Dumping Syndrome After Bariatric Surgery: Advanced Nutritional Perspectives and Integrated Pharmacological Management
Abstract
1. Introduction
2. The Aetiology of Dumping Syndrome
2.1. Bariatric Surgery: A Cornerstone in Dumping Syndrome Pathogenesis
2.2. Alternative Aetiologies: Beyond Bariatric Surgery
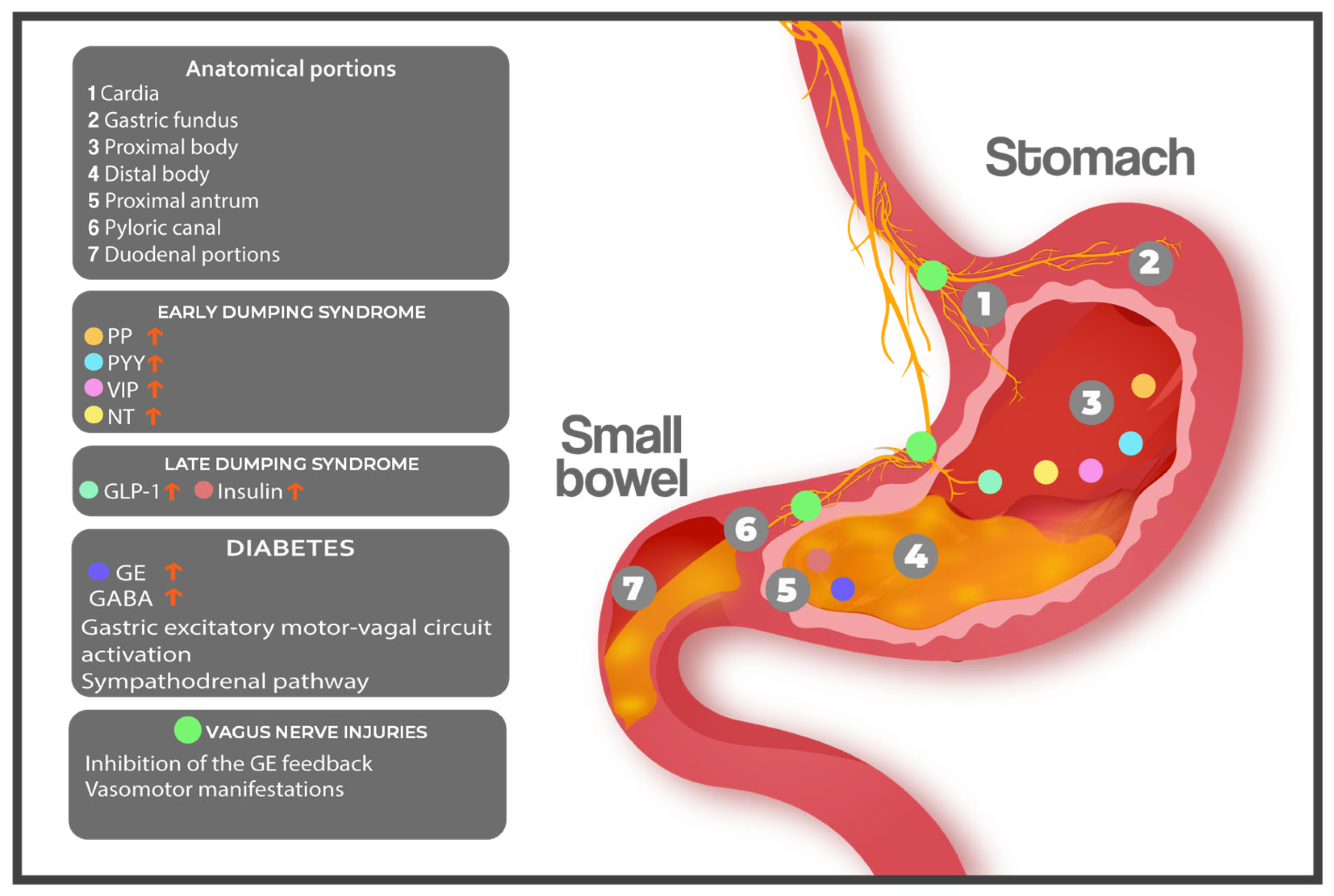
3. The Pathophysiological Landscape of Dumping Syndrome: From Mechanical Alterations to Hormonal Storms
4. Diagnostic Approach to Dumping Syndrome: Clinical Criteria, Functional Testing, and Diagnostic Challenges
5. Therapeutic Approach to Post-Bariatric Dumping Syndrome: From Nutrition to Pharmacotherapy
5.1. Pathophysiological Principles Guiding Nutritional Intervention
5.1.1. Nutritional Intervention for Early Dumping Syndrome
5.1.2. Nutritional Intervention for Late Dumping Syndrome
5.2. Fundamental Dietary Strategies and Specific Considerations
5.3. Towards Precision Nutrition: Individualisation and Advanced Tools
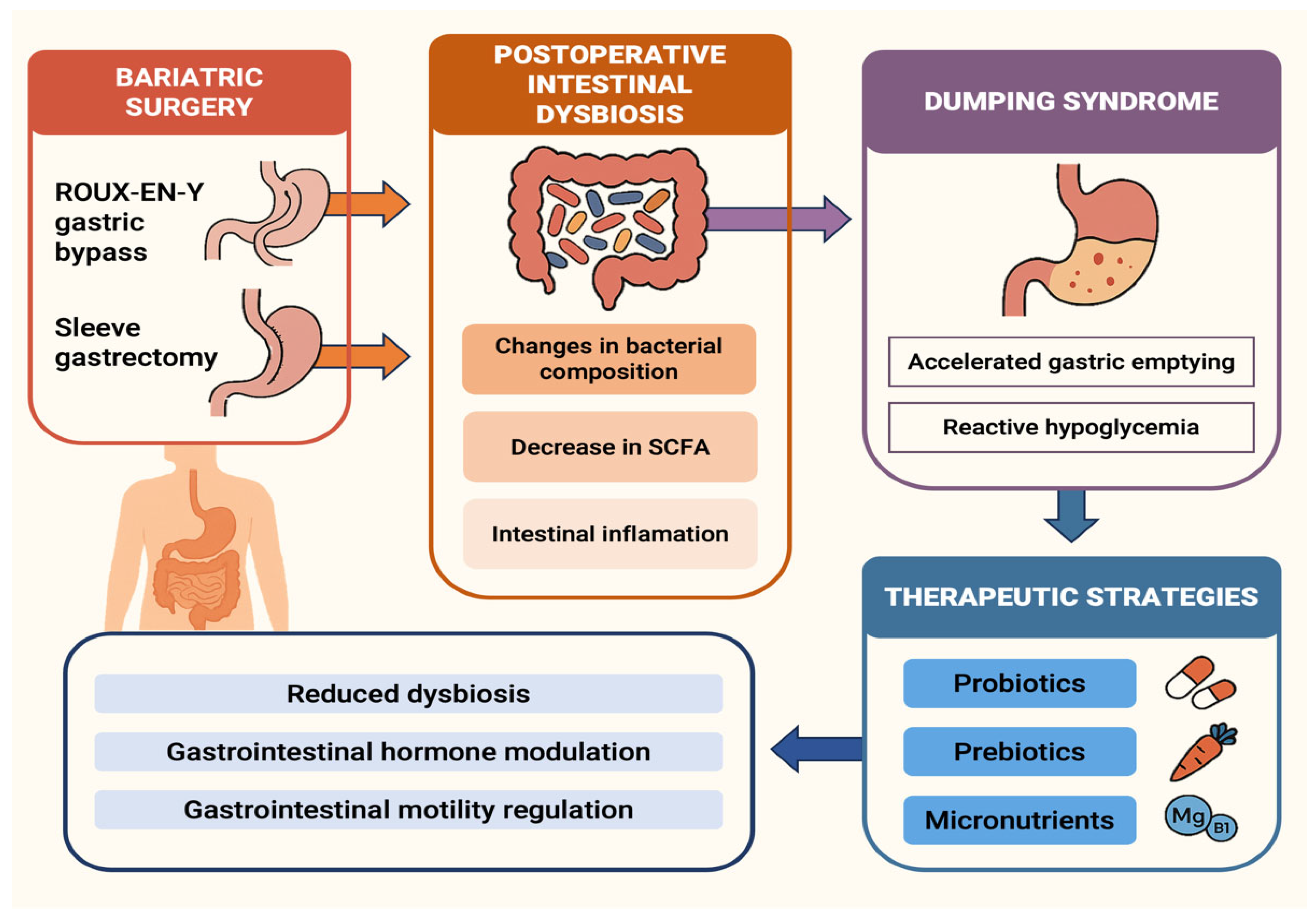
5.4. The Gut Microbiota Axis and Its Nutritional Modulation
5.5. Transition to Pharmacotherapy
5.6. Pharmacotherapy Options: Mechanisms and Evidence
5.7. Revisiting Surgical Alternatives in Refractory Cases
6. Modulation of Gut Microbiota and Micronutrient Balance in Post-Bariatric Patients: Emerging Links with Dumping Syndrome and Therapeutic Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rives-Lange, C.; Poghosyan, T.; Rassy, N.; Carette, C.; Phan, A.; Goeau-Brissonnière, M.; de Castelbajac, F.; Merazka, A.; Czernichow, S. The future of bariatric surgery research: A worldwide mapping of registered trials. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2022, 23, e13433. [Google Scholar] [CrossRef]
- Kermansaravi, M.; Chiappetta, S.; Shahabi Shahmiri, S.; Varas, J.; Parmar, C.; Lee, Y.; Dang, J.T.; Shabbir, A.; Hashimoto, D.; Davarpanah Jazi, A.H.; et al. International expert consensus on the current status and future prospects of artificial intelligence in metabolic and bariatric surgery. Sci. Rep. 2025, 15, 9312. [Google Scholar] [CrossRef]
- Hsu, J.L.; Farrell, T.M. Updates in Bariatric Surgery. Am. Surg. 2024, 90, 925–933. [Google Scholar] [CrossRef]
- Salas-Parra, R.D.; Smolkin, C.; Choksi, S.; Pryor, A.D. Bariatric Surgery: Current Trends and Newer Surgeries. Gastrointest. Endosc. Clin. N. Am. 2024, 34, 609–626. [Google Scholar] [CrossRef]
