Nutritional Status and Dietary Challenges in Patients with Systemic Sclerosis: A Comprehensive Review
Abstract
1. Introduction
2. The GI Tract
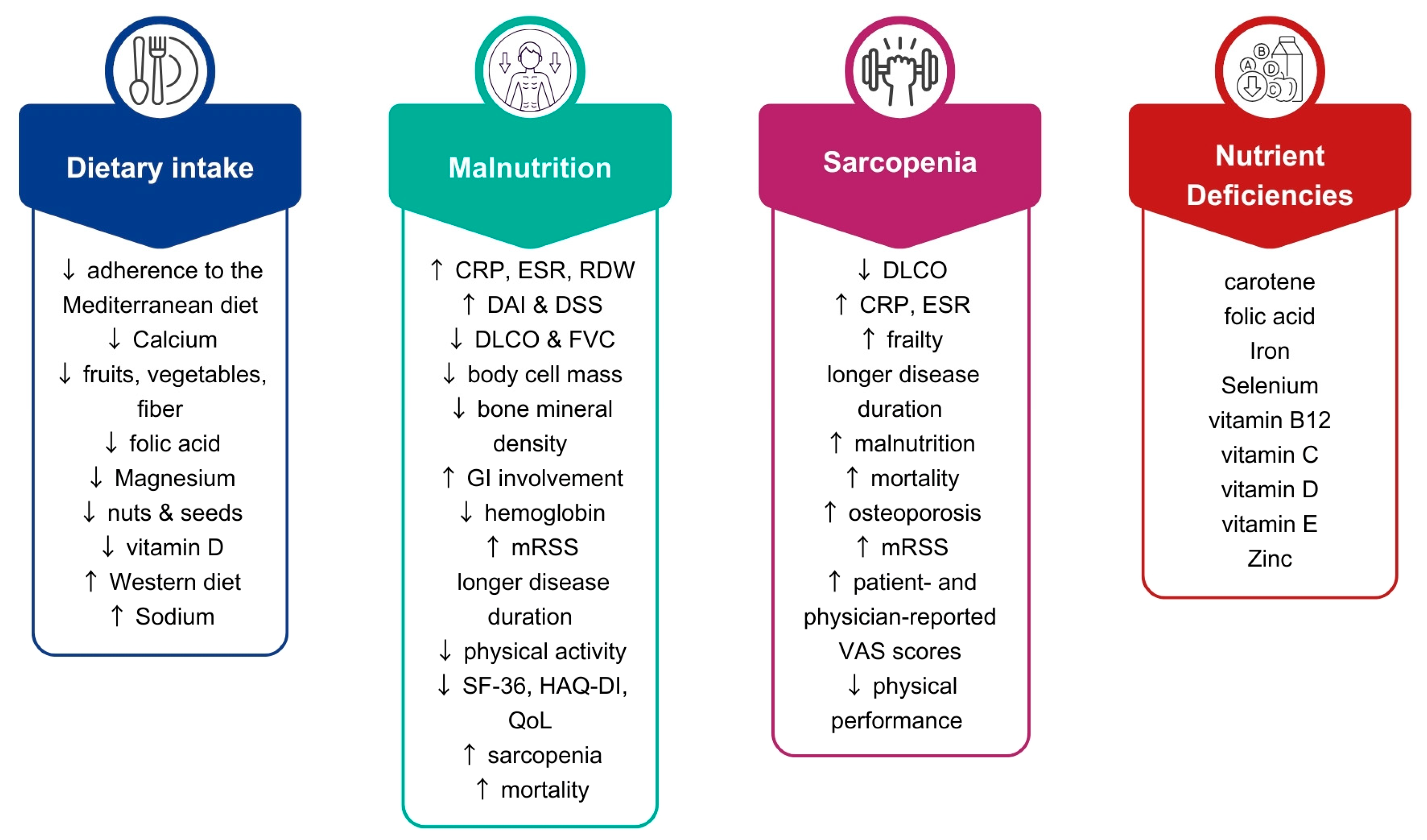
3. Dietary Intake of Patients with SSc
4. Malnutrition in SSc
Possible Factors Underlying Malnutrition and GI Involvement
5. Sarcopenia in SSc
Frailty in SSc
6. Nutrient Deficiencies
7. Specific Nutrients and Dietary Patterns
8. Nutrition Recommendations by Scientific Societies
9. Conclusions
10. Future Directions and Limitations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIP | Autoimmune Protocol |
| ANA | Antinuclear antibodies |
| BADL | Basic activities of daily living |
| BMI | Body mass index |
| CONUT | Controlling Nutritional Status |
| CRP | C-reactive protein |
| DAI | Disease activity index |
| DLCO | Diffusing capacity of the lung for carbon monoxide |
| DSS | Disease severity score |
| dSSc | Diffuse Systemic Sclerosis |
| ENA | Extractable nuclear antigen |
| ESPEN | European Society of Clinical Nutrition and Metabolism |
| ESR | Erythrocyte sedimentation rate |
| EULAR | European League Against Rheumatism |
| FODMAP | Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols |
| FVC | Forced vital capacity |
| GAVE | Gastric antral vascular ectasia |
| GERD | Gastroesophageal reflux disease |
| GI | Gastrointestinal |
| GLIM | Global Leadership Initiative on Malnutrition |
| HAQ-DI | Heath assessment questionnaire disability index |
| IADL | Instrumental activities of daily living |
| IL-6 | Interleukin-6 |
| ILD | Interstitial lung disease |
| lSSc | Limited cutaneous Systemic Sclerosis |
| MedDiet | Mediterranean diet |
| MMF | Mycophenolate mofetil |
| mRSS | Modified Rodnan Skin Score |
| MUST | Malnutrition Universal Screening Tool |
| ONS | Oral nutrition supplements |
| PCI | Pneumatosis cystoides intestinalis |
| PhA | Phase angle |
| QoL | Quality of life |
| RDW | Red-cell-distribution-width |
| SARC-F | Strength, assistance with walking, rise from a chair, climb stairs, and falls |
| SARC-F + EBM | Strength, assistance with walking, rise from a chair, climb stairs, and falls adjusted for age and body mass |
| SF-36 | 36-item short form health survey |
| SGA | Subjective Global Assessment |
| SIBO | Small intestinal bacterial overgrowth |
| SS | Sjögren’s syndrome |
| SSc | Systemic sclerosis |
| SSc-ILD | SSc-associated interstitial lung disease |
| TNF-α | TNF-α: Tumor-necrosis factor-α |
| UCLA SCTC GIT 2.0 | UCLA Scleroderma Clinical Trials Consortium Gastrointestinal Tract 2.0 |
| VAS | Visual analogue scale |
References
- Volkmann, E.R.; McMahan, Z.H.; Smith, V.; Jouneau, S.; Miede, C.; Alves, M.; Herrick, A.L. SENSCIS Trial Investigators Risk of Malnutrition in Patients With Systemic Sclerosis-Associated Interstitial Lung Disease Treated With Nintedanib in the Randomized, Placebo-Controlled SENSCIS Trial. Arthritis Care Res. 2023, 75, 2501–2507. [Google Scholar] [CrossRef]
- Tian, J.; Kang, S.; Zhang, D.; Huang, Y.; Zhao, M.; Gui, X.; Yao, X.; Lu, Q. Global, Regional, and National Incidence and Prevalence of Systemic Sclerosis. Clin. Immunol. 2023, 248, 109267. [Google Scholar] [CrossRef]
- Argibay, A.; Novo, I.; Ávila, M.; Diéguez González, P.; Estévez Gil, M.; Maure, B.; Gimena, B.; Vázquez Triñanes, C.; Rivera Gallego, A. AB0545 Gastrointestinal Involvement In Systemic Sclerosis. Ann. Rheum. Dis. 2020, 79, 1569. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Khanna, D. Gastrointestinal Involvement in Systemic Sclerosis. In Handbook of Systemic Autoimmune Diseases; Elsevier: Amsterdam, The Netherlands, 2017; Volume 13, pp. 243–261. [Google Scholar]
- Sjogren, R.W. Gastrointestinal Motility Disorders in Scleroderma. Arthritis Rheum. 1994, 37, 1265–1282. [Google Scholar] [CrossRef]
- Miller, J.B.; Gandhi, N.; Clarke, J.; McMahan, Z. Gastrointestinal Involvement in Systemic Sclerosis: An Update. J. Clin. Rheumatol. 2018, 24, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, A.; Akesson, A.; Akesson, B. Dietary Intake and Nutritional Status in Patients with Systemic Sclerosis. Ann. Rheum. Dis. 1992, 51, 1143–1148. [Google Scholar] [CrossRef]
- Baron, M.; Hudson, M.; Tatibouet, S.; Steele, R.; Lo, E.; Gravel, S.; Gyger, G.; El Sayegh, T.; Pope, J.; Fontaine, A.; et al. The Canadian Systemic Sclerosis Oral Health Study: Orofacial Manifestations and Oral Health-Related Quality of Life in Systemic Sclerosis Compared with the General Population. Rheumatology 2014, 53, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Shreiner, A.B.; Murray, C.; Denton, C.; Khanna, D. Gastrointestinal Manifestations of Systemic Sclerosis. J. scleroderma Relat. Disord. 2016, 1, 247. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, E.R.; McMahan, Z. Gastrointestinal Involvement in Systemic Sclerosis: Pathogenesis, Assessment and Treatment. Curr. Opin. Rheumatol. 2022, 34, 328–336. [Google Scholar] [CrossRef]
- Chatterjee, S. Nutritional Implications of GI-Related Scleroderma. Pract. Gastroenterol. 2016, 40, 35–46. [Google Scholar]
- Siegert, E.; March, C.; Otten, L.; Makowka, A.; Preis, E.; Buttgereit, F.; Riemekasten, G.; Müller-Werdan, U.; Norman, K. Prevalence of Sarcopenia in Systemic Sclerosis: Assessing Body Composition and Functional Disability in Patients with Systemic Sclerosis. Nutrition 2018, 55–56, 51–55. [Google Scholar] [CrossRef]
- Corallo, C.; Fioravanti, A.; Tenti, S.; Pecetti, G.; Nuti, R.; Giordano, N. Sarcopenia in Systemic Sclerosis: The Impact of Nutritional, Clinical, and Laboratory Features. Rheumatol. Int. 2019, 39, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, F.; Skally, M.; O’Hanlon, C.; Foley, M.; Houlihan, J.; Gaughan, L.; Smith, O.; Moore, B.; Cunneen, S.; Sweeney, E.; et al. Food for Thought. Malnutrition Risk Associated with Increased Risk of Healthcare-Associated Infection. J. Hosp. Infect. 2019, 101, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, S.; Woods, R.T. Malnutrition and Quality of Life in Older People: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2013, 12, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Hax, V.; Tessari, J.; Pena, E.; Santo, R.C.d.E.; Dos Santos, L.P.; Gasparin, A.A.; Bredemeier, M.; Fighera, T.M.; Spritzer, P.M.; Xavier, R.M.; et al. Physical Frailty in Patients with Systemic Sclerosis. Semin. Arthritis Rheum. 2022, 56, 152077. [Google Scholar] [CrossRef]
- Barison, C.; Piovani, E.; Moschetti, L.; Pedretti, E.; Lazzaroni, M.G.; Franceschini, F.; Airò, P. Frailty Assessment in Patients with Systemic Sclerosis. Clin. Exp. Rheumatol. 2025, 43, 1408–1413. [Google Scholar] [CrossRef]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and Its Role in Immunity and Inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef]
