Multifaceted Nutrition Intervention for Frail Elderly in the Community: Protocol of a Randomized Controlled Trial (The MINUTE Study)
Abstract
1. Introduction
2. Methods
2.1. Aims
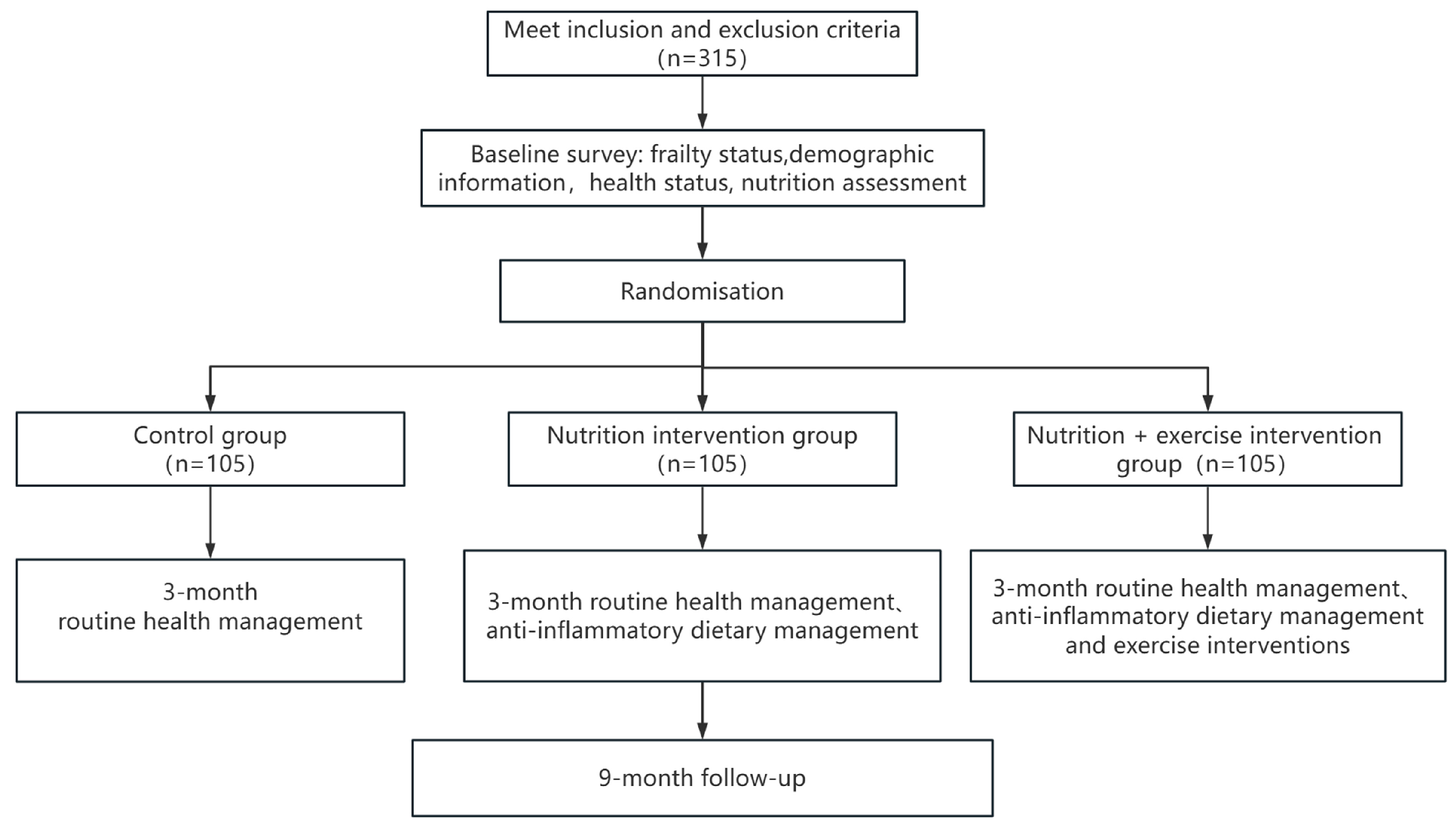
2.2. Study Design
2.3. Recruitment of Participants
- (1)
- Initial contact and information dissemination: Community physicians and neighborhood committees will identify potential participants who meet the basic age criteria (aged 65 years or older). They will provide these individuals with detailed information about the study, including its purpose, procedures, risks, and benefits. This information will be delivered through flyers, community announcements, and face-to-face consultations.