- Adams, T.D.; Meeks, H.; Fraser, A.; Davidson, L.E.; Holmen, J.; Newman, M.; Ibele, A.R.; Richards, N.; Hunt, S.C.; Kim, J. Long-term all-cause and cause-specific mortality for four bariatric surgery procedures. Obesity 2023, 31, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, E.; Arts, J.; Karamanolis, G.; Laurenius, A.; Siquini, W.; Suzuki, H.; Ukleja, A.; Van Beek, A.; Vanuytsel, T.; Bor, S.; et al. International consensus on the diagnosis and management of dumping syndrome. Nat. Rev. Endocrinol. 2020, 16, 448–466. [Google Scholar] [CrossRef] [PubMed]
- Masclee, G.M.C.; Masclee, A.A.M. Dumping Syndrome: Pragmatic Treatment Options and Experimental Approaches for Improving Clinical Outcomes. Clin. Exp. Gastroenterol. 2023, 16, 197–211. [Google Scholar] [CrossRef] [PubMed]
- D’hoedt, A.; Vanuytsel, T. Dumping syndrome after bariatric surgery: Prevalence, pathophysiology and role in weight reduction—A systematic review. Acta Gastro-Enterol. Belg. 2023, 86, 417–427. [Google Scholar] [CrossRef]
- Goyal, R.K.; Cristofaro, V.; Sullivan, M.P. Rapid gastric emptying in diabetes mellitus: Pathophysiology and clinical importance. J. Diabetes Complicat. 2019, 33, 107414. [Google Scholar] [CrossRef]
- Wang, P.T.; Wellington, J.; Koch, K.L. Clinical features and gastric myoelectrical activity in patients with idiopathic and post-surgical rapid gastric emptying who present with unexplained chronic nausea. Neurogastroenterol. Motil. 2021, 33, e13988. [Google Scholar] [CrossRef]
- Davis, J.; Camilleri, M.; Eckert, D.; Burton, D.; Joyner, M.; Acosta, A. Physical activity is associated with accelerated gastric emptying and increased ghrelin in obesity. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2020, 32, e13879. [Google Scholar] [CrossRef]
- Tack, J.; Raymenants, K.; Van de Bruaene, C.; Scarpellini, E. Dumping syndrome: Update on pathophysiology, diagnosis, and management. Neurogastroenterol. Motil. 2025, 37, e14962. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Garvey, W.T.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures—2019 update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg. Obes. Relat. Dis. 2020, 16, 175–247. [Google Scholar] [CrossRef] [PubMed]
- Wauters, L.; Vanuytsel, T. Applications of peptide hormone ligands for the treatment of dumping and short bowel syndrome. Curr. Opin. Pharmacol. 2018, 43, 118–123. [Google Scholar] [CrossRef]
- Hong, S.; Park, B.; Noh, H.; Choi, D.-J. Herbal Medicine for Dumping Syndrome: A Systematic Review and Meta-Analysis. Integr. Cancer Ther. 2019, 18, 1534735419873404. [Google Scholar] [CrossRef]
- Guarino, D.; Moriconi, D.; Mari, A.; Rebelos, E.; Colligiani, D.; Baldi, S.; Anselmino, M.; Ferrannini, E.; Nannipieri, M. Postprandial hypoglycaemia after Roux-en-Y gastric bypass in individuals with type 2 diabetes. Diabetologia 2019, 62, 178–186. [Google Scholar] [CrossRef]
