Preventive Effects of an Opuntia stricta var. dillenii Extract on Lipid Metabolism in a High-Fat High-Fructose Diet-Induced Obesity Animal Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Opuntia stricta var. dillenii Extract
2.3. Animals, Diets, and Experimental Design
2.4. Serum Parameters
2.5. Adipocyte Morphometric Analysis
2.6. Immunoblotting for Protein Expression Measurement
2.7. Enzyme Activity in Adipose Tissue
2.8. Statistical Analysis
3. Results
3.1. Food and Energy Intake, Total Body Weight, Adipose Pad Weights, and White Adipose Index
3.2. Serum Biochemical Parameters
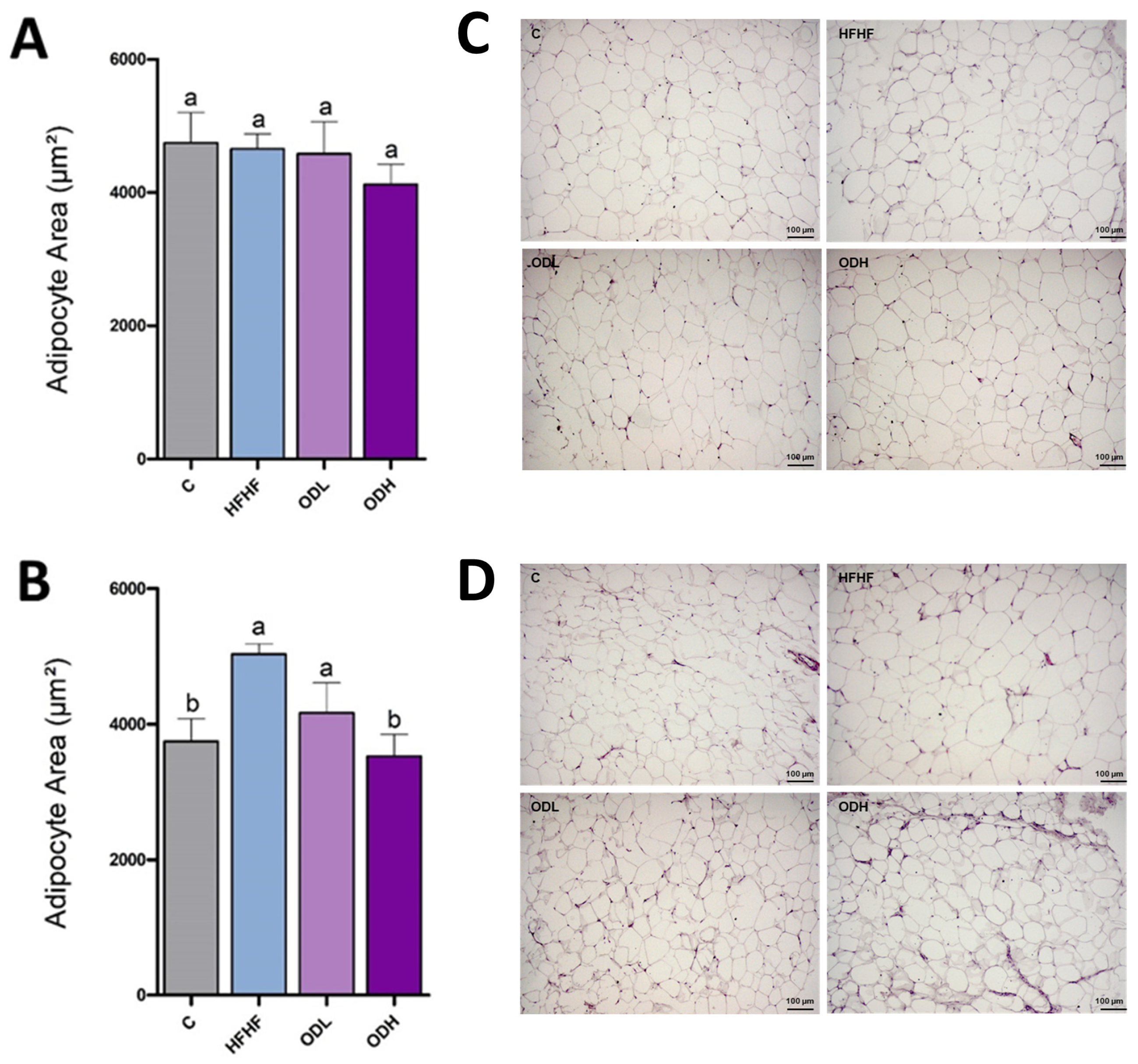
3.3. Histological Analysis
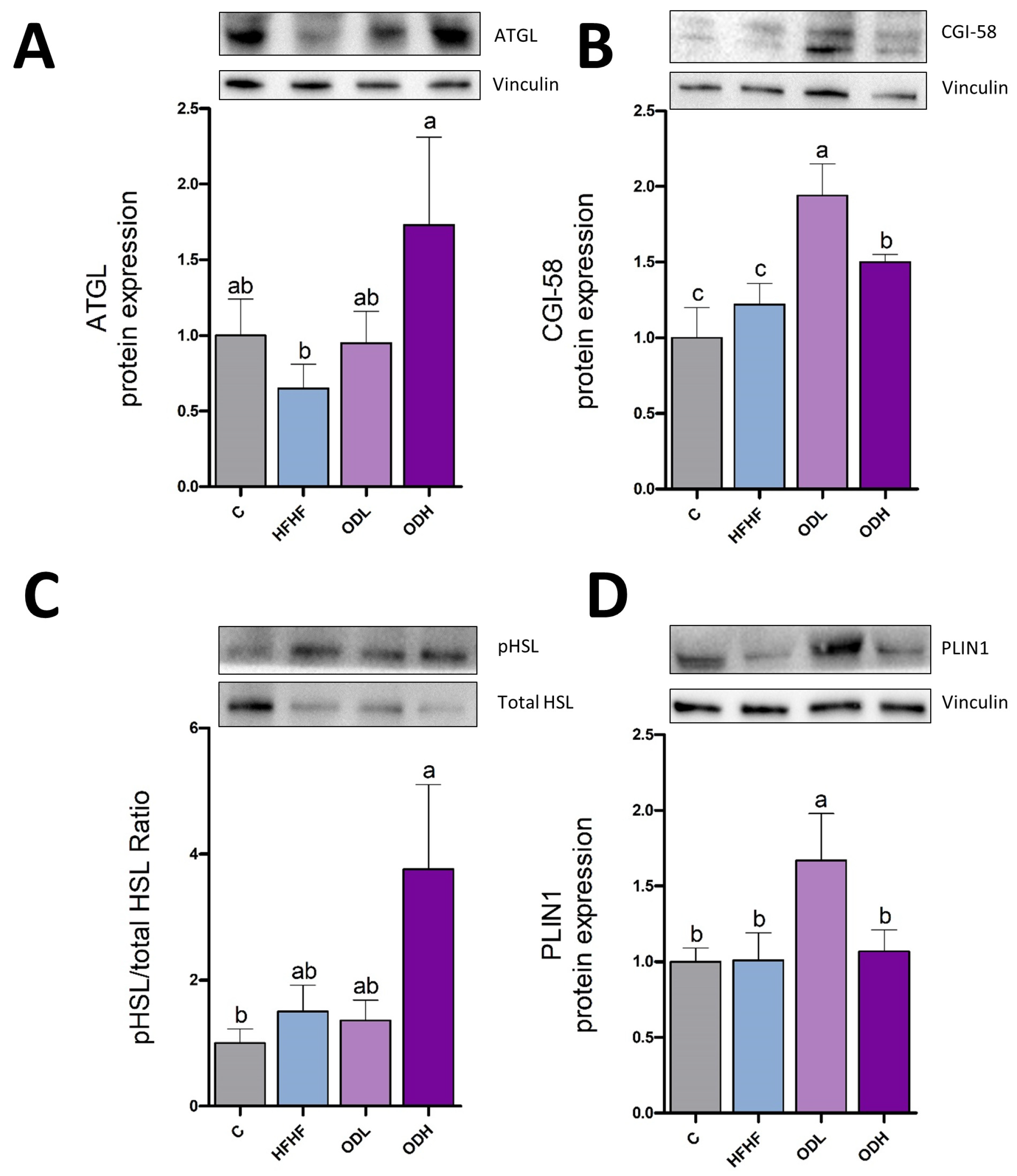
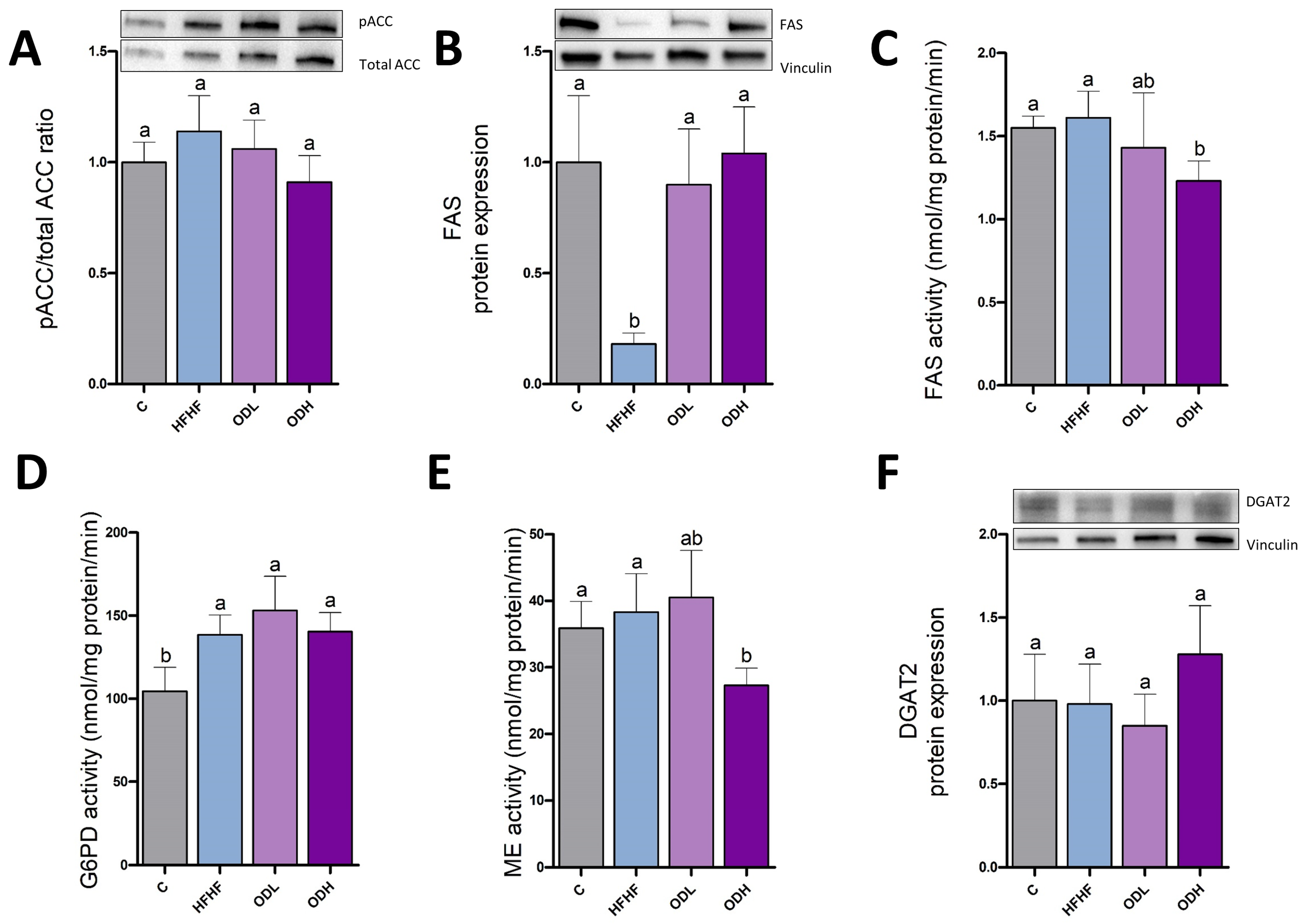
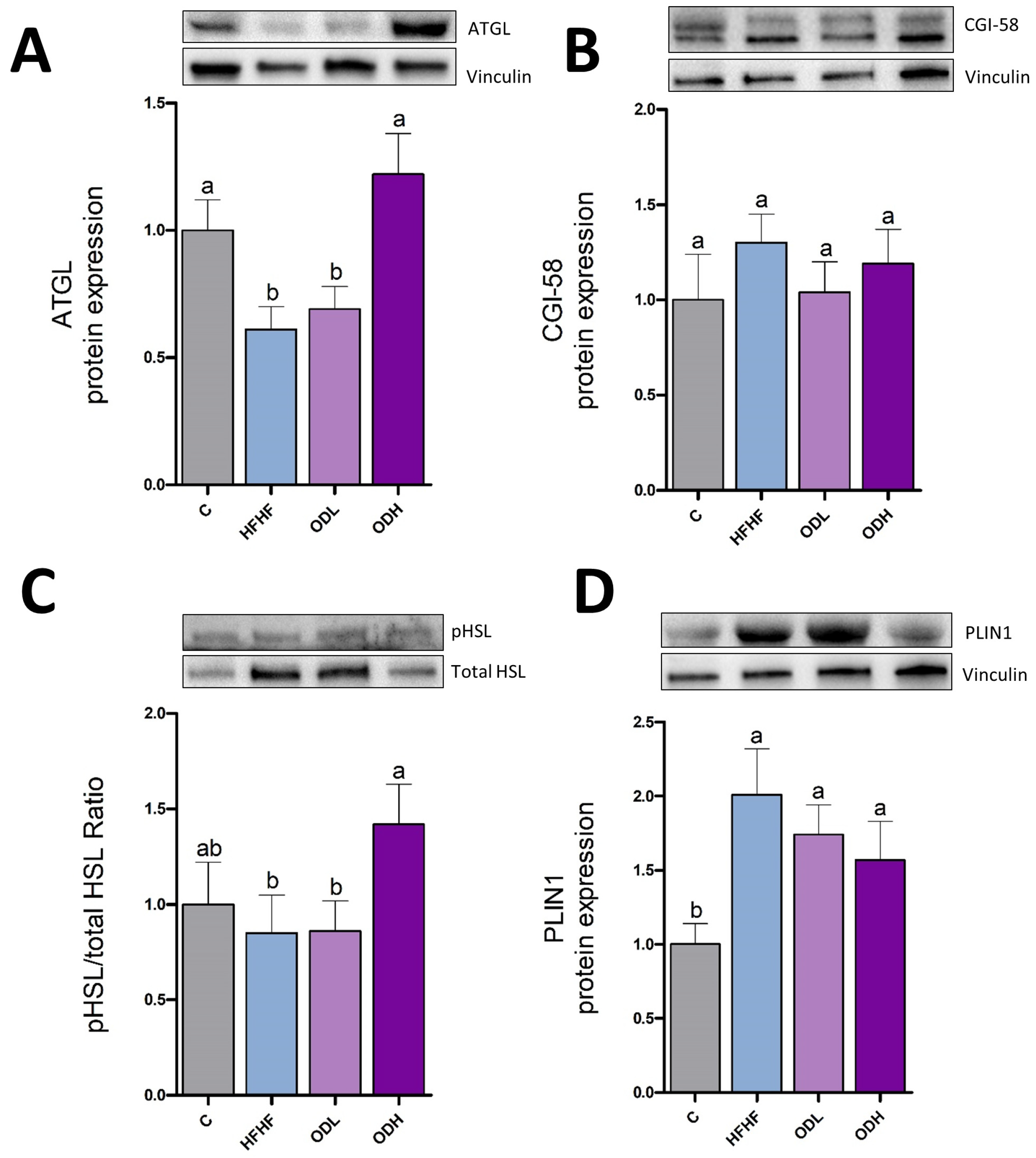
3.4. Protein Expression and Enzymatic Activities in Epididymal Tissue
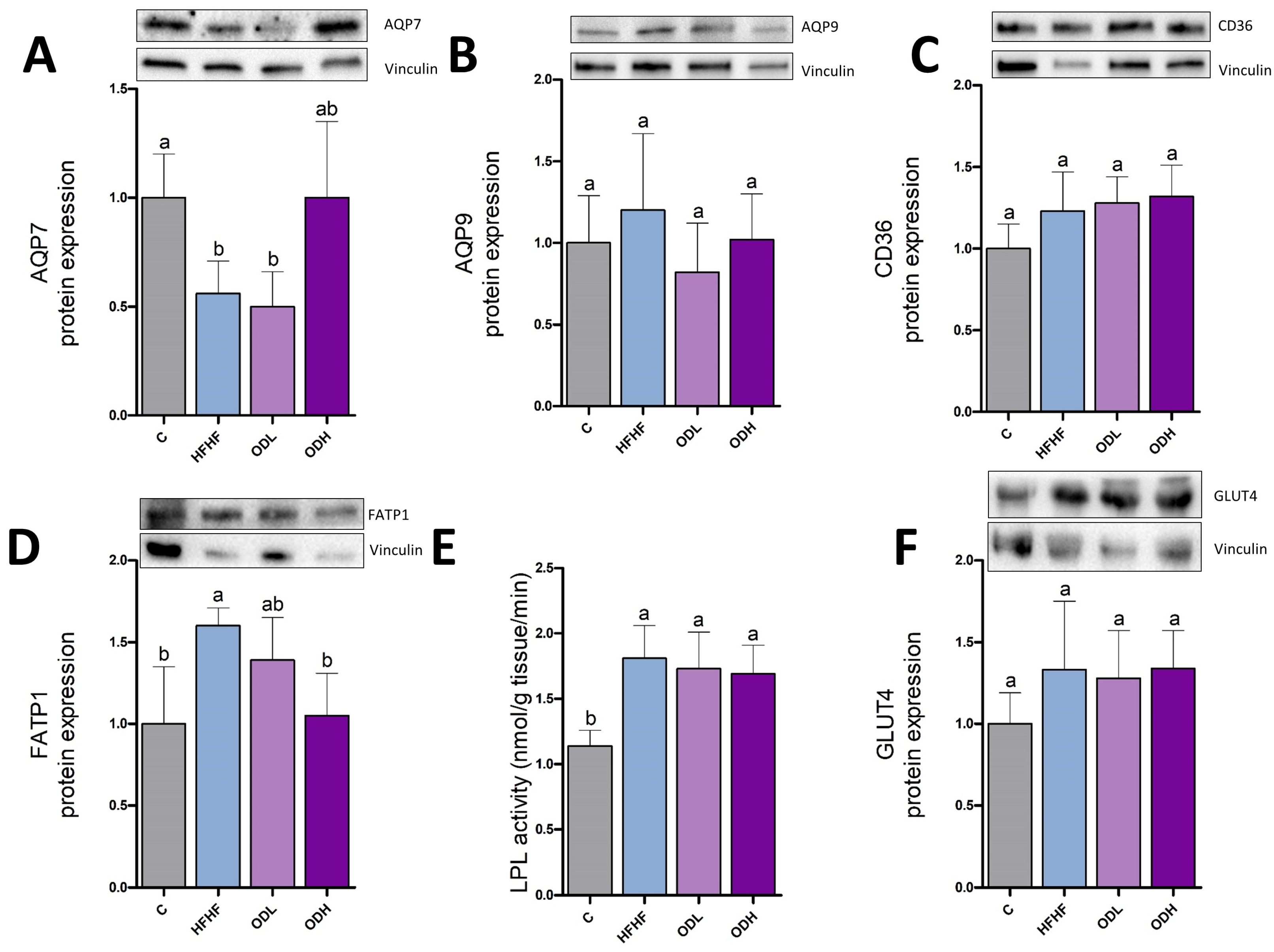
3.5. Protein Expression and Enzymatic Activities in Subcutaneous Tissue
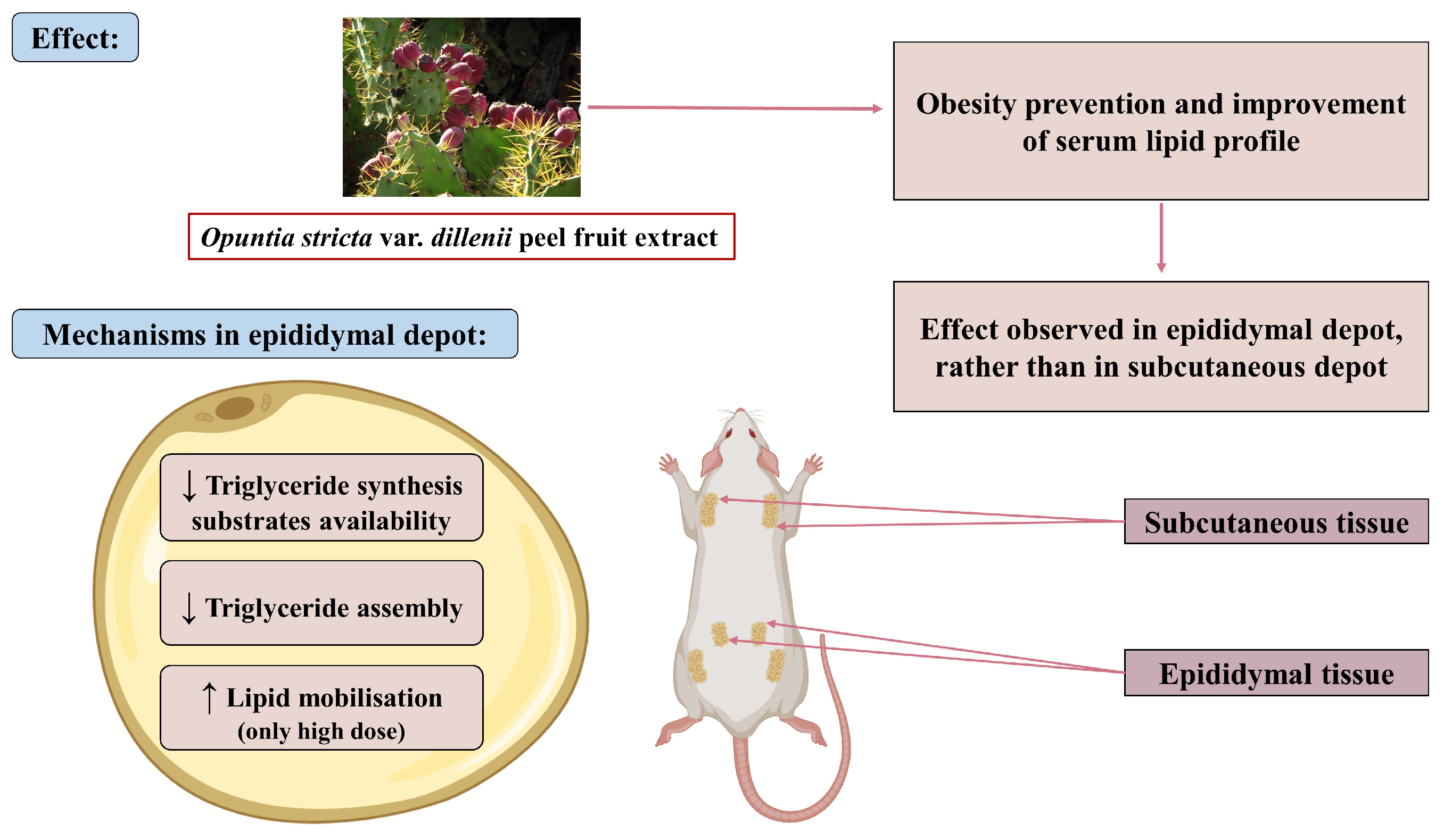
