Luteolin Alleviates Ulcerative Colitis in Mice by Modulating Gut Microbiota and Plasma Metabolism
Highlights
- Luteolin exhibited anti-inflammatory effects and restored the intestinal barrier in a UC mouse model.
- Luteolin modulated the gut microbiota, enhancing beneficial bacteria and reducing pathogenic species.
- Luteolin affected key metabolites, offering insights into UC pathogenesis and therapeutic targets.
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Data and Sample Collection
2.3. Histopathological Analysis
2.4. Real-Time qPCR Validation
2.5. Immunohistochemistry
2.6. Enzyme-Linked Immunosorbent Assay
2.7. Western Blot
2.8. Gut Microbiota Analysis
2.9. Analysis of Plasma Metabolomics
2.10. Statistical Analysis of Plasma Metabolomics
2.11. Statistical Analysis
3. Results
3.1. Luteolin Exerts Protective Effects Against DSS-Induced Colitis in Mice
3.2. Anti-Inflammatory Effect of Luteolin
3.3. Luteolin Influences the Recovery of the Colon Barrier
3.4. Regulatory Effects of Luteolin on Gut Microbiota
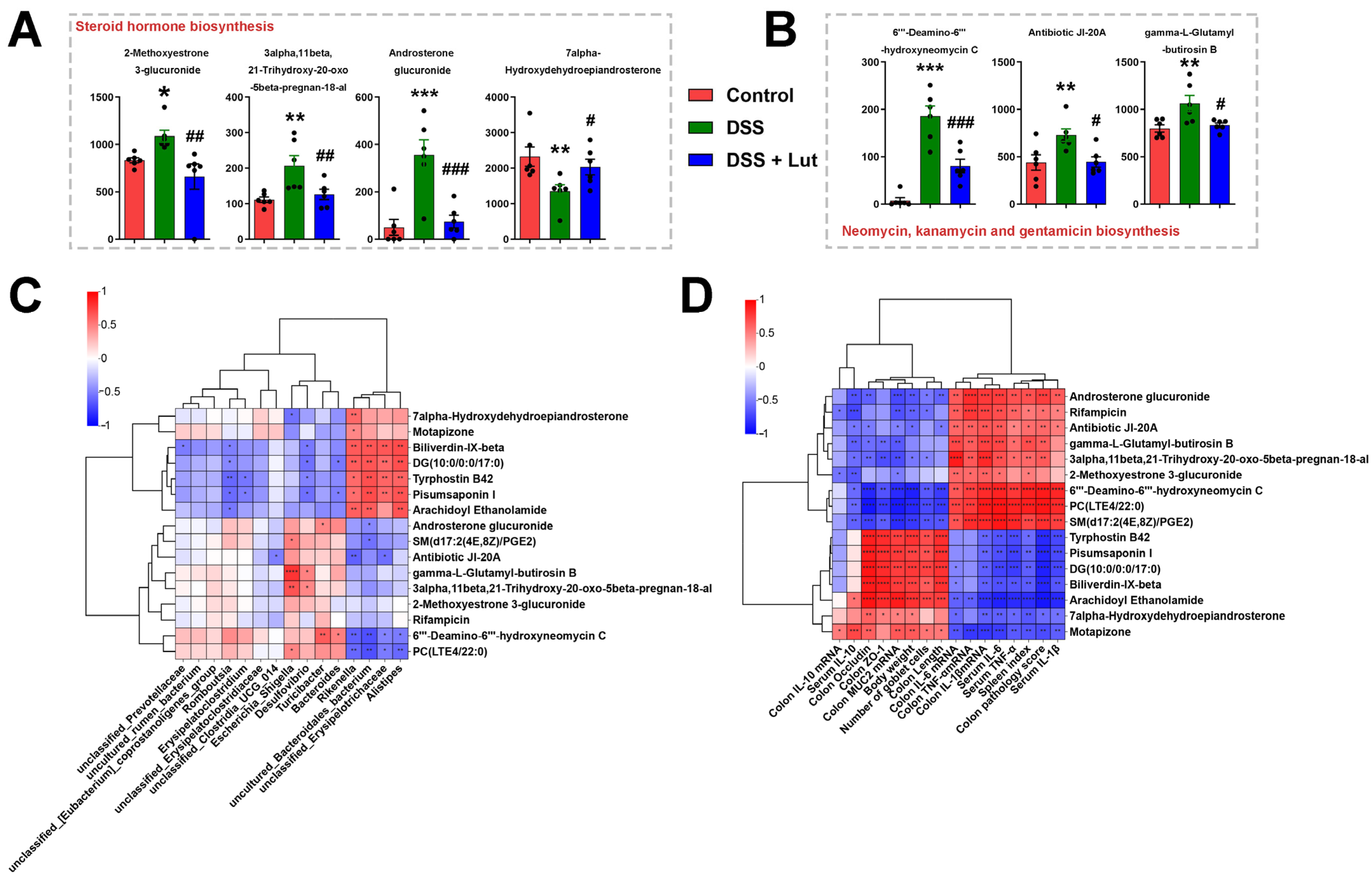
3.5. Analysis of Intergroup Differences in Intestinal Microbial Communities and Their Correlation with Colonic Inflammatory Manifestations
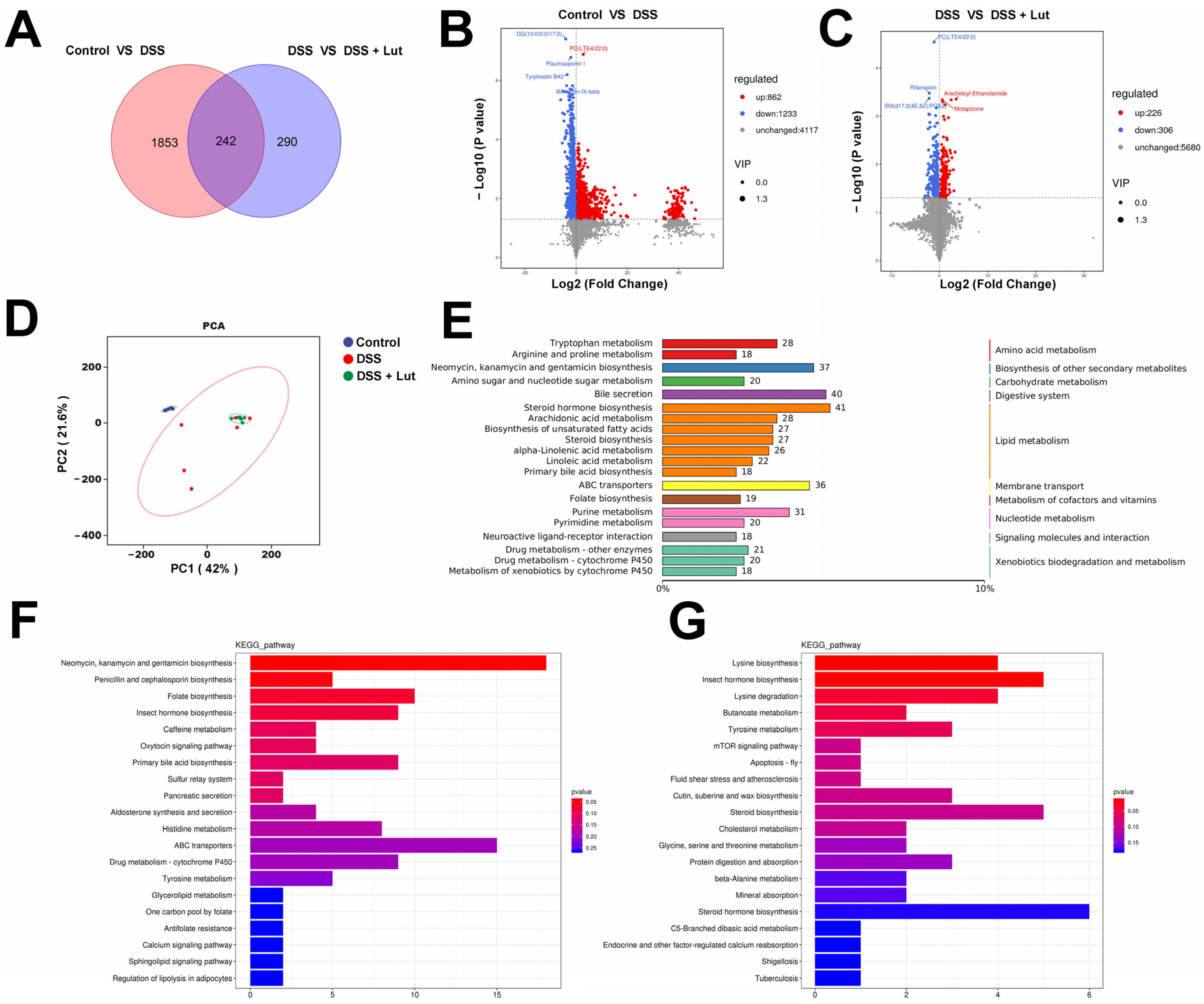
3.6. Lut Changes the Plasma Metabolic Profiles of Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feakins, R.M. Ulcerative colitis or Crohn’s disease? Pitfalls and problems. Histopathology 2014, 64, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Hodson, R. Inflammatory bowel disease. Nature 2016, 540, S97. [Google Scholar] [CrossRef]
- Voelker, R. What Is Ulcerative Colitis? JAMA 2024, 331, 716. [Google Scholar] [CrossRef] [PubMed]
- Burri, E.; Maillard, M.H.; Schoepfer, A.M.; Seibold, F.; Van Assche, G.; Riviere, P.; Laharie, D.; Manz, M. Treatment Algorithm for Mild and Moderate-to-Severe Ulcerative Colitis: An Update. Digestion 2020, 101 (Suppl. S1), 2–15. [Google Scholar] [CrossRef] [PubMed]
- Kucharzik, T.; Koletzko, S.; Kannengiesser, K.; Dignass, A. Ulcerative Colitis-Diagnostic and Therapeutic Algorithms. Dtsch. Arztebl. Int. 2020, 117, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Osterman, M.T. Biologic Therapy for Ulcerative Colitis. Gastroenterol. Clin. N. Am. 2020, 49, 717–729. [Google Scholar] [CrossRef]
