Edible Insects as Future Proteins: Nutritional Value, Functional Properties, Bioactivities, and Safety Perspectives
Abstract
1. Introduction
2. Nutritional Characteristics of Edible Insects and Their Protein Fractions
2.1. Nutritional Characteristics of Edible Insects Subsection
| Bombyx mori | Hermetia illucens | Acheta domesticus | Tenebrio molitor | Locusta migratoria | Omphisa fuscidentalis | Formicidae | ||
|---|---|---|---|---|---|---|---|---|
| Proximate composition | Proteins (%) | 48.70–58.00 | 41.44 | 64.38–70.75 | 47.70–49.08 | 55–65 | 42–67 | 29.89–39.09 |
| Fat (%) | 30.10–35.00 | 35.69 | 18.55–22.8 | 35.17–37.7 | 10–20 | 11.29 | 55.9 | |
| Fiber (%) | 2.00 | 0.08 | - | 5.00–14.96 | 3–7 | - | 3–5 | |
| Ash (%) | 4.00–8.60 | 7.87 | 3.57–5.10 | 2.36–3.00 | 3–6 | 5.72 | 1.39 | |
| Carbohydrates (%) | 1.00 | 12.85 | 2.60 | 7.09–7.10 | 5–10 | - | - | |
| Energy (kJ/kg) | 23,236.74 | - | 19,057.89 | 22,863.14 | 4000–5000 | 3500–4500 | - | |
| Minerals (mg/100 g) | Calcium | 158.00 | 2295.00 | 132.14–210.00 | 44.36–47.18 | 65.6 | 129.5 | 88 |
| Potassium | - | 478.00 | 1126.62 | 761.54–895.01 | 349.8 | 536.5 | 262 | |
| Magnesium | 207.00 | 220.00 | 80.00–109.42 | 210.24–221.54 | 39.4 | 104.7 | 106 | |
| Phosphorous | 474.00 | 547.00 | 780.00–957.79 | 697.44–748.03 | 266.9 | - | 169 | |
| Sodium | - | 204.00 | 435.06 | 125.38–140.94 | 515.9 | 103.5 | - | |
| Iron | 26.00 | 27.00 | 6.27–11.23 | 5.41–5.51 | 3.45 | - | 5.7 | |
| Zinc | 23.00 | 6.90 | 18.64–21.79 | 11.41–13.65 | 8.22 | 14.005 | 10.9 | |
| Manganese | 0.71 | 13.06 | 2.97–3.73 | 0.92–1.36 | 1.07 | - | 4.18 | |
| Copper | 0.15 | 1.12 | 0.85–2.01 | 1.60–1.64 | 1.69 | 2.7 | 1.11 | |
| Selenium | 0.15 | 0.07 | 0.60 | 0.03–0.07 | 0.0099 | 0.044 | - | |
| Vitamins | Retinol (μg/100 g) | - | 118.00 | 24.33 | - | 0.01–0.1 | - | 0.01–0.05 |
| α-Tocopherol (IU/kg) | - | 80.39 | 63.96–81.00 | - | 1–5 | 0.5–2 | 1–3 | |
| Ascorbic acid (mg/100 g) | - | - | 9.74 | 3.15–6.15 | 1–5 | 1–3 | 1–4 | |
| Thiamin (mg/100 g) | - | - | 0.13 | 0.31–0.63 | 0.2–0.5 | 0.1–0.3 | 0.1–0.4 | |
| Riboflavin (mg/100 g) | - | - | 11.07 | 0.41–2.13 | 0.3–0.7 | 0.1–0.4 | 0.1–0.5 | |
| Niacin (mg/100 g) | 0.95 | - | 12.59 | 10.59–10.68 | 1–2 | 0.5–1.5 | 2.02 | |
| Pantothenic acid (mg/100 g) | - | - | 7.47 | 3.72–6.88 | 0.5–1 | - | - | |
| Biotin (μg/100 g) | - | - | 55.19 | 78.74–94.87 | - | - | ||
| Folic acid (mg/100 g) | - | - | 0.49 | 0.30–0.41 | 0.01–0.05 | - | 0.73 | |
| Reference | [11] | [22] | [11] | [11] | [23,24] | [25] | [26,27] | |
| Source | Picture | Amino Acid Ratio | Reference |
|---|---|---|---|
| Bombyx mori |  | 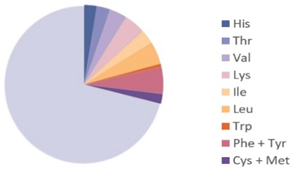 | [9] |
| Hermetia illucens |  | 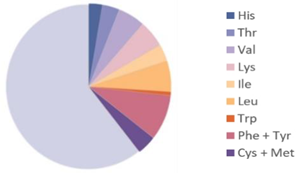 | [28] |
| Tenebrio molitor |  |  | [9] |
| Acheta domesticus |  | 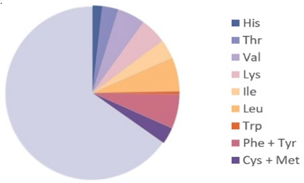 | [9] |
| Locusta migratoria |  |  | [24] |
| Omphisa fuscidentalis |  |  | [29] |
| Formicidae |  | 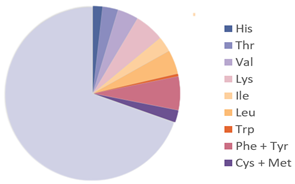 | [27] |
| Amino Acids Required in Human Nutrition (FAO) |  | 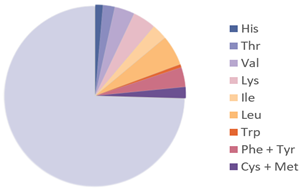 | [28] |
2.2. Common Edible Insects
2.2.1. Bombyx Mori Pupae
2.2.2. Acheta Domesticus
2.2.3. Tenebrio molitor
2.2.4. Hermetia Illucens
2.2.5. Locusta Migratoria
2.2.6. Formicidae
2.2.7. Omphisa Fuscidentalis
3. Functional Properties of Insect Proteins
3.1. Solubility
3.2. Water- and Oil-Holding Capacities
3.3. Emulsifying Properties
3.4. Foaming Properties
3.5. Gelation Properties
| Functional Characteristics | Influencing Factors | Source | Influencing Effect | Reference |
|---|---|---|---|---|
| Solubility | pH | Gryllodes sigillatus | The solubility exceeded 30% under acidic (pH 3) and neutral (pH 7) conditions, increasing to 50–90% in alkaline environments (pH > 7). | [82] |
| Solubility | Ionic Strength | Tenebrio molitor | Upon the addition of 0.1 M NaCl, the protein solubility reached 100%. However, at 1 M NaCl, the protein solubility decreased to 94.7%, although it remained higher than that under salt-free conditions (62.1%). | [83] |
| Solubility | pH | Tenebrio molitor | Protein solubility is lowest at pH 4–6 (29.6%) and reaches its highest at pH 11 (68.6%). | [83] |
| Solubility | Ultrasound frequency | Bombyx mori | Under the treatment at 40 kHz, the solubility of insect protein reached 30%, compared to 19.12% in the control group. The best solubility was achieved under the triple-frequency treatment (22/28/40 kHz), reaching 41.16%. | [114] |
| Solubility | Temperature | Bombyx mori | Under high-temperature conditions (50 °C) combined with triple-frequency ultrasound (22/28/40 kHz), the protein solubility reached 41.16%, representing a 115.27% increase compared to that at 20 °C (19.12%). | [114] |
| Solubility | Extraction technique | Hermetia illucens | The solubility was highest with the organic solvent extraction method (5.24%), followed by the cold-press defatting method (4.42%), while the water extraction method had the lowest solubility (4.16%). | [86] |
| Solubility | Amino acid composition | Tenebrio molitor Protaetia brevitarsis | The high proportion of hydrophobic amino acids in Tenebrio molitor protein (valine: 1.13%) reduces its solubility. In contrast, the salt-soluble protein of Protaetia brevitarsis is rich in lysine (2.35%) and glutamic acid (6.47%), enhancing hydrophilic interactions and improving solubility. | [85] |
| Solubility | Treatment method | Tenebrio molitor | Cold atmospheric plasma induced more substantial alterations in protein composition, leading to a reduction in protein solubility. | [94] |
| Emulsion | Extraction technique | Protaetia brevitarsis | The emulsifying capacity of protein extracted by the salt-soluble method (88.5%) was significantly higher than that obtained by the water-soluble method (75.2%). | [85] |
| Emulsion | Extraction technique | Hermetia illucens | The protein extracted by the cold-press method exhibited the highest emulsifying capacity (85%), followed by the organic solvent extraction method (76%), while the water-extracted protein showed the lowest emulsifying capacity (60%). | [86] |
| Emulsion | Temperature | Tenebrio molitor | Temperature was 55 °C, the EA was 28.94%, and the ES was 64.97%. Temperature was 75 °C, the EA was 37.87%, and the ES was 78.03%. Temperature was 95 °C, the EA was 38.00%, and the ES was 65.16%. | [115] |
| Emulsion | Temperature | Sphenarium purpurascens Charpentier | Temperature was 60 °C, and the EC was 20.33%. Temperature was 90 °C, and the EC was 18.5%. | [114] |
| Emulsion | pH | Acheta domesticus | At pH 6, the EC and ES were the lowest. At pH 12, the EC was 38.58%, and the ES was 33.33%. | [96] |
| Foaming | pH | Blaptica dubia | Under pH conditions of 3, 5, 7, and 10, foam formation occurred only at pH 5, with a half-life of 5 min, indicating that neutral to slightly acidic conditions are most favorable for foaming. | [84] |
| Foaming | Temperature | Tenebrio molitor | Temperature was 55 °C, the FC was 6.50%, and the FS was 94.30%. Temperature was 75 °C, the FC was 8.19%, and the FS was 93.82%. Temperature was 95 °C, the FC was 3.58%, and the FS was 97.37%. | [115] |
| Foaming | pH | Acheta domesticus | At pH 4, the FC was 14.05%, and the FS was 5.11%. At pH 6, the FC was 9.10%, and the FS was 6.01%. | [96] |
| Foaming | Ionic Strength | Gryllus assimilis | When the concentration of NaCl is 0.3 M, the foaming property is 1150%, and the FS is 25%. When the concentration of NaCl is 0.5 M, the foaming property is 1170% and the FS is 35%. | [102] |
| Foaming | Temperature | Gryllus assimilis | The foaming property of the untreated protein is 190%. The foaming property of the protein heat-treated at 75 °C is 970%, and that of the protein heat-treated at 95 °C is 1070%. | [102] |
| Foaming | pH | Hermetia illucens | At pH 6, the FC was 34.37%, and the FS was 23.81%. At pH 10, the FC was 5.26%, and the FS was 10%. | [116] |
| Gelation | Protein concentration | Zophobas morio Blaptica dubia | At a protein supernatant concentration of 3%, none of the insect proteins formed a gel, whereas at a concentration of 30%, all insect proteins formed a gel. | [84] |
| Gelation | pH | Acheta domesticus | At a protein concentration of 3% w/v, only the supernatant of Acheta domesticus could form a gel under the condition of pH = 7. At a protein concentration of 30% w/v, the supernatants of Acheta domesticus were all capable of forming gels at pH = 7/10. | [84] |
| Gelation | pH | Tenebrio molitor | pH 5.5: The gel relies more on hydrogen bonding, resulting in a more particulate structure with poor homogeneity. pH 7.5: Enhanced electrostatic repulsion promotes protein unfolding, leading to a denser gel with the highest storage modulus. | [117] |
| Gelation | Treatment method | Hermetia illucens | Ultrasonic treatment significantly improved protein gel properties, with the maximum particle size of 245.3 nm, the highest surface hydrophobicity of 617.9, the optimal elastic modulus of 2900 Pa, and the densest microstructure (pore size of 0.54 μm). | [110] |
| OHC | Treatment method | Acheta domesticus | Under PEF treatment conditions, the OHC significantly increased, with a maximum enhancement of 4.13 g oil/g. | [118] |
| OHC | Treatment method | Tenebrio molitor | Under HHP treatment conditions, the OHC of insect hydrolysates doubled, increasing from 1.21 g oil/g to 2.42 g oil/g. | [118] |
| OHC | Temperature | Tenebrio molitor | Temperature was 55 °C, and the OHC was 1.62 g oil/g. Temperature was 75 °C, and the OHC was 1.66 g oil/g. Temperature was 95 °C, and the OHC was 1.74 g oil/g. | [115] |
| OHC | Temperature | Sphenarium purpurascens Charpentier | Temperature was 60 °C, and the OHC was 2.79 g oil/g. Temperature was 90 °C, and the OHC was 2.16 g oil/g. | [114] |
| WHC | Treatment method | Tenebrio molitor | After defatting, the WHC of Tenebrio molitor powder increased from 1.24–1.31 g water/g to 1.97–2.02 g water/g. | [116] |
| WHC | pH | Gryllus assimilis | As the pH deviates further from the isoelectric point, the WHC increases. The isoelectric point of cricket protein is 3.85, with a WHC of 1.73 g water/g at pH 5.5 and 1.82 g water/g at pH 7.0. | [119] |
| WHC | Particle size | Protaetia brevitarsis | Particle size was 40 mesh, and the WHC was 4.42 ± 0.01 g water/g. Particle size was 100 mesh, and the WHC was 4.78 ± 0.10 g water/g. Under ultrafine grinding treatment conditions, the WHC was 5.07 ± 0.11 g water/g. | [120] |
| WHC | pH | Acheta domesticus | At pH 4, the WHC was the lowest. At pH 12, the WHC was 0.24–0.26 g water/g. | [96] |
4. Bioactivities of Insect Protein-Derived Hydrolysates and Peptides
4.1. Antioxidant Activity
4.2. Anti-Hypertensive Activity
4.3. Anti-Diabetic Activity
4.4. Antimicrobial Activity
| Bioactivities | Source | Processing Method | Influence | Reference |
|---|---|---|---|---|
| Antioxidant | Tenebrio molitor | Ethanol treatment | The 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity of untreated mealworm protein was approximately 34%, whereas that of the protein treated with 20% ethanol reached the highest level (38%). | [122] |