- Vavricka, S.R.; Greuter, T. Gastroparesis and Dumping Syndrome: Current Concepts and Management. J. Clin. Med. 2019, 8, 1127. [Google Scholar] [CrossRef] [PubMed]
- van Furth, A.M.; van den Broek, M.; Emous, M.; de Heide, L.J.M.; Kuipers, F.; van Beek, A.P. Cholecystectomy increases the risk of dumping syndrome and postbariatric hypoglycemia after bariatric surgery. Surg. Obes. Relat. Dis. 2020, 16, 1939–1947. [Google Scholar] [CrossRef]
- Aarts, E.O.; Mahawar, K. From the Knife to the Endoscope—A History of Bariatric Surgery. Curr. Obes. Rep. 2020, 9, 348–363. [Google Scholar] [CrossRef]
- Eisenberg, D.; Shikora, S.A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.V.; de Luca, M.; Faria, S.L.; Goodpaster, K.P.S.; Haddad, A.; et al. 2022 American Society of Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) Indications for Metabolic and Bariatric Surgery. Obes. Surg. 2023, 33, 3–14. [Google Scholar] [CrossRef]
- Bettini, S.; Belligoli, A.; Fabris, R.; Busetto, L. Diet approach before and after bariatric surgery. Rev. Endocr. Metab. Disord. 2020, 21, 297–306. [Google Scholar] [CrossRef]
- Van de Velde, F.; Lapauw, B. Late dumping syndrome or postprandial reactive hypoglycaemic syndrome after bariatric surgery. Nat. Rev. Endocrinol. 2021, 17, 317–318. [Google Scholar] [CrossRef]
- Perdomo, C.M.; Cohen, R.V.; Sumithran, P.; Clément, K.; Frühbeck, G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet 2023, 401, 1116–1130. [Google Scholar] [CrossRef]
- Buchwald, H.; Buchwald, J.N. Metabolic (Bariatric and Nonbariatric) Surgery for Type 2 Diabetes: A Personal Perspective Review. Diabetes Care 2019, 42, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chiang, J.; Ghanem, O.; Ferzli, G. Decision-making Considerations in Revisional Bariatric Surgery. Surg. Laparosc. Endosc. Percutan. Tech. 2024, 34, 400–406. [Google Scholar] [CrossRef]
- Tsenteradze, T.; Fayyaz, F.; Ekhator, C.; Ahmed, I.; Oliveira Souza Lima, S.R.; Daher, O.A.; Bakht, D.; Arif, H.; Bellegarde, S.B.; Anika, N.N.; et al. Navigating Bariatric Surgery: Understanding and Managing Short-Term and Long-Term Complications. Cureus 2023, 15, e48580. [Google Scholar] [CrossRef]
- Souza, N.M.M.; Santos, A.C.O.; Santa-Cruz, F.; Guimarães, H.; Silva, L.M.L.; de-Lima, D.S.C.; Ferraz, Á.A.B.; Kreimer, F. Nutritional impact of bariatric surgery: A comparative study of Roux-en-Y Gastric Bypass and Sleeve gastrectomy between patients from the public and private health systems. Rev. Col. Bras. Cir. 2020, 47, e20202404. [Google Scholar] [CrossRef]
