Postprandial Aminoacidemia Following the Ingestion of Alternative and Sustainable Proteins in Humans: A Narrative Review
Highlights
- Currently, the production and consumption of proteins derived from alternative sources (plant, fungi, insect, and algae) are increasing; therefore, investigating the protein quality of these protein sources is quintessential.
- Amount of protein, amino acid profile, and the rate of digestion of protein sources mainly influences the postprandial aminoacid kinetic parameters.
- Analysis of the postprandial plasma amino acid responses after ingesting alternative proteins would serves as a springboard to assess their muscle anabolic potential in both health and disease.
- Future work will need to focus on investigating the postprandial AA kinetics and muscle anabolic effects after ingesting protein blends, specific AA fortified proteins, whole foods, and mixed meals derived from alternative protein sources on different age groups, genders, populations, and health conditions.
Abstract
1. Introduction
2. Methods
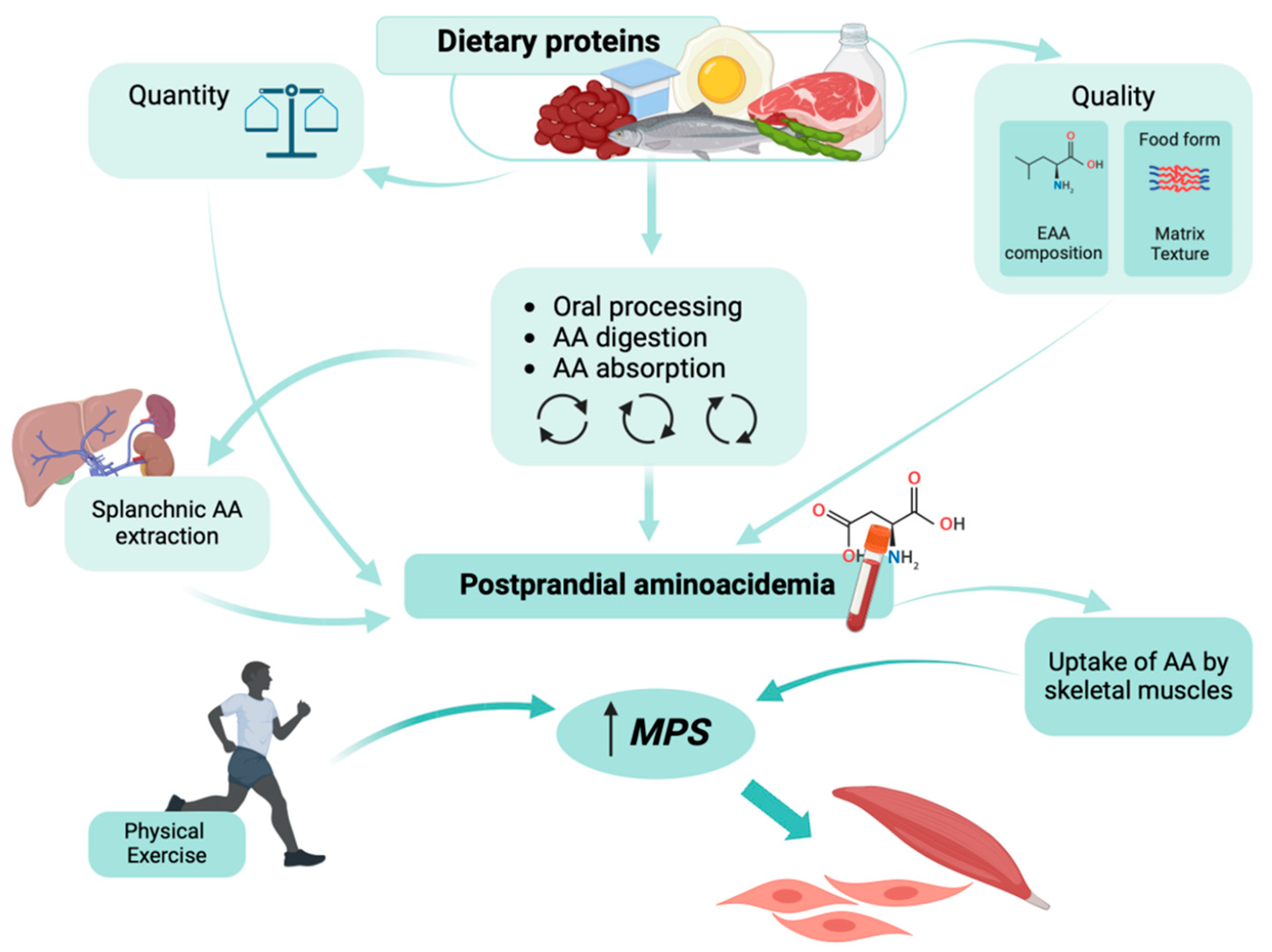
3. Protein Ingestion and Muscle Anabolism
4. Postprandial Aminoacidemia Following the Ingestion of Sustainable Protein Sources
4.1. Postprandial Aminoacidemia Following the Ingestion of Plant-Derived Proteins
| Alternative Protein Source | Name of Protein | Study Participant | Duration | Intervention Products in Protocol | Postprandial Aminoacidemia |
|---|---|---|---|---|---|
| Plant | Soy protein [30] | 18 men; 22.8 ± 3.9 years | 3 h | 21.4 g of whey protein hydrolysate , 22.2 g of soy protein isolate, and 21.9 g of casein |
|
| Soy protein [85] | 30 men; 71 ± 5 years | 4 h | 20 g or 40 g of soy protein isolate, and 20 g or 40 g of whey protein isolate |
| |
| Potato protein [89] | 24 Men; 24 ± 4 years | 5 h | 30 g of potato protein concentrate and 30 g of milk protein concentrate |
| |
| Plant-derived protein blend(wheat, corn, and pea) [92] | 24 males; 24 ± 4 years | 5 h | 30 g of plant-derived protein blend, and 30 g milk protein |
| |
| Fungi | Mycoprotein [96] | 20 men; 22 ± 1 years | 4 h | 70 g of mycoprotein, and 31 g of milk protein. Both proteins contains equal amounts of leucine (2.5 g) |
|
| Mycoprotein [97] | 24 male; 21 ± 2 years | 4 h | 70 g of mycoprotein whole food matrix (MYC), and 38.2 g of a protein concentrate obtained from mycoprotein. Both proteins contain equal amounts of leucine (2.5 g) |
| |
| Blend of mycoprotein and pea protein [98] | 33 men and women; 21 ± 1 years | 4 h | 25g of mycoprotein (MYC), 25 g of pea protein (PEA), 25 g of mycoprotein and pea protein blend (BLEND) |
| |
| Insects | Lesser mealworm-derived protein [99] | 24 men; 23 ± 3 years | 5 h | 30 g of lesser mealworm protein and 30 g of milk protein |
|
| Lesser mealworm protein isolate [100] | 6 men; 18–30 years | 2 h | 30.5 g of lesser mealworm protein isolate, 27.8 g of whey protein isolate, and 28.7 g ofsoy protein isolate |
| |
| Cricket protein powder [101] | 50 samples; 18–30 years | 4 h | Cricket protein powder (0.25 g/kg fat-free mass), whey protein concentrate, and pea protein isolate (0.25 g/kg fat-free mass) |
| |
| Cricket-derived protein [102] | 24 males; 23 ± 4 years | 5 h | 25 g of cricket-derived protein and 25 g of beef-derived protein |
| |
| Algae | Spirulina and chlorella [103] | 36 male: 22 ± 3 years | 4 h | 25 g of spirulina, 25 g of chlorella, and 25 g of mycoprotein (MYC) |
|
4.2. Postprandial Aminoacidemia Following the Ingestion of Fungi-Derived Protein
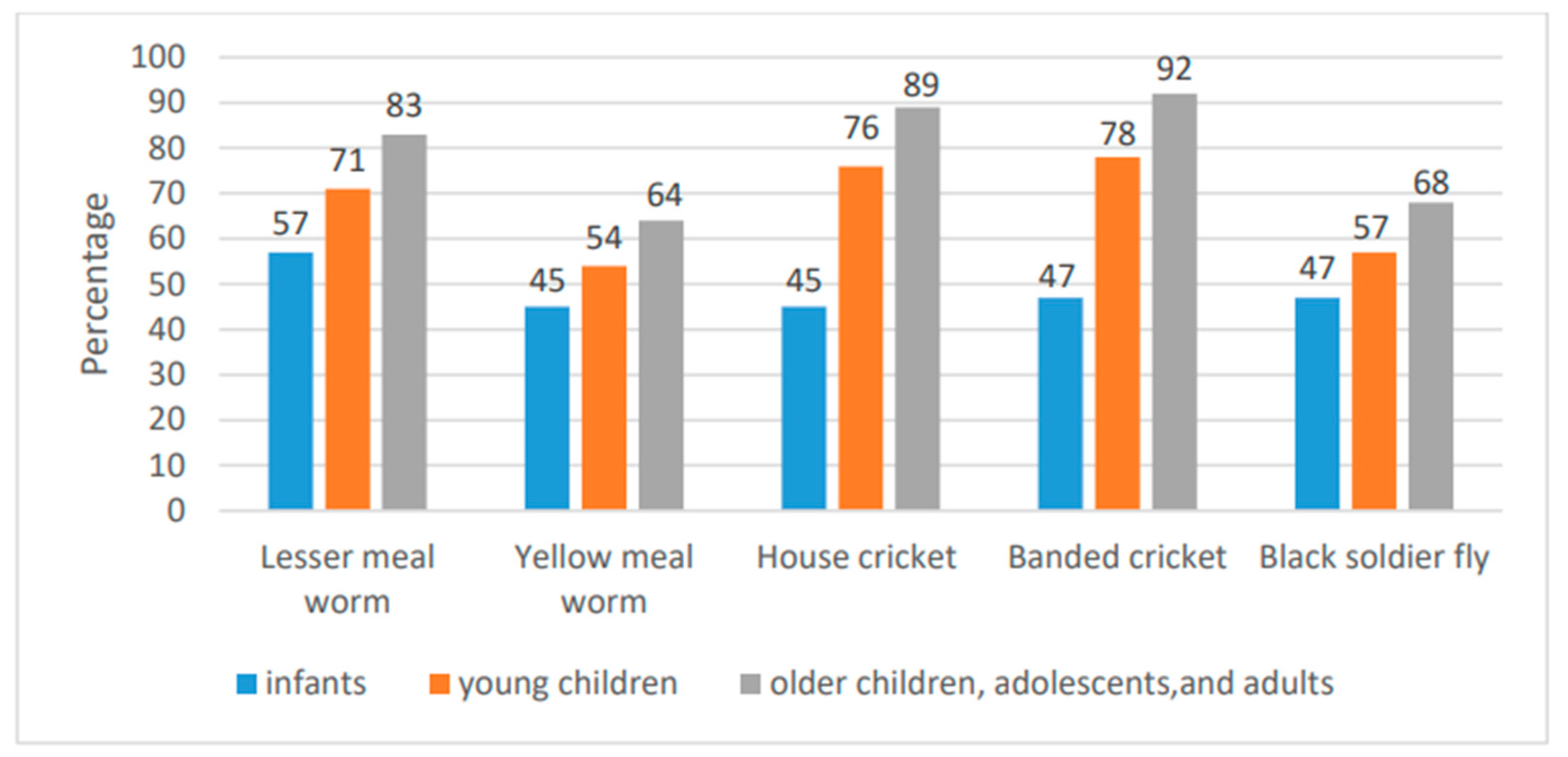
4.3. Postprandial Aminoacidemia Following the Ingestion of Insect-Derived Protein
4.4. Postprandial Aminoacidemia Following the Ingestion of Algae-Derived Protein
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations, Population Division. World Population Prospects 2019: Ten Key Findings; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, S.J.; Campbell, B.M.; Ingram, J.S. Climate change and food systems. Annu. Rev. Environ. Resour. 2012, 37, 195–222. [Google Scholar] [CrossRef]