- Black, R.E. Global Distribution and Disease Burden Related to Micronutrient Deficiencies. Nestle Nutr. Inst. Workshop Ser. 2014, 78, 21–28. [Google Scholar] [CrossRef]
- Vinarov, Z.; Abdallah, M.; Agundez, J.A.G.; Allegaert, K.; Basit, A.W.; Braeckmans, M.; Ceulemans, J.; Corsetti, M.; Griffin, B.T.; Grimm, M.; et al. Impact of Gastrointestinal Tract Variability on Oral Drug Absorption and Pharmacokinetics: An UNGAP Review. Eur. J. Pharm. Sci. 2021, 162, 105812. [Google Scholar] [CrossRef]
- Andréasson, K.; Neringer, K.; Wuttge, D.M.; Henrohn, D.; Marsal, J.; Hesselstrand, R. Mycophenolate Mofetil for Systemic Sclerosis: Drug Exposure Exhibits Considerable Inter-Individual Variation-a Prospective, Observational Study. Arthritis Res. Ther. 2020, 22, 230. [Google Scholar] [CrossRef]
- Jung, S.; Martin, T.; Schmittbuhl, M.; Huck, O. The Spectrum of Orofacial Manifestations in Systemic Sclerosis: A Challenging Management. Oral Dis. 2017, 23, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhu, J.; Zhu, Y.; Zhang, X.; Wu, R.; Li, S.; Su, Y. Oral Manifestations of Patients with Systemic Sclerosis: A Meta-Analysis for Case-Controlled Studies. BMC Oral Health 2021, 21, 250. [Google Scholar] [CrossRef]
- Benz, K.; Baulig, C.; Knippschild, S.; Strietzel, F.P.; Hunzelmann, N.; Jackowski, J. Prevalence of Oral and Maxillofacial Disorders in Patients with Systemic Scleroderma-A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 5238. [Google Scholar] [CrossRef]
- Nota, A.; Pittari, L.; Paggi, M.; Abati, S.; Tecco, S. Correlation between Bruxism and Gastroesophageal Reflux Disorder and Their Effects on Tooth Wear. A Systematic Review. J. Clin. Med. 2022, 11, 1107. [Google Scholar] [CrossRef]
- Sura, L.; Madhavan, A.; Carnaby, G.; Crary, M.A. Dysphagia in the Elderly: Management and Nutritional Considerations. Clin. Interv. Aging 2012, 7, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Detopoulou, P.; Grammatikopoulou, M.G.; Lytra, E.; Pylarinou, I.; Panoutsopoulos, G. Determination of the International Dysphagia Diet Standardization Initiative Level of Commercially Available Oral Nutritional Supplements. Clin. Nutr. ESPEN 2025, 67, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Lahcene, M.; Oumnia, N.; Matougui, N.; Boudjella, M.; Tebaibia, A.; Touchene, B. Esophageal Dysmotility in Scleroderma: A Prospective Study of 183 Cases. Gastroenterol. Clin. Biol. 2009, 33, 466–469. [Google Scholar] [CrossRef]
- Pardali, E.C.; Kontouli, K.-M.; Manolakis, A.; Detopoulou, P.; Argyriou, K.; Tsakmaki, I.A.; Simopoulou, T.; Katsiari, C.G.; Goulis, D.G.; Kapsoritakis, A.; et al. Frequency of Dysphagia among Patients Submitted to a Rheumatology Department: A Cross-Sectional Analysis Based on the EAT-10 Questionnaire. Rheumatol. Int. 2025, 45, 196. [Google Scholar] [CrossRef]
- Adarsh, M.; Sharma, S.K.; Prasad, K.K.; Dhir, V.; Singh, S.; Sinha, S.K. Esophageal Manometry, Esophagogastroduodenoscopy, and Duodenal Mucosal Histopathology in Systemic Sclerosis. JGH Open 2019, 3, 206–209. [Google Scholar] [CrossRef]
- Lahcene, M.; Oumnia, N.; Matougui, N.; Boudjella, M.; Tebaibia, A.; Touchene, B. Esophageal Involvement in Scleroderma: Clinical, Endoscopic, and Manometric Features. ISRN Rheumatol. 2011, 2011, 325826. [Google Scholar] [CrossRef][Green Version]
- Denaxas, K.; Ladas, S.D.; Karamanolis, G.P. Evaluation and Management of Esophageal Manifestations in Systemic Sclerosis. Ann. Gastroenterol. 2018, 31, 165–170. [Google Scholar] [CrossRef]
- Benfaremo, D.; Pacenti, N.; Galli, F.L.; Colangelo, A.; Moroncini, G. POS0564 Self-Reported Oropharyngeal Dysphagia and Swallowing-Related Quality of Life in People with Systemic Sclerosis: Preliminary Results of a Cross-Sectional Study. Ann. Rheum. Dis. 2025, 84, 766–767. [Google Scholar] [CrossRef]
- Keller, J.; Layer, P. The Pathophysiology of Malabsorption. Viszeralmedizin 2014, 30, 150–154. [Google Scholar] [CrossRef]
- Freedberg, D.E.; Kim, L.S.; Yang, Y.-X. The Risks and Benefits of Long-Term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology 2017, 152, 706–715. [Google Scholar] [CrossRef]
- Hess, M.W.; Hoenderop, J.G.J.; Bindels, R.J.M.; Drenth, J.P.H. Systematic Review: Hypomagnesaemia Induced by Proton Pump Inhibition. Aliment. Pharmacol. Ther. 2012, 36, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.R.; Schneider, J.L.; Zhao, W.; Corley, D.A. Proton Pump Inhibitor and Histamine 2 Receptor Antagonist Use and Vitamin B12 Deficiency. JAMA 2013, 310, 2435–2442. [Google Scholar] [CrossRef]
- University of Michigan Health. Malnutrition for Scleroderma. Available online: https://www.uofmhealth.org/conditions-treatments/rheumatology/nutrition-scleroderma (accessed on 30 August 2025).
- Gemmell, S.; Gemmell, B. Nutritional Needs in Scleroderma; Scleroderma Australia Inc.: Melton, VIC, Australia, 2021. [Google Scholar]
- Tandaipan, J.L.; Castellví, I. Systemic Sclerosis and Gastrointestinal Involvement. Rev. Colomb. Reumatol. (English Ed.) 2020, 27, 44–54. [Google Scholar] [CrossRef]
- Hung, E.W.; Mayes, M.D.; Sharif, R.; Assassi, S.; Machicao, V.I.; Hosing, C.; St Clair, E.W.; Furst, D.E.; Khanna, D.; Forman, S.; et al. Gastric Antral Vascular Ectasia and Its Clinical Correlates in Patients with Early Diffuse Systemic Sclerosis in the SCOT Trial. J. Rheumatol. 2013, 40, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.; Hummers, L.K.; Pasricha, P.J.; McMahan, Z.H. Gastroparesis in Systemic Sclerosis: A Detailed Analysis Using Whole-Gut Scintigraphy. Rheumatology 2022, 61, 4503–4508. [Google Scholar] [CrossRef]
- Parrado, R.H.; Lemus, H.N.; Coral-Alvarado, P.X.; Quintana López, G. Gastric Antral Vascular Ectasia in Systemic Sclerosis: Current Concepts. Int. J. Rheumatol. 2015, 2015, 762546. [Google Scholar] [CrossRef]
- Camilleri, M.; Parkman, H.P.; Shafi, M.A.; Abell, T.L.; Gerson, L. Clinical Guideline: Management of Gastroparesis. Am. J. Gastroenterol. 2013, 108, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Morrisroe, K.; Hansen, D.; Stevens, W.; Sahhar, J.; Ngian, G.-S.; Hill, C.; Roddy, J.; Walker, J.; Proudman, S.; Nikpour, M. Gastric Antral Vascular Ectasia in Systemic Sclerosis: A Study of Its Epidemiology, Disease Characteristics and Impact on Survival. Arthritis Res. Ther. 2022, 24, 103. [Google Scholar] [CrossRef] [PubMed]
- Tuveri, M.; Borsezio, V.; Gabbas, A.; Mura, G. Gastric Antral Vascular Ectasia--an Unusual Cause of Gastric Outlet Obstruction: Report of a Case. Surg. Today 2007, 37, 503–505. [Google Scholar] [CrossRef]
- University of Michigan Health System. Gastroparesis Nutrition Therapy. Available online: https://www.med.umich.edu/1libr/Gastro/GastroparesisNutrition.pdf (accessed on 30 August 2025).