- (2)
- Screening and pre-selection: Potential participants will be invited to attend a preliminary screening session at the Community Health Service Center. During this session, community physicians will conduct a brief health assessment and administer the FRAIL scale to determine the frailty status. Individuals who are assessed as frail by the FRAIL scale will be considered for further evaluation.
- (3)
- Informed consent and detailed assessment: Eligible individuals will be provided with a detailed explanation of the study procedures and will be asked to provide written informed consent. Following consent, a comprehensive baseline assessment will be conducted by trained healthcare professionals. This assessment includes a review of medical history, physical examination, and evaluation of functional and mental health status.
- (1)
- Assessed as frailty by the FRAIL scale. The FRAIL scale is a simple 5-item screening tool where frailty is defined as meeting 3 or more of the following criteria: fatigue, resistance (inability to climb one flight of stairs), ambulation (inability to walk 100 m), illnesses (≥5 comorbidities), and loss of weight (>5% in the past year).
- (2)
- Aged 65 years or older.
- (3)
- Able to provide written informed consent, after receiving a detailed explanation of the study purpose, procedures, risks, and benefits.
- (1)
- Major comorbidities: History of major comorbidities (e.g., stroke, myocardial infarction, active cancer, or severe cardiocerebrovascular disease) that may confound study results or pose risks to participants.
- (2)
- Significant functional impairments: Participants with disability, dementia, severe visual or hearing impairments, or physical activity impairment will be excluded. These impairments will be assessed through standardized functional tests (e.g., Mini-Mental State Examination for cognitive function, visual acuity tests, and hearing assessments) and physical performance tests (e.g., gait speed and balance tests). The exclusion will be based on the potential for these impairments to limit participants’ ability to complete the study protocol or accurately report outcomes.
- (3)
- Moderate to severe mental health conditions: Participants with moderate to severe anxiety or depression will be excluded. Mental health status will be evaluated using validated screening tools (e.g., the Geriatric Depression Scale and the State-Trait Anxiety Inventory). The exclusion will be based on the potential for these conditions to affect participants’ ability to comply with the study protocol or accurately report outcomes.
2.4. Randomization, Allocation Concealment, and Blinding
3. Intervention
3.1. Control Group
3.2. Multifaceted Nutrition Intervention
3.3. Multifaceted Nutrition and Exercise Combined Intervention
- (1)
- Initial supervised instruction: Prior to starting the home-based program, each participant will attend a one-on-one or small-group session with a trained exercise specialist. During this session, the specialist will demonstrate each exercise, ensure the participant can perform it with correct form, and provide personalized feedback.
- (2)
- Structured guidance materials: Participants will receive a detailed, illustrated exercise manual and a training wheel chart (Figure 3) that clearly outlines the weekly schedule, sets, and repetitions, making the program easy to follow at home.
- (3)
- Ongoing remote support: Participants will have weekly access to an exercise specialist via an online platform or telephone to ask questions, report difficulties, and receive motivational support.
- (4)
- Adherence monitoring: Participants will be asked to maintain a simple exercise diary to log their completed sessions. This will be reviewed weekly by the research team to monitor adherence and provide timely reminders if needed.
- (5)
- Integration with community healthcare: To leverage the existing community healthcare network, community hospitals and family physicians will be engaged to provide ongoing supervision. During their routine weekly interactions or health consultations, they will specifically inquire about the participants’ exercise progress, record their adherence, and offer encouragement. This integrates the intervention into the participants’ regular healthcare, providing an additional layer of personal contact and accountability.