| Antioxidant | Hermetia illucens | Ultrasound-assisted enzymatic hydrolysis | Under the conditions of enzymatic hydrolysis time of 80 min and temperature of 50 °C, the hydrolysate treated with ultrasound has the highest hydroxyl radical scavenging rate, which is 72%. | [123] |
| Antioxidant | Gryllus assimilis | Enzymatic hydrolysis | The enzymatic hydrolysis using FlavourzymeTM 500 L alone exhibited the most remarkable positive effect on the antioxidant properties of proteins. Its IC50 values for DPPH and ABTS radical scavenging activities were 455 and 71 µg/mL, respectively. | [31] |
| Antioxidant | Gryllodes sigillatus | Heat treatment | The peptides obtained after the treatment exhibited the highest scavenging activity against ABTS and DPPH free radicals, with EC50 values of 2.75 and 6.91 μg/mL, respectively. | [100] |
| Antioxidant | Spodoptera littoralis | Enzymatic hydrolysis | The hydrolysates obtained by simulated gastrointestinal digestion (IC50 = 320 μg/mL) and mucosal enzyme digestion (IC50 = 211 μg/mL) exhibited strong antioxidant activity in vitro. | [124] |
| Antioxidant | Hermetia illucens | Maillard reaction | The ABTS+ radical scavenging activity of Hermetia illucens larva-glucose conjugate produced at 90 °C showed significantly higher scavenging activity, which varied with the reaction time (reaching a maximum of 55% at 10 h). | [125] |
| Antioxidant | Hermetia illucens | Ultrasound-assisted enzymatic hydrolysis | The antioxidant activity of hydrolysates obtained by multi-frequency swept-frequency ultrasound (SFU mode, 40 ± 2 kHz) pretreatment combined with Alcalase enzymatic hydrolysis of Hermetia illucens larva meat protein (HILMP) was significantly enhanced: the hydroxyl radical scavenging rate reached 72.3%. | [136] |
| Anti-hypertensive | Grasshopper | Fermentation | Cricket powder fermented with Lactobacillus NRRL B-50572 for 24 h exhibited an ACE inhibitory rate of 23.47% and an IC50 value of 970 μg /mL. | [130] |
| Anti-hypertensive | Gryllus assimilis | Enzymatic hydrolysis | The peptides obtained from protein hydrolyzed by the binary enzyme mixture of FlavourzymeTM 500 L and AlcalaseTM 2.4 L exhibited the highest ACE inhibitory rate, reaching 50.84%. | [139] |
| Anti-hypertensive | Gryllodes sigillatus | Enzymatic hydrolysis | Cricket protein hydrolysates (DH 60–85%) and their digested products exhibit strong ACE inhibitory activity (inhibition rate > 90%, IC50 as low as 51 μg/mL), showing potential for improving hypertension. | [152] |
| Anti-hypertensive | Musca domestica | Extraction with water | The water extract of Musca domestica larvae exhibits significant ACE inhibitory activity (IC50 = 430 μg/mL), demonstrating potential for improving hypertension. | [153] |
| Anti-hypertensive | Bombyx mori | Ultrasonic treatment | Silkworm pupae protein was treated with ultrasonic waves at a power of 410 W/100 mL, followed by hydrolysis with Alcalase for 32 min. The hydrolysate with the highest ACE inhibitory activity (IC50 = 4.9 μg /mL) was obtained at a hydrolysis time of 50 min. | [132] |
| Anti-hypertensive | Bombyx mori | Enzymatic hydrolysis | The hydrolysate of silkworm larva protein isolate (SLPI) digested by gastrointestinal enzymes in vitro exhibits strong ACE inhibitory activity, with an IC50 value of 8.3 µg/mL, indicating its potential as an anti-hypertensive active ingredient. | [133] |
| Anti-diabetic | Tenebrio molitor | Ultrasonic treatment | Experimental results indicated that after the protein was subjected to 15-min ultrasonic pretreatment (US15) followed by 1.5-h trypsin hydrolysis, the α-glucosidase inhibition rate exceeded 85% and remained consistently high thereafter. | [137] |
| Anti-diabetic | Tenebrio molitor | Enzymatic hydrolysis | Peptides with DPP-IV and α-glucosidase inhibitory activities were isolated and identified from enzymatically hydrolyzed Tenebrio molitor protein. The results showed that peptides with molecular weights between 500 and 1600 Da exhibited the strongest DPP-IV inhibitory capacity (IC50 = 910 μg/mL). | [138] |
| Anti-diabetic | Tenebrio molitor | Aqueous extract | The hot extract of Tenebrio molitor showed strong α-amylase (IC50 = 410 μg/mL) and α-glucosidase inhibition (IC50 = 7400 μg/mL), while the cold extract was more effective against lipase (IC50 = 430 μg/mL). | [154] |
| Anti-diabetic | Bombyx mori Protaetia brevitarsis Caelifera Gryllus bimaculatus Tenebrio molitor Allomyrina dichotoma | Cordyceps fermentation | Fermented insects exhibit anti-diabetic effects by promoting glucose absorption. | [155] |
| Anti-diabetic | Gryllus assimilis | Enzymatic hydrolysis | FlavourzymeTM 500 L: NeutraseTM 0.8 L, 1:1—α-amylase inhibition: 55.40 ± 2.93%; α-glucosidase inhibition: 17.07 ± 1.32%; IC50: 1990 μg/mL (α-amylase) and 6210 μg/mL (α-glucosidase). | [139] |
| Anti-diabetic | Bombyx mori | Enzymatic hydrolysis | The protein samples hydrolyzed by Flavourzyme and Alcalase exhibited the highest bioactivity, with an α-glucosidase inhibition rate of approximately 40%. | [140] |
| Antimicrobial | bumblebee | Immunization with E. coli and isolation of peptide from the hemolymph | Combination treatment (low-dose hymenoptaecin with 1.25 μM abaecin) resulted in a statistically significant reduction in CFU counts compared with either peptide alone (p < 0.05) | [147] |
| Antimicrobial | Galleria mellonella | Immunization with viable E. coli D31 and isolation of peptide from the hemolymph | Eight defensive peptides were isolated and identified from the hemolymph of Galleria mellonella larvae under immune challenge, five of which are newly discovered with diverse antimicrobial activity profiles, and the Gm defensin-like peptide shows the strongest activity. | [147] |
| Antimicrobial | Apis cerana | Recombinant proteins are expressed in the baculovirus-insect cell system and purified by Strep-tag affinity chromatography. | Apis cerana venom serine protease inhibitor inhibits serine proteases (trypsin IC50 = 1.37 ± 0.20 μg/mL; proteinase K IC50 = 1.14 ± 0.07 μg/mL; plasmin IC50 = 2.24 ± 0.51 μg/mL), and exerts broad antimicrobial activity (B. thuringiensis MIC50 = 8.47 ± 0.67 μg/mL; E. coli MIC50 = 16.80 ± 1.26 μg/mL; B. bassiana IC50 = 9.80 ± 1.19 μg/mL). | [156] |
| Antimicrobial | Tribolium castaneum | The TcPaSK peptide was chemically synthesized based on an extended sequence of Tribolium castaneum insect defensin 3. | The minimum inhibitory concentration (MIC) range of TcPaSK against Staphylococcus aureus is 16–32 µg/mL, indicating its effective inhibition of bacterial growth. Notably, this concentration range is significantly lower than the toxic concentration towards mammalian cells (>100 µg/mL), which demonstrates the peptide’s specific targeting of bacteria. | [148] |
| Anti-microbial | Tribolium castaneum | Tribolium castaneum Defensin 1 was synthesized by solid-phase synthesis. | Tribolium castaneum Defensin 1 at a concentration of 12.5 μg/mL could increase the survival rate of nematodes infected with S. aureus from 22% to 87%. | [150] |
5. Allergenicity of Insect Proteins
5.1. Clinical Reactions to Allergens
5.2. Allergens in Edible Insect Proteins
5.3. Reducing Allergenicity
6. Application of Insect Protein
6.1. The Application of Insect Protein in Food
6.2. The Application of Insect Protein in Medicine
6.3. The Application of Insect Protein in Agriculture
6.4. Insect Quality and Consumer Acceptance
7. Challenges and Prospects
7.1. Legislative Challenges
7.2. Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Assatory, A.; Vitelli, M.; Rajabzadeh, A.R.; Legge, R.L. Dry Fractionation Methods for Plant Protein, Starch and Fiber Enrichment: A Review. Trends Food Sci. Technol. 2019, 86, 340–351. [Google Scholar] [CrossRef]
- Alexandre, V.; Even, P.C.; Larue-Achagiotis, C.; Blouin, J.-M.; Blachier, F.; Benamouzig, R.; Tomé, D.; Davila, A.-M. Lactose malabsorption and colonic fermentations alter host metabolism in rats. Br. J. Nutr. 2013, 110, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Poore, J.; Nemecek, T. Reducing Food’s Environmental Impacts through Producers and Consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Peñalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association Between Dietary Factors and Mortality from Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA 2017, 317, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.-V.; Wang, H.-C.; Chen, S.-H.; Lee, J.-M. The Threshold Effect of Overfishing on Global Fishery Outputs: International Evidence from a Sustainable Fishery Perspective. Fishes 2023, 8, 71. [Google Scholar] [CrossRef]
- Zainab, H.B.; Aminu, U.I.; Mustapha, I.; Adam, S.T. Proximate Analysis and Antinutritional Factors of Water Melon Seeds (Citrullus lenatus). Int. J. Biochem. Res. Rev. 2021, 30, 41–47. [Google Scholar] [CrossRef]
- Gravel, A.; Doyen, A. The Use of Edible Insect Proteins in Food: Challenges and Issues Related to Their Functional Properties. Innov. Food Sci. Emerg. Technol. 2020, 59, 102272. [Google Scholar] [CrossRef]
- Ojha, S.; Bekhit, A.E.-D.; Grune, T.; Schlüter, O.K. Bioavailability of Nutrients from Edible Insects. Curr. Opin. Food Sci. 2021, 41, 240–248. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Nakagaki, B.J.; Defoliart, G.R. Comparison of Diets for Mass-Rearing Acheta domesticus (Orthoptera: Gryllidae) as a Novelty Food, and Comparison of Food Conversion Efficiency with Values Reported for Livestock. J. Econ. Entomol. 1991, 84, 891–896. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Potential and Challenges of Insects as an Innovative Source for Food and Feed Production. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- de Castro, R.J.S.; Ohara, A.; dos Aguilar, J.G.S.; Domingues, M.A.F. Nutritional, Functional and Biological Properties of Insect Proteins: Processes for Obtaining, Consumption and Future Challenges. Trends Food Sci. Technol. 2018, 76, 82–89. [Google Scholar] [CrossRef]
- Hasnan, F.F.B.; Feng, Y.; Sun, T.; Parraga, K.; Schwarz, M.; Zarei, M. Insects as Valuable Sources of Protein and Peptides: Production, Functional Properties, and Challenges. Foods 2023, 12, 4243. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; FitzGerald, R.J. Unlocking the Biological Potential of Proteins from Edible Insects through Enzymatic Hydrolysis: A Review. Innov. Food Sci. Emerg. Technol. 2017, 43, 239–252. [Google Scholar] [CrossRef]
- Altomare, A.A.; Baron, G.; Aldini, G.; Carini, M.; D’Amato, A. Silkworm pupae as source of high-value edible proteins and of bioactive peptides. Food Sci. Nutr. 2020, 8, 2652–2661. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Rochow, V.B.; Gahukar, R.T.; Ghosh, S.; Jung, C. Chemical Composition, Nutrient Quality and Acceptability of Edible Insects Are Affected by Species, Developmental Stage, Gender, Diet, and Processing Method. Foods 2021, 10, 1036. [Google Scholar] [CrossRef]
- El-Husseini, A.E.-D.; Bredt, D.S. Protein Palmitoylation: A Regulator of Neuronal Development and Function. Nat. Rev. Neurosci. 2002, 3, 791–802. [Google Scholar] [CrossRef]
- Tzompa-Sosa, D.A.; Yi, L.; van Valenberg, H.J.F.; van Boekel, M.A.J.S.; Lakemond, C.M.M. Insect Lipid Profile: Aqueous versus Organic Solvent-Based Extraction Methods. Food Res. Int. 2014, 62, 1087–1094. [Google Scholar] [CrossRef]