- Moran, D.; Wall, E. Livestock production and greenhouse gas emissions: Defining the problem and specifying solutions. Anim. Front. 2011, 1, 19–25. [Google Scholar] [CrossRef]
- Aiking, H. Future protein supply. Trends Food Sci. Technol. 2011, 22, 112–120. [Google Scholar] [CrossRef]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.D.; Castel, V.; Rosales, M.; Rosales, M.; de Haan, C. Livestock’s Long Shadow: Environmental Issues and Options; Food & Agriculture Org.: Rome, Italy, 2006. [Google Scholar]
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef] [PubMed]
- Zhong, V.W.; Allen, N.B.; Greenland, P.; Carnethon, M.R.; Ning, H.; Wilkins, J.T.; Lloyd-Jones, D.M.; Van Horn, L. Protein foods from animal sources, incident cardiovascular disease and all-cause mortality: A substitution analysis. Int. J. Epidemiol. 2021, 50, 223–233. [Google Scholar] [CrossRef]
- González, N.; Marquès, M.; Nadal, M.; Domingo, J.L. Meat consumption: Which are the current global risks? A review of recent (2010–2020) evidences. Food Res. Int. 2020, 137, 109341. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, W.A.; Viaene, J. Ethical challenges for livestock production: Meeting consumer concerns about meat safety and animalwelfare. J. Agric. Environ. Ethics 2000, 12, 141–151. [Google Scholar] [CrossRef]
- Burlingame, B.; Dernini, S. Sustainable Diets and Biodiversity; Food & Agriculture Org.: Rome, Italy, 2010. [Google Scholar]
- Gil, M.; Rudy, M.; Duma-Kocan, P.; Stanisławczyk, R.; Krajewska, A.; Dziki, D.; Hassoon, W.H. Sustainability of Alternatives to Animal Protein Sources, a Comprehensive Review. Sustainability 2024, 16, 7701. [Google Scholar] [CrossRef]
- Yimam, M.A.; Andreini, M.; Carnevale, S.; Muscaritoli, M. The role of algae, fungi, and insect-derived proteins and bioactive peptides in preventive and clinical nutrition. Front. Nutr. 2024, 11, 1461621. [Google Scholar] [CrossRef] [PubMed]
- Aiking, H.; de Boer, J. The next protein transition. Trends Food Sci. Technol. 2020, 105, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Alsaffar, A.A. Sustainable diets: The interaction between food industry, nutrition, health and the environment. Food Sci. Technol. Int. 2016, 22, 102–111. [Google Scholar] [CrossRef]
- Godfray, H. Meat: The Future Series-Alternative Proteins; World Economic Forum: Geneva, Switzerland, 2019. [Google Scholar]
- Pyett, S.; de Vet, E.; Trindade, L.; van Zanten, H.; Fresco, L. Chickpeas, Crickets and Chlorella: Our Future Proteins; Wageningen Food & Biobased Research: Wageningen, The Netherlands, 2019. [Google Scholar]
- Boland, M.J.; Rae, A.N.; Vereijken, J.M.; Meuwissen, M.P.; Fischer, A.R.; van Boekel, M.A.; Rutherfurd, S.M.; Gruppen, H.; Moughan, P.J.; Hendriks, W.H. The future supply of animal-derived protein for human consumption. Trends Food Sci. Technol. 2013, 29, 62–73. [Google Scholar] [CrossRef]
- Michel, F.; Hartmann, C.; Siegrist, M. Consumers’ associations, perceptions and acceptance of meat and plant-based meat alternatives. Food Qual. Prefer. 2021, 87, 104063. [Google Scholar] [CrossRef]
- Grover, Z.; Ee, L.C. Protein energy malnutrition. Pediatr. Clin. 2009, 56, 1055–1068. [Google Scholar] [CrossRef]
- Antonio, J. High-protein diets in trained individuals. Res. Sports Med. 2019, 27, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Lonnie, M.; Hooker, E.; Brunstrom, J.M.; Corfe, B.M.; Green, M.A.; Watson, A.W.; Williams, E.A.; Stevenson, E.J.; Penson, S.; Johnstone, A.M. Protein for life: Review of optimal protein intake, sustainable dietary sources and the effect on appetite in ageing adults. Nutrients 2018, 10, 360. [Google Scholar] [CrossRef]