- Jin, M.Y.; Wu, X.Y.; Li, M.Y.; Li, X.T.; Huang, R.M.; Sun, Y.M.; Xu, Z.L. Noni (Morinda citrifolia L.) Fruit Polysaccharides Regulated IBD Mice via Targeting Gut Microbiota: Association of JNK/ERK/NF-κB Signaling Pathways. J. Agric. Food Chem. 2021, 69, 10151–10162. [Google Scholar] [CrossRef]
- Guo, X.Y.; Liu, X.J.; Hao, J.Y. Gut microbiota in ulcerative colitis: Insights on pathogenesis and treatment. J. Dig. Dis. 2020, 21, 147–159. [Google Scholar] [CrossRef]
- Iacob, S.; Iacob, D.G.; Luminos, L.M. Intestinal Microbiota as a Host Defense Mechanism to Infectious Threats. Front. Microbiol. 2018, 9, 3328. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, B.S. Role of the gut microbiota in human nutrition and metabolism. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. S4), 9–17. [Google Scholar] [CrossRef]
- Sun, C.Y.; Yang, N.; Zheng, Z.L.; Liu, D.; Xu, Q.L. T helper 17 (Th17) cell responses to the gut microbiota in human diseases. Biomed. Pharmacother. 2023, 161, 114483. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, J.; Liu, R.; Zhang, F.; Wen, S.; Liu, Y.; Ren, W.; Zhang, X.; Shang, Y.; Gao, M.; et al. Predominance of Escherichia-Shigella in Gut Microbiome and Its Potential Correlation with Elevated Level of Plasma Tumor Necrosis Factor Alpha in Patients with Tuberculous Meningitis. Microbiol. Spectr. 2022, 10, e192622. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Guan, X.; Ding, W.; Luo, Y.; Wang, W.; Bu, W.; Song, J.; Tan, X.; Sun, E.; Ning, Q.; et al. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2021, 166, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Zhang, X.; Tong, L.; Liu, Q.; Liang, X.; Bu, Y.; Gong, P.; Liu, T.; Zhang, L.; Xia, Y.; et al. Effect of Extracellular Vesicles Derived from Lactobacillus plantarum Q7 on Gut Microbiota and Ulcerative Colitis in Mice. Front. Immunol. 2021, 12, 777147. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; He, J.; Zhang, Z.; Li, J.; Zou, H.; Tan, X.; Wang, Y.; Yao, Y.; Xiong, W. Propionic Acid Driven by the Lactobacillus johnsonii Culture Supernatant Alleviates Colitis by Inhibiting M1 Macrophage Polarization by Modulating the MAPK Pathway in Mice. J. Agric. Food Chem. 2023, 71, 14951–14966. [Google Scholar] [CrossRef]
- Luo, S.; Wen, R.; Wang, Q.; Zhao, Z.; Nong, F.; Fu, Y.; Huang, S.; Chen, J.; Zhou, L.; Luo, X. Rhubarb Peony Decoction ameliorates ulcerative colitis in mice by regulating gut microbiota to restoring Th17/Treg balance. J. Ethnopharmacol. 2019, 231, 39–49. [Google Scholar] [CrossRef]
