Effects of Homocysteine Circulating Levels on Human Spontaneous Fertility and In Vitro Fertilization Outcomes: A Literature Review
Abstract
1. Introduction
2. Methodology
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Extraction
2.4. Quality Assessment and Limitations
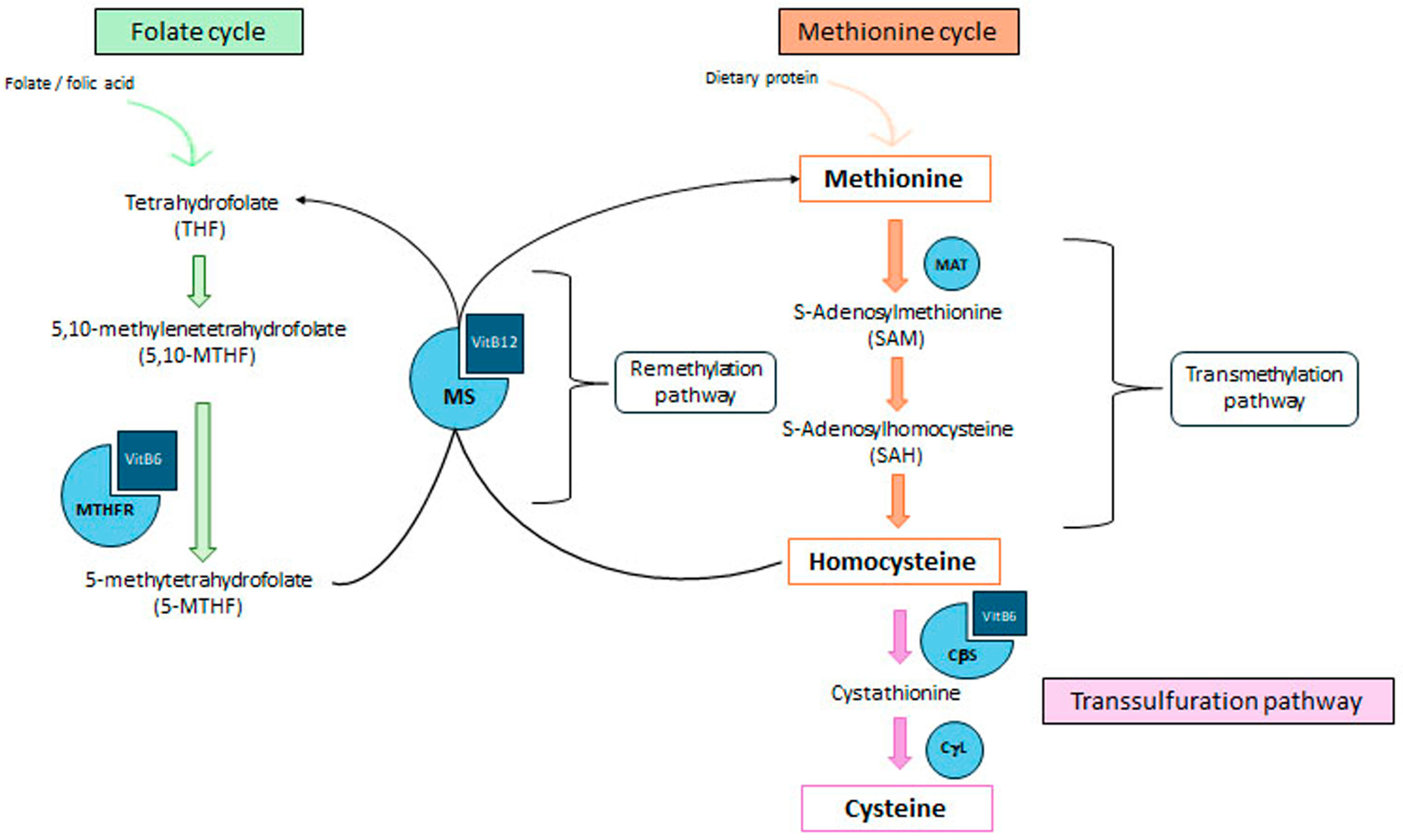
3. Metabolism of Homocysteine
4. Hyperhomocysteinemia
4.1. Nutritional Defects
4.2. Defective Function of Enzymes Involved in Hcy Catabolism
5. Homocysteine and Male Fertility
5.1. Dietary Intake/Supplementation of Folates and Vitamins
5.2. Genetic Polymorphisms of Enzymes Involved in Met and Folate Cycles
5.2.1. MTHFR 677C>T Polymorphism
5.2.2. MTHFR 1298A>C Polymorphism
6. Homocysteine and Female Fertility
7. Hcy and In Vitro Fertilization (IVF)
8. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CβS | Cystathionine β-synthase |
| Cys | Cysteine |
| DNA | Deoxyribonucleic acid |
| FF | Follicular fluid |
| FSH | Follicle-stimulating hormone |
| GnRH | Gonadotropin-releasing hormone |
| HHcy | Hyperhomocysteinemia |
| Hcy | Homocysteine |
| IVF | In vitro fertilization |
| MAT | Methionine adenosyltransferase |
| Met | Methionine |
| MS | Methionine synthase |
| MTHFR | 5,10-Methylenetetrahydrofolate reductase |
| NO | Nitric oxide |
| PCOS | Polycystic ovary syndrome |
| RIF | Repeated implantation failure |
| RNA | Ribonucleic acid |
| SAH | S-adenosylhomocysteine |
| SAM | S-adenosylmethionine |
| SNP | Single nucleotide polymorphism |
| 5-MTHF | 5-methyltetrahydrofolate |
| THF | Tetrahydrofolate |
References
- Cetin, I.; Berti, C.; Calabrese, S. Role of Micronutrients in the Periconceptional Period. Hum. Reprod. Update 2010, 16, 80–95. [Google Scholar] [CrossRef]
- Forges, T.; Monnier-Barbarino, P.; Alberto, J.M.; Guéant-Rodriguez, R.M.; Daval, J.L.; Guéant, J.L. Impact of Folate and Homocysteine Metabolism on Human Reproductive Health. Hum. Reprod. Update 2007, 13, 225–238. [Google Scholar] [CrossRef]
- Perła-Kaján, J.; Jakubowski, H. Dysregulation of Epigenetic Mechanisms of Gene Expression in the Pathologies of Hyperhomocysteinemia. Int. J. Mol. Sci. 2019, 20, 3140. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.A.; Loscalzo, J. The Treatment of Hyperhomocysteinemia. Annu. Rev. Med. 2009, 60, 39–54. [Google Scholar] [CrossRef]
- Jakubowski, H. Protein N-Homocysteinylation and Colorectal Cancer. Trends Cancer 2019, 5, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H. Homocysteine Modification in Protein Structure/Function and Human Disease. Physiol. Rev. 2019, 99, 555–604. [Google Scholar] [CrossRef] [PubMed]
- Mudd, S.H.; Finkelstein, J.D.; Refsum, H.; Ueland, P.M.; Malinow, M.R.; Lentz, S.R.; Jacobsen, D.W.; Brattström, L.; Wilcken, B.; Wilcken, D.E.; et al. Homocysteine and Its Disulfide Derivatives: A Suggested Consensus Terminology. Arter. Thromb. Vasc. Biol. 2000, 20, 1704–1706. [Google Scholar] [CrossRef]
- Glowacki, R.; Jakubowski, H. Cross-Talk between Cys34 and Lysine Residues in Human Serum Albumin Revealed by N-Homocysteinylation. J. Biol. Chem. 2004, 279, 10864–10871. [Google Scholar] [CrossRef]
- Sikora, M.; Marczak, Ł.; Perła-Kajan, J.; Jakubowski, H. Sex Affects N-Homocysteinylation at Lysine Residue 212 of Albumin in Mice. Sci. Rep. 2019, 9, 2669. [Google Scholar] [CrossRef]
- Jakubowski, H. The Molecular Basis of Homocysteine Thiolactone-Mediated Vascular Disease. Clin. Chem. Lab. Med. 2007, 45, 1704–1716. [Google Scholar] [CrossRef]
- Jakubowski, H. Quality Control in tRNA Charging -- Editing of Homocysteine. Acta Biochim. Pol. 2011, 58, 149–163. [Google Scholar] [CrossRef]
- Fowler, B. Homocysteine: Overview of Biochemistry, Molecular Biology, and Role in Disease Processes. Semin. Vasc. Med. 2005, 5, 77–86. [Google Scholar] [CrossRef]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The Metabolism and Significance of Homocysteine in Nutrition and Health. Nutr. Metab. 2017, 14, 78. [Google Scholar] [CrossRef]
- Verhoef, P.; van Vliet, T.; Olthof, M.R.; Katan, M.B. A High-Protein Diet Increases Postprandial but Not Fasting Plasma Total Homocysteine Concentrations: A Dietary Controlled, Crossover Trial in Healthy Volunteers. Am. J. Clin. Nutr. 2005, 82, 553–558. [Google Scholar] [CrossRef]
- McRae, M.P. Betaine Supplementation Decreases Plasma Homocysteine in Healthy Adult Participants: A Meta-Analysis. J. Chiropr. Med. 2013, 12, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Škovierová, H.; Vidomanová, E.; Mahmood, S.; Sopková, J.; Drgová, A.; Červeňová, T.; Halašová, E.; Lehotský, J. The Molecular and Cellular Effect of Homocysteine Metabolism Imbalance on Human Health. Int. J. Mol. Sci. 2016, 17, 1733. [Google Scholar] [CrossRef]
- Jacobsen, D.W.; Gatautis, V.J.; Green, R.; Robinson, K.; Savon, S.R.; Secic, M.; Ji, J.; Otto, J.M.; Taylor, L.M. Total Plasma Homocysteine: The Mediator/Marker Controversy Continues. 1994. Clin. Chem. 2009, 55, 1742–1743. [Google Scholar] [CrossRef] [PubMed]
- Fokkema, M.R.; Weijer, J.M.; Dijck-Brouwer, D.A.; van Doormaal, J.J.; Muskiet, F.A. Influence of Vitamin-Optimized Plasma Homocysteine Cutoff Values on the Prevalence of Hyperhomocysteinemia in Healthy Adults. Clin. Chem. 2001, 47, 1001–1007. [Google Scholar] [CrossRef]
