Role of Epigallocatechin Gallate in Selected Malignant Neoplasms in Women
Abstract
1. Introduction
1.1. Bioavailability and Metabolic Biodegradation Pathways of EGCG
1.2. Basic Epidemiological Data on Selected Malignant Neoplasms in Women
2. Anticancer Activity of EGCG
2.1. Breast Cancer
2.1.1. Anti-Breast Cancer Activity of EGCG
2.1.2. Combined Action of EGCG Against Breast Cancer
2.1.3. EGCG-Based Nanosystems for Breast Cancer Treatment
2.2. Cervical Cancer
2.2.1. Anti-Cervical Cancer Activity of EGCG
2.2.2. Combined Action of EGCG Against Cervical Cancer
2.2.3. EGCG-Based Nanosystems for Cervical Cancer Treatment
2.3. Endometrial Cancer
Anti-Endometrial Cancer Activity of EGCG
2.4. Ovarian Cancer
2.4.1. Anti-Ovarian Cancer Activity of EGCG
2.4.2. Combined Action of EGCG Against Ovarian Cancer
2.4.3. EGCG-Based Nanosystems for Ovarian Cancer Treatment
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Bizuayehu, H.M.; Ahmed, K.Y.; Kibret, G.D.; Dadi, A.F.; Belachew, S.A.; Bagade, T.; Tegegne, T.K.; Venchiarutti, R.L.; Kibret, K.T.; Hailegebireal, A.H.; et al. Global disparities of cancer and its projected burden in 2050. JAMA Netw. Open 2024, 7, e2443198. [Google Scholar] [CrossRef] [PubMed]
- Farhoosh, R.; Golmovahhed, G.A.; Khodaparast, M.H.H. Antioxidant activity of various extracts of old tea leaves and black tea wastes (Camellia sinensis L.). Food Chem. 2007, 100, 231–236. [Google Scholar] [CrossRef]
- Pan, X.; Niu, G.; Liu, H. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem. Eng. Process. 2003, 42, 129–133. [Google Scholar] [CrossRef]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Dai, J.; Sameen, D.E.; Zeng, Y.; Li, S.; Qin, W.; Liu, Y. An overview of tea polyphenols as bioactive agents for food packaging applications. LWT 2022, 167, 113845. [Google Scholar] [CrossRef]
- Wang, H.; Provan, G.J.; Helliwell, K. Tea flavonoids: Their functions, utilisation and analysis. Trends Food Sci. Technol. 2000, 11, 152–160. [Google Scholar] [CrossRef]
- Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin gallate: A review of its beneficial properties to prevent metabolic syndrome. Nutrients 2015, 7, 5443–5468. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Antoniewicz, J.; Mruk, H.; Janda, K. Health benefits and chemical composition of Matcha green tea: A review. Molecules 2020, 26, 85. [Google Scholar] [CrossRef] [PubMed]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green tea (Camellia sinensis): A review of its phytochemistry, pharmacology, and toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Liu, C.; Dong, S.L.; Ou, C.S.; Lu, J.L.; Ye, J.H.; Liang, Y.R.; Zheng, X.Q. Anticarcinogenic potentials of tea catechins. Front. Nutr. 2022, 9, 1060783. [Google Scholar] [CrossRef]
- Zeb, F.; Naqeeb, H.; Osaili, T.; Faris, M.E.; Ismail, L.C.; Obaid, R.S.; Naja, F.; Radwan, H.; Hasan, H.; Hashim, M.; et al. Molecular crosstalk between polyphenols and gut microbiota in cancer prevention. Nutr. Res. 2024, 124, 21–42. [Google Scholar] [CrossRef]
- Upadhyay, P.K.; Singh, S.; Vishwakarma, V.K. Natural polyphenols in cancer management: Promising role, mechanisms, and chemistry. Curr. Pharm. Biotechnol. 2024, 25, 694–712. [Google Scholar] [CrossRef]
- Liu, Z.; Vincken, J.P.; de Bruijn, W.J.C. Tea phenolics as prebiotics. Trends Food Sci. Technol. 2022, 127, 156–168. [Google Scholar] [CrossRef]
- Chu, C.; Deng, J.; Man, Y.; Qu, Y. Green tea extracts epigallocatechin-3-gallate for different treatments. Biomed. Res. Int. 2017, 2017, 5615647. [Google Scholar] [CrossRef]
- Rice-Evans, C. Implications of the mechanisms of action of tea polyphenols as antioxidants in vitro for chemoprevention in humans. Exp. Biol. Med. 1999, 220, 262–266. [Google Scholar] [CrossRef]
- Lee, M.J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Chen, L.; Lee, M.J.; Li, H.; Yang, C.S. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab. Dispos. 1997, 25, 1045–1050. [Google Scholar] [PubMed]
- Yang, C.S.; Sang, S.; Lambert, J.D.; Lee, M.J. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol. Nutr. Food Res. 2008, 52, S139–S151. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.H.; Cai, Y.; Alberts, D.S.; Hakim, I.; Dorr, R.; Shahi, F.; Crowell, J.A.; Yang, C.S.; Hara, Y. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol. Biomark. Prev. 2001, 10, 53–58. [Google Scholar]
- Chow, H.H.S.; Cai, Y.; Hakim, I.A.; Crowell, J.A.; Shahi, F.; Brooks, C.A.; Dorr, R.T.; Hara, Y.; Alberts, D.S. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin. Cancer Res. 2003, 9, 3312–3319. [Google Scholar] [PubMed]
- Chow, H.H.S.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Ranger-Moore, J.; Chew, W.M.; Celaya, C.A.; Rodney, S.R.; Hara, Y.; Alberts, D.S. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of polyphenon E in healthy individuals. Clin. Cancer Res. 2005, 11, 4627–4633. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, 5239. [Google Scholar] [CrossRef]
- Nakagawa, K.; Nakayama, K.; Nakamura, M.; Sookwong, P.; Tsuduki, T.; Niino, H.; Kimura, F.; Miyazawa, T. Effects of co-administration of tea epigallocatechin-3-gallate (EGCG) and caffeine on absorption and metabolism of EGCG in humans. Biosci. Biotechnol. Biochem. 2009, 73, 2014–2017. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001. [Google Scholar] [CrossRef]
- Chen, T.; Yang, C.S. Biological fates of tea polyphenols and their interactions with microbiota in the gastrointestinal tract: Implications on health effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 2691–2709. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Kohri, T.; Matsumoto, N.; Yamakawa, M.; Suzuki, M.; Nanjo, F.; Hara, Y.; Oku, N. Metabolic fate of (−)-[4-(3)H]epigallocatechin gallate in rats after oral administration. J. Agric. Food Chem. 2001, 49, 4102–4112. [Google Scholar] [CrossRef] [PubMed]
- Marín, V.; Burgos, V.; Pérez, R.; Maria, D.A.; Pardi, P.; Paz, C. The potential role of epigallocatechin-3-gallate (EGCG) in breast cancer treatment. Int. J. Mol. Sci. 2023, 24, 10737. [Google Scholar] [CrossRef] [PubMed]