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, Y.; Pitchumoni, C.S. Obesity, Obesities and Gastrointestinal Cancers. Dis. Mon. 2023, 69, 101592. [Google Scholar] [CrossRef]
- World Health Organization, Regional Office for Europe. WHO European Regional Obesity Report 2022; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2025. [Google Scholar]
- World Health Organization, Americas Region (PAHO). Nine Latin American and Caribbean Countries Intensify Efforts to Curb Obesity with PAHO Support; World Health Organization, Americas Region: Washington, DC, USA, 2025. [Google Scholar]
- World Health Organization. Obesity and Overweight; World Health Organization: Geneva, Switzerland, 2025. [Google Scholar]
- Ramirez-Rodriguez, Y.; Martinez-Huelamo, M.; Pedraza-Chaverri, J.; Ramirez, V.; Martinez-Taguena, N.; Trujillo, J. Ethnobotanical, Nutritional and Medicinal Properties of Mexican Drylands Cactaceae Fruits: Recent Findings and Research Opportunities. Food Chem. 2020, 312, 126073. [Google Scholar] [CrossRef]
- Rahimi, P.; Abedimanesh, S.; Mesbah-Namin, S.A.; Ostadrahimi, A. Betalains, the Nature-Inspired Pigments, in Health and Diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 2949–2978. [Google Scholar] [CrossRef] [PubMed]
