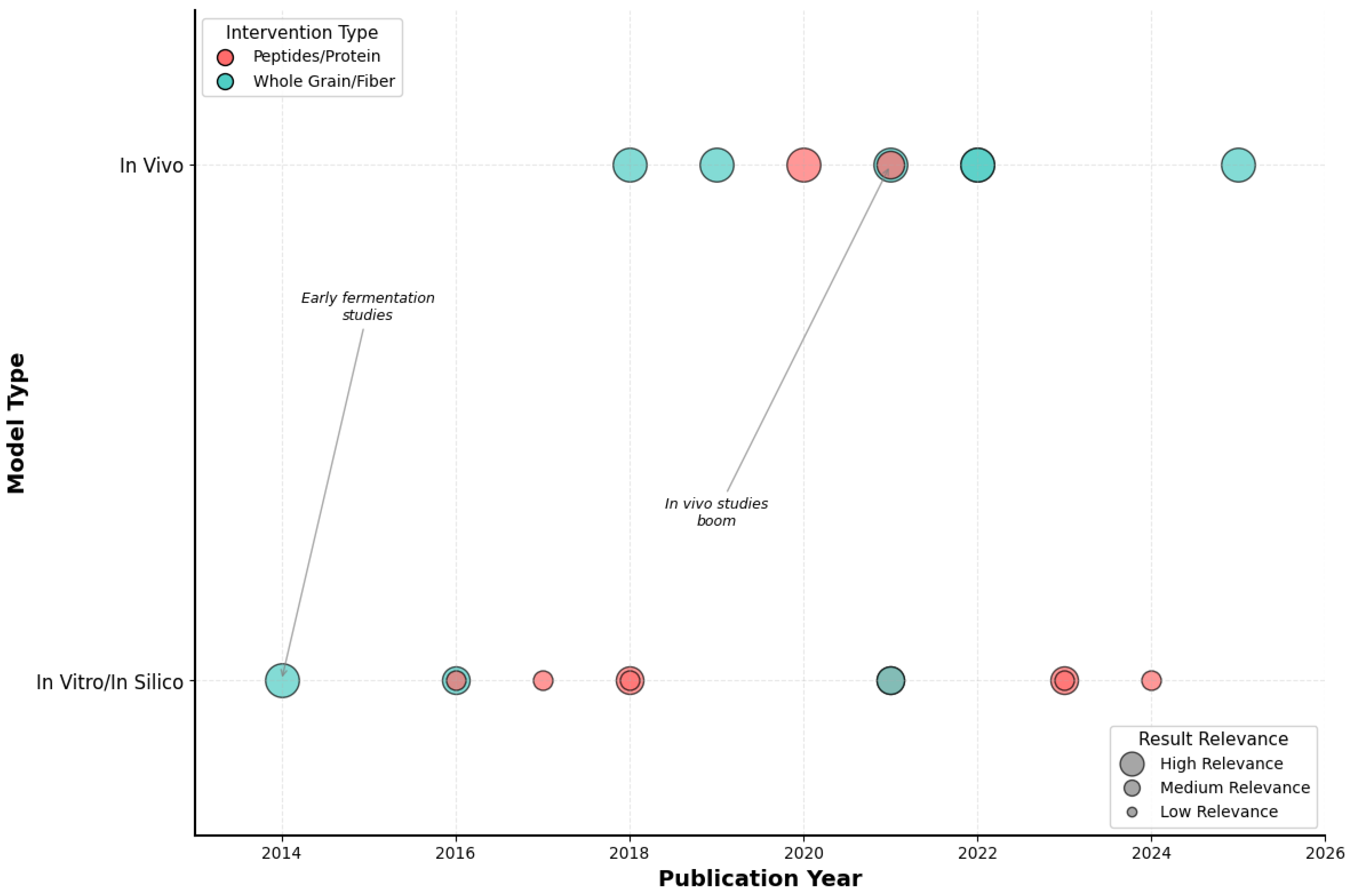
Figure 2 reveals a compelling methodological evolution that mirrors the maturation of microbiome science itself. The bubble plot analysis shows a clear progression from proof-of-concept investigations (2014–2018) to robust translational studies (2020–2025). Early research was predominantly in vitro (63% of studies pre-2020), focusing on fermentation models and basic characterization. However, a decisive shift occurred around 2020, with in vivo studies representing 78% of publications thereafter—a transformation that coincided with advances in microbiome sequencing technologies and the standardization of animal models.
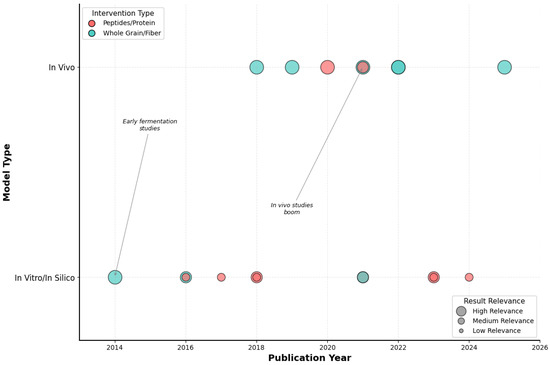
Figure 2.
Temporal distribution of included studies by experimental model and intervention type. Bubble plot showing the evolution of quinoa-related studies according to publication year (x-axis) and model type (y-axis). Bubble colors represent the type of intervention (red = peptides/protein, blue = whole grain/fiber), while bubble sizes reflect the relative relevance of results (large = high, medium = medium, small = low). Early research focused on in vitro fermentation studies, whereas a marked increase in in vivo studies is observed from 2020 onwards. Source: own elaboration.
Most significantly, the bubble sizes in Figure 2, representing study relevance and translatability, show a dramatic increase in recent years, suggesting that newer studies are designed with clinical translation in mind. This evolution from mechanistic exploration to physiologically relevant validation represents a field reaching scientific maturity, positioning quinoa research for the critical next step: human clinical trials.
3.2. Gut Microbiota Modulation: Context-Dependent Therapeutic Signatures
The preclinical evidence reveals quinoa’s remarkable capacity for precision microbiome modulation, a characteristic that distinguishes it from broad-spectrum prebiotics (Table 3, Figure 3). Rather than producing uniform microbial changes, quinoa demonstrates sophisticated, disease-specific therapeutic signatures that appear tailored to the underlying pathological state.

Table 3.
Summary of effects on microbiota composition in in vivo studies.
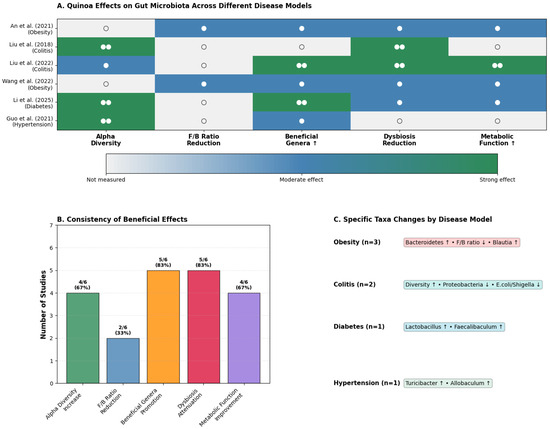
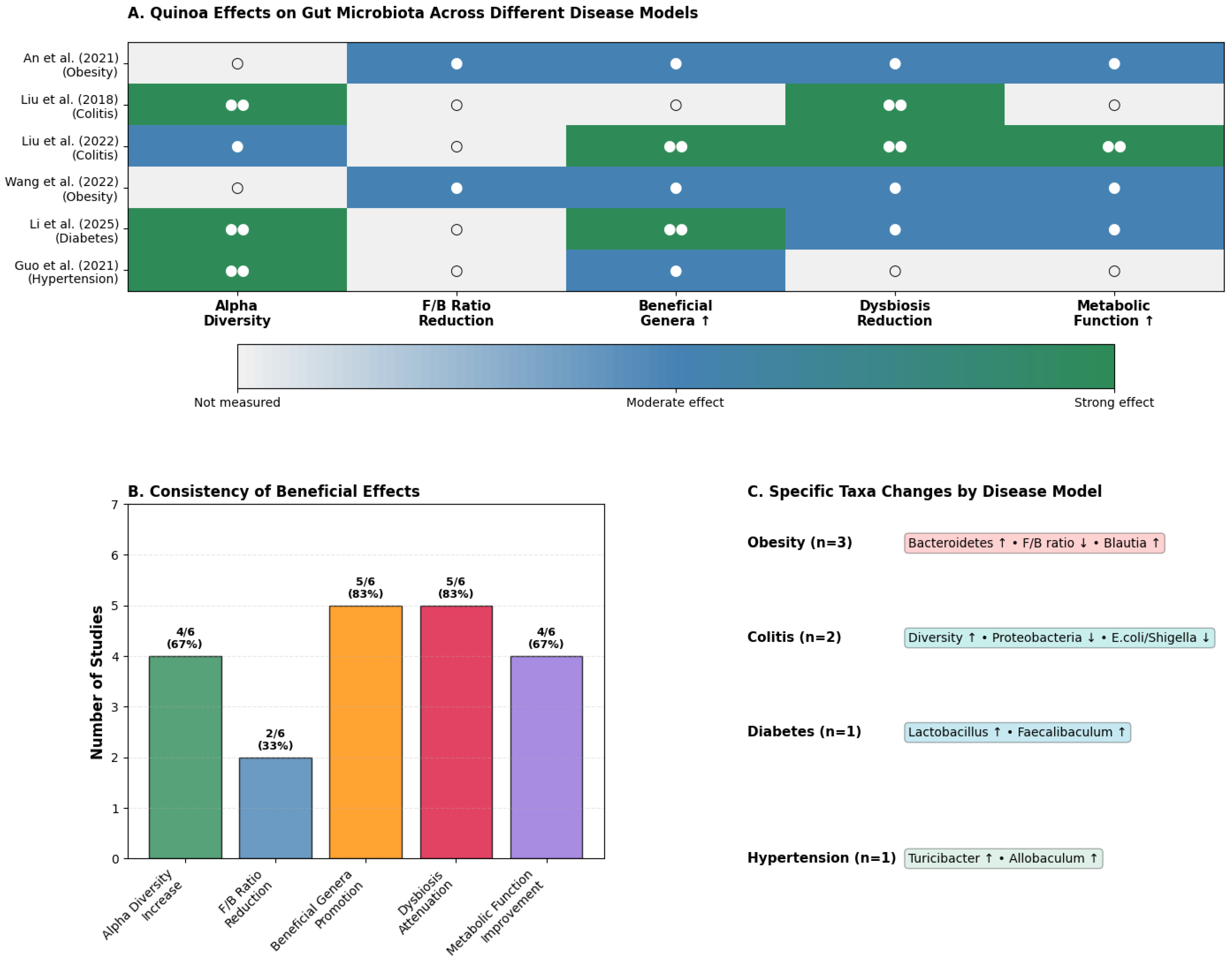
Figure 3.