- Vargas, E.J.; Abu Dayyeh, B.K.; Storm, A.C.; Bazerbachi, F.; Matar, R.; Vella, A.; Kellogg, T.; Stier, C. Endoscopic management of dumping syndrome after Roux-en-Y gastric bypass: A large international series and proposed management strategy. Gastrointest. Endosc. 2020, 92, 91–96. [Google Scholar] [CrossRef]
- Andrade, L.; Chiote, I.; Santos-Cruz, A.; Brito-Costa, A.; Mendes, L.; Silva-Nunes, J.; Pereira, J. Protein Intake, Adherence to Vitamin-Mineral Supplementation, and Dumping Syndrome in Patients Undergoing One Anastomosis Gastric Bypass. Obes. Surg. 2021, 31, 3557–3564. [Google Scholar] [CrossRef] [PubMed]
- Binnetoğlu, K. Nutrition and Patient Follow-Up in Bariatric Surgery. Eurasian J. Med. 2023, 55, S70–S74. [Google Scholar] [CrossRef] [PubMed]
- Watson, L.E.; Xie, C.; Wang, X.; Li, Z.; Phillips, L.K.; Sun, Z.; Jones, K.L.; Horowitz, M.; Rayner, C.K.; Wu, T. Gastric Emptying in Patients with Well-Controlled Type 2 Diabetes Compared with Young and Older Control Subjects Without Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 3311–3319. [Google Scholar] [CrossRef]
- Xie, C.; Huang, W.; Wang, X.; Trahair, L.G.; Pham, H.T.; Marathe, C.S.; Young, R.L.; Jones, K.L.; Horowitz, M.; Rayner, C.K.; et al. Gastric emptying in health and type 2 diabetes: An evaluation using a 75 g oral glucose drink. Diabetes Res. Clin. Pract. 2021, 171, 108610. [Google Scholar] [CrossRef] [PubMed]
- Perelló, M.; Dickson, S.L.; Zigman, J.M.; Leggio, L. Ghrelin Nomenclature Consensus Group Toward a consensus nomenclature for ghrelin, its non-acylated form, liver expressed antimicrobial peptide 2 and growth hormone secretagogue receptor. J. Neuroendocrinol. 2023, 35, e13224. [Google Scholar] [CrossRef]
- Dons-Jensen, A.; Horup, S.S.; Hvas, A.-M.; Vestergaard, E.T.; Johansen, R.F. Ghrelin, growth hormone and insulin-like growth Factor-I levels in people with protein C deficiency. Scand. J. Clin. Lab. Investig. 2022, 82, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Lawal, A.; Barboi, A.; Krasnow, A.; Hellman, R.; Jaradeh, S.; Massey, B.T. Rapid gastric emptying is more common than gastroparesis in patients with autonomic dysfunction. Am. J. Gastroenterol. 2007, 102, 618–623. [Google Scholar] [CrossRef]
- Dong, W.-Y.; Zhu, X.; Tang, H.-D.; Huang, J.-Y.; Zhu, M.-Y.; Cheng, P.-K.; Wang, H.; Wang, X.-Y.; Wang, H.; Mao, Y.; et al. Brain regulation of gastric dysfunction induced by stress. Nat. Metab. 2023, 5, 1494–1505. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Manduca, A.; Lake, D.S.; Fidler, J.; Edwards, P.; Grimm, R.C.; Zinsmeister, A.R.; Riederer, S.J. Gastric motor disturbances in patients with idiopathic rapid gastric emptying. Neurogastroenterol. Motil. 2011, 23, 617-e252. [Google Scholar] [CrossRef]