- Takagaki, A.; Nanjo, F. Metabolism of (−)-epigallocatechin gallate by rat intestinal flora. J. Agric. Food Chem. 2010, 58, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Bresciani, L.; Brindani, N.; Ludwig, I.A.; Pereira-Caro, G.; Angelino, D.; Llorach, R.; Calani, L.; Brighenti, F.; Clifford, M.N.; et al. Phenyl-γ-valerolactones and phenylvaleric acids, the main colonic metabolites of flavan-3-ols: Synthesis, analysis, bioavailability, and bioactivity. Nat. Prod. Rep. 2019, 36, 714–752. [Google Scholar] [CrossRef]
- van der Hooft, J.J.J.; de Vos, R.C.H.; Mihaleva, V.; Bino, R.J.; Ridder, L.; de Roo, N.; Jacobs, D.M.; van Duynhoven, J.P.M.; Vervoort, J. Structural elucidation and quantification of phenolic conjugates present in human urine after tea intake. Anal. Chem. 2012, 84, 7263–7271. [Google Scholar] [CrossRef]
- Zagami, P.; Carey, L.A. Triple negative breast cancer: Pitfalls and progress. NPJ Breast Cancer 2022, 8, 95. [Google Scholar] [CrossRef]
- Laganà, A.S.; Chiantera, V.; Gerli, S.; Proietti, S.; Lepore, E.; Unfer, V.; Carugno, J.; Favilli, A. Preventing persistence of HPV infection with natural molecules. Pathogens 2023, 12, 416. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef]
- Walcher, L.; Kistenmacher, A.K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer stem cells—Origins and biomarkers: Perspectives for targeted personalized therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef]
- Medema, J.P. Cancer stem cells: The challenges ahead. Nat. Cell Biol. 2013, 15, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Naujokat, C.; McKee, D.L. The “big five” phytochemicals targeting cancer stem cells: Curcumin, EGCG, sulforaphane, resveratrol and genistein. Curr. Med. Chem. 2021, 28, 4321–4342. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Calderón Iglesias, R.; Ruiz, R.; Aparicio, S.; Crespo, J.; Dzul Lopez, L.; Giampieri, F.; Battino, M. The use of natural compounds for the targeting and chemoprevention of ovarian cancer. Cancer Lett. 2017, 411, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Awajan, D.; Alqudah, A.; Alsawwaf, R.; Althunibat, R.; Abu AlRoos, M.; Al Safadi, A.; Abu Asab, S.; Hadi, R.W.; Al Kury, L.T. Targeting cancer hallmarks with epigallocatechin gallate (EGCG): Mechanistic basis and therapeutic targets. Molecules 2024, 29, 1373. [Google Scholar] [CrossRef]
- Hung, S.W.; Li, Y.; Chen, X.; Chu, K.O.; Zhao, Y.; Liu, Y.; Guo, X.; Man, G.C.W.; Wang, C.C. Green tea epigallocatechin-3-gallate regulates autophagy in male and female reproductive cancer. Front. Pharmacol. 2022, 13, 906746. [Google Scholar] [CrossRef]
- Min, K.; Kwon, T.K. Anticancer effects and molecular mechanisms of epigallocatechin-3-gallate. Integr. Med. Res. 2014, 3, 16–24. [Google Scholar] [CrossRef]
- Ferrari, E.; Bettuzzi, S.; Naponelli, V. The potential of epigallocatechin gallate (EGCG) in targeting autophagy for cancer treatment: A narrative review. Int. J. Mol. Sci. 2022, 23, 6075. [Google Scholar] [CrossRef]
- Alam, M.; Ali, S.; Ashraf, G.M.; Bilgrami, A.L.; Yadav, D.K.; Hassan, M.I. Epigallocatechin 3-gallate: From green tea to cancer therapeutics. Food Chem. 2022, 379, 132135. [Google Scholar] [CrossRef]
- Rady, I.; Mohamed, H.; Rady, M.; Siddiqui, I.A.; Mukhtar, H. Cancer preventive and therapeutic effects of EGCG, the major polyphenol in green tea. Egypt. J. Basic Appl. Sci. 2018, 5, 1–23. [Google Scholar] [CrossRef]
- Huang, Y.J.; Wang, K.L.; Chen, H.Y.; Chiang, Y.F.; Hsia, S.M. Protective effects of epigallocatechin gallate (EGCG) on endometrial, breast, and ovarian cancers. Biomolecules 2020, 10, 1481. [Google Scholar] [CrossRef]
- Cai, J.; Qiao, Y.; Chen, L.; Lu, Y.; Zheng, D. Regulation of the Notch signaling pathway by natural products for cancer therapy. J. Nutr. Biochem. 2024, 123, 109483. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhang, Z.; Han, Y.; Wang, J.; Wang, Y.; Chen, X.; Shao, Y.; Cheng, Y.; Zhou, W.; Lu, X.; et al. A review on anti-cancer effect of green tea catechins. J. Funct. Foods 2020, 74, 104172. [Google Scholar] [CrossRef]
- Suganuma, M.; Saha, A.; Fujiki, H. New cancer treatment strategy using combination of green tea catechins and anticancer drugs. Cancer Sci. 2011, 102, 317–323. [Google Scholar] [CrossRef]
- Fujiki, H.; Sueoka, E.; Watanabe, T.; Suganuma, M. Synergistic enhancement of anticancer effects on numerous human cancer cell lines treated with the combination of EGCG, other green tea catechins, and anticancer compounds. J. Cancer Res. Clin. Oncol. 2015, 141, 1511–1522. [Google Scholar] [CrossRef]
- Dai, W.; Ruan, C.; Zhang, Y.; Wang, J.; Han, J.; Shao, Z.; Sun, Y.; Liang, J. Bioavailability enhancement of EGCG by structural modification and nano-delivery: A review. J. Funct. Foods 2020, 65, 103732. [Google Scholar] [CrossRef]
- Huang, C.Y.; Han, Z.; Li, X.; Xie, H.H.; Zhu, S.S. Mechanism of EGCG promoting apoptosis of MCF-7 cell line in human breast cancer. Oncol. Lett. 2017, 14, 3623–3627. [Google Scholar] [CrossRef]
- Mittal, A.; Pate, M.; Wylie, R.; Tollefsbol, T.; Katiyar, S. EGCG down-regulates telomerase in human breast carcinoma MCF-7 cells, leading to suppression of cell viability and induction of apoptosis. Int. J. Oncol. 2004, 24, 703–710. [Google Scholar] [CrossRef]
- Moradzadeh, M.; Hosseini, A.; Erfanian, S.; Rezaei, H. Epigallocatechin-3-gallate promotes apoptosis in human breast cancer T47D cells through down-regulation of PI3K/AKT and telomerase. Pharmacol. Rep. 2017, 69, 924–928. [Google Scholar] [CrossRef]
- Sheng, J.; Shi, W.; Guo, H.; Long, W.; Wang, Y.; Qi, J.; Liu, J.; Xu, Y. The inhibitory effect of (−)-epigallocatechin-3-gallate on breast cancer progression via reducing SCUBE2 methylation and DNMT activity. Molecules 2019, 24, 2899. [Google Scholar] [CrossRef]
- Zan, L.; Chen, Q.; Zhang, L.; Li, X. Epigallocatechin gallate (EGCG) suppresses growth and tumorigenicity in breast cancer cells by downregulation of miR-25. Bioengineered 2019, 10, 374–382. [Google Scholar] [CrossRef]