- Perucini-Avendano, M.; Nicolas-Garcia, M.; Jimenez-Martinez, C.; Perea-Flores, M.d.J.; Gomez-Patino, M.B.; Arrieta-Baez, D.; Davila-Ortiz, G. Cladodes: Chemical and Structural Properties, Biological Activity, and Polyphenols Profile. Food Sci. Nutr. 2021, 9, 4007–4017. [Google Scholar] [CrossRef]
- Madrigal-Santillan, E.; Portillo-Reyes, J.; Madrigal-Bujaidar, E.; Sanchez-Gutierrez, M.; Izquierdo-Vega, J.A.; Izquierdo-Vega, J.; Delgado-Olivares, L.; Vargas-Mendoza, N.; Alvarez-Gonzalez, I.; Morales-Gonzalez, A.; et al. Opuntia Spp. in Human Health: A Comprehensive Summary on its Pharmacological, Therapeutic and Preventive Properties. Part 2. Plants 2022, 11, 2333. [Google Scholar] [CrossRef]
- Sanchez-Tapia, M.; Aguilar-Lopez, M.; Perez-Cruz, C.; Pichardo-Ontiveros, E.; Wang, M.; Donovan, S.M.; Tovar, A.R.; Torres, N. Nopal (Opuntia Ficus Indica) Protects from Metabolic Endotoxemia by Modifying Gut Microbiota in Obese Rats Fed High Fat/Sucrose Diet. Sci. Rep. 2017, 7, 4716. [Google Scholar] [CrossRef]