- Srivastava, S. Emerging Insights into the Metabolic Alterations in Aging Using Metabolomics. Metabolites 2019, 9, 301. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, Z.; Xu, C.; Kan, S.; Chen, D. Disturbances of the Gut Microbiota and Microbiota-Derived Metabolites in Inflammatory Bowel Disease. Nutrients 2022, 14, 5140. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Hu, X.L.; Yuan, Z.W.; Xiao, Y.; Ji, P.; Wei, Y.M.; Hua, Y.L. Pulsatilla decoction improves DSS-induced colitis via modulation of fecal-bacteria-related short-chain fatty acids and intestinal barrier integrity. J. Ethnopharmacol. 2023, 300, 115741. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Du, H.; Zhang, M.; Xu, H.; Pu, X.; Chen, Q.; Luo, R.; Hu, Y.; Wang, Y.; Tu, H.; et al. Anti-inflammatory effect of Rhein on ulcerative colitis via inhibiting PI3K/Akt/mTOR signaling pathway and regulating gut microbiota. Phytother. Res. 2022, 36, 2081–2094. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Xiong, Y.; Han, M.; Li, Z.; Peng, L.; Zhang, H.; Zou, Q.; Wu, L.; Ye, Q.; Liao, L. Characterization and Quantification of Luteolin-Metal Complexes in Aqueous Extract of Lonicerae Japonicae Flos and Huangshan Wild Chrysanthemum. Int. J. Anal. Chem. 2021, 2021, 6677437. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, A.K.; Kumar, S.A.; Dharmalingam, P.; Ganapasam, S. Luteolin, a bioflavonoid inhibits azoxymethane-induced colon carcinogenesis: Involvement of iNOS and COX-2. Pharmacogn. Mag. 2014, 10, S306–S310. [Google Scholar] [CrossRef]
- Cao, S.Y.; Ye, S.J.; Wang, W.W.; Wang, B.; Zhang, T.; Pu, Y.Q. Progress in active compounds effective on ulcerative colitis from Chinese medicines. Chin. J. Nat. Med. 2019, 17, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Yi, T.; Li, Y.; Zhang, W.; Wang, X.; Liu, J.; Fan, Y.; Ji, J.; Xu, L. Luteolin attenuates lupus nephritis by regulating macrophage oxidative stress via HIF-1α pathway. Eur. J. Pharmacol. 2023, 953, 175823. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.J.; Shi, J.Z.; He, Y.Y.; Hao, J.J.; Zhang, H.Y.; Luo, D.M.; Song, J.K.; Yan, Y.; Xie, X.M.; Du, G.H.; et al. Luteolin alleviates cognitive impairment in Alzheimer’s disease mouse model via inhibiting endoplasmic reticulum stress-dependent neuroinflammation. Acta Pharmacol. Sin. 2022, 43, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.R.; Feng, T.; Zhang, D.; Zhi, L.L.; Zhang, J.T.; Liu, X.F.; Pan, Y.; Xu, J.W.; Cui, W.J.; Dong, L. Luteolin alleviated neutrophilic asthma by inhibiting IL-36γ secretion-mediated MAPK pathways. Pharm. Biol. 2023, 61, 165–176. [Google Scholar] [CrossRef]