- Kang, S.S.; Wong, P.W.; Malinow, M.R. Hyperhomocyst(e)Inemia as a Risk Factor for Occlusive Vascular Disease. Annu. Rev. Nutr. 1992, 12, 279–298. [Google Scholar] [CrossRef] [PubMed]
- Hak, A.E.; Polderman, K.H.; Westendorp, I.C.; Jakobs, C.; Hofman, A.; Witteman, J.C.; Stehouwer, C.D. Increased Plasma Homocysteine after Menopause. Atherosclerosis 2000, 149, 163–168. [Google Scholar] [CrossRef]
- Berger, P.B.; Herrmann, R.R.; Dumesic, D.A. The Effect of Estrogen Replacement Therapy on Total Plasma Homocysteine in Healthy Postmenopausal Women. Mayo Clin. Proc. 2000, 75, 18–23. [Google Scholar] [CrossRef]
- Lussana, F.; Zighetti, M.L.; Bucciarelli, P.; Cugno, M.; Cattaneo, M. Blood Levels of Homocysteine, Folate, Vitamin B6 and B12 in Women Using Oral Contraceptives Compared to Non-Users. Thromb. Res. 2003, 112, 37–41. [Google Scholar] [CrossRef]
- Roopnarinesingh, R.; Jackson, B.; Osman, Z.; Harrison, R.; Mayne, P. Homocysteine in Assisted Reproduction: Does Oestradiol Influence Homocysteine Levels? J. Obstet. Gynaecol. 2006, 26, 59–62. [Google Scholar] [CrossRef]
- Jakubowski, H. Homocysteine Editing, Thioester Chemistry, Coenzyme A, and the Origin of Coded Peptide Synthesis †. Life 2017, 7, 6. [Google Scholar] [CrossRef]
- Gurda, D.; Handschuh, L.; Kotkowiak, W.; Jakubowski, H. Homocysteine Thiolactone and N-Homocysteinylated Protein Induce pro-Atherogenic Changes in Gene Expression in Human Vascular Endothelial Cells. Amino Acids 2015, 47, 1319–1339. [Google Scholar] [CrossRef]
- van Guldener, C.; Stehouwer, C.D. Hyperhomocysteinemia, Vascular Pathology, and Endothelial Dysfunction. Semin. Thromb. Hemost. 2000, 26, 281–289. [Google Scholar] [CrossRef]
- Obeid, R.; Herrmann, W. Homocysteine and Lipids: S-Adenosyl Methionine as a Key Intermediate. FEBS Lett. 2009, 583, 1215–1225. [Google Scholar] [CrossRef]
- Hay, G.; Clausen, T.; Whitelaw, A.; Trygg, K.; Johnston, C.; Henriksen, T.; Refsum, H. Maternal Folate and Cobalamin Status Predicts Vitamin Status in Newborns and 6-Month-Old Infants. J. Nutr. 2010, 140, 557–564. [Google Scholar] [CrossRef]
- Irizarry, M.C.; Gurol, M.E.; Raju, S.; Diaz-Arrastia, R.; Locascio, J.J.; Tennis, M.; Hyman, B.T.; Growdon, J.H.; Greenberg, S.M.; Bottiglieri, T. Association of Homocysteine with Plasma Amyloid β Protein in Aging and Neurodegenerative Disease. Neurology 2005, 65, 1402–1408. [Google Scholar] [CrossRef]
- Kruman, I.I.; Culmsee, C.; Chan, S.L.; Kruman, Y.; Guo, Z.; Penix, L.; Mattson, M.P. Homocysteine Elicits a DNA Damage Response in Neurons That Promotes Apoptosis and Hypersensitivity to Excitotoxicity. J. Neurosci. 2000, 20, 6920–6926. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Skoumas, J.; Masoura, C.; Toutouzas, P.; Stefanadis, C. Effect of Exposure to Secondhand Smoke on Markers of Inflammation: The ATTICA Study. Am. J. Med. 2004, 116, 145–150. [Google Scholar] [CrossRef]
- Refsum, H.; Smith, A.D.; Ueland, P.M.; Nexo, E.; Clarke, R.; McPartlin, J.; Johnston, C.; Engbaek, F.; Schneede, J.; McPartlin, C.; et al. Facts and Recommendations about Total Homocysteine Determinations: An Expert Opinion. Clin. Chem. 2004, 50, 3–32. [Google Scholar] [CrossRef]
- Ho, C.; Quay, T.; Devlin, A.; Lamers, Y. Prevalence and Predictors of Low Vitamin B6 Status in Healthy Young Adult Women in Metro Vancouver. Nutrients 2016, 8, 538. [Google Scholar] [CrossRef]
- Lonn, E. Rationale, Design and Baseline Characteristics of a Large, Simple, Randomized Trial of Combined Folic Acid and Vitamins B6 and B12 in High-Risk Patients: The Heart Outcomes Prevention Evaluation (HOPE)-2 Trial. Can. J. Cardiol. 2006, 22, 47–53. [Google Scholar] [CrossRef]
- Lonn, E.; Yusuf, S.; Arnold, M.J.; Sheridan, P.; Pogue, J.; Micks, M.; McQueen, M.J.; Probstfield, J.; Fodor, G.; Held, C.; et al. Homocysteine Lowering with Folic Acid and B Vitamins in Vascular Disease. The Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. N. Engl. J. Med. 2006, 354, 1567–1577. [Google Scholar] [CrossRef]
- Miraglia, N.; Dehay, E. Folate Supplementation in Fertility and Pregnancy: The Advantages of (6S)5-Methyltetrahydrofolate. Altern. Ther. Health Med. 2022, 28, 12–17. [Google Scholar]
- Menezo, Y.; Elder, K.; Clement, A.; Clement, P. Folic Acid, Folinic Acid, 5 Methyl TetraHydroFolate Supplementation for Mutations That Affect Epigenesis through the Folate and One-Carbon Cycles. Biomolecules 2022, 12, 197. [Google Scholar] [CrossRef]
- Kamen, B.A.; Smith, A.K. A Review of Folate Receptor Alpha Cycling and 5-Methyltetrahydrofolate Accumulation with an Emphasis on Cell Models in Vitro. Adv. Drug Deliv. Rev. 2004, 56, 1085–1097. [Google Scholar] [CrossRef]
- Laraqui, A.; Allami, A.; Carrié, A.; Coiffard, S.; Benkouka, F.; Benjouad, A.; Bendriss, A.; Kadiri, N.; Bennouar, N.; Benomar, A.; et al. Influence of Methionine Synthase (A2756G) and Methionine Synthase Reductase (A66G) Polymorphisms on Plasma Homocysteine Levels and Relation to Risk of Coronary Artery Disease. Acta Cardiol. 2006, 61, 51–61. [Google Scholar] [CrossRef]
- Li, W.-X.; Cheng, F.; Zhang, A.-J.; Dai, S.-X.; Li, G.-H.; Lv, W.-W.; Zhou, T.; Zhang, Q.; Zhang, H.; Zhang, T.; et al. Folate Deficiency and Gene Polymorphisms of MTHFR, MTR and MTRR Elevate the Hyperhomocysteinemia Risk. Clin. Lab. 2017, 63, 523–533. [Google Scholar] [CrossRef]