3.4. Quality Control of the Intervention
- (1)
- To ensure the fidelity and successful implementation of the intervention while prioritizing the safety of the elderly, we will mobilize the involvement of multiple institutions. Nutrition experts offer personalized consultation and guidance on dietary needs, while exercise specialists provide tailored recommendations at varying intensity levels. Community hospitals contribute by offering examination venues and equipment, and family doctors are tasked with ensuring the safety of the elderly. Additionally, staff from senior living communities or care centers assist in the project’s execution, and the elderly actively engage in feedback and communication, sharing their thoughts and experiences.
- (2)
- Three manuals (“Nutrition Intervention Manual for Older Adults”, “the Staff Manual for the Nutrition Intervention Program for Older Adults”, and “Exercise Intervention Manual”) will be used to implement and manage this complex intervention. The manuals detail the responsibilities of study personnel at each stage of the project. The manuals also describe in detail the work processes for implementing each component of the intervention, i.e., who, when, how, and to what extent specific intervention elements are to be delivered.
- (3)
- Throughout the implementation process, project executors will conduct regular interviews and exchanges with the elderly, promptly conveying their thoughts and feedback to the project leaders. Periodic meetings will be held to consolidate the opinions of all stakeholders involved in the project, allowing for the continuous optimization and improvement of any emerging issues.
- (4)
- We will employ a comprehensive set of measures to collect process indicators and ensure adherence to the intervention, as follows:
- Participant activity diary: Records of frail individuals completing nutritional or exercise sessions as prescribed are maintained.
- Satisfaction survey: Frail older adults will be asked to rate their satisfaction with the weekly interventions using smiley faces.
- Online check-ins: Daily check-in frequency and time periods are recorded by designated personnel, detailing the specific daily meals and exercise routines.
- Lecture logs: Records of attendance at each online and offline lecture are maintained, forming a participant log.
- Urinary sodium monitoring: Urine samples from frail elderly individuals will be collected before and after the intervention to monitor urinary sodium levels, assessing dietary adherence.
4. Outcomes
4.1. Primary Outcome
4.2. Secondary Outcomes
4.3. Biological Sample Collection and Handling
4.4. Adverse Events
4.5. Sample Size Estimation
4.6. Statistical Analyses
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Feng, J.; Hong, G.L.; Qian, W.R.; Hu, R.F.; Shi, G.M. Aging in China: An International and Domestic Comparative Study. Sustainability 2020, 12, 5086. [Google Scholar] [CrossRef]
- Peng, D. Negative population growth and population ageing in China. China Popul. Dev. Stud. 2023, 7, 95–103. [Google Scholar] [CrossRef]
- Liu, X.; Yang, X. Research Progress on Frailty in Elderly People. Clin. Interv. Aging 2024, 19, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Umegaki, H. Hospital-associated complications in frail older adults. Nagoya J. Med. Sci. 2024, 86, 181–188. [Google Scholar]
- Xi, J.Y.; Ming, R.Q.; Wang, Y.J.; Fu, Y.B.; Zhang, Z.; Zhang, J.; Bai, J.J.; Xiang, Y.N.; Lin, X.; Gu, J.; et al. Current and predicted disease burden in middle aged and elderly population aged 55 years and above in Shenzhen, 2016–2030. Zhonghua Liu Xing Bing Xue Za Zhi 2024, 45, 1550–1558. [Google Scholar] [PubMed]