- Sen, T.; Moulik, S.; Dutta, A.; Choudhury, P.R.; Banerji, A.; Das, S.; Roy, M.; Chatterjee, A. Multifunctional effect of epigallocatechin-3-gallate (EGCG) in downregulation of gelatinase-A (MMP-2) in human breast cancer cell line MCF-7. Life Sci. 2009, 84, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Mao, L.; Xu, P.; Zheng, X.; Hackman, R.M.; Mackenzie, G.G.; Wang, Y. Suppressing glucose metabolism with epigallocatechin-3-gallate (EGCG) reduces breast cancer cell growth in preclinical models. Food Funct. 2018, 9, 5682–5696. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.W.; Makey, K.L.; Tucker, K.B.; Chinchar, E.; Mao, X.; Pei, I.; Thomas, E.Y.; Miele, L. EGCG, a major green tea catechin suppresses breast tumor angiogenesis and growth via inhibiting the activation of HIF-1α and NFκB, and VEGF expression. Vasc. Cell 2013, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Xu, M.; Zhong, W.; Cui, Z.; Liu, F.; Zhou, K.; Li, X. EGCG decreases the expression of HIF-1α and VEGF and cell growth in MCF-7 breast cancer cells. J. BUON 2014, 19, 435–439. [Google Scholar]
- Mineva, N.D.; Paulson, K.E.; Naber, S.P.; Yee, A.S.; Sonenshein, G.E. Epigallocatechin-3-gallate inhibits stem-like inflammatory breast cancer cells. PLoS ONE 2013, 8, e73464. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Singh, A.K.; Sharma, A.; Warren, J.; Gaddipati, J.P.; Maheshwari, R.K. Green tea polyphenols and its constituent epigallocatechin gallate inhibits proliferation of human breast cancer cells in vitro and in vivo. Cancer Lett. 2007, 245, 232–241. [Google Scholar] [CrossRef]
- Braicu, C.; Gherman, C.D.; Irimie, A.; Berindan-Neagoe, I. Epigallocatechin-3-gallate (EGCG) inhibits cell proliferation and migratory behaviour of triple negative breast cancer cells. J. Nanosci. Nanotechnol. 2013, 13, 632–637. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, X.; Rieger-Christ, K.M.; Summerhayes, I.C.; Wazer, D.E.; Paulson, K.E.; Yee, A.S. Suppression of Wnt signaling by the green tea compound (–)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells. J. Biol. Chem. 2006, 281, 10865–10875. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Passi, N.; Maheshwari, R.K. Green tea polyphenol and epigallocatechin gallate induce apoptosis and inhibit invasion in human breast cancer cells. Cancer Biol. Ther. 2007, 6, 1938–1943. [Google Scholar] [CrossRef]
- Bigelow, R.L.H.; Cardelli, J.A. The green tea catechins, (−)-epigallocatechin-3-gallate (EGCG) and (−)-epicatechin-3-gallate (ECG), inhibit HGF/Met signaling in immortalized and tumorigenic breast epithelial cells. Oncogene 2006, 25, 1922–1930. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Y.; Liu, M.; Yan, Q.; Zhao, W.; Yang, P.; Gao, Q.; Wei, J.; Zhao, W.; Ma, L. Epigallocatechin gallate inhibits cell growth and regulates miRNA expression in cervical carcinoma cell lines infected with different high-risk human papillomavirus subtypes. Exp. Ther. Med. 2018, 17, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Tudoran, O.; Soritau, O.; Balacescu, O.; Balacescu, L.; Braicu, C.; Rus, M.; Gherman, C.; Virag, P.; Irimie, F.; Berindan-Neagoe, I. Early transcriptional pattern of angiogenesis induced by EGCG treatment in cervical tumour cells. J. Cell. Mol. Med. 2012, 16, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Panji, M.; Behmard, V.; Zare, Z.; Malekpour, M.; Nejadbiglari, H.; Yavari, S.; Nayerpour dizaj, T.; Safaeian, A.; Maleki, N.; Abbasi, M.; et al. Suppressing effects of green tea extract and epigallocatechin-3-gallate (EGCG) on TGF-β-induced epithelial-to-mesenchymal transition via ROS/Smad signaling in human cervical cancer cells. Gene 2021, 794, 145774. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Cao, J.; Xie, L.; Shi, X. Cell growth inhibition and gene expression regulation by (−)-epigallocatechin-3-gallate in human cervical cancer cells. Arch. Pharm. Res. 2009, 32, 1309–1315. [Google Scholar] [CrossRef]
- Kedhari Sundaram, M.; Haque, S.; Somvanshi, P.; Bhardwaj, T.; Hussain, A. Epigallocatechin gallate inhibits HeLa cells by modulation of epigenetics and signaling pathways. 3 Biotech. 2020, 10, 484. [Google Scholar] [CrossRef]
- Sharma, C.; Nusri, Q.E.A.; Begum, S.; Javed, E.; Rizvi, T.A.; Hussain, A. (−)-Epigallocatechin-3-gallate induces apoptosis and inhibits invasion and migration of human cervical cancer cells. Asian Pac. J. Cancer Prev. 2012, 13, 4815–4822. [Google Scholar] [CrossRef]
- Noguchi, M.; Yokoyama, M.; Watanabe, S.; Uchiyama, M.; Nakao, Y.; Hara, K.; Iwasaka, T. Inhibitory effect of the tea polyphenol, (−)-epigallocatechin gallate, on growth of cervical adenocarcinoma cell lines. Cancer Lett. 2006, 234, 135–142. [Google Scholar] [CrossRef]
- Yokoyama, M.; Noguchi, M.; Nakao, Y.; Pater, A.; Iwasaka, T. The tea polyphenol, (−)-epigallocatechin gallate effects on growth, apoptosis, and telomerase activity in cervical cell lines. Gynecol. Oncol. 2004, 92, 197–204. [Google Scholar] [CrossRef]
- Sabanayagam, R.; Krishnamoorthy, S.; Gnanagurusamy, J.; Muruganatham, B.; Muthusami, S. EGCG attenuate EGF triggered matrix abundance and migration in HPV positive and HPV negative cervical cancer cells. Med. Oncol. 2023, 40, 261. [Google Scholar] [CrossRef]
- Manohar, M.; Fatima, I.; Saxena, R.; Chandra, V.; Sankhwar, P.L.; Dwivedi, A. (−)-Epigallocatechin-3-gallate induces apoptosis in human endometrial adenocarcinoma cells via ROS generation and p38 MAP kinase activation. J. Nutr. Biochem. 2013, 24, 940–947. [Google Scholar] [CrossRef]
- Dann, J.M.; Sykes, P.H.; Mason, D.R.; Evans, J.J. Regulation of vascular endothelial growth factor in endometrial tumour cells by resveratrol and EGCG. Gynecol. Oncol. 2009, 113, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hou, Y.; Han, G.; Yang, Y.; Wang, S.; Lv, X.; Gao, M. S100A4/NF-κB axis mediates the anticancer effect of epigallocatechin-3-gallate in platinum-resistant ovarian cancer. iScience 2024, 27, 108885. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.W.; Bae, S.M.; Kim, Y.-W.; Lee, J.M.; Namkoong, S.E.; Lee, I.P.; Kim, S.H.; Kim, C.K.; Ahn, W.S. Anticancer effects of (−)-epigallocatechin-3-gallate on ovarian carcinoma cell lines. Gynecol. Oncol. 2004, 94, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Bae, S.M.; Lee, J.M.; Namkoong, S.E.; Han, S.J.; Lee, B.R.; Lee, I.P.; Kim, S.H.; Lee, Y.J.; Kim, C.K.; et al. Activity of green tea polyphenol epigallocatechin-3-gallate against ovarian carcinoma cell lines. Cancer Res. Treat. 2004, 36, 315. [Google Scholar] [CrossRef]