- Kuwelker, S.; Muthyala, A.; O’Connor, M.; Bharucha, A.E. Clinical features and disturbances of gastrointestinal transit in patients with rapid gastric emptying. Neurogastroenterol. Motil. 2020, 32, e13779. [Google Scholar] [CrossRef]
- Febo-Rodriguez, L.; Chumpitazi, B.P.; Sher, A.C.; Shulman, R.J. Gastric accommodation: Physiology, diagnostic modalities, clinical relevance, and therapies. Neurogastroenterol. Motil. 2021, 33, e14213. [Google Scholar] [CrossRef]
- Jalleh, R.J.; Plummer, M.P.; Marathe, C.S.; Umapathysivam, M.M.; Quast, D.R.; Rayner, C.K.; Jones, K.L.; Wu, T.; Horowitz, M.; Nauck, M.A. Clinical Consequences of Delayed Gastric Emptying With GLP-1 Receptor Agonists and Tirzepatide. J. Clin. Endocrinol. Metab. 2024, 110, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.K.; Guo, Y.; Mashimo, H. Advances in the physiology of gastric emptying. Neurogastroenterol. Motil. 2019, 31, e13546. [Google Scholar] [CrossRef]
- Richardson, J.; Dezfuli, G.; Mangel, A.W.; Gillis, R.A.; Vicini, S.; Sahibzada, N. CNS sites controlling the gastric pyloric sphincter: Neuroanatomical and functional study in the rat. J. Comp. Neurol. 2023, 531, 1562–1581. [Google Scholar] [CrossRef]
- Mussa, B.M.; Sood, S.; Verberne, A.J. Implication of neurohormonal-coupled mechanisms of gastric emptying and pancreatic secretory function in diabetic gastroparesis. World J. Gastroenterol. 2018, 24, 3821–3833. [Google Scholar] [CrossRef] [PubMed]
- Veldman, F.; Hawinkels, K.; Keszthelyi, D. Efficacy of vagus nerve stimulation in gastrointestinal disorders: A systematic review. Gastroenterol. Rep. 2025, 13, goaf009. [Google Scholar] [CrossRef] [PubMed]
- Ly, T.; Oh, J.Y.; Sivakumar, N.; Shehata, S.; La Santa Medina, N.; Huang, H.; Liu, Z.; Fang, W.; Barnes, C.; Dundar, N.; et al. Sequential appetite suppression by oral and visceral feedback to the brainstem. Nature 2023, 624, 130–137. [Google Scholar] [CrossRef]
- Mori, H.; Verbeure, W.; Schol, J.; Carbone, F.; Tack, J. Gastrointestinal hormones and regulation of gastric emptying. Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Gastrointestinal Hormones and Regulation of Gastric Emptying. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 3–10. [Google Scholar] [CrossRef]
- Barakat, G.M.; Ramadan, W.; Assi, G.; Khoury, N.B.E. Satiety: A gut–brain–relationship. J. Physiol. Sci. 2024, 74, 11. [Google Scholar] [CrossRef]
