Association Between the Jiangnan Diet and Mild Cognitive Impairment Among the Elderly
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
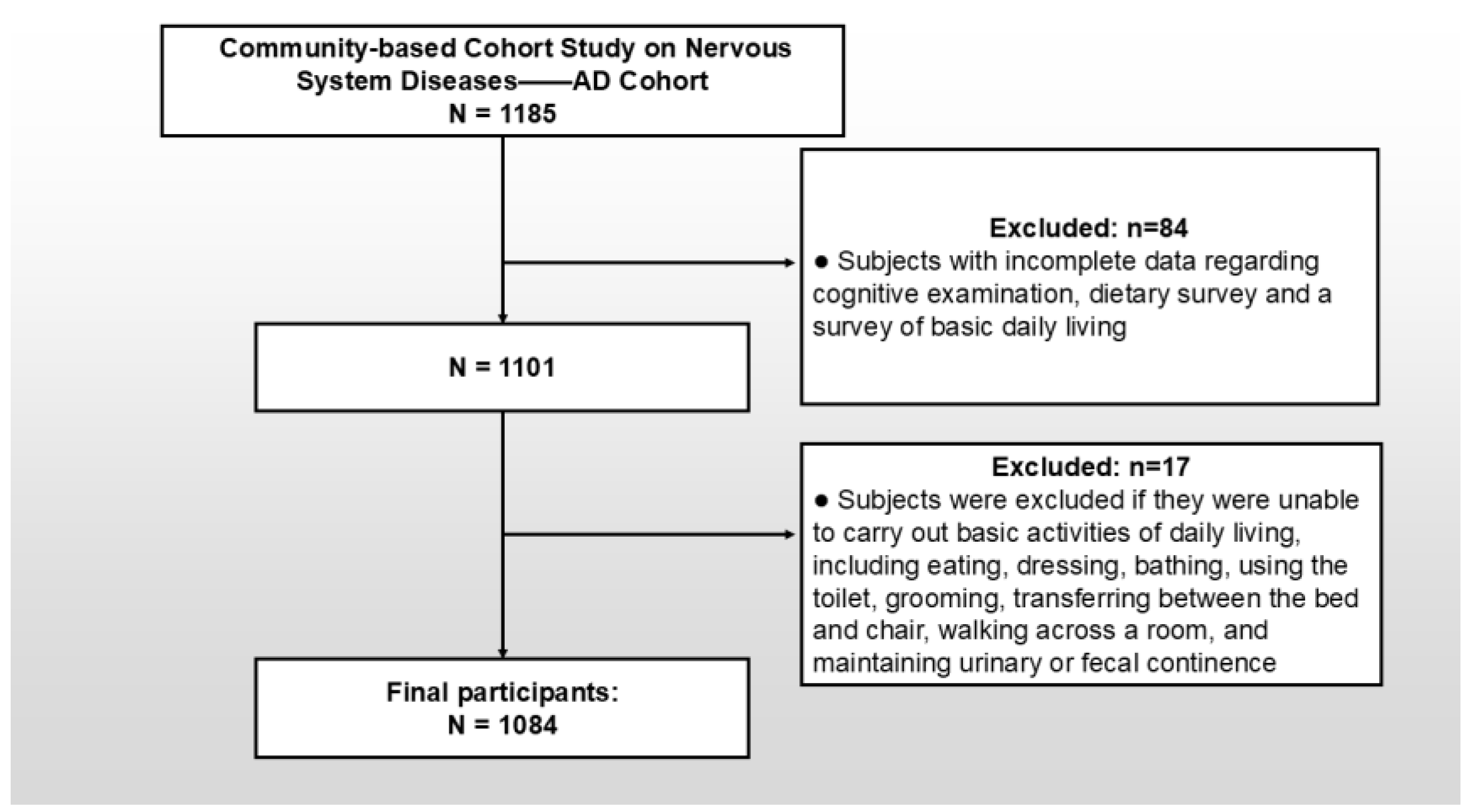
2.2. Target Population and Sampling Strategy
- Age ≥ 55 years;
- Permanent residency (defined as ≥5 years’ residency) amidst the target community;
- Nonexistence of comorbidities potentially confounding cognitive assessments, including congenital/acquired intellectual disability, severe psychiatric disorders (e.g., schizophrenia or bipolar disorder), or uncorrectable visual/auditory impairments.
