Integrating Nutrition, Inflammation, and Immunity: The CALLY Index as a Novel Prognostic Biomarker in Acute Geriatric Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Clinical and Multidimensional Assessment
2.3. Statistical Analysis
3. Results
3.1. A Descriptive Analysis of the Study Population
3.2. CALLY Index, Clinical and Biochemical Parameters
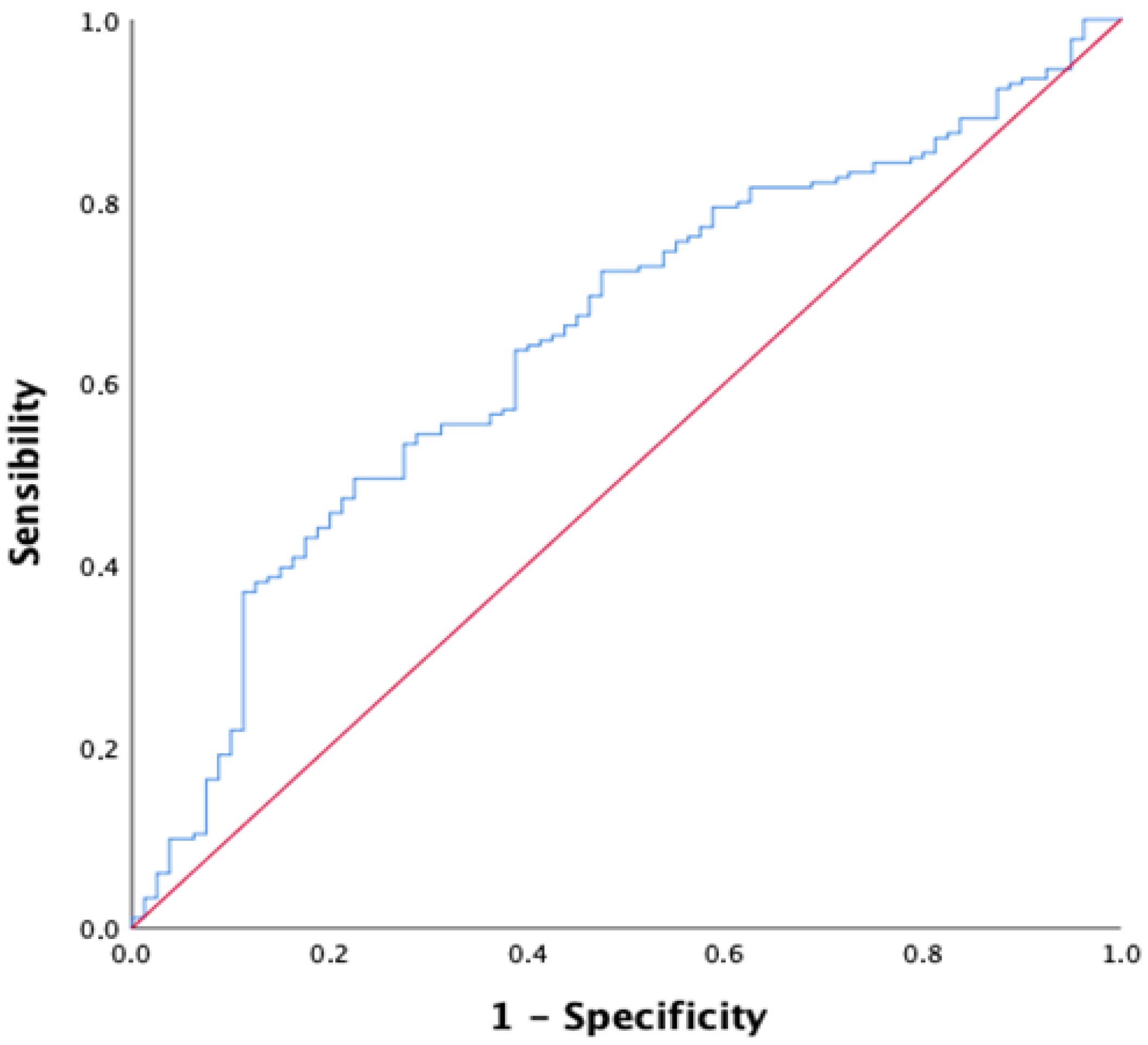
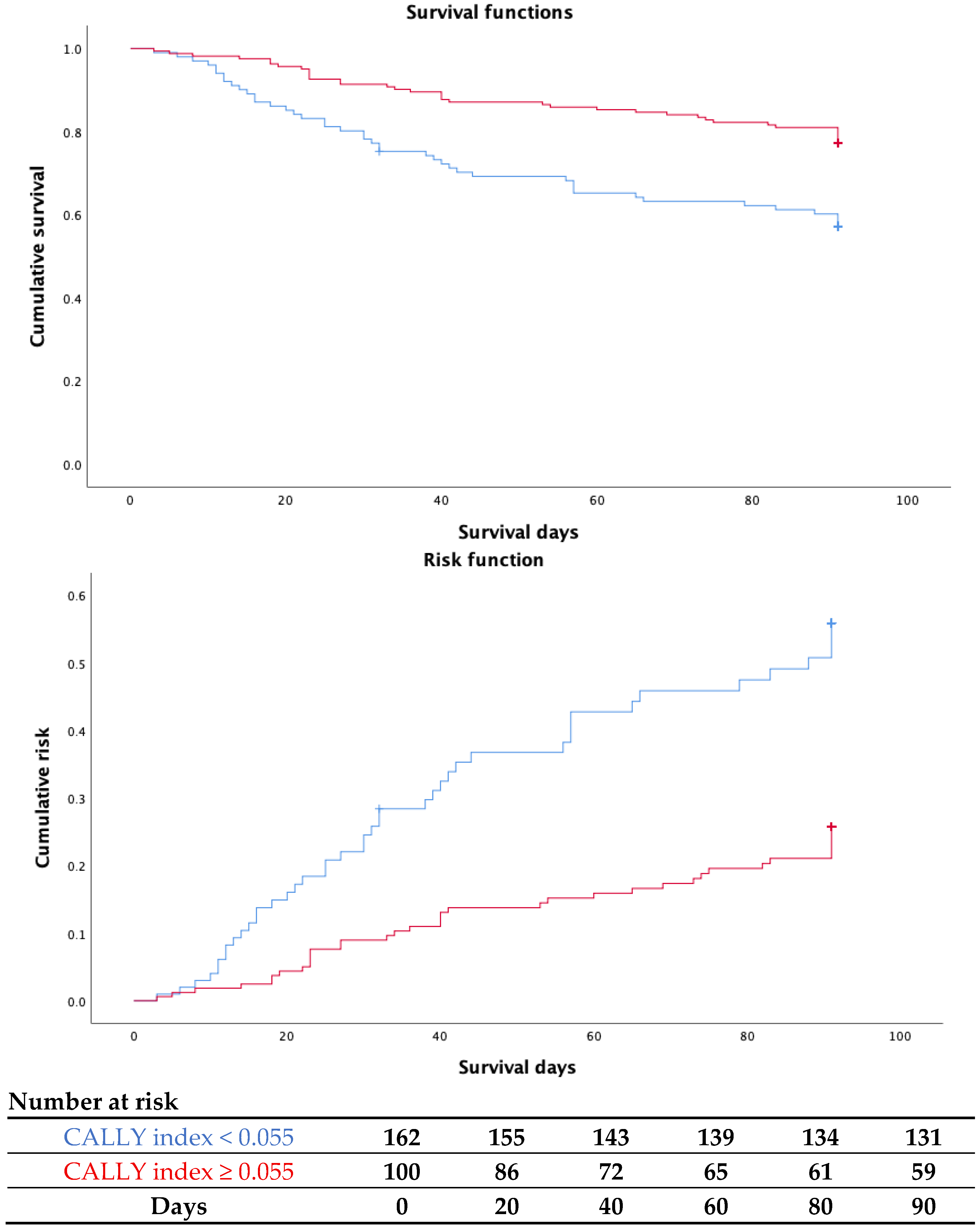
3.3. CALLY Index for Short-Term Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calderón-Larrañaga, A.; Vetrano, D.L.; Ferrucci, L.; Mercer, S.W.; Marengoni, A.; Onder, G.; Eriksdotter, M.; Fratiglioni, L. Multimorbidity and Functional Impairment-Bidirectional Interplay, Synergistic Effects and Common Pathways. J. Intern. Med. 2019, 285, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, F.; Keller, B.; Gressies, C.; Schuetz, P. Inflammation and Nutrition: Friend or Foe? Nutrients 2023, 15, 1159. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Hahn, F.; Mähringer-kunz, A.; Stoehr, F.; Gairing, S.J.; Michel, M.; Foerster, F.; Weinmann, A.; Galle, P.R.; Mittler, J.; et al. Immunonutritive Scoring for Patients with Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization: Evaluation of the CALLY Index. Cancers 2021, 13, 5018. [Google Scholar] [CrossRef] [PubMed]
- Matsui, S.; Kato, Y.; Ohgi, K.; Ashida, R.; Yamada, M.; Otsuka, S.; Uesaka, K.; Sugiura, T. Prognostic Impact of the CALLY Index in Patients with Resectable Pancreatic Cancer. Surg. Oncol. Insight 2025, 2, 100119. [Google Scholar] [CrossRef]
- Aoyama, T.; Maezawa, Y.; Hashimoto, I.; Hara, K.; Tamagawa, A.; Kazama, K.; Kato, A.; Cho, H.; Nakazono, M.; Numata, M.; et al. The CRP-Albumin-Lymphocyte (CALLY) Index Is an Independent Prognostic Factor for Gastric Cancer Patients Who Receive Curative Treatment. Anticancer Res. 2024, 44, 1629–1636. [Google Scholar] [CrossRef]
- Sarıdaş, A.; Çetinkaya, R. The Prognostic Value of the CALLY Index in Sepsis: A Composite Biomarker Reflecting Inflammation, Nutrition, and Immunity. Diagnostics 2025, 15, 1026. [Google Scholar] [CrossRef]