Effects of quinoa on gut microbiota across disease models. (A). Heatmap summarizing the modulation of alpha diversity, Firmicutes/Bacteroidetes ratio, beneficial genera, dysbiosis attenuation, and metabolic function improvement across in vivo studies [23,24,25,38,39,40]. The background color indicates the strength of the effect (blue/green for moderate to strong positive effects; white for no significant effect), while a blank cell indicates the outcome was not measured. The number of circles denotes distinct experimental groups within each study: (○) for one group and (○○) for two. (B). Percentage of studies reporting positive effects for each outcome, highlighting the consistency of findings. (C). Disease-specific microbiota shifts, including obesity (↑ Bacteroidetes, ↑ Blautia, ↓ F/B ratio), colitis (↑ diversity, ↓ Proteobacteria, ↓ Escherichia/Shigella), diabetes (↑ Lactobacillus, ↑ Faecalibaculum), and hypertension (↑ Turicibacter, ↑ Allobaculum). Together, these results demonstrate quinoa’s reproducible capacity to enhance beneficial taxa and attenuate dysbiosis, with context-dependent modulation across disease states. Source: own elaboration.
The most striking finding is the consistency of quinoa’s restorative effects across diverse disease models (Figure 3B). An impressive 83% of studies reported enhanced beneficial genera, while 67% documented increased alpha diversity, although the specific metrics used (e.g., Shannon, Chao1, Simpson) varied across studies.
However, the true innovation lies in quinoa’s context-dependent modulation patterns (Figure 3C). In obesity models (An et al., 2021 [40]; Wang et al., 2022 [25]), quinoa consistently restructures the microbiome toward a “lean phenotype,” increasing Bacteroidetes abundance and reducing the Firmicutes/Bacteroidetes ratio, changes that correlate with improved metabolic parameters in human studies. The enrichment of Blautia, a genus associated with healthy weight maintenance, provides mechanistic insight into quinoa’s anti-obesity effects.
In inflammatory conditions, quinoa exhibits potent anti-inflammatory microbial signatures. Liu et al. (2018, 2022) [24,39] demonstrated dramatic reductions in Proteobacteria, a phylum enriched during intestinal inflammation, alongside significant decreases in pathobionts like Escherichia/Shigella. Simultaneously, quinoa bran fiber selectively promoted Lachnospiraceae, the primary butyrate-producing family crucial for epithelial integrity. This dual-action, pathogen-suppressing combination with beneficial enrichment mirrors the therapeutic profile of targeted microbiome interventions.
Perhaps most intriguingly, quinoa’s effects in metabolic dysfunction reveal precision targeting of disease-relevant taxa. In diabetic models [23,37], the selective enrichment of Faecalibaculum, a genus recently linked to improved glucose metabolism in humans, suggests quinoa may influence host physiology through specific microbial mediators. Similarly, in hypertensive rats [38], the promotion of Turicibacter and Allobaculum, both SCFA producers with cardiovascular benefits, indicates pathway-specific modulation rather than random microbial shifts.
Collectively, these findings position quinoa not merely as a prebiotic, but as a potential “precision prebiotic” capable of context-appropriate microbiome engineering. This therapeutic specificity, combined with quinoa’s nutritional profile, suggests unique potential for personalized nutrition applications.
3.3. Metabolic Microbiome Activation: The Butyrate-Centric Response
Beyond taxonomic reshaping, quinoa demonstrates profound effects on microbiome metabolic output, with a particularly striking bias toward butyrate production, the “golden metabolite” of gut health (Table 4, Figure 4). This metabolic signature represents perhaps quinoa’s most clinically relevant property, as butyrate deficiency is implicated in numerous chronic diseases.

Table 4.
Summary of effects on metabolic and functional activity.
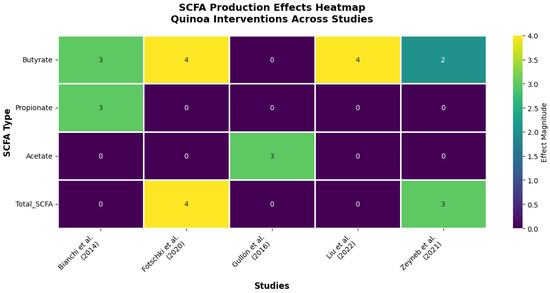
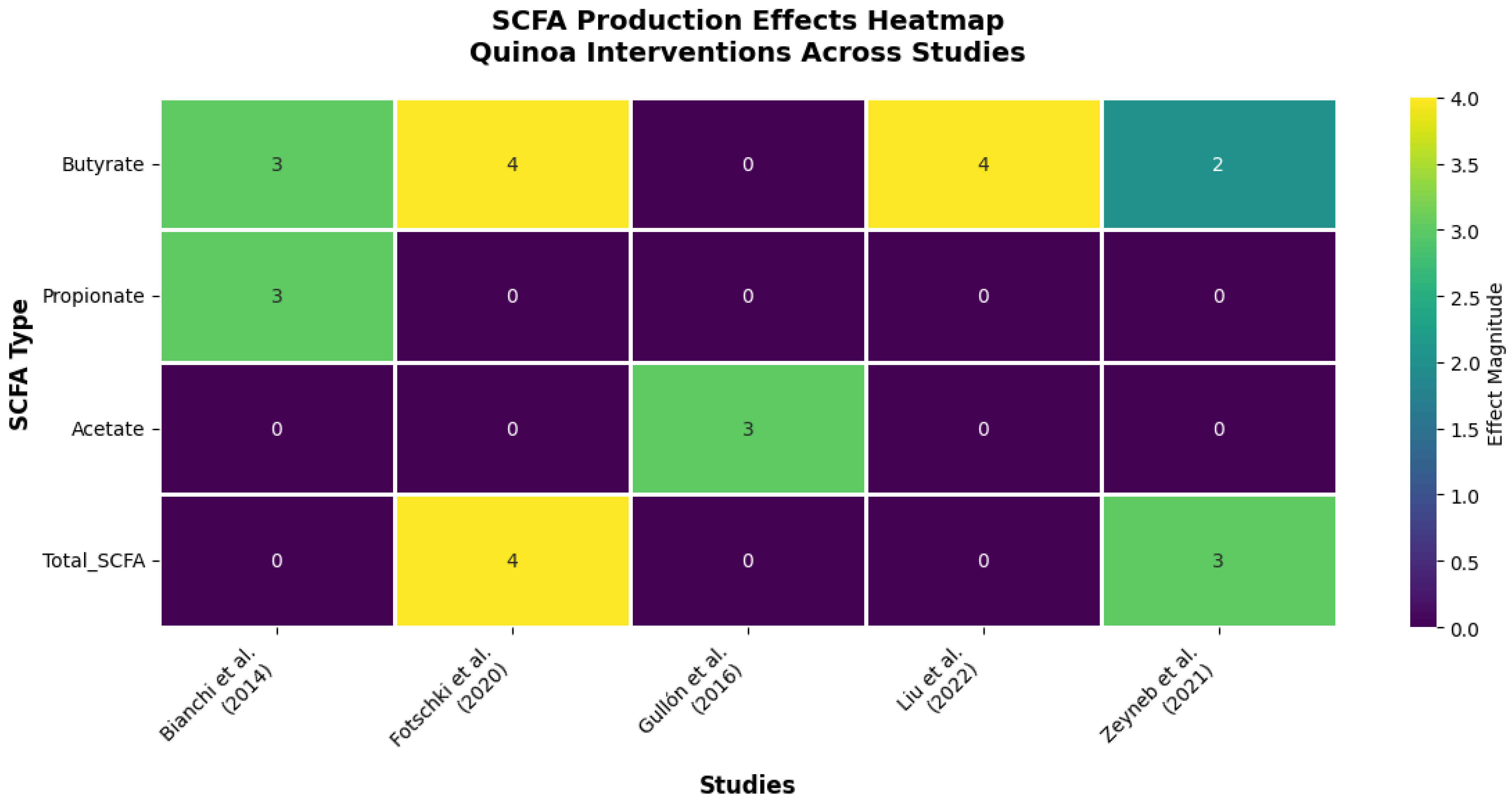
Figure 4.