- Chen, R.; Xu, P.; Song, P.P.; Wang, M.F.; He, J.J. China has faster pace than Japan in population aging in next 25 years. Biosci. Trends 2019, 13, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.B.; Feng, Q.S. Aging and health in China. Front. Public Health 2022, 10, 998769. [Google Scholar] [CrossRef]
- Wang, M.Y.; Sung, H.C.; Liu, J.Y. Population Aging and Its Impact on Human Wellbeing in China. Front. Public Health 2022, 10, 883566. [Google Scholar] [CrossRef]
- Trong, N.T.; Dong, N.T.; Ly, P.T. Population aging and economic growth: Evidence from ASEAN countries. Cogent Bus. Manag. 2024, 11, 2298055. [Google Scholar] [CrossRef]
- Zheng, P.P.; Guo, Z.L.; Du, X.J.; Yang, H.M.; Wang, Z.J. Prevalence of Disability among the Chinese Older Population: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 1656. [Google Scholar] [CrossRef]
- Fan, L.; Hou, X.Y.; Liu, Y.; Chen, S.; Wang, Q.; Du, W. Catastrophic Health Expenditure Associated with Frailty in Community-Dwelling Chinese Older Adults: A Prospective Cohort Analysis. Front. Public Health 2021, 9, 718910. [Google Scholar] [CrossRef]
- Groce, N.E. Global disability: An emerging issue. Lancet Glob. Health 2018, 6, e724–e725. [Google Scholar] [CrossRef]
- Jin, H.Y.; Liu, X.; Xue, Q.L.; Chen, S.; Wu, C. The Association between Frailty and Healthcare Expenditure among Chinese Older Adults. J. Am. Med. Dir. Assoc. 2020, 21, 780–785. [Google Scholar] [CrossRef]
- The Lancet Public Health. Disability—A neglected issue in public health. Lancet Public Health 2021, 6, e346. [Google Scholar] [CrossRef]
- Eidam, A.; Bauer, J.M.; Benzinger, P. Prevention of frailty. Z. Gerontol. Geriatr. 2024, 57, 435–441. [Google Scholar] [CrossRef]
- Khan, K.T.; Hemati, K.; Donovan, A.L. Geriatric Physiology and the Frailty Syndrome. Anesthesiol. Clin. 2019, 37, 453–474. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 2021, 50, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Pradana, A.A.; Bai, D.; Hidayat, A.T.; Lin, C.J.; Lee, S.C. Cost of illness analysis of frailty for older adults: A systematic review and meta-analysis. Eur. Geriatr. Med. 2025, 16, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, G.; Gao, H.; Wu, Q.; Meng, J.; Wang, F.; Jiang, S.; Chen, M.; Xu, W.; Zhang, Y.; et al. Geriatric syndromes, chronic inflammation, and advances in the management of frailty: A review with new insights. Biosci. Trends 2023, 17, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Snijders, T.; Leenders, M.; de Groot, L.; van Loon, L.J.C.; Verdijk, L.B. Muscle mass and strength gains following 6 months of resistance type exercise training are only partly preserved within one year with autonomous exercise continuation in older adults. Exp. Gerontol. 2019, 121, 71–78. [Google Scholar] [CrossRef]
- Marzuca-Nassr, G.N.; Alegría-Molina, A.; SanMartín-Calísto, Y.; Artigas-Arias, M.; Huard, N.; Sapunar, J.; Salazar, L.A.; Verdijk, L.B.; van Loon, L.J.C. Muscle Mass and Strength Gains Following Resistance Exercise Training in Older Adults 65–75 Years and Older Adults Above 85 Years. Int. J. Sport Nutr. Exerc. Metab. 2024, 34, 11–19. [Google Scholar] [CrossRef]
- Bernabei, R.; Landi, F.; Calvani, R.; Cesari, M.; Del Signore, S.; Anker, S.D.; Bejuit, R.; Bordes, P.; Cherubini, A.; Cruz-Jentoft, A.J.; et al. Multicomponent intervention to prevent mobility disability in frail older adults: Randomised controlled trial (SPRINTT project). BMJ 2022, 377, e068788. [Google Scholar] [CrossRef]
- Kwan, R.Y.C.; Liu, J.; Sin, O.S.K.; Fong, K.N.K.; Qin, J.; Wong, J.C.Y.; Lai, C. Effects of Virtual Reality Motor-Cognitive Training for Older People with Cognitive Frailty: Multicentered Randomized Controlled Trial. J. Med. Internet Res. 2024, 26, e57809. [Google Scholar] [CrossRef]