- Kouřimská, L.; Adámková, A. Nutritional and Sensory Quality of Edible Insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Bukkens, S.G.F. The Nutritional Value of Edible Insects. Ecol. Food Nutr. 2021, 36, 287–319. [Google Scholar] [CrossRef]
- Lu, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Sinpru, B.; Sroichak, T.; Archa, P.; Thongpea, S.; Paengkoum, S.; Purba, R.A.P.; et al. Nutritional Composition of Black Soldier Fly Larvae (Hermetia illucens L.) and Its Potential Uses as Alternative Protein Sources in Animal Diets: A Review. Insects 2022, 13, 831. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of Frozen and Dried Formulations from Migratory Locust (Locusta migratoria) as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06667. [Google Scholar] [CrossRef]
- Clarkson, C.; Mirosa, M.; Birch, J. Potential of Extracted Locusta Migratoria Protein Fractions as Value-Added Ingredients. Insects 2018, 9, 20. [Google Scholar] [CrossRef]
- Sheileja, T.; Shantibala, T.; Singh, K.M. Nutritive Value of Bamboo Worm Omphisa fuscidentalis (Lepidoptera: Crambidae): An Edible Insect as Protein Rich Food. Pharma Innov. J. 2022, 11, 2229–2233. [Google Scholar]
- Xu, N.; Yu, J.; Zhang, F.; Wu, S.; Zou, C.; Wang, Q.; Wang, Y. Colony Composition and Nutrient Analysis of Polyrhachis Dives Ants, a Natural Prey of the Chinese Pangolin (Manis pentadactyla). Zoo Biol. 2022, 41, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Li, D.; Feng, F.; Ren, Y. Nutritional Composition of Polyrhachis vicina Roger (Edible Chinese Black Ant). Songklanakarin J. Sci. Technol. 2006, 28, 107–114. [Google Scholar]
- Caligiani, A.; Marseglia, A.; Leni, G.; Baldassarre, S.; Maistrello, L.; Dossena, A.; Sforza, S. Composition of Black Soldier Fly Prepupae and Systematic Approaches for Extraction and Fractionation of Proteins, Lipids and Chitin. Food Res. Int. 2018, 105, 812–820. [Google Scholar] [CrossRef]
- Sornkhwan, T.; Sansenya, S.; Chumanee, S.; Sricheewin, C. Evaluation of Biological Activity and Amino Acid Profile of Protein from Thaiedible Insects. Food Res. 2024, 8, 301–308. [Google Scholar] [CrossRef]
- Bessa, L.W.; Pieterse, E.; Marais, J.; Hoffman, L.C. Why for Feed and Not for Human Consumption? The Black Soldier Fly Larvae. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2747–2763. [Google Scholar] [CrossRef]
- de Matos, F.M.; Novelli, P.K.; de Castro, R.J.S. Enzymatic hydrolysis of black cricket (Gryllus assimilis) proteins positively affects their antioxidant properties. J. Food Sci. 2021, 86, 571–578. [Google Scholar] [CrossRef]
- Queiroz, L.S.; Regnard, M.; Jessen, F.; Mohammadifar, M.A.; Sloth, J.J.; Petersen, H.O.; Ajalloueian, F.; Brouzes, C.M.C.; Fraihi, W.; Fallquist, H.; et al. Physico-Chemical and Colloidal Properties of Protein Extracted from Black Soldier Fly (Hermetia illucens) Larvae. Int. J. Biol. Macromol. 2021, 186, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Tomotake, H.; Katagiri, M.; Yamato, M. Silkworm Pupae (Bombyx mori) Are New Sources of High Quality Protein and Lipid. J. Nutr. Sci. Vitaminol. 2010, 56, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Yamakawa, M. Moricin, a Novel Type of Antibacterial Peptide Isolated from the Silkworm, Bombyx mori (∗). J. Biol. Chem. 1995, 270, 29923–29927. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.-H.; Zhang, S.-Z.; Zhu, L.-B.; Wang, J.; Liu, Y.-X.; Wang, Y.-L.; Kong, X.; You, L.-L.; Toufeeq, S.; Liu, S.-H.; et al. The Digestive Proteinase Trypsin, Alkaline A Contributes to Anti-BmNPV Activity in Silkworm (Bombyx mori). Dev. Comp. Immunol. 2021, 119, 104035. [Google Scholar] [CrossRef]
- Al-Qazzaz, M.F.; Ismail, D.B. Insect Meal as a Source of Protein in Animal Diet. Anim. Nutr. Feed. Technol. 2016, 16, 527–547. [Google Scholar] [CrossRef]
- Wang, D.; Bai, Y.; Li, J.; Zhang, C. Nutritional Value of the Field Cricket (Gryllus testaceus Walker). Insect Sci. 2004, 11, 275–283. [Google Scholar] [CrossRef]
- Hammer, L.; Moretti, D.; Abbühl-Eng, L.; Kandiah, P.; Hilaj, N.; Portmann, R.; Egger, L. Mealworm Larvae (Tenebrio molitor) and Crickets (Acheta domesticus) Show High Total Protein In Vitro Digestibility and Can Provide Good-to-Excellent Protein Quality as Determined by In Vitro DIAAS. Front. Nutr. 2023, 10, 1150581. [Google Scholar] [CrossRef]
- Montowska, M.; Kowalczewski, P.Ł.; Rybicka, I.; Fornal, E. Nutritional Value, Protein and Peptide Composition of Edible Cricket Powders. Food Chem. 2019, 289, 130–138. [Google Scholar] [CrossRef]
- Liu, P.; Piao, X.S.; Thacker, P.A.; Zeng, Z.K.; Li, P.F.; Wang, D.; Kim, S.W. Chito-oligosaccharide reduces diarrhea incidence and attenuates the immune response of weaned pigs challenged with Escherichia coli K88. J. Anim. Sci. 2010, 88, 3871–3879. [Google Scholar] [CrossRef]
- Xie, B.; Zhu, Y.; Chu, X.; Pokharel, S.S.; Qian, L.; Chen, F. Research Progress and Production Status of Edible Insects as Food in China. Foods 2024, 13, 1986. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, X.; Zhao, M.; He, Z.; Sun, L.; Wang, C.; Ding, W. Edible Insects in China: Utilization and Prospects. Insect Sci. 2018, 25, 184–198. [Google Scholar] [CrossRef]
- John, N.K.; Glaston, M.K.; Simon, M.N.; Monicah, A. Effect of Processing Methods on the In Vitro Protein Digestibility and Vitamin Content of Edible Winged Termite (Macrotermes subhylanus) and Grasshopper (Ruspolia differens). Food Bioprocess Technol. 2019, 3, 778–782. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Pinckaers, P.J.M.; van Loon, J.J.A.; van Loon, L.J.C. Consideration of Insects as a Source of Dietary Protein for Human Consumption. Nutr. Rev. 2017, 75, 1035–1045. [Google Scholar] [CrossRef]
- Davis, G.R.F. Essential Dietary Amino Acids for Growth of Larvae of the Yellow Mealworm, Tenebrio molitor L. J. Nutr. 1975, 105, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Z.; Zhang, W.; Qiao, H.; Wen, P.; Zhang, Y. Proteomic Analysis, Purification and Characterization of a New Milk-Clotting Protease from Tenebrio Molitor Larvae. J. Funct. Foods 2022, 89, 104944. [Google Scholar] [CrossRef]
- Contreras, E.; Rausell, C.; Real, M.D. Proteome Response of Tribolium Castaneum Larvae to Bacillus Thuringiensis Toxin Producing Strains. PLoS ONE 2013, 8, e55330. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Lee, S.Y.; Kurata, S.; Natori, S.; Lee, B.L. Purification and Molecular Cloning of cDNA for an Inducible Antibacterial Protein from Larvae of the Coleopteran, Tenebrio molitor. J. Biochem. 1994, 116, 53–58. [Google Scholar] [CrossRef]
- Wang, S.; Shao, B.; Ye, X.; Rao, P. Purification and Characterization of a Chitinase from Peanut (Arachis hypogaea L.). J. Food Biochem. 2008, 32, 32–45. [Google Scholar] [CrossRef]
- Qadeer, S.; Khan, M.A.; Shahzad, Q.; Azam, A.; Ansari, M.S.; Rakha, B.A.; Ejaz, R.; Husna, A.; Duman, J.; Akhter, S. Efficiency of beetle (Dendroides canadensis) recombinant antifreeze protein for buffalo semen freezability and fertility. Theriogenology 2016, 86, 1662–1669. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Li, J.; Ma, X. Use of Hermetia illucens larvae as a dietary protein source: Effects on growth performance, carcass traits, and meat quality in finishing pigs. Meat Sci. 2019, 158, 107837. [Google Scholar] [CrossRef]
- Ravi, H.K.; Vian, M.A.; Tao, Y.; Degrou, A.; Costil, J.; Trespeuch, C.; Chemat, F. Alternative Solvents for Lipid Extraction and Their Effect on Protein Quality in Black Soldier Fly (Hermetia illucens) Larvae. J. Clean. Prod. 2019, 238, 117861. [Google Scholar] [CrossRef]
- Marco, M.D.; Martínez, S.; Hernandez, F.; Madrid, J.; Gai, F.; Rotolo, L.; Belforti, M.; Bergero, D.; Katz, H.; Dabbou, S.; et al. Nutritional value of two insect larval meals (Tenebrio molitor and Hermetia illucens) for broiler chickens: Apparent nutrient digestibility, apparent ileal amino acid digestibility and apparent metabolizable energy. Anim. Feed. Sci. Technol. 2015, 209, 211–218. [Google Scholar] [CrossRef]
- Altmann, B.A.; Neumann, C.; Velten, S.; Liebert, F.; Mörlein, D. Meat Quality Derived from High Inclusion of a Micro-Alga or Insect Meal as an Alternative Protein Source in Poultry Diets: A Pilot Study. Foods 2018, 7, 34. [Google Scholar] [CrossRef]
- Kim, W.; Bae, S.; Park, K.; Lee, S.; Choi, Y.; Han, S.; Koh, Y. Biochemical characterization of digestive enzymes in the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). J. Asia-Pac. Entomol. 2010, 14, 11–14. [Google Scholar] [CrossRef]
- Van Moll, L.; Wouters, M.; De Smet, J.; De Vooght, L.; Delputte, P.; Van Der Borght, M.; Cos, P. In-Depth Biological Characterization of Two Black Soldier Fly Anti-Pseudomonas Peptides Reveals LPS-Binding and Immunomodulating Effects. mSphere 2023, 8, e0045423. [Google Scholar] [CrossRef] [PubMed]
- da Jantzen Silva Lucas, A.; de Menegon Oliveira, L.; da Rocha, M.; Prentice, C. Edible Insects: An Alternative of Nutritional, Functional and Bioactive Compounds. Food Chem. 2020, 311, 126022. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Bußler, S.; Schlüter, O.K. Food Waste Valorisation and Circular Economy Concepts in Insect Production and Processing. Waste Manag. 2020, 118, 600–609. [Google Scholar] [CrossRef]
- Ye, S.-J.; Park, H.-J.; Baik, M.-Y. Modification of plant proteins as alternatives to animal proteins: A review. Food Sci. Biotechnol. 2024, 34, 349–363. [Google Scholar] [CrossRef]
- Ruiz, V.; SandovalTrujillo, H.; QuirinoBarreda, T.; SnchezHerrera, K.; DazGarca, R.; CalvoCarrillo, C. Chemical composition and amino acids content of five species of edible Grasshoppers from Mexico. Emir. J. Food Agric. 2015, 27, 654–658. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, W.; Zhao, X.; Yu, Z.; Guo, H.; Yang, Y.; Zhang, J.; Moussian, B. Apolipophorin-II/I Contributes to Cuticular Hydrocarbon Transport and Cuticle Barrier Construction in Locusta migratoria. Front. Physiol. 2020, 11, 790. [Google Scholar] [CrossRef]
- Hatle, J.D.; Maslikova, V.; Short, C.A.; Bracey, D.; Darmanjian, M.; Morningstar, S.; Reams, B.; Mashanov, V.S.; Jahan-Mihan, A.; Hahn, D.A. Protein Storage and Reproduction Increase in Grasshoppers on a Diet Matched to the Amino Acids of Egg Yolk Protein. J. Exp. Biol. 2022, 225, jeb244450. [Google Scholar] [CrossRef] [PubMed]
- Çabuk, B.; Yılmaz, B. Fortification of traditional egg pasta (erişte) with edible insects: Nutritional quality, cooking properties and sensory characteristics evaluation. J. Food Sci. Technol. 2020, 57, 2750–2757. [Google Scholar] [CrossRef] [PubMed]
- Bolton, B. Synopsis and Classification of the Formicidae. Mem. Am. Entomol. Inst. 2003, 71, 1–370. [Google Scholar]
- Silva, E.J.; Camargo, R.d.S.; Forti, L.C.; Travaglini, R.V. Protein content of leaf-cutting ant queens before the nuptial flight and during the post-claustral phase. Rev. Bras. Entomol. 2014, 58, 333–336. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.-M.; Li, T.; Liu, X.; Xu, Z.-H. Characterization and Phylogenetic Implication of Complete Mitochondrial Genome of the Medicinal Ant Formica sinae (Hymenoptera: Formicidae): Genomic Comparisons in Formicidae. J. Med. Entomol. 2022, 5, 1971–1979. [Google Scholar] [CrossRef]
- Cao, T.-T.; Ma, J.-L.; Zhang, Y.; Peng, J.-W.; Lin, H. Efficacy of Formic Acid in Combination with cDMARDs in Rheumatoid Arthritis. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 4366–4375. [Google Scholar] [CrossRef]
