Effectiveness of Interventions to Improve Malnutrition Among Older Adults Living with Frailty Who Are Discharged from the Acute Setting: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
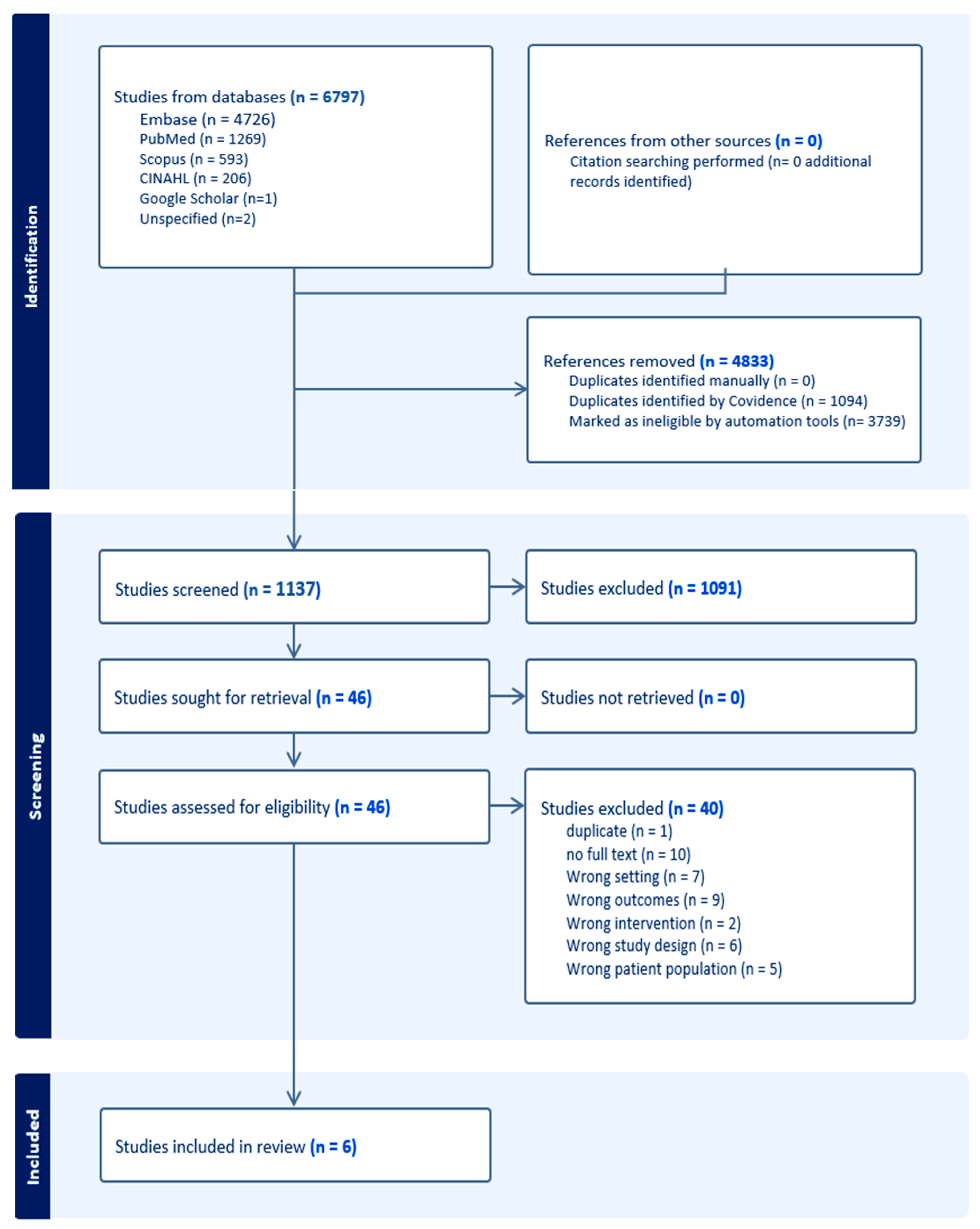
2.3. Search Strategy and Data Extraction
2.4. Quality Assessment
3. Results
3.1. Study Characteristics
3.2. Frailty
3.3. Characteristics of the Dietary Intervention and Control Groups
3.4. Nutrition Status
3.5. Dietary Intake
3.6. Impact of Nutrition Intervention on Frailty
3.7. Quality of Life and Readmissions
4. Discussion
4.1. Statement of Principal Findings
4.2. Strengths and Limitations of the Review
4.3. Implications of Results for Practice, Policy, and Future Research
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Pirlich, M.; Schütz, T.; Kemps, M.; Luhman, N.; Minko, N.; Lübke, H.J.; Rossnagel, K.; Willich, S.N.; Lochs, H. Social risk factors for hospital malnutrition. Nutrition 2005, 21, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.; et al. Diagnostic criteria for malnutrition–An ESPEN consensus statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Ligthart-Melis, G.C.; Luiking, Y.C.; Kakourou, A.; Cederholm, T.; Maier, A.B.; de van der Schueren, M.A.E. Frailty, Sarcopenia, and Malnutrition Frequently (Co-)occur in Hospitalized Older Adults: A Systematic Review and Meta-analysis. J. Am. Med. Dir. Assoc. 2020, 21, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Odlund Olin, A.; Koochek, A.; Ljungqvist, O.; Cederholm, T. Nutritional status, well-being and functional ability in frail elderly service f lat residents. Eur. J. Clin. Nutr. 2005, 59, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Haboubi, N. Assessment and management of nutrition in older people and its importance to health. Clin. Interv. Aging 2010, 5, 207–216. [Google Scholar] [CrossRef]
- Cass, A.R.; Charlton, K.E. Prevalence of hospital-acquired malnutrition and modifiable determinants of nutritional deterioration during inpatient admissions: A systematic review of the evidence. J. Hum. Nutr. Diet. 2022, 35, 1043–1058. [Google Scholar] [CrossRef]
- Lee, D.R.; Kawas, C.H.; Gibbs, L.; Corrada, M.M. Prevalence of frailty and factors associated with frailty in individuals aged 90 and older: The 90+ Study. J. Am. Geriatr. Soc. 2016, 64, 2257–2262. [Google Scholar] [CrossRef]
- Aspell, N.; O’Sullivan, M.; O’Shea, E.; Irving, K.; Duffy, C.; Gorman, R.; Warters, A. Predicting admission to long-term care and mortality among community-based, dependent older people in Ireland. Int. J. Geriatr. Psychiatry 2019, 34, 999–1007. [Google Scholar] [CrossRef]
- Kelly, S.; O’Brien, I.; Smuts, K.; O’Sullivan, M.; Warters, A. Prevalence of frailty among community dwelling older adults in receipt of low level home support: A cross-sectional analysis of the North Dublin Cohort. BMC Geriatr. 2017, 17, 121. [Google Scholar] [CrossRef] [PubMed]
- Griffin, A.; O’Neill, A.; O’Connor, M.; Ryan, D.; Tierney, A.; Galvin, R. The prevalence of malnutrition and impact on patient outcomes among older adults presenting at an Irish emergency department: A secondary analysis of the OPTI-MEND trial. BMC Geriatr. 2020, 20, 455. [Google Scholar] [CrossRef]
- Sullivan, E.S.; Rice, N. National Malnutrition Screening Survey Republic of Ireland 2023; Irish Society for Clinical Nutrition & Metabolism: Dublin, Ireland, 2024. [Google Scholar]