- Kwan, R.Y.C.; Liu, J.Y.W.; Fong, K.N.K.; Qin, J.; Leung, P.K.; Sin, O.S.K.; Hon, P.Y.; Suen, L.W.; Tse, M.K.; Lai, C.K. Feasibility and Effects of Virtual Reality Motor-Cognitive Training in Community-Dwelling Older People with Cognitive Frailty: Pilot Randomized Controlled Trial. JMIR Serious Games 2021, 9, e28400. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Biello, F.; Maggiora, P.M.; Bruna, R.; Burrafato, G.; Cappelli, M.; Varughese, F.; Martini, V.; Platini, F.; Deambrogi, C.; et al. A randomized clinical study on the impact of Comprehensive Geriatric Assessment (CGA) based interventions on the quality of life of elderly, frail, onco-hematologic patients candidate to anticancer therapy: Protocol of the ONCO-Aging study. BMC Geriatr. 2021, 21, 320. [Google Scholar] [CrossRef] [PubMed]
- Jyväkorpi, S.K.; Ramel, A.; Strandberg, T.E.; Piotrowicz, K.; Błaszczyk-Bębenek, E.; Urtamo, A.; Rempe, H.M.; Geirsdóttir, Ó.; Vágnerová, T.; Billot, M.; et al. The sarcopenia and physical frailty in older people: Multi-component treatment strategies (SPRINTT) project: Description and feasibility of a nutrition intervention in community-dwelling older Europeans. Eur. Geriatr. Med. 2021, 12, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Gasana, J.; O’Keeffe, T.; Withers, T.M.; Greaves, C.J. A systematic review and meta-analysis of the long-term effects of physical activity interventions on objectively measured outcomes. BMC Public Health 2023, 23, 1697. [Google Scholar] [CrossRef]
- Cereda, E.; Pisati, R.; Rondanelli, M.; Caccialanza, R. Whey Protein, Leucine- and Vitamin-D-Enriched Oral Nutritional Supplementation for the Treatment of Sarcopenia. Nutrients 2022, 14, 1524. [Google Scholar] [CrossRef] [PubMed]
- Artaza-Artabe, I.; Sáez-López, P.; Sánchez-Hernández, N.; Fernández-Gutierrez, N.; Malafarina, V. The relationship between nutrition and frailty: Effects of protein intake, nutritional supplementation, vitamin D and exercise on muscle metabolism in the elderly. A systematic review. Maturitas 2016, 93, 89–99. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Weng, X.; Yang, X.; Bao, J.; Liao, S.; Xi, Y.; Song, X.; Guo, G. Effects of the Vivifrail-B multicomponent exercise program based on society ecosystems theory on physical function in community-dwelling frail older adults: A randomized controlled trial. Exp. Gerontol. 2025, 200, 112670. [Google Scholar] [CrossRef]
- Gaston, S.; Billman, E.; Han, L.; Drover, D. Frailty assessment utilization around the globe-a systematic review. J. Frailty Aging 2025, 14, 100088. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.M.; Chang, P.; Wang, Z.; Yang, B.; Ye, H.F. Association of the dietary inflammation index with frailty in middle-aged and older adults: A systematic review and meta-analysis. Front. Nutr. 2025, 12, 1607110. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef]
- Li, Q.; Yang, H.; Song, S.; Liu, J.; Wang, Z.; Wang, J. Bioactive Components in Whole Grains for the Regulation of Skeletal Muscle Function. Foods 2022, 11, 2752. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, B.; Zahiragic, L.; Bierwagen, A.; Kabisch, S.; Groener, J.B.; Nowotny, P.J.; Fleitmann, A.K.; Herder, C.; Pacini, G.; Erlund, I.; et al. Low-energy diets differing in fibre, red meat and coffee intake equally improve insulin sensitivity in type 2 diabetes: A randomised feasibility trial. Diabetologia 2015, 58, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Casas-Herrero, Á.; Sáez de Asteasu, M.L.; Antón-Rodrigo, I.; Sánchez-Sánchez, J.L.; Montero-Odasso, M.; Marín-Epelde, I.; Ramón-Espinoza, F.; Zambom-Ferraresi, F.; Petidier-Torregrosa, R.; Elexpuru-Estomba, J.; et al. Effects of Vivifrail multicomponent intervention on functional capacity: A multicentre, randomized controlled trial. J. Cachexia Sarcopenia Muscle 2022, 13, 884–893. [Google Scholar] [CrossRef]