- Wang, F.; Chang, Z.; Fan, Q.; Wang, L. Epigallocatechin-3-gallate inhibits the proliferation and migration of human ovarian carcinoma cells by modulating p38 kinase and matrix metalloproteinase-2. Mol. Med. Rep. 2014, 9, 1085–1089. [Google Scholar] [CrossRef]
- Qin, J.; Fu, M.; Wang, J.; Huang, F.; Liu, H.; Huangfu, M.; Yu, D.; Liu, H.; Li, X.; Guan, X.; et al. PTEN/AKT/mTOR signaling mediates anticancer effects of epigallocatechin-3-gallate in ovarian cancer. Oncol. Rep. 2020, 43, 1885–1896. [Google Scholar] [CrossRef]
- Rao, S.D.; Pagidas, K. Epigallocatechin-3-gallate, a natural polyphenol, inhibits cell proliferation and induces apoptosis in human ovarian cancer cells. Anticancer Res. 2010, 30, 2519–2523. [Google Scholar]
- Zhang, Z.; Zhang, Q.; Yu, Y.; Su, S. Epigallocatechin gallate inhibits ovarian cancer cell growth and induces cell apoptosis via activation of FOXO3A. Vitr. Cell. Dev. Biol. Anim. 2023, 59, 739–746. [Google Scholar] [CrossRef]
- Spinella, F.; Rosanò, L.; Di Castro, V.; Decandia, S.; Albini, A.; Nicotra, M.R.; Natali, P.G.; Bagnato, A. Green tea polyphenol epigallocatechin-3-gallate inhibits the endothelin axis and downstream signaling pathways in ovarian carcinoma. Mol. Cancer Ther. 2006, 5, 1483–1492. [Google Scholar] [CrossRef]
- Baker, K.M.; Bauer, A.C. Green tea catechin, EGCG, suppresses PCB 102-induced proliferation in estrogen-sensitive breast cancer cells. Int. J. Breast Cancer 2015, 2015, 163591. [Google Scholar] [CrossRef]
- Zhang, M.; Holman, C.D.J.; Huang, J.P.; Xie, X. Green tea and the prevention of breast cancer: A case-control study in Southeast China. Carcinogenesis 2006, 28, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Ogunleye, A.A.; Xue, F.; Michels, K.B. Green tea consumption and breast cancer risk or recurrence: A meta-analysis. Breast Cancer Res. Treat. 2010, 119, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Martel, F. The role of EGCG in breast cancer prevention and therapy. Mini Rev. Med. Chem. 2021, 21, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Gianfredi, V.; Vannini, S.; Moretti, M.; Villarini, M.; Bragazzi, N.L.; Izzotti, A.; Nucci, D. Sulforaphane and epigallocatechin gallate restore estrogen receptor expression by modulating epigenetic events in the breast cancer cell line MDA-MB-231: A systematic review and meta-analysis. J. Nutrigenet. Nutr. 2017, 10, 126–135. [Google Scholar] [CrossRef]
- Bimonte, S.; Cascella, M.; Barbieri, A.; Arra, C.; Cuomo, A. Current shreds of evidence on the anticancer role of EGCG in triple negative breast cancer: An update of the current state of knowledge. Infect. Agents Cancer 2020, 15, 2. [Google Scholar] [CrossRef]
- Meeran, S.M.; Patel, S.N.; Chan, T.H.; Tollefsbol, T.O. A novel prodrug of epigallocatechin-3-gallate: Differential epigenetic HTERT repression in human breast cancer cells. Cancer Prev. Res. 2011, 4, 1243–1254. [Google Scholar] [CrossRef]
- Giró-Perafita, A.; Rabionet, M.; Planas, M.; Feliu, L.; Ciurana, J.; Ruiz-Martínez, S.; Puig, T. EGCG-derivative G28 shows high efficacy inhibiting the mammosphere-forming capacity of sensitive and resistant TNBC models. Molecules 2019, 24, 1027. [Google Scholar] [CrossRef]
- Chen, D.; Pamu, S.; Cui, Q.; Chan, T.H.; Dou, Q.P. Novel epigallocatechin gallate (EGCG) analogs activate AMP-activated protein kinase pathway and target cancer stem cells. Bioorg. Med. Chem. 2012, 20, 3031–3037. [Google Scholar] [CrossRef]
- Sartippour, M.R.; Pietras, R.; Marquez-Garban, D.C.; Chen, H.-W.; Heber, D.; Henning, S.M.; Sartippour, G.; Zhang, L.; Lu, M.; Weinberg, O.; et al. The combination of green tea and tamoxifen is effective against breast cancer. Carcinogenesis 2006, 27, 2424–2433. [Google Scholar] [CrossRef]
- Guo, S.; Yang, S.; Taylor, C.; Sonenshein, G.E. Green tea polyphenol epigallocatechin-3 gallate (EGCG) affects gene expression of breast cancer cells transformed by the carcinogen 7,12-dimethylbenz[a]anthracene. J. Nutr. 2005, 135, 2978S–2986S. [Google Scholar] [CrossRef]
- Belguise, K.; Guo, S.; Sonenshein, G.E. Activation of FOXO3a by the green tea polyphenol epigallocatechin-3-gallate induces estrogen receptor α expression reversing invasive phenotype of breast cancer cells. Cancer Res. 2007, 67, 5763–5770. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhao, D.; Elliott, S.; Zhao, W.; Curiel, T.; Beckman, B.; Burow, M. Epigallocatechin-3 gallate induces growth inhibition and apoptosis in human breast cancer cells through survivin suppression. Int. J. Oncol. 2007, 31, 705–711. [Google Scholar] [CrossRef] [PubMed]
- De Amicis, F.; Russo, A.; Avena, P.; Santoro, M.; Vivacqua, A.; Bonofiglio, D.; Mauro, L.; Aquila, S.; Tramontano, D.; Fuqua, S.A.; et al. In vitro mechanism for downregulation of ER-α expression by epigallocatechin gallate in ER+/PR+ human breast cancer cells. Mol. Nutr. Food Res. 2013, 57, 840–853. [Google Scholar] [CrossRef] [PubMed]
- Hallman, K.; Aleck, K.; Quigley, M.; Dwyer, B.; Lloyd, V.; Szmyd, M.; Dinda, S. The regulation of steroid receptors by epigallocatechin-3-gallate in breast cancer cells. Breast Cancer 2017, 9, 365–373. [Google Scholar] [CrossRef]
- Ranzato, E.; Magnelli, V.; Martinotti, S.; Waheed, Z.; Cain, S.M.; Snutch, T.P.; Marchetti, C.; Burlando, B. Epigallocatechin-3-gallate elicits Ca2+ spike in MCF-7 breast cancer cells: Essential role of Cav3.2 channels. Cell Calcium 2014, 56, 285–295. [Google Scholar] [CrossRef]