- Kariu, T.; Hamada, N.; Lakshmyya, K. Luteolin inhibits Porphyromonas gingivalis growth and alleviates alveolar bone destruction in experimental murine periodontitis. Biosci. Biotechnol. Biochem. 2023, 88, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, L.; Luo, H. Luteolin ameliorates dextran sulfate sodium-induced colitis in mice possibly through activation of the Nrf2 signaling pathway. Int. Immunopharmacol. 2016, 40, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Du, P.; Du, Y.; Zhao, D.; Cai, Y.; Yang, Q.; Guo, Z. Luteolin alleviates inflammation and modulates gut microbiota in ulcerative colitis rats. Life Sci. 2021, 269, 119008. [Google Scholar] [CrossRef]
- Liu, X.; Ren, X.; Zhou, L.; Liu, K.; Deng, L.; Qing, Q.; Li, J.; Zhi, F.; Li, M. Tollip Orchestrates Macrophage Polarization to Alleviate Intestinal Mucosal Inflammation. J. Crohns Colitis 2022, 16, 1151–1167. [Google Scholar] [CrossRef] [PubMed]
- Alex, P.; Zachos, N.C.; Nguyen, T.; Gonzales, L.; Chen, T.E.; Conklin, L.S.; Centola, M.; Li, X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm. Bowel Dis. 2009, 15, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Yang, L.; Zhang, X.; Ji, P.; Hua, Y.; Wei, Y. Mechanism of Huang-lian-Jie-du decoction and its effective fraction in alleviating acute ulcerative colitis in mice: Regulating arachidonic acid metabolism and glycerophospholipid metabolism. J. Ethnopharmacol. 2020, 259, 112872. [Google Scholar] [CrossRef]
- Yang, S.; Han, H.; Li, J.; Zhang, Y.; Zhao, J.; Wei, H.; Hasi, T.; Lv, H.; Zhao, X.; Quan, K. Transcriptomic analysis of gene expression in normal goat ovary and intersex goat gonad. Reprod. Domest. Anim. 2021, 56, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Yang, S.; Xiao, L.; Yang, S.; Yan, Z.; Wang, F.; Ma, X.; Zhang, L.; Zhang, Y.; Hu, J.; et al. Melatonin promotes progesterone secretion in sheep luteal cells by regulating autophagy via the AMPK/mTOR pathway. Theriogenology 2024, 214, 342–351. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, Y.; Wu, N.; Yao, Y.; Xu, M.; Du, H.; Tu, Y. The anti-inflammatory activity of peptides from simulated gastrointestinal digestion of preserved egg white in DSS-induced mouse colitis. Food Funct. 2018, 9, 6444–6454. [Google Scholar] [CrossRef]