- Ba, C.E.B.; Jones, C.W.; Braz, V.A.; Swor, R.A.; Richmond, N.L.; Hwang, K.S.; Hollowell, A.G.; Weaver, M.A.; Platts-Mills, T.F. Risk factors for malnutrition among older adults in the emergency department: A Multicenter study. J. Am. Geriatr. Soc. 2017, 65, 1741–1747. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef]
- Beck, A.M.; Ovesen, L.; Schroll, M. Home-made oral supplement as nutritional support of old nursing home residents, who are undernourished or at risk of undernutrition based on the MNA. A pilot trial. Aging Clin. Exp. Res. 2002, 14, 212–215. [Google Scholar] [CrossRef]
- Singh, F.; Bernstein, M.A.; Ryan, A.D.; O’Neill, E.F.; Clements, K.M.; Evans, W.J. The effect of oral nutritional supplements on habitual dietary quality and quantity in frail elders. J. Nutr. Health Aging 2000, 4, 5–12. [Google Scholar]
- Parsons, E.L.; Stratton, R.J.; Cawood, A.L.; Smith, T.R.; Elia, M. Oral nutritional supplements in a randomised trial are more effective than dietary advice at improving quality of life in malnourished care home residents. Clin. Nutr. 2017, 36, 134–142. [Google Scholar] [CrossRef]
- Van Wymelbeke, V.; Brondel, L.; Bon, F.; Martin-Pfitzenmeyer, I.; Manckoundia, P. An innovative brioche enriched in protein and energy improves the nutritional status of malnourished nursing home residents compared to oral nutritional supplement and usual breakfast: FARINE+ project. Clin. Nutr. ESPEN 2016, 15, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.O.; Jeong, Y.; Park, Y.; Bae, J.S.; Kwon, Y.; Cho, M.; Yoo, C.H.; Lee, K.E. Reinforcement effects of social network intervention during nutritional supplementation in frail older adults. Gerontology 2021, 67, 620–632. [Google Scholar] [CrossRef]
- Nykänen, I.; Rissanen, T.H.; Sulkava, R.; Hartikainen, S. Effects of individual dietary counseling as part of a comprehensive geriatric assessment (CGA) on nutritional status: A population-based intervention study. J. Nutr. Health Aging 2014, 18, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, N.; Alvi, L.H.; Williams, A. Long-term support referrals to enhance food security and well-being in older adults: Texas physicians and nurses on what works. J. Public Health 2024, 32, 421–433. [Google Scholar] [CrossRef]
- Smoliner, C.; Norman, K.; Scheufele, R.; Hartig, W.; Pirlich, M.; Lochs, H. Effects of food fortification on nutritional and functional status in frail elderly nursing home residents at risk of malnutrition. Nutrition 2008, 24, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Integrated Care for Older People: Guidelines on Community-Level Interventions to Manage Declines in Intrinsic Capacity; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Beck, A.M.; Kjær, S.; Hansen, B.S.; Storm, R.L.; Thal-Jantzen, K.; Bitz, C. Follow-up home visits with registered dietitians have a positive effect on the functional and nutritional status of geriatric medical patients after discharge: A randomized controlled trial. Clin. Rehabil. 2013, 27, 483–493. [Google Scholar] [CrossRef]
- Beck, A.; Andersen, U.T.; Leedo, E.; Jensen, L.L.; Martins, K.; Quvang, M.; Rask, K.Ø.; Vedelspang, A.; Rønholt, F. Does adding a dietician to the liaison team after discharge of geriatric patients improve nutritional outcome: A randomised controlled trial. Clin. Rehabil. 2015, 29, 1117–1128. [Google Scholar] [CrossRef]