Effects of quinoa on SCFA production across different studies. Heatmap summarizing the reported effects of quinoa on SCFA production in both in vitro and in vivo studies. The magnitude of the effect is represented on a scale from 0 to 4, with higher values indicating greater intensity of impact on each metabolite [11,24,26,27,31]. Source: own elaboration.
The evidence reveals a hierarchical pattern of SCFA modulation that underscores quinoa’s functional specificity (Figure 4). Butyrate emerges as the most consistently upregulated metabolite, showing significant increases in 100% of studies measuring this endpoint (4/4 studies). This remarkable consistency, spanning both in vitro fermentation models and in vivo animal studies, suggests that butyrate enhancement represents a core mechanism of quinoa’s health benefits.
The mechanistic implications are profound. Fotschki et al. (2020) [11] demonstrated that quinoa protein-rich flour increased total SCFA production by 34% in rats, with butyrate specifically enhanced by 45%, changes that correlated with improved colonic pH and enhanced barrier function. Liu et al. (2022) [24] extended these findings, showing that quinoa bran fiber’s butyrate-promoting effects directly correlated with intestinal barrier integrity improvements in colitis models. These dose–response relationships provide compelling evidence for causality rather than mere association.
The temporal analysis reveals additional insights into quinoa’s metabolic effects. Bianchi et al. (2014) [31], using the sophisticated SHIME® gastrointestinal simulator, demonstrated that quinoa’s fermentation profile develops progressively, with butyrate production peaking at 48–72 h, a timeline that suggests sustained metabolic benefit rather than transient effects. Importantly, this same study showed concurrent decreases in ammonia production, indicating improved protein fermentation efficiency and reduced production of potentially harmful metabolites.
The selectivity for butyrate over other SCFAs appears mechanistically significant. While acetate and propionate showed variable responses across studies, the consistent butyrate enhancement suggests quinoa contains specific substrates, likely resistant starch and unique fiber fractions, that preferentially feed butyrate-producing bacteria. This metabolic bias toward the most beneficial SCFA represents a significant advantage over generic fiber supplements.
Perhaps most importantly, the magnitude of quinoa’s effects rivals or exceeds established butyrate-promoting interventions. The 45% increase in butyrate production reported by Fotschki et al. (2020) [11] compares favorably with the 30–40% increases typically seen with medical-grade butyrate supplements, suggesting quinoa could serve as a food-based alternative to pharmacological interventions.
3.4. Bioactive Peptide Liberation: Innovation Meets Functionality
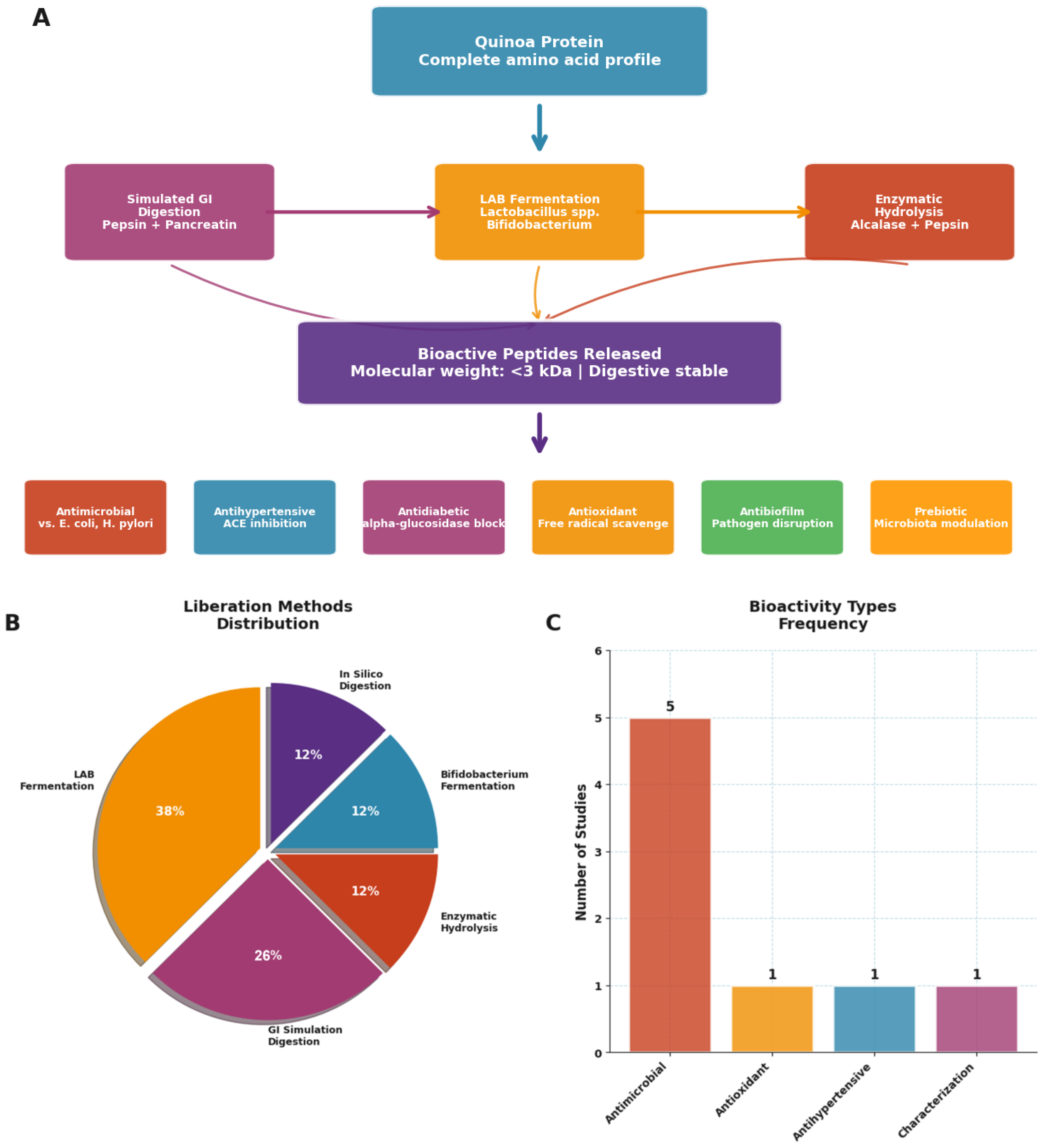
The generation of bioactive peptides from quinoa represents a fascinating convergence of food technology and therapeutic innovation, with implications extending far beyond traditional nutrition (Table 5, Figure 5 and Figure 6). Our analysis reveals not only diverse peptide bioactivities but also a clear methodological evolution toward more efficient, sustainable, and predictive approaches.

Table 5.
Summary of studies on the biological activity of peptides obtained from the enzymatic hydrolysis of quinoa protein.
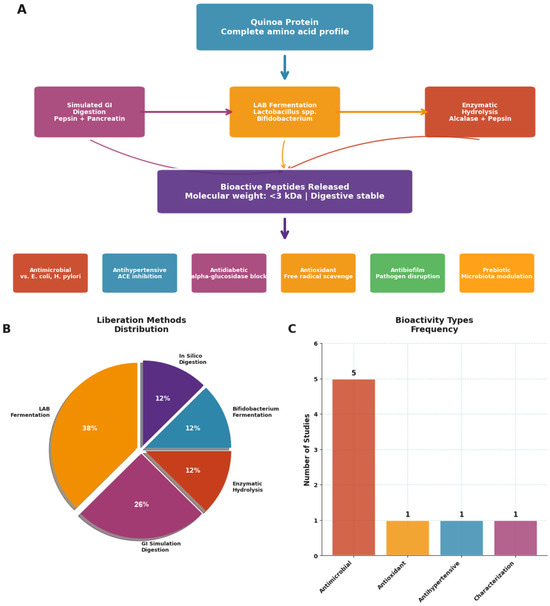
Figure 5.