- Tatiya-Aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Immune response and inflammatory pathway of ulcerative colitis. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wang, X.J.; Su, Y.L.; Wang, Q.; Huang, S.W.; Pan, Z.F.; Chen, Y.P.; Liang, J.J.; Zhang, M.L.; Xie, X.Q.; et al. Baicalein ameliorates ulcerative colitis by improving intestinal epithelial barrier via AhR/IL-22 pathway in ILC3s. Acta Pharmacol. Sin. 2022, 43, 1495–1507. [Google Scholar] [CrossRef]
- Wei, J.; Chen, C.; Feng, J.; Zhou, S.; Feng, X.; Yang, Z.; Lu, H.; Tao, H.; Li, L.; Xv, H.; et al. Muc2 mucin O-glycosylation interacts with enteropathogenic Escherichia coli to influence the development of ulcerative colitis based on the NF-kB signaling pathway. J. Transl. Med. 2023, 21, 793. [Google Scholar] [CrossRef]
- Blonski, W.; Buchner, A.M.; Lichtenstein, G.R. Treatment of ulcerative colitis. Curr. Opin. Gastroenterol. 2014, 30, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.; Liang, B.; Jiang, C.; Ni, H.; Wang, L. Luteolin inhibits IL-1β-induced in flammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed. Pharmacother. 2019, 109, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhang, P.; Li, H.; Wang, Y.; Huang, Y.; Fan, L.; Ma, X.; Qian, X.; Xi, J. Cerium-Luteolin Nanocomplexes in Managing Inflammation-Related Diseases by Antioxidant and Immunoregulation. ACS Nano 2024, 18, 6229–6242. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Jin, W.; Lin, J.; Wang, Z.; Ma, Y.; Zhang, W.; Zhu, Y.; Hu, Y.; Qu, Q.; Guo, S. Forsythia suspensa polyphenols regulate macrophage M1 polarization to alleviate intestinal inflammation in mice. Phytomedicine 2024, 125, 155336. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.M.; Wang, Q.M.; Huang, B.Y.; Mai, C.T.; Wang, C.L.; Wang, T.T.; Zhang, X.J. Dioscin ameliorates murine ulcerative colitis by regulating macrophage polarization. Pharmacol. Res. 2021, 172, 105796. [Google Scholar] [CrossRef]
- Radziszewska, M.; Smarkusz-Zarzecka, J.; Ostrowska, L.; Pogodzinski, D. Nutrition and Supplementation in Ulcerative Colitis. Nutrients 2022, 14, 2469. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Mao, C.; Wang, X.; Li, L.; Tong, H.; Mao, J.; Ji, D.; Lu, T.; Hao, M.; Huang, Z.; et al. The Anti-colitis Effect of Schisandra chinensis Polysaccharide Is Associated with the Regulation of the Composition and Metabolism of Gut Microbiota. Front. Cell Infect Microbiol. 2020, 10, 519479. [Google Scholar] [CrossRef]