- Dafalla, A.I.; Mhalhal, T.R.; Hiscocks, K.; Heath, J.; Sayegh, A.I. The Vagus Nerve and the Celiaco-mesenteric Ganglia Participate in the Feeding Responses Evoked by Non-sulfated Cholecystokinin-8 in Male Sprague Dawley Rats. Endocr. Res. 2020, 45, 73–83. [Google Scholar] [CrossRef]
- Assan, D.; Mustapha, U.F.; Chen, H.; Li, Z.; Peng, Y.; Li, G. The Roles of Neuropeptide Y (Npy) and Peptide YY (Pyy) in Teleost Food Intake: A Mini Review. Life 2021, 11, 547. [Google Scholar] [CrossRef]
- Graybeal, A.J.; Willis, J.L.; Morales-Marroquin, E.; Tinsley, G.M.; Messiah, S.E.; Shah, M. Emerging evidence of the relationship between fat-free mass and ghrelin, glucagon-like peptide-1, and peptide-YY. Nutrition 2022, 103, 111815. [Google Scholar] [CrossRef]
- Yang, C.-H.; Onda, D.-A.; Oakhill, J.S.; Scott, J.W.; Galic, S.; Loh, K. Regulation of Pancreatic β-Cell Function by the NPY System. Endocrinology 2021, 162, bqab070. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Akiba, Y.; Kaunitz, J.D. Recent advances in vasoactive intestinal peptide physiology and pathophysiology: Focus on the gastrointestinal system. F1000Research 2019, 8, 1629. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, S.; Qiu, S.; Fiorentino, F.; Simillis, C.; Rasheed, S.; Tekkis, P.; Kontovounisios, C. The role of Neurotensin and its receptors in non-gastrointestinal cancers: A review. Cell Commun. Signal. CCS 2020, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Al-Missri, M.Z.; Jialal, I. Physiology, Motilin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Brierley, D.I.; de Lartigue, G. Reappraising the role of the vagus nerve in GLP-1-mediated regulation of eating. Br. J. Pharmacol. 2022, 179, 584–599. [Google Scholar] [CrossRef]
- Papamargaritis, D.; le Roux, C.W. Do Gut Hormones Contribute to Weight Loss and Glycaemic Outcomes after Bariatric Surgery? Nutrients 2021, 13, 762. [Google Scholar] [CrossRef]
- Dimitriadis, G.K.; Randeva, M.S.; Miras, A.D. Potential Hormone Mechanisms of Bariatric Surgery. Curr. Obes. Rep. 2017, 6, 253–265. [Google Scholar] [CrossRef]
- Delgado-Aros, S.; Kim, D.-Y.; Burton, D.D.; Thomforde, G.M.; Stephens, D.; Brinkmann, B.H.; Vella, A.; Camilleri, M. Effect of GLP-1 on gastric volume, emptying, maximum volume ingested, and postprandial symptoms in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G424–G431. [Google Scholar] [CrossRef]
- Maselli, D.B.; Camilleri, M. Effects of GLP-1 and Its Analogs on Gastric Physiology in Diabetes Mellitus and Obesity. Adv. Exp. Med. Biol. 2021, 1307, 171–192. [Google Scholar] [CrossRef]