- Vomero, N.D.; Colpo, E. Nutritional Care in Peptic Ulcer. Arq. Bras. Cir. Dig. 2014, 27, 298–302. [Google Scholar] [CrossRef]
- Tian, X.-P.; Zhang, X. Gastrointestinal Complications of Systemic Sclerosis. World J. Gastroenterol. 2013, 19, 7062–7068. [Google Scholar] [CrossRef]
- Sakkas, L.I.; Simopoulou, T.; Daoussis, D.; Liossis, S.-N.; Potamianos, S. Intestinal Involvement in Systemic Sclerosis: A Clinical Review. Dig. Dis. Sci. 2018, 63, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Tauber, M.; Avouac, J.; Benahmed, A.; Barbot, L.; Coustet, B.; Kahan, A.; Allanore, Y. Prevalence and Predictors of Small Intestinal Bacterial Overgrowth in Systemic Sclerosis Patients with Gastrointestinal Symptoms. Clin. Exp. Rheumatol. 2014, 32, S82–S87. [Google Scholar]
- Marie, I.; Ducrotté, P.; Denis, P.; Menard, J.-F.; Levesque, H. Small Intestinal Bacterial Overgrowth in Systemic Sclerosis. Rheumatology 2009, 48, 1314–1319. [Google Scholar] [CrossRef]
- Montoro-Huguet, M.A.; Belloc, B.; Domínguez-Cajal, M. Small and Large Intestine (I): Malabsorption of Nutrients. Nutrients 2021, 13, 1254. [Google Scholar] [CrossRef]
- Tan, T.C.; Noviani, M.; Leung, Y.Y.; Low, A.H.L. The Microbiome and Systemic Sclerosis: A Review of Current Evidence. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101687. [Google Scholar] [CrossRef]
- Andréasson, K.; Alrawi, Z.; Persson, A.; Jönsson, G.; Marsal, J. Intestinal Dysbiosis Is Common in Systemic Sclerosis and Associated with Gastrointestinal and Extraintestinal Features of Disease. Arthritis Res. Ther. 2016, 18, 278. [Google Scholar] [CrossRef]
- Kaneko, M.; Sasaki, S.; Teruya, S.; Ozaki, K.; Ishimaru, K.; Terai, E.; Nakayama, H.; Watanabe, T. Pneumatosis Cystoides Intestinalis in Patients with Systemic Sclerosis: A Case Report and Review of 39 Japanese Cases. Case Rep. Gastrointest. Med. 2016, 2016, 2474515. [Google Scholar] [CrossRef]
- Alastal, Y. Gastrointestinal Manifestations Associated with Systemic Sclerosis: Results from the Nationwide Inpatient Sample. Ann. Gastroenterol. 2017, 30, 498. [Google Scholar] [CrossRef]
- Healy, M.; Claghorn, K.V.B.; Van Horn, A. Low Fiber Diet for Diarrhea. OncoLink. Available online: https://www.oncolink.org/support/nutrition-and-cancer/during-and-after-treatment/low-fiber-diet-for-diarrhea (accessed on 30 August 2025).
- Shah, S.C.; Day, L.W.; Somsouk, M.; Sewell, J.L. Meta-Analysis: Antibiotic Therapy for Small Intestinal Bacterial Overgrowth. Aliment. Pharmacol. Ther. 2013, 38, 925–934. [Google Scholar] [CrossRef]
- Polkowska-Pruszyńska, B.; Gerkowicz, A.; Rawicz-Pruszyński, K.; Krasowska, D. Gut Microbiome in Systemic Sclerosis: A Potential Therapeutic Target. Postepy Dermatol. Alergol. 2020, 39, 101–109. [Google Scholar] [CrossRef]
- Parodi, A.; Sessarego, M.; Greco, A.; Bazzica, M.; Filaci, G.; Setti, M.; Savarino, E.; Indiveri, F.; Savarino, V.; Ghio, M. Small Intestinal Bacterial Overgrowth in Patients Suffering From Scleroderma: Clinical Effectiveness of Its Eradication. Am. J. Gastroenterol. 2008, 103, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Vidal Neira, L.F.; Piscoya Arbañil, J.; Rolando Castañedá, T.; Aita Arroyo, G.; Frias Coronado, V.; Garcia-Calderon, J.H. Digestive Involvement in Progressive Systemic Sclerosis. Arq. Gastroenterol. 1988, 25, 8–22. [Google Scholar] [PubMed]
- Emmanuel, A. Current Management of the Gastrointestinal Complications of Systemic Sclerosis. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 461–472. [Google Scholar] [CrossRef]
- Caserta, L.; de Magistris, L.; Secondulfo, M.; Caravelli, G.; Riegler, G.; Cuomo, G.; D’Angelo, S.; Naclerio, C.; Valentini, G.; Carratù, R. Assessment of Intestinal Permeability and Orocecal Transit Time in Patients with Systemic Sclerosis: Analysis of Relationships with Epidemiologic and Clinical Parameters. Rheumatol. Int. 2003, 23, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, E.R.; Chang, Y.-L.; Barroso, N.; Furst, D.E.; Clements, P.J.; Gorn, A.H.; Roth, B.E.; Conklin, J.L.; Getzug, T.; Borneman, J.; et al. Association of Systemic Sclerosis With a Unique Colonic Microbial Consortium. Arthritis Rheumatol. 2016, 68, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Huang, Y.-H. An Unusual Etiology of Diarrhea: Primary Intestinal Lymphangiectasia. Rev. Esp. Enfermedades Dig. 2025. [Google Scholar] [CrossRef]
- Wang, S.J.; Lan, J.L.; Chen, D.Y.; Chen, Y.H.; Hsieh, T.Y.; Lin, W.Y. Colonic Transit Disorders in Systemic Sclerosis. Clin. Rheumatol. 2001, 20, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Suresh, N.; Karanth, R.; Cheah, R.; Casey, J.; Jayne, D.G.; Del Galdo, F. Systemic Sclerosis and Anorectal Dysfunction: The Leeds Experience. J. Scleroderma Relat. Disord. 2024, 9, 210–215. [Google Scholar] [CrossRef]
- Omair, M.A.; Lee, P. Effect of Gastrointestinal Manifestations on Quality of Life in 87 Consecutive Patients with Systemic Sclerosis. J. Rheumatol. 2012, 39, 992–996. [Google Scholar] [CrossRef]
- Engel, A.F.; Kamm, M.A.; Talbot, I.C. Progressive Systemic Sclerosis of the Internal Anal Sphincter Leading to Passive Faecal Incontinence. Gut 1994, 35, 857–859. [Google Scholar] [CrossRef]
- Hansi, N.; Thoua, N.; Carulli, M.; Chakravarty, K.; Lal, S.; Smyth, A.; Herrick, A.; Ogunbiyi, O.; Shaffer, J.; Mclaughlin, J.; et al. Consensus Best Practice Pathway of the UK Scleroderma Study Group: Gastrointestinal Manifestations of Systemic Sclerosis. Clin. Exp. Rheumatol. 2014, 32, S214–S221. [Google Scholar]
- Burlui, A.M.; Cardoneanu, A.; Macovei, L.A.; Rezus, C.; Boiculese, L.V.; Graur, M.; Rezus, E. Diet in Scleroderma: Is There a Need for Intervention? Diagnostics 2021, 11, 2118. [Google Scholar] [CrossRef] [PubMed]
- Samm, D.-A.A.; Macoustra, A.R.; Crane, R.K.; McWilliams, L.M.; Proudman, S.M.; Chapple, L.-A.S. A Survey of Australian Rheumatologists’ Perspectives of Nutrition Needs in Systemic Sclerosis. J. Scleroderma Relat. Disord. 2023, 8, 203–209. [Google Scholar] [CrossRef]
- Bitarafan, S.; Harirchian, M.-H.; Nafissi, S.; Sahraian, M.-A.; Togha, M.; Siassi, F.; Saedisomeolia, A.; Alipour, E.; Mohammadpour, N.; Chamary, M.; et al. Dietary Intake of Nutrients and Its Correlation with Fatigue in Multiple Sclerosis Patients. Iran. J. Neurol. 2014, 13, 28–32. [Google Scholar]
- Natalello, G.; Bosello, S.L.; Campochiaro, C.; Abignano, G.; De Santis, M.; Ferlito, A.; Karadağ, D.T.; Padula, A.A.; Cavalli, G.; D’Agostino, M.A.; et al. Adherence to the Mediterranean Diet in Italian Patients With Systemic Sclerosis: An Epidemiologic Survey. ACR Open Rheumatol. 2024, 6, 14–20. [Google Scholar] [CrossRef]
- Samm, D.; Macoustra, A.; Crane, R.; Proudman, S.; McWilliams, L.; Chapple, L. P136 Views of Nutrition in Patients with Scleroderma. Clin. Nutr. ESPEN 2023, 54, 547. [Google Scholar] [CrossRef]
- Baron, M.; Bernier, P.; Côté, L.F.; Delegge, M.H.; Falovitch, G.; Friedman, G.; Gornitsky, M.; Hoffer, J.; Hudson, M.; Khanna, D.; et al. Screening and Therapy for Malnutrition and Related Gastro-Intestinal Disorders in Systemic Sclerosis: Recommendations of a North American Expert Panel. Clin. Exp. Rheumatol. 2010, 28, S42–S46. [Google Scholar]
- Hvas, C.L.; Harrison, E.; Eriksen, M.K.; Herrick, A.L.; McLaughlin, J.T.; Lal, S. Nutritional Status and Predictors of Weight Loss in Patients with Systemic Sclerosis. Clin. Nutr. ESPEN 2020, 40, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Paolino, S.; Pacini, G.; Schenone, C.; Patanè, M.; Sulli, A.; Sukkar, S.G.; Lercara, A.; Pizzorni, C.; Gotelli, E.; Cattelan, F.; et al. Nutritional Status and Bone Microarchitecture in a Cohort of Systemic Sclerosis Patients. Nutrients 2020, 12, 1632. [Google Scholar] [CrossRef] [PubMed]
- Caimmi, C.; Caramaschi, P.; Venturini, A.; Bertoldo, E.; Vantaggiato, E.; Viapiana, O.; Ferrari, M.; Lippi, G.; Frulloni, L.; Rossini, M. Malnutrition and Sarcopenia in a Large Cohort of Patients with Systemic Sclerosis. Clin. Rheumatol. 2018, 37, 987–997. [Google Scholar] [CrossRef]
- Rosato, E.; Gigante, A.; Gasperini, M.L.; Proietti, L.; Muscaritoli, M. Assessing Malnutrition in Systemic Sclerosis With Global Leadership Initiative on Malnutrition and European Society of Clinical Nutrition and Metabolism Criteria. JPEN J. Parenter. Enteral Nutr. 2021, 45, 618–624. [Google Scholar] [CrossRef]