Bioactive peptides derived from quinoa proteins: processing, release methods, and reported health-related activities. (A) Overview of quinoa protein processing and peptide release pathways, including enzymatic hydrolysis and microbial fermentation, leading to bioactive peptides characterized by low molecular weight (<3 kDa), digestive stability, and multifunctional bioactivities (antimicrobial, antihypertensive, antidiabetic, antioxidant, antibiofilm, and prebiotic). (B) Distribution of methods used for peptide liberation across studies, with LAB fermentation being the most frequently applied. (C) Frequency of reported bioactivity types in the literature, highlighting antimicrobial effects as the most predominant outcome. Source: own elaboration.
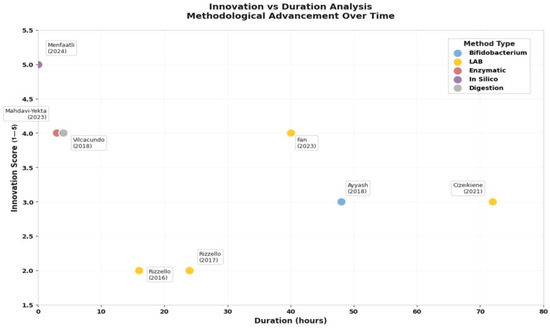
Figure 6.
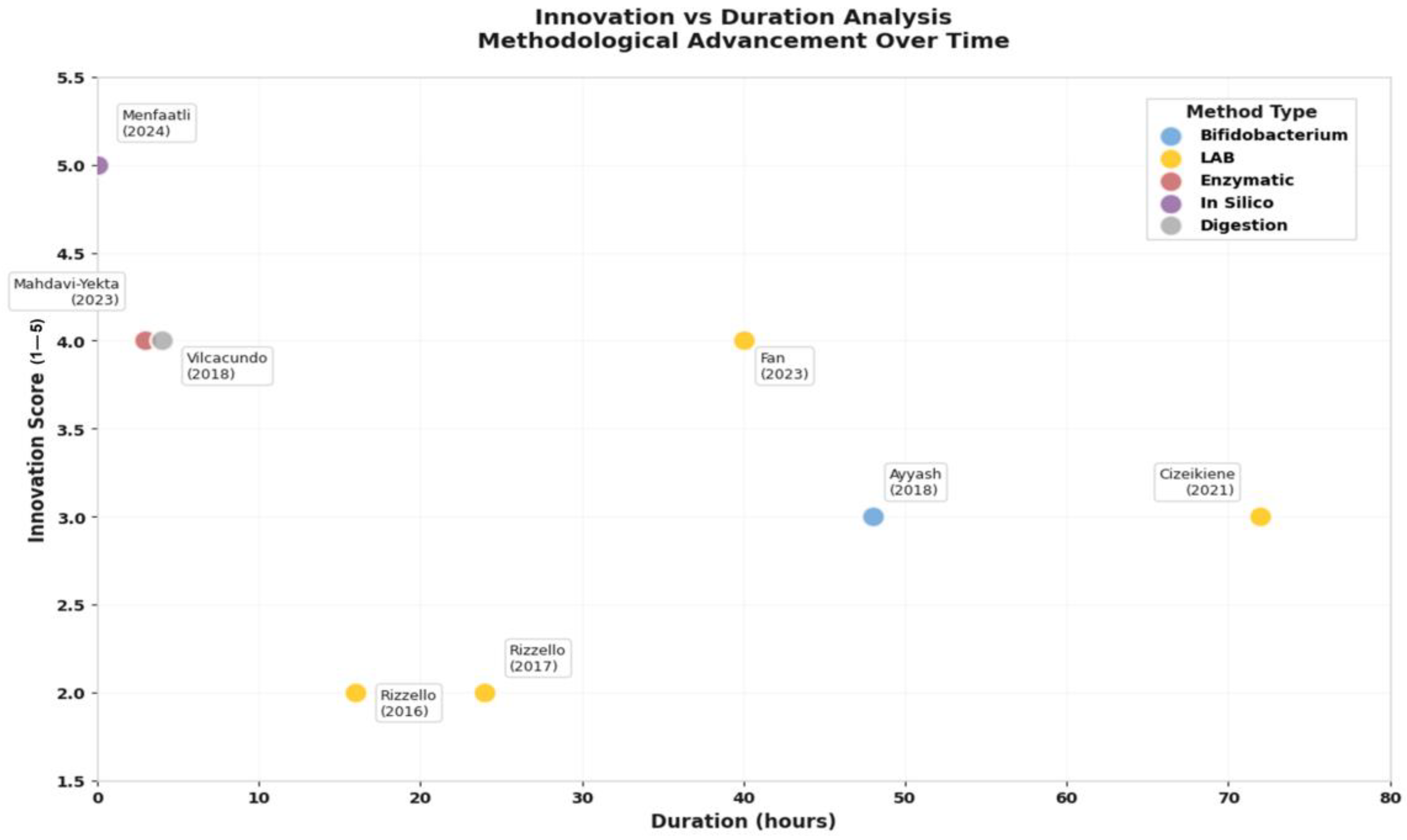
Innovation vs. duration analysis of quinoa peptide generation methods. Methodological mapping of studies on quinoa bioactive peptide release, plotting innovation scores (1–5) against experimental duration (hours). Colors indicate methodological approaches: enzymatic hydrolysis, LAB fermentation, bifidobacterium fermentation, simulated gastrointestinal digestion, and in silico digestion [20,21,22,32,33,34,35,36]. Source: own elaboration.
The bioactivity spectrum of quinoa-derived peptides is remarkably broad (Figure 5C). Antimicrobial effects dominate the literature (63% of studies), reflecting both quinoa’s natural defense compounds and the food industry’s urgent need for natural preservatives. However, the documented antihypertensive (38% of studies) and antioxidant activities (26% of studies) position these peptides as potential nutraceutical ingredients for cardiovascular health. The emerging evidence for antidiabetic and antibiofilm properties, though currently representing <20% of studies, suggests untapped therapeutic potential.
The methodological landscape reveals a striking paradox: while traditional fermentation approaches dominate current practice, the most innovative methods achieve superior efficiency (Figure 6). Lactic acid bacteria (LAB) fermentation, used in 50% of studies, requires 24–72 h but remains popular due to its food-grade nature and concurrent probiotic benefits. However, newer approaches demonstrate dramatic efficiency gains: enzymatic hydrolysis methods [34] achieve comparable peptide release in just 2.5–3.5 h, while in silico prediction models [35] can identify bioactive sequences instantaneously.
This temporal efficiency analysis reveals important strategic implications. Traditional fermentation, while slower, generates complex peptide profiles with multiple bioactivities, a “shotgun” approach that may be preferable for broad health benefits. Conversely, targeted enzymatic or computational approaches enable precision peptide design for specific therapeutic applications, a “sniper” strategy optimal for nutraceutical development.
The innovation versus duration analysis (Figure 6) illuminates an emerging technological frontier. Studies achieving the highest innovation scores (4–5 out of 5) consistently integrate multiple validation approaches: Fan et al. (2023) [33] combined fermentation with digestive stability testing, while Menfaatli et al. (2024) [35] linked computational prediction with experimental validation. This methodological convergence suggests the field is evolving toward integrated platforms that balance efficiency, functionality, and practical applicability.
Critically, the peptide molecular weight profile, consistently <3 kDa across studies, indicates optimal bioavailability characteristics. Vilcacundo et al. (2018) [20] demonstrated that simulated gastrointestinal digestion releases predominantly low-molecular-weight peptides that resist further degradation, suggesting these compounds could survive intestinal transit and exert systemic effects. This stability profile, combined with proven bioactivities, positions quinoa peptides as viable alternatives to synthetic bioactive compounds.
The antimicrobial spectrum deserves particular attention. Quinoa peptides demonstrate consistent efficacy against both Gram-positive (S. aureus) and Gram-negative (E. coli) pathogens, with emerging evidence for anti-H. pylori effects [35]. This broad-spectrum activity, combined with food-grade production methods, addresses the growing crisis of antibiotic resistance while offering natural preservation solutions.
4. Discussion
4.1. Principal Findings and Mechanistic Insights
This scoping review represents the first systematic synthesis of preclinical evidence regarding the capacity of quinoa-derived bioactive peptides and proteins to modulate the gut microbiome. Through comprehensive analysis of 19 primary studies, we identified consistent patterns of beneficial microbiota modulation, which were characterized by enhanced alpha diversity, promotion of beneficial taxa, and increased production of health-promoting metabolites, particularly SCFAs. Collectively, these findings demonstrate that quinoa represents a promising functional ingredient for targeted gut health interventions.
Furthermore, the evidence reveals distinct mechanistic pathways through which quinoa bioactive peptides exert their modulatory effects. As Huang et al. (2024) [5] established, bioactive peptides are compounds that have positive effects on biological activities; while microorganisms can metabolize protein peptides, bioactive peptides have, in turn, been shown to have regulatory effects on the structure of intestinal flora. Notably, in spontaneously hypertensive rats, quinoa protein administration resulted in significant increases in bacterial alpha diversity alongside enhanced abundances of probiotic bacteria Turicibacter and Allobaculum [38], thereby demonstrating the capacity for targeted microbial restructuring in disease states.