- He, Q.; Cao, Y.; Fan, X.; Li, B.; He, Q.; Zhang, H. Long-Term Prognostic Value of CRP–Albumin–Lymphocyte Index in Elderly Patients with Heart Failure with Preserved Ejection Fraction. Exp. Gerontol. 2025, 204, 112744. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, T.T.; Deng, C.J.; Jiang, Z.H.; Yang, Y.; Hou, X.G.; Yan, T.; Wang, S.; Feng, Y.J.; Zheng, Y.Y.; et al. Association between the C-Reactive Protein-Albumin-Lymphocyte (CALLY) Index and Adverse Clinical Outcomes in CAD Patients after PCI: Findings of a Real-World Study. Rev. Cardiovasc. Med. 2024, 25, 111. [Google Scholar] [CrossRef]
- Güven, B.; Deniz, M.F.; Geylan, N.A.; Kültürsay, B.; Dönmez, A.; Bulat, Z.; Gül, Ö.B.; Kaya, M.; Oktay, V. A Novel Indicator of All-Cause Mortality in Acute Coronary Syndrome: The CALLY Index. Biomark. Med. 2025, 19, 287–294. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, X.; Xu, Y.; Li, Y.; Dai, Q.; Chang, X. Lower CALLY Index Levels Indicate Higher Poor Functional Outcome Risk in Acute Ischemic Stroke Patients Treated with Endovascular Thrombectomy. Front. Aging Neurosci. 2025, 17, 1587861. [Google Scholar] [CrossRef]
- Augustine, T.; Li, Y.; Hu, Z.; Hu, T.; Wang, T.; Luo, X. Association between the C-Reactive Protein-Albumin-Lymphocyte Index and All-Cause Mortality in Chinese Older Adults: A National Cohort Study Based on CLHLS from 2014 to 2018. Front. Nutr. 2025, 12, 1575470. [Google Scholar] [CrossRef]
- Bhullar, K.K.; Singh, N. From Concept to Cure: The Life and Legacy of Scipione Riva-Rocci. Cureus 2024, 16, e70436. [Google Scholar] [CrossRef] [PubMed]
- Katz, S. Assessing Self-Maintenance: Activities of Daily Living, Mobility, and Instrumental Activities of Daily Living. J. Am. Geriatr. Soc. 1983, 31, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.L. The Mini Nutritional Assessment (MNA) and Its Use in Grading the Nutritional State of Elderly Patients. Nutrition 1999, 15, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.D.; Paradis, C.F.; Houck, P.R.; Mazumdar, S.; Stack, J.A.; Rifai, A.H.; Mulsant, B.; Reynolds, C.F. Rating Chronic Medical Illness Burden in Geropsychiatric Practice and Research: Application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992, 41, 237–248. [Google Scholar] [CrossRef]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef]