- Liesivuori, J. Formic Acid. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 659–661. ISBN 978-0-12-386455-0. [Google Scholar]
- Ricke, S.C.; Dittoe, D.K.; Richardson, K.E. Formic Acid as an Antimicrobial for Poultry Production: A Review. Front. Vet. Sci. 2020, 7, 563. [Google Scholar] [CrossRef]
- Hefetz, A.; Blum, M.S. Biosynthesis of Formic Acid by the Poison Glands of Formicine Ants. Biochim. Biophys. Acta BBA Gen. Subj. 1978, 543, 484–496. [Google Scholar] [CrossRef]
- Deng, H.; Mitsuno, H.; Kanzaki, R.; Nakamoto, T. Gas Phase Odorant Detection by Insect Olfactory Receptor. IEEE Sens. J. 2021, 21, 21184–21191. [Google Scholar] [CrossRef]
- Wheeler, D.E.; Martinez, T. Storage Proteins in Ants (Hymenoptera: Formicidae). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1995, 112, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, N.T.; Chhay, T.; Keo, S.; Lertpatarakomol, R.; Kajaysri, J.; Kang, K.; Miech, P.; Plötz, M.; Mitchaothai, J. Proximate Composition of Thai and Cambodian Ready-to-Eat Insects. J. Food Qual. 2021, 2021, 9731464. [Google Scholar] [CrossRef]
- Kurdi, P.; Chaowiwat, P.; Weston, J.; Hansawasdi, C. Studies on Microbial Quality, Protein Yield, and Antioxidant Properties of Some Frozen Edible Insects. Int. J. Food Sci. 2021, 2021, 5580976. [Google Scholar] [CrossRef] [PubMed]
- Chantakun, K.; Petcharat, T.; Wattanachant, S.; Karim, M.S.B.A.; Kaewthong, P. Fatty Acid Profile and Thermal Behavior of Fat-Rich Edible Insect Oils Compared to Commonly Consumed Animal and Plant Oils. Food Sci. Anim. Resour. 2024, 4, 790–804. [Google Scholar] [CrossRef]
- Tungjitwitayakul, J.; Singtripop, T.; Nettagul, A.; Oda, Y.; Tatun, N.; Sekimoto, T.; Sakurai, S. Identification, characterization, and developmental regulation of two storage proteins in the bamboo borer Omphisa fuscidentalis. J. Insect Physiol. 2008, 5, 62–76. [Google Scholar] [CrossRef]
- Mishyna, M.; Keppler, J.K.; Chen, J. Techno-Functional Properties of Edible Insect Proteins and Effects of Processing. Curr. Opin. Colloid Interface Sci. 2021, 56, 101508. [Google Scholar] [CrossRef]
- Thongkaew, C.; Singthong, J.; Klangsinsirikul, S. Properties of Insect Protein Concentrate and Potential Application in Seasoned Rice Noodles. Food Sci. Technol. Int. 2022, 30, 307–316. [Google Scholar] [CrossRef]
- Vihinen, M. Solubility of proteins. ADMET DMPK 2020, 8, 391–399. [Google Scholar] [CrossRef]
- Kim, T.-K.; Lee, M.H.; Yu, M.-H.; Yong, H.I.; Jang, H.W.; Jung, S.; Choi, Y.-S. Thermal Stability and Rheological Properties of Heat-Induced Gels Prepared Using Edible Insect Proteins in a Model System. LWT 2020, 134, 110270. [Google Scholar] [CrossRef]
- Hall, F.G.; Jones, O.G.; O’Haire, M.E.; Liceaga, A.M. Functional Properties of Tropical Banded Cricket (Gryllodes sigillatus) Protein Hydrolysates. Food Chem. 2017, 224, 414–422. [Google Scholar] [CrossRef]
- Yi, L.; Van Boekel, M.A.J.S.; Lakemond, C.M.M. Extracting Tenebrio molitor Protein While Preventing Browning: Effect of pH and NaCl on Protein Yield. J. Insects Food Feed 2017, 3, 21–32. [Google Scholar] [CrossRef]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A.J.S. Extraction and Characterisation of Protein Fractions from Five Insect Species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef]
- Kim, T.-K.; Yong, H.I.; Jeong, C.H.; Han, S.G.; Kim, Y.-B.; Paik, H.-D.; Choi, Y.-S. Technical Functional Properties of Water- and Salt-soluble Proteins Extracted from Edible Insects. Food Sci. Anim. Resour. 2019, 39, 643–654. [Google Scholar] [CrossRef]
- Kim, T.-K.; Lee, J.-H.; Yong, H.I.; Kang, M.-C.; Cha, J.Y.; Chun, J.Y.; Choi, Y.-S. Effects of Defatting Methods on the Physicochemical Properties of Proteins Extracted from Hermetia illucens Larvae. Foods 2022, 11, 1400. [Google Scholar] [CrossRef]
- Villaseñor, V.M.; Enriquez-Vara, J.N.; Urías-Silva, J.E.; Mojica, L. Edible Insects: Techno-Functional Properties Food and Feed Applications and Biological Potential. Food Rev. Int. 2022, 38, 866–892. [Google Scholar] [CrossRef]
- Chen, M.J.; Lin, C.W. Factors Affecting the Water-Holding Capacity of Fibrinogen/Plasma Protein Gels Optimized by Response Surface Methodology. J. Food Sci. 2002, 67, 2579–2582. [Google Scholar] [CrossRef]
- Han, X.; Li, B.; Puolanne, E.; Heinonen, M. Hybrid Sausages Using Pork and Cricket Flour: Texture and Oxidative Storage Stability. Foods 2023, 12, 1262. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M.; Rybczyńska, K.; Jakubczyk, A. Selected Species of Edible Insects as a Source of Nutrient Composition. Food Res. Int. 2015, 77, 460–466. [Google Scholar] [CrossRef]
- Aremu, M.; Olaofe, O.; Akintayo, E. Functional properties of some Nigerian varieties of legume seed flours and flour concentration effect on foaming and gelation properties. J. Food Technol. 2007, 5, 109–115. [Google Scholar]
- Sosa, E.F.; Thompson, C.; Chaves, M.G.; Acevedo, B.A.; Avanza, M.V. Legume Seeds Treated by High Hydrostatic Pressure: Effect on Functional Properties of Flours. Food Bioprocess Technol. 2020, 13, 323–340. [Google Scholar] [CrossRef]
- Aryee, A.N.A.; Agyei, D.; Udenigwe, C.C. 2—Impact of Processing on the Chemistry and Functionality of Food Proteins. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2018; pp. 27–45. ISBN 978-0-08-100722-8. [Google Scholar]
- Bußler, S.; Rumpold, B.A.; Fröhling, A.; Jander, E.; Rawel, H.M.; Schlüter, O.K. Cold Atmospheric Pressure Plasma Processing of Insect Flour from Tenebrio molitor: Impact on Microbial Load and Quality Attributes in Comparison to Dry Heat Treatment. Innov. Food Sci. Emerg. Technol. 2016, 36, 277–286. [Google Scholar] [CrossRef]
- Dong, X.; Woo, M.W.; Quek, S.Y. The Physicochemical Properties, Functionality, and Digestibility of Hempseed Protein Isolate as Impacted by Spray Drying and Freeze Drying. Food Chem. 2024, 433, 137310. [Google Scholar] [CrossRef]
- Ndiritu, A.K.; Kinyuru, J.N.; Gichuhi, P.N.; Kenji, G.M. Effects of NaCl and pH on the functional properties of edible crickets (Acheta domesticus) protein concentrate. J. Food Meas. Charact. 2019, 13, 1788–1796. [Google Scholar] [CrossRef]
- Amarowicz, R. Modification of emulsifying properties of food proteins by enzymatic hydrolysis. Eur. J. Lipid Sci. Technol. 2010, 11, 695–696. [Google Scholar] [CrossRef]
- Gould, J.; Wolf, B. Interfacial and Emulsifying Properties of Mealworm Protein at the Oil/Water Interface. Food Hydrocoll. 2018, 77, 57–65. [Google Scholar] [CrossRef]
- Stone, A.K.; Tanaka, T.; Nickerson, M.T. Protein Quality and Physicochemical Properties of Commercial Cricket and Mealworm Powders. J. Food Sci. Technol. 2019, 56, 3355–3363. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, E.; Baraniak, B.; Karaś, M. Identification of Antioxidant and Anti-Inflammatory Peptides Obtained by Simulated Gastrointestinal Digestion of Three Edible Insects Species (Gryllodes sigillatus, Tenebrio molitor, Schistocerca gragaria). Int. J. Food Sci. Technol. 2018, 53, 2542–2551. [Google Scholar] [CrossRef]
- Dickinson, E. Food Emulsions and Foams: Stabilization by Particles. Curr. Opin. Colloid Interface Sci. 2010, 15, 40–49. [Google Scholar] [CrossRef]
- Santiago, L.A.; Fadel, O.M.; Tavares, G.M. How Does the Thermal-Aggregation Behavior of Black Cricket Protein Isolate Affect Its Foaming and Gelling Properties? Food Hydrocoll. 2021, 110, 106169. [Google Scholar] [CrossRef]
- Nikolić, K.; Tepavcevic, J. Functional Properties of Insect Proteins. AIDASCO Rev. 2024, 2, 26–31. [Google Scholar] [CrossRef]
- Kavle, R.R.; Nolan, P.J.; Bekhit, A.E.D.A.; Carne, A.; Morton, J.D.; Agyei, D. Physicochemical Characteristics, Techno-Functionalities, and Amino Acid Profile of Prionoplus Reticularis (Huhu) Larvae and Pupae Protein Extracts. Foods 2023, 12, 417. [Google Scholar] [CrossRef]
- Ma, Z.; Mondor, M.; Goycoolea Valencia, F.; Hernández-Álvarez, A.J. Current State of Insect Proteins: Extraction Technologies, Bioactive Peptides and Allergenicity of Edible Insect Proteins. Food Funct. 2023, 14, 8129–8156. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, E.; Pečová, M.; Pankiewicz, U. Impact of Mealworm Powder (Tenebrio molitor) Fortification on Ice Cream Quality. Sustainability 2023, 15, 16041. [Google Scholar] [CrossRef]
- Bresciani, A.; Cardone, G.; Jucker, C.; Savoldelli, S.; Marti, A. Technological Performance of Cricket Powder (Acheta domesticus L.) in Wheat-Based Formulations. Insects 2022, 13, 546. [Google Scholar] [CrossRef] [PubMed]
- Scholliers, J.; Steen, L.; Fraeye, I. Gelation of a Combination of Insect and Pork Proteins as Affected by Heating Temperature and Insect: Meat Ratio. Food Res. Int. 2020, 137, 109703. [Google Scholar] [CrossRef]
- Kim, T.-K.; Lee, M.H.; Yong, H.I.; Kang, M.-C.; Jung, S.; Choi, Y.-S. Porcine Myofibrillar Protein Gel with Edible Insect Protein: Effect of pH-Shifting. LWT 2022, 154, 112629. [Google Scholar] [CrossRef]
- Kumar, S.; Queiroz, L.S.; Marie, R.; Nascimento, L.G.L.; Mohammadifar, M.A.; De Carvalho, A.F.; Brouzes, C.M.C.; Fallquist, H.; Fraihi, W.; Casanova, F. Gelling Properties of Black Soldier Fly (Hermetia illucens) Larvae Protein after Ultrasound Treatment. Food Chem. 2022, 386, 132826. [Google Scholar] [CrossRef]
- Boonarsa, P.; Nakagawa, K.; Banlue, K.; Siriamornpun, S. Enhancement of Nutritional and Textural Properties of Meat Analogues Using Silkworm Pupae Powder via Freeze Alignment Technique. Food Chem. X 2025, 27, 102370. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, G.H.; Kim, H.E.; Kim, M.J.; Chin, K.B. Evaluation of Gelation Properties of Salt-Soluble Proteins Extracted from Protaetia brevitarsis Larvae and Tenebrio molitor Larvae and Application to Pork Myofibrillar Protein Gel System. Food Sci. Anim. Resour. 2023, 43, 1031–1043. [Google Scholar] [CrossRef]
- Krawczyk, A.; Fernández-López, J.; Zimoch-Korzycka, A. Insect Protein as a Component of Meat Analogue Burger. Foods 2024, 13, 1806. [Google Scholar] [CrossRef]
- Torruco-Uco, J.G.; Hernández-Santos, B.; Herman-Lara, E.; Martínez-Sánchez, C.E.; Juárez-Barrientos, J.M.; Rodríguez-Miranda, J. Chemical, functional and thermal characterization, and fatty acid profile of the edible grasshopper (Sphenarium purpurascens Ch.). Eur. Food Res. Technol. 2019, 245, 285–292. [Google Scholar] [CrossRef]
- Binder, M.; Mahler, V.; Hayek, B.; Sperr, W.R.; Schöller, M.; Prozell, S.; Wiedermann, G.; Valent, P.; Valenta, R.; Duchêne, M. Molecular and Immunological Characterization of Arginine Kinase from the Indianmeal Moth, Plodia Interpunctella, a Novel Cross-Reactive Invertebrate Pan-Allergen. J. Immunol. 2001, 167, 5470–5477. [Google Scholar] [CrossRef] [PubMed]
- Mintah, B.K.; He, R.; Agyekum, A.A.; Dabbour, M.; Golly, M.K.; Ma, H. Edible Insect Protein for Food Applications: Extraction, Composition, and Functional Properties. J. Food Process. Eng. 2020, 43, e13362. [Google Scholar] [CrossRef]
- Klost, M.; Ramirez-Huerta, M.I.; Drusch, S. Heat-Induced Gelation of Protein from Mealworm (Tenebrio molitor): Influence of pH and Zinc Concentration. Food Hydrocoll. Health 2022, 2, 100105. [Google Scholar] [CrossRef]