- Tu, S.H.; Ku, C.Y.; Ho, C.T.; Chen, C.S.; Huang, C.S.; Lee, C.H.; Chen, L.C.; Pan, M.H.; Chang, H.W.; Chang, C.H.; et al. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits nicotine- and estrogen-induced α9-nicotinic acetylcholine receptor upregulation in human breast cancer cells. Mol. Nutr. Food Res. 2011, 55, 455–466. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, G.; Fan, B.; Zhou, Y.; Zhou, X.; Wei, L.; Zhang, J. Green tea (−)-epigallocatechin-3-gallate down-regulates VASP expression and inhibits breast cancer cell migration and invasion by attenuating Rac1 activity. Eur. J. Pharmacol. 2009, 606, 172–179. [Google Scholar] [CrossRef]
- Pan, M.H.; Lin, C.C.; Lin, J.K.; Chen, W.J. Tea polyphenol (−)-epigallocatechin 3-gallate suppresses heregulin-β1-induced fatty acid synthase expression in human breast cancer cells by inhibiting phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase cascade signaling. J. Agric. Food Chem. 2007, 55, 5030–5037. [Google Scholar] [CrossRef]
- Sen, T.; Chatterjee, A. Epigallocatechin-3-gallate (EGCG) downregulates EGF-induced MMP-9 in breast cancer cells: Involvement of integrin receptor α5β1 in the process. Eur. J. Nutr. 2011, 50, 465–478. [Google Scholar] [CrossRef]
- Sen, T.; Dutta, A.; Chatterjee, A. Epigallocatechin-3-gallate (EGCG) downregulates gelatinase-B (MMP-9) by involvement of FAK/ERK/NFκB and AP-1 in the human breast cancer cell line MDA-MB-231. Anticancer Drugs 2010, 21, 632–644. [Google Scholar] [CrossRef]
- Moreira, L.; Araújo, I.; Costa, T.; Correia-Branco, A.; Faria, A.; Martel, F.; Keating, E. Quercetin and epigallocatechin gallate inhibit glucose uptake and metabolism by breast cancer cells by an estrogen receptor-independent mechanism. Exp. Cell Res. 2013, 319, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Hong, O.Y.; Noh, E.M.; Jang, H.Y.; Lee, Y.R.; Lee, B.K.; Jung, S.H.; Kim, J.S.; Youn, H.J. Epigallocatechin gallate inhibits the growth of MDA-MB-231 breast cancer cells via inactivation of the β-catenin signaling pathway. Oncol. Lett. 2017, 14, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhu, W.; Jia, L.; Sun, X.; Chen, G.; Zhao, X.; Li, X.; Meng, X.; Kong, L.; Xing, L.; et al. Phase I study of topical epigallocatechin-3-gallate (EGCG) in patients with breast cancer receiving adjuvant radiotherapy. Br. J. Radiol. 2016, 89, 20150665. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhu, W.; Zhao, X.; Li, X.; Zhou, Z.; Zheng, M.; Meng, X.; Kong, L.; Zhang, S.; He, D.; et al. Efficacy of epigallocatechin-3-gallate in preventing dermatitis in patients with breast cancer receiving postoperative radiotherapy. JAMA Dermatol. 2022, 158, 779. [Google Scholar] [CrossRef]
- Zhu, W.; Jia, L.; Chen, G.; Zhao, H.; Sun, X.; Meng, X.; Zhao, X.; Xing, L.; Yu, J.; Zheng, M. Epigallocatechin-3-gallate ameliorates radiation-induced acute skin damage in breast cancer patients undergoing adjuvant radiotherapy. Oncotarget 2016, 7, 48607–48613. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Wu, J.M. Suppression of cell proliferation and gene expression by combinatorial synergy of EGCG, resveratrol and γ-tocotrienol in estrogen receptor-positive MCF-7 breast cancer cells. Int. J. Oncol. 2008, 33, 851–859. [Google Scholar] [CrossRef]
- Eddy, S.F.; Kane, S.E.; Sonenshein, G.E. Trastuzumab-resistant HER2-driven breast cancer cells are sensitive to epigallocatechin-3 gallate. Cancer Res. 2007, 67, 9018–9023. [Google Scholar] [CrossRef]
- Wang, S.; Chen, R.; Zhong, Z.; Shi, Z.; Chen, M.; Wang, Y. Epigallocatechin-3-gallate potentiates the effect of curcumin in inducing growth inhibition and apoptosis of resistant breast cancer cells. Am. J. Chin. Med. 2014, 42, 1279–1300. [Google Scholar] [CrossRef]
- Zeng, L.; Holly, J.M.P.; Perks, C.M. Effects of physiological levels of the green tea extract epigallocatechin-3-gallate on breast cancer cells. Front. Endocrinol. 2014, 5, 61. [Google Scholar] [CrossRef]
- Scandlyn, M.J.; Stuart, E.C.; Somers-Edgar, T.J.; Menzies, A.R.; Rosengren, R.J. A new role for tamoxifen in oestrogen receptor-negative breast cancer when it is combined with epigallocatechin gallate. Br. J. Cancer 2008, 99, 1056–1063. [Google Scholar] [CrossRef]
- Chisholm, K.; Bray, B.J.; Rosengren, R.J. Tamoxifen and epigallocatechin gallate are synergistically cytotoxic to MDA-MB-231 human breast cancer cells. Anticancer Drugs 2004, 15, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Steed, K.L.; Jordan, H.R.; Tollefsbol, T.O. SAHA and EGCG promote apoptosis in triple-negative breast cancer cells, possibly through the modulation of CIAP2. Anticancer Res. 2020, 40, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.A.; Jordan, H.R.; Tollefsbol, T.O. Effects of SAHA and EGCG on growth potentiation of triple-negative breast cancer cells. Cancers 2018, 11, 23. [Google Scholar] [CrossRef]
- Somers-Edgar, T.J.; Scandlyn, M.J.; Stuart, E.C.; Le Nedelec, M.J.; Valentine, S.P.; Rosengren, R.J. The combination of epigallocatechin gallate and curcumin suppresses ERα-breast cancer cell growth in vitro and in vivo. Int. J. Cancer 2008, 122, 1966–1971. [Google Scholar] [CrossRef]
- Bimonte, S.; Cascella, M.; Barbieri, A.; Arra, C.; Cuomo, A. Shining a light on the effects of the combination of (–)-epigallocatechin-3-gallate and tapentadol on the growth of human triple-negative breast cancer cells. Vivo 2019, 33, 1463–1468. [Google Scholar] [CrossRef]
- Luo, T.; Wang, J.; Yin, Y.; Hua, H.; Jing, J.; Sun, X.; Li, M.; Zhang, Y.; Jiang, Y. (−)-Epigallocatechin gallate sensitizes breast cancer cells to paclitaxel in a murine model of breast carcinoma. Breast Cancer Res. 2010, 12, R8. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; Tessaro, L.; Lima, A.K.O.; Velloso, I.P.S.; Conte-Junior, C.A. Recent progress in nanotechnology improving the therapeutic potential of polyphenols for cancer. Nutrients 2023, 15, 3136. [Google Scholar] [CrossRef]
- Zeng, L.; Yan, J.; Luo, L.; Ma, M.; Zhu, H. Preparation and characterization of (−)-epigallocatechin-3-gallate (EGCG)-loaded nanoparticles and their inhibitory effects on human breast cancer MCF-7 cells. Sci. Rep. 2017, 7, 45521. [Google Scholar] [CrossRef]