- Kramer, C.S.; Groenendijk, I.; Beers, S.; Wijnen, H.H.; Van de Rest, O.; De Groot, L.C. The association between malnutrition and physical performance in older adults: A systematic review and meta-analysis of observational studies. Curr. Dev. Nutr. 2022, 6, nzac007. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, S.; Liu, W.; Han, K.; Jia, W.; Liu, M.; He, Y. Malnutrition is an independent risk factor for low health-related quality of life among centenarians. Front. Med. 2021, 8, 729928. [Google Scholar] [CrossRef]
- Dent, E.; Wright, O.R.; Woo, J.; Hoogendijk, E.O. Malnutrition in older adults. Lancet 2023, 401, 951–966. [Google Scholar] [CrossRef]
- Reyes, B.; Diaz, S.; Engstrom, G.; Ouslander, J. Adherence to care transitions recommendations among high-risk hospitalized older patients. J. Am. Geriatr. Soc. 2021, 69, 1638–1645. [Google Scholar] [CrossRef]
- Skivington, K.; Matthews, L.; Simpson, S.A.; Craig, P.; Baird, J.; Blazeby, J.M.; Boyd, K.A.; Craig, N.; French, D.P.; McIntosh, E.; et al. A New Framework for Developing and Evaluating Complex Interventions: Update of Medical Research Council Guidance. BMJ 2021, 374, n2061. [Google Scholar] [CrossRef] [PubMed]
- Manzano, A. The Craft of Interviewing in Realist Evaluation. Evaluation 2016, 22, 342–360. [Google Scholar] [CrossRef]
- Vivanti, A.; Isenring, E.; Baumann, S.; Powrie, D.; O’NEill, M.; Clark, D.; Courtice, S.; Campbell, K.; Ferguson, M. Emergency department malnutrition screening and support model improves outcomes in a pilot randomised controlled trial. Emerg. Med. J. 2015, 32, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Thompson, C.H.; Kaambwa, B.; Shahi, R.; Hakendorf, P.; Miller, M. Investigation of the benefits of early malnutrition screening with telehealth follow up in elderly acute medical admissions. QJM Int. J. Med. 2017, 110, 639–647. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.P.M.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Cochrane Collaboration: London, UK, 2024. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. Short Physical Performance Battery (SPPB); APA PsycTests: Washington, DC, USA, 1994. [Google Scholar]
- Vellas, B.; Villars, H.; Abellan, G.; Soto, M.E.; Rolland, Y.; Guigoz, Y.; Morley, J.E.; Chumlea, W.; Salva, A.; Rubenstein, L.Z.; et al. Overview of the MNA®—Its History and Challenges. J. Nutr. Health Aging 2006, 10, 456–465. [Google Scholar]
- Ferguson, M.; Capra, S.; Bauer, J.; Banks, M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 1999, 15, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Guigoz, Y.; Lauque, S.; Vellas, B.J. Identifying the elderly at risk for malnutrition. The mini nutritional assessment. Clin. Geriatr. Med. 2002, 18, 737–757. [Google Scholar] [CrossRef]
- Thorsdottir, I.; Gunnarsdottir, I.; Eriksen, B. Screening method evaluated by nutritional status measurements can be used to detect malnourishment in chronic obstructive pulmonary disease. J. Am. Diet. Assoc. 2001, 101, 648–654. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Blondal, B.S.; Geirsdottir, O.G.; Halldorsson, T.I.; Beck, A.M.; Jonsson, P.V.; Ramel, A. HOMEFOOD randomised trial–six-month nutrition therapy in discharged older adults reduces hospital readmissions and length of stay at hospital up to 18 months of follow-up. J. Nutr. Health Aging 2023, 27, 632–640. [Google Scholar] [CrossRef]