2.3. Data Gathering
2.4. Cognitive Function Evaluation
2.5. Dietary Pattern and Energy Evaluation
2.6. Assessment of Covariates
2.7. Statistical Analyses
3. Results
3.1. Establishment of Dietary Pattern
3.2. Basic Information
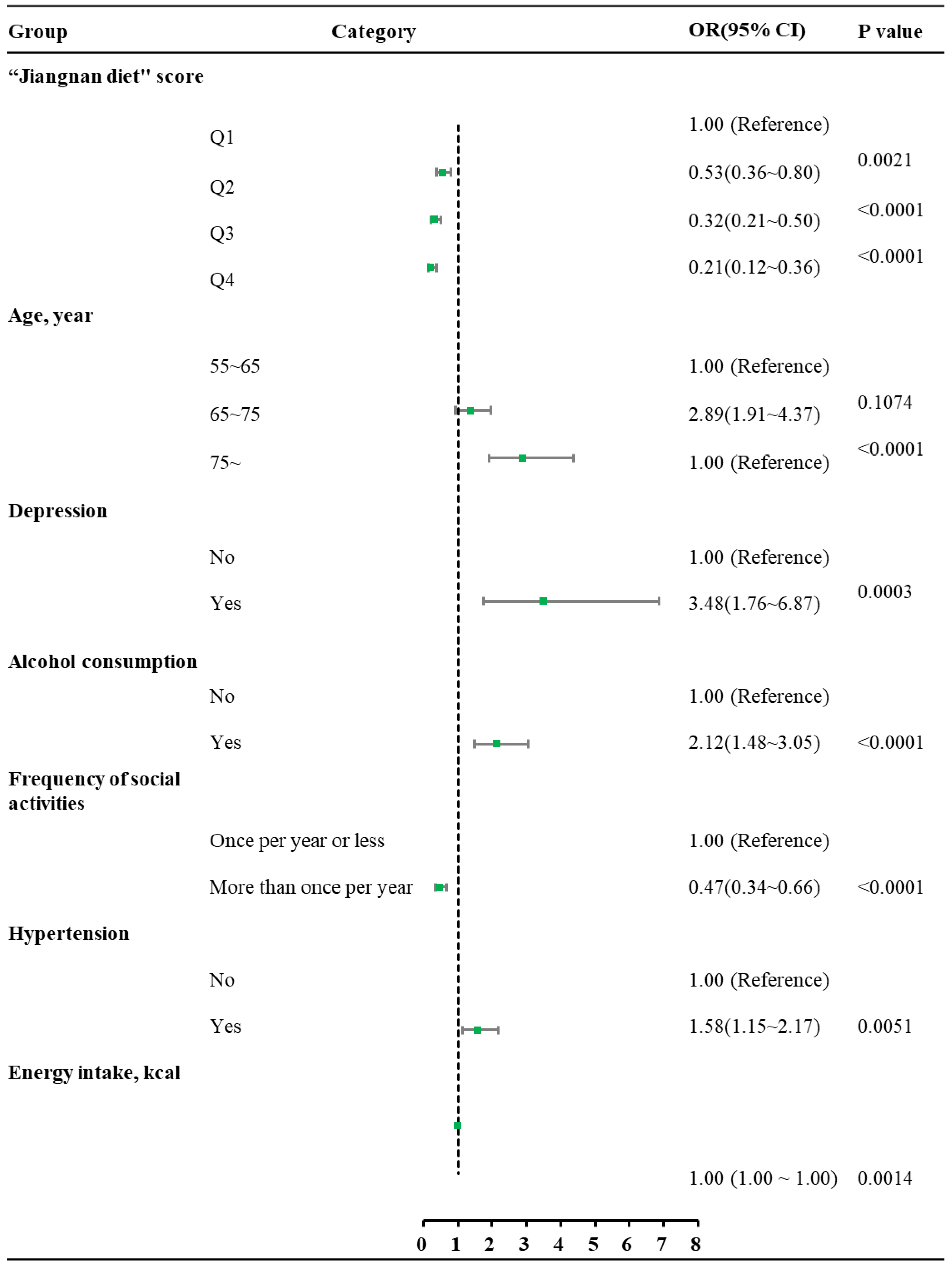
3.3. Dietary Pattern and Its Association with Mild Cognitive Impairment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| BMI | Body Mass Index |
| CI | Confidence interval |
| FCTs | Converted FFQ Food Composition Tables |
| FFQ | Food Frequency Questionnaire |
| GDS | Geriatric Depression Scale |
| MCI | Mild cognitive impairment |
| MIND | Mediterranean-DASH Intervention for Neurodegenerative Delay |
| MMSE | Mini-Mental State Examination |
| MOCA | Montreal Cognitive Assessment |
| OR | Odds Ratio |
| PSQI | Pittsburgh Sleep Quality Index |
| WHO | World Health Organization |
References
- United Nations. World Population Prospects 2024; United Nations: New York, NY, USA, 2024. [Google Scholar]
- National Bureau of Statistics of China. Statistical Bulletin of the People’s Republic of China on National Economic and Social Development; National Bureau of Statistics of China: Beijing, China, 2024. [Google Scholar]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Cao, Y.; Yu, F.; Lyu, Y.; Lu, X. Promising candidates from drug clinical trials: Implications for clinical treatment of Alzheimer’s disease in China. Front. Neurol. 2022, 13, 1034243. [Google Scholar] [CrossRef]
- World Health Organization. Dementia; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Gifford, K.A.; Liu, D.; Romano, R.R.; Jones, R.N.; Jefferson, A.L. Development of a subjective cognitive decline questionnaire using item response theory: A pilot study. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2015, 1, 429–439. [Google Scholar] [CrossRef]
- Petersen, R.C.; Roberts, R.O.; Knopman, D.S.; Boeve, B.F.; Geda, Y.E.; Ivnik, R.J.; Smith, G.E.; Jack, C.R.J. Mild cognitive impairment: Ten years later. Arch Neurol. 2009, 66, 1447–1455. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, P.; Zou, Z.; Li, X.; Cao, M.; Ma, J.; Jing, J. Status of Cardiovascular Health in Chinese Children and Adolescents. JACC Asia 2022, 2, 87–100. [Google Scholar] [CrossRef]
- Huang, L.; Tao, Y.; Chen, H.; Chen, X.; Shen, J.; Zhao, C.; Xu, X.; He, M.; Zhu, D.; Zhang, R.; et al. Mediterranean-Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay (MIND) Diet and Cognitive Function and its Decline: A Prospective Study and Meta-analysis of Cohort Studies. Am. J. Clin. Nutr. 2023, 118, 174–182. [Google Scholar] [CrossRef]