- Lancha, A.H., Jr.; Zanella, R., Jr.; Tanabe, S.G.O.; Andriamihaja, M.; Blachier, F. Dietary protein supplementation in the elderly for limiting muscle mass loss. Amino Acids 2017, 49, 33–47. [Google Scholar] [CrossRef]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition; FAO Food Nutrition Paper; FAO: Rome, Italy, 2013; Volume 92, pp. 1–66. [Google Scholar]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition; FAO Food Nutrition Paper; FAO: Rome, Italy, 2011; Volume 92, pp. 1–66. [Google Scholar]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Boirie, Y.; Gachon, P.; Beaufrere, B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am. J. Clin. Nutr. 1997, 65, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Mittendorfer, B.; Wolf, S.E.; Wolfe, R.R. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am. J. Physiol.-Endocrinol. Metab. 1999, 277, E513–E520. [Google Scholar] [CrossRef] [PubMed]
- Groen, B.B.; Horstman, A.M.; Hamer, H.M.; de Haan, M.; van Kranenburg, J.; Bierau, J.; Poeze, M.; Wodzig, W.K.; Rasmussen, B.B.; van Loon, L.J. Post-Prandial Protein Handling: You Are What You Just Ate. PLoS ONE 2015, 10, e0141582. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.-P.; Maubois, J.-L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Kobayashi, H.; Sheffield-Moore, M.; Mittendorfer, B.; Wolfe, R.R. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am. J. Clin. Nutr. 2003, 78, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Church, D.D.; Hirsch, K.R.; Park, S.; Kim, I.-Y.; Gwin, J.A.; Pasiakos, S.M.; Wolfe, R.R.; Ferrando, A.A. Essential amino acids and protein synthesis: Insights into maximizing the muscle and whole-body response to feeding. Nutrients 2020, 12, 3717. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Patterson, B.W.; Mittendorfer, B. Human muscle protein turnover—Why is it so variable? J. Appl. Physiol. 2011, 110, 480–491. [Google Scholar] [CrossRef]
- Fujita, S.; Dreyer, H.C.; Drummond, M.J.; Glynn, E.L.; Cadenas, J.G.; Yoshizawa, F.; Volpi, E.; Rasmussen, B.B. Nutrient signalling in the regulation of human muscle protein synthesis. J. Physiol. 2007, 582, 813–823. [Google Scholar] [CrossRef]
- Koopman, R.; Saris, W.H.; Wagenmakers, A.J.; Van Loon, L.J. Nutritional interventions to promote post-exercise muscle protein synthesis. Sports Med. 2007, 37, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; Jacques, P.F. Protein intake and human health: Implications of units of protein intake. Adv. Nutr. 2021, 12, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005, 19, 1–22. [Google Scholar] [CrossRef]
- Guillet, C.; Prod’homme, M.; Balage, M.; Gachon, P.; Giraudet, C.; Morin, L.; Grizard, J.; Boirie, Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. 2004, 18, 1586–1587. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am. J. Clin. Nutr. 2005, 82, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Morley, J.E.; Schols, A.M.; Ferrucci, L.; Cruz-Jentoft, A.J.; Dent, E.; Baracos, V.E.; Crawford, J.A.; Doehner, W.; Heymsfield, S.B. Sarcopenia: A time for action. An SCWD position paper. J. Cachexia Sarcopenia Muscle 2019, 10, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Paulussen, K.J.; McKenna, C.F.; Beals, J.W.; Wilund, K.R.; Salvador, A.F.; Burd, N.A. Anabolic resistance of muscle protein turnover comes in various shapes and sizes. Front. Nutr. 2021, 8, 615849. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-related loss of muscle mass and function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.J. Invited review: Aging and sarcopenia. J. Appl. Physiol. 2003, 95, 1717–1727. [Google Scholar] [CrossRef]
- Siparsky, P.N.; Kirkendall, D.T.; Garrett Jr, W.E. Muscle changes in aging: Understanding sarcopenia. Sports Health 2014, 6, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Walrand, S.; Beelen, M.; Gijsen, A.P.; Kies, A.K.; Boirie, Y.; Saris, W.H.; van Loon, L.J. Dietary protein digestion and absorption rates and the subsequent postprandial muscle protein synthetic response do not differ between young and elderly men. J. Nutr. 2009, 139, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Robinson, M.J.; Fry, J.L.; Tang, J.E.; Glover, E.I.; Wilkinson, S.B.; Prior, T.; Tarnopolsky, M.A.; Phillips, S.M. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 2009, 89, 161–168. [Google Scholar] [CrossRef]