- Blondal, B.S.; Geirsdottir, O.G.; Beck, A.M.; Halldorsson, T.I.; Jónsson, P.V.; Sveinsdottir, K.; Ramel, A. HOMEFOOD randomized trial—Beneficial effects of 6-month nutrition therapy on body weight and physical function in older adults at risk for malnutrition after hospital discharge. Eur. J. Clin. Nutr. 2023, 77, 45–54. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Hsu, L.-L.; Hsu, C.-C.; Hsieh, T.-J.; Su, S.-C.; Peng, Y.-W.; Guo, T.-M.; Kang, Y.-W.; Pan, W.-H. Dietary education with customised dishware and food supplements can reduce frailty and improve mental well-being in elderly people: A single-blind randomized controlled study. Asia Pac. J. Clin. Nutr. 2018, 27, 1018–1030. [Google Scholar]
- Andersen, A.L.; Houlind, M.B.; Nielsen, R.L.; Jørgensen, L.M.; Bengaard, A.K.; Bornæs, O.; Juul-Larsen, H.G.; Hansen, N.M.; Brøchner, L.D.; Hansen, R.G.; et al. Effectiveness of a multidisciplinary and transitional nutritional intervention compared with standard care on health-related quality of life among acutely admitted medical patients aged ≥ 65 years with malnutrition or risk of malnutrition: A randomized controlled trial. Clin. Nutr. ESPEN 2024, 61, 52–62. [Google Scholar]
- Lee, M.-C.; Wu, T.-Y.; Chen, Y.-M.; Hsiao, S.-H.; Tsai, C.-Y. Post-acute care for frail older people decreases 90-day emergency room visits, readmissions and mortality: An interventional study. PLoS ONE 2023, 18, e0279654. [Google Scholar] [CrossRef] [PubMed]
- Dorner, B.; Friedrich, E.K. Position of the Academy of Nutrition and Dietetics: Individualized nutrition approaches for older adults: Long-term care, post-acute care, and other settings. J. Acad. Nutr. Diet. 2018, 118, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Kong, J.; Underwood, C.; Petocz, P.; Hirani, V.; Dawson, B.; O’Leary, F. Systematic review andmeta-analysis of the effect of protein and amino acid supplements in older adults with acute or chronic conditions. Br. J. Nutr. 2018, 119, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Myint, M.W.W.; Wu, J.; Wong, E.; Chan, S.P.; To, T.S.J.; Chau, M.W.R.; Ting, K.H.; Fung, P.M.; Au, K.S.D. Clinical benefits of oral nutri-tional supplementation for elderly hip fracture patients: A single blind randomised controlled trial. Age Ageing 2013, 42, 39–45. [Google Scholar] [CrossRef]
- Williams, C.M.; Driver, L.T.; Older, J.; Dickerson, J.W. A controlledtrial of sip-feed supplements in elderly orthopaedic patients. Eur. J. Clin. Nutr. 1989, 43, 267–274. [Google Scholar]
- Bouillanne, O.; Curis, E.; Hamon-Vilcot, B.; Nicolis, I.; Chrétien, P.; Schauer, N.; Vincent, J.P.; Cynober, L.; Aussel, C. Impact of proteinpulse feeding on lean mass in malnourished and at-risk hospi-talized elderly patients: A randomized controlled trial. Clin. Nutr. 2013, 32, 186–192. [Google Scholar] [CrossRef]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Tribolet, P.; Bregenzer, T.; Braun, N.; et al. Individualised Nutritional Support in Medical Inpatients at Nutritional Risk: A Randomised Clinical Trial. Lancet 2019, 10188, 2312–2321. [Google Scholar] [CrossRef]
- Rasmussen, N.M.; Belqaid, K.; Lugnet, K.; Nielsen, A.L.; Rasmussen, H.H.; Beck, A.M. Effectiveness of multidisciplinary nutritional support in older hospitalised patients: A systematic review and meta-analyses. Clin. Nutr. ESPEN 2018, 27, 44–52. [Google Scholar] [CrossRef]
- Avenell, A.; Smith, T.O.; Curtain, J.P.; Mak, J.C.; Myint, P.K. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst. Rev. 2016, 11, CD001880. [Google Scholar] [CrossRef] [PubMed]
- Duncan, D.G.; Beck, S.J.; Hood, K.; Johansen, A. Using dietetic assistants to improve the outcome of hip fracture: A randomised controlled trial of nutritional support in an acute trauma ward. Age Ageing 2006, 35, 148–153. [Google Scholar] [CrossRef] [PubMed]