- Mendes, M.I.S.; Colaço, H.G.; Smith, D.E.C.; Ramos, R.J.J.F.; Pop, A.; Van Dooren, S.J.M.; Tavares De Almeida, I.; Kluijtmans, L.A.J.; Janssen, M.C.H.; Rivera, I.; et al. Reduced Response of Cystathionine Beta-Synthase (CBS) to S-Adenosylmethionine (SAM): Identification and Functional Analysis of CBS Gene Mutations in Homocystinuria Patients. J. Inher. Metab. Disea. 2014, 37, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Karimian, M.; Colagar, A.H. Association of C677T Transition of the Human Methylenetetrahydrofolate Reductase (MTHFR) Gene with Male Infertility. Reprod. Fertil. Dev. 2016, 28, 785–794. [Google Scholar] [CrossRef]
- Karimian, M.; Hosseinzadeh Colagar, A. Methionine Synthase A2756G Transition Might Be a Risk Factor for Male Infertility: Evidences from Seven Case-Control Studies. Mol. Cell. Endocrinol. 2016, 425, 1–10. [Google Scholar] [CrossRef]
- Long, S.; Goldblatt, J. MTHFR Genetic Testing: Controversy and Clinical Implications. Aust. Fam. Physician 2016, 45, 237–240. [Google Scholar] [PubMed]
- McNulty, H.; Dowey, L.R.C.; Strain, J.J.; Dunne, A.; Ward, M.; Molloy, A.M.; McAnena, L.B.; Hughes, J.P.; Hannon-Fletcher, M.; Scott, J.M. Riboflavin Lowers Homocysteine in Individuals Homozygous for the MTHFR 677C->T Polymorphism. Circulation 2006, 113, 74–80. [Google Scholar] [CrossRef]
- Guéant, J.-L.; Guéant-Rodriguez, R.-M.; Anello, G.; Bosco, P.; Brunaud, L.; Romano, C.; Ferri, R.; Romano, A.; Candito, M.; Namour, B. Genetic Determinants of Folate and Vitamin B12 Metabolism: A Common Pathway in Neural Tube Defect and Down Syndrome? Clin. Chem. Lab. Med. 2003, 41, 1473–1477. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhao, R.; Shen, M.; Ye, J.; Li, X.; Huang, Y.; Hua, L.; Wang, Z.; Li, J. Role of Genetic Mutations in Folate-Related Enzyme Genes on Male Infertility. Sci. Rep. 2015, 5, 15548. [Google Scholar] [CrossRef]
- Sperandeo, M.P.; de Franchis, R.; Andria, G.; Sebastio, G. A 68-Bp Insertion Found in a Homocystinuric Patient Is a Common Variant and Is Skipped by Alternative Splicing of the Cystathionine Beta-Synthase mRNA. Am. J. Hum. Genet. 1996, 59, 1391–1393. [Google Scholar]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12, Folate, and the Methionine Remethylation Cycle—Biochemistry, Pathways, and Regulation. J. Inher. Metab. Disea. 2019, 42, 673–685. [Google Scholar] [CrossRef]
- Kelly, T.L.J.; Li, E.; Trasler, J.M. 5-Aza-2′-Deoxycytidine Induces Alterations in Murine Spermatogenesis and Pregnancy Outcome. J. Androl. 2003, 24, 822–830. [Google Scholar] [CrossRef]
- Klein, R.; Pfitzer, P. Flow Cytometry of Postmortem Human Testicular Tissue in Cases of Atherosclerosis. Cytometry 1984, 5, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.J.; Carvalho, F.; Sousa, M.; Barros, A. Genomic Imprinting in Disruptive Spermatogenesis. Lancet 2004, 363, 1700–1702. [Google Scholar] [CrossRef]
- Singh, K.; Jaiswal, D. One-Carbon Metabolism, Spermatogenesis, and Male Infertility. Reprod. Sci. 2013, 20, 622–630. [Google Scholar] [CrossRef]
- Holm, J.; Hansen, S.I.; Høier-Madsen, M.; Christensen, T.B.; Nichols, C.W. Characterization of a High-Affinity Folate Receptor in Normal and Malignant Human Testicular Tissue. Biosci. Rep. 1999, 19, 571–580. [Google Scholar] [CrossRef]
- Murphy, L.E.; Mills, J.L.; Molloy, A.M.; Qian, C.; Carter, T.C.; Strevens, H.; Wide-Swensson, D.; Giwercman, A.; Levine, R.J. Folate and Vitamin B12 in Idiopathic Male Infertility. Asian J. Androl. 2011, 13, 856–861. [Google Scholar] [CrossRef]
- Malm, J.; Birn, H.; Frohm, B.; Hansen, S.; Høier-Madsen, M.; Holm, J. A Minor Fraction of a High-affinity Folate Binding Protein from the Epididymis Is Associated with Membranous Vesicles and Spermatozoa in Human Semen. Int. J. Androl. 2005, 28, 267–274. [Google Scholar] [CrossRef]
- Chen, Q.; Ng, V.; Mei, J.; Chia, S.E. Comparison of seminal vitamin B12, folate, reactive oxygen species and various sperm parameters between fertile and infertile males. Wei Sheng Yan Jiu 2001, 30, 80–82. [Google Scholar]
- Boxmeer, J.C.; Smit, M.; Utomo, E.; Romijn, J.C.; Eijkemans, M.J.C.; Lindemans, J.; Laven, J.S.E.; Macklon, N.S.; Steegers, E.A.P.; Steegers-Theunissen, R.P.M. Low Folate in Seminal Plasma Is Associated with Increased Sperm DNA Damage. Fertil. Steril. 2009, 92, 548–556. [Google Scholar] [CrossRef]
- Cosentino, M.J.; Pakyz, R.E.; Fried, J. Pyrimethamine: An Approach to the Development of a Male Contraceptive. Proc. Natl. Acad. Sci. USA 1990, 87, 1431–1435. [Google Scholar] [CrossRef]
- Kalla, N.R.; Saggar, S.K.; Puri, R.; Mehta, U. Regulation of Male Fertility by Pyrimethamine in Adult Mice. Res. Exp. Med. 1997, 197, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.L.J.; Neaga, O.R.; Schwahn, B.C.; Rozen, R.; Trasler, J.M. Infertility in 5,10-Methylenetetrahydrofolate Reductase (MTHFR)-Deficient Male Mice Is Partially Alleviated by Lifetime Dietary Betaine Supplementation1. Biol. Reprod. 2005, 72, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Said, T.M. Role of Caspases in Male Infertility. Hum. Reprod. Update 2004, 10, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Sanocka, D.; Jędrzejczak, P.; Szumała-Kaękol, A.; Frączek, M.; Kurpisz, M. Male Genital Tract Inflammation: The Role of Selected Interleukins in Regulation of Pro-Oxidant and Antioxidant Enzymatic Substances in Seminal Plasma. J. Androl. 2003, 24, 448–455. [Google Scholar] [CrossRef]