- Bounihi, A.; Bitam, A.; Bouazza, A.; Yargui, L.; Koceir, E.A. Fruit Vinegars Attenuate Cardiac Injury Via Anti-Inflammatory and Anti-Adiposity Actions in High-Fat Diet-Induced Obese Rats. Pharm. Biol. 2017, 55, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Helies-Toussaint, C.; Fouche, E.; Naud, N.; Blas-Y-Estrada, F.; Del Socorro Santos-Diaz, M.; Negre-Salvayre, A.; Barba de la Rosa, A.P.; Gueraud, F. Opuntia Cladode Powders Inhibit Adipogenesis in 3 T3-F442A Adipocytes and a High-Fat-Diet Rat Model by Modifying Metabolic Parameters and Favouring Faecal Fat Excretion. BMC Complement. Med. Ther. 2020, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Shirazinia, R.; Rahimi, V.B.; Kehkhaie, A.R.; Sahebkar, A.; Rakhshandeh, H.; Askari, V.R. Opuntia Dillenii: A Forgotten Plant with Promising Pharmacological Properties. J. Pharmacopunct. 2019, 22, 16–27. [Google Scholar] [CrossRef]
- Besne-Eseverri, I.; Trepiana, J.; Gomez-Zorita, S.; Antunes-Ricardo, M.; Cano, M.P.; Portillo, M.P. Beneficial Effects of Opuntia Spp. on Liver Health. Antioxidants 2023, 12, 1174. [Google Scholar] [CrossRef]
- The Food and Agriculture Organization of the United Nations and the International Center for Agricultural Research in the Dry Areas. Crop Ecology, Cultivation and Uses of Cactus Pear, 1st ed.; FAO: Rome, Italy, 2017; p. 244. [Google Scholar]
- Manzur-Valdespino, S.; Ramírez-Moreno, E.; Arias-Rico, J.; Jaramillo-Morales, O.A.; Calderón-Ramos, Z.G.; Delgado-Olivares, L.; Córdoba-Díaz, M.; Córdoba-Díaz, D.; Cruz-Cansino, N.D.S. Opuntia ficus-indica L. Mill Residues—Properties and Application Possibilities in Food Supplements. Appl. Sci. 2020, 10, 3260. [Google Scholar] [CrossRef]
- Gheribi, R.; Habibi, Y.; Khwaldia, K. Prickly Pear Peels as a Valuable Resource of Added-Value Polysaccharide: Study of Structural, Functional and Film Forming Properties. Int. J. Biol. Macromol. 2019, 126, 238–245. [Google Scholar] [CrossRef]
- United Nations. United Nations Development Programme (UNDP). 2025. Available online: https://www.undp.org/sustainable-development-goals (accessed on 1 September 2025).