- van der Meij, B.S.; Wijnhoven, H.A.; Lee, J.S.; Houston, D.K.; Hue, T.; Harris, T.B.; Kritchevsky, S.B.; Newman, A.B.; Visser, M. Poor appetite and dietary intake in community-dwelling older adults. J. Am. Geriatr. Soc. 2017, 65, 2190–2197. [Google Scholar] [CrossRef]
- Shahar, D.R.; Yu, B.; Houston, D.K.; Kritchevsky, S.B.; Lee, J.S.; Rubin, S.M.; Sellmeyer, D.E.; Tylavsky, F.A.; Harris, T.B.; Health, Aging and Body Composition Study. Dietary factors in relation to daily activity energy expenditure and mortality among older adults. JNHA-J. Nutr. Health Aging 2009, 13, 414–420. [Google Scholar] [CrossRef]
- Dean, M.; Raats, M.M.; Grunert, K.G.; Lumbers, M. Factors influencing eating a varied diet in old age. Public Health Nutr. 2009, 12, 2421–2427. [Google Scholar] [CrossRef]
- Mathewson, S.L.; Azevedo, P.S.; Gordon, A.L.; Phillips, B.E.; Greig, C.A. Overcoming protein-energy malnutrition in older adults in the residential care setting: A narrative review of causes and interventions. Ageing Res. Rev. 2021, 70, 101401. [Google Scholar] [CrossRef]
- Trabal, J.; Farran-Codina, A. Effects of dietary enrichment with conventional foods onenergy and protein intake in older adults: A systematic review. Nutr. Rev. 2015, 73, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Munk, T.; Svendsen, J.A.; Knudsen, A.W.; Østergaard, T.B.; Thomsen, T.; Olesen, S.S.; Rasmussen, H.H.; Beck, A.M. A multimodal nutritional intervention after discharge improves quality of life and physical function in older patients e a randomized controlled trial. Clin. Nutr. 2021, 40, 5500–5510. [Google Scholar] [CrossRef]
- Nykänen, I.; Rissanen, T.H.; Sulkava, R.; Hartikainen, S. Effects of individual dietary counseling as part of a comprehensive geriatric assessment (CGA) on frailty status: A population based intervention study. J. Clin. Gerontol. Geriatr. 2012, 3, 89–93. [Google Scholar] [CrossRef]
- Rydwik, E.; Frandin, K.; Akner, G. Effects of a physical training and nutritional intervention program in frail elderly people regarding habitual physical activity level and activities of daily living-A randomized controlled pilot study. Arch. Gerontol. Geriatr. 2010, 51, 283–289. [Google Scholar] [CrossRef]
- Schuetz, P.; Sulo, S.; Walzer, S.; Vollmer, L.; Brunton, C.; Kaegi-Braun, N.; Stanga, Z.; Mueller, B.; Gomes, F. Cost savings associated with nutritional support in medical inpatients: An economic model based on data from a systematic review of randomised trials. BMJ Open. 2021, 11, e046402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, S.; Shafrin, J.; Kerr, K.W.; Schuetz, P. Health economic value of postacute oral nutritional supplementation in older adult medical patients at risk for malnutrition: A US-based modelling approach. BMJ Open. 2024, 14, e086787. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Philipson, T.J.; Snider, J.T.; Lakdawalla, D.N.; Stryckman, B.; Goldman, D.P. Impact of oral nutritional supplementation on hospital outcomes. Am. J. Manag. Care 2013, 19, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Mullin, G.E.; Fan, L.; Sulo, S.; Partridge, J. The association between oral nutritional supplements and 30-day hospital readmissions of malnourished patients at a US academic medical center. J. Acad. Nutr. Diet. 2019, 119, 1168–1175. [Google Scholar] [CrossRef]