- Mannozzi, C.; Foligni, R.; Mozzon, M.; Aquilanti, L.; Cesaro, C.; Isidoro, N.; Osimani, A. Nonthermal technologies affecting techno-functional properties of edible insect-derived proteins, lipids, and chitin: A literature review. Innov. Food Sci. Emerg. Technol. 2023, 88, 103453. [Google Scholar] [CrossRef]
- Betoret, E.; Betoret, N.; Vidal, D.; Fito, P. Functional Foods Development: Trends and Technologies. Trends Food Sci. Technol. 2011, 22, 498–508. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Chen, S.-C.; Wang, Q.-L.; Liu, C.-Q.; Xiao, J.-H.; Huang, D.-W. Effects of Traditional Grinding and Superfine Grinding Technologies on the Properties and Volatile Components of Protaetia Brevitarsis Larvae Powder. LWT Food Sci. Technol. 2023, 173, 114307. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Hoon Lee, J.; Kim, Y.-J.; Kim, T.-K.; Song, K.-M.; Choi, Y.-S. Effect of Ethanol Treatment on the Structural, Techno-Functional, and Antioxidant Properties of Edible Insect Protein Obtained from Tenebrio molitor Larvae. Food Chem. 2024, 437, 137852. [Google Scholar] [CrossRef]
- Mintah, B.K.; He, R.; Dabbour, M.; Agyekum, A.A.; Xing, Z.; Golly, M.K.; Ma, H. Sonochemical Action and Reaction of Edible Insect Protein: Influence on Enzymolysis Reaction-kinetics, free-Gibbs, Structure, and Antioxidant Capacity. J. Food Biochem. 2019, 43, e12982. [Google Scholar] [CrossRef]
- Vercruysse, L.; Smagghe, G.; Beckers, T.; Camp, J.V. Antioxidative and ACE Inhibitory Activities in Enzymatic Hydrolysates of the Cotton Leafworm, Spodoptera Littoralis. Food Chem. 2009, 114, 38–43. [Google Scholar] [CrossRef]
- Mshayisa, V.V.; Van Wyk, J. Hermetia illucens Protein Conjugated with Glucose via Maillard Reaction: Antioxidant and Techno-Functional Properties. Int. J. Food Sci. 2021, 2021, 5572554. [Google Scholar] [CrossRef] [PubMed]
- Pattarayingsakul, W.; Nilavongse, A.; Reamtong, O.; Chittavanich, P.; Mungsantisuk, I.; Mathong, Y.; Prasitwuttisak, W.; Panbangred, W. Angiotensin-Converting Enzyme Inhibitory and Antioxidant Peptides from Digestion of Larvae and Pupae of Asian Weaver Ant, Oecophylla smaragdina, Fabricius. J. Sci. Food Agric. 2017, 97, 3133–3140. [Google Scholar] [CrossRef] [PubMed]
- Igić, R. Four Decades of Ocular Renin-Angiotensin and Kallikrein-Kinin Systems (1977–2017). Exp. Eye Res. 2018, 166, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Devi, W.D.; Bonysana, R.; Kapesa, K.; Rai, A.K.; Mukherjee, P.K.; Rajashekar, Y. Potential of edible insects as source of functional foods: Biotechnological approaches for improving functionality. Syst. Microbiol. Biomanuf. 2022, 2, 461–472. [Google Scholar] [CrossRef]
- Staljanssens, D.; Van Camp, J.; Herregods, G.; Dhaenens, M.; Deforce, D.; Van de Voorde, J.; Smagghe, G. Antihypertensive Effect of Insect Cells: In Vitro and in Vivo Evaluation. Peptides 2011, 32, 526–530. [Google Scholar] [CrossRef]
- Mendoza-Salazar, A.; Santiago-López, L.; Torres-Llanez, M.J.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Liceaga, A.M.; González-Córdova, A.F. In Vitro Antioxidant and Antihypertensive Activity of Edible Insects Flours (Mealworm and Grasshopper) Fermented with Lactococcus lactis Strains. Fermentation 2021, 7, 153. [Google Scholar] [CrossRef]
- Zhang, H.N.; Li, Y.H.; Huang, X.Z.; Li, J.; Luo, Y.H. Preparation of Silkworm Pupa Peptides and Its Antihypertensive Activity in Spontaneously Hypertensive Rats. Lat. Am. J. Pharm. 2012, 31, 1155–1160. [Google Scholar]
- Jia, J.; Wu, Q.; Yan, H.; Gui, Z. Purification and Molecular Docking Study of a Novel Angiotensin-I Converting Enzyme (ACE) Inhibitory Peptide from Alcalase Hydrolysate of Ultrasonic-Pretreated Silkworm Pupa (Bombyx mori) Protein. Process. Biochem. 2015, 50, 876–883. [Google Scholar] [CrossRef]
- Wu, Q.-Y.; Jia, J.-Q.; Tan, G.-X.; Xu, J.-L.; Gui, Z. Physicochemical properties of silkworm larvae protein isolate and gastrointestinal hydrolysate bioactivities. Afr. J. Biotechnol. 2011, 10, 6145–6153. [Google Scholar]
- Stella, R.; Peggion, C.; Bergantin, C.; Biancotto, G.; Frosini, M.; Dreassi, E.; Marcolongo, P.; Aloisi, A.M.; Pessina, F. Serum Metabolomics and Proteomics to Study the Antihypertensive Effect of Protein Extracts from Tenebrio mlitor. Nutrients 2022, 14, 3288. [Google Scholar] [CrossRef]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- Matheswaran, P.; Raja, L.; Banu, G. Antioxidant and Anti-Inflammatory Efficacy of Functional Proteins Obtained from Seven Edible Insects. Int. J. Entomol. Res. 2019, 4, 24–31. [Google Scholar]
- Rivero-Pino, F.; Javier Espejo-Carpio, F.; Perez-Galvez, R.; Guadix, A.; Guadix, E.M. Effect of Ultrasound Pretreatment and Sequential Hydrolysis on the Production of Tenebrio molitor Antidiabetic Peptides. Food Bioprod. Process. 2020, 123, 217–224. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Guadix, A.; Guadix, E.M. Identification of Novel Dipeptidyl Peptidase IV and α-Glucosidase Inhibitory Peptides from Tenebrio molitor. Food Funct. 2021, 12, 873–880. [Google Scholar] [CrossRef]
- de Matos, F.M.; Zanetti, G. Production of Black Cricket Protein Hydrolysates with α-Amylase, α-Glucosidase and Angiotensin I-Converting Enzyme Inhibitory Activities Using a Mixture of Proteases. Biocatal. Agric. Biotechnol. 2022, 39, 102276. [Google Scholar] [CrossRef]
- Yoon, S.; Wong, N.A.K.; Chae, M.; Auh, J.-H. Comparative Characterization of Protein Hydrolysates from Three Edible Insects: Mealworm Larvae, Adult Crickets, and Silkworm Pupae. Foods 2019, 8, 563. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Masson, F.; Zaidman-Rémy, A.; Heddi, A. Antimicrobial peptides and cell processes tracking endosymbiont dynamics. Philos. Trans. R. Soc. B Biol. Sci. 2016, 37, 20150298. [Google Scholar] [CrossRef]
- Bulet, P.; Stöcklin, R. Insect antimicrobial peptides: Structures, properties and gene regulation. Protein Pept. Lett. 2005, 12, 3–11. [Google Scholar] [CrossRef]
- Hultmark, D.; Steiner, H.; Rasmuson, T.; Boman, H.G. Insect Immunity. Purification and Properties of Three Inducible Bactericidal Proteins from Hemolymph of Immunized Pupae of Hyalophora cecropia. Eur. J. Biochem. 1980, 10, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.Y.; Chowdhury, M.; Huang, Y.D.; Yu, X.Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, E.; Podsiadlowski, L.; Muhammed, M.; Vilcinskas, A. Diversity, Evolution and Medical Applications of Insect Antimicrobial Peptides. Philos. Trans. R. Soc. B-Biol. Sci. 2016, 371, 20150290. [Google Scholar] [CrossRef]
- Rahnamaeian, M.; Cytryńska, M.; Zdybicka-Barabas, A.; Dobslaff, K.; Wiesner, J.; Twyman, R.M.; Zuchner, T.; Sadd, B.M.; Regoes, R.R.; Schmid-Hempel, P.; et al. Insect Antimicrobial Peptides Show Potentiating Functional Interactions against Gram-Negative Bacteria. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150293. [Google Scholar] [CrossRef]
- Robles-Fort, A.; García-Robles, I.; Fernando, W.; Hoskin, D.W.; Rausell, C.; Real, M.D. Dual Antimicrobial and Antiproliferative Activity of TcPaSK Peptide Derived from a Tribolium castaneum Insect Defensin. Microorganisms 2021, 9, 222. [Google Scholar] [CrossRef]
- Bertrams, W.; Lindhauer, N.S.; Rieke, M.C.; Paas, A.; Hoffmann, K.; Greene, B.; Visekruna, A.; Vilcinskas, A.; Seidel, K.; Schmeck, B. Tribolium castaneum defensin 1 kills Moraxella catarrhalis in an in vitro infection model but does not harm commensal bacteria. Virulence 2020, 12, 1003–1010. [Google Scholar] [CrossRef]
- Rajamuthiah, R.; Jayamani, E.; Conery, A.L.; Fuchs, B.B.; Kim, W.; Johnston, T.; Vilcinskas, A.; Ausubel, F.M.; Mylonakis, E. A Defensin from the Model Beetle Tribolium castaneum Acts Synergistically with Telavancin and Daptomycin against Multidrug Resistant Staphylococcus Aureus. PLoS ONE 2015, 10, e0128576. [Google Scholar] [CrossRef]
- Makarova, O.; Rodríguez-Rojas, A.; Eravci, M.; Weise, C.; Dobson, A.; Johnston, P.; Rolff, J. Antimicrobial Defence and Persistent Infection in Insects Revisited. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2016, 371, 20150296. [Google Scholar] [CrossRef]
- Hall, F.; Johnson, P.E.; Liceaga, A. Effect of Enzymatic Hydrolysis on Bioactive Properties and Allergenicity of Cricket (Gryllodes sigillatus) Protein. Food Chem. 2018, 262, 39–47. [Google Scholar] [CrossRef]
- Li, H.; Inoue, A.; Taniguchi, S.; Yukutake, T.; Suyama, K.; Nose, T.; Maeda, I. Multifunctional Biological Activities of Water Extract of Housefly Larvae (Musca domestica). PharmaNutrition 2017, 5, 119–126. [Google Scholar] [CrossRef]
- Anusha, S.; Negi, P.S. Processing of Silkworm (Bombyx Mori) Pupae Waste and Mealworm (Tenebrio molitor) Larvae: Chemical Characterization of Extracts Rich in Anti-Oxidant, Anti-Diabetic, and Anti-Obesity Activity. Biomass Convers. Biorefin. 2025, in press. [Google Scholar] [CrossRef]
- Kim, B.S.; Turk, A.; Lee, S.; Lee, H.H.; Kim, M.H.; Jeong, S.Y.; Kwon, E.-B.; Hwang, B.Y.; Lee, M.K. Anti-Diabetic Activity of Cordyceps-Fermented Edible Insects by the Promotion of Glucose Absorption. J. Med. Food 2025, 28, 105–111. [Google Scholar] [CrossRef]
- Yang, J.; Lee, K.S.; Kim, B.Y.; Choi, Y.S.; Yoon, H.J.; Jia, J.; Jin, B.R. Anti-Fibrinolytic and Anti-Microbial Activities of a Serine Protease Inhibitor from Honeybee (Apis cerana) Venom. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 201, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Emilia, M.; Magdalena, C.; Weronika, G.; Julia, W.; Danuta, K.; Jakub, S.; Krzysztof, K. IgE-based analysis of sensitization and cross-reactivity to yellow mealworm and edible insect allergens before their widespread dietary introduction. Sci. Rep. 2025, 15, 1466. [Google Scholar] [CrossRef] [PubMed]
- De Gier, S.; Verhoeckx, K. Insect (Food) Allergy and Allergens. Mol. Immunol. 2018, 100, 82–106. [Google Scholar] [CrossRef]
- Barennes, H.; Phimmasane, M.; Rajaonarivo, C. Insect Consumption to Address Undernutrition, a National Survey on the Prevalence of Insect Consumption among Adults and Vendors in Laos. PLoS ONE 2015, 10, e0136458. [Google Scholar] [CrossRef]
- Yew, K.L.; Kok, V.S.L. Exotic Food Anaphylaxis and the Broken Heart: Sago Worm and Takotsubo Cardiomyopathy. Med. J. Malays. 2012, 67, 540–541. [Google Scholar]
- Ji, K.-M.; Zhan, Z.-K.; Chen, J.-J.; Liu, Z.-G. Anaphylactic Shock Caused by Silkworm Pupa Consumption in China. Allergy 2008, 63, 1407–1408. [Google Scholar] [CrossRef]
- Choi, G.-S.; Shin, Y.S.; Kim, J.E.; Ye, Y.-M.; Park, H.-S. Five Cases of Food Allergy to Vegetable Worm (Cordyceps sinensis) Showing Cross-Reactivity with Silkworm Pupae. Allergy 2010, 65, 1196–1197. [Google Scholar] [CrossRef]
- Ayuso, R.; Reese, G.; Leong-Kee, S.; Plante, M.; Lehrer, S.B. Molecular Basis of Arthropod Cross-Reactivity: IgE-Binding Cross-Reactive Epitopes of Shrimp, House Dust Mite and Cockroach Tropomyosins. Int. Arch. Allergy Immunol. 2002, 129, 38–48. [Google Scholar] [CrossRef]
- Pennisi, E. All in the (Bigger) Family. Science 2015, 347, 220–221. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.; Tham, E.H.; Lee, B.W. An Update on Shellfish Allergy. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 236–242. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, L.; Wangorsch, A.; Zoccatelli, G. Allergens from Edible Insects: Cross-Reactivity and Effects of Processing. Curr. Allergy Asthma Rep. 2021, 21, 35. [Google Scholar] [CrossRef]