- Díaz-Delgado, G.L.; Rodríguez-Rodríguez, E.M.; Ríos, D.; Cano, M.P.; Lobo, M.G. Morphological Characterization of Opuntia Accessions from Tenerife (Canary Islands, Spain) using UPOV Descriptors. Horticulturae 2024, 10, 662. [Google Scholar] [CrossRef]
- Gomez-Lopez, I.; Lobo-Rodrigo, G.; Portillo, M.P.; Cano, M.P. Ultrasound-Assisted “Green” Extraction (UAE) of Antioxidant Compounds (Betalains and Phenolics) from Opuntia Stricta Var. Dilenii’s Fruits: Optimization and Biological Activities. Antioxidants 2021, 10, 1786. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, E.; Reynes, B.; Diaz-Rua, R.; Ceresi, E.; Oliver, P.; Palou, A. The Intake of High-Fat Diets Induces the Acquisition of Brown Adipocyte Gene Expression Features in White Adipose Tissue. Int. J. Obes. 2015, 39, 1619–1629. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.; Fernandez-Quintela, A.; Churruca, I.; Rodriguez, V.M.; Simon, E.; Portillo, M.P. Hepatomegaly Induced by Trans-10,Cis-12 Conjugated Linoleic Acid in Adult Hamsters Fed an Atherogenic Diet is Not Associated with Steatosis. J. Am. Coll. Nutr. 2009, 28, 43–49. [Google Scholar] [CrossRef]
- Mambrini, S.P.; Menichetti, F.; Ravella, S.; Pellizzari, M.; De Amicis, R.; Foppiani, A.; Battezzati, A.; Bertoli, S.; Leone, A. Ultra-Processed Food Consumption and Incidence of Obesity and Cardiometabolic Risk Factors in Adults: A Systematic Review of Prospective Studies. Nutrients 2023, 15, 2583. [Google Scholar] [CrossRef]
- Borah, A.K.; Sharma, P.; Singh, A.; Kalita, K.J.; Saha, S.; Chandra Borah, J. Adipose and Non-Adipose Perspectives of Plant Derived Natural Compounds for Mitigation of Obesity. J. Ethnopharmacol. 2021, 280, 114410. [Google Scholar] [CrossRef]
- Chen, X.; Pan, S.; Li, F.; Xu, X.; Xing, H. Plant-Derived Bioactive Compounds and Potential Health Benefits: Involvement of the Gut Microbiota and its Metabolic Activity. Biomolecules 2022, 12, 1871. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; P, N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; K, S.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Ahmad, B.; Friar, E.P.; Vohra, M.S.; Garrett, M.D.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Mechanisms of Action for the Anti-Obesogenic Activities of Phytochemicals. Phytochemistry 2020, 180, 112513. [Google Scholar] [CrossRef]
- Gomez-Lopez, I.; Eseberri, I.; Cano, M.P.; Portillo, M.P. Anti-Obesity Effect of Different Opuntia Stricta Var. Dillenii’s Prickly Pear Tissues and Industrial by-Product Extracts in 3T3-L1 Mature Adipocytes. Nutrients 2024, 16, 499. [Google Scholar] [CrossRef]
- Besne-Eseverri, I.; Trepiana, J.; Boutaleb, L.; Martin, M.A.; Krisa, S.; Lobo, M.G.; Cano, M.P.; Portillo, M.P. Anti-Steatotic Effect of Opuntia Stricta Var. Dillenii Prickly Pear Extracts on Murine and Human Hepatocytes. Int. J. Mol. Sci. 2025, 26, 2864. [Google Scholar] [CrossRef]
- Li, J.; Yu, X.; Pan, W.; Unger, R.H. Gene Expression Profile of Rat Adipose Tissue at the Onset of High-Fat-Diet Obesity. Am. J. Physiol. Endocrinol. Metab. 2002, 282, 1334. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and Metabolic Health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Feboli, A.; Laurentiz, A.C.; Soares, S.C.S.; Augusto, J.G.; Anjos, L.A.; Magalhaes, L.G.; Filardi, R.S.; Laurentiz, R.S. Ovicidal and Larvicidal Activity of Extracts of Opuntia Ficus-Indica Against Gastrointestinal Nematodes of Naturally Infected Sheep. Vet. Parasitol. 2016, 226, 65–68. [Google Scholar] [CrossRef]