- Witard, O.C.; Jackman, S.R.; Breen, L.; Smith, K.; Selby, A.; Tipton, K.D. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am. J. Clin. Nutr. 2014, 99, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Medawar, E.; Huhn, S.; Villringer, A.; Veronica Witte, A. The effects of plant-based diets on the body and the brain: A systematic review. Transl. Psychiatry 2019, 9, 226. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Aiking, H. Protein production: Planet, profit, plus people? Am. J. Clin. Nutr. 2014, 100, 483S–489S. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Clark, M. Global diets link environmental sustainability and human health. Nature 2014, 515, 518–522. [Google Scholar] [CrossRef]
- Guzmán-Luna, P.; Mauricio-Iglesias, M.; Flysjö, A.; Hospido, A. Analysing the interaction between the dairy sector and climate change from a life cycle perspective: A review. Trends Food Sci. Technol. 2021, 126, 168–179. [Google Scholar] [CrossRef]
- Wilkinson, S.B.; Tarnopolsky, M.A.; MacDonald, M.J.; MacDonald, J.R.; Armstrong, D.; Phillips, S.M. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am. J. Clin. Nutr. 2007, 85, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Beals, J.W.; Sukiennik, R.A.; Nallabelli, J.; Emmons, R.S.; Van Vliet, S.; Young, J.R.; Ulanov, A.V.; Li, Z.; Paluska, S.A.; De Lisio, M. Anabolic sensitivity of postprandial muscle protein synthesis to the ingestion of a protein-dense food is reduced in overweight and obese young adults. Am. J. Clin. Nutr. 2016, 104, 1014–1022. [Google Scholar] [CrossRef]
- Pennings, B.; Groen, B.B.; van Dijk, J.-W.; de Lange, A.; Kiskini, A.; Kuklinski, M.; Senden, J.M.; Van Loon, L.J. Minced beef is more rapidly digested and absorbed than beef steak, resulting in greater postprandial protein retention in older men. Am. J. Clin. Nutr. 2013, 98, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M. Nutrient-rich meat proteins in offsetting age-related muscle loss. Meat Sci. 2012, 92, 174–178. [Google Scholar] [CrossRef]
- Robinson, M.J.; Burd, N.A.; Breen, L.; Rerecich, T.; Yang, Y.; Hector, A.J.; Baker, S.K.; Phillips, S.M. Dose-dependent responses of myofibrillar protein synthesis with beef ingestion are enhanced with resistance exercise in middle-aged men. Appl. Physiol. Nutr. Metab. 2013, 38, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Symonsi, T.; Sheffield-Moore, M.; Mamerow, M.; Wolfe, R.; Paddon-Jones, D. The anabolic response to resistance exercise and a protein-rich meal is not diminished by age. J. Nutr. Health Aging 2011, 15, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.; Witard, O.C. Characterising the muscle anabolic potential of dairy, meat and plant-based protein sources in older adults. Proc. Nutr. Soc. 2018, 77, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, M.; Maselli, P.; Nucara, A. Relationship between digestibility and secondary structure of raw and thermally treated legume proteins: A Fourier transform infrared (FT-IR) spectroscopic study. Amino Acids 2012, 43, 911–921. [Google Scholar] [CrossRef]
- Gilani, G.S.; Cockell, K.A.; Sepehr, E. Effects of antinutritional factors on protein digestibility and amino acid availability in foods. J. AOAC Int. 2005, 88, 967–987. [Google Scholar] [CrossRef]
- Berrazaga, I.; Micard, V.; Gueugneau, M.; Walrand, S. The role of the anabolic properties of plant-versus animal-based protein sources in supporting muscle mass maintenance: A critical review. Nutrients 2019, 11, 1825. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Bhandari, B.; Cichero, J.; Prakash, S. Gastrointestinal digestion of dairy and soy proteins in infant formulas: An in vitro study. Food Res. Int. 2015, 76, 348–358. [Google Scholar] [CrossRef]
- WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition; WHO Technical Report Series 935; WHO: Geneva, Switzerland, 2007; pp. 1–265. [Google Scholar]
- Pinckaers, P.J.; Trommelen, J.; Snijders, T.; van Loon, L.J. The anabolic response to plant-based protein ingestion. Sports Med. 2021, 51 (Suppl. 1), 59–74. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, S.; Burd, N.A.; van Loon, L.J. The skeletal muscle anabolic response to plant-versus animal-based protein consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef]