| Group | Intervention Components | Personnel | Frequency |
|---|---|---|---|
| Control Group | 1. Routine community health lectures
| Trained project staff | Monthly |
| Multifaceted Nutrition Intervention | 1. Daily dietary guidance
| Trained nutrition specialists | Weekly |
2. Personalized nutritional consultations
| Intelligent app/programs | At any time | |
3. Health education
| Trained nutrition specialists | Every two weeks | |
| Multifaceted Nutrition and Exercise Combined Intervention | Includes all components from the Multifaceted Nutrition Intervention group, PLUS: 4. Exercise guidance
| Trained sports specialists | Weekly |
| No. | Activities | Content (Lecture Theme) |
| The first month | ||
| 1 | Lecture 1 | Nutritional Status and Common Health Issues in the Elderly |
| 2 | Lecture 2 | Unlocking the Health Code: Understanding Nutrients |
| Qualitative interviews: satisfaction survey and feedback | ||
| The second month | ||
| 3 | Lecture 3 | Unlocking the Health Code: Recognizing the Nutritional Value of Foods |
| 4 | Lecture 4 | Focus on Nutritional Screening: How the Elderly Can Identify Malnutrition |
| Qualitative interviews: satisfaction survey and feedback | ||
| The third month | ||
| 5 | Lecture 5 | How the Elderly Should Plan Their Three Meals a Day |
| 6 | Lecture 6 | To Live Longer, Overcome ‘Physical Frailty’—A New Perspective on Elderly Health Management |
| Qualitative interviews: satisfaction survey and feedback | ||
| The fourth month | ||
| 7 | Lecture 7 | A Healthy Life, ‘Muscle’ is Essential—Nutritional Management of Sarcopenia |
| 8 | Lecture 8 | Scientific Diet: Helping You ‘Keep Your Memory’ |
| Qualitative interviews: satisfaction survey and feedback | ||
| Measurements | Time Points | Instrument/Method of Assessment | Outcome Variables | |||
|---|---|---|---|---|---|---|
| Baseline | 3 Months | 6 Months | 12 Months | |||
| Physical examination | ||||||
| Height | Yes | Yes | Yes | Yes | Stadiometer | BMI |
| Weight | Yes | Yes | Yes | Yes | Lever scale | |
| Waist/Calf circumference | Yes | Yes | Yes | Yes | Tape | Waist/Calf circumference |
| Sebum | Yes | Yes | Yes | Yes | Sebaceous pliers | Body fat percentage |
| Body composition analysis | Yes | Yes | Yes | Yes | Body composition analyzer/bioelectrical impedance analysis technique | Body fat mass, body fat percentage, defatted body weight, muscle mass, body water, protein, inorganic salts, and other data |
| (Strength of one’s) Grip | Yes | Yes | Yes | Yes | Grip strength meter/adjust for palm size, measure with index finger second joint at a right angle, maximum effort | Grip strength measurements |
| Simple Physical Performance Based Assessment (SPPB) | ||||||
| Balance testing | Yes | Yes | Yes | Yes | Timekeeping device/includes balancing in 3 different difficulty positions: feet together, half tandem standing, and tandem standing. | Test scores |
| 3 or 4 meter pace test | Yes | Yes | Yes | Yes | Timekeeping device/walk at maximum speed. Record the shortest of two trials. | Test scores |
| Timed Sit-Stand Test | Yes | Yes | Yes | Yes | Timekeeping device/assess lower limb strength in elders by timing 5 rapid sit-to-stand cycles with a stopwatch. | Test scores |
| Frailty status | ||||||
| Fried Frailty Phenotype | Yes | Yes | Yes | Yes | Scale questionnaire | Scale score |