- Halim, M.A.; Degerblad, M.; Sundbom, M.; Karlbom, U.; Holst, J.J.; Webb, D.-L.; Hellström, P.M. Glucagon-Like Peptide-1 Inhibits Prandial Gastrointestinal Motility Through Myenteric Neuronal Mechanisms in Humans. J. Clin. Endocrinol. Metab. 2018, 103, 575–585. [Google Scholar] [CrossRef]
- Quercia, I.; Dutia, R.; Kotler, D.P.; Belsley, S.; Laferrère, B. Gastrointestinal changes after bariatric surgery. Diabetes Metab. 2014, 40, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.P.R.; Gama, L.A.; Beckmann, A.P.S.; Pinto, L.A.; de Miranda, J.R.A.; Marques, R.G.; Américo, M.F. Gastric plication surgery changes gastrointestinal and metabolic parameters in an obesity-induced high-fat diet model. Neurogastroenterol. Motil. 2024, 36, e14717. [Google Scholar] [CrossRef]
- van Furth, A.M.; de Heide, L.J.M.; Emous, M.; Veeger, N.; van Beek, A.P. Dumping Syndrome and Postbariatric Hypoglycemia: Supporting Evidence for a Common Etiology. Surg. Obes. Relat. Dis. 2021, 17, 1912–1918. [Google Scholar] [CrossRef]
- Fanni, G.; Katsogiannos, P.; Nandi Jui, B.; Sundbom, M.; Hetty, S.; Pereira, M.J.; Eriksson, J.W. Response of multiple hormones to glucose and arginine challenge in T2DM after gastric bypass. Endocr. Connect. 2022, 11, e220172. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, Y.; Shen, Q.; Zhang, W.; Yao, Q.; Yang, Y. Prevalence and risk factors for symptoms suggestive of hypoglycemia and early dumping syndrome after sleeve gastrectomy. Surg. Obes. Relat. Dis. 2019, 15, 1439–1446. [Google Scholar] [CrossRef]
- Ceppa, E.P.; Ceppa, D.P.; Omotosho, P.A.; Dickerson, J.A.; Park, C.W.; Portenier, D.D. Algorithm to diagnose etiology of hypoglycemia after Roux-en-Y gastric bypass for morbid obesity: Case series and review of the literature. Surg. Obes. Relat. Dis. 2012, 8, 641–647. [Google Scholar] [CrossRef]
- Tsai, C.; Steffen, R.; Kessler, U.; Merki, H.; Zehetner, J. Short-term outcomes of endoscopic gastro-jejunal revisions for treatment of dumping syndrome after Roux-En-Y gastric bypass. Surg. Endosc. 2020, 34, 3626–3632. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Kornrich, D.B.; Krasner, H.; Eckardt, S.; Ahmad, Z.; Braslow, A.; Broggelwirth, B. Prevalence of Dumping Syndrome After Laparoscopic Sleeve Gastrectomy and Comparison with Laparoscopic Roux-en-Y Gastric Bypass. Obes. Surg. 2019, 29, 1506–1513. [Google Scholar] [CrossRef]
- Papamargaritis, D.; Koukoulis, G.; Sioka, E.; Zachari, E.; Bargiota, A.; Zacharoulis, D.; Tzovaras, G. Dumping symptoms and incidence of hypoglycaemia after provocation test at 6 and 12 months after laparoscopic sleeve gastrectomy. Obes. Surg. 2012, 22, 1600–1606. [Google Scholar] [CrossRef]