- Pinckaers, P.J.; Weijzen, M.E.; Houben, L.H.; Zorenc, A.H.; Kouw, I.W.; Groot, L.C.D.; Verdijk, L.B.; Snijders, T.; Loon, L.J.V. The muscle protein synthetic response following ingestion of corn protein, milk protein and their protein blend in young males. Curr. Dev. Nutr. 2020, 4, 651. [Google Scholar] [CrossRef]
- Reidy, P.T.; Walker, D.K.; Dickinson, J.M.; Gundermann, D.M.; Drummond, M.J.; Timmerman, K.L.; Fry, C.S.; Borack, M.S.; Cope, M.B.; Mukherjea, R. Protein blend ingestion following resistance exercise promotes human muscle protein synthesis. J. Nutr. 2013, 143, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Reidy, P.T.; Walker, D.K.; Dickinson, J.M.; Gundermann, D.M.; Drummond, M.J.; Timmerman, K.L.; Cope, M.B.; Mukherjea, R.; Jennings, K.; Volpi, E. Soy-dairy protein blend and whey protein ingestion after resistance exercise increases amino acid transport and transporter expression in human skeletal muscle. J. Appl. Physiol. 2014, 116, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.S.; Phillips, B.E.; Wilkinson, D.J.; Limb, M.C.; Rankin, D.; Mitchell, W.K.; Kobayashi, H.; Greenhaff, P.L.; Smith, K.; Atherton, P.J. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. Am. J. Physiol.-Endocrinol. Metab. 2015, 308, E1056–E1065. [Google Scholar] [CrossRef] [PubMed]
- Churchward-Venne, T.A.; Breen, L.; Di Donato, D.M.; Hector, A.J.; Mitchell, C.J.; Moore, D.R.; Stellingwerff, T.; Breuille, D.; Offord, E.A.; Baker, S.K. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: A double-blind, randomized trial. Am. J. Clin. Nutr. 2014, 99, 276–286. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Burd, N.A.; Mitchell, C.J.; West, D.W.; Philp, A.; Marcotte, G.R.; Baker, S.K.; Baar, K.; Phillips, S.M. Supplementation of a suboptimal protein dose with leucine or essential amino acids: Effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J. Physiol. 2012, 590, 2751–2765. [Google Scholar] [CrossRef]
- Wall, B.T.; Hamer, H.M.; de Lange, A.; Kiskini, A.; Groen, B.B.; Senden, J.M.; Gijsen, A.P.; Verdijk, L.B.; van Loon, L.J. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin. Nutr. 2013, 32, 412–419. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Bukhari, S.S.; Phillips, B.E.; Limb, M.C.; Cegielski, J.; Brook, M.S.; Rankin, D.; Mitchell, W.K.; Kobayashi, H.; Williams, J.P. Effects of leucine-enriched essential amino acid and whey protein bolus dosing upon skeletal muscle protein synthesis at rest and after exercise in older women. Clin. Nutr. 2018, 37, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L. Plant Versus Animal-Based Proteins to Support Muscle Conditioning; Gatorade Sports Science Institute: Valhalla, NY, USA, 2021. [Google Scholar]
- Gorissen, S.H.; Crombag, J.J.; Senden, J.M.; Waterval, W.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Bhattarai, R.R.; Dhital, S.; Gidley, M.J. Interactions among macronutrients in wheat flour determine their enzymic susceptibility. Food Hydrocoll. 2016, 61, 415–425. [Google Scholar] [CrossRef]
- Zahir, M.; Fogliano, V.; Capuano, E. Food matrix and processing modulate in vitro protein digestibility in soybeans. Food Funct. 2018, 9, 6326–6336. [Google Scholar] [CrossRef]
- Yang, Y.; Churchward-Venne, T.A.; Burd, N.A.; Breen, L.; Tarnopolsky, M.A.; Phillips, S.M. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr. Metab. 2012, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.M.; Buman, M.P.; Dickinson, J.M.; Ransdell, L.B.; Johnston, C.S.; Wharton, C.M. No Significant Differences in Muscle Growth and Strength Development When Consuming Soy and Whey Protein Supplements Matched for Leucine Following a 12 Week Resistance Training Program in Men and Women: A Randomized Trial. Int. J. Environ. Res. Public Health 2020, 17, 3871. [Google Scholar] [CrossRef]