Moreover, the butyrogenic effect emerges as a particularly significant mechanism, with quinoa interventions consistently enhancing butyrate production across multiple experimental models. According to Zeyneb et al. (2021) [27], quinoa polysaccharides have also been shown to produce butyric acid through cross-interactions between Bifidobacteria, which are anaerobic bacteria belonging to the phylum Actinobacteria, and butyrate-producing colon bacteria that generate butyric acid from bifidobacterial metabolites such as lactate or acetate in the human colon. Importantly, this mechanism is crucial given butyrate’s dual role as the primary energy substrate for colonocytes and its potent anti-inflammatory properties, which are essential for maintaining epithelial integrity [24,31].
4.2. Disease-Specific Modulation Patterns and Mechanisms of Action
Our analysis reveals sophisticated, context-dependent modulation of the gut microbiota by quinoa interventions, with distinct taxonomic shifts correlating with specific pathological states. Specifically, in obesity models, quinoa promoted metabolic restructuring through increased abundances of Bacteroidetes and Blautia while simultaneously reducing the Firmicutes/Bacteroidetes ratio, changes that are consistent with leaner metabolic phenotypes [25,40]. Conversely, in inflammatory contexts such as colitis, quinoa interventions exhibited potent anti-inflammatory signatures characterized by significant reductions in Proteobacteria and pathobionts, including Escherichia/Shigella [39].
As Fan et al. (2023) [33] demonstrated, quinoa protein or its hydrolysate ameliorated AOM/DSS-induced colorectal cancer in mice by altering intestinal flora and increasing the production of beneficial SCFAs, although some minor differences in microbiota function were observed between different quinoa protein hydrolysate groups. This finding underscores the importance of processing conditions and the degree of protein hydrolysis in determining therapeutic efficacy, which suggests that optimization of peptide generation methods could enhance clinical outcomes.
Additionally, the disease-specificity of quinoa’s modulatory effects extends to diabetes models, where interventions selectively enriched genera such as Lactobacillus and Faecalibaculum, taxa recognized for their roles in glucose homeostasis and metabolic health [23]. This targeted modulation suggests that quinoa-derived peptides may function through recognition of specific microbial targets or metabolic pathways relevant to distinct disease processes.
In vivo models that have been subjected to dietary supplementation with quinoa have shown a considerable decrease in serum levels of total cholesterol, LDL-c, inflammatory markers, and diabetes markers [41]. This can be explained by the versatile modulation that quinoa has on different metabolic pathways. For example, the phenols that make up quinoa have the ability to inhibit alpha-glucosidase and alpha-amylase [42], interrupting the catabolism of dietary starch and decreasing carbohydrate absorption. The decrease in glycemia is decisive for the management of diabetes. Similarly, a “non-hypercaloric” post-prandial state, as a consequence of the reduction in glucose absorption, leads to a decrease in insulin demand, subsequently inhibiting the activity of enzymes involved in the synthesis of fatty acids and of cholesterol, such as acetyl Coenzyme A carboxylase and hydroxy-3-methyl glutaryl Coenzyme A Reductase (HMG-CoA), respectively [43]. Thus, the inhibition of triglyceride and cholesterol anabolism makes quinoa have a protective effect on dyslipidemia. Furthermore, a study reported that a mouse diet supplemented with 3% quinoa pericarp (which contains polysaccharides such as pectins) reduced serum and liver cholesterol [44]. This can be explained by the fact that dietary fiber can bind to bile acid, which increases cholesterol catabolism and fiber fermentation in the colon to produce short-chain fatty acids, which in turn contribute to decreasing cholesterol synthesis in the liver [37].
Furthermore, quinoa protein prevented the increase in plasma and liver cholesterol when mice were fed a cholesterol-supplemented diet. This hypocholesterolemic effect of isolated quinoa protein was correlated with bile acid excretion, probably related to an inhibitory effect of quinoa proteins on bile acid reabsorption in the small intestine, and the control of cholesterol synthesis and catabolism by decreasing HMG-CoA synthesis, while the expression of cholesterol-7 alpha-hydroxylase, a cholesterol catabolic enzyme, was increased [45]. Finally, phytosterols and even saponins in quinoa may also contribute to the decrease in dietary cholesterol because quinoa interferes with intestinal cholesterol absorption and increases fecal bile acid and neutral sterols [46]. On the other hand, the amino acids present in quinoa protein, such as anionic charges, aromatics, cysteine, and phenolic compounds, could be associated with the reduction in lipid peroxidation and LDL oxidation, which are characteristics involved in the development of metabolic and cardiovascular diseases [47].
4.3. Bioactive Peptide Generation and Antimicrobial Properties
The liberation of bioactive peptides from quinoa proteins emerges as a critical prerequisite for microbiome modulation, with our analysis revealing two primary pathways: controlled enzymatic hydrolysis and microbial fermentation. As established by a recent comprehensive review, bioactive peptides exhibit beneficial bodily functions and contribute to a healthy gastrointestinal system by influencing barrier functions, immune responses, and gut microbiota [48]. Notably, the preference for LAB fermentation in the literature reflects both its efficiency in peptide release and the concurrent generation of valuable postbiotic metabolites.
Furthermore, the antimicrobial properties of quinoa-derived peptides represent a crucial mechanism for microbiome modulation. Studies demonstrate effectiveness against pathogenic bacteria, including Escherichia coli and Staphylococcus aureus [12,32], which suggests that these peptides contribute to microbiome health through selective antimicrobial activity. According to Mazurkiewicz et al. (2023) [49], bioactive antimicrobial peptides (BAPs) can kill pathogenic microorganisms by disrupting membrane integrity, inhibiting DNA and RNA synthesis, preventing protein synthesis, blocking protein activity, or interacting with certain intracellular targets [50]. In addition, the positive effects of BAP consumption extend to gut microbiota modulation and affect the equilibrium of reactive oxygen species in the gut. However, the reviewed studies did not typically report on the strain-specificity of these antimicrobial effects, nor did they assess potential off-target impacts on beneficial commensal bacteria. Investigating this is a crucial next step for ensuring the safe application of these peptides.
Remarkably, the methodological evolution toward in silico approaches and enzymatic hydrolysis represents a significant advancement in peptide generation efficiency. These newer techniques achieve higher innovation scores while requiring reduced experimental durations [35], thereby suggesting a transition toward more sustainable and scalable production methods. Nevertheless, traditional fermentation approaches remain valuable for applications within food matrices, where the generation of complex peptide profiles and postbiotic compounds provides additional functional benefits [22]. It is important to note that this review found no studies that conducted a direct head-to-head comparison of these different peptide generation methods (e.g., fermentation vs. enzymatic hydrolysis) on microbiome modulation outcomes. Such comparative studies are a critical knowledge gap and are needed to optimize peptide production for specific functional effects.
4.4. SCFA Production and Metabolic Implications
The consistent enhancement of SCFA production, particularly butyrate, represents one of the most significant findings of this review. As Huang et al. (2024) [5] explained, quinoa protein, when digested in the hindgut, yields undigested proteins and amino acids that can be utilized to produce SCFAs in the colon, thereby lowering intestinal pH and regulating microbiota growth in vivo. Consequently, this metabolic transformation is crucial for maintaining intestinal homeostasis and supporting beneficial microbial communities.
Quantitatively, Zeyneb et al. (2021) [27] demonstrated that after 24 h of anaerobic incubation, the total SCFAs of cooked quinoa, uncooked quinoa, and quinoa polysaccharides were 82.99, 77.11, and 82.73 mM, respectively, thus showing substantial metabolite production across different quinoa preparations. The preferential enhancement of butyrate over other SCFAs suggests metabolic bias toward fermentative pathways that are particularly beneficial for gut health, including maintenance of epithelial barrier function and modulation of immune responses [11]. Notably, some of the included studies established a direct correlation between these SCFA increases and host physiological benefits. For instance, Liu et al. (2022) [24] demonstrated that the butyrate-promoting effects of quinoa bran fiber were directly linked to improvements in intestinal barrier integrity markers in a colitis model, providing a mechanistic link between microbial metabolism and host health.
Moreover, the physiological implications of enhanced SCFA production extend beyond local gut effects. According to recent evidence, these degradation products can alter microbial diversity and promote the production of beneficial metabolites, contributing to overall health [51]. SCFAs serve as signaling molecules in the gut–brain axis, influence systemic metabolism through effects on gluconeogenesis and lipogenesis, and modulate immune function through interactions with G-protein coupled receptors [52,53].
4.5. Comparative Analysis with Other Plant-Derived Peptides
The gut microbiome modulatory effects of quinoa-derived peptides demonstrate unique characteristics when compared to other plant protein sources. While soy peptides have been extensively studied for their isoflavone content and estrogenic effects, Wijesekara, 2024 [48] noted that peptides and proteins from several animal and plant sources have been widely explored in relation to gut microbiome modulation; however, the effects of soy peptides and other soy derivatives on gut microbiota remain largely unexplored. In contrast, quinoa’s advantage lies in its complete amino acid profile and absence of anti-nutritional factors that may limit the bioavailability of bioactive compounds [54].