- Eckart, A.; Struja, T.; Kutz, A.; Baumgartner, A.; Baumgartner, T.; Zurfluh, S.; Neeser, O.; Huber, A.; Stanga, Z.; Mueller, B.; et al. Relationship of Nutritional Status, Inflammation, and Serum Albumin Levels During Acute Illness: A Prospective Study. Am. J. Med. 2020, 133, 713–722.e7. [Google Scholar] [CrossRef]
- Xu, L.; Li, C.; Aiello, A.E.; Langa, K.M.; Dowd, J.B.; Stebbins, R.C.; Meier, H.C.S.; Jiang, Z.; Noppert, G.A.; Li, G. Compositional Analysis of Lymphocytes and Their Relationship with Health Outcomes: Findings from the Health and Retirement Study. Immun. Ageing 2025, 22, 12. [Google Scholar] [CrossRef]
- Ma, Y.C.; Ju, Y.M.; Cao, M.Y.; Yang, D.; Zhang, K.X.; Liang, H.; Leng, J.Y. Exploring the Relationship between Malnutrition and the Systemic Immune-Inflammation Index in Older Inpatients: A Study Based on Comprehensive Geriatric Assessment. BMC Geriatr. 2024, 24, 19. [Google Scholar] [CrossRef]
- Parmar, P.A.; Sharma, S.; Kakadiya, J.P.; Lakkad, D. Neutrophil-to-Lymphocyte Ratio as a Novel Predictor of Sarcopenia in Maintenance Hemodialysis Patients: A Cross-Sectional Study Exploring Associations across Body Composition Categories. BMC Musculoskelet. Disord. 2025, 26, 39. [Google Scholar] [CrossRef]
- Navarro-Martínez, R.; Cauli, O. Lymphocytes as a Biomarker of Frailty Syndrome: A Scoping Review. Diseases 2021, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Zidar, D.A.; Al-Kindi, S.G.; Liu, Y.; Krieger, N.I.; Perzynski, A.T.; Osnard, M.; Nmai, C.; Anthony, D.D.; Lederman, M.M.; Freeman, M.L.; et al. Association of Lymphopenia with Risk of Mortality among Adults in the US General Population. JAMA Netw. Open 2019, 2, e1916526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zeng, X.; He, F.; Huang, X. Inflammatory Biomarkers of Frailty: A Review. Exp. Gerontol. 2023, 179, 112253. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Q.; Ke, X.; Xu, Y.; Xu, B.; Zhang, K.; Zhu, W.; Lian, X.; Liu, L.; Guo, Z. Higher CALLY Index Levels Indicate Lower Sarcopenia Risk among Middle-Aged and Elderly Community Residents as well as Hospitalized Patients. Sci. Rep. 2024, 14, 24591. [Google Scholar] [CrossRef]