- Messina, M.; Lynch, H.; Dickinson, J.M.; Reed, K.E. No difference between the effects of supplementing with soy protein versus animal protein on gains in muscle mass and strength in response to resistance exercise. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Mobley, C.B.; Haun, C.T.; Roberson, P.A.; Mumford, P.W.; Romero, M.A.; Kephart, W.C.; Anderson, R.G.; Vann, C.G.; Osburn, S.C.; Pledge, C.D. Effects of whey, soy or leucine supplementation with 12 weeks of resistance training on strength, body composition, and skeletal muscle and adipose tissue histological attributes in college-aged males. Nutrients 2017, 9, 972. [Google Scholar] [CrossRef]
- Pinckaers, P.J.M.; Hendriks, F.K.; Hermans, W.J.H.; Goessens, J.P.B.; Senden, J.M.; Van Kranenburg, J.M.X.; Wodzig, W.K.H.W.; Snijders, T.; van Loon, L.J.C. Potato Protein Ingestion Increases Muscle Protein Synthesis Rates at Rest and during Recovery from Exercise in Humans. Med. Sci. Sports Exerc. 2022, 54, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, S.Y.; Bahniwal, R.; Holloway, T.M.; Lim, C.; McLeod, J.C.; McGlory, C.; Baker, S.K.; Phillips, S.M. Potato Protein Isolate Stimulates Muscle Protein Synthesis at Rest and with Resistance Exercise in Young Women. Nutrients 2020, 12, 1235. [Google Scholar] [CrossRef] [PubMed]
- Pinckaers, P.J.; Smeets, J.S.; Kouw, I.W.; Goessens, J.P.; Gijsen, A.P.; de Groot, L.C.; Verdijk, L.; van Loon, L.J.; Snijders, T. Post-prandial muscle protein synthesis rates following the ingestion of pea-derived protein do not differ from ingesting an equivalent amount of milk-derived protein in healthy, young males. Eur. J. Nutr. 2024, 63, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Pinckaers, P.J.M.; Kouw, I.W.; Gorissen, S.H.; Houben, L.H.; Senden, J.M.; Wodzig, W.K.; de Groot, L.C.; Verdijk, L.B.; Snijders, T.; van Loon, L.J. The Muscle Protein Synthetic Response to the Ingestion of a Plant-Derived Protein Blend Does Not Differ from an Equivalent Amount of Milk Protein in Healthy Young Males. J. Nutr. 2022, 152, 2734–2743. [Google Scholar] [CrossRef] [PubMed]
- Bos, C.; Gaudichon, C.; Pueyo, M.E.; Morens, C.; Tomé, D.; Metges, C.C.; Petzke, K.J.; Everwand, J.; Benamouzig, R. Postprandial kinetics of dietary amino acids are the main determinant of their metabolism after soy or milk protein ingestion in humans. J. Nutr. 2003, 133, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Fouillet, H.; Mariotti, F.; Gaudichon, C.; Bos, C.; Tome, D. Peripheral and splanchnic metabolism of dietary nitrogen are differently affected by the protein source in humans as assessed by compartmental modeling. J. Nutr. 2002, 132, 125–133. [Google Scholar] [CrossRef]
- Kaplan, A.; Zelicha, H.; Tsaban, G.; Meir, A.Y.; Rinott, E.; Kovsan, J.; Novack, L.; Thiery, J.; Ceglarek, U.; Burkhardt, R. Protein bioavailability of Wolffia globosa duckweed, a novel aquatic plant–A randomized controlled trial. Clin. Nutr. 2019, 38, 2576–2582. [Google Scholar] [CrossRef] [PubMed]
- Monteyne, A.J.; Coelho, M.O.; Porter, C.; Abdelrahman, D.R.; Jameson, T.S.; Jackman, S.R.; Blackwell, J.R.; Finnigan, T.J.; Stephens, F.B.; Dirks, M.L. Mycoprotein ingestion stimulates protein synthesis rates to a greater extent than milk protein in rested and exercised skeletal muscle of healthy young men: A randomized controlled trial. Am. J. Clin. Nutr. 2020, 112, 318–333. [Google Scholar] [CrossRef]
- West, S.; Monteyne, A.J.; Whelehan, G.; Abdelrahman, D.R.; Murton, A.J.; Finnigan, T.J.A.; Blackwell, J.R.; Stephens, F.B.; Wall, B.T. Mycoprotein ingestion within or without its wholefood matrix results in equivalent stimulation of myofibrillar protein synthesis rates in resting and exercised muscle of young men. Br. J. Nutr. 2023, 130, 20–32. [Google Scholar] [CrossRef] [PubMed]
- West, S.; Monteyne, A.J.; Whelehan, G.; van der Heijden, I.; Abdelrahman, D.R.; Murton, A.J.; Finnigan, T.J.; Stephens, F.B.; Wall, B.T. Ingestion of mycoprotein, pea protein, and their blend support comparable postexercise myofibrillar protein synthesis rates in resistance-trained individuals. Am. J. Physiol.-Endocrinol. Metab. 2023, 325, E267–E279. [Google Scholar] [CrossRef] [PubMed]