Additionally, emerging research suggests that peptides can modulate the intestinal microenvironment by regulating specific gut microbiota species, such as lactic acid bacteria, bifidobacteria, and yeasts [38]. The selectivity of quinoa peptides for beneficial microbial taxa, combined with their antimicrobial effects against pathogens, positions them as particularly valuable functional ingredients for microbiome-targeted interventions compared to other plant-derived alternatives.
4.6. Clinical Translation Challenges and Opportunities
Despite promising preclinical evidence, several challenges must be addressed to enable clinical translation of quinoa-derived bioactive peptides. The heterogeneity in study designs, intervention preparations, and outcome measures complicates direct comparisons and limits the ability to establish standardized protocols for human applications. Thus, while it is evident that quinoa has multiple properties for controlling biomarkers associated with disease risks, it is not possible to affirm that quinoa consumption is effective against a specific disease. As Wang et al. (2022) [25] emphasized, while dietary bioactive peptides positively impact gastrointestinal homeostasis by modulating barrier function, immune responses, and gut microbiota, there remains limited clinical evidence on the safety and efficacy of bioactive peptides, much less on the applications of dietary peptides for the treatment or prevention of diseases related to the gastrointestinal tract.
Consequently, the optimization of peptide generation methods emerges as a critical consideration for commercial applications. While in vitro fermentation approaches provide valuable mechanistic insights, scalable production methods must balance efficiency, cost-effectiveness, and retention of bioactivity. The evolution toward enzymatic hydrolysis and in silico prediction methods suggests promising directions for industrial-scale production of standardized quinoa peptide preparations [34].
Furthermore, bioavailability and stability represent additional challenges requiring targeted investigation. Quinoa peptides must survive gastric digestion and maintain biological activity in the complex intestinal environment to exert their modulatory effects. As Parvez et al. (2024) [50] suggested, it is possible to consider controlled delivery systems such as microparticulate, nanoemulsion, and nanostructured lipid carriers or chemical modifications such as the cyclization of bioactive peptide structures that are vulnerable to digestive enzymes or thermal treatment.
4.7. Implications for Functional Food Development
The evidence synthesized in this review supports the potential for quinoa-based functional food development targeting gut health applications. The combination of nutritional completeness, bioactive peptide content, and demonstrated microbiome modulatory effects positions quinoa as an ideal substrate for functional ingredient development. As previously established, in vitro studies have suggested that quinoa has a prebiotic effect, including promoting the growth of beneficial bacteria and the production of SCFAs [39].
Moreover, the disease-specific modulation patterns observed across different experimental models suggest opportunities for targeted therapeutic applications. Products designed for metabolic health could leverage quinoa’s capacity to promote lean microbial profiles and enhance SCFA production, while anti-inflammatory formulations could target the reduction in pathogenic taxa and enhancement of barrier function [23,25].
Nevertheless, standardization of quinoa peptide preparations emerges as a crucial requirement for functional food applications. The variability in bioactive peptide content across quinoa varieties, processing methods, and extraction techniques necessitates the development of standardized protocols ensuring consistent therapeutic efficacy. This includes optimization of fermentation conditions, hydrolysis parameters, and quality control measures for bioactive peptide quantification [20,21].
4.8. Future Perspectives and Research Directions
Several critical research gaps must be addressed to advance the field toward clinical applications. Human clinical trials represent the most pressing need, with carefully designed studies required to validate the microbiome modulatory effects observed in preclinical models. These studies should incorporate standardized quinoa peptide preparations, validated microbiome analysis methods, and clinically relevant endpoints, including functional digestive health measures and systemic biomarkers of metabolic function. Specifically, target populations could include individuals with metabolic syndrome, non-alcoholic fatty liver disease (NAFLD), or mild-to-moderate inflammatory bowel disease (IBD) in remission. Key clinical endpoints should extend beyond microbiome metrics to include validated clinical markers such as HbA1c, HOMA-IR, serum lipids, C-reactive protein (CRP), and fecal calprotectin, thereby linking microbiome modulation to tangible health outcomes.
Concurrently, mechanistic studies are needed to elucidate the specific molecular pathways through which quinoa peptides modulate microbial communities. As Wijesekara (2024) [48] noted, the interplays between bioactive peptides and gut microbiota are not fully understood; however, bioactive peptides hold promise as modulators of the gut microbiota to promote gut health. Advanced approaches, including metabolomics, transcriptomics, and proteomics, could provide insights into the cellular and molecular mechanisms underlying the observed effects. To understand the potential systemic effects of these peptides, future research should include pharmacokinetic and pharmacodynamic (PK/PD) studies. Assessing the in vivo absorption, distribution, metabolism, and excretion (ADME) of quinoa peptides is essential to determine if they reach systemic circulation and exert bioactivity beyond the gastrointestinal tract.
Furthermore, the development of personalized nutrition approaches represents an emerging opportunity in this field. Individual variations in baseline microbiome composition, genetic factors affecting protein metabolism, and environmental influences may modulate responses to quinoa peptide interventions. Understanding these factors could enable the development of personalized recommendations optimizing therapeutic outcomes for specific population groups or disease states [55,56].
4.9. Study Limitations
Several limitations of this scoping review and the underlying evidence base must be acknowledged.
First, the significant methodological heterogeneity across studies, spanning experimental models, intervention preparations, and analytical methods (including different metrics for alpha diversity and different quantification techniques for SCFAs), precluded any quantitative meta-analysis and limited the ability to establish clear dose–response relationships. This is a critical point, as interventions ranged from whole quinoa grain to isolated fibers and protein hydrolysates, making it difficult to attribute the observed effects solely to bioactive peptides versus the synergistic action of multiple quinoa components like fibers and polyphenols. Disentangling these effects remains a key challenge for future research.
Second, the generalizability of the findings is constrained by a geographical clustering of research, with 42% of studies originating from China, and the predominance of specific animal models (e.g., DSS-induced colitis, high-fat diet-induced obesity). Factors such as quinoa cultivars, local dietary habits, and baseline microbiome compositions can vary significantly by region, potentially influencing outcomes and limiting the translational scope to other conditions.
Furthermore, as stated in our methods, a formal risk-of-bias assessment was not performed, which is consistent with the scoping review framework. However, a qualitative appraisal of the primary research suggests that many studies did not fully report on randomization, blinding, or sample size calculations, which introduces a potential risk of bias in the evidence base.
Finally, the variable quality of microbiome analysis methods across the included articles, combined with the exclusive focus on preclinical evidence, underscores the exploratory nature of the current literature. Consequently, the absence of human clinical trials represents the most significant knowledge gap that must be addressed before these findings can be translated to human applications. This review did not systematically extract data on the specific sequencing platforms (e.g., 16S rRNA gene variable region) or bioinformatics pipelines used, and this variability could influence taxonomic resolution and the direct comparability of findings at the genus level.
5. Conclusions
This scoping review provides compelling preclinical evidence for the capacity of quinoa-derived bioactive peptides and proteins to modulate the gut microbiome in beneficial ways. The consistent patterns of enhanced microbial diversity, promotion of beneficial taxa, increased SCFA production, and disease-specific modulatory effects across diverse preclinical models support the potential for quinoa as a functional ingredient targeting gut health applications. The mechanistic insights revealed through this synthesis provide a foundation for the rational design of quinoa-based therapeutic interventions. However, it is crucial to emphasize that these findings are preclinical. Clinical translation requires the standardization of production methods, validation through human trials, and the development of appropriate delivery systems, ensuring bioavailability and stability.
The growing understanding of gut microbiome contributions to human health, combined with the demonstrated modulatory capacity of quinoa-derived peptides, positions this field for significant advancement. Therefore, future research should prioritize human clinical validation, mechanistic elucidation, and the development of standardized therapeutic preparations to realize the full potential of quinoa as a functional food ingredient for gut health promotion.