- Dupont, R.; Longué, M.; Galinier, A.; Cinq Frais, C.; Ingueneau, C.; Astudillo, L.; Arlet, P.; Adoue, D.; Alric, L.; Prévot, G.; et al. Impact of Micronutrient Deficiency & Malnutrition in Systemic Sclerosis: Cohort Study and Literature Review. Autoimmun. Rev. 2018, 17, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Szabo, I.; Petcu, A.; Chicinas, D.; Rednic, V.; Tamas, M.M.; Muntean, L.; Filipescu, I.; Simon, S.P.; Pamfil, C.; Rednic, S. SAT0343 Nutritional Status of Systemic Sclerosis Patients: A Pilot Study. Ann. Rheum. Dis. 2020, 79, 1117. [Google Scholar] [CrossRef]
- Bagnato, G.; Pigatto, E.; Bitto, A.; Pizzino, G.; Irrera, N.; Abignano, G.; Ferrera, A.; Sciortino, D.; Wilson, M.; Squadrito, F.; et al. The PREdictor of MAlnutrition in Systemic Sclerosis (PREMASS) Score: A Combined Index to Predict 12 Months Onset of Malnutrition in Systemic Sclerosis. Front. Med. 2021, 8, 651748. [Google Scholar] [CrossRef]
- Rivet, V.; Riviere, S.; Goulabchand, R.; Suzon, B.; Henneton, P.; Partouche, L.; Rullier, P.; Quellec, A.L.; Konate, A.; Schiffmann, A.; et al. High Prevalence of Malnutrition in Systemic Sclerosis: Results from a French Monocentric Cross-Sectional Study. Nutrition 2023, 116, 112171. [Google Scholar] [CrossRef]
- Baron, M.; Hudson, M.; Steele, R.; Canadian Scleroderma Research Group. Malnutrition Is Common in Systemic Sclerosis: Results from the Canadian Scleroderma Research Group Database. J. Rheumatol. 2009, 36, 2737–2743. [Google Scholar] [CrossRef] [PubMed]
- Cano Garcia, L.; Redondo, R.; Al Mashhadani, N.; Ordoñez Cañizares, M.D.C.; Mena-Vázquez, N.; Manrique Arija, S. AB0820 The Risk of Malnutrition Is a Problem Associated with Systemic Sclerosis. Ann. Rheum. Dis. 2023, 82, 1623. [Google Scholar] [CrossRef]
- Caporali, R.; Caccialanza, R.; Bonino, C.; Klersy, C.; Cereda, E.; Xoxi, B.; Crippa, A.; Rava, M.L.; Orlandi, M.; Bonardi, C.; et al. Disease-Related Malnutrition in Outpatients with Systemic Sclerosis. Clin. Nutr. 2012, 31, 666–671. [Google Scholar] [CrossRef]
- Cereda, E.; Codullo, V.; Klersy, C.; Breda, S.; Crippa, A.; Rava, M.L.; Orlandi, M.; Bonardi, C.; Fiorentini, M.L.; Caporali, R.; et al. Disease-Related Nutritional Risk and Mortality in Systemic Sclerosis. Clin. Nutr. 2014, 33, 558–561. [Google Scholar] [CrossRef]
- Cruz-Domínguez, M.P.; García-Collinot, G.; Saavedra, M.A.; Montes-Cortes, D.H.; Morales-Aguilar, R.; Carranza-Muleiro, R.A.; Vera-Lastra, O.L.; Jara, L.J. Malnutrition Is an Independent Risk Factor for Mortality in Mexican Patients with Systemic Sclerosis: A Cohort Study. Rheumatol. Int. 2017, 37, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Doerfler, B.; Allen, T.S.; Southwood, C.; Brenner, D.; Hirano, I.; Sheean, P. Medical Nutrition Therapy for Patients With Advanced Systemic Sclerosis (MNT PASS): A Pilot Intervention Study. J. Parenter. Enter. Nutr. 2017, 41, 678–684. [Google Scholar] [CrossRef]
- Fairley, J.L.; Hansen, D.; Quinlivan, A.; Proudman, S.; Sahhar, J.; Ngian, G.-S.; Walker, J.; Host, L.V.; Morrisroe, K.; Stevens, W.; et al. Frequency and Implications of Malnutrition in Systemic Sclerosis. Rheumatology 2025, 64, 1251–1260. [Google Scholar] [CrossRef]
- Gajdecki, J.; Stępień, B.; Gajdecka, K.; Brzezińska, O.; Nadel, A.; Makowska, J.; Opinc-Rosiak, A. Controlling Nutritional Status Score as a Sensitive Instrument for Malnutrition Screening in Systemic Sclerosis—A Retrospective Study. Rheumatol. Int. 2025, 45, 80. [Google Scholar] [CrossRef]
- Krause, L.; Becker, M.O.; Brueckner, C.S.; Bellinghausen, C.-J.; Becker, C.; Schneider, U.; Haeupl, T.; Hanke, K.; Hensel-Wiegel, K.; Ebert, H.; et al. Nutritional Status as Marker for Disease Activity and Severity Predicting Mortality in Patients with Systemic Sclerosis. Ann. Rheum. Dis. 2010, 69, 1951–1957. [Google Scholar] [CrossRef]
- Molfino, A.; ML, G.; Gigante, A.; Rosato, E.; Muscaritoli, M. Left Ventricular Mass Index as Potential Surrogate of Muscularity in Patients With Systemic Sclerosis Without Cardiovascular Disease. JPEN J. Parenter. Enteral Nutr. 2021, 45, 1302–1308. [Google Scholar] [CrossRef]
- Murtaugh, M.A.; Frech, T.M. Nutritional Status and Gastrointestinal Symptoms in Systemic Sclerosis Patients. Clin. Nutr. 2013, 32, 130–135. [Google Scholar] [CrossRef]
- Ortiz-Santamaria, V.; Puig, C.; Soldevillla, C.; Barata, A.; Cuquet, J.; Recasens, A. Nutritional Support in Patients with Systemic Sclerosis. Reumatol. Clin. 2014, 10, 283–287. [Google Scholar] [CrossRef]
- Preis, E.; Franz, K.; Siegert, E.; Makowka, A.; March, C.; Riemekasten, G.; Cereda, E.; Norman, K. The Impact of Malnutrition on Quality of Life in Patients with Systemic Sclerosis. Eur. J. Clin. Nutr. 2018, 72, 504–510. [Google Scholar] [CrossRef]
- Rosato, E.; Gigante, A.; Colalillo, A.; Pellicano, C.; Alunni Fegatelli, D.; Muscaritoli, M. GLIM-Diagnosed Malnutrition Predicts Mortality and Risk of Hospitalization in Systemic Sclerosis: A Retrospective Study. Eur. J. Intern. Med. 2023, 117, 103–110. [Google Scholar] [CrossRef]
- Rosato, E.; Gigante, A.; Pellicano, C.; Colalillo, A.; Alunni-Fegatelli, D.; Muscaritoli, M. Phase Angle, Nutritional Status, and Mortality in Systemic Sclerosis: An Exploratory Pilot Study. Nutrition 2023, 107, 111946. [Google Scholar] [CrossRef]
- Spanjer, M.J.; Bultink, I.E.M.; de van der Schueren, M.A.E.; Voskuy, A.E. Prevalence of Malnutrition and Validation of Bioelectrical Impedance Analysis for the Assessment of Body Composition in Patients with Systemic Sclerosis. Rheumatology 2017, 56, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Tas Kilic, D.; Akdogan, A.; Kilic, L.; Sari, A.; Erden, A.; Armagan, B.; Kilickaya, M.; Kalyoncu, U.; Turhan, T.; Kiraz, S.; et al. Evaluation of Vitamin B12 Deficiency and Associated Factors in Patients With Systemic Sclerosis. J. Clin. Rheumatol. 2018, 24, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Türk, İ.; Cüzdan, N.; Çiftçi, V.; Arslan, D.; MC, D.; Unal, İ. Malnutrition, Associated Clinical Factors, and Depression in Systemic Sclerosis: A Cross-Sectional Study. Clin. Rheumatol. 2020, 39, 57–67. [Google Scholar] [CrossRef]
- Wojteczek, A.; Dardzińska, J.; Ziętkiewicz, M.; Smoleńska, Ż.; Czuszyńska, Z.; De Cock, D.; Zdrojewski, Z.; Małgorzewicz, S.; Chmielewski, M. High-Protein Nutritional Supplements Improve Nutritional Status in Malnourished Patients with Systemic Sclerosis. Nutrients 2024, 16, 2622. [Google Scholar] [CrossRef] [PubMed]
- Wojteczek, A.; Dardzińska, J.A.; Małgorzewicz, S.; Gruszecka, A.; Zdrojewski, Z. Prevalence of Malnutrition in Systemic Sclerosis Patients Assessed by Different Diagnostic Tools. Clin. Rheumatol. 2020, 39, 227–232. [Google Scholar] [CrossRef]
- Yalcinkaya, Y.; Erturk, Z.; Unal, A.U.; Kaymaz Tahra, S.; Pehlivan, O.; Atagunduz, P.; Direskeneli, H.; Inanç, N. The Assessment of Malnutrition and Severity of Gastrointestinal Disease by Using Symptom-Based Questionnaires in Systemic Sclerosis: Is It Related to Severe Organ Involvement or Capillary Rarefaction at Microcirculation? Clin. Exp. Rheumatol. 2020, 38, 127–131. [Google Scholar]
- Chang, R.W.S.; Richardson, R. Nutritional Assessment Using a Microcomputer 2. Programme Evaluation. Clin. Nutr. 1984, 3, 75–82. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic Criteria for Malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- Ren, Q.; Ding, K.; Jiang, W.; Zhu, W.; Gao, Y. Molecular Crosstalk and Potential Causal Mechanisms of Rheumatoid Arthritis and Sarcopenia Co-Morbidity: A Gene Integration Analysis. Exp. Gerontol. 2025, 203, 112729. [Google Scholar] [CrossRef]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of Interleukin-6 and Tumor Necrosis Factor-Alpha with Muscle Mass and Muscle Strength in Elderly Men and Women: The Health ABC Study. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef]
- Hunzelmann, N.; Moinzadeh, P.; Genth, E.; Krieg, T.; Lehmacher, W.; Melchers, I.; Meurer, M.; Müller-Ladner, U.; Olski, T.M.; Pfeiffer, C.; et al. High Frequency of Corticosteroid and Immunosuppressive Therapy in Patients with Systemic Sclerosis despite Limited Evidence for Efficacy. Arthritis Res. Ther. 2009, 11, R30. [Google Scholar] [CrossRef]