| Tilburg Frailty Indicator | Yes | Yes | Yes | Yes | Scale questionnaire | Scale score |
| Frailty trajectories | Yes | Yes | Yes | Yes | Changes in frailty trajectories before and after the intervention, as measured by the Frailty Index | |
| Frailty recovery rates | Yes | Yes | Yes | Yes | Difference in frailty scores before and after intervention | |
| Nutritional status | ||||||
| Malnutrition risk assessment | Yes | Yes | Yes | Yes | Scale questionnaire | Scale score |
| Nutritional literacy assessment for the elderly | Yes | Yes | Yes | Yes | Scale questionnaire | Scale score |
| Metabolomics | ||||||
| Serum protein | Yes | Yes | Yes | Physiological and biochemical tests | Serum total protein, albumin, and transferrin | |
| Urine 8-hydroxyguanosine (8-oxo-Gsn) | Yes | Yes | Yes | Physiological and biochemical tests | ||
| Serum inflammatory factors | Yes | Yes | Yes | Physiological and biochemical tests | ||
| Changes in serum differential metabolites | Yes | Yes | Utilize nontargeted metabolomics to assess serum metabolite changes pre- and post-intervention, elucidating the intervention’s mechanism | |||
| Microbiota | ||||||
| Changes in gut microbiota | Yes | Yes | Using the 16S sequencing method to detect changes in gut microbiota and differential bacterial genera among groups | |||
| Comprehensive geriatric assessment | ||||||
| Comprehensive geriatric assessment | Yes | Yes | Yes | Yes | Comprehensive Geriatric Assessment Questionnaire (CGAQ) | Physical assessment, functional assessment, medication review, social functioning assessment, environmental assessment, etc. |
| Intrinsic capacity | ||||||
| Intrinsic capacity | Yes | Yes | Yes | Yes | Scale questionnaire | 5-dimensional structure |
| State of health | ||||||
| EuroQol Five Dimensions Questionnaire | Yes | Yes | Yes | Yes | EuroQol Five Dimensions Questionnaire | Mobility, self-care, usual activities, pain/discomfort, and anxiety/depression |
| Sleep conditions | ||||||
| Pittsburgh Sleep Quality Index | Yes | Yes | Yes | Yes | Pittsburgh Sleep Quality Index | Rate the quality of sleep in the last 1 month |
| Participants’ satisfaction | ||||||
| Participants’ satisfaction | Yes | Yes | Scale questionnaire | |||
| Cognition function | ||||||
| Cognition function | Yes | Yes | Yes | Yes | Mini-Mental State Examination (MMSE) | |
| Adverse event | ||||||
| Adverse event | Yes | Yes | Yes | Yes | Serious, unexpected, and supervised adverse events | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Zhang, H.; Sun, M.; Ma, Y.; Tu, Y.; Tian, J.; Fan, R.; Zhu, W.; Zhang, Z. Multifaceted Nutrition Intervention for Frail Elderly in the Community: Protocol of a Randomized Controlled Trial (The MINUTE Study). Nutrients 2025, 17, 3213. https://doi.org/10.3390/nu17203213
Han Y, Zhang H, Sun M, Ma Y, Tu Y, Tian J, Fan R, Zhu W, Zhang Z. Multifaceted Nutrition Intervention for Frail Elderly in the Community: Protocol of a Randomized Controlled Trial (The MINUTE Study). Nutrients. 2025; 17(20):3213. https://doi.org/10.3390/nu17203213
Chicago/Turabian StyleHan, Yaxin, Haohao Zhang, Meng Sun, Yuxin Ma, Yahui Tu, Jiajing Tian, Rui Fan, Wenli Zhu, and Zhaofeng Zhang. 2025. "Multifaceted Nutrition Intervention for Frail Elderly in the Community: Protocol of a Randomized Controlled Trial (The MINUTE Study)" Nutrients 17, no. 20: 3213. https://doi.org/10.3390/nu17203213
APA StyleHan, Y., Zhang, H., Sun, M., Ma, Y., Tu, Y., Tian, J., Fan, R., Zhu, W., & Zhang, Z. (2025). Multifaceted Nutrition Intervention for Frail Elderly in the Community: Protocol of a Randomized Controlled Trial (The MINUTE Study). Nutrients, 17(20), 3213. https://doi.org/10.3390/nu17203213