- Chen, H.; Dhana, K.; Huang, Y.; Huang, L.; Tao, Y.; Liu, X.; Melo Van Lent, D.; Zheng, Y.; Ascherio, A.; Willett, W.; et al. Association of the Mediterranean Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay (MIND) Diet With the Risk of Dementia. JAMA Psychiatry 2023, 80, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Chinese Nutrition Society. Dietary Guidelines for Chinese Residents (2022); People’s Medical Publishing House: Beijing, China, 2022. [Google Scholar]
- Hua, J.; Fan, X.; Su, J.; Chen, L.; Qin, Y.; Cui, L.; Zhou, J.; Tao, R. Research Progress on Healthy Dietary Patterns and Cardiovascular Diseases. Chin. J. Prev. Control Chronic Dis. 2023, 12. [Google Scholar]
- Luo, Y.; Wang, J.; Sun, L.; Gu, W.; Zong, G.; Song, B.; Shen, C.; Zhou, P.; Chen, Y.; Wu, Y.; et al. Isocaloric-restricted Mediterranean Diet and Chinese Diets High or Low in Plants in Adults with Prediabetes. J. Clin. Endocrinol. Metab. 2022, 107, 2216–2227. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, X.; Song, Q.; Cui, X.; Shi, Z.; Zang, J.; Su, J.; Sun, X. Jiangnan dietary pattern actively prevents muscle mass loss: Based on a cohort study. J. Hum. Nutr. Diet. 2022, 35, 957–967. [Google Scholar] [CrossRef]
- He, M.; Su, D.; Zhang, R.; Xu, P.; Han, D.; Huang, L.; Zou, Y. Sex Disparity in the Nutrition-Related Determinants of Mild Cognitive Impairment: A Case–Control Study. Nutrients 2025, 17, 248. [Google Scholar] [CrossRef]
- Peng, J.; Li, X.; Wang, J.; Li, F.; Gao, J.; Deng, Y.; Li, B.; Li, T.; Li, Y.; Tang, S.; et al. Association between plant-based dietary patterns and cognitive function in middle-aged and older residents of China. J. Alzheimers Dis. 2024, 103, 282–292. [Google Scholar] [CrossRef]
- Smith, L.; Lopez Sanchez, G.F.; Veronese, N.; Soysal, P.; Oh, H.; Kostev, K.; Rahmati, M.; Butler, L.; Gibson, P.; Keyes, H.; et al. Association of Fruit and Vegetable Consumption with Mild Cognitive Impairment in Low- and Middle-Income Countries. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2023, 78, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Li, S.; Chen, J.; Li, Y.; Wu, Y.; Zhang, D. Association of dietary ω-3 and ω-6 fatty acids intake with cognitive performance in older adults: National Health and nutrition examination Survey (NHANES) 2011–2014. Nutr. J. 2020, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, J.; Qiu, J.; Li, Y.; Wang, J.; Jiao, J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: A dose-response meta-analysis of 21 cohort studies. Am. J. Clin. Nutr. 2016, 103, 330–340. [Google Scholar] [CrossRef]
- Shukui, H.; Yitao, R.; Xin, M.; Panpan, S.; Jinxiang, M.; Ziyu, Z.; Hongru, C. Analysis of Disease Burden Trends and Forecast of Alzheimer’s Disease and Other Dementias among the Elderly in China from 1992 to 2021. Chin. Gen. Pract. 2025, 8, 996–1003. [Google Scholar]
- Hao, W.; Na, L.; Jianan, Y.; Jin, P.; Weiwei, G.; Xiaoyan, Z.; Yunqi, G.; Pinyuan, D.; Min, Y. Trends in incidence and mortality of Alzheimer’s disease in Zhejiang Province from 2003 to 2017. China Prev. Med. J. 2022, 3, 227–239. [Google Scholar]