- Hermans, W.J.; Senden, J.M.; Churchward-Venne, T.A.; Paulussen, K.J.; Fuchs, C.J.; Smeets, J.S.; van Loon, J.J.; Verdijk, L.B.; van Loon, L.J. Insects are a viable protein source for human consumption: From insect protein digestion to postprandial muscle protein synthesis in vivo in humans: A double-blind randomized trial. Am. J. Clin. Nutr. 2021, 114, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Vangsoe, M.T.; Thogersen, R.; Bertram, H.C.; Heckmann, L.-H.L.; Hansen, M. Ingestion of insect protein isolate enhances blood amino acid concentrations similar to soy protein in a human trial. Nutrients 2018, 10, 1357. [Google Scholar] [CrossRef] [PubMed]
- Lanng, S.K.; Oxfeldt, M.; Pedersen, S.S.; Johansen, F.T.; Risikesan, J.; Lejel, T.; Bertram, H.C.; Hansen, M. Influence of protein source (cricket, pea, whey) on amino acid bioavailability and activation of the mTORC1 signaling pathway after resistance exercise in healthy young males. Eur. J. Nutr. 2023, 62, 1295–1308. [Google Scholar] [CrossRef]
- Dai, J.; Lov, J.; Martin-Arrowsmith, P.W.; Gritsas, A.; Churchward-Venne, T.A. The acute effects of insect vs. beef-derived protein on postprandial plasma aminoacidemia, appetite hormones, appetite sensations, and energy intake in healthy young men. Eur. J. Clin. Nutr. 2022, 76, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, I.; West, S.; Monteyne, A.J.; Finnigan, T.J.; Abdelrahman, D.R.; Murton, A.J.; Stephens, F.B.; Wall, B.T. Algae Ingestion Increases Resting and Exercised Myofibrillar Protein Synthesis Rates to a Similar Extent as Mycoprotein in Young Adults. J. Nutr. 2023, 153, 3406–3417. [Google Scholar] [CrossRef]
- Wang, B.; Shi, Y.; Lu, H.; Chen, Q. A critical review of fungal proteins: Emerging preparation technology, active efficacy and food application. Trends Food Sci. Technol. 2023, 141, 104178. [Google Scholar] [CrossRef]
- Monteyne, A.J.; Coelho, M.O.C.; Porter, C.; Abdelrahman, D.R.; Jameson, T.S.O.; Finnigan, T.J.A.; Stephens, F.B.; Dirks, M.L.; Wall, B.T. Branched-Chain Amino Acid Fortification Does Not Restore Muscle Protein Synthesis Rates following Ingestion of Lower- Compared with Higher-Dose Mycoprotein. J. Nutr. 2020, 150, 2931–2941. [Google Scholar] [CrossRef]
- Saeed, F.; Afzaal, M.; Khalid, A.; Shah, Y.A.; Ateeq, H.; Islam, F.; Akram, N.; Ejaz, A.; Nayik, G.A.; Shah, M.A. Role of mycoprotein as a non-meat protein in food security and sustainability: A review. Int. J. Food Prop. 2023, 26, 683–695. [Google Scholar] [CrossRef]
- Edwards, D.; Cummings, J. The protein quality of mycoprotein. Proc. Nutr. Soc. 2010, 69, E331. [Google Scholar] [CrossRef]
- Dunlop, M.V.; Kilroe, S.P.; Bowtell, J.L.; Finnigan, T.J.A.; Salmon, D.L.; Wall, B.T. Mycoprotein represents a bioavailable and insulinotropic non-animal-derived dietary protein source: A dose-response study. Br. J. Nutr. 2017, 118, 673–685. [Google Scholar] [CrossRef]
- Gorissen, S.H.; Burd, N.A.; Hamer, H.M.; Gijsen, A.P.; Groen, B.B.; Van Loon, L.J. Carbohydrate coingestion delays dietary protein digestion and absorption but does not modulate postprandial muscle protein accretion. J. Clin. Endocrinol. Metab. 2014, 99, 2250–2258. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Wall, B.T.; Kiskini, A.; de Lange, A.; Groen, B.B.; Bakker, J.A.; Gijsen, A.P.; Verdijk, L.B.; van Loon, L.J. Carbohydrate co-ingestion with protein does not further augment post-prandial muscle protein accretion in older men. Nutr. Metab. 2013, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Beelen, M.; Stellingwerff, T.; Pennings, B.; Saris, W.H.; Kies, A.K.; Kuipers, H.; Van Loon, L.J. Coingestion of carbohydrate with protein does not further augment postexercise muscle protein synthesis. Am. J. Physiol.-Endocrinol. Metab. 2007, 293, E833–E842. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.A.; Geiselman, P.J.; Lovejoy, J.; Greenway, F.; Volaufova, J.; Martin, C.K.; Arnett, C.; Ortego, L. Effects of consuming mycoprotein, tofu or chicken upon subsequent eating behaviour, hunger and safety. Appetite 2006, 46, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Burley, V.; Paul, A.; Blundell, J. Influence of a high-fibre food (myco-protein^*) on appetite: Effects on satiation (within meals) and satiety (following meals). Eur. J. Clin. Nutr. 1993, 47, 409. [Google Scholar] [PubMed]