- Reda, T.H.; Atsbha, M.K. Nutritional Composition, Antinutritional Factors, Antioxidant Activities, Functional Properties, and Sensory Evaluation of Cactus Pear (Opuntia Ficus-Indica) Seeds Grown in Tigray Region, Ethiopia. Int. J. Food Sci. 2019, 2019, 5697052. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Choi, S.M.; Lim, S.H.; Choi, C. Betanin from Beetroot (Beta Vulgaris L.) Regulates Lipid Metabolism and Promotes Fat Browning in 3T3-L1 Adipocytes. Pharmaceuticals 2023, 16, 1727. [Google Scholar] [CrossRef] [PubMed]
- Hadipour, E.; Taleghani, A.; Tayarani-Najaran, N.; Tayarani-Najaran, Z. Biological Effects of Red Beetroot and Betalains: A Review. Phytother. Res. 2020, 34, 1847–1867. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, C.; Torres, N.; Gutierrez-Uribe, J.A.; Noriega, L.G.; Torre-Villalvazo, I.; Leal-Diaz, A.M.; Antunes-Ricardo, M.; Marquez-Mota, C.; Ordaz, G.; Chavez-Santoscoy, R.A.; et al. The Effect of Isorhamnetin Glycosides Extracted from Opuntia Ficus-Indica in a Mouse Model of Diet Induced Obesity. Food Funct. 2015, 6, 805–815. [Google Scholar] [CrossRef]
- Bouhrim, M.; Ouassou, H.; Choukri, M.; Mekhfi, H.; Ziyyat, A.; Abdelkhaleq, L.; Aziz, M.; Bnouham, M. Hepatoprotective Effect of Opuntia Dillenii Seed Oil on CCl 4 Induced Acute Liver Damage in Rat. Asian Pac. J. Trop. Biomed. 2018, 8, 254. [Google Scholar] [CrossRef]
- Ma, H.; You, G.; Cui, F.; Chen, L.; Yang, X.; Chen, L.; Lu, H.; Zhang, W. Effects of a Low-Fat Diet on the Hepatic Expression of Adiponectin and its Receptors in Rats with NAFLD. Ann. Hepatol. 2015, 14, 108–117. [Google Scholar] [CrossRef]
- Cesaro, A.; De Michele, G.; Fimiani, F.; Acerbo, V.; Scherillo, G.; Signore, G.; Rotolo, F.P.; Scialla, F.; Raucci, G.; Panico, D.; et al. Visceral Adipose Tissue and Residual Cardiovascular Risk: A Pathological Link and New Therapeutic Options. Front. Cardiovasc. Med. 2023, 10, 1187735. [Google Scholar] [CrossRef]
- Ameer, F.; Scandiuzzi, L.; Hasnain, S.; Kalbacher, H.; Zaidi, N. De Novo Lipogenesis in Health and Disease. Metabolism 2014, 63, 895–902. [Google Scholar] [CrossRef]
- da Silva, I.V.; Soveral, G. Aquaporins in Obesity. Adv. Exp. Med. Biol. 2023, 1398, 289–302. [Google Scholar] [CrossRef]
- Wilk, P.; Kuska, K.; Wator, E.; Malecki, P.H.; Wos, K.; Tokarz, P.; Dubin, G.; Grudnik, P. Structural Characterization of Glycerol Kinase from the Thermophilic Fungus Chaetomium Thermophilum. Int. J. Mol. Sci. 2020, 21, 9570. [Google Scholar] [CrossRef]
- Huang, S.; Czech, M.P. The GLUT4 Glucose Transporter. Cell. Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef]
- Chitraju, C.; Walther, T.C.; Farese, R.V.J. The Triglyceride Synthesis Enzymes DGAT1 and DGAT2 have Distinct and Overlapping Functions in Adipocytes. J. Lipid Res. 2019, 60, 1112–1120. [Google Scholar] [CrossRef]
- Daquinag, A.C.; Gao, Z.; Fussell, C.; Immaraj, L.; Pasqualini, R.; Arap, W.; Akimzhanov, A.M.; Febbraio, M.; Kolonin, M.G. Fatty Acid Mobilization from Adipose Tissue is Mediated by CD36 Posttranslational Modifications and Intracellular Trafficking. JCI Insight 2021, 6, e147057. [Google Scholar] [CrossRef] [PubMed]





| Compound | Content (µg of Compound/g Dry Weight) |
|---|---|
| Piscidic acid | 19,269 ± 382 |
| Betanin | 5160 ± 88 |
| Isobetanin | 3038 ± 95 |
| 2′-O-apiosyl-4-O-phyllocactin | 1372 ± 102 |
| Isorhamnetin glucoxyl-rhamnosyl-pentoside (IG2) | 989 ± 21 |
| Neobetanin | 429 ± 11 |
| Quercetin glycoside 2 (QG2) | 245 ± 9 |
| Quercetin-3-O-rhamnosyl-rutinoside (QG3) | 202 ± 6 |