- Sookrung, N.; Chaicumpa, W.; Tungtrongchitr, A.; Vichyanond, P.; Bunnag, C.; Ramasoota, P.; Tongtawe, P.; Sakolvaree, Y.; Tapchaisri, P. Periplaneta Americana Arginine Kinase as a Major Cockroach Allergen among Thai Patients with Major Cockroach Allergies. Environ. Health Perspect. 2006, 114, 875–880. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Lou, H.; Wang, C.; Ni, M.; Yu, D.; Zhang, L.; Kang, L. Hexamerin-2 Protein of Locust as a Novel Allergen in Occupational Allergy. J. Asthma Allergy 2022, 15, 145–155. [Google Scholar] [CrossRef]
- Han, X.-Y.; Yang, H.; Rao, S.-T.; Liu, G.-Y.; Hu, M.-J.; Zeng, B.-C.; Cao, M.-J.; Liu, G.-M. The Maillard Reaction Reduced the Sensitization of Tropomyosin and Arginine Kinase from Scylla Paramamosain, Simultaneously. J. Agric. Food Chem. 2018, 66, 2934–2943. [Google Scholar] [CrossRef]
- Liu, G.-M.; Li, B.; Yu, H.-L.; Cao, M.-J.; Cai, Q.-F.; Lin, J.-W.; Su, W.-J. Induction of Mud Crab (Scylla paramamosain) Tropomyosin and Arginine Kinase Specific Hypersensitivity in BALB/c Mice. J. Sci. Food Agric. 2012, 92, 232–238. [Google Scholar] [CrossRef]
- Liu, G.; Hu, M.; Sun, L.-C.; Han, X.; Liu, Q.; Alcocer, M.; Fei, D.; Cao, M.-J.; Liu, G.-M. Allergenicity and Oral Tolerance of Enzymatic Cross-Linked Tropomyosin Evaluated Using Cell and Mouse Models. J. Agric. Food Chem. 2017, 65, 2205–2213. [Google Scholar] [CrossRef]
- Delfino, D.; Prandi, B.; Calcinai, L.; Ridolo, E.; Dellafiora, L.; Pedroni, L.; Nicoletta, F.; Cavazzini, D.; Tedeschi, T.; Folli, C. Molecular Characterization of the Allergenic Arginine Kinase from the Edible Insect Hermetia illucens (Black Soldier Fly). Mol. Nutr. Food Res. 2024, 68, e2300911. [Google Scholar] [CrossRef]
- Azzi, A.; Clark, S.A.; Ellington, W.R.; Chapman, M.S. The role of phosphagen specificity loops in arginine kinase. Protein Sci. 2004, 13, 575–585. [Google Scholar] [CrossRef]
- Jeong, K.Y.; Han, I.-S.; Lee, J.Y.; Park, K.H.; Lee, J.-H.; Park, J.-W. Role of Tropomyosin in Silkworm Allergy. Mol. Med. Rep. 2017, 15, 3264–3270. [Google Scholar] [CrossRef] [PubMed]
- Verhoeckx, K.C.M.; van Broekhoven, S.; den Hartog-Jager, C.F.; Gaspari, M.; de Jong, G.A.H.; Wichers, H.J.; van Hoffen, E.; Houben, G.F.; Knulst, A.C. House Dust Mite (Der p 10) and Crustacean Allergic Patients May React to Food Containing Yellow Mealworm Proteins. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2014, 65, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, C.; Nebbia, S.; Cirrincione, S.; Brussino, L.; Giorgis, V.; Romito, A.; Marchese, C.; Manfredi, M.; Marengo, E.; Giuffrida, M.G.; et al. Thermal Processing of Insect Allergens and IgE Cross-Recognition in Italian Patients Allergic to Shrimp, House Dust Mite and Mealworm. Food Res. Int. 2021, 148, 110567. [Google Scholar] [CrossRef]
- Kamemura, N.; Sugimoto, M.; Tamehiro, N.; Adachi, R.; Tomonari, S.; Watanabe, T.; Mito, T. Cross-Allergenicity of Crustacean and the Edible Insect Gryllus bimaculatus in Patients with Shrimp Allergy. Mol. Immunol. 2019, 106, 127–134. [Google Scholar] [CrossRef]
- Barre, A.; Pichereaux, C.; Simplicien, M.; Burlet-Schiltz, O.; Benoist, H.; Rougé, P. A Proteomic- and Bioinformatic-Based Identification of Specific Allergens from Edible Insects: Probes for Future Detection as Food Ingredients. Foods 2021, 10, 280. [Google Scholar] [CrossRef]
- Leni, G.; Tedeschi, T.; Faccini, A.; Pratesi, F.; Folli, C.; Puxeddu, I.; Migliorini, P.; Gianotten, N.; Jacobs, J.; Depraetere, S.; et al. Shotgun Proteomics, in-Silico Evaluation and Immunoblotting Assays for Allergenicity Assessment of Lesser Mealworm, Black Soldier Fly and Their Protein Hydrolysates. Sci. Rep. 2020, 10, 1228. [Google Scholar] [CrossRef]
- Abdelmoteleb, M.; Palmer, L.K.; Marsh, J.; Johnson, P.; Goodman, R.E. Bioinformatics and Proteomics Evaluations of Potential IgE Cross-Reactive Proteins in Novel Edible Insects and Shrimp for Food Safety. J. Allergy Clin. Immunol. 2019, 143, AB237. [Google Scholar] [CrossRef]
- Carriço-Sá, B.; Teixeira, C.S.S.; Villa, C.; Mendes, E.; Ferreira, I.M.; Mafra, I.; Costa, J. Protein Extraction from Edible Insects: Implications for IgE-Binding Capacity. Food Chem. 2025, 468, 142453. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; la Rosa, T.G.; Paz, S.M. la Edible Insects as a Source of Biopeptides and Their Role in Immunonutrition. Food Funct. 2024, 15, 2789–2798. [Google Scholar] [CrossRef]
- He, W.; He, K.; Sun, F.; Mu, L.; Liao, S.; Li, Q.; Yi, J.; Liu, Z.; Wu, X. Effect of Heat, Enzymatic Hydrolysis and Acid-Alkali Treatment on the Allergenicity of Silkworm Pupa Protein Extract. Food Chem. 2021, 343, 128461. [Google Scholar] [CrossRef]
- Boukil, A.; Perreault, V.; Chamberland, J.; Mezdour, S.; Pouliot, Y.; Doyen, A. High Hydrostatic Pressure-Assisted Enzymatic Hydrolysis Affect Mealworm Allergenic Proteins. Molecules 2020, 25, 2685. [Google Scholar] [CrossRef]
- Dong, X.; Wang, J.; Raghavan, V. Impact of Microwave Processing on the Secondary Structure, in-Vitro Protein Digestibility and Allergenicity of Shrimp (Litopenaeus vannamei) Proteins. Food Chem. 2021, 337, 127811. [Google Scholar] [CrossRef]
- Hall, F.; Liceaga, A. Effect of Microwave-Assisted Enzymatic Hydrolysis of Cricket (Gryllodes sigillatus) Protein on ACE and DPP-IV Inhibition and Tropomyosin-IgG Binding. J. Funct. Foods 2020, 64, 103634. [Google Scholar] [CrossRef]
- Broekman, H.C.H.P.; Knulst, A.C.; de Jong, G.; Gaspari, M.; den Hartog Jager, C.F.; Houben, G.F.; Verhoeckx, K.C.M. Is Mealworm or Shrimp Allergy Indicative for Food Allergy to Insects? Mol. Nutr. Food Res. 2017, 61, 1601061. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Kim, J.-H.; Ji, D.-S.; Lee, C.-H. Effects of Heating Time and Temperature on Functional Properties of Proteins of Yellow Mealworm Larvae (Tenebrio molitor L.). Food Sci. Anim. Resour. 2019, 39, 296–308. [Google Scholar] [CrossRef]
- van Broekhoven, S.; Bastiaan-Net, S.; de Jong, N.W.; Wichers, H.J. Influence of Processing and in Vitro Digestion on the Allergic Cross-Reactivity of Three Mealworm Species. Food Chem. 2016, 196, 1075–1083. [Google Scholar] [CrossRef]
- Nath, N.; Baset, B.; Shoshe, N.; Belal, S.; Aktaruzzaman, M.D.; Uddin, M.N. Growth Performance and Feed Conversion Efficiency of Crossbred Heifers. Iran. J. Appl. Anim. Sci. 2016, 6, 511. [Google Scholar]
- Wang, Y.; Liu, C.; Lang, H.; Hu, Z.; Wang, X.; Yang, Z.; Wang, Z.; Guo, Z.; Jiang, L. Effects of Microwave on the Structural and Emulsifying Properties and Interfacial Properties of Oxidized Soybean Protein Aggregates. Food Chem. X 2023, 19, 100861. [Google Scholar] [CrossRef]
- Gazikalović, I.; Mijalković, J.; Šekuljica, N.; Jakovetić Tanasković, S.; Đukić Vuković, A.; Mojović, L.; Knežević-Jugović, Z. Synergistic Effect of Enzyme Hydrolysis and Microwave Reactor Pretreatment as an Efficient Procedure for Gluten Content Reduction. Foods 2021, 10, 2214. [Google Scholar] [CrossRef]
- Hall, F.G.; Liceaga, A.M. Isolation and Proteomic Characterization of Tropomyosin Extracted from Edible Insect Protein. Food Chem. Mol. Sci. 2021, 3, 100049. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Lu, Q.; Kong, L.; Ge, W. Effect of Microwave Heating on Physicochemical Properties, Protein Composition and Structure, and Micromorphology of Camel and Bovine Milk Samples. J. Food Compos. Anal. 2023, 122, 105468. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Guo, X.; Cao, Y.; Qu, G.; Li, Q.; Gao, Y.; Yu, X. Microwave Pretreatment Effects on the Aroma Precursors, Sensory Characteristics and Flavor Profiles of Fragrant Rapeseed Oil. Food Chem. X 2024, 22, 101381. [Google Scholar] [CrossRef]
- Siddique, I.J.; Salema, A.A.; Antunes, E.; Vinu, R. Technical Challenges in Scaling up the Microwave Technology for Biomass Processing. Renew. Sustain. Energy Rev. 2022, 153, 111767. [Google Scholar] [CrossRef]
- De Marchi, L.; Mainente, F.; Leonardi, M.; Scheurer, S.; Wangorsch, A.; Mahler, V.; Pilolli, R.; Sorio, D.; Zoccatelli, G. Allergenicity Assessment of the Edible Cricket Acheta domesticus in Terms of Thermal and Gastrointestinal Processing and IgE Cross-Reactivity with Shrimp. Food Chem. 2021, 359, 129878. [Google Scholar] [CrossRef]
- Liceaga, A.M. Approaches for Utilizing Insect Protein for Human Consumption: Effect of Enzymatic Hydrolysis on Protein Quality and Functionality. Ann. Entomol. Soc. Am. 2019, 112, 529–532. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B. Can Insects Help to Ease the Problem of World Food Shortage. Search 1975, 6, 261–262. [Google Scholar]
- Deroy, O.; Reade, B.; Spence, C. The Insectivore’s Dilemma, and How to Take the West out of It. Food Qual. Prefer. 2015, 44, 44–55. [Google Scholar] [CrossRef]
- Hartmann, C.; Shi, J.; Giusto, A.; Siegrist, M. The Psychology of Eating Insects: A Cross-Cultural Comparison between Germany and China. Food Qual. Prefer. 2015, 44, 148–156. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Alvi, T.; Sameen, A.; Khan, S.; Blinov, A.V.; Nagdalian, A.A.; Mehdizadeh, M.; Adli, D.N.; Onwezen, M. Consumer Acceptance of Alternative Proteins: A Systematic Review of Current Alternative Protein Sources and Interventions Adapted to Increase Their Acceptability. Sustainability 2022, 14, 15370. [Google Scholar] [CrossRef]
- van Huis, A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Kowalski, S.; Mikulec, A.; Mickowska, B.; Skotnicka, M.; Mazurek, A. Wheat Bread Supplementation with Various Edible Insect Flours. Influence of Chemical Composition on Nutritional and Technological Aspects. LWT 2022, 159, 113220. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Cardinali, F.; Roncolini, A.; Garofalo, C.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foligni, R.; Raffaelli, N.; et al. Bread enriched with cricket powder (Acheta domesticus): A technological, microbiological and nutritional evaluation. Innov. Food Sci. Emerg. Technol. 2018, 48, 150–163. [Google Scholar] [CrossRef]
- da Rosa Machado, C.; Thys, R.C.S. Cricket Powder (Gryllus assimilis) as a New Alternative Protein Source for Gluten-Free Breads. Innov. Food Sci. Emerg. Technol. 2019, 56, 102180. [Google Scholar] [CrossRef]
- Homann, A.M.; Ayieko, M.A.; Konyole, S.O.; Roos, N. Acceptability of biscuits containing 10% cricket (Acheta domesticus) compared to milk biscuits among 5-10-year-old Kenyan schoolchildren. J. Insects Food Feed. 2017, 3, 95–104. [Google Scholar] [CrossRef]
- Akande, A.O.; Jolayemi, O.S.; Adelugba, V.A.; Akande, S.T. Silkworm Pupae (Bombyx mori) and Locusts as Alternative Protein Sources for High-Energy Biscuits. J. Asia-Pac. Entomol. 2020, 23, 234–241. [Google Scholar] [CrossRef]
- Ortolá, M.D.; Martínez-Catalá, M.; Yuste Del Carmen, A.; Castelló, M.L. Physicochemical and sensory properties of biscuits formulated with Tenebrio molitor and Alphitobius diaperinus flours. J. Texture Stud. 2022, 53, 540–549. [Google Scholar] [CrossRef]
- García-Segovia, P.; Igual, M.; Noguerol, A.T.; Martínez-Monzó, J. Use of Insects and Pea Powder as Alternative Protein and Mineral Sources in Extruded Snacks. Eur. Food Res. Technol. 2020, 246, 703–712. [Google Scholar] [CrossRef]