- Rumpold, B.A.; Schlüter, O. Insect-based protein sources and their potential for human consumption: Nutritional composition and processing. Anim. Front. 2015, 5, 20–24. [Google Scholar]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Churchward-Venne, T.A.; Pinckaers, P.J.; van Loon, J.J.; van Loon, L.J. Consideration of insects as a source of dietary protein for human consumption. Nutr. Rev. 2017, 75, 1035–1045. [Google Scholar] [CrossRef]
- Miglietta, P.P.; De Leo, F.; Ruberti, M.; Massari, S. Mealworms for food: A water footprint perspective. Water 2015, 7, 6190–6203. [Google Scholar] [CrossRef]
- Malla, N.; Nørgaard, J.V.; Lærke, H.N.; Heckmann, L.-H.L.; Roos, N. Some insect species are good-quality protein sources for children and adults: Digestible indispensable amino acid score (DIAAS) determined in growing pigs. J. Nutr. 2022, 152, 1042–1051. [Google Scholar] [CrossRef]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Ramírez, J.; Mondragón-Portocarrero, A.C.; Rodríguez, J.A.; Lorenzo, J.M.; Santos, E.M. Algae as a potential source of protein meat alternatives. Front. Nutr. 2023, 10, 1254300. [Google Scholar] [CrossRef]
- Darragh, A.J.; Hodgkinson, S.M. Quantifying the digestibility of dietary protein. J. Nutr. 2000, 130, 1850S–1856S. [Google Scholar] [PubMed]
- Weijzen, M.E.; van Gassel, R.J.; Kouw, I.W.; Trommelen, J.; Gorissen, S.H.; van Kranenburg, J.; Goessens, J.P.; van de Poll, M.C.; Verdijk, L.B.; van Loon, L.J. Ingestion of free amino acids compared with an equivalent amount of intact protein results in more rapid amino acid absorption and greater postprandial plasma amino acid availability without affecting muscle protein synthesis rates in young adults in a double-blind randomized trial. J. Nutr. 2022, 152, 59–67. [Google Scholar] [PubMed]
- Van Vliet, S.; Shy, E.L.; Abou Sawan, S.; Beals, J.W.; West, D.W.; Skinner, S.K.; Ulanov, A.V.; Li, Z.; Paluska, S.A.; Parsons, C.M. Consumption of whole eggs promotes greater stimulation of postexercise muscle protein synthesis than consumption of isonitrogenous amounts of egg whites in young men. Am. J. Clin. Nutr. 2017, 106, 1401–1412. [Google Scholar] [CrossRef]
- Elliot, T.A.; Cree, M.G.; Sanford, A.P.; Wolfe, R.R.; Tipton, K.D. Milk ingestion stimulates net muscle protein synthesis following resistance exercise. Med. Sci. Sports Exerc. 2006, 38, 667–674. [Google Scholar] [CrossRef]
- Hermans, W.J.; Fuchs, C.J.; Hendriks, F.K.; Houben, L.H.; Senden, J.M.; Verdijk, L.B.; van Loon, L.J. Cheese ingestion increases muscle protein synthesis rates both at rest and during recovery from exercise in healthy, young males: A randomized parallel-group trial. J. Nutr. 2022, 152, 1022–1030. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yimam, M.A.; Andreini, M.; Carnevale, S.; Muscaritoli, M. Postprandial Aminoacidemia Following the Ingestion of Alternative and Sustainable Proteins in Humans: A Narrative Review. Nutrients 2025, 17, 211. https://doi.org/10.3390/nu17020211
Yimam MA, Andreini M, Carnevale S, Muscaritoli M. Postprandial Aminoacidemia Following the Ingestion of Alternative and Sustainable Proteins in Humans: A Narrative Review. Nutrients. 2025; 17(2):211. https://doi.org/10.3390/nu17020211
Chicago/Turabian StyleYimam, Mohammed Ahmed, Martina Andreini, Sara Carnevale, and Maurizio Muscaritoli. 2025. "Postprandial Aminoacidemia Following the Ingestion of Alternative and Sustainable Proteins in Humans: A Narrative Review" Nutrients 17, no. 2: 211. https://doi.org/10.3390/nu17020211
APA StyleYimam, M. A., Andreini, M., Carnevale, S., & Muscaritoli, M. (2025). Postprandial Aminoacidemia Following the Ingestion of Alternative and Sustainable Proteins in Humans: A Narrative Review. Nutrients, 17(2), 211. https://doi.org/10.3390/nu17020211