- Das, A.; Haque, I.; Ray, P.; Ghosh, A.; Dutta, D.; Quadir, M.; De, A.; Gunewardena, S.; Chatterjee, I.; Banerjee, S.; et al. CCN5 activation by free or encapsulated EGCG is required to render triple-negative breast cancer cell viability and tumor progression. Pharmacol. Res. Perspect. 2021, 9, e00753. [Google Scholar] [CrossRef]
- Wang, X.; He, J.; Jiang, S.; Gao, Y.; Zhang, L.K.; Yin, L.; You, R.; Guan, Y.Q. Multi-ligand modified PC@DOX-PA/EGCG micelles effectively inhibit the growth of ER+, PR+ or HER2+ breast cancer. J. Mater. Chem. B 2022, 10, 418–429. [Google Scholar] [CrossRef]
- Ramadass, S.K.; Anantharaman, N.V.; Subramanian, S.; Sivasubramanian, S.; Madhan, B. Paclitaxel/epigallocatechin gallate coloaded liposome: A synergistic delivery to control the invasiveness of MDA-MB-231 breast cancer cells. Colloids Surf. B Biointerfaces 2015, 125, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Mony, U.; Vijaykumar, D.K.; Koyakutty, M.; Paul-Prasanth, B.; Menon, D. Sequential release of epigallocatechin gallate and paclitaxel from PLGA-casein core/shell nanoparticles sensitizes drug-resistant breast cancer cells. Nanomedicine 2015, 11, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- de Pace, R.C.C.; Liu, X.; Sun, M.; Nie, S.; Zhang, J.; Cai, Q.; Gao, W.; Pan, X.; Fan, Z.; Wang, S. Anticancer activities of (−)-epigallocatechin-3-gallate encapsulated nanoliposomes in MCF7 breast cancer cells. J. Liposome Res. 2013, 23, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Kulhari, H.; Pooja, D.; Gudem, S.; Bhargava, S.; Shukla, R.; Sistla, R. Encapsulation of biophenolic phytochemical EGCG within lipid nanoparticles enhances its stability and cytotoxicity against cancer. Chem. Phys. Lipids 2016, 198, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Pooja, D.; Kulhari, H.; Gudem, S.; Ravuri, H.G.; Bhargava, S.; Ramakrishna, S. Bombesin conjugated solid lipid nanoparticles for improved delivery of epigallocatechin gallate for breast cancer treatment. Chem. Phys. Lipids 2019, 224, 104770. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Lu, J.L.; Liang, Y.R.; Li, Q.S. Suppressive effects of EGCG on cervical cancer. Molecules 2018, 23, 2334. [Google Scholar] [CrossRef]
- Aarthy, M.; Panwar, U.; Singh, S.K. Structural dynamic studies on identification of EGCG analogues for the inhibition of human papillomavirus E7. Sci. Rep. 2020, 10, 8661. [Google Scholar] [CrossRef]
- Grandi, G.; Botticelli, L.; Fraia, P.D.; Babalini, C.; Masini, M.; Unfer, V. The association of four natural molecules—EGCG, folic acid, vitamin B12, and HA—To counteract HPV cervical lesions: A case report. J. Pers. Med. 2023, 13, 567. [Google Scholar] [CrossRef]
- Calcagno, M.; Incocciati, B.; Di Fraia, L.; Unfer, V. Counteracting HPV cervical and anal infection through dietary supplementation of EGCG, folic acid, vitamin B12 and hyaluronic acid: Clinical case reports. J. Clin. Med. 2024, 13, 3597. [Google Scholar] [CrossRef]
- Miyoshi, N.; Tanabe, H.; Suzuki, T.; Saeki, K.; Hara, Y. Applications of a standardized green tea catechin preparation for viral warts and human papilloma virus-related and unrelated cancers. Molecules 2020, 25, 2588. [Google Scholar] [CrossRef]
- Chu, Y.W.; Liu, S.T.; Yang, Y.L.; Huang, S.M.; Wang, W.M. The cytotoxic mechanism of epigallocatechin gallate on proliferative HaCaT keratinocytes. J. Biomed. Sci. 2017, 24, 55. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.K.W.; Kehoe, S.T.; Woodman, C.B.J.; Dawson, C.W. The major constituent of green tea, epigallocatechin-3-gallate (EGCG), inhibits the growth of HPV18-infected keratinocytes by stimulating proteasomal turnover of the E6 and E7 oncoproteins. Pathogens 2021, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Muthusami, S.; Prabakaran, D.S.; An, Z.; Yu, J.R.; Park, W.Y. EGCG suppresses Fused Toes Homolog protein through p53 in cervical cancer cells. Mol. Biol. Rep. 2013, 40, 5587–5596. [Google Scholar] [CrossRef]
- Asif Siddiqui, F.; Naim, M.; Islam, N. Apoptotic effect of green tea polyphenol (EGCG) on cervical carcinoma cells. Diagn. Cytopathol. 2011, 39, 482–488. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Nag, D.; Ganguli, A.; Das, A.; Ghosh Dastidar, D.; Chakrabarti, G. Theaflavin and epigallocatechin-3-gallate synergistically induce apoptosis through inhibition of PI3K/Akt signaling upon depolymerizing microtubules in HeLa cells. J. Cell. Biochem. 2019, 120, 5987–6003. [Google Scholar] [CrossRef]
- Pal, D.; Sur, S.; Roy, R.; Mandal, S.; Kumar Panda, C. Epigallocatechin gallate in combination with eugenol or amarogentin shows synergistic chemotherapeutic potential in cervical cancer cell line. J. Cell. Physiol. 2019, 234, 825–836. [Google Scholar] [CrossRef]
- McDonnell, A.M.; Pyles, H.M.; Diaz-Cruz, E.S.; Barton, C.E. Enoxacin and epigallocatechin gallate (EGCG) act synergistically to inhibit the growth of cervical cancer cells in culture. Molecules 2019, 24, 1580. [Google Scholar] [CrossRef]
- Kilic, U.; Sahin, K.; Tuzcu, M.; Basak, N.; Orhan, C.; Elibol-Can, B.; Kilic, E.; Sahin, F.; Kucuk, O. Enhancement of cisplatin sensitivity in human cervical cancer: Epigallocatechin-3-gallate. Front. Nutr. 2015, 1, 28. [Google Scholar] [CrossRef]
- Yokoyama, M.; Noguchi, M.; Nakao, Y.; Ysunaga, M.; Yamasaki, F.; Iwasaka, T. Antiproliferative effects of the major tea polyphenol, (−)-epigallocatechin gallate and retinoic acid in cervical adenocarcinoma. Gynecol. Oncol. 2008, 108, 326–331. [Google Scholar] [CrossRef]
- Raish, M.; Husain, S.Z.A.; Bae, S.M.; Han, S.J.; Park, C.H.; Shin, J.C.; Ahn, W.S. Photodynamic therapy in combination with green tea polyphenol EGCG enhances antitumor efficacy in human papillomavirus 16 (E6/E7) immortalized tumor cells. J. Appl. Res. 2010, 10, 58–67. [Google Scholar]