- Luo, L.; Li, M.; Xi, Y.; Hu, J.; Hu, W. C-Reactive Protein–Albumin–Lymphocyte Index as a Feasible Nutrition–Immunity–Inflammation Marker of the Outcome of All-Cause and Cardiovascular Mortality in Elderly. Clin. Nutr. ESPEN 2024, 63, 346–353. [Google Scholar] [CrossRef]
- Kregel, H.R.; Murphy, P.B.; Attia, M.; Meyer, D.E.; Morris, R.S.; Onyema, E.C.; Adams, S.D.; Wade, C.E.; Harvin, J.A.; Kao, L.S.; et al. The Geriatric Nutritional Risk Index as a Predictor of Complications in Geriatric Trauma Patients. J. Trauma Acute Care Surg. 2022, 93, 195–199. [Google Scholar] [CrossRef]
- Lo Buglio, A.; Bellanti, F.; Capurso, C.; Vendemiale, G. Controlling Nutritional Status (CONUT) Score as a Predictive Marker in Hospitalized Frail Elderly Patients. J. Pers. Med. 2023, 13, 1119. [Google Scholar] [CrossRef]

| All (n = 264) | Women (n = 167) | Men (n = 97) | p | |
|---|---|---|---|---|
| Age (years) | 88.02 ± 6.37 | 88.62 ± 6.47 | 86.98 ± 6.07 | 0.044 |
| Activities of Daily Living (ADL, n) | 2.19 ± 2.02 | 2.16 ± 1.94 | 2.24 ± 2.16 | 0.778 |
| Instrumental ADL (IADL, n) | 1.42 ± 2.03 | 1.37 ± 2.01 | 1.51 ± 2.07 | 0.607 |
| Mini Nutritional Assessment (MNA, n) | 17.33 ± 8.06 | 17.18 ± 8.62 | 17.58 ± 7.06 | 0.715 |
| Cumulative Illness Rating Scale for Geriatrics (CIRS-G, n) | 9.70 ± 5.41 | 9.94 ± 5.35 | 9.31 ± 5.51 | 0.419 |
| Number of drugs (n) | 7.07 ± 3.03 | 7.08 ± 2.99 | 7.05 ± 3.12 | 0.922 |
| Systolic Blood Pressure (SPB, mmHg) | 127.30 ± 26.93 | 129.36 ± 28.31 | 123.79 ± 24.13 | 0.106 |
| Diastolic Blood Pressure (DPB, mmHg) | 70.02 ± 13.34 | 71.12 ± 13.95 | 68.16 ± 12.08 | 0.083 |
| Peripheral Oxygen Saturation (SpO2, %) | 94.25 ± 11.19 | 94.43 ± 10.39 | 94.00 ± 12.33 | 0.814 |
| quick Sequential Organ Failure Assessment (qSOFA, n) | 1.50 ± 0.62 | 1.56 ± 0.62 | 1.42 ± 0.60 | 0.084 |
| Hemoglobin (Hb, g/dL) | 11.38 ± 2.05 | 11.31 ± 2.05 | 11.51 ± 2.04 | 0.443 |
| White Blood Cell Count (WBC, ×103/μL) | 10.00 ± 6.52 | 9.73 ± 4.73 | 10.47 ± 8.79 | 0.373 |