- Huang, Q.; Jia, X.; Zhang, J.; Huang, F.; Wang, H.; Zhang, B.; Wang, L.; Jiang, H.; Wang, Z. Diet—Cognition Associations Differ in Mild Cognitive Impairment Subtypes. Nutrients 2021, 13, 1341. [Google Scholar] [CrossRef]
- Jia, X.; Wang, Z.; Huang, F.; Su, C.; Du, W.; Jiang, H.; Wang, H.; Wang, J.; Wang, F.; Su, W.; et al. A comparison of the Mini-Mental State Examination (MMSE) with the Montreal Cognitive Assessment (MoCA) for mild cognitive impairment screening in Chinese middle-aged and older population: A cross-sectional study. BMC Psychiatry 2021, 21, 485. [Google Scholar] [CrossRef]
- Lu, J.; Li, D.; Li, F.; Zhou, A.; Wang, F.; Zuo, X.; Jia, X.; Song, H.; Jia, J. Montreal Cognitive Assessment in Detecting Cognitive Impairment in Chinese Elderly Individuals: A Population-Based Study. J. Geriatr. Psychiatry Neurol. 2011, 24, 184–190. [Google Scholar] [CrossRef]
- Winblad, B.; Palmer, K.; Kivipelto, M.; Jelic, V.; Fratiglioni, L.; Wahlund, L.O.; Nordberg, A.; Bäckman, L.; Albert, M.; Almkvist, O.; et al. Mild cognitive impairment—Beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004, 256, 240–246. [Google Scholar] [CrossRef]
- Julayanont, P.; Brousseau, M.; Chertkow, H.; Phillips, N.; Nasreddine, Z.S. Montreal Cognitive Assessment Memory Index Score (MoCA-MIS) as a Predictor of Conversion from Mild Cognitive Impairment to Alzheimer’s Disease. J. Am. Geriatr. Soc. 2014, 62, 679–684. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zou, Y.; Su, D.; Zhao, D.; Zhou, M.; Xu, P.; Zhang, R. Relationship of Composite Dietary Antioxidant Index vs. Alcohol Consumption with Mild Cognitive Impairment in the Elderly. Nutrients 2025, 17, 2111. [Google Scholar] [CrossRef]
- He, M.; Zou, Y.; Huang, L.; Zhao, D.; Han, D.; Su, D.; Zhang, R. The Nutritional Status of Vitamin D and Its Influencing Factors among Adults in Zhejiang Province in 2018. J. Hyg. Res. 2022, 51, 844–848. [Google Scholar]
- Zhao, D.; Gong, Y.; Huang, L.; Lv, R.; Gu, Y.; Ni, C.; Zhu, D.; Yang, M.; Rong, S.; Zhang, R.; et al. Validity of food and nutrient intakes assessed by a food frequency questionnaire among Chinese adults. Nutr. J. 2024, 23, 23. [Google Scholar] [CrossRef]
- Wei, J.; Zhu, X.; Liu, J.; Gao, Y.; Liu, X.; Wang, K.; Zheng, X. Estimating global prevalence of mild cognitive impairment and dementia in elderly with overweight, obesity, and central obesity: A systematic review and meta-analysis. Off. J. Int. Assoc. Study Obes. 2025, 26, e13882. [Google Scholar] [CrossRef]
- WS/T 428–2013; Adult Weight Assessment. National Health and Family Planning Commission of the People’s Republic of China. Standards Press of China: Beijing, China, 2013.