- Herrero, M.; Lamirande, E.; Gagnon, C. Nitric Oxide Is a Signaling Molecule in Spermatozoa. Curr. Pharm. Des. 2003, 9, 419–425. [Google Scholar] [CrossRef]
- Revelli, A.; Costamagna, C.; Moffa, F.; Aldieri, E.; Ochetti, S.; Bosia, A.; Massobrio, M.; Lindblom, B.; Ghigo, D. Signaling Pathway of Nitric Oxide-Induced Acrosome Reaction in Human Spermatozoa1. Biol. Reprod. 2001, 64, 1708–1712. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, E.; De Angelis, F.; Gazzano, E.; Hassanpour, H.; Bertagna, A.; Aldieri, E.; Revelli, A.; Ghigo, D. Nitric Oxide Stimulates Human Sperm Motility via Activation of the Cyclic GMP/Protein Kinase G Signaling Pathway. Reproduction 2011, 141, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Bentivoglio, G.; Melica, F.; Cristoforoni, P. Folinic Acid in the Treatment of Human Male Infertility. Fertil. Steril. 1993, 60, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.Y.; Merkus, H.M.W.M.; Thomas, C.M.G.; Menkveld, R.; Zielhuis, G.A.; Steegers-Theunissen, R.P.M. Effects of Folic Acid and Zinc Sulfate on Male Factor Subfertility: A Double-Blind, Randomized, Placebo-Controlled Trial. Fertil. Steril. 2002, 77, 491–498. [Google Scholar] [CrossRef]
- Ebisch, I.M.W.; Van Heerde, W.L.; Thomas, C.M.G.; Van Der Put, N.; Wong, W.Y.; Steegers-Theunissen, R.P.M. C677T Methylenetetrahydrofolate Reductase Polymorphism Interferes with the Effects of Folic Acid and Zinc Sulfate on Sperm Concentration. Fertil. Steril. 2003, 80, 1190–1194. [Google Scholar] [CrossRef]
- Ebisch, I.M.W.; Peters, W.H.M.; Thomas, C.M.G.; Wetzels, A.M.M.; Peer, P.G.M.; Steegers-Theunissen, R.P.M. Homocysteine, Glutathione and Related Thiols Affect Fertility Parameters in the (Sub)Fertile Couple. Hum. Reprod. 2006, 21, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Bezold, G.; Lange, M.; Peter, R.U. Homozygous Methylenetetrahydrofolate Reductase C677T Mutation and Male Infertility. N. Engl. J. Med. 2001, 344, 1172–1173. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, Y.Y.; Wu, S.; Tang, Y.D.; Rao, X.D.; Xiong, L.; Tan, M.; Deng, M.Z.; Liu, H. Association between C677T and A1298C Polymorphisms of the MTHFR Gene and Risk of Male Infertility: A Meta-Analysis. Genet. Mol. Res. 2016, 15, 10–4238. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Z.; Zhang, M.; Gong, R.; Xu, Y.; Wang, B. Association of the Methylenetetrahydrofolate Reductase Gene C677T Polymorphism with the Risk of Male Infertility: A Meta-Analysis. Ren. Fail. 2016, 38, 185–193. [Google Scholar] [CrossRef]
- Hong, H.; Hu, Y.; Yu, X.; Zhou, L.; Lv, M.; Sun, Y.; Ren, W.; Zhou, D. Associations of C677T Polymorphism in Methylenetetrahydrofolate Reductase (MTHFR) Gene with Male Infertility Risk: A Meta-Analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 212, 101–109. [Google Scholar] [CrossRef]
- Tüttelmann, F.; Rajpert-De Meyts, E.; Nieschlag, E.; Simoni, M. Gene Polymorphisms and Male Infertility--a Meta-Analysis and Literature Review. Reprod. Biomed. Online 2007, 15, 643–658. [Google Scholar] [CrossRef]
- Wu, W.; Shen, O.; Qin, Y.; Lu, J.; Niu, X.; Zhou, Z.; Lu, C.; Xia, Y.; Wang, S.; Wang, X. Methylenetetrahydrofolate Reductase C677T Polymorphism and the Risk of Male Infertility: A Meta-Analysis. Int. J. Androl. 2012, 35, 18–24. [Google Scholar] [CrossRef]
- Rai, V.; Kumar, P. Methylenetetrahydrofolate Reductase C677T Polymorphism and Risk for Male Infertility in Asian Population. Ind. J. Clin. Biochem. 2017, 32, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.-J.; Zhang, Y.-P.; Ren, P.-W.; Yang, B.; Deng, S.; Peng, Z.-F.; Liu, L.-R.; Wei, W.; Dong, Q. Contribution of MTR A2756G Polymorphism and MTRR A66G Polymorphism to the Risk of Idiopathic Male Infertility. Medicine 2019, 98, e18273. [Google Scholar] [CrossRef] [PubMed]
- Han, L.-J.; He, X.-F.; Ye, X.-H. Methylenetetrahydrofolate Reductase C677T and A1298C Polymorphisms and Male Infertility Risk: An Updated Meta-Analysis. Medicine 2020, 99, e23662. [Google Scholar] [CrossRef]
- Li, F.; Qi, J.; Li, L.; Yan, T. MTHFR C677T, MTHFR A1298C, MTRR A66G and MTR A2756G Polymorphisms and Male Infertility Risk: A Systematic Review and Meta-Analysis. Reprod. Biol. Endocrinol. 2024, 22, 133. [Google Scholar] [CrossRef]
- Singh, K.; Singh, S.K.; Sah, R.; Singh, I.; Raman, R. Mutation C677T in the Methylenetetrahydrofolate Reductase Gene Is Associated with Male Infertility in an Indian Population1. Int. J. Androl. 2005, 28, 115–119. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, H.C.; Jeong, Y.-M.; Chung, T.-G.; Kim, H.-J.; Kim, N.K.; Lee, S.-H.; Lee, S. MTHFR C677T Polymorphism Associates with Unexplained Infertile Male Factors. J. Assist. Reprod. Genet. 2005, 22, 361–368. [Google Scholar] [CrossRef]