- Stoops, J.; Vandeweyer, D.; Crauwels, S.; Verreth, C.; Boeckx, H.; Van Der Borght, M.; Claes, J.; Lievens, B.; Van Campenhout, L. Minced Meat-like Products from Mealworm Larvae (Tenebrio molitor and Alphitobius diaperinus): Microbial Dynamics during Production and Storage. Innov. Food Sci. Emerg. Technol. 2017, 41, 1–9. [Google Scholar] [CrossRef]
- Park, Y.-S.; Choi, Y.-S.; Hwang, K.-E.; Kim, T.-K.; Lee, C.-W.; Shin, D.-M.; Han, S.G. Physicochemical Properties of Meat Batter Added with Edible Silkworm Pupae (Bombyx mori) and Transglutaminase. Korean J. Food Sci. Anim. Resour. 2017, 37, 351–359. [Google Scholar] [CrossRef]
- Choi, B.D.; Wong, N.A.K.; Auh, J.-H. Defatting and Sonication Enhances Protein Extraction from Edible Insects. Korean J. Food Sci. Anim. Resour. 2017, 37, 955–961. [Google Scholar] [CrossRef]
- En, A.; Kiin-Kabari, D. Nutritional Composition and Microbiology of Some Edible Insects Commonly Eaten in Africa, Hurdles and Future Prospects: A Critical Review. J. Food Microbiol. Saf. Hyg. 2016, 1, 107. [Google Scholar] [CrossRef]
- Cázares-Samaniego, P.J.; Castillo, C.G.; Ramos-López, M.A.; González-Chávez, M.M. Volatilome and Essential Oil of Ulomoides dermestoides: A Broad-Spectrum Medical Insect. Molecules 2021, 26, 6311. [Google Scholar] [CrossRef] [PubMed]
- Pirintsos, S.; Panagiotopoulos, A.; Bariotakis, M.; Daskalakis, V.; Lionis, C.; Sourvinos, G.; Karakasiliotis, I.; Kampa, M.; Castanas, E. From Traditional Ethnopharmacology to Modern Natural Drug Discovery: A Methodology Discussion and Specific Examples. Molecules 2022, 27, 4060. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Cruz-Monterrosa, R.G.; Liceaga, A.M. Beyond Human Nutrition of Edible Insects: Health Benefits and Safety Aspects. Insects 2022, 13, 1007. [Google Scholar] [CrossRef] [PubMed]
- Guiné, R.P.F.; Florença, S.G.; Costa, C.A.; Correia, P.M.R.; Boustani, N.M.; Matran, I.; Jakšić, K.; Chuck-Hernández, C.; Bartkiene, E.; Djekic, I.; et al. Consumers’ Perceptions about Edible Insects’ Nutritional Value and Health Effects: Study Involving 14 Countries. Animals 2024, 14, 1631. [Google Scholar] [CrossRef]
- Quah, Y.; Tong, S.-R.; Bojarska, J.; Giller, K.; Tan, S.-A.; Ziora, Z.M.; Esatbeyoglu, T.; Chai, T.-T. Bioactive Peptide Discovery from Edible Insects for Potential Applications in Human Health and Agriculture. Molecules 2023, 28, 1233. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Pang, L.; Li, J.-J.; Zhang, C.; Li, J.-X.; Zhang, X.; Mao, T.; Wu, D.-T.; Ma, X.-Y.; Geng, F.-N.; et al. Characterization and Diabetic Wound Healing Benefits of Protein-Polysaccharide Complexes Isolated from an Animal Ethno-Medicine Periplaneta americana L. Int. J. Biol. Macromol. 2022, 195, 466–474. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Che, J.; Fan, H.; Liu, Q.; Xia, H. The Inhibitory Effect of Periplaneta americana L. on Hepatocellular Carcinoma: Explore the Anti-Hepatocellular Carcinoma Active Site and Its Mechanism of Action. J. Ethnopharmacol. 2022, 291, 114884. [Google Scholar] [CrossRef]
- Yu, W.; Ying, H.; Tong, F.; Zhang, C.; Quan, Y.; Zhang, Y. Protective Effect of the Silkworm Protein 30Kc6 on Human Vascular Endothelial Cells Damaged by Oxidized Low Density Lipoprotein (Ox-LDL). PLoS ONE 2013, 8, e68746. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, K.P.; Lee, D.Y.; Kim, Y.T.; Baek, S.; Yoon, M.S. Inhibitory Effect of Modified Silkworm Pupae Oil in PDGF-BB-Induced Proliferation and Migration of Vascular Smooth Muscle Cells. Food Sci. Biotechnol. 2020, 29, 1091–1099. [Google Scholar] [CrossRef]
- Wang, W.; Xu, L.; Zou, Y.; Pang, D.; Shi, W.; Mu, L.; Li, E.; Lan, D.; Wang, Y.; Liao, S. Comprehensive Identification of Principal Lipid Classes and Tocochromanols in Silkworm (Antheraea pernyi and Bombyx mori) Pupae Oils. Eur. J. Lipid Sci. Technol. 2019, 122, 1900280. [Google Scholar] [CrossRef]
- Fasakin, E.A.; Balogun, A.M.; Ajayi, O.O. Evaluation of Full-Fat and Defatted Maggot Meals in the Feeding of Clariid Catfish Clarias Gariepinus Fingerlings. Aquac. Res. 2003, 34, 733–738. [Google Scholar] [CrossRef]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the Use of Insects in the Diet of Farmed Fish: Past and Future. Anim. Feed Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Ssepuuya, G.; Namulawa, V.; Mbabazi, D.; Mugerwa, S.; Fuuna, P.; Nampijja, Z.; Ekesi, S.; Fiaboe, K.K.M.; Nakimbugwe, D. Use of insects for fish and poultry compound feed in sub-Saharan Africa—A systematic review. J. Insects Food Feed 2017, 3, 289–302. [Google Scholar] [CrossRef]
- Benzertiha, A.; Kierończyk, B.; Rawski, M.; Kołodziejski, P.; Bryszak, M.; Józefiak, D. Insect Oil as An Alternative to Palm Oil and Poultry Fat in Broiler Chicken Nutrition. Animals 2019, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Muros, M.-J.; Barroso, F.G.; Manzano-Agugliaro, F. Insect Meal as Renewable Source of Food for Animal Feeding: A Review. J. Clean. Prod. 2014, 65, 16–27. [Google Scholar] [CrossRef]
- Khan, S.H. Recent Advances in Role of Insects as Alternative Protein Source in Poultry Nutrition. J. Appl. Anim. Res. 2018, 46, 1144–1157. [Google Scholar] [CrossRef]
- Zentek, J.; Buchheit-Renko, S.; Ferrara, F.; Vahjen, W.; Van Kessel, A.G.; Pieper, R. Nutritional and Physiological Role of Medium-Chain Triglycerides and Medium-Chain Fatty Acids in Piglets. Anim. Health Res. Rev. 2011, 12, 83–93. [Google Scholar] [CrossRef]
- Onyango, C.; Noetzold, H.; Bley, T.; Henle, T. Proximate Composition and Digestibility of Fermented and Extruded Uji from Maize–Finger Millet Blend. LWT Food Sci. Technol. 2004, 37, 827–832. [Google Scholar] [CrossRef]
- Fukuda, E.P.; Cox, J.R.; Wickersham, T.A.; Drewery, M.L. Evaluation of Black Soldier Fly Larvae (Hermetia illucens) as a Protein Supplement for Beef Steers Consuming Low-Quality Forage. Transl. Anim. Sci. 2022, 6, txac018. [Google Scholar] [CrossRef]
- Miech, P.; Lindberg, J.; Berggren, Å.; Ty, C.; Jansson, A. Apparent faecal digestibility and nitrogen retention in piglets fed whole and peeled Cambodian field cricket meal. J. Insects Food Feed. 2017, 3, 279–288. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-Art on Use of Insects as Animal Feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Zuidhof, M.J.; Molnar, C.L.; Morley, F.M.; Wray, T.L.; Robinson, F.E.; Khan, B.A.; Al-Ani, L.; Goonewardene, L.A. Nutritive Value of House Fly (Musca domestica) Larvae as a Feed Supplement for Turkey Poults. Anim. Feed Sci. Technol. 2003, 105, 225–230. [Google Scholar] [CrossRef]
- Kröger, T.; Dupont, J.; Büsing, L.; Fiebelkorn, F. Acceptance of Insect-Based Food Products in Western Societies: A Systematic Review. Front. Nutr. 2021, 8, 759885. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.S.G.; Fischer, A.R.H.; Tinchan, P.; Stieger, M.; Steenbekkers, L.P.A.; van Trijp, H.C.M. Insects as Food: Exploring Cultural Exposure and Individual Experience as Determinants of Acceptance. Food Qual. Prefer. 2015, 42, 78–89. [Google Scholar] [CrossRef]
- Andrijana, A.; Marija, M.; Marija, B.; Vojana, O. Survey on Public Acceptance of Insects as Novel Food in a Non-EU Country: A Case Study of Serbia. J. Insects Food Feed. 2023, 10, 91–106. [Google Scholar] [CrossRef]
- Tilami, S.K.; Jurkaninová, L.; Kotíková, Z.; Škvorová, P.; Ferusová, Ž.; Kouřimská, L. Quality and Nutritional Evaluation of Bakery Products Enriched with Cricket Meal. Appl. Food Res. 2025, 5, 101303. [Google Scholar] [CrossRef]
- Pozharliev, R.; De Angelis, M.; Rossi, D.; Bagozzi, R.; Amatulli, C. I Might Try It: Marketing Actions to Reduce Consumer Disgust toward Insect-Based Food. J. Retail. 2023, 99, 149–167. [Google Scholar] [CrossRef]
- Modlinska, K.; Adamczyk, D.; Goncikowska, K.; Maison, D.; Pisula, W. The Effect of Labelling and Visual Properties on the Acceptance of Foods Containing Insects. Nutrients 2020, 12, 2498. [Google Scholar] [CrossRef]
- Rapid Risk Assessment: What Is the Risk to Consumers from Consumption of the Seven Edible Insects’ Products Currently Available in the UK Market?|Food Standards Agency. Available online: https://www.food.gov.uk/research/novel-and-non-traditional-foods-additives-and-processes/rapid-risk-assessment-what-is-the-risk-to-consumers-from-consumption-of-the-seven-edible-insects-products-currently-available-in (accessed on 3 October 2022).
- Ros-Baró, M.; Sánchez-Socarrás, V.; Santos-Pagès, M.; Bach-Faig, A.; Aguilar-Martínez, A. Consumers’ Acceptability and Perception of Edible Insects as an Emerging Protein Source. Int. J. Environ. Res. Public Health 2022, 19, 15756. [Google Scholar] [CrossRef]
- Hartmann, C.; Siegrist, M. Insects as food: Perception and acceptance Findings from current research. Ernahrungs Umsch. 2017, 64, 44–50. [Google Scholar] [CrossRef]
- Modlinska, K.; Adamczyk, D.; Pisula, W. The Impact of a Safety Certificate on the Evaluation of Food Products Containing Insects. J. Consum. Prot. Food Saf. 2025; in press. [Google Scholar] [CrossRef]
- Okaiyeto, S.A.; Yu, S.-H.; Deng, L.-Z.; Wang, Q.-H.; Sutar, P.P.; Wang, H.; Zhao, J.-H.; Mujumdar, A.S.; Ni, J.-B.; Lv, W.; et al. How to Enhance the Acceptability of Insects Food—A Review. Food Front. 2024, 5, 311–328. [Google Scholar] [CrossRef]
- Tso, R.; Lim, A.J.; Forde, C.G. A Critical Appraisal of the Evidence Supporting Consumer Motivations for Alternative Proteins. Foods 2020, 10, 24. [Google Scholar] [CrossRef]
- Naranjo-Guevara, N.; Stroh, B.; Floto-Stammen, S. Packaging Communication as a Tool to Reduce Disgust with Insect-Based Foods: Effect of Informative and Visual Elements. Foods 2023, 12, 3606. [Google Scholar] [CrossRef] [PubMed]
- Belluco, S.; Halloran, A.; Ricci, A. New protein sources and food legislation: The case of edible insects and EU law. Food Secur. 2017, 9, 803–814. [Google Scholar] [CrossRef]
- Imathiu, S. Benefits and food safety concerns associated with consumption of edible insects. NFS J. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No. 999/2001 of the European Parliament and of the Council Laying Down Rules for the Prevention, Control and Eradication of Certain Transmissible Spongiform Encephalopathies. 2001. Available online: https://eur-lex.europa.eu/legal-content/EN/LSU/?uri=oj:JOL_2001_147_R_0001_01 (accessed on 3 October 2025).
- European Commission. Regulation (EC) No. 893/2017. 2017. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32017R0893 (accessed on 3 October 2025).
- European Commission. Regulation (EU) 2021/1925. 2021. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32021R1925 (accessed on 3 October 2025).
- European Commission. Regulation (EU) 2015/2283 of the European Parliament and of the Council on Novel Foods. 2015. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX%3A32015R2283 (accessed on 3 October 2025).
- European Commission. Regulation (EC) No. 178/2002 of the European Parliament and of the Council Laying Down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying Down Procedures in Matters of Food Safety. 2002. Available online: https://m.book118.com/html/2024/1213/6045111005011011.shtm (accessed on 3 October 2025).
- European Commission. Regulation (EC) No. 183/2005. 2005. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32005R0183 (accessed on 3 October 2025).