- Huang, F.; Wang, H.; Wang, Z.; Zhang, J.; Du, W.; Jia, X.; Wang, L.; Zhang, B. Is geriatric depression scale a valid instrument to screen depression in Chinese community-dwelling elderly? BMC Geriatr. 2021, 21, 310. [Google Scholar] [CrossRef]
- Liu, X.; Tang, M.; Hu, L.; Wang, A.; Wu, H.; Zhao, G.; Gao, C.; Li, W. Reliability and validity of the Pittsburgh sleep quality index. Chin. J. Psychiatry 1996, 29, 103–107. [Google Scholar]
- Buysse, D.J.; Reynolds, C.F.R.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Hypertension; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Luo, L.; Li, H.; Luo, J. Detection of cognitive impairment and its relationship with physical activity levels in Chinese elderly: A cross-sectional analysis based on data from the 2018 China Health and Retirement Longitudinal Study. J. Math. Med. 2025, 38, 90–97. [Google Scholar]
- Wang, J.; Lin, X.; Bloomgarden, Z.T.; Ning, G. The Jiangnan diet, a healthy diet pattern for Chinese. J. Diabetes 2020, 12, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, G.; Ren, Q.; Li, M. Comparison and Evaluation of Fatty Acid Components and Contents in Eight Different Vegetable Oils. Modern Food 2025, 7, 175–178. [Google Scholar]
- Loef, M.; Walach, H. The omega-6/omega-3 ratio and dementia or cognitive decline: A systematic review on human studies and biological evidence. J. Nutr. Gerontol. Geriatr. 2013, 32, 1–23. [Google Scholar] [CrossRef]
- Zhao, Z. New Archaeobotanic Data for the Study of the Origins of Agriculture in China. Curr. Anthropol. 2011, 52, S295–S306. [Google Scholar] [CrossRef]
- Welty, F.K.; Daher, R.; Garelnabi, M. Fish and Omega-3 Fatty Acids: Sex and Racial Differences in Cardiovascular Outcomes and Cognitive Function. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 89–107. [Google Scholar] [CrossRef]
- Blasko, I. Interaction of ω-3 fatty acids with B vitamins in slowing the progression of brain atrophy: Identifying the elderly at risk. Am. J. Clin. Nutr. 2015, 102, 7–8. [Google Scholar] [CrossRef]
- Gordon, S.; Hoey, L.; McNulty, H.; Keenan, J.; Pangilinan, F.; Brody, L.C.; Ward, M.; Strain, J.J.; McAnena, L.; McCann, A.; et al. Associations of one-carbon metabolism, related B-vitamins and ApoE genotype with cognitive function in older adults: Identification of a novel gene-nutrient interaction. BMC Med. 2025, 23, 440. [Google Scholar] [CrossRef]
- Plevin, D.; Galletly, C. The neuropsychiatric effects of vitamin C deficiency: A systematic review. BMC Psychiatry 2020, 20, 315. [Google Scholar] [CrossRef]
- Fangfang, H.; Qiong, W.; Shuai, Z.; Xiao, H.; Jingya, Z.; Guodong, S.; Yan, Z. Vegetable and Fruit Intake, Its Patterns, and Cognitive Function: Cross-Sectional Findings among Older Adults in Anhui, China. J. Nutr. Health Aging 2022, 26, 529–536. [Google Scholar] [CrossRef]
- Sun, F.; Zhao, L.; Jin, Q.; Wang, Q.; Jin, S.; Xie, J.; Xu, J.; Yin, M.; Jin, C.; Wang, J. The impact of salt consumption on cardiometabolic and cognitive health in aged female rats. Sci. Rep. 2024, 14, 25363. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, S.; Wang, C.; Fan, H.; Zou, Q.; Pu, Y.; Cai, Z. Dietary salt promotes cognition impairment through GLP-1R/mTOR/p70S6K signaling pathway. Sci. Rep. 2024, 14, 7970. [Google Scholar] [CrossRef]