- Gava, M.M.; Kayaki, E.A.; Bianco, B.; Teles, J.S.; Christofolini, D.M.; Pompeo, A.C.L.; Glina, S.; Barbosa, C.P. Polymorphisms in Folate-Related Enzyme Genes in Idiopathic Infertile Brazilian Men. Reprod. Sci. 2011, 18, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Gava, M.M.; De Oliveira Chagas, E.; Bianco, B.; Christofolini, D.M.; Pompeo, A.C.L.; Glina, S.; Barbosa, C.P. Methylenetetrahydrofolate Reductase Polymorphisms Are Related to Male Infertility in Brazilian Men. Genet. Test. Mol. Biomark. 2011, 15, 153–157. [Google Scholar] [CrossRef]
- Lee, H.-C.; Jeong, Y.-M.; Lee, S.H.; Cha, K.Y.; Song, S.-H.; Kim, N.K.; Lee, K.W.; Lee, S. Association Study of Four Polymorphisms in Three Folate-Related Enzyme Genes with Non-Obstructive Male Infertility. Hum. Reprod. 2006, 21, 3162–3170. [Google Scholar] [CrossRef]
- Stuppia, L.; Gatta, V.; Scarciolla, O.; Colosimo, A.; Guanciali-Franchi, P.; Calabrese, G.; Palka, G. The Methylenetethrahydrofolate Reductase (MTHFR) C677T Polymorphism and Male Infertility in Italy. J. Endocrinol. Investig. 2003, 26, 620–622. [Google Scholar] [CrossRef]
- Ullah, M.; Liu, D.D.; Thakor, A.S. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience 2019, 15, 421–438. [Google Scholar] [CrossRef]
- Muñoz-Moran, E.; Dieguez-Lucena, J.; Fernandez-Arcas, N.; Peran-Mesa, S.; Reyes-Engel, A. Genetic Selection and Folate Intake during Pregnancy. Lancet 1998, 352, 1120–1121. [Google Scholar] [CrossRef]
- Isotalo, P.A.; Wells, G.A.; Donnelly, J.G. Neonatal and Fetal Methylenetetrahydrofolate Reductase Genetic Polymorphisms: An Examination of C677T and A1298C Mutations. Am. J. Hum. Genet. 2000, 67, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Engel, A. Implications on Human Fertility of the 677C→T and 1298A→C Polymorphisms of the MTHFR Gene: Consequences of a Possible Genetic Selection. Mol. Hum. Reprod. 2002, 8, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Najafipour, R.; Moghbelinejad, S.; Aleyasin, A.; Jalilvand, A. Effect of B9 and B12 Vitamin Intake on Semen Parameters and Fertility of Men with MTHFR Polymorphisms. Andrology 2017, 5, 704–710. [Google Scholar] [CrossRef]
- Paracchini, V.; Garte, S.; Taioli, E. MTHFR C677T Polymorphism, GSTM1 Deletion and Male Infertility: A Possible Suggestion of a Gene–Gene Interaction? Biomarkers 2006, 11, 53–60. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Li, L.; Li, Q.; Ren, K.; Sun, X.; Li, J. Metformin Treatment and Homocysteine: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 798. [Google Scholar] [CrossRef]
- Shen, O.; Liu, R.; Wu, W.; Yu, L.; Wang, X. Association of the Methylenetetrahydrofolate Reductase Gene A1298C Polymorphism with Male Infertility: A Meta-Analysis. Ann. Hum. Genet. 2012, 76, 25–32. [Google Scholar] [CrossRef]
- Gupta, N.; Sarkar, S.; David, A.; Gangwar, P.K.; Gupta, R.; Khanna, G.; Sankhwar, S.N.; Khanna, A.; Rajender, S. Significant Impact of the MTHFR Polymorphisms and Haplotypes on Male Infertility Risk. PLoS ONE 2013, 8, e69180. [Google Scholar] [CrossRef]
- Shi, T.-L.; Wu, Y.; Li, Y.; Chen, Z.-F.; Ma, Y.-N.; Zhang, Z.-T.; Zhang, Y.-H.; Zhang, L. The Relevance of MTHFR C677T, A1298C, and MTRR A66G Polymorphisms with Response to Male Infertility in Asians: A Meta-Analysis. Medicine 2019, 98, e14283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yin, G.; Liu, J.; Liang, Y.; Li, Y.; Zhao, J.; Zhang, L.; Wang, B.; Tang, N. Association between MTHFR A1298C Polymorphism and Male Infertility: A Meta-Analysis. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 2017, 37, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Vanselow, J.; Pohland, R.; Furbass, R. Promoter-2-Derived Expression in Bovine Granulosa Cells Coincides with Gene-Specific DNA Hypo-Methylation. Mol. Cell. Endocrinol. 2005, 233, 57–64. [Google Scholar] [CrossRef]
- Sinclair, K.D.; Allegrucci, C.; Singh, R.; Gardner, D.S.; Sebastian, S.; Bispham, J.; Thurston, A.; Huntley, J.F.; Rees, W.D.; Maloney, C.A.; et al. DNA Methylation, Insulin Resistance, and Blood Pressure in Offspring Determined by Maternal Periconceptional B Vitamin and Methionine Status. Proc. Natl. Acad. Sci. USA 2007, 104, 19351–19356. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of Oxidative Stress in Female Reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef]
- Calhaz-Jorge, C. Peritoneal Fluid Concentrations of Interleukin-8 in Patients with Endometriosis Depend on the Severity of the Disorder and Are Higher in the Luteal Phase. Hum. Reprod. 2003, 18, 593–597. [Google Scholar] [CrossRef]
- Gmyrek, G.B.; Sozanski, R.; Jerzak, M.; Chrobak, A.; Wickiewicz, D.; Skupnik, A.; Sieradzka, U.; Fortuna, W.; Gabrys, M.; Chelmonska-Soyta, A. Evaluation of Monocyte Chemotactic Protein-1 Levels in Peripheral Blood of Infertile Women with Endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 122, 199–205. [Google Scholar] [CrossRef]
- Thaler, C.J.; Budiman, H.; Ruebsamen, H.; Nagel, D.; Lohse, P. Effects of the Common 677C>T Mutation of the 5,10-Methylenetetrahydrofolate Reductase (MTHFR) Gene on Ovarian Responsiveness to Recombinant Follicle-Stimulating Hormone. Am. J. Reprod. Immunol. 2006, 55, 251–258. [Google Scholar] [CrossRef]