- Guan, W.J.; Yuan, J.J.; Li, H.M.; Gao, Y.H.; Huang, Y.; Chen, C.L.; Chen, R.C.; Zhong, N.S. Proteobacteria community compositions correlate with bronchiectasis severity. Int. J. Tuberc. Lung Dis. 2018, 22, 1095–1105. [Google Scholar] [CrossRef]
- Baltazar-Diaz, T.A.; Gonzalez-Hernandez, L.A.; Aldana-Ledesma, J.M.; Pena-Rodriguez, M.; Vega-Magana, A.N.; Zepeda-Morales, A.; Lopez-Roa, R.I.; Del, T.S.; Martinez-Lopez, E.; Salazar-Montes, A.M.; et al. Escherichia/Shigella, SCFAs, and Metabolic Pathways—The Triad That Orchestrates Intestinal Dysbiosis in Patients with Decompensated Alcoholic Cirrhosis from Western Mexico. Microorganisms 2022, 10, 1231. [Google Scholar] [CrossRef]
- Zheng, L.; Luo, M.; Kuang, G.; Liu, Y.; Liang, D.; Huang, H.; Yi, X.; Wang, C.; Wang, Y.; Xie, Q.; et al. Capsular Polysaccharide from Bacteroides fragilis Protects Against Ulcerative Colitis in an Undegraded Form. Front. Pharmacol. 2020, 11, 570476. [Google Scholar] [CrossRef] [PubMed]
- Zamani, S.; Hesam, S.S.; Zali, M.R.; Asadzadeh, A.H.; Sarabi, A.A.; Bokaie, S.; Nomanpour, B.; Sechi, L.A.; Feizabadi, M.M. Detection of enterotoxigenic Bacteroides fragilis in patients with ulcerative colitis. Gut Pathog. 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xing, H.; Bolotnikov, G.; Kramer, M.; Gotzmann, N.; Knippschild, U.; Kissmann, A.K.; Rosenau, F. Enriched Aptamer Libraries in Fluorescence-Based Assays for Rikenella microfusus-Specific Gut Microbiome Analyses. Microorganisms. 2023, 11, 2266. [Google Scholar] [CrossRef] [PubMed]
- Li, M.X.; Li, M.Y.; Lei, J.X.; Wu, Y.Z.; Li, Z.H.; Chen, L.M.; Zhou, C.L.; Su, J.Y.; Huang, G.X.; Huang, X.Q.; et al. Huangqin decoction ameliorates DSS-induced ulcerative colitis: Role of gut microbiota and amino acid metabolism, mTOR pathway and intestinal epithelial barrier. Phytomedicine 2022, 100, 154052. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, N.; Zhang, X.; Shahzad, K.; Qi, R.; Zhang, Z.; Lu, Z.; Lu, Y.; Yu, X.; Zafar, M.H.; et al. Mixed silage with Chinese cabbage waste enhances antioxidant ability by increasing ascorbate and aldarate metabolism through rumen Prevotellaceae UCG-004 in Hu sheep. Front. Microbiol. 2022, 13, 978940. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cao, Q.; Ma, B.; Xia, Y.; Liu, M.; Tian, J.; Chen, J.; Su, C.; Duan, X. Probiotic powder ameliorates colorectal cancer by regulating Bifidobacterium animalis, Clostridium cocleatum, and immune cell composition. PLoS ONE 2023, 18, e277155. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Eslami, M.; Yousefi, B.; Kokhaei, P.; Hemati, M.; Nejad, Z.R.; Arabkari, V.; Namdar, A. Importance of probiotics in the prevention and treatment of colorectal cancer. J. Cell. Physiol. 2019, 234, 17127–17143. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.; Thomson, J.M.; Fox, J.G.; El-Omar, E.M.; Hold, G.L. Could Helicobacter organisms cause inflammatory bowel disease? FEMS Immunol. Med. Microbiol. 2011, 61, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Aktas, M.; Wessel, M.; Hacker, S.; Klusener, S.; Gleichenhagen, J.; Narberhaus, F. Phosphatidylcholine biosynthesis and its significance in bacteria interacting with eukaryotic cells. Eur. J. Cell Biol. 2010, 89, 888–894. [Google Scholar] [CrossRef]
- Stremmel, W. Mucosal protection by phosphatidylcholine as new therapeutic concept in ulcerative colitis. Z. Gastroenterol. 2013, 51, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Ota, Y.; Tamegai, H.; Kudo, F.; Kuriki, H.; Koike-Takeshita, A.; Eguchi, T.; Kakinuma, K. Butirosin-biosynthetic gene cluster from Bacillus circulans. J. Antibiot. 2000, 53, 1158–1167. [Google Scholar] [CrossRef]
- Toda, S.; Nakagawa, S.; Naito, T.; Kawaguchi, H. Aminoglycoside antibiotics. XV Chemical conversion of neomycin B to paromomycin I, 6‴-deamino-6‴-hydroxyneomycin B and 6‴-deamino-6‴-hydroxy-paromomycin I. J. Antibiot. 1983, 36, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Milara, J.; Navarro, A.; Almudever, P.; Lluch, J.; Morcillo, E.J.; Cortijo, J. Oxidative stress-induced glucocorticoid resistance is prevented by dual PDE3/PDE4 inhibition in human alveolar macrophages. Clin. Exp. Allergy 2011, 41, 535–546. [Google Scholar] [CrossRef]
- Schermuly, R.T.; Inholte, C.; Ghofrani, H.A.; Gall, H.; Weissmann, N.; Weidenbach, A.; Seeger, W.; Grimminger, F. Lung vasodilatory response to inhaled iloprost in experimental pulmonary hypertension: Amplification by different type phosphodiesterase inhibitors. Respir. Res. 2005, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Soliman, E.; Henderson, K.L.; Danell, A.S.; Van Dross, R. Arachidonoyl-ethanolamide activates endoplasmic reticulum stress-apoptosis in tumorigenic keratinocytes: Role of cyclooxygenase-2 and novel J-series prostamides. Mol. Carcinog. 2016, 55, 117–130. [Google Scholar] [CrossRef]