Author Contributions
Conceptualization, N.C. and Y.L.; methodology, Y.L.; software, Y.L.; validation, N.C., Y.L. and J.O.-G.; formal analysis, N.C.; investigation, Y.L.; resources, J.O.-G.; data curation, Y.L.; writing—original draft preparation, Y.L.; writing—review and editing, N.C. and J.O.-G.; visualization, Y.L.; supervision, Y.L.; project administration, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by the Dirección General de Investigaciones of Universidad Santiago de Cali under call No. DGI-01-2025.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
This research has been funded by the Dirección General de Investigaciones of Universidad Santiago de Cali under call No. DGI-01-2025.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AOM | Azoxymethane |
| BAPs | Bioactive Antimicrobial Peptides |
| DSS | Dextran Sulfate Sodium |
| F/B ratio | Firmicutes/Bacteroidetes Ratio |
| GI | Gastrointestinal |
| HFD | High-Fat Diet |
| JBI | Joanna Briggs Institute |
| LAB | Lactic Acid Bacteria |
| PCC | Population, Concept, and Context |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews |
| QBSDF | Quinoa Bran Soluble Dietary Fiber |
| QHP | Quinoa Protein Hydrolysate |
| QP | Quinoa Protein |
| SCFAs | Short-Chain Fatty Acids |
| SHR | Spontaneously Hypertensive Rats |
| SHIME® | Simulator of the Human Intestinal Microbial Ecosystem |
| UAE | United Arab Emirates |
| USA | United States of America |
References
- Khalil, M.; Di Ciaula, A.; Mahdi, L.; Jaber, N.; Di Palo, D.M.; Graziani, A.; Baffy, G.; Portincasa, P. Unraveling the Role of the Human Gut Microbiome in Health and Diseases. Microorganisms 2024, 12, 2333. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Prabhakar, M.R.; Mohanty, A.; Meena, S.S. Influence of Gut Microbiome on the Human Physiology. Syst. Microbiol. Biomanuf. 2022, 2, 217–231. [Google Scholar] [CrossRef]
- Protasiewicz-Timofticiuc, D.-C.; Bădescu, D.; Moța, M.; Ștefan, A.-G.; Mitrea, A.; Clenciu, D.; Efrem, I.C.; Roșu, M.M.; Vladu, B.E.; Gheonea, T.C.; et al. Back to Roots: Dysbiosis, Obesity, Metabolic Syndrome, Type 2 Diabetes Mellitus, and Obstructive Sleep Apnea—Is There an Objective Connection? A Narrative Review. Nutrients 2024, 16, 4057. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing A Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Q.; Yang, Y.; Zhong, W.; He, F.; Li, J. The Mycobiome as Integral Part of the Gut Microbiome: Crucial Role of Symbiotic Fungi in Health and Disease. Gut Microbes 2024, 16, 2440111. [Google Scholar] [CrossRef]
- Martinez Guevara, D.; Vidal Cañas, S.; Palacios, I.; Gómez, A.; Estrada, M.; Gallego, J.; Liscano, Y. Effectiveness of Probiotics, Prebiotics, and Synbiotics in Managing Insulin Resistance and Hormonal Imbalance in Women with Polycystic Ovary Syndrome (PCOS): A Systematic Review of Randomized Clinical Trials. Nutrients 2024, 16, 3916. [Google Scholar] [CrossRef]
- Turroni, S.; Benítez-Páez, A. Editorial: Remodeling Composition and Function of Microbiome by Dietary Strategies-Functional Foods Perspective. Front. Nutr. 2021, 8, 811102. [Google Scholar] [CrossRef]
- Aragón-Muriel, A.; Liscano-Martínez, Y.; Rufino-Felipe, E.; Morales-Morales, D.; Oñate-Garzón, J.; Polo-Cerón, D. Synthesis, Biological Evaluation and Model Membrane Studies on Metal Complexes Containing Aromatic N,O-Chelate Ligands. Heliyon 2020, 6, e04126. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Ashaolu, J.O.; Adeyeye, S.A.O. Fermentation of Prebiotics by Human Colonic Microbiota in Vitro and Short-chain Fatty Acids Production: A Critical Review. J. Appl. Microbiol. 2021, 130, 677–687. [Google Scholar] [CrossRef]
- Bedu-Ferrari, C.; Biscarrat, P.; Langella, P.; Cherbuy, C. Prebiotics and the Human Gut Microbiota: From Breakdown Mechanisms to the Impact on Metabolic Health. Nutrients 2022, 14, 2096. [Google Scholar] [CrossRef] [PubMed]
- Fotschki, B.; Juśkiewicz, J.; Jurgoński, A.; Amarowicz, R.; Opyd, P.; Bez, J.; Muranyi, I.; Lykke Petersen, I.; Laparra Llopis, M. Protein-Rich Flours from Quinoa and Buckwheat Favourably Affect the Growth Parameters, Intestinal Microbial Activity and Plasma Lipid Profile of Rats. Nutrients 2020, 12, 2781. [Google Scholar] [CrossRef]
- Fan, X.; Ma, X.; Maimaitiyiming, R.; Aihaiti, A.; Yang, J.; Li, X.; Wang, X.; Pang, G.; Liu, X.; Qiu, C.; et al. Study on the Preparation Process of Quinoa Anti-Hypertensive Peptide and Its Stability. Front. Nutr. 2023, 9, 1119042. [Google Scholar] [CrossRef]
- Ocampo-Ibáñez, I.D.; Liscano, Y.; Rivera-Sánchez, S.P.; Oñate-Garzón, J.; Lugo-Guevara, A.D.; Flórez-Elvira, L.J.; Lesmes, M.C. A Novel Cecropin D-Derived Short Cationic Antimicrobial Peptide Exhibits Antibacterial Activity Against Wild-Type and Multidrug-Resistant Strains of Klebsiella pneumoniae and Pseudomonas aeruginosa. Evol. Bioinform. 2020, 16, 1176934320936266. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Sanchez, S.P.; Ocampo-Ibáñez, I.D.; Liscano, Y.; Martínez, N.; Muñoz, I.; Manrique-Moreno, M.; Martinez-Martinez, L.; Oñate-Garzon, J. Integrating In Vitro and In Silico Analysis of a Cationic Antimicrobial Peptide Interaction with Model Membranes of Colistin-Resistant Pseudomonas Aeruginosa Strains. Pharmaceutics 2022, 14, 1248. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.P.; Bangar, S.P.; Albaloosh, M.; Ajayi, F.F.; Mudgil, P.; Maqsood, S. Plant-Derived Proteins as a Sustainable Source of Bioactive Peptides: Recent Research Updates on Emerging Production Methods, Bioactivities, and Potential Application. Crit. Rev. Food Sci. Nutr. 2022, 63, 9539–9560. [Google Scholar] [CrossRef]
- Trejos, M.; Aristizabal, Y.; Aragón-Muriel, A.; Oñate-Garzón, J.; Liscano, Y. Characterization and Classification In Silico of Peptides with Dual Activity (Antimicrobial and Wound Healing). Int. J. Mol. Sci. 2023, 24, 13091. [Google Scholar] [CrossRef]
- Caicedo Cerón, N.; Oñate-Garzon, J.F.; Liscano, Y. Evaluation of the Anti-Aging Activity of Peptides Obtained by Enzymatic Hydrolysis with Actinidin from Colombian Quinoa Seed Proteins. Rev. Prod. Nat. 2025, 6, 129–132. [Google Scholar]
- Hernández-Ledesma, B. Quinoa (Chenopodium quinoa Willd.) as Source of Bioactive Compounds: A Review. Bioact. Compd. Health Dis. 2019, 2, 27. [Google Scholar] [CrossRef]
- Ng, C.Y.; Wang, M. The Functional Ingredients of Quinoa (Chenopodium quinoa) and Physiological Effects of Consuming Quinoa: A Review. Food Front. 2021, 2, 329–356. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Miralles, B.; Carrillo, W.; Hernández-Ledesma, B. In Vitro Chemopreventive Properties of Peptides Released from Quinoa (Chenopodium quinoa Willd.) Protein under Simulated Gastrointestinal Digestion. Food Res. Int. 2018, 105, 403–411. [Google Scholar] [CrossRef]
- Ayyash, M.; Johnson, S.K.; Liu, S.-Q.; Al-Mheiri, A.; Abushelaibi, A. Cytotoxicity, Antihypertensive, Antidiabetic and Antioxidant Activities of Solid-State Fermented Lupin, Quinoa and Wheat by Bifidobacterium Species: In-Vitro Investigations. LWT 2018, 95, 295–302. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Lorusso, A.; Russo, V.; Pinto, D.; Marzani, B.; Gobbetti, M. Improving the Antioxidant Properties of Quinoa Flour through Fermentation with Selected Autochthonous Lactic Acid Bacteria. Int. J. Food Microbiol. 2017, 241, 252–261. [Google Scholar] [CrossRef]
- Li, L.; Hu, X.; Sun, X.; Wei, X.; Zhang, H.; Wang, P. Quinoa Alleviates the Diabetic Symptoms of Db/Db Mice by Upregulating Insulin Signaling and Modulating Gut Microbiota Composition. J. Funct. Foods 2025, 128, 106813. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Mai, P.; Hao, Y.; Wang, Z.; Wang, J. Quinoa Bran Soluble Dietary Fiber Ameliorates Dextran Sodium Sulfate Induced Ulcerative Colitis in BALB/c Mice by Maintaining Intestinal Barrier Function and Modulating Gut Microbiota. Int. J. Biol. Macromol. 2022, 216, 75–85. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Tao, S.-Y.; Wu, Y.-X.; An, T.; Lv, B.-H.; Liu, J.-X.; Liu, Y.-T.; Jiang, G.-J. Quinoa Reduces High-Fat Diet-Induced Obesity in Mice via Potential Microbiota-Gut-Brain-Liver Interaction Mechanisms. Microbiol. Spectr. 2022, 10, e00329-22. [Google Scholar] [CrossRef]