- Güler-Yüksel, M.; Hoes, J.N.; Bultink, I.E.M.; Lems, W.F. Glucocorticoids, Inflammation and Bone. Calcif. Tissue Int. 2018, 102, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Tada, M.; Mandai, K.; Hidaka, N.; Inui, K.; Nakamura, H. Glucocorticoid Use Is an Independent Risk Factor for Developing Sarcopenia in Patients with Rheumatoid Arthritis: From the CHIKARA Study. Clin. Rheumatol. 2020, 39, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Pardali, E.C.; Klonizakis, M.; Goulis, D.G.; Papadopoulou, S.K.; Cholevas, C.; Giaginis, C.; Tsigalou, C.; Bogdanos, D.P.; Grammatikopoulou, M.G. Sarcopenia in Rheumatic Diseases: A Hidden Issue of Concern. Diseases 2025, 13, 134. [Google Scholar] [CrossRef]
- Ajdynan, M.; Melkonyan, N.; Zhuravleva, N.; Guryanova, E.; Diomidova, V. AB0865 Body Composition Assessment in Patients with Systemic Scleroderma. Ann. Rheum. Dis. 2023, 82, 1645–1646. [Google Scholar]
- Hax, V.; Do Espirito Santo, R.C.; Dos Santos, L.P.; Farinon, M.; de Oliveira, M.S.; Três, G.L.; Gasparin, A.A.; De Andrade, N.P.B.; Bredemeier, M.; Xavier, R.M.; et al. Practical Screening Tools for Sarcopenia in Patients with Systemic Sclerosis. PLoS ONE 2021, 16, e0245683. [Google Scholar] [CrossRef]
- Paolino, S.; Goegan, F.; Cimmino, M.A.; Casabella, A.; Pizzorni, C.; Patanè, M.; Schenone, C.; Tomatis, V.; Sulli, A.; Gotelli, E.; et al. Advanced Microvascular Damage Associated with Occurence of Sarcopenia in Systemic Sclerosis Patients: Results from a Retrospective Cohort Study. Clin. Exp. Rheumatol. 2020, 38 (Suppl. 1), 65–72. [Google Scholar]
- Pardali, E.C.; Kontouli, K.-M.; Gkouvi, A.; Tsakmaki, I.A.; Patrikiou, E.; Karapli, M.; Liaskos, C.; Liapis, N.M.; Syrmou, V.; Alexiou, I.; et al. Screening and Diagnosis of Sarcopenia in Rheumatic and Musculoskeletal Diseases: Findings from a Cross-Sectional Study. Rheumatol. Int. 2025, 45, 67. [Google Scholar] [CrossRef]
- Rincón, I.D.R.; Alak, M.; Alsina, G.; Quevedo, P.; Rivero, M.; Duartes, D. Sarcopenia in Systemic Sclerosis. Arthritis Rheumatol. 2019, 71, 1657. [Google Scholar]
- Sangaroon, A.; Foocharoen, C.; Theerakulpisut, D.; Srichompoo, K.; Mahakkanukrauh, A.; Suwannaroj, S.; Seerasaporn, P.; Pongchaiyakul, C. Prevalence and Clinical Association of Sarcopenia among Thai Patients with Systemic Sclerosis. Sci. Rep. 2022, 12, 18198. [Google Scholar] [CrossRef] [PubMed]
- Sari, A.; Esme, M.; Aycicek, G.S.; Armagan, B.; Kilic, L.; Ertenli, A.I.; Halil, M.G.; Akdogan, A. Evaluating Skeletal Muscle Mass with Ultrasound in Patients with Systemic Sclerosis. Nutrition 2021, 84, 110999. [Google Scholar] [CrossRef] [PubMed]
- Yuce Inel, T.; Dervis Hakim, G.; Birlik, M. Assessment of Factors Related to Sarcopenia in Patients with Systemic Sclerosis. J. Clin. Med. 2025, 14, 1573. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European Consensus on Definition and Diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Ishii, S.; Tanaka, T.; Shibasaki, K.; Ouchi, Y.; Kikutani, T.; Higashiguchi, T.; Obuchi, S.P.; Ishikawa-Takata, K.; Hirano, H.; Kawai, H.; et al. Development of a Simple Screening Test for Sarcopenia in Older Adults. Geriatr. Gerontol. Int. 2014, 14, 93–101. [Google Scholar] [CrossRef]
- Omair, M.A.; Pagnoux, C.; McDonald-Blumer, H.; Johnson, S.R. Low Bone Density in Systemic Sclerosis. A Systematic Review. J. Rheumatol. 2013, 40, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Atteritano, M.; Sorbara, S.; Bagnato, G.; Miceli, G.; Sangari, D.; Morgante, S.; Visalli, E.; Bagnato, G. Bone Mineral Density, Bone Turnover Markers and Fractures in Patients with Systemic Sclerosis: A Case Control Study. PLoS ONE 2013, 8, e66991. [Google Scholar] [CrossRef]
- Shahin, A.A.; Zayed, H.S.; Sayed, S.; Gomaa, W. Bone Mineral Density in Patients with Systemic Sclerosis and Its Association with Hand Involvement. Egypt. Rheumatol. 2013, 35, 233–238. [Google Scholar] [CrossRef][Green Version]
- Hongkanjanapong, S.; Pongkulkiat, P.; Mahakkanukrauh, A.; Suwannaroj, S.; Foocharoen, C. Clinical Outcomes and Associated Factors with Mortality in Systemic Sclerosis Patients with Sarcopenia. Am. J. Med. Sci. 2025, 369, 35–43. [Google Scholar] [CrossRef]
- Barazzoni, R.; Bischoff, S.C.; Boirie, Y.; Busetto, L.; Cederholm, T.; Dicker, D.; Toplak, H.; Van Gossum, A.; Yumuk, V.; Vettor, R. Sarcopenic Obesity: Time to Meet the Challenge. Clin. Nutr. 2018, 37, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Guler, S.A.; Kwan, J.M.; Winstone, T.A.; Milne, K.M.; Dunne, J.V.; Wilcox, P.G.; Ryerson, C.J. Severity and Features of Frailty in Systemic Sclerosis-Associated Interstitial Lung Disease. Respir. Med. 2017, 129, 1–7. [Google Scholar] [CrossRef]
- Ito, K.; Naganawa, T.; Ohara, R.; Dosoden, N.; Sawada, M.; Ito, Y.; Watanabe, N.; Umeda, A.; Akamatsu, K.; Kurumizawa, M.; et al. ABS0639 Clinical Characteristics of Physical Frailty in Patients with Systemic Sclerosis: An Analysis Using a Japanese Single-Center Cohort. Ann. Rheum. Dis. 2025, 84, 2303. [Google Scholar] [CrossRef]
- Opriș-Belinski, D.; Cobilinschi, C.O.; Caraiola, S.; Ungureanu, R.; Cotae, A.-M.; Grințescu, I.M.; Cobilinschi, C.; Andrei, A.C.; Țincu, R.; Ene, R.; et al. Trace Element Deficiency in Systemic Sclerosis-Too Much Effort for Some Traces? Nutrients 2024, 16, 2053. [Google Scholar] [CrossRef] [PubMed]
- Westerman, M.P. Anemia and Scleroderma. Arch. Intern. Med. 1968, 122, 39. [Google Scholar] [CrossRef]
- Sari, A.; Gill, A.; Nihtyanova, S.I.; Ong, V.H.; Denton, C.P. THU0398 Unexplained Iron Deficiency Is Frequent in Systemic Sclerosis. Ann. Rheum. Dis. 2018, 77, 413–414. [Google Scholar] [CrossRef]
- Herrick, A.L.; Worthington, H.; Rieley, F.; Clark, D.; Schofield, D.; Braganza, J.M.; Jayson, M.I. Dietary Intake of Micronutrient Antioxidants in Relation to Blood Levels in Patients with Systemic Sclerosis—PubMed. J. Rheumatol. 1996, 23, 650–653. [Google Scholar]
- Ruiter, G.; Lanser, I.J.; de Man, F.S.; van der Laarse, W.J.; Wharton, J.; Wilkins, M.R.; Howard, L.S.; Vonk-Noordegraaf, A.; Voskuyl, A.E. Iron Deficiency in Systemic Sclerosis Patients with and without Pulmonary Hypertension. Rheumatology 2014, 53, 285–292. [Google Scholar] [CrossRef]
- Läubli, J.; Dobrota, R.; Maurer, B.; Jordan, S.; Misselwitz, B.; Fox, M.; Distler, O. Impaired Micronutrients and Prealbumin in Patients with Established and Very Early Systemic Sclerosis. Clin. Exp. Rheumatol. 2020, 38 (Suppl. 1), 120–126. [Google Scholar]
- Hughes, M.; Cooper, G.J.S.; Wilkinson, J.; New, P.; Guy, J.M.; Herrick, A.L. Abnormalities of Selenium but Not of Copper Homeostasis May Drive Tissue Fibrosis in Patients with Systemic Sclerosis. Rheumatology 2015, 54, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.; Hax, V.; Monticielo, O.; Macedo, T.F.; Barreto, R.K.M.; Marcondes, N.A.; Chakr, R. Dualities of the Vitamin D in Systemic Sclerosis: A Systematic Literature Review. Adv. Rheumatol. 2021, 61, 34. [Google Scholar] [CrossRef]
- Spronk, I.; Kullen, C.; Burdon, C.; O’Connor, H. Relationship between Nutrition Knowledge and Dietary Intake. Br. J. Nutr. 2014, 111, 1713–1726. [Google Scholar] [CrossRef]
- Gkiouras, K.; Grammatikopoulou, M.G.; Myrogiannis, I.; Papamitsou, T.; Rigopoulou, E.I.; Sakkas, L.I.; Bogdanos, D.P. Efficacy of N-3 Fatty Acid Supplementation on Rheumatoid Arthritis’ Disease Activity Indicators: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. Crit. Rev. Food Sci. Nutr. 2024, 64, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The Omega-6/Omega-3 Fatty Acid Ratio: Health Implications. Oléagineux Corps Gras Lipides 2010, 17, 267–275. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Khalili, A.; Roodi, P.B.; Saeedy, S.A.G.; Najafi, S.; Keshavarz Mohammadian, M.; Djafarian, K. Selenium Supplementation Decreases CRP and IL-6 and Increases TNF-Alpha: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Trace Elem. Med. Biol. 2023, 79, 127199. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Pizzol, D.; Smith, L.; Dominguez, L.J.; Barbagallo, M. Effect of Magnesium Supplementation on Inflammatory Parameters: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 679. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Sugioka, Y.; Tada, M.; Okano, T.; Mamoto, K.; Inui, K.; Habu, D.; Koike, T. Monounsaturated Fatty Acids Might Be Key Factors in the Mediterranean Diet That Suppress Rheumatoid Arthritis Disease Activity: The TOMORROW Study. Clin. Nutr. 2018, 37, 675–680. [Google Scholar] [CrossRef]