- Orio, F.; Palomba, S.; Di Biase, S.; Colao, A.; Tauchmanova, L.; Savastano, S.; Labella, D.; Russo, T.; Zullo, F.; Lombardi, G. Homocysteine Levels and C677T Polymorphism of Methylenetetrahydrofolate Reductase in Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Palep-Singh, M.; Picton, H.M.; Yates, Z.R.; Barth, J.H.; Balen, A.H. Plasma Homocysteine Concentrations and the Single Nucleotide Polymorphisms in the Methionine Synthase Gene (MTR 2756A>G): Associations with the Polycystic Ovary Syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 138, 180–186. [Google Scholar] [CrossRef]
- Loverro, G.; Lorusso, F.; Mei, L.; Depalo, R.; Cormio, G.; Selvaggi, L. The Plasma Homocysteine Levels Are Increased in Polycystic Ovary Syndrome. Gynecol. Obstet. Investig. 2002, 53, 157–162. [Google Scholar] [CrossRef]
- Chang, H.; Xie, L.; Ge, H.; Wu, Q.; Wen, Y.; Zhang, D.; Zhang, Y.; Ma, H.; Gao, J.; Wang, C.C.; et al. Effects of Hyperhomocysteinaemia and Metabolic Syndrome on Reproduction in Women with Polycystic Ovary Syndrome: A Secondary Analysis. Reprod. Biomed. Online 2019, 38, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Yaralı, H.; Yıldırır, A.; Aybar, F.; Kabakçı, G.; Bükülmez, O.; Akgül, E.; Oto, A. Diastolic Dysfunction and Increased Serum Homocysteine Concentrations May Contribute to Increased Cardiovascular Risk in Patients with Polycystic Ovary Syndrome. Fertil. Steril. 2001, 76, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Schachter, M. Insulin Resistance in Patients with Polycystic Ovary Syndrome Is Associated with Elevated Plasma Homocysteine. Hum. Reprod. 2003, 18, 721–727. [Google Scholar] [CrossRef]
- Vrbíková, J.; Tallová, J.; Bicíková, M.; Dvoráková, K.; Hill, M.; Stárka, L. Plasma Thiols and Androgen Levels in Polycystic Ovary Syndrome. Clin. Chem. Lab. Med. 2003, 41, 216–221. [Google Scholar] [CrossRef]
- Bayraktar, F.; Dereli, D.; Özgen, A.G.; Yilmaz, C. Plasma Homocysteine Levels in Polycystic Ovary Syndrome and Congenital Adrenal Hyperplasia. Endocr. J. 2004, 51, 601–608. [Google Scholar] [CrossRef]
- Wijeyaratne, C.N.; Nirantharakumar, K.; Balen, A.H.; Barth, J.H.; Sheriff, R.; Belchetz, P.E. Plasma Homocysteine in Polycystic Ovary Syndrome: Does It Correlate with Insulin Resistance and Ethnicity? Clin. Endocrinol. 2004, 60, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Kandel, P.; Baidya, S.; Rajkarnikar, S.; Niraula, A.; Tuladhar, E.T.; Bhattarai, A.; Raut, M.; Dubey, R.K.; Koirala, P. High Serum Homocysteine among Women with Polycystic Ovarian Syndrome Visiting an Infertility Clinic of a Tertiary Care Centre. JNMA J. Nepal. Med. Assoc. 2024, 62, 82–84. [Google Scholar] [CrossRef]
- Hemati, T.; Moghadami-Tabrizi, N.; Davari-Tanha, F.; Salmanian, B.; Javadian, P. High Plasma Homocysteine and Insulin Resistance in Patients with Polycystic Ovarian Syndrome. Iran. J. Reprod. Med. 2011, 9, 223–228. [Google Scholar]
- Asanidze, E.; Kristesashvili, J.; Parunashvili, N.; Urjumelashvili, M.; Tsetskhladze, Z.; Asanidze, A. Hyperhomocysteinemia and Pregnancy Outcomes in Women with Polycystic Ovary Syndrome: A Case-Control Study. Int. J. Reprod. Biomed. 2023, 21, 167–174. [Google Scholar] [CrossRef]
- Zhang, B.; Qi, X.; Zhao, Y.; Li, R.; Zhang, C.; Chang, H.-M.; Pang, Y.; Qiao, J. Elevated CD14++CD16+ Monocytes in Hyperhomocysteinemia-Associated Insulin Resistance in Polycystic Ovary Syndrome. Reprod. Sci. 2018, 25, 1629–1636. [Google Scholar] [CrossRef]
- Kilic-Okman, T.; Guldiken, S.; Kucuk, M. Relationship between Homocysteine and Insulin Resistance in Women with Polycystic Ovary Syndrome. Endocr. J. 2004, 51, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Biri, A.; Bukan, N.; Karakoç, A.; Sancak, B.; Törüner, F.; Paşaoğlu, H. Levels of Lipoprotein and Homocysteine in Non-Obese and Obese Patients with Polycystic Ovary Syndrome. Gynecol. Endocrinol. 2005, 20, 258–263. [Google Scholar] [CrossRef]
- Sills, E.S.; Genton, M.G.; Perloe, M.; Schattman, G.L.; Bralley, J.A.; Tucker, M.J. Plasma Homocysteine, Fasting Insulin, and Androgen Patterns among Women with Polycystic Ovaries and Infertility. J. Obstet. Gynaecol. 2001, 27, 163–168. [Google Scholar] [CrossRef]
- Badawy, A.; State, O.; El Gawad, S.S.A.; El Aziz, O.A. Plasma Homocysteine and Polycystic Ovary Syndrome: The Missed Link. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 131, 68–72. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, Y.-Y.; Liu, L.; Qiao, Y.-N.; Geng, H.-R.; Lin, Y.; Xu, W.; Cao, J.; Zhao, J.-Y. Homocysteine Inhibits Pro-Insulin Receptor Cleavage and Causes Insulin Resistance via Protein Cysteine-Homocysteinylation. Cell Rep. 2021, 37, 109821. [Google Scholar] [CrossRef]
- Mondal, K.; Chakraborty, P.; Kabir, S.N. Hyperhomocysteinemia and Hyperandrogenemia Share PCSK9-LDLR Pathway to Disrupt Lipid Homeostasis in PCOS. Biochem. Biophys. Res. Commun. 2018, 503, 8–13. [Google Scholar] [CrossRef]
- Li, T.; Dong, G.; Kang, Y.; Zhang, M.; Sheng, X.; Wang, Z.; Liu, Y.; Kong, N.; Sun, H. Increased Homocysteine Regulated by Androgen Activates Autophagy by Suppressing the Mammalian Target of Rapamycin Pathway in the Granulosa Cells of Polycystic Ovary Syndrome Mice. Bioengineered 2022, 13, 10875–10888. [Google Scholar] [CrossRef] [PubMed]