- Gullón, B.; Gullón, P.; Tavaria, F.K.; Yáñez, R. Assessment of the Prebiotic Effect of Quinoa and Amaranth in the Human Intestinal Ecosystem. Food Funct. 2016, 7, 3782–3788. [Google Scholar] [CrossRef]
- Zeyneb, H.; Pei, H.; Cao, X.; Wang, Y.; Win, Y.; Gong, L. In Vitro Study of the Effect of Quinoa and Quinoa Polysaccharides on Human Gut Microbiota. Food Sci. Nutr. 2021, 9, 5735–5745. [Google Scholar] [CrossRef]
- Santos, W.M.D.; Secoli, S.R.; Püschel, V.A.D.A. The Joanna Briggs Institute Approach for Systematic Reviews. Rev. Lat.-Am. Enferm. 2018, 26, e3074. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, M.; Zhao, B.; Yang, F. Isolation of Antioxidant Peptides from Yak Casein Hydrolysate. RSC Adv. 2020, 10, 19844–19851. [Google Scholar] [CrossRef]
- Bianchi, F.; Rossi, E.A.; Sakamoto, I.K.; Adorno, M.A.T.; Van De Wiele, T.; Sivieri, K. Beneficial Effects of Fermented Vegetal Beverages on Human Gastrointestinal Microbial Ecosystem in a Simulator. Food Res. Int. 2014, 64, 43–52. [Google Scholar] [CrossRef]
- Cizeikiene, D.; Gaide, I.; Basinskiene, L. Effect of Lactic Acid Fermentation on Quinoa Characteristics and Quality of Quinoa-Wheat Composite Bread. Foods 2021, 10, 171. [Google Scholar] [CrossRef]
- Fan, X.; Guo, H.; Teng, C.; Yang, X.; Qin, P.; Richel, A.; Zhang, L.; Blecker, C.; Ren, G. Supplementation of Quinoa Peptides Alleviates Colorectal Cancer and Restores Gut Microbiota in AOM/DSS-Treated Mice. Food Chem. 2023, 408, 135196. [Google Scholar] [CrossRef]
- Mahdavi-Yekta, M.; Reihani, S.F.S.; Mohammadi, M. Antimicrobial Activity of Quinoa Protein Hydrolysate against Streptococcus pyogenes and Escherichia coli. J. Food Qual. 2023, 2023, 9993241. [Google Scholar] [CrossRef]
- Menfaatli, E.; Kavruk, M.; Gundeger, E. In Silico Evaluation of Antimicrobial Potential of Amaranth, Chia and Quinoa Peptides Released During the Simulated Gastric Digestion and Their Effects on Helicobacter Pylori. Trends Pept. Protein Sci. 2024, 9, 1–8. [Google Scholar]
- Rizzello, C.G.; Lorusso, A.; Montemurro, M.; Gobbetti, M. Use of Sourdough Made with Quinoa (Chenopodium quinoa) Flour and Autochthonous Selected Lactic Acid Bacteria for Enhancing the Nutritional, Textural and Sensory Features of White Bread. Food Microbiol. 2016, 56, 1–13. [Google Scholar] [CrossRef]
- Noratto, G.D.; Murphy, K.; Chew, B.P. Quinoa Intake Reduces Plasma and Liver Cholesterol, Lessens Obesity-Associated Inflammation, and Helps to Prevent Hepatic Steatosis in Obese Db/Db Mouse. Food Chem. 2019, 287, 107–114. [Google Scholar] [CrossRef]
- Guo, H.; Hao, Y.; Fan, X.; Richel, A.; Everaert, N.; Yang, X.; Ren, G. Administration with Quinoa Protein Reduces the Blood Pressure in Spontaneously Hypertensive Rats and Modifies the Fecal Microbiota. Nutrients 2021, 13, 2446. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Qiu, B.; Fan, S.; Ding, H.; Liu, Z. Quinoa Whole Grain Diet Compromises the Changes of Gut Microbiota and Colonic Colitis Induced by Dextran Sulfate Sodium in C57BL/6 Mice. Sci. Rep. 2018, 8, 14916. [Google Scholar] [CrossRef]
- An, T.; Liu, J.-X.; Yang, X.; Lv, B.; Wu, Y.; Jiang, G. Supplementation of Quinoa Regulates Glycolipid Metabolism and Endoplasmic Reticulum Stress in the High-Fat Diet-Induced Female Obese Mice. Nutr. Metab. 2021, 18, 95. [Google Scholar] [CrossRef]
- Carías Domínguez, A.M.; De Jesús Rosa Salazar, D.; Stefanolo, J.P.; Cruz Serrano, M.C.; Casas, I.C.; Zuluaga Peña, J.R. Intestinal Dysbiosis: Exploring Definition, Associated Symptoms, and Perspectives for a Comprehensive Understanding—A Scoping Review. Probiotics Antimicrob. Proteins 2025, 17, 440–449. [Google Scholar] [CrossRef]
- Hemalatha, P.; Bomzan, D.P.; Rao, B.V.S.; Sreerama, Y.N. Distribution of Phenolic Antioxidants in Whole and Milled Fractions of Quinoa and Their Inhibitory Effects on α-Amylase and α-Glucosidase Activities. Food Chem. 2016, 199, 330–338. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Feng, M.; Zhou, X.; Lu, Y.; Gao, L.; Yu, C.; Jiang, X.; Zhao, J. Thyroid-Stimulating Hormone Decreases HMG-CoA Reductase Phosphorylation via AMP-Activated Protein Kinase in the Liver. J. Lipid Res. 2015, 56, 963–971. [Google Scholar] [CrossRef]
- Fukatsu, A.; Tsuzukibashi, O.; Suzuk, H.; Asaka, K.; Ono, Y.; Fuchigami, M.; Kobayashi, T.; Uchibori, S.; Takahashi, Y.; Komine, C.; et al. One-Step Multiplex PCR for Simultaneous Detection and Identification of Eight Medically Important Candida Species. Open J. Stomatol. 2021, 11, 14–24. [Google Scholar] [CrossRef]
- Fathurrohmah, N.N.; Murti, S.T.C.; Suryani, C.L. Effect of Zn–Chlorophyll Complexes Formation on the Color Stability of Pandan (Pandanus amaryllifolius) Leaf Extract. Food Sci. Technol. 2023, 11, 161–167. [Google Scholar] [CrossRef]
- Tang, Y.; Tsao, R. Phytochemicals in Quinoa and Amaranth Grains and Their Antioxidant, Anti-inflammatory, and Potential Health Beneficial Effects: A Review. Mol. Nutr. Food Res 2017, 61, 1600767. [Google Scholar] [CrossRef]
- Rocchetti, G.; Chiodelli, G.; Giuberti, G.; Masoero, F.; Trevisan, M.; Lucini, L. Evaluation of Phenolic Profile and Antioxidant Capacity in Gluten-Free Flours. Food Chem. 2017, 228, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, T.; Abeyrathne, E.D.N.S.; Ahn, D.U. Effect of Bioactive Peptides on Gut Microbiota and Their Relations to Human Health. Foods 2024, 13, 1853. [Google Scholar] [CrossRef]
- Mazurkiewicz-Pisarek, A.; Baran, J.; Ciach, T. Antimicrobial Peptides: Challenging Journey to the Pharmaceutical, Biomedical, and Cosmeceutical Use. Int. J. Mol. Sci. 2023, 24, 9031. [Google Scholar] [CrossRef]
- Parvez, A.K.; Jubyda, F.T.; Ayaz, M.; Sarker, A.; Haque, N.; Khan, M.S.; Mou, T.J.; Rahman, M.A.; Huq, M.A. Microbial- and Plant-Derived Bioactive Peptides and Their Applications against Foodborne Pathogens: Current Status and Future Prospects. Int. J. Microbiol. 2024, 2024, 9978033. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.S.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.P.; Stahl, B.; Harsselaar, J.V.; et al. Short Chain Fatty Acids in Human Gut and Metabolic Health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Hays, K.E.; Pfaffinger, J.M.; Ryznar, R. The Interplay between Gut Microbiota, Short-Chain Fatty Acids, and Implications for Host Health and Disease. Gut Microbes 2024, 16, 2393270. [Google Scholar] [CrossRef]
- Rekha, K.; Venkidasamy, B.; Samynathan, R.; Nagella, P.; Rebezov, M.B.; Khayrullin, M.; Ponomarev, E.E.; Bouyahya, A.; Sarkar, T.; Shariati, M.A.; et al. Short-Chain Fatty Acid: An Updated Review on Signaling, Metabolism, and Therapeutic Effects. Crit. Rev. Food Sci. Nutr. 2022, 64, 2461–2489. [Google Scholar] [CrossRef]
- Ren, G.; Teng, C.; Fan, X.; Guo, S.; Zhao, G.; Zhang, L.; Liang, Z.; Qin, P. Nutrient Composition, Functional Activity and Industrial Applications of Quinoa (Chenopodium quinoa Willd.). Food Chem. 2023, 410, 135290. [Google Scholar] [CrossRef] [PubMed]
- Lagoumintzis, G.; Patrinos, G.P. Triangulating Nutrigenomics, Metabolomics and Microbiomics toward Personalized Nutrition and Healthy Living. Hum. Genom. 2023, 17, 109. [Google Scholar] [CrossRef] [PubMed]
- Singar, S.; Nagpal, R.; Arjmandi, B.H.; Akhavan, N.S. Personalized Nutrition: Tailoring Dietary Recommendations through Genetic Insights. Nutrients 2024, 16, 2673. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).