- Mohammed, A.T.; Khattab, M.; Ahmed, A.M.; Turk, T.; Sakr, N.; M Khalil, A.; Abdelhalim, M.; Sawaf, B.; Hirayama, K.; Huy, N.T. The Therapeutic Effect of Probiotics on Rheumatoid Arthritis: A Systematic Review and Meta-Analysis of Randomized Control Trials. Clin. Rheumatol. 2017, 36, 2697–2707. [Google Scholar] [CrossRef]
- Grammatikopoulou, M.G.; Marakis, G.; Gkiouras, K.; Athanatou, D.; Maraki, M.I.; Bogdanos, D.P. Fly Me to the Immune: Immunonutrition in Rheumatic Diseases. Mediterr. J. Rheumatol. 2023, 34, 30. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Talotta, R.; Rapisarda, F.; D’Amico, V.; Laganà, M.; Malaguti, M.; Campennì, A.; Cannavò, S.; Hrelia, S.; Ruggeri, R.M. Unlocking the Power of the Mediterranean Diet: Two in One-Dual Benefits for Rheumatic and Thyroid Autoimmune Diseases. Nutrients 2025, 17, 1383. [Google Scholar] [CrossRef]
- Vranou, P.; Gkoutzourelas, A.A.; Athanatou, D.; Zafiriou, E.; Grammatikopoulou, M.G.; Bogdanos, D.P. Let Food Be Thy Medicine: The Case of The Mediterranean Diet in Rheumatoid Arthritis. Mediterr. J. Rheumatol. 2020, 31, 325–329. [Google Scholar] [CrossRef]
- Pardali, E.C.; Gkouvi, A.; Gkouskou, K.K.; Manolakis, A.C.; Tsigalou, C.; Goulis, D.G.; Bogdanos, D.P.; Grammatikopoulou, M.G. Autoimmune Protocol Diet: A Personalized Elimination Diet for Patients with Autoimmune Diseases. Metab. Open 2025, 25, 100342. [Google Scholar] [CrossRef]
- Cano-Garcia, L.; Garcia Studer, A.; Ortiz-Márquez, F.; Manrique-Arija, S.; Mena-Vázquez, N.; Fernández-Nebro, A. ABS0465 Impact of Adherence to the Mediterranean Diet on the Severity and Functionality of Patient with Systemic Sclerosis. Ann. Rheum. Dis. 2025, 84, 2288–2289. [Google Scholar] [CrossRef]
- De Luca, G.; Natalello, G.; Abignano, G.; Campochiaro, C.; Temiz Karadağ, D.; De Santis, M.; Gremese, E.; Bosello, S.L.; Dagna, L. FRI0233 IImpact and Adherence to the Mediterranean Diet in Systemic Sclerosis Italian Patients: Correlation with Gastrointestinal Symptoms, Mood Disturbances and Quality of Life. Ann. Rheum. Dis. 2020, 79, 700. [Google Scholar] [CrossRef]
- Frech, T.M.; Khanna, D.; Maranian, P.; Frech, E.J.; Sawitzke, A.D.; Murtaugh, M.A. Probiotics for the Treatment of Systemic Sclerosis-Associated Gastrointestinal Bloating/ Distention. Clin. Exp. Rheumatol. 2011, 29, S22–S25. [Google Scholar] [PubMed]
- Low, A.H.L.; Teng, G.G.; Pettersson, S.; de Sessions, P.F.; Ho, E.X.P.; Fan, Q.; Chu, C.W.; Law, A.H.N.; Santosa, A.; Lim, A.Y.N.; et al. A Double-Blind Randomized Placebo-Controlled Trial of Probiotics in Systemic Sclerosis Associated Gastrointestinal Disease. Semin. Arthritis Rheum. 2019, 49, 411–419. [Google Scholar] [CrossRef]
- Marighela, T.F.; Arismendi, M.I.; Marvulle, V.; Brunialti, M.K.C.; Salomão, R.; Kayser, C. Effect of Probiotics on Gastrointestinal Symptoms and Immune Parameters in Systemic Sclerosis: A Randomized Placebo-Controlled Trial. Rheumatology 2019, 58, 1985–1990. [Google Scholar] [CrossRef]
- Marie, I.; Leroi, A.-M.; Gourcerol, G.; Levesque, H.; Ménard, J.-F.; Ducrotte, P. Fructose Malabsorption in Systemic Sclerosis. Medicine 2015, 94, e1601. [Google Scholar] [CrossRef]
- Marie, I.; Leroi, A.; Gourcerol, G.; Levesque, H.; Menard, J.; Ducrotte, P. Lactose Malabsorption in Systemic Sclerosis. Aliment. Pharmacol. Ther. 2016, 44, 1123–1133. [Google Scholar] [CrossRef]
- Nguyen, A.D.; Andréasson, K.; McMahan, Z.H.; Bukiri, H.; Howlett, N.; Lagishetty, V.; Lee, S.M.; Jacobs, J.P.; Volkmann, E.R. Gastrointestinal Tract Involvement in Systemic Sclerosis: The Roles of Diet and the Microbiome. Semin. Arthritis Rheum. 2023, 60, 152185. [Google Scholar] [CrossRef]
- Wojteczek, A.; Ziętkiewicz, M.; Małgorzewicz, S.; Zdrojewski, Z. FRI0430 Effect of Oral Nutritional Intervention on Nutritional Status in Patients with Systemic Sclerosis. Ann. Rheum. Dis. 2018, 77, 745. [Google Scholar] [CrossRef]
- Harrison, E.; Herrick, A.L.; Dibb, M.; McLaughlin, J.T.; Lal, S. Long-Term Outcome of Patients with Systemic Sclerosis Requiring Home Parenteral Nutrition. Clin. Nutr. 2015, 34, 991–996. [Google Scholar] [CrossRef]
- Fragkos, K.C.; Sebepos-Rogers, G.; Rahman, F. When Is Parenteral Nutrition Indicated in the Hospitalized, Acutely Ill Patient? Curr. Opin. Gastroenterol. 2020, 36, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.R.; Cawood, A.L.; Walters, E.R.; Guildford, N.; Stratton, R.J. Ready-Made Oral Nutritional Supplements Improve Nutritional Outcomes and Reduce Health Care Use—A Randomised Trial in Older Malnourished People in Primary Care. Nutrients 2020, 12, 517. [Google Scholar] [CrossRef]
- Keane, N.; Ghannam, A.; KC, F.; Rahman, F. Oral, Enteral and Parenteral Nutritional Therapies in Scleroderma: A Systematic Review. Clin. Nutr. ESPEN 2022, 51, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Hartl, W.H.; Jauch, K.W.; Parhofer, K.; Rittler, P. Working group for developing the guidelines for parenteral nutrition of The German Association for Nutritional Medicine Complications and Monitoring—Guidelines on Parenteral Nutrition, Chapter 11. Ger. Med. Sci. 2009, 7, Doc17. [Google Scholar] [CrossRef] [PubMed]
- Romanazzo, S.; Rometsch, C.; Marangoni, A.; Guiducci, S.; Cosci, F. Psychological Features of Systemic Sclerosis: Results from an Observational Study. Front. Med. 2024, 11, 1473587. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.S.; Guerra, R.S.; Fonseca, I.; Pichel, F.; Ferreira, S.; Amaral, T.F. Financial Impact of Sarcopenia on Hospitalization Costs. Eur. J. Clin. Nutr. 2016, 70, 1046–1051. [Google Scholar] [CrossRef]
- Mitropoulos, A.; Boström, C.; Mattsson, M.; Kouidi, E.; Dimitroulas, T.; Liem, S.I.E.; Vlieland, T.P.M.V.; de Vries-Bouwstra, J.K.; Jacobsen, S.; Cuomo, G.; et al. Exploring the Effects of a Combined Exercise Programme on Pain and Fatigue Outcomes in People with Systemic Sclerosis: Study Protocol for a Large European Multi-Centre Randomised Controlled Trial. Trials 2022, 23, 962. [Google Scholar] [CrossRef]
| GI Tract Involvement | Clinical Manifestations |
|---|---|
| Oral cavity | Microstomia, xerostomia, dental caries, periodontal disease, dystrophic calcinosis, bone/joint involvement; worsened by coexisting Sjögren’s syndrome; reflux increases acidity, dental wear. |
| Esophagus | Dysmotility disorder, decreased peristalsis, hypotensive LES, dysphagia, regurgitation, heartburn, GERD—up to 76% prevalence. |
| Stomach | Gastritis, gastroparesis, gastric ulcers, GAVE. Symptoms: abdominal pain, bloating, early satiety, nausea. GAVE linked to anemia/bleeding. |
| Small intestine | Dysmotility (40–88%), pseudo-obstruction, SIBO (weight loss, malabsorption, dysbiosis), rare PCI, possible celiac disease. |
| Colon | Hypomotility (20–50%), colonic dilatation, diarrhea (SIBO, malabsorption), constipation, fecal incontinence (anorectal dysfunction, internal anal sphincter involvement). |
| First Author | Recruitment | Sample | BC Method Applied | Malnutrition Criteria Applied | Obesity (%) | Underweight (%) | Nutritional Risk (Other Tools) (%) | Low FFMI (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Duration | Size (N) | Men/Women Ratio | Low | Moderate | High | ||||||
| Andréasson [55] | Skane University Hospital, Lund | 2014–5 | 98 | NR | - | MUST | - | - | 80.6MUST | 19.4MUST | - | |
| Bagnato [84] | University Hospital of Messina and Padova and University of Leeds | NR | 159 | 11/148 | - | MUST, ESPEN | - | - | Discovery cohort 45MUST, validation cohort 47MUST, 84.3ESPEN | Discovery cohort 37MUST, validation cohort 34MUST | Discovery cohort 18MUST, validation cohort 18MUST | - |
| 15.7ESPEN | ||||||||||||
| Baron [86] | 15 centers, Canada | 2004–8 | 586 | 76/510 | - | MUST | - | - | 70.1MUST | 12.5MUST | 17.4MUST | - |
| Burlui [72] | Dept of Rheumatology and Rehabilitation, “Grigore T. Popa” University of Medicine | NR | 42 | 6/36 | - | MUST | - | - | 73.81MUST | 11.9MUST | 14.3MUST | - |
| Caimmi [80] | Verona Medical School | 2016 | 141 | 22/119 | DXA | ESPEN, MUST | - | - | 79.4MUST, 90.8ESPEN | 12.8MUST | 7.8MUST | - |
| 9.2ESPEN | ||||||||||||
| Cano-Garcia [87] | Regional Universitario de Málaga | NR | 52 | 1/51 | - | ΜΝA | - | - | 64.5MNA | 32.7MNA | 1.9MNA | - |
| Caporali [88] | Rheumatology Unit, Research Hospital Fondazione IRCCS Policlinico San Matteo | 2007–8 | 160 | 20/140 | - | BMI, weight loss | - | - | 85 | 15 | - | - |
| Cereda [89] | Rheumatology Unit, Fondazione IRCCS Policlinico San Matteo | 2007–8 | 160 | 20/140 | - | MUST | - | 3.1 | 45.6MUST | 30MUST | 24.4MUST | - |