- Wulffelé, M.G.; Kooy, A.; Lehert, P.; Bets, D.; Ogterop, J.C.; Borger Van Der Burg, B.; Donker, A.J.M.; Stehouwer, C.D.A. Effects of Short-term Treatment with Metformin on Serum Concentrations of Homocysteine, Folate and Vitamin B12 in Type 2 Diabetes Mellitus: A Randomized, Placebo-controlled Trial. J. Intern. Med. 2003, 254, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, Z.; Yang, X.; Pan, H.; Zhang, C.; Li, X.; Bai, Y.; Wang, F. The Effect of Metformin on Homocysteine Levels in Patients with Polycystic Ovary Syndrome: A Systematic Review and Meta-analysis. J. Obstet. Gynaecol. 2021, 47, 1804–1816. [Google Scholar] [CrossRef] [PubMed]
- Kilicdag, E.B.; Bagis, T.; Tarim, E.; Aslan, E.; Erkanli, S.; Simsek, E.; Haydardedeoglu, B.; Kuscu, E. Administration of B-Group Vitamins Reduces Circulating Homocysteine in Polycystic Ovarian Syndrome Patients Treated with Metformin: A Randomized Trial. Hum. Reprod. 2005, 20, 1521–1528. [Google Scholar] [CrossRef]
- Sfakianoudis, K.; Zikopoulos, A.; Grigoriadis, S.; Seretis, N.; Maziotis, E.; Anifandis, G.; Xystra, P.; Kostoulas, C.; Giougli, U.; Pantos, K.; et al. The Role of One-Carbon Metabolism and Methyl Donors in Medically Assisted Reproduction: A Narrative Review of the Literature. Int. J. Mol. Sci. 2024, 25, 4977. [Google Scholar] [CrossRef]
- Ocal, P.; Ersoylu, B.; Cepni, I.; Guralp, O.; Atakul, N.; Irez, T.; Idil, M. The Association between Homocysteine in the Follicular Fluid with Embryo Quality and Pregnancy Rate in Assisted Reproductive Techniques. J. Assist. Reprod. Genet. 2012, 29, 299–304. [Google Scholar] [CrossRef]
- D’Elia, P.Q.; Dos Santos, A.A.; Bianco, B.; Barbosa, C.P.; Christofolini, D.M.; Aoki, T. MTHFR Polymorphisms C677T and A1298C and Associations with IVF Outcomes in Brazilian Women. Reprod. Biomed. Online 2014, 28, 733–738. [Google Scholar] [CrossRef]
- Lu, Y.; Xia, Z. Diminished Ovarian Reserve Is Associated with Metabolic Disturbances and Hyperhomocysteinemia in Women with Infertility. J. Obstet. Gynaecol. 2023, 43, 2282722. [Google Scholar] [CrossRef]
- Ma, J.-Y.; Li, S.; Chen, L.-N.; Schatten, H.; Ou, X.-H.; Sun, Q.-Y. Why Is Oocyte Aneuploidy Increased with Maternal Aging? J. Genet. Genom. 2020, 47, 659–671. [Google Scholar] [CrossRef]
- Hasbargen, U.; Lohse, P.; Thaler, C.J. The Number of Dichorionic Twin Pregnancies Is Reduced by the Common MTHFR 677C→T Mutation. Hum. Reprod. 2000, 15, 2659–2662. [Google Scholar] [CrossRef]
- Revelli, A.; Piane, L.D.; Casano, S.; Molinari, E.; Massobrio, M.; Rinaudo, P. Follicular Fluid Content and Oocyte Quality: From Single Biochemical Markers to Metabolomics. Reprod. Biol. Endocrinol. 2009, 7, 40. [Google Scholar] [CrossRef]
- Berker, B.; Kaya, C.; Aytac, R.; Satiroglu, H. Homocysteine Concentrations in Follicular Fluid Are Associated with Poor Oocyte and Embryo Qualities in Polycystic Ovary Syndrome Patients Undergoing Assisted Reproduction. Hum. Reprod. 2009, 24, 2293–2302. [Google Scholar] [CrossRef]
- Akamine, K.; Mekaru, K.; Gibo, K.; Nagata, C.; Nakamura, R.; Oishi, S.; Miyagi, M.; Heshiki, C.; Aoki, Y. Impact of the One-carbon Metabolism on Oocyte Maturation, Fertilization, Embryo Quality, and Subsequent Pregnancy. Reprod. Med. Biol. 2021, 20, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Razi, Y.; Eftekhar, M.; Fesahat, F.; Dehghani Firouzabadi, R.; Razi, N.; Sabour, M.; Razi, M.H. Concentrations of Homocysteine in Follicular Fluid and Embryo Quality and Oocyte Maturity in Infertile Women: A Prospective Cohort. J. Obstet. Gynaecol. 2021, 41, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, T.; Horozal, P.E.; Karakulak, A.; Timucin, E.; Dattilo, M. Follicular Homocysteine as a Marker of Oocyte Quality in PCOS and the Role of Micronutrients. J. Assist. Reprod. Genet. 2023, 40, 1933–1941. [Google Scholar] [CrossRef]
- Steegers-Theunissen, R.P.M.; Steegers, E.A.P.; Thomas, C.M.G.; Hollanders, H.M.G.; Peereboom-Stegeman, J.H.J.C.; Trijbels, F.J.M.; Eskes, T.K.A.B. Study on the Presence of Homocysteine in Ovarian Follicular Fluid. Fertil. Steril. 1993, 60, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Szymański, W.; Kazdepka-Ziemińska, A. Effect of homocysteine concentration in follicular fluid on a degree of oocyte maturity. Ginekol. Pol. 2003, 74, 1392–1396. [Google Scholar]
- Ogawa, S.; Ota, K.; Takahashi, T.; Yoshida, H. Impact of Homocysteine as a Preconceptional Screening Factor for In Vitro Fertilization and Prevention of Miscarriage with Folic Acid Supplementation Following Frozen-Thawed Embryo Transfer: A Hospital-Based Retrospective Cohort Study. Nutrients 2023, 15, 3730. [Google Scholar] [CrossRef]
- Cooney, C.A.; Dave, A.A.; Wolff, G.L. Maternal Methyl Supplements in Mice Affect Epigenetic Variation and DNA Methylation of Offspring. J. Nutr. 2002, 132, 2393S–2400S. [Google Scholar] [CrossRef] [PubMed]