- Oladimeji, O.; Akinyelu, J.; Singh, M. Co-polymer functionalised gold nanoparticles show efficient mitochondrial targeted drug delivery in cervical carcinoma cells. J. Biomed. Nanotechnol. 2020, 16, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, M.; Ciebiera, M.; Nowicka, G.; Łoziński, T.; Ali, M.; Al-Hendy, A. Epigallocatechin gallate for the treatment of benign and malignant gynecological diseases—Focus on epigenetic mechanisms. Nutrients 2024, 16, 559. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.M.; Wu, A.H. Green and black tea in relation to gynecologic cancers. Mol. Nutr. Food Res. 2011, 55, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Aussem, A.; Ludwig, K. The potential for reducing Lynch syndrome cancer risk with nutritional Nrf2 activators. Nutr. Cancer 2021, 73, 404–419. [Google Scholar] [CrossRef]
- Man, G.C.W.; Wang, J.; Song, Y.; Wong, J.H.; Zhao, Y.; Lau, T.S.; Leung, K.T.; Chan, T.H.; Wang, H.; Kwong, J.; et al. Therapeutic potential of a novel prodrug of green tea extract in induction of apoptosis via ERK/JNK and Akt signaling pathway in human endometrial cancer. BMC Cancer 2020, 20, 964. [Google Scholar] [CrossRef]
- Wang, J.; Man, G.C.W.; Chan, T.H.; Kwong, J.; Wang, C.C. A prodrug of green tea polyphenol (–)-epigallocatechin-3-gallate (Pro-EGCG) serves as a novel angiogenesis inhibitor in endometrial cancer. Cancer Lett. 2018, 412, 10–20. [Google Scholar] [CrossRef]
- Wang, C.C.; Xu, H.; Man, G.C.W.; Zhang, T.; Chu, K.O.; Chu, C.Y.; Cheng, J.T.Y.; Li, G.; He, Y.X.; Qin, L.; et al. Prodrug of green tea epigallocatechin-3-gallate (Pro-EGCG) as a potent anti-angiogenesis agent for endometriosis in mice. Angiogenesis 2013, 16, 59–69. [Google Scholar] [CrossRef]
- Bimonte, S.; Cascella, M. The potential roles of epigallocatechin-3-gallate in the treatment of ovarian cancer: Current state of knowledge. Drug Des. Dev. Ther. 2020, 14, 4245–4250. [Google Scholar] [CrossRef]
- Rodriguez Torres, S.; Gresseau, L.; Benhamida, M.; Fernandez-Marrero, Y.; Annabi, B. Epigallocatechin-3-gallate prevents the acquisition of a cancer stem cell phenotype in ovarian cancer tumorspheres through the inhibition of Src/JAK/STAT3 signaling. Biomedicines 2023, 11, 1000. [Google Scholar] [CrossRef]
- Trudel, D.; Labbé, D.P.; Araya-Farias, M.; Doyen, A.; Bazinet, L.; Duchesne, T.; Plante, M.; Grégoire, J.; Renaud, M.C.; Bachvarov, D.; et al. A two-stage, single-arm, phase II study of EGCG-enriched green tea drink as a maintenance therapy in women with advanced stage ovarian cancer. Gynecol. Oncol. 2013, 131, 357–361. [Google Scholar] [CrossRef]
- Kiselev, V.I.; Ashrafyan, L.A.; Muyzhnek, E.L.; Gerfanova, E.V.; Antonova, I.B.; Aleshikova, O.I.; Sarkar, F.H. A new promising way of maintenance therapy in advanced ovarian cancer: A comparative clinical study. BMC Cancer 2018, 18, 904. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.M.; Soprano, K.J.; Weinstein, K.; Fong, D. Epigallocatechin-3-gallate delivers hydrogen peroxide to induce death of ovarian cancer cells and enhances their cisplatin susceptibility. J. Cell. Physiol. 2006, 207, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Yang, J.; Shen, L.; Chen, X. Inhibitory effect of epigallocatechin gallate on ovarian cancer cell proliferation associated with aquaporin 5 expression. Arch. Gynecol. Obstet. 2012, 285, 459–467. [Google Scholar] [CrossRef]
- Chen, H.; Landen, C.N.; Li, Y.; Alvarez, R.D.; Tollefsbol, T.O. Epigallocatechin gallate and sulforaphane combination treatment induce apoptosis in paclitaxel-resistant ovarian cancer cells through hTERT and Bcl-2 down-regulation. Exp. Cell Res. 2013, 319, 697–706. [Google Scholar] [CrossRef]
- Chen, H.; Landen, C.N.; Li, Y.; Alvarez, R.D.; Tollefsbol, T.O. Enhancement of cisplatin-mediated apoptosis in ovarian cancer cells through potentiating G2/M arrest and P21 upregulation by combinatorial epigallocatechin gallate and sulforaphane. J. Oncol. 2013, 2013, 872957. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, P.; Wang, P.; Yang, C.S.; Wang, X.; Feng, Q. EGCG enhances cisplatin sensitivity by regulating expression of the copper and cisplatin influx transporter CTR1 in ovary cancer. PLoS ONE 2015, 10, e0125402. [Google Scholar] [CrossRef]
- Mazumder, M.E.H.; Beale, P.; Chan, C.; Yu, J.Q.; Huq, F. Epigallocatechin gallate acts synergistically in combination with cisplatin and designed trans-palladiums in ovarian cancer cells. Anticancer Res. 2012, 32, 4851–4860. [Google Scholar]
- Yunos, N.M.; Beale, P.; Yu, J.Q.; Huq, F. Synergism from sequenced combinations of curcumin and epigallocatechin-3-gallate with cisplatin in the killing of human ovarian cancer cells. Anticancer Res. 2011, 31, 1131–1140. [Google Scholar]
- Sharma, T.; Singh, D.; Mahapatra, A.; Mahapatra, P.; Sahoo, S.; Sahoo, S.K. Advancements in clinical translation of flavonoid nanoparticles for cancer treatment. OpenNano 2022, 8, 100074. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Semin. Cancer Biol. 2021, 69, 200–211. [Google Scholar] [CrossRef]
- Dobrzynska, M.; Napierala, M.; Florek, E. Flavonoid nanoparticles: A promising approach for cancer therapy. Biomolecules 2020, 10, 1268. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yang, Y.; Wang, C.; Liu, M.; Wang, J.; Qiao, S.; Jiang, P.; Sun, C.; Jiang, S. Epigallocatechin-3-gallate at the nanoscale: A new strategy for cancer treatment. Pharm. Biol. 2024, 62, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jiang, Z.; Ma, L.; Huang, Q. Advances in nanodelivery of green tea catechins to enhance the anticancer activity. Molecules 2021, 26, 3301. [Google Scholar] [CrossRef] [PubMed]
- Granja, A.; Pinheiro, M.; Reis, S. Epigallocatechin gallate nanodelivery systems for cancer therapy. Nutrients 2016, 8, 307. [Google Scholar] [CrossRef]
- Alizadeh, L.; Alizadeh, E.; Zarebkohan, A.; Ahmadi, E.; Rahmati-Yamchi, M.; Salehi, R. AS1411 aptamer-functionalized chitosan-silica nanoparticles for targeted delivery of epigallocatechin gallate to the SKOV-3 ovarian cancer cell lines. J. Nanopart. Res. 2020, 22, 5. [Google Scholar] [CrossRef]
- Chuan, D.; Mu, M.; Hou, H.; Zhao, N.; Li, J.; Tong, A.; Zou, B.; Chen, H.; Han, B.; Guo, G. Folic acid-functionalized tea polyphenol as a tumor-targeting nanodrug delivery system. Mater. Des. 2021, 206, 109805. [Google Scholar] [CrossRef]