- Sulo, S.; Vargas, J.; Gomez, G.; Misas, J.D.; Serralde-Zúñiga, A.E.; Correia, M.I.T. Hospital nutrition care informs potential cost-savings for healthcare: A budget impact analysis. Clin. Nutr. ESPEN 2021, 42, 195–200. [Google Scholar] [CrossRef]
- Wong, A.; Huang, Y.; Banks, M.D.; Sowa, P.M.; Bauer, J.D. A Cost–Consequence Analysis of Nutritional Interventions Used in Hospital Settings for Older Adults with or at Risk of Malnutrition. Healthcare 2024, 12, 1041. [Google Scholar] [CrossRef] [PubMed]
- Riley, K.; Sulo, S.; Dabbous, F.; Partridge, J.; Kozmic, S.; Landow, W.; VanDerBosch, G.; Falson, M.K.; Sriram, K. Reducing hospitalizations and costs: A home health nutrition-focused quality improvement program. J. Parenter. Enter. Nutr. 2020, 44, 58–68. [Google Scholar] [CrossRef]
- Hall, B.T.; Englehart, M.S.; Blaseg, K.; Wessel, K.; Stawicki, S.P.; Evans, D.C. Implementation of a dietitian-led enteral nutrition support clinic results in quality improvement, reduced readmissions, and cost savings. Nutr. Clin. Pract. 2014, 29, 649–655. [Google Scholar] [CrossRef]
- Mendonça, N.; Avgerinou, C.; Çavdar, S.; Cederholm, T.; Cruz-Jentoft, A.J.; Torbahn, G.; Sieber, C.; Visser, M. Critical outcomes to be included in the Core Outcome Set for nutritional intervention studies in older adults with malnutrition or at risk of malnutrition: A modified Delphi Study. Eur. J. Clin. Nutr. 2024, 78, 663–669. [Google Scholar] [CrossRef]
- Abuzied, Y. A practical guide to the kaizen approach as a quality improvement tool. Glob. J. Qual. Saf. Healthc. 2022, 5, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Christoff, P. Running PDSA cycles. Curr. Probl. Pediatr. Adolesc. Health Care 2018, 48, 198–201. [Google Scholar] [CrossRef]
- Wakai, T.; Simasek, M.; Nakagawa, U.; Saijo, M.; Fetters, M.D. Screenings during well-child visits in primary care: A quality improvement study. J. Am. Board Fam. Med. 2018, 31, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Vordenberg, S.E.; Smith, M.A.; Diez, H.L.; Remington, T.L.; Bostwick, J.R. Using the plan-do-study-act (PDSA) model for continuous quality improvement of an established simulated patient program. Innov. Pharm. 2018, 9, 1. [Google Scholar] [CrossRef] [PubMed]
| Domain | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Adults aged ≥ 65 years living with frailty and identified as malnourished at discharge from acute care settings | Individuals < 65 years or without frailty and malnutrition |
| Intervention | Nutrition-based interventions (e.g., ONS, dietary counselling, meal programmes) | Interventions without a nutrition component |
| Comparator | Standard care or other active interventions | No comparator group |
| Outcomes | Primary; change in nutritional status. Secondary; physical function, frailty score, food intake, quality of life, hospital readmission, mortality and stakeholders’ perspectives on the model of nutritional care (e.g., acceptability, feasibility, perceived benefits and challenges, experiences, and priorities of patients, caregivers, clinicians, and decision-makers). | No relevant outcomes reported |
| Setting | Discharged from acute care settings (e.g., hospital wards, emergency departments) | Long-term care residents, nursing home patients, or those not discharged from acute care |
| Study Design | Randomised controlled trials (RCTs) or studies with a randomised design | Non-randomised studies, observational studies, case reports |