- Boyama, B.A.; Cepni, I.; Imamoglu, M.; Oncul, M.; Tuten, A.; Yuksel, M.A.; Kervancioglu, M.E.; Kaleli, S.; Ocal, P. Homocysteine in Embryo Culture Media as a Predictor of Pregnancy Outcome in Assisted Reproductive Technology. Gynecol. Endocrinol. 2016, 32, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Murto, T.; Skoog Svanberg, A.; Yngve, A.; Nilsson, T.K.; Altmäe, S.; Wånggren, K.; Salumets, A.; Stavreus-Evers, A. Folic Acid Supplementation and IVF Pregnancy Outcome in Women with Unexplained Infertility. Reprod. Biomed. Online 2014, 28, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Murto, T.; Kallak, T.K.; Hoas, A.; Altmäe, S.; Salumets, A.; Nilsson, T.K.; Skoog Svanberg, A.; Wånggren, K.; Yngve, A.; Stavreus-Evers, A. Folic Acid Supplementation and Methylenetetrahydrofolate Reductase (MTHFR) Gene Variations in Relation to in Vitro Fertilization Pregnancy Outcome. Acta Obstet. Gynecol. Scand. 2015, 94, 65–71. [Google Scholar] [CrossRef]
- Laanpere, M.; Altmäe, S.; Kaart, T.; Stavreus-Evers, A.; Nilsson, T.K.; Salumets, A. Folate-Metabolizing Gene Variants and Pregnancy Outcome of IVF. Reprod. Biomed. Online 2011, 22, 603–614. [Google Scholar] [CrossRef]
- Palomares, A.R.; Ruiz-Galdon, M.; Liu, K.; Reyes-Engel, A.; Rodriguez-Wallberg, K.A. Profiling the Influence of Gene Variants Related to Folate-Mediated One-Carbon Metabolism on the Outcome of In Vitro Fertilization (IVF) with Donor Oocytes in Recipients Receiving Folic Acid Fortification. Int. J. Mol. Sci. 2022, 23, 11298. [Google Scholar] [CrossRef]
- Ye, F.; Zhang, S.; Qi, Q.; Zhou, J.; Du, Y.; Wang, L. Association of MTHFR 677C>T Polymorphism with Pregnancy Outcomes in IVF/ICSI-ET Recipients with Adequate Synthetic Folic Acid Supplementation. Biosci. Trends 2022, 16, 282–290. [Google Scholar] [CrossRef]
- Haggarty, P.; McCallum, H.; McBain, H.; Andrews, K.; Duthie, S.; McNeill, G.; Templeton, A.; Haites, N.; Campbell, D.; Bhattacharya, S. Effect of B Vitamins and Genetics on Success of In-Vitro Fertilisation: Prospective Cohort Study. Lancet 2006, 367, 1513–1519. [Google Scholar] [CrossRef]
- Dobson, A.T.; Davis, R.M.; Rosen, M.P.; Shen, S.; Rinaudo, P.F.; Chan, J.; Cedars, M.I. Methylenetetrahydrofolate Reductase C677T and A1298C Variants Do Not Affect Ongoing Pregnancy Rates Following IVF. Hum. Reprod. 2006, 22, 450–456. [Google Scholar] [CrossRef]
- Cirillo, M.; Fucci, R.; Rubini, S.; Coccia, M.E.; Fatini, C. 5-Methyltetrahydrofolate and Vitamin B12 Supplementation Is Associated with Clinical Pregnancy and Live Birth in Women Undergoing Assisted Reproductive Technology. Int. J. Environ. Res. Public Health 2021, 18, 12280. [Google Scholar] [CrossRef] [PubMed]
- Manzur, N.F.; Gluska, H.; Feferkorn, I.; Skvirsky, S.; Ben-Shlomo, I.; Wiener-Megnazi, Z. Homocysteine Serum Levels Correlate with the Number of Failed IVF Cycles Even When within Normal Range. Arch. Gynecol. Obstet. 2023, 307, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Nowak, I.; Bylińska, A.; Wilczyńska, K.; Wiśniewski, A.; Malinowski, A.; Wilczyński, J.R.; Radwan, P.; Radwan, M.; Barcz, E.; Płoski, R.; et al. The Methylenetetrahydrofolate Reductase c.c.677 C>T and c.c.1298 A>C Polymorphisms in Reproductive Failures: Experience from an RSA and RIF Study on a Polish Population. PLoS ONE 2017, 12, e0186022. [Google Scholar] [CrossRef] [PubMed]
- Azem, F.; Many, A.; Ben Ami, I.; Yovel, I.; Amit, A.; Lessing, J.B.; Kupferminc, M.J. Increased Rates of Thrombophilia in Women with Repeated IVF Failures. Hum. Reprod. 2004, 19, 368–370. [Google Scholar] [CrossRef]
- Cho, S.H.; Kim, J.H.; An, H.J.; Kim, J.O.; Kim, Y.R.; Lee, W.S.; Kim, N.K. Association of Methionine Synthase (Rs1805087), Methionine Synthase Reductase (Rs1801394), and Methylenetetrahydrofolate Dehydrogenase 1 (Rs2236225) Genetic Polymorphisms with Recurrent Implantation Failure. Hum. Fertil. 2021, 24, 161–168. [Google Scholar] [CrossRef]
- Zeng, X.-T.; Lu, J.-T.; Tang, X.-J.; Weng, H.; Luo, J. Association of Methionine Synthase Rs1801394 and Methionine Synthase Reductase Rs1805087 Polymorphisms with Meningioma in Adults: A Meta-Analysis. Biomed. Rep. 2014, 2, 432–436. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Revelli, A.; Nuzzo, A.M.; Moretti, L.; Arduino, S.; Roero, S.; Scali, R.; Scali, L.; Gennarelli, G.; Gigliotti, F.; Gatto, M.; et al. Effects of Homocysteine Circulating Levels on Human Spontaneous Fertility and In Vitro Fertilization Outcomes: A Literature Review. Nutrients 2025, 17, 3211. https://doi.org/10.3390/nu17203211
Revelli A, Nuzzo AM, Moretti L, Arduino S, Roero S, Scali R, Scali L, Gennarelli G, Gigliotti F, Gatto M, et al. Effects of Homocysteine Circulating Levels on Human Spontaneous Fertility and In Vitro Fertilization Outcomes: A Literature Review. Nutrients. 2025; 17(20):3211. https://doi.org/10.3390/nu17203211
Chicago/Turabian StyleRevelli, Alberto, Anna Maria Nuzzo, Laura Moretti, Silvana Arduino, Sofia Roero, Roberto Scali, Lorenzo Scali, Gianluca Gennarelli, Francesca Gigliotti, Marlisa Gatto, and et al. 2025. "Effects of Homocysteine Circulating Levels on Human Spontaneous Fertility and In Vitro Fertilization Outcomes: A Literature Review" Nutrients 17, no. 20: 3211. https://doi.org/10.3390/nu17203211
APA StyleRevelli, A., Nuzzo, A. M., Moretti, L., Arduino, S., Roero, S., Scali, R., Scali, L., Gennarelli, G., Gigliotti, F., Gatto, M., & Rolfo, A. (2025). Effects of Homocysteine Circulating Levels on Human Spontaneous Fertility and In Vitro Fertilization Outcomes: A Literature Review. Nutrients, 17(20), 3211. https://doi.org/10.3390/nu17203211