- Bae, K.H.; Tan, S.; Yamashita, A.; Ang, W.X.; Gao, S.J.; Wang, S.; Chung, J.E.; Kurisawa, M. Hyaluronic acid-green tea catechin micellar nanocomplexes: Fail-safe cisplatin nanomedicine for the treatment of ovarian cancer without off-target toxicity. Biomaterials 2017, 148, 41–53. [Google Scholar] [CrossRef]
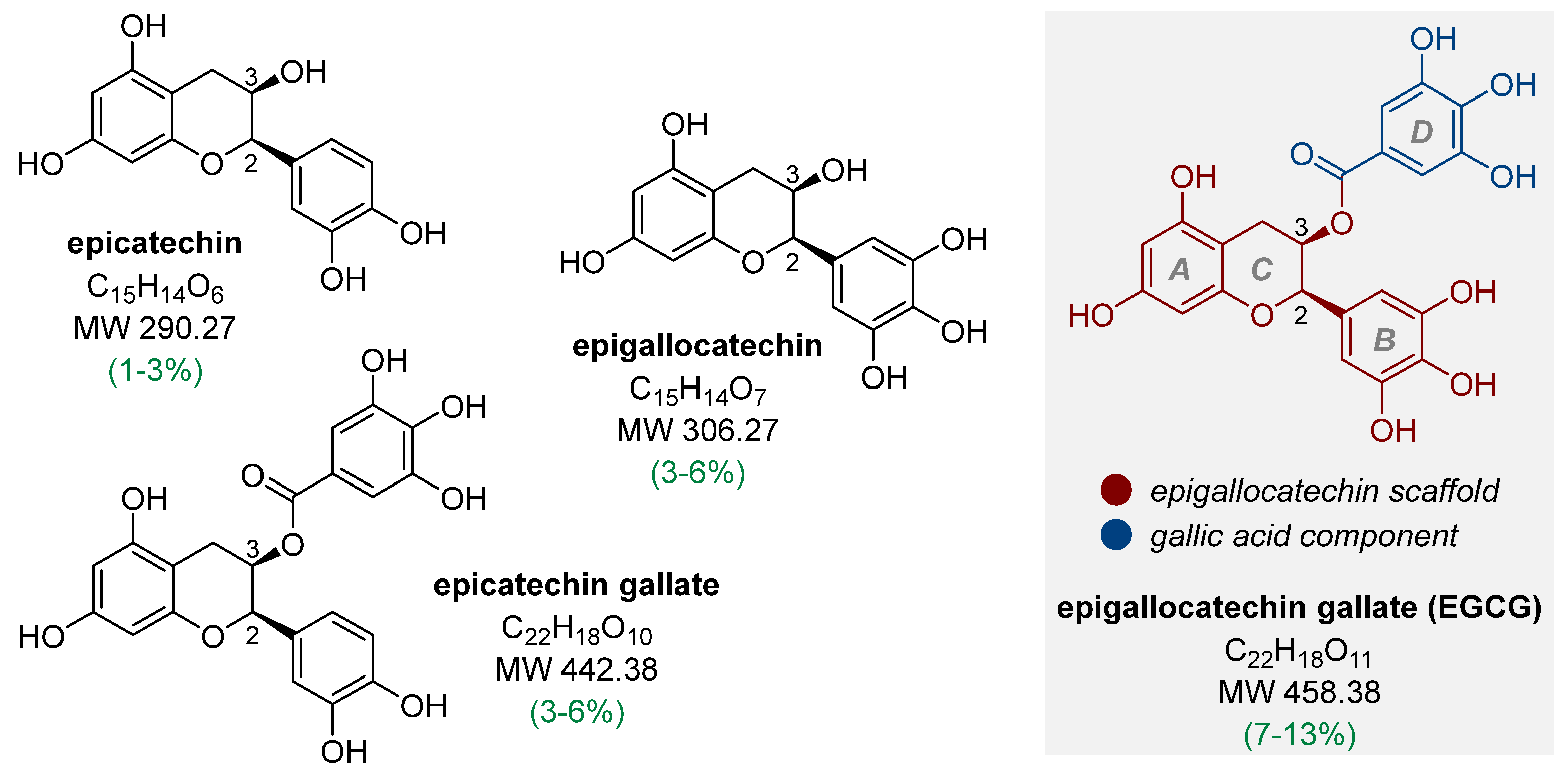
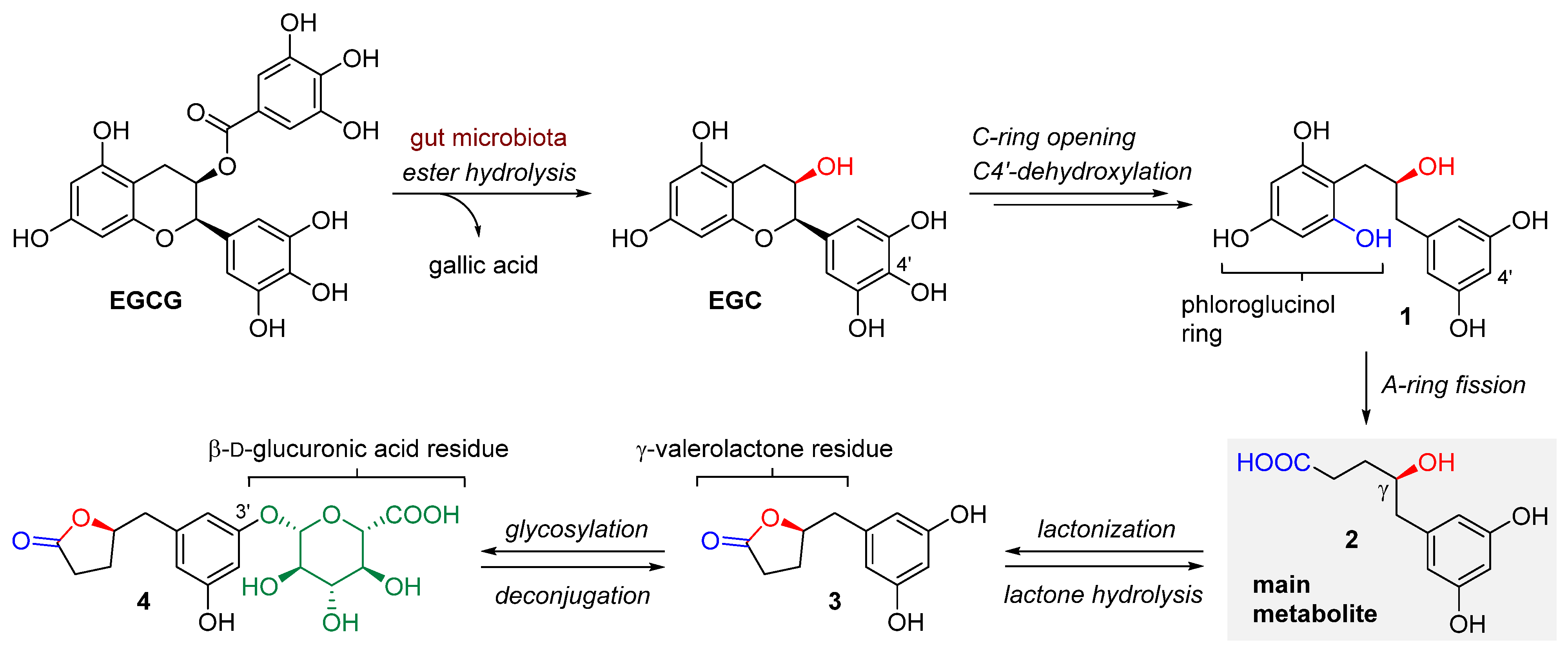
| Cancer Type | Cancer Cell Lines | In Vitro EGCG Concentration | In Vivo Tests | Anticancer Effects | Ref. |
|---|---|---|---|---|---|
| Breast cancer | MCF-7 | 2, 4, 8, 16, 32, 64, 128 μM | Inhibition of proliferation, promotion of apoptosis | [56] | |
| MCF-7 | 10–100 μg/mL | Inhibition of reproductive potential and colony formation, reduction in cell viability, promotion of apoptosis | [57] | ||
| T47D | 10–80 μM | Reduction in cell viability, modulation of gene expression | [58] | ||
| MCF-7, MDA-MB 231 | 20 μM | Inhibition of the progression of cancer | [59] | ||
| MCF-7 | 0.5–20 μg/mL | CB-17 SCID mice (6–8 weeks old); EGCG dose: 100 mg/kg, oral gavage | Inhibition of cell viability, induction of apoptosis, disruption of the cell cycle in the G2/M phase, inhibition of tumor growth | [60] | |
| MCF-7 | 20 μM | Reduction in MMP-2 activity | [61] | ||
| 4T1 | 10–320 μM | BALB/c mice (4 weeks old); EGCG dose: 5, 10, 20 mg/kg, intraperitoneal injections | Reduction in cell growth, induction of apoptosis, promotion of mitochondrial depolarization, inhibition of glucose metabolism, reduction in tumor mass | [62] | |
| E0771, MCF-7, MDA-MB-231 | 10, 50 μg/mL | C57BL/6 mice (7 weeks old); EGCG dose: 50–100 mg/kg, drinking water | Impact on cancer cells and tumor blood vessels, reduction in tumor mass | [63] | |
| MCF-7 | 25, 50, 100 mg/L | Reduction in cell proliferation and growth | [64] | ||
| SUM-149, SUM-190 | 5, 10, 20, 40, 60, 80, 160 µg/mL | NOD/SCID mice (6 weeks old); EGCG dose: 16.5 mg/kg, intraperitoneal injections | Reduction in cell growth, invasiveness, and survival, reduction in the growth of existing tumors | [65] | |
| MDA-MB-231 | 1–200 μg/mL | NCr-nu/nu mice (5 weeks old); EGCG dose: 1 mg in 100 μL of autoclaved distilled water | Inhibition of cell invasiveness, induction of apoptosis, delay in the occurrence of cancer, reduction in tumor mass | [66] | |
| Hs578T | 10 μM | Inhibition of proliferation, migration, and invasiveness | [67] | ||
| MDA-MB-231 | 0.5 μg/mL | Inhibition of invasion | [68] | ||
| MDA-MB-231 | 50, 80 μg/mL | Inhibition of invasiveness, induction of apoptosis | [69] | ||
| MDA-MB-231 | 0.07–20 μM | Inhibition of Met signaling, blocking invasiveness | [70] | ||
| Cervical cancer | C33A, CaSki, HeLa, SiHa | 10, 20, 40, 60, 80, 100 µg/mL | Inhibition of cell growth, probably due to regulation of miRNA expression | [71] | |
| HeLa | 10 μM | Inhibition of proliferation, adhesion, spread and invasiveness | [72] | ||
| HeLa, SiHa | 20, 40, 60, 80, 100 μM | Inhibition of cell viability | [73] | ||
| CaSki, HeLa | 10, 25, 50, 100 µM | Cell cycle arrest, apoptosis induction | [74] | ||
| HeLa | 50 µM | Modulation of epigenetic factors and signaling pathways (MAPK, PI3K, Wnt) | [75] | ||
| HeLa | 1–100 μM | Inhibition of cell growth, invasion, and migration | [76] | ||
| OMC-4, TMCC-1 | 50–100 μM | Induction of apoptosis, inhibition of telomerase activity, dysregulation of the cell cycle | [77] | ||
| HeLa, ME180, SiHa, TMCC-1 | 50, 100 μM | Induction of apoptosis, inhibition of telomerase activity | [78] | ||
| C33A, ME180 | 50 μM | Reduction in matrix abundance and cell migration | [79] | ||
| Endometrial cancer | Ishikawa | 50, 75, 100, 125, 150 μM | Inhibition of cell proliferation, induction of apoptosis, modulation of pro- and anti-apoptotic proteins, generation of ROS | [80] | |
| Ishikawa, RL952 | 100 μM | Reduction in VEGF | [81] | ||
| Ovarian cancer | A2780/DDP, SKOV3/DDP | 0–40 μM | BALB/c mice (5 weeks old); EGCG dose: 50 mg/kg, intraperitoneal injections | Inhibition of cell proliferation and motility, induction of apoptosis, inhibition of tumor growth | [82] |
| OVCAR-3, PA-1, SKOV-3 | 10–100 μM | Inhibition of cell growth, cell cycle arrest, induction of apoptosis | [83] | ||
| OVCAR-3, PA-1, SKOV-3 | 10–100 μM | Inhibition of cell growth, cell cycle arrest, induction of apoptosis | [84] | ||
| OVCAR-3 | 0–200 μM | Inhibition of cell proliferation and migration | [85] | ||
| CAOV-3, OVCAR-3, SKOV3 | 5, 10, 20, 40, 80 µg/mL | BALB/c mice (4–5 weeks old); EGCG dose: 10, 30, 50 mg/kg | Inhibition of cell proliferation and induction of apoptosis; increased inhibition of tumor growth compared to paclitaxel (50 mg/kg EGCG vs. 5 mg/kg paclitaxel) | [86] | |
| SKOV-3 | 20, 30, 40, 50 µg/mL | Inhibition of cell proliferation and viability, DNA damage, induction of apoptosis | [87] | ||
| A2780, SKOV3 | 0–20 nM | BALB/c mice; EGCG dose: 200 mg/kg, intratumoral injection | Inhibition of cell migration and survival, acceleration of apoptosis, inhibition of tumor growth | [88] | |
| HEY, OVCA 433 | 10, 20, 40 μM | Athymic (nu+/nu+) mice (4–6 weeks old); EGCG source: green tea leaves (12.4 g/L), drinking fluid | Inhibition of cell growth, induction of apoptosis, inhibition of angiogenesis and invasiveness; reduction in tumor growth | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markowska, A.; Antoszczak, M.; Markowska, J.; Huczyński, A. Role of Epigallocatechin Gallate in Selected Malignant Neoplasms in Women. Nutrients 2025, 17, 212. https://doi.org/10.3390/nu17020212
Markowska A, Antoszczak M, Markowska J, Huczyński A. Role of Epigallocatechin Gallate in Selected Malignant Neoplasms in Women. Nutrients. 2025; 17(2):212. https://doi.org/10.3390/nu17020212
Chicago/Turabian StyleMarkowska, Anna, Michał Antoszczak, Janina Markowska, and Adam Huczyński. 2025. "Role of Epigallocatechin Gallate in Selected Malignant Neoplasms in Women" Nutrients 17, no. 2: 212. https://doi.org/10.3390/nu17020212
APA StyleMarkowska, A., Antoszczak, M., Markowska, J., & Huczyński, A. (2025). Role of Epigallocatechin Gallate in Selected Malignant Neoplasms in Women. Nutrients, 17(2), 212. https://doi.org/10.3390/nu17020212