- Steffens, D.C. Late-Life Depression, Antidepressant Treatment, and Cognition: The Short Haul and the Long Haul. Am. J. Psychiatry 2024, 181, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Fitzpatrick, A.L.; Rapp, S.R.; Nahin, R.L.; Williamson, J.D.; Lopez, O.L.; DeKosky, S.T.; Kuller, L.H.; Mackey, R.H.; Mukamal, K.J.; et al. Alcohol Consumption and Risk of Dementia and Cognitive Decline Among Older Adults with or Without Mild Cognitive Impairment. JAMA Netw. Open. 2019, 2, e1910319. [Google Scholar] [CrossRef]
- Chen, Y.; Grodstein, F.; Capuano, A.W.; Wang, T.; Bennett, D.A.; James, B.D. Late-life social activity and subsequent risk of dementia and mild cognitive impairment. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2025, 21, e14316. [Google Scholar] [CrossRef]
- Wei, C.; Zhao, J.; Hu, R.; Wei, X. Association between depressive status and mild cognitive impairment in middle-aged and elderly Chinese adults from CHARLS study. Front. Psychiatry 2025, 16, 1516341. [Google Scholar] [CrossRef]
- Shajahan, S.; Peters, R.; Carcel, C.; Woodward, M.; Harris, K.; Anderson, C.S. Hypertension and Mild Cognitive Impairment: State-of-the-Art Review. Am. J. Hypertens. 2024, 37, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Sindi, S.; Pérez, L.M.; Vetrano, D.L.; Triolo, F.; Kåreholt, I.; Sjöberg, L.; Darin-Mattsson, A.; Kivipelto, M.; Inzitari, M.; Calderón-Larrañaga, A. Sleep disturbances and the speed of multimorbidity development in old age: Results from a longitudinal population-based study. BMC Med. 2020, 18, 382. [Google Scholar] [CrossRef]
- Zafar, J.; Malik, N.I.; Atta, M.; Makhdoom, I.F.; Ullah, I.; Manzar, M.D. Loneliness may mediate the relationship between depression and the quality of life among elderly with mild cognitive impairment. Psychogeriatrics 2021, 21, 805–812. [Google Scholar] [CrossRef]
- Aiken-Morgan, A.T.; Capuano, A.W.; Wilson, R.S.; Barnes, L.L. Changes in Body Mass Index and Incident Mild Cognitive Impairment Among African American Older Adults. J. Gerontol. 2024, 79, glad263. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Zeng, Q.; Luo, X.; Xu, T.; Shen, Z.; Xu, X.; Wang, C.; Li, K.; Huang, P.; Li, X.; et al. Effects of Cigarette Smoking on Resting-State Functional Connectivity of the Nucleus Basalis of Meynert in Mild Cognitive Impairment. Front. Aging Neurosci. 2021, 13, 755630. [Google Scholar] [CrossRef]

| Food Groups | Food Items |
|---|---|
| Rice and rice products | Rice, rice flour, etc. |
| Wheat and wheat products | Bread, steamed buns, noodles, dumplings, etc. |
| Whole grains and miscellaneous | Whole-wheat bread, buckwheat, corn, millet, sorghum, coix seed, rye, mung beans, adzuki beans, pinto beans, kidney beans, etc. |
| Tubers | Sweet potatoes, potatoes, taros, yams, etc. |
| Soybeans and soybean products | Dried soybeans (soybeans, green soybeans, or black soybeans), soy milk, soybean flour, tofu, tofu skin, etc. |