| Cruz-Domínguez [90] | Hospital (NOD) in Mexico City | 2005–14 | 147 | NR | - | Chang index | - | - | Survivors 64Chang, Deceased 32Chang | Mild: Survivors 4.7Chang, Deceased 5.3Chang; Moderate: Survivors 18.8Chang, Deceased 10.5Chang | Survivors 12.5Chang, Deceased 52.6Chang | - |
| Doerfler [91] | Multiple sites, N. America, Europe | NR | 14 | 12/2 | DXA | abPGSGA | - | - | 83abPGSGA * | 17abPGSGA * | - | |
| Dupont [82] | Toulouse University Hospital | 2011–6 | 82 | 20/62 | - | HAS, MUST | - | - | 83HAS, 79MUST | 6MUST | 15MUST | - |
| 17HAS | ||||||||||||
| Fairley [92] | Multicenter trial, Australia | 2007–23 | 1903 | 272/1631 | - | GLIM, MUST | - | - | 43.3MUST, 20.9GLIM | 25.9MUST, 46GLIM | 30.7MUST, 33.1GLIM | - |
| Gajdecki [93] | Rheumatology Dept, Medical University of Łódź | 2018–23 | 44 | 34/10 | - | CONUT, MUST | 4.9 | 9.8 | 65.9MUST, 38.6CONUT | 19.5MUST, light: 43.2CONUT, moderate: 43.2CONUT | 14.6MUST, 20.9GLIM, 0CONUT | - |
| Hax [16] | Hospital de Clínicas, Porto Alegre | 2019 | 94 | 7/87 | - | ESPEN | - | - | 85.1ESPEN | 14.9ESPEN | - | |
| Hvas [78] | Rheumatology specialist center, Salford | NR | 168 | 31/137 | BIA | BMI, MUST | 11 | 7 | 74MUST | 14MUST | 12MUST | - |
| Krause [94] | Dept of Rheumatology & Clinical Immunology, Charitéplatz | NR | 124 | 20/104 | BIA | BMI, PhA | 4.8 | 13.7 | 44.4PhA | 33.8PhA | 21.8PhA | - |
| Molfino [95] | Scleroderma Unit, Dept of Translational & Precision Medicine, Azienda Policlinico Umberto I, Sapienza University | NR | 64 | 11/55 | BIA | MUST, FFMI, BMI | NR | NR | 61MUST | 12.5MUST | 26.5MUST | 28.2 |
| Murtaugh [96] | University of Utah SSc Center | NR | 24 | 4/20 | - | MUST, SGA | - | - | 50SGA, 62.5MUST | 37.5SGA, 8.3MUST | 12.5SGA, 29.2MUST | - |
| Ortiz-Santamaria [97] | Hospital Universitario de Granollers | NR | 9 | 1/8 | - | MUST | NR | 0 | 87.5MUST | 12.5MUST | - | |
| Paolino [79] | Scleroderma Clinic, Rheumatology Division, Genova University | NR | 36 | 0/36 | DXA | ESPEN | NR | - | 63.8ESPEN | 36.1ESPEN | NR | |
| Preis [98] | Dept of Rheumatology & Clinical Immunology, Charité—University Medicine Berlin | 2013–4 | 129 | 12/117 | - | BMI, MUST | NR | 6.2 | 74.4MUST | 14.7MUST | 10.9MUST | - |
| Rivet [85] | Dept of Internal Medicine, Saint Eloi University Hospital | 1985–2019 | 119 | 18/101 | - | HAS, MUST | - | - | 40.8HAS, 41.7MUST | 34.2HAS, 20MUST | 25HAS, 38.3MUST | - |
| Rosato [81] | Dept of Translational & Precision Medicine, Sapienza University | 2018 | 102 | 16/86 | BIA | MUST, ESPEN, GLIM, FFMI | - | - | 69.6MUST, 83.4GLIM 91.2ESPEN | 12.7MUST, 12.7GLIM | 17.6MUST, 3.9GLIM | 21.6 |
| 8.8ESPEN | ||||||||||||
| Rosato [99] | Dept of Translational & Precision Medicine, Sapienza University | 2017–21 | 101 | 15/86 | - | BMI, GLIM | - | - | 78.2GLIM | 12.9GLIM | 8.9GLIM | - |
| Rosato [100] | Dept of Translational & Precision Medicine, Sapienza University | ΝR | 104 | 16/88 | BIA | MUST, GLIM | - | - | 69.2MUST, 79.8GLIM | 10.6MUST, 11.5GLIM | 20.2MUST, 8.7GLIM | - |
| Spanjer [101] | Dept of Rheumatology, Amsterdam Rheumatology & Immunology Center | 2013–4 | 72 | 21/51 | BIA, DXA | FFMI, BMI | 11.1 | 4.2 | 91.7ESPEN | 8.3ESPEN | 20.8 | |
| Szabo [83] | County Emergency Clinical Hospital Cluj-Napoca | NR | 75 | NR | - | MUST | - | - | 93MUST | 6MUST | 1MUST | - |
| Tas Kilic [102] | Ankara Numune Training & Research Hospital, Ankara | NR | 62 | 6/56 | - | BMI, MUST | 24.2 | 11.3 | 74.2MUST | 25.8MUST | - | |
| Türk [103] | Division of Rheumatology, Cukurova University, Adana | 2016–7 | 98 | 15/83 | - | MUST | - | - | 61.2MUST | 15.3MUST | 23.5MUST | - |
| Pardali (in press) | Dept of Rheumatology & Clinical Immunology, Larissa University Hospital | 2022–5 | 29 | 4/25 | Skinfolds | GLIM, SGA | 20.7 | 24.1 | 82.8SGA, 27.6GLIM | 6.9SGA, 37.9GLIM | 10.4SGA, 34.5GLIM | NR |
| Volkmann [1] | UK hospital (NOD) | 2015–7 | 576 | 143/433 | - | MUST | - | 10.6 | Νintedanib 91.7MUST; control 87.2MUST * | Νintedanib 5.9MUST; control 8MUST * | Νintedanib 2.4MUST; control 4.9MUST * | - |
| Wojteczek [104,105] | Clinical University Centre, Gdańsk | 2013–4 | 56 | 9/47 | BIA | ESPEN, GLIM, 7-SGA, SNAQ | 12.5 | 5.4 | 82.1ESPEN, 76.87-SGA, 83.9SNAQ, 37.5GLIM | 21.4 7-SGA, 50GLIM | 1.87-SGA, 12.5GLIM | 73.2 |
| 16.1SNAQ, 17.9ESPEN | ||||||||||||
| Yalcinkaya [106] | Division of Rheumatology, Marmara University | NR | 134 | 10/124 | - | BMI, MUST | NR | 3.6 | 85MUST | 9MUST | 6MUST | - |
| Malnutrition Assessment Method | Components | Limitations of Methods When Applied in SSc |
|---|---|---|
| abPG-SGA | Patient-reported intake, symptoms, weight, activity. |
|
| BMI | Weight/height. |
|
| Chang index | Nutritional risk using parameters like weight loss, BMI, GI symptoms, functional status. |
|
| CONUT | Albumin, total cholesterol, lymphocyte count. |
|
| ESPEN guidelines |
|
|
| FMI | Fat mass adjusted for height. |
|
| GLIM | Phenotypic (weight loss, low BMI, reduced muscle mass) + etiologic (inflammation, reduced intake/assimilation). |
|
| HAS | Weight loss, BMI, acute disease effect. |
|
| MNA |
|
|
| MUST | BMI, unintentional weight loss, acute disease effect. |
|
| PhA | Cell membrane integrity, body composition. |
|
| SGA | Clinical judgment integrating history and physical exam. |
|
| SNAQ |
|
|
| First Author | Sample | Sarcopenia | |||
|---|---|---|---|---|---|
| N | Prevalence (%) | Diagnostic Criteria | Sarcopenia Assessment Methods | Muscle Mass Assessment Methods | |
| Ajdynan [115] | 43 | 33.3 | EWGSOP 2019 | SMI ‡, HGS, sit-to-stand | DXA |
| Caimmi [80] | 140 | 20.7 | SMI | SMI ‡ | DXA |
| Corallo [13] | 62 | 42 | EWGSOP 2010 | SMI ‡, HGS | DXA |
| Doerfler [91] | 13 | 54 | SMI | SMI ‡ | DXA |
| Hax [116] | 94 | 15.9/22.3/ 21.3/21.3/36.2 | EWGSOP 2019/SARC-F/ SARC-CalF/SARC-F+EBM/Ishii | SMI ‡, HGS, 4MWS, SPPB | DXA |
| Paolino [117] | 43 | 23.3 | EWGSOP 2010 | SMI ‡ | DXA |
| Pardali [118] | 17 | 52.9 | EWGSOP 2010 | FFMI ¤, HGS | Skinfold thickness |
| Rincón [119] | 27 | 33.3 | EWGSOP 2010 | SMI ‡, HGS, 4MWS | DXA |
| Sangaroon [120] | 180 | 22.8 | AWGS 2019 | SMI ‡, FFMI ¤, HGS, 6MWS | DXA |
| Sari [121] | 93 | 10.7 | EWGSOP 2010 | ASMI †, HGS | BIA |
| Siegert [12] | 129 | 22.5 | EWGSOP 2010 | SMI ‡, HGS | BIA |
| Yuce Inel [122] | 80 | 20/5/20/8.8 | SARC-F/SMI ‡/SMMI Š/FFMI ¤ | 4MWS, sit-to-stand | BIA |
| Key Points | Details/Criteria |
|---|---|
| Dietary practices | Educate patients on safe dietary modifications. Prevent overly restrictive diets that worsen malnutrition or deficiencies. Support individualized dietary advice rather than patient-led restrictions [73]. |
| Dietetic referral & service delivery model | Refer to a dietitian ideally at diagnosis, at symptom change, and periodically thereafter. A dietitian should have SSc expertise/experience; multidisciplinary care is optimal [73,77]. Provide dietetic input through written resources, face-to-face, telehealth, phone calls, online resources, and group sessions [73]. |
| Symptom screening & monitoring | Screen all patients with SSc at baseline and monitor regularly, as this strongly affects nutrition and QoL [73]. Early intervention reduces complications [73]. BMI < 18.5 kg/m2 suggests PEM [73]. |
| Weight loss & malnutrition | Substantial weight loss over 3–6 months may indicate inadequate intake [38]. Implement systematic malnutrition screening (MUST or similar). Monitor weight regularly [38,73]. Rule out bacterial overgrowth and gastroparesis [38]. Screen for malabsorption, PBC, pancreatic insufficiency [71]. Increase healthy fats (olive/canola/peanut oil, nuts, seeds, avocado, fatty fish, coconut milk, oil-based dressings) [39,71]. Use calorie-dense smoothies (fruit + yogurt + milk + nut butter + oil + protein powder). Increase protein (marinate meats to soften; use broths; add protein powders to smoothies, cereal, yogurt) [39] and add high-protein supplements [71]/Eat every 2 h for maximal intake [38]. Enteral feeding if possible (jejunal/gastrostomy). Parenteral nutrition if intestinal failure. Multidisciplinary approach (rheumatology, gastroenterology, dietetics) [71]. |