| Language | Any language with a full English translation or full English text | Studies without English abstract or inaccessible full text |
| Author | Participants | Sex | Mean Age | Healthcare Setting | Comorbidities |
|---|---|---|---|---|---|
| [44] | N = 106 > 65 years IG: n = 53 CG: n = 53 | IG: 71.7% F CG: 52.8% F | IG: 83.3 ± 6.7 CG: 81.8 ± 6.0 | Discharge home from the hospital within a day of recruitment | N/A |
| [45] | N = 106 > 65 years IG: n = 53 CG: n = 53 | IG: 71.7% F CG: 52.8% F | IG: 83.3 ± 6.7 CG: 81.8 ± 6.0 | Patients were discharged home to independent living from the hospital | N/A |
| [46] | N = 37 pre-frail or frail Modified L. Fried criteria; without severe disease (e.g., cancers under treatment, immobilisation, or severe arthritis), diagnosed dementia, mental illness, or an inability to communicate | N = 17 M N = 20 F | 74 | Hospital | Hypertension Control: 6 (60%) Group 2: 6 (75%) Group 3: 5 (55.6%) Group 4: 6 (66.7%) Diabetes Control: 2 (20%) Group 2: 3 (37.5%) Group 3: 2 (22.2%) Group 4: 3 (33.3%) |
| [33] | N = 24 >60 years screening positive for risk of malnutrition using the malnutrition screening tool excluding category 1, nursing home resident or under dietetics already | 42% M | 79.0 ± 7.7 | Emergency Department | N/A |
| [47] | N = 130 IG n = 65 CG n = 65 | CG: n (%): 26(36.9) n (%): 26(36.9) IG: | CG: 80.2 (7.1) IG: 79.2 (7.0) | Emergency Department | CG: Abnormal clinical and laboratory finding n(%): 8 (13.1), Diseases of respiratory system: 23 (37.7), Other diseases 30 (49.2) IG: Abnormal clinical and laboratory finding n(%): 12 (21.8), Diseases of respiratory system: 18 (32.7), Other diseases 25 (45.5) |
| [48] | N = 254 | IG M: 101 (49.3%) CG M:28 (57.1%) | IG: 87.6 ± 6.0 CG: 85.2 ± 6.0 | Emergency Department | The most prevalent diagnosis was dementia (40.5%) for the PAC group and CKD (73.5%) for the control group (p < 0.001) |
| Study | Nutritional Measure | Control (CG) | Intervention (IG) |
|---|---|---|---|
| [44,45] | ISNST score | 4.5 ± 1.3 | 5.1 ± 1.7 |
| BMI (kg/m2) | 26.9 ± 5.3 | 28.5 ± 6.5 | |
| Fat-free mass (kg) | 49.1 ± 11.9 | 48.1 ± 10.2 | |
| [46] | BMI (kg/m2) | 24.6 ± 1.1 | 25.5 ± 0.9 (multinutrient)/25.5 ± 1.1 (multinutrient + soy)/28.4 ± 1.2 (nutrition education) |
| MNA-SF (% normal) | 100% | 100%/88.9%/100% | |
| [33] | MST ≥ 2 | – | 88% |
| [47] | Body weight (kg) | 70.9 ± 19.4 | 69.5 ± 15.9 |
| [48] | MNA (% normal) | 29.8% | 8.9% |
| Study | Frailty/Functional Measure | Control (CG) | Intervention (IG) |
|---|---|---|---|
| [44,45] | Handgrip strength (kg) | 21.5 ± 8.5 | 19.7 ± 6.8 |
| SPPB score | 2.4 ± 2.0 | 2.5 ± 1.8 | |
| Pre-frail (%) | 80 | 87.5/77.8/88.9 | |
| [46] | Frail (Fried’s criteria) | 62.5% | 71.9% |
| [33] | Frailty (CFS) Mild Moderate Severe | 32.6% 28.6%38.8% | 14.2% 35.1%50.7% |
| [47] | ADL dependence IADL dependence | 5 (10.2%) 0 (0%) | 28 (13.7%) 7 (3.4%) |
| Study (Ref.) | Intervention Team | Screening Tools | Physical Function Measures | Intervention Characteristics | Control Group | Follow-Up Care and Duration | Primary Outcome(s) | Secondary Outcome(s) |
|---|---|---|---|---|---|---|---|---|
| [44] | Clinical nutritionists | MNA-FF, ISNST | SPPB | Individual therapy: 5 in-person + 3 phone calls; home-delivered energy/protein-rich meals (1 hot meal + 2 snacks/day, with/without ONS) | Nutrition recommendations for older adults; encouraged Meals on Wheels (MOW) | Community follow-up; home visits; 1-, 6-, 12-, and 18 months post-discharge | Hospital readmissions and Length of stay | Mortality and need for long-term care residence |