| Vegetables | Fresh legumes, tomatoes, peppers, carrots, melons, vegetables, leafy greens, Chinese cabbage and other leafy vegetables, cruciferous vegetables, other fresh or frozen vegetables, green onions and garlic, fungi and algae, dried vegetables |
| Fruits | Orange fruits, watermelons, melons, other melon fruits, berry fruits, figs, all other fresh/frozen fruits, all other dried fruits except preserved fruits |
| Milk and milk products | Whole liquid milk, low-fat liquid milk/skim liquid milk, whole milk powder, low-fat milk powder/skim milk powder, yogurt, cheese, ice cream |
| Meat | Chicken, duck, goose, pigeon, quail, meat, lean pork, fatty pork, beef, lamb, other non-processed meat, processed meat products |
| Seafood | Marine fish, marine shrimp and crabs, and other seafood |
| Freshwater aquatic products | Freshwater fish, freshwater shrimps, and crabs |
| Eggs | Fresh eggs (eggs, duck eggs, goose eggs, quail eggs), salted eggs, salted duck eggs, salted goose eggs, pine eggs |
| Nuts | Peanuts, melon seeds, pumpkin seeds, seaweeds, melon seeds, other melon seeds |
| Sault | Sault |
| Oil | Vegetable oil and animal oil |
| Vegetable oil | Rapeseed oil, peanut oil, corn oil, rice bran oil |
| Dietary Pattern | Influence Coefficient | Root Test Value | % of Variance | Cumulative% |
|---|---|---|---|---|
| “Animal products” pattern | 5.32 | 33.272 | 33.272 | |
| Freshwater aquatic products | 0.712 | |||
| Meat | 0.688 | |||
| Nuts | 0.654 | |||
| Fruits | 0.648 | |||
| Oil | −0.074 | |||
| Vegetable oil | −0.060 | |||
| Sault | 0.100 | |||
| “Whole Grains-tubers” pattern | 2.41 | 15.052 | 48.324 | |
| Whole grains and miscellaneous | 0.808 | |||
| Tubers | 0.775 | |||
| Vegetables | 0.685 | |||
| Soybeans and soybean products | 0.615 | |||
| Oil | −0.029 | |||
| Vegetable oil | −0.022 | |||
| Sault | −0.063 | |||
| “High in salt and oil” pattern | 1.23 | 7.677 | 56.001 | |
| Oil | 0.956 | |||
| Vegetable oil | 0.955 | |||
| Sault | 0.755 | |||
| “Wheat-based foods” pattern | 0.97 | 6.070 | 62.071 | |
| Wheat and wheat products | 0.616 |
| Variables | Total, N | MCI, N (%) | Normal, N (%) | χ2 | p |
|---|---|---|---|---|---|
| Total | 1084 | 267 (24.6) | 817 (75.4) | ||
| “Animal products” pattern | 27.09 | <0.0001 | |||
| Q1 | 276 (25.5) | 93 (34.8) | 183 (22.4) | ||
| Q2 | 269 (24.8) | 76 (28.5) | 193 (23.6) | ||
| Q3 | 268 (24.7) | 44 (16.5) | 224 (27.4) | ||
| Q4 | 271 (25) | 54 (20.2) | 217 (26.6) | ||
| “Whole Grains-tubers” pattern | 24.86 | <0.0001 | |||
| Q1 | 276 (25.5) | 95 (35.6) | 181 (22.2) | ||
| Q2 | 269 (24.8) | 71 (26.6) | 198 (24.2) | ||
| Q3 | 269 (24.8) | 49 (18.4) | 220 (26.9) | ||
| Q4 | 270 (24.9) | 52 (19.5) | 218 (26.7) | ||
| “High in salt and oil” pattern | 33.53 | <0.0001 | |||
| Q1 | 277 (25.6) | 87 (32.6) | 190 (23.3) | ||
| Q2 | 268 (24.7) | 84 (31.5) | 184 (22.5) | ||
| Q3 | 270 (24.9) | 61 (22.9) | 209 (25.6) | ||
| Q4 | 269 (24.8) | 35 (13.1) | 234 (28.6) | ||
| “Wheat-based foods” pattern | 27.18 | <0.0001 | |||
| Q1 | 276 (25.5) | 58 (21.7) | 218 (26.7) | ||
| Q2 | 270 (24.9) | 42 (15.7) | 228 (27.9) | ||
| Q3 | 268 (24.7) | 89 (33.3) | 179 (21.9) | ||
| Q4 | 270 (24.9) | 78 (29.2) | 192 (23.5) | ||