| 5″-O-E-sinapoyl-2-apiosyl-phyllocactin | 183 ± 37 |
| Composition by Energy (%) | Standard Diet | High-Fat High-Fructose Diet (HFHF) |
|---|---|---|
| Protein | 20.3 | 20.0 |
| Carbohydrate | 63.9 | 40.0 |
| Fructose | - | 10.0 |
| Lipid | 15.8 | 40.0 |
| Total | 100.0 | 20.0 |
| Energy (kcal/g) | 3.9 | 4.5 |
| C | HFHF | ODL | ODH | ANOVA | |
|---|---|---|---|---|---|
| Food intake (g/day) | 19.3 ± 0.3 a | 20.4 ± 0.4 a | 20.1 ± 0.6 a | 19.5 ± 0.2 a | NS |
| Energy intake (kcal/day) | 76.6 ± 1.1 b | 91.7 ± 1.8 a | 90.1 ± 2.5 a | 87.6 ± 0.7 a | p < 0.05 |
| Body weight (g) | 425.0 ± 7.4 b | 473.5 ± 10.6 a | 459.0 ± 12.0 a | 460.0 ± 8.0 a | p < 0.05 |
| Body weight gain (g) | 232.3 ± 9.9 b | 281.4 ± 12.3 a | 267.1 ± 13.6 ab | 266.1 ± 10.8 a | p < 0.05 |
| Total white AT weight (g) | 40.7 ± 2.2 c | 61.0 ± 2.3 a | 50.5 ± 4.0 b | 53.0 ± 3.3 b | p < 0.05 |
| Subcutaneous AT weight (g) | 13.4 ± 0.7 b | 19.0 ± 0.9 a | 16.5 ± 1.2 a | 16.6 ± 1.3 a | p < 0.05 |
| Visceral AT weight (g) | 30.8 ± 2.8 b | 43.7 ± 1.4 a | 36.4 ± 3.2 b | 36.4 ± 2.3 b | p < 0.05 |
| Epidydimal AT weight (g) | 10.0 ± 0.6 c | 16.5 ± 0.8 a | 14.6 ± 1.4 ab | 14.3 ± 1.0 b | p < 0.05 |
| Perirenal AT weight (g) | 12.8 ± 0.9 b | 19.1 ± 0.9 a | 17.8 ± 1.1 b | 17.4 ± 1.2 a | p < 0.05 |
| Mesenteric AT weight (g) | 4.5 ± 0.4 c | 6.3 ± 0.4 a | 5.6 ± 0.5 ab | 5.4 ± 0.2 b | p < 0.05 |
| White adipose index (%) | 9.6 ± 0.5 c | 12.7 ± 0.3 a | 11.1 ± 0.7 bc | 11.5 ± 0.6 ab | p < 0.05 |
| C | HFHF | ODL | ODH | ANOVA | |
|---|---|---|---|---|---|
| Triglycerides (mg/dL) | 100.4 ± 2.1 a | 93.7 ± 5.1 ab | 92.3 ± 2.4 b | 46.7 ± 9.9 c | p < 0.05 |
| Total Cholesterol (mg/dL) | 63.6 ± 5.4 c | 123.0 ± 4.3 a | 106.4 ± 6.4 b | 119.7 ± 2.5 ab | p < 0.05 |
| HDL Cholesterol (mg/dL) | 13.5 ± 0.7 b | 13.1 ± 0.4 b | 12.5 ± 1.5 b | 20.3 ± 1.8 a | p < 0.05 |
| Non-HDL Cholesterol (mg/dL) | 50.1 ± 5.5 c | 109.4 ± 4.6 a | 92.4 ± 5.8 b | 99.6 ± 2.5 ab | p < 0.05 |
| NEFA (mmol/L) | 0.9 ± 0.1 a | 0.8 ± 0.1 a | 0.7 ± 0.1 a | 0.7 ± 0.1 a | NS |
| Leptin (ng/mL) | 2.0 ± 0.5 a | 1.8 ± 0.3 a | 1.5 ± 0.3 a | 1.5 ± 0.2 a | NS |
| Adiponectin (ng/mL) | 4.7 ± 0.1 a | 4.7 ± 0.1 a | 4.7 ± 0.1 a | 4.7 ± 0.2 a | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-García, I.; Fernández-Quintela, A.; Oliver, P.; Picó, C.; Cano, M.P.; Portillo, M.P.; Trepiana, J. Preventive Effects of an Opuntia stricta var. dillenii Extract on Lipid Metabolism in a High-Fat High-Fructose Diet-Induced Obesity Animal Model. Nutrients 2025, 17, 3178. https://doi.org/10.3390/nu17193178
Gómez-García I, Fernández-Quintela A, Oliver P, Picó C, Cano MP, Portillo MP, Trepiana J. Preventive Effects of an Opuntia stricta var. dillenii Extract on Lipid Metabolism in a High-Fat High-Fructose Diet-Induced Obesity Animal Model. Nutrients. 2025; 17(19):3178. https://doi.org/10.3390/nu17193178
Chicago/Turabian StyleGómez-García, Iker, Alfredo Fernández-Quintela, Paula Oliver, Catalina Picó, M. Pilar Cano, María P. Portillo, and Jenifer Trepiana. 2025. "Preventive Effects of an Opuntia stricta var. dillenii Extract on Lipid Metabolism in a High-Fat High-Fructose Diet-Induced Obesity Animal Model" Nutrients 17, no. 19: 3178. https://doi.org/10.3390/nu17193178
APA StyleGómez-García, I., Fernández-Quintela, A., Oliver, P., Picó, C., Cano, M. P., Portillo, M. P., & Trepiana, J. (2025). Preventive Effects of an Opuntia stricta var. dillenii Extract on Lipid Metabolism in a High-Fat High-Fructose Diet-Induced Obesity Animal Model. Nutrients, 17(19), 3178. https://doi.org/10.3390/nu17193178