| Lymphocyte count (×109/L) | 1.36 ± 1.08 | 1.29 ± 0.69 | 1.48 ± 1.54 | 0.182 |
| Serum Albumin (g/dL) | 3.19 ± 0.47 | 3.21 ± 0.48 | 3.15 ± 0.45 | 0.374 |
| C-reactive Protein (CRP, mg/dL) | 6.79 ± 7.59 | 6.42 ± 7.03 | 7.42 ± 8.46 | 0.305 |
| Creatinine (mg/dL) | 1.21 ± 0.74 | 1.10 ± 0.66 | 1.41 ± 0.83 | 0.001 |
| Glucose (mg/dL) | 111.29 ± 49.73 | 107.06 ± 37.57 | 118.34 ± 64.80 | 0.089 |
| Total Cholesterol (mg/dL) | 140.92 ± 42.71 | 147.42 ± 45.11 | 130.14 ± 36.31 | 0.023 |
| Triglycerides (mg/dL) | 110.25 ± 68.22 | 111.66 ± 59.30 | 108.00 ± 81.08 | 0.767 |
| CALLY index | 0.60 ± 1.48 | 0.60 ± 1.55 | 0.59 ± 1.35 | 0.990 |
| Sample Population (n = 264) | CALLY Index < 0.055 (n = 101) | CALLY Index > 0.055 (n = 163) | p | |
|---|---|---|---|---|
| Age (years) | 88.02 ± 6.37 | 87.85 ± 7.00 | 88.12 ± 5.96 | 0.732 |
| Sex (M/F) | 97/167 | 40/61 | 57/106 | 0.448 |
| Activities of Daily Living (ADL, n) | 2.19 ± 2.02 | 1.74 ± 1.88 | 2.46 ± 2.07 | 0.007 |
| Instrumental ADL (IADL, n) | 1.42 ± 2.03 | 1.11 ± 1.75 | 1.60 ± 2.16 | 0.064 |
| Mini Nutritional Assessment (MNA, n) | 18.10 ± 14.24 | 16.12 ± 8.43 | 18.05 ± 7.77 | 0.079 |
| Cumulative Illness Rating Scale for Geriatrics (CIRS-G, n) | 9.70 ± 5.41 | 9.78 ± 5.70 | 9.66 ± 5.26 | 0.874 |
| Number of drugs (n) | 7.07 ± 3.03 | 6.80 ± 3.19 | 7.24 ± 2.93 | 0.250 |
| Systolic Blood Pressure (SPB, mmHg) | 127.30 ± 26.93 | 116.41 ± 21.24 | 133.92 ± 27.90 | <0.001 |
| Diastolic Blood Pressure (DPB, mmHg) | 70.02 ± 13.34 | 66.33 ± 11.47 | 72.26 ± 13.93 | <0.001 |
| Peripheral Oxygen Saturation (SpO2, %) | 94.25 ± 11.19 | 93.08 ± 13.78 | 94.82 ± 9.73 | 0.372 |
| Quick Sequential Organ Failure Assessment (qSOFA, n) | 1.50 ± 0.62 | 1.71 ± 0.71 | 1.38 ± 0.52 | <0.001 |
| Hemoglobin (Hb, g/dL) | 11.38 ± 2.05 | 11.17 ± 2.02 | 11.52 ± 2.05 | 0.173 |
| White Blood Cell Count (WBC, ×103/μL) | 10.00 ± 6.52 | 11.12 ± 5.55 | 9.31 ± 6.98 | 0.028 |
| Lymphocyte count (×109/L) | 1.36 ± 1.08 | 0.98 ± 0.51 | 1.59 ± 1.26 | <0.001 |
| Serum Albumine (g/dL) | 3.19 ± 0.47 | 2.94 ± 0.46 | 3.34 ± 0.41 | <0.001 |