| Region | 3.13 | 0.077 | |||
| Urban | 554 (51.1) | 149 (55.8) | 405 (49.6) | ||
| Rural | 530 (48.9) | 118 (44.2) | 412 (50.4) | ||
| Gender | 0.01 | 0.932 | |||
| Male | 514 (47.4) | 126 (47.2) | 388 (47.5) | ||
| Female | 570 (52.6) | 141 (52.8) | 429 (52.5) | ||
| Age, year | 43.57 | <0.001 | |||
| 55~65 | 372 (34.3) | 63 (23.6) | 309 (37.8) | ||
| 65~75 | 479 (44.2) | 110 (41.2) | 369 (45.2) | ||
| 75~ | 233 (21.5) | 94 (35.2) | 139 (17.0) | ||
| Educational level | 4.6 | 0.032 | |||
| Junior school or below | 927 (86.5) | 236 (90.4) | 691 (85.2) | ||
| Senior high school or above | 145 (13.5) | 25 (9.6) | 120 (14.8) | ||
| Occupation | 0.14 | 0.710 | |||
| Employed or re-employed after retirement or seeking employment | 166 (15.3) | 39 (14.6) | 127 (15.5) | ||
| Retired or unemployed | 918 (84.7) | 228 (85.4) | 690 (84.5) | ||
| Marriage | 1.11 | 0.293 | |||
| Married | 919 (84.8) | 221 (82.8) | 698 (85.4) | ||
| Unmarried/divorced/widowed | 165 (15.2) | 46 (17.2) | 119 (14.6) | ||
| BMI (kg/m2) | 0.46 | 0.498 | |||
| <24.0 | 614 (56.6) | 156 (58.4) | 458 (56.1) | ||
| ≥24 | 470 (43.4) | 111 (41.6) | 359 (43.9) | ||
| Frequency of social activities | 24.04 | <0.001 | |||
| Once per year or less | 275 (25.4) | 98 (36.7) | 177 (21.7) | ||
| More than once per year | 809 (74.6) | 169 (63.3) | 640 (78.3) | ||
| Depression | 20.09 | <0.001 | |||
| No | 1041 (96.0) | 244 (91.4) | 797 (97.6) | ||
| Yes | 43 (4.0) | 23 (8.6) | 20 (2.5) | ||
| Sleep disturbances | 6.10 | 0.014 | |||
| No | 585 (54.1) | 126 (47.6) | 459 (56.3) | ||
| Yes | 496 (45.9) | 139 (52.5) | 357 (43.8) | ||
| Smoking | 2.58 | 0.108 | |||
| No | 882 (81.5) | 208 (78.2) | 674 (82.6) | ||
| Yes | 200 (18.5) | 58 (21.8) | 142 (17.4) | ||
| Alcohol consumption | 10.89 | 0.012 | |||
| No | 874 (80.6) | 197 (73.8) | 677 (82.9) | ||
| Yes | 210(19.4) | 70(26.2) | 140(17.1) | ||
| Hypertension | 15.58 | <0.001 | |||
| No | 499 (46.0) | 95 (35.6) | 404 (49.5) | ||
| Yes | 585 (54.0) | 172 (64.4) | 413 (50.6) | ||
| Diabetes | 2.71 | 0.100 | |||
| No | 988 (91.6) | 238 (89.1) | 750 (92.4) | ||
| Yes | 91 (8.4) | 29 (10.9) | 62 (7.6) | ||
| Energy intake, kcal * | 1517.5 ± 1282.4 | 1528.8 ± 1495 | 1513.8 ± 1205.8 | −0.15 | 0.882 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Zou, Y.; Zhang, R.; Su, D.; Xu, P. Association Between the Jiangnan Diet and Mild Cognitive Impairment Among the Elderly. Nutrients 2025, 17, 3189. https://doi.org/10.3390/nu17203189
He M, Zou Y, Zhang R, Su D, Xu P. Association Between the Jiangnan Diet and Mild Cognitive Impairment Among the Elderly. Nutrients. 2025; 17(20):3189. https://doi.org/10.3390/nu17203189
Chicago/Turabian StyleHe, Mengjie, Yan Zou, Ronghua Zhang, Danting Su, and Peiwei Xu. 2025. "Association Between the Jiangnan Diet and Mild Cognitive Impairment Among the Elderly" Nutrients 17, no. 20: 3189. https://doi.org/10.3390/nu17203189
APA StyleHe, M., Zou, Y., Zhang, R., Su, D., & Xu, P. (2025). Association Between the Jiangnan Diet and Mild Cognitive Impairment Among the Elderly. Nutrients, 17(20), 3189. https://doi.org/10.3390/nu17203189