| [45] | Clinical nutritionists | ISNST | SPPB | Nutrition counselling for community-dwelling older adults | Nutrition recommendations + encouraged MOW | Community/primary care setting; 6 months | Energy- and protein intake, body weight and physical function | Anthropometric measurements, nutritional status, muscular strength, dietary intake, exercise, and reported food-related digestion issues, such as diarrhoea, nausea, constipation, or stomach pain. |
| [46] | Registered dietitian, trained researchers | MNA-SF, MST | Grip strength, gait speed | Four groups: (1) Daily food guide; (2) + micronutrient supplements; (3) + micronutrients + soy protein; (4) + individualised nutrition education, customised dishware, food supplements | Daily food guide | Community/primary care setting; 1 month and 3 months | Dietary intake | Comprehensive geriatric assessment including a nutritional status assessment, modified L. Fried’s frailty assessment, and depressive symptoms assessment |
| [33] | Registered dietitian, MDT (nurses, geriatricians, PT, OT, speech therapists, pharmacists, social workers) | Not reported | Not specified | Individualised dietary counselling | No information on standard care | Yes, post-discharge; 12 weeks | Body weight, quality of life, depression, falls history and days of hospital admissions | |
| [47] | Registered dietitian, MDT | MNA-SF | STS test | Individualised dietary counselling | Standard care: daily dietary records validated by dietitian | Yes, post-discharge; 8 and 16 weeks after discharge | Health-related quality of life | Intake of energy and protein, body weight, well-being, hand grip strength, frailty |
| [48] | Registered dietitian, MDT | Not reported | CFS | Individualised dietary counselling | Dietary counselling during hospitalisation | Yes, post-discharge; 90 days | Emergency room visits, readmissions, and mortality |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarier, C.; Walsh, S.; Bowers, S.; O’Connor, M.; Mohamed, A.; Keller, H.; Ford, K.L.; Galvin, R.; Griffin, A. Effectiveness of Interventions to Improve Malnutrition Among Older Adults Living with Frailty Who Are Discharged from the Acute Setting: A Systematic Review. Nutrients 2025, 17, 3181. https://doi.org/10.3390/nu17193181
Sarier C, Walsh S, Bowers S, O’Connor M, Mohamed A, Keller H, Ford KL, Galvin R, Griffin A. Effectiveness of Interventions to Improve Malnutrition Among Older Adults Living with Frailty Who Are Discharged from the Acute Setting: A Systematic Review. Nutrients. 2025; 17(19):3181. https://doi.org/10.3390/nu17193181
Chicago/Turabian StyleSarier, Cerenay, Siobhan Walsh, Sheila Bowers, Margaret O’Connor, Ahmed Mohamed, Heather Keller, Katherine L. Ford, Rose Galvin, and Anne Griffin. 2025. "Effectiveness of Interventions to Improve Malnutrition Among Older Adults Living with Frailty Who Are Discharged from the Acute Setting: A Systematic Review" Nutrients 17, no. 19: 3181. https://doi.org/10.3390/nu17193181
APA StyleSarier, C., Walsh, S., Bowers, S., O’Connor, M., Mohamed, A., Keller, H., Ford, K. L., Galvin, R., & Griffin, A. (2025). Effectiveness of Interventions to Improve Malnutrition Among Older Adults Living with Frailty Who Are Discharged from the Acute Setting: A Systematic Review. Nutrients, 17(19), 3181. https://doi.org/10.3390/nu17193181