- Meijer, N.; Safitri, R.A.; Tao, W.; Hoek-Van den Hil, E.F. Review: European Union Legislation and Regulatory Framework for Edible Insect Production—Safety Issues. Animal 2025, 19, 101468. [Google Scholar] [CrossRef]
- Baiano, A. Edible Insects: An Overview on Nutritional Characteristics, Safety, Farming, Production Technologies, Regulatory Framework, and Socio-Economic and Ethical Implications. Trends Food Sci. Technol. 2020, 100, 35–50. [Google Scholar] [CrossRef]
- Piha, S.; Pohjanheimo, T.; Lähteenmäki-Uutela, A.; Křečková, Z.; Otterbring, T. The Effects of Consumer Knowledge on the Willingness to Buy Insect Food: An Exploratory Cross-Regional Study in Northern and Central Europe. Food Qual. Prefer. 2018, 70, 1–10. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Marimuthu, S.B.; Meijer, N. Regulations on Insects as Food and Feed: A Global Comparison. J. Insects Food Feed 2021, 7, 849–856. [Google Scholar] [CrossRef]
- Azagoh, C.; Ducept, F.; Garcia, R.; Rakotozafy, L.; Cuvelier, M.-E.; Keller, S.; Lewandowski, R.; Mezdour, S. Extraction and Physicochemical Characterization of Tenebrio molitor Proteins. Food Res. Int. 2016, 88, 24–31. [Google Scholar] [CrossRef]
- Bußler, S.; Rumpold, B.A.; Jander, E.; Rawel, H.M.; Schlüter, O.K. Recovery and Techno-Functionality of Flours and Proteins from Two Edible Insect Species: Meal Worm (Tenebrio molitor) and Black Soldier Fly (Hermetia illucens) Larvae. Heliyon 2016, 2, e00218. [Google Scholar] [CrossRef]
- Womeni, H.; Tiencheu, B.; Linder, M.; Nabayo, E.; Tenyang, N.; Mbiapo, F.; Villeneuve, P.; Fanni, J.; Parmentier, M. Nutritional value and effect of cooking, drying and storage process on some functional properties of Rhynchophorus phoenicis. Int. J. Life Sci. Pharma Res. 2012, 2, L203–L219. [Google Scholar]
- Zhao, X.; Vázquez-Gutiérrez, J.L.; Johansson, D.P.; Landberg, R.; Langton, M. Yellow Mealworm Protein for Food Purposes—Extraction and Functional Properties. PLoS ONE 2016, 11, e0147791. [Google Scholar] [CrossRef]
- Veldkamp, T.; van Duinkerken, G.; van Huis, A.; Lakemond, C.M.M.; Ottevanger, E.; Bosch, G.; van Boekel, T. Insects as a Sustainable Feed Ingredient in Pig and Poultry Diets: A Feasibility Study; Wageningen UR Livestock Research: Lelystad, The Netherlands, 2012. [Google Scholar]
- Li, T.; Khan, S.; Wei, M.; Li, H.; Wen, T.; Guo, J.; Jin, D. Utilizing Black Soldier Fly Larvae to Improve Bioconversion and Reduce Pollution: A Sustainable Method for Efficient Treatment of Mixed Wastes of Wet Distiller Grains and Livestock Manure. Molecules 2023, 28, 5735. [Google Scholar] [CrossRef]
- Morella, P.; Lambán, M.P.; Royo, J.; Sánchez, J.C. Vertical Farming Monitoring: How Does It Work and How Much Does It Cost? Sensors 2023, 23, 3502. [Google Scholar] [CrossRef]
- Zhan, S.; Fang, G.; Cai, M.; Kou, Z.; Xu, J.; Cao, Y.; Bai, L.; Zhang, Y.; Jiang, Y.; Luo, X.; et al. Genomic Landscape and Genetic Manipulation of the Black Soldier Fly Hermetia illucens, a Natural Waste Recycler. Cell Res. 2020, 30, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Omotoso, O.T. Nutritional Quality, Functional Properties and Anti-Nutrient Compositions of the Larva of Cirina Forda (Westwood) (Lepidoptera: Saturniidae). J. Zhejiang Univ. Sci. B. 2006, 7, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, D.; Wilkinson, K.A.; Treilhou, M.; Téné, N.; Castillo, D.; Sauvain, M. Prospecting Peptides Isolated from Black Soldier Fly (Diptera: Stratiomyidae) With Antimicrobial Activity Against Helicobacter Pylori (Campylobacterales: Helicobacteraceae). J. Insect Sci. 2019, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.-G.; Lee, H.-Y.; Kim, J.-H.; Kang, Y.-R.; Moon, D.-I.; Seo, M.-Y.; Back, H.-I.; Kim, S.-Y.; Oh, M.-R.; Park, S.-H.; et al. Effects of Male Silkworm Pupa Powder on the Erectile Dysfunction by Chronic Ethanol Consumption in Rats. Lab. Anim. Res. 2012, 28, 83–90. [Google Scholar] [CrossRef]
- Gere, A.; Székely, G.; Kovács, S.; Kókai, Z.; Sipos, L. Readiness to Adopt Insects in Hungary: A Case Study. Food Qual. Prefer. 2017, 59, 81–86. [Google Scholar] [CrossRef]
- Berger, S.; Bärtsch, C.; Schmidt, C.; Christandl, F.; Wyss, A.M. When Utilitarian Claims Backfire: Advertising Content and the Uptake of Insects as Food. Front. Nutr. 2018, 5, 88. [Google Scholar] [CrossRef]
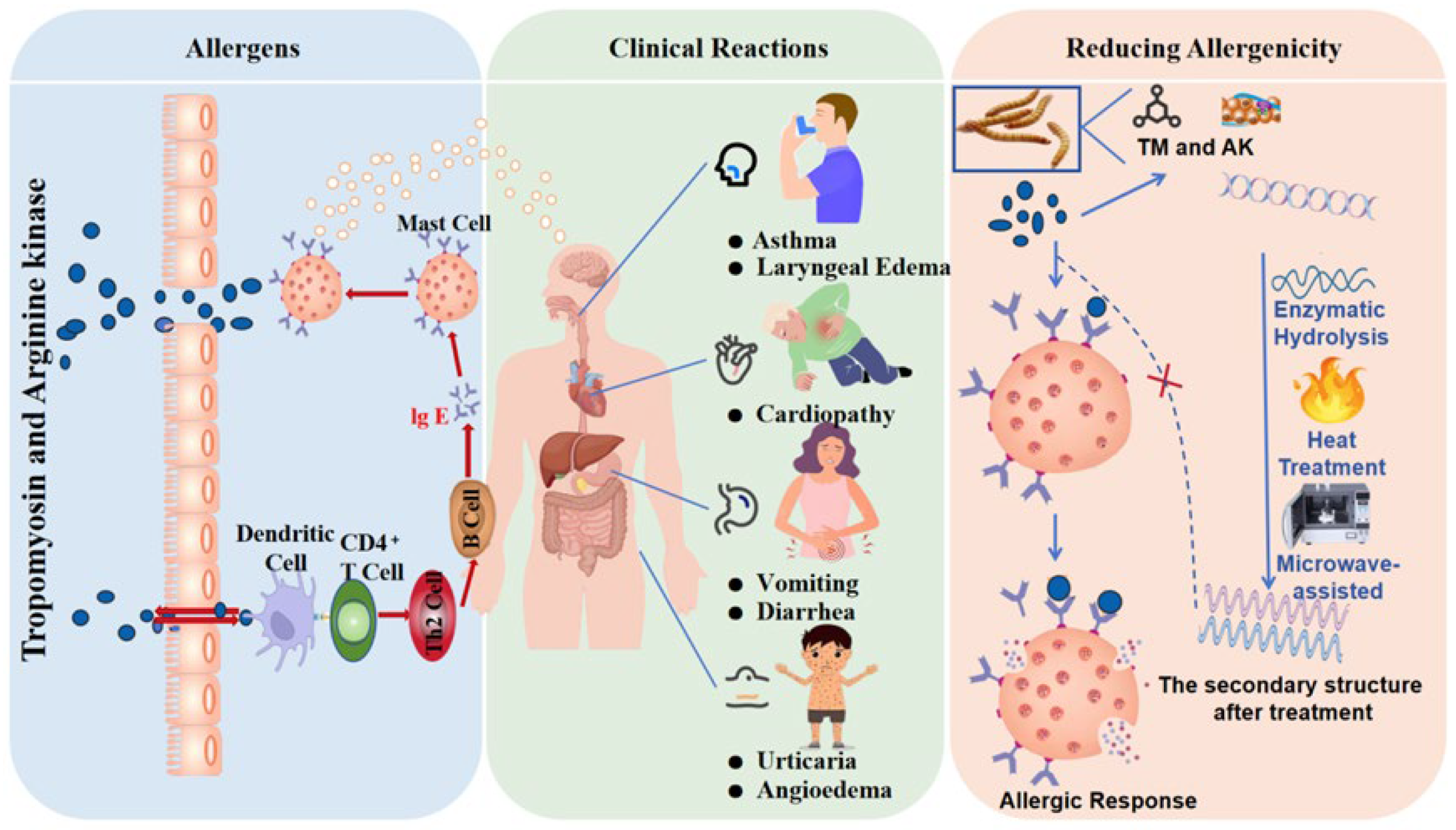
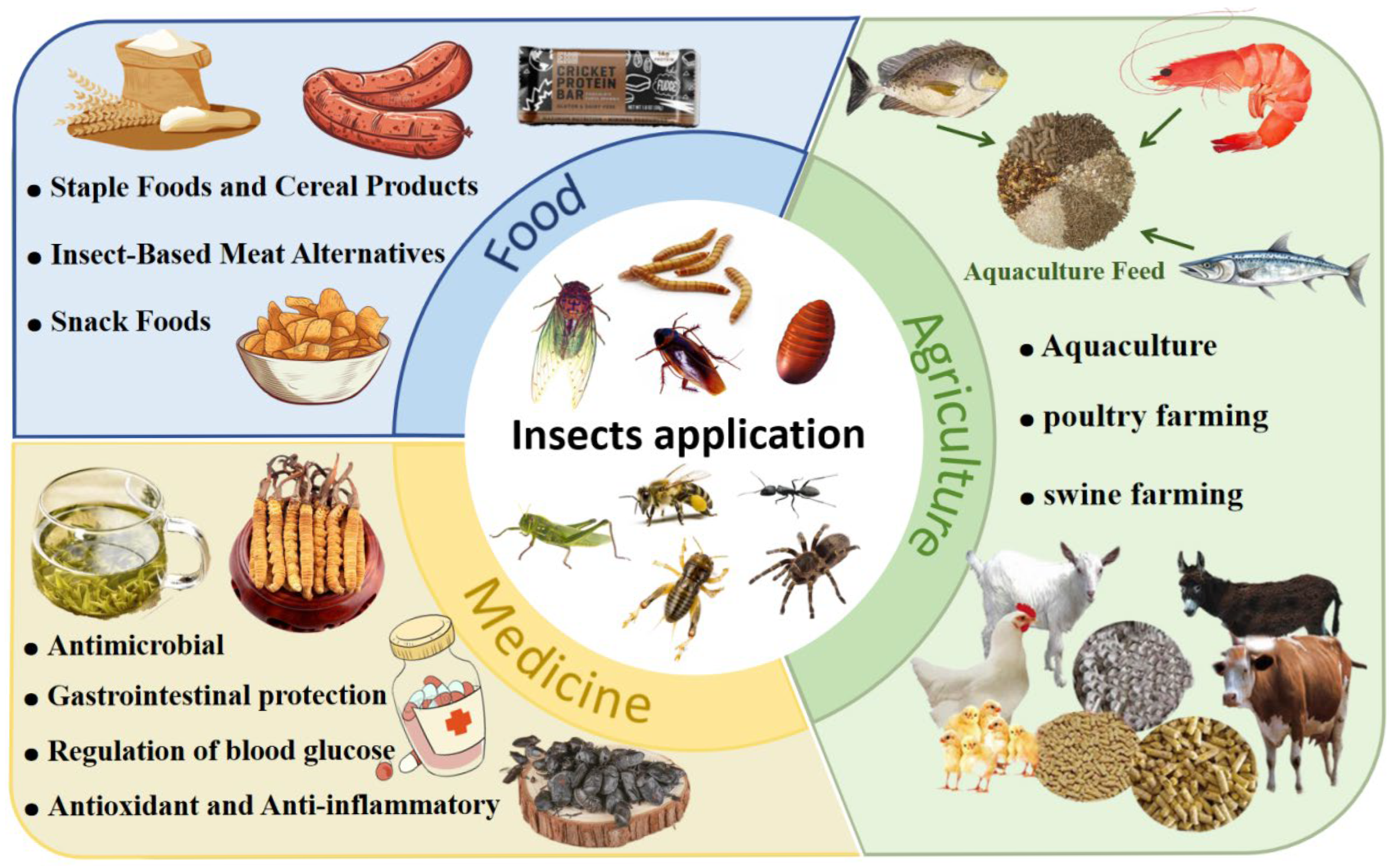
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Feng, M.; Wei, T.; Pan, F.; Zhao, L.; Zhao, L. Edible Insects as Future Proteins: Nutritional Value, Functional Properties, Bioactivities, and Safety Perspectives. Nutrients 2025, 17, 3165. https://doi.org/10.3390/nu17193165
Xu X, Feng M, Wei T, Pan F, Zhao L, Zhao L. Edible Insects as Future Proteins: Nutritional Value, Functional Properties, Bioactivities, and Safety Perspectives. Nutrients. 2025; 17(19):3165. https://doi.org/10.3390/nu17193165
Chicago/Turabian StyleXu, Xinyan, Mengmeng Feng, Tongwei Wei, Fei Pan, Liang Zhao, and Lei Zhao. 2025. "Edible Insects as Future Proteins: Nutritional Value, Functional Properties, Bioactivities, and Safety Perspectives" Nutrients 17, no. 19: 3165. https://doi.org/10.3390/nu17193165
APA StyleXu, X., Feng, M., Wei, T., Pan, F., Zhao, L., & Zhao, L. (2025). Edible Insects as Future Proteins: Nutritional Value, Functional Properties, Bioactivities, and Safety Perspectives. Nutrients, 17(19), 3165. https://doi.org/10.3390/nu17193165