| Labs for screening to detect deficiencies and malnutrition | Hemoglobin (Iron, folate, B12), serum carotene (fat malabsorption), serum folate (↑ in bacterial overgrowth, not valid with supplements), serum albumin (<35 g/L → rule out PEM, but not sensitive/specific) [77]. Protein status: total protein, albumin, pre-albumin. Vitamin/mineral deficiencies: Iron, ferritin, TIBC, Zinc, B12. Malabsorption/SIBO: folate, carotene, vitamin D [38], serum methylmalonic acid, Zinc, vitamin D, vitamin K or prothrombin time, breath tests (C14 xylose = better, hydrogen breath = more available and often abnormal in SSc with bacterial overgrowth) [77]. |
| Other screening | Ask about GI symptoms, oral health (teeth, chewing, taste), saliva production, depressive symptoms. Assess severity of malnutrition [77]. |
| General diet recommendations | Eat small, frequent meals every 3–4 h (or every 2 h if underweight). Choose fresh, minimally processed foods with short ingredient lists [38]. Avoid artificial ingredients, preservatives, and hydrogenated oils. Use adapted utensils/kitchen tools; pre-cut or frozen fruit/vegetables; stock frozen meals; occupational therapy support [39]. |
| Inflammation | Add antioxidant-rich herbs/spices: basil, rosemary, oregano, cinnamon, ginger, paprika, cayenne, turmeric, curry powder [38], and colored fruits/vegetables (dark green, orange, yellow, red, purple, blue) [38]. Limit added sugars; natural sugars in fruit/dairy are usually fine unless GI distress occurs. Watch for hidden sugars (sucrose, cane juice, fructose, syrups, honey, molasses) [38]. Include omega-3s (fatty fish, ground flaxseed, walnuts). Eat vitamin E-rich foods (nuts, seeds, olive oil). Consider vitamin D3 [38]. Consider OTC multivitamin/mineral with Ζinc, Ιron, vitamins A, D, E, K, folate, and B12. Extra supplementation may be required if deficiencies are present. Probiotics may improve bloating/distention [38]. |
| Bone health | Calcium sources: Dairy, bone broths, leafy greens, nuts, tahini, tinned salmon/sardines. Magnesium sources: Nuts, seeds, greens, dark chocolate. Vitamin D: Sunlight + supplements (dose per individual needs). Seek professional advice [39]. |
| Water | Drink filtered water only (avoid plastic exposure). Use glass/stainless steel. Daily target = half body weight in ounces (e.g., 150 lb → 75 oz) [38]. |
| Daily intake | Fruit: Two–three/day; choose colorful fresh/frozen fruits; avoid gas-causing (FODMAP). Vegetables: Five–seven/day; colorful, fresh/frozen preferred; avoid high-FODMAP veggies. Proteins: Lean meats, fish (8–12 oz fatty fish weekly), eggs, nuts, beans if tolerated; avoid processed/fried meats. Milk/dairy: Two–three/day; low-fat, Greek or lactose-free if sensitive; avoid regular lactose if symptomatic. Whole grains: Three–six/day; 100% whole grains ≥3 g fiber; avoid refined flour/wheat if FODMAP-sensitive. Fats & oils: One–two/day; olive, peanut, canola, nuts, avocado; avoid trans fats, limit butter/shortening [38]. |
| Swallowing | Refer to a speech pathologist when oropharyngeal swallowing problems are encountered [77]. Eat slowly and chew well. Use soft/pureed foods (mashed potatoes, soups, casseroles). Dip dry foods (bread, biscuits) in liquids. Drink fluids between bites. Blend/mince foods, add sauces/oils to moisten. Seek speech pathologist input [39]. Use smoothies, blended fruits/vegetables, yogurt, cottage cheese, scrambled eggs, soft meats with sauces, pasta dishes. Add whey protein or meal replacement powders to smoothies [38]. |
| Xerostomia | Rule out Sjögren’s (serology ± biopsy). Treat with biotin, artificial saliva, or trial of pilocarpine 5 mg or Evoxac before meals [71]. |
| Gastroparesis | If early satiety, nausea, vomiting → do radionuclide gastric emptying study, refer to gastroenterologist. Pro-motility agents might help [77]. |
| Reflux/heartburn | Eat small, frequent meals (avoid overfilling). Do not eat 2–3 h before bedtime. Avoid trigger foods (citrus, tomato, fried/greasy foods, coffee, garlic, onions, peppermint, gas-producing foods like beans, broccoli, raw peppers, raw onions, spicy foods, carbonated drinks, alcohol). If overweight in midsection → weight loss may help. Use a wedge pillow or elevate head of bed to reduce regurgitation [38,39]. Pharmacologic: PPIs first-line; increase dose or add H2 blocker if refractory. Consider prokinetics if delayed emptying. Surgery (fundoplication) not generally recommended due to motility disorder [71,77]. |
| Malabsorption (bacterial overgrowth) | Trial of selective antibiotic (10–21 days). If relapses: repeated monthly courses or continuous therapy. Consider probiotics after antibiotics. For refractory small bowel involvement: octreotide 50 mcg SC at bedtime (but costly, parenteral, and may impair gastric emptying/pancreatic secretions) [39,71]. |
| Diarrhea | Treat the identified cause. Antibiotics for SIBO, pancreatic enzyme replacement if insufficient. Bile acid sequestrants if bile acid malabsorption. Consider probiotics. Symptomatic antidiarrheals if no reversible cause found [71]. Opt for soluble fiber (banana, apple, oats, prunes) [39]. |
| Decreased GI motility/constipation | Engage in regular exercise (e.g., walking). Eat a high-fiber diet (whole grains, fruits, vegetables). Hydrate [38,39,71]. Increase daily fluid intake [38,39]. Take a probiotic or yogurt with active cultures [38]. Room-temperature drinks if Raynaud’s [39]. |
| Incontinence (fecal soilage) | Conservative: Bowel retraining, diet modification. Medical: Antidiarrheals if loose stools. Advanced: Sacral nerve stimulation, sphincter repair (variable long-term success) [71]. |
| Severe refractory cases | If all else fails → enteral nutrition (preferred) via jejunostomy; parenteral nutrition if necessary (but risk of sepsis, thrombosis, liver failure). Decision requires coordination (rheumatologist, gastroenterologist, dietitian) [71]. |
| Fatigue | Eat small, frequent meals for steady energy. Stay hydrated. Engage in 30–60 min daily moderate exercise (walking, biking, pool exercise, pilates, yoga, tai chi). Get 7–8 h of sleep nightly. If Iron is low, discuss supplementation with a doctor. Take Iron pills with vitamin C-containing juice to improve absorption [38]. |
| Poor circulation/Raynaud’s | Exercise regularly to improve blood flow. For finger ulcers → eat animal proteins rich in Zinc and Iron (beef, pork) to promote wound healing [38]. |
| Tight, thickened skin | Eat vitamin E-rich foods (nuts, seeds, wheat germ, olive/canola/peanut oils). Consider a biotin 5 mg supplement for skin and nail health [38]. |
| FODMAP [38] | Oligosaccharides (FOS/GOS): Avoid wheat/rye grains, legumes, onion, garlic, asparagus, cabbage, broccoli, artichokes, leeks, shallots. Choose gluten-free grains, corn, rice, oatmeal, celery, spinach, white potatoes. Disaccharides (Lactose): Avoid milk, cheese, yogurt, ice cream, custard. Choose lactose-free dairy, unsweetened almond or rice milk. Monosaccharides (Excess fructose): Avoid apples, pears, watermelon, mango, honey, agave, high fructose corn syrup, dried fruits. Choose berries, citrus, ripe banana, kiwi, pineapple, rhubarb. Polyols (Sugar alcohols): Avoid apricot, plum, peach, prunes, avocado, mushrooms, and “diet/sugar-free” products with sorbitol, mannitol, xylitol, maltitol. No safe alternatives listed—best avoided. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardali, E.C.; Gkouvi, A.; Grammatikopoulou, M.G.; Mitropoulos, A.; Cholevas, C.; Poulimeneas, D.; Klonizakis, M. Nutritional Status and Dietary Challenges in Patients with Systemic Sclerosis: A Comprehensive Review. Nutrients 2025, 17, 3144. https://doi.org/10.3390/nu17193144
Pardali EC, Gkouvi A, Grammatikopoulou MG, Mitropoulos A, Cholevas C, Poulimeneas D, Klonizakis M. Nutritional Status and Dietary Challenges in Patients with Systemic Sclerosis: A Comprehensive Review. Nutrients. 2025; 17(19):3144. https://doi.org/10.3390/nu17193144
Chicago/Turabian StylePardali, Eleni C., Arriana Gkouvi, Maria G. Grammatikopoulou, Alexandros Mitropoulos, Christos Cholevas, Dimitrios Poulimeneas, and Markos Klonizakis. 2025. "Nutritional Status and Dietary Challenges in Patients with Systemic Sclerosis: A Comprehensive Review" Nutrients 17, no. 19: 3144. https://doi.org/10.3390/nu17193144
APA StylePardali, E. C., Gkouvi, A., Grammatikopoulou, M. G., Mitropoulos, A., Cholevas, C., Poulimeneas, D., & Klonizakis, M. (2025). Nutritional Status and Dietary Challenges in Patients with Systemic Sclerosis: A Comprehensive Review. Nutrients, 17(19), 3144. https://doi.org/10.3390/nu17193144