| C-reactive Protein (CRP, mg/dL) | 6.79 ± 7.59 | 13.98 ± 7.46 | 2.34 ± 2.65 | <0.001 |
| Creatinine (mg/dL) | 1.21 ± 0.74 | 1.30 ± 0.84 | 1.16 ± 0.67 | 0.137 |
| Glucose (mg/dL) | 111.29 ± 49.73 | 116.50 ± 50.14 | 107.99 ± 49.36 | 0.197 |
| Total Cholesterol (mg/dL) | 140.92 ± 42.71 | 127.50 ± 34.68 | 148.02 ± 44.98 | 0.008 |
| Triglycerides (mg/dL) | 110.25 ± 68.22 | 117.20 ± 70.77 | 106.57 ± 66.96 | 0.400 |
| Variable | B | OR | 95% CI | p-Value |
|---|---|---|---|---|
| Age (years) | 0.053 | 1.055 | 0.99–1.12 | 0.087 |
| Sex (F vs. M) | −0.310 | 0.733 | 0.36–1.51 | 0.400 |
| Number of drugs | 0.067 | 1.069 | 0.94–1.21 | 0.294 |
| CIRS-G | 0.041 | 1.042 | 0.98–1.11 | 0.185 |
| MNA | −0.050 | 0.952 | 0.91–0.99 | 0.030 |
| Hemoglobin (g/dL) | 0.052 | 1.053 | 0.88–1.26 | 0.577 |
| qSOFA | 0.312 | 1.367 | 0.75–2.49 | 0.309 |
| CALLY index (<0.055) | −0.805 | 0.447 | 0.22–0.93 | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancinetti, F.; Guazzarini, A.G.; Gaspari, M.; Croce, M.F.; Serra, R.; Mecocci, P.; Boccardi, V. Integrating Nutrition, Inflammation, and Immunity: The CALLY Index as a Novel Prognostic Biomarker in Acute Geriatric Care. Nutrients 2025, 17, 3192. https://doi.org/10.3390/nu17203192
Mancinetti F, Guazzarini AG, Gaspari M, Croce MF, Serra R, Mecocci P, Boccardi V. Integrating Nutrition, Inflammation, and Immunity: The CALLY Index as a Novel Prognostic Biomarker in Acute Geriatric Care. Nutrients. 2025; 17(20):3192. https://doi.org/10.3390/nu17203192
Chicago/Turabian StyleMancinetti, Francesca, Anna Giulia Guazzarini, Martina Gaspari, Michele Francesco Croce, Rocco Serra, Patrizia Mecocci, and Virginia Boccardi. 2025. "Integrating Nutrition, Inflammation, and Immunity: The CALLY Index as a Novel Prognostic Biomarker in Acute Geriatric Care" Nutrients 17, no. 20: 3192. https://doi.org/10.3390/nu17203192
APA StyleMancinetti, F., Guazzarini, A. G., Gaspari, M., Croce, M. F., Serra, R., Mecocci, P., & Boccardi, V. (2025). Integrating Nutrition, Inflammation, and Immunity: The CALLY Index as a Novel Prognostic Biomarker in Acute Geriatric Care. Nutrients, 17(20), 3192. https://doi.org/10.3390/nu17203192