- Turcotte, C.; Chouinard, F.; Lefebvre, J.S.; Flamand, N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J. Leukoc. Biol. 2015, 97, 1049–1070. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, Y.; Ding, G.; Heiney, K.M.; Huang, S.; Zhang, A. Role of COX-2/mPGES-1/prostaglandin E2 cascade in kidney injury. Mediators Inflamm. 2015, 2015, 147894. [Google Scholar] [CrossRef]
- Gasparitsch, M.; Schieber, A.; Schaubeck, T.; Keller, U.; Cattaruzza, M.; Lange-Sperandio, B. Tyrphostin AG490 reduces inflammation and fibrosis in neonatal obstructive nephropathy. PLoS ONE 2019, 14, e226675. [Google Scholar] [CrossRef]
- Gyurkovska, V.; Stefanova, T.; Dimitrova, P.; Danova, S.; Tropcheva, R.; Ivanovska, N. Tyrosine kinase inhibitor tyrphostin AG490 retards chronic joint inflammation in mice. Inflammation 2014, 37, 995–1005. [Google Scholar] [CrossRef] [PubMed]
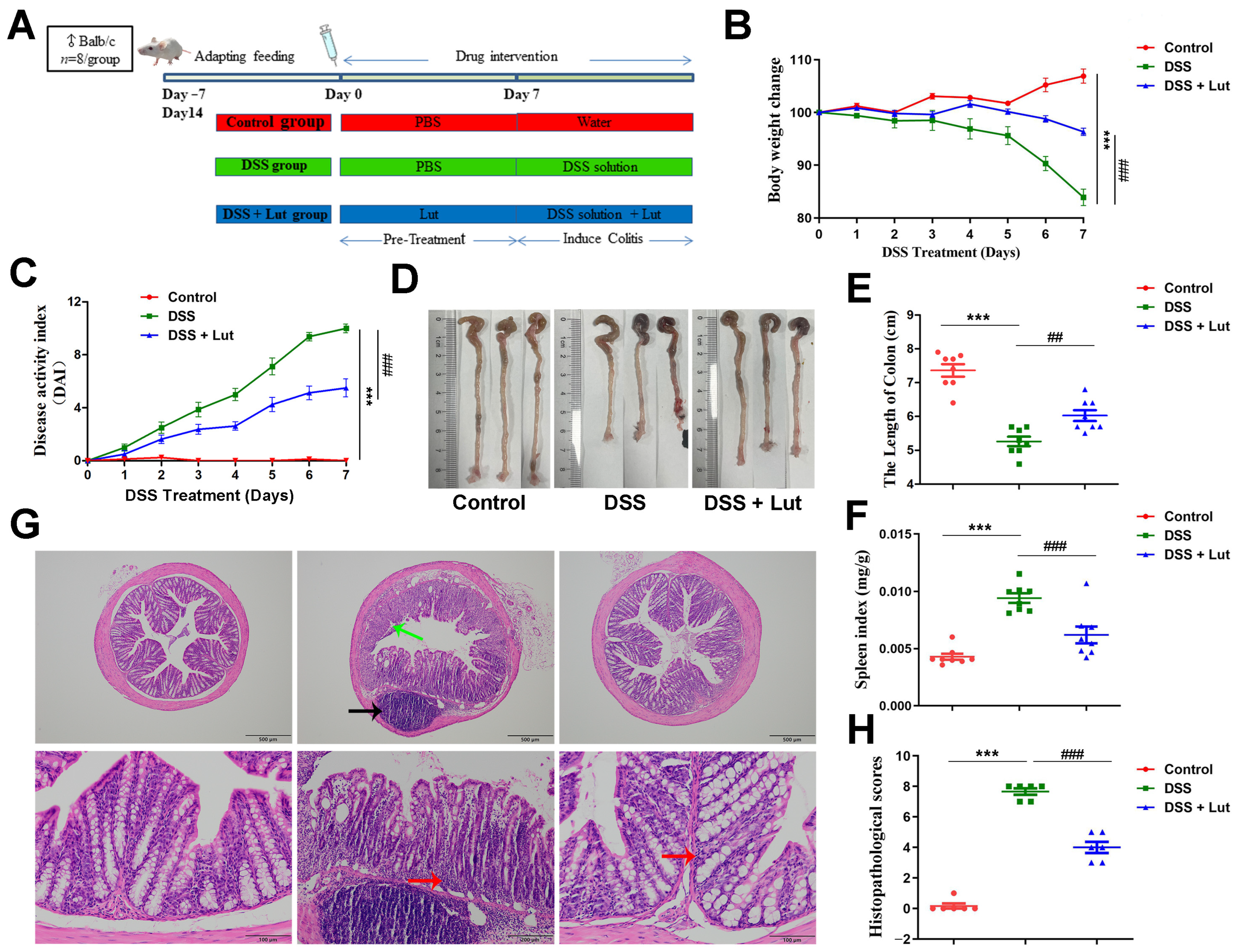
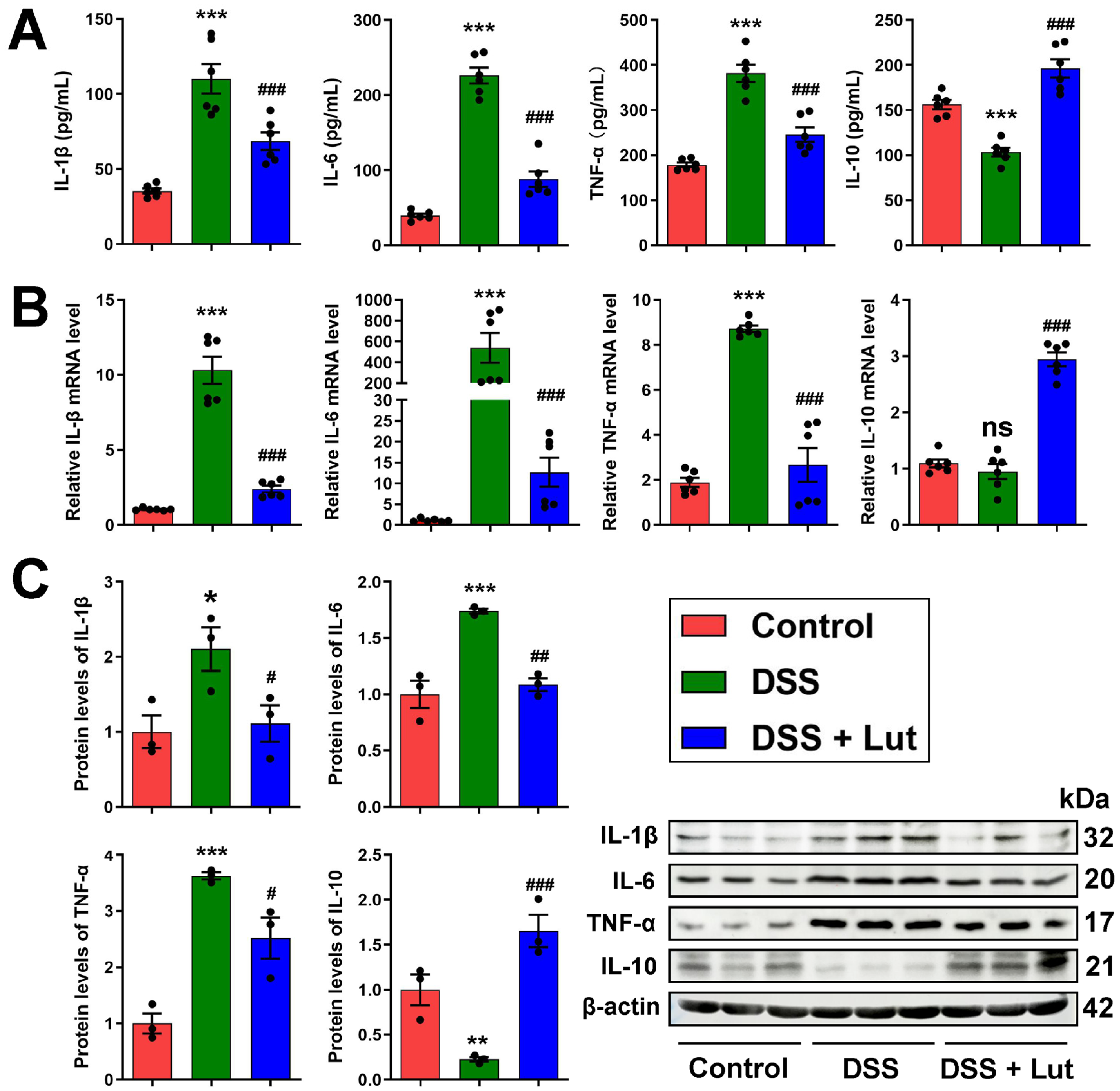
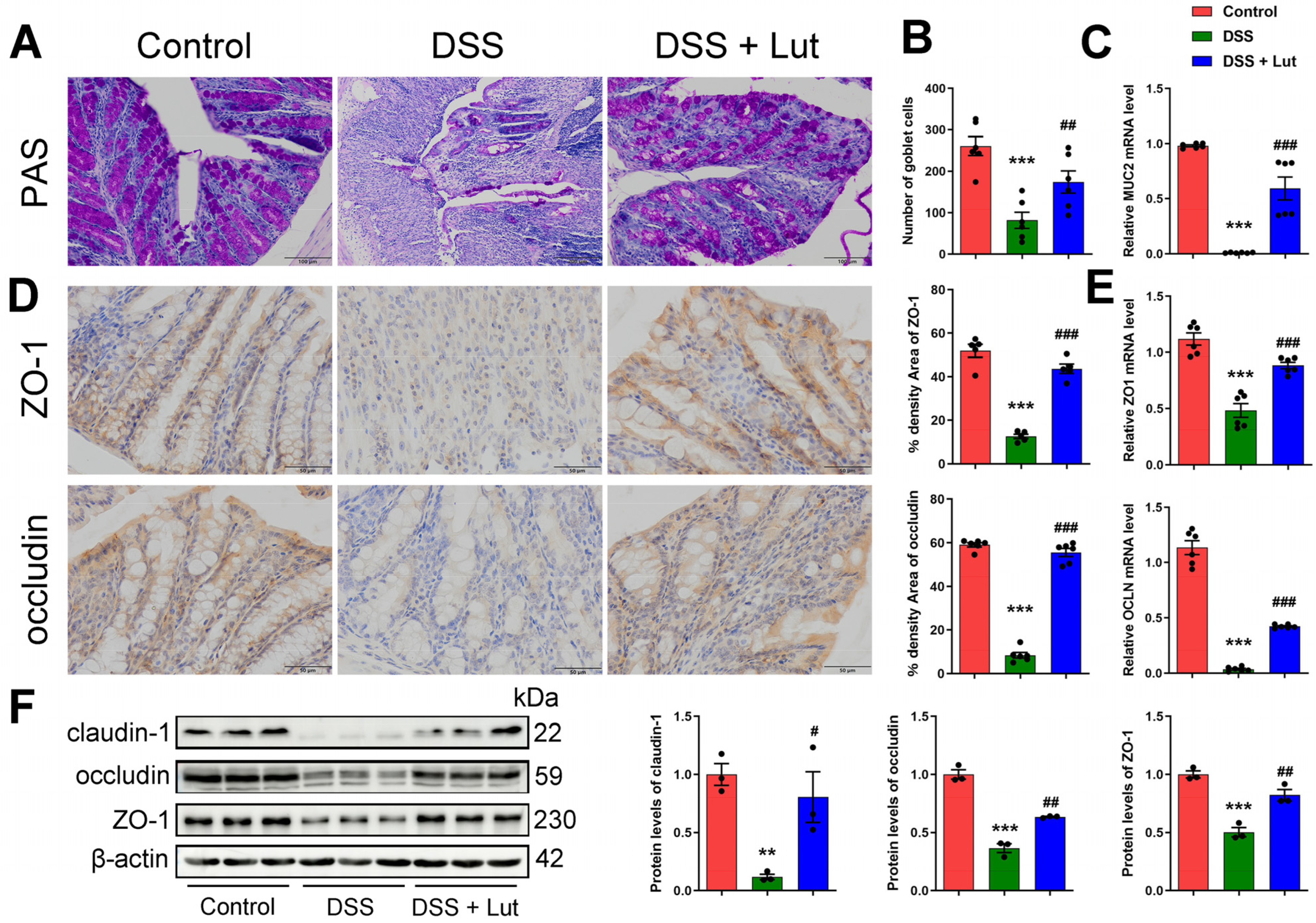

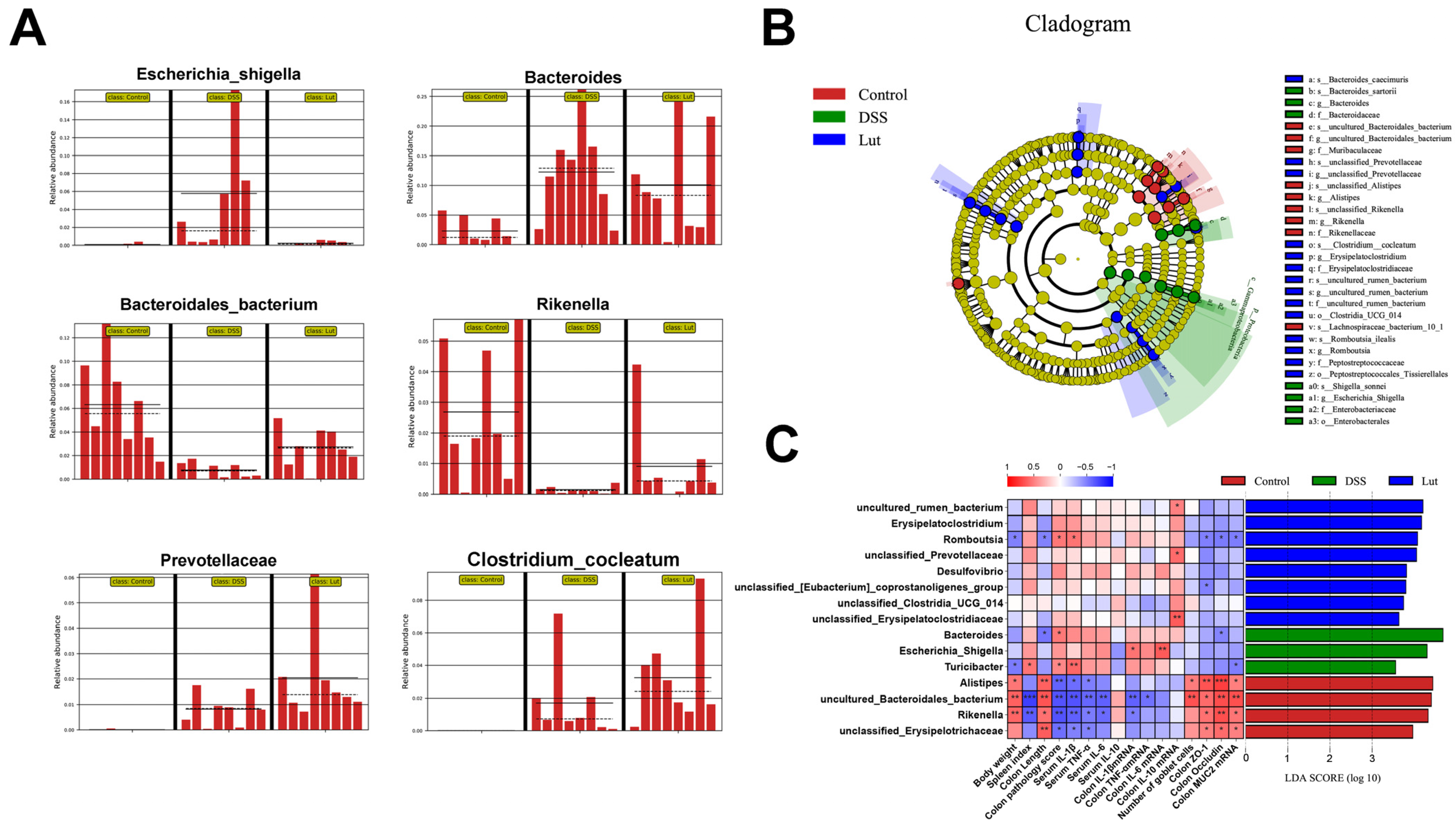
| Score | Weight Loss | Stool Characteristics | Degree of Hematochezia |
|---|---|---|---|
| 0 | <1% | Normal | Negative |
| 1 | 1–5% | Formed but adherent | Weak positive |
| 2 | 5–10% | Semi-formed/soft | Positive |
| 3 | 10–20% | Liquid-like consistency but does not adhere to the anus | Strong positive |
| 4 | >20% | Adheres to the anus | Visible blood in stool |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Duan, H.; Yan, Z.; Xue, C.; Niu, T.; Cheng, W.; Zhang, Y.; Zhao, X.; Hu, J.; Zhang, L. Luteolin Alleviates Ulcerative Colitis in Mice by Modulating Gut Microbiota and Plasma Metabolism. Nutrients 2025, 17, 203. https://doi.org/10.3390/nu17020203
Yang S, Duan H, Yan Z, Xue C, Niu T, Cheng W, Zhang Y, Zhao X, Hu J, Zhang L. Luteolin Alleviates Ulcerative Colitis in Mice by Modulating Gut Microbiota and Plasma Metabolism. Nutrients. 2025; 17(2):203. https://doi.org/10.3390/nu17020203
Chicago/Turabian StyleYang, Shuai, Hongwei Duan, Zhenxing Yan, Chen Xue, Tian Niu, Wenjing Cheng, Yong Zhang, Xingxu Zhao, Junjie Hu, and Lihong Zhang. 2025. "Luteolin Alleviates Ulcerative Colitis in Mice by Modulating Gut Microbiota and Plasma Metabolism" Nutrients 17, no. 2: 203. https://doi.org/10.3390/nu17020203
APA StyleYang, S., Duan, H., Yan, Z., Xue, C., Niu, T., Cheng, W., Zhang, Y., Zhao, X., Hu, J., & Zhang, L. (2025). Luteolin Alleviates Ulcerative Colitis in Mice by Modulating Gut Microbiota and Plasma Metabolism. Nutrients, 17(2), 203. https://doi.org/10.3390/nu17020203