- Yamauchi, S.; Orita, H.; Chen, J.; Egawa, H.; Yoshimoto, Y.; Kubota, A.; Matsui, R.; Yube, Y.; Kaji, S.; Oka, S.; et al. Long-term outcomes of postgastrectomy syndrome after total laparoscopic distal gastrectomy using the augmented rectangle technique. World J. Gastrointest. Surg. 2022, 14, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xue, Y.; Zheng, Z.; Liu, X.; Xin, C.; Huang, Y.; Yin, J.; Zhang, J. Safety and efficacy of augmented-rectangle technique versus delta-shaped anastomosis for treating gastric cancer in total laparoscopic distal gastrectomy. Langenbeck’s Arch. Surg. 2023, 408, 260. [Google Scholar] [CrossRef]
- Samigullin, A.; Weihrauch, J.; Otto, M.; Rech, A.; Buchenberger, S.; Morcos, M.; Humpert, P.M. Postprandial Symptoms in a Mixed-Meal-Test after Bariatric Surgery: Clinical Experience and a Critical Review of Dumping Syndrome Definition and Management. Obes. Facts 2025, 18, 31–38. [Google Scholar] [CrossRef]
- van Beek, A.P.; Emous, M.; Laville, M.; Tack, J. Dumping syndrome after esophageal, gastric or bariatric surgery: Pathophysiology, diagnosis, and management. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2017, 18, 68–85. [Google Scholar] [CrossRef]
- Nofal, M.; Yousef, A.; Alkhawaldeh, I.; Al-Jafari, M.; Zuaiter, S.; Zein Eddin, S. Dumping Syndrome after Bariatric Surgery. Ann. Ital. Chir. 2024, 95, 522–533. [Google Scholar] [CrossRef]
- Laurenius, A.; Werling, M.; le Roux, C.W.; Fändriks, L.; Olbers, T. Dumping symptoms is triggered by fat as well as carbohydrates in patients operated with Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2017, 13, 1159–1164. [Google Scholar] [CrossRef]
- Suhl, E.; Anderson-Haynes, S.-E.; Mulla, C.; Patti, M.-E. Medical nutrition therapy for post-bariatric hypoglycemia: Practical insights. Surg. Obes. Relat. Dis. 2017, 13, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Sanaka, M.; Yamamoto, T.; Anjiki, H.; Nagasawa, K.; Kuyama, Y. Effects of agar and pectin on gastric emptying and post-prandial glycaemic profiles in healthy human volunteers. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1151–1155. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Migdanis, A.; Koukoulis, G.D.; Chougias, D.; Migdanis, I.; Armeni, E.; Kanellakis, S.; Manouras, A.; Kapsoritakis, A.; Potamianos, S. The Effect of Fat Supplementation on the Appearance of Symptoms Associated with Dumping Syndrome in Patients Having Undergone Gastric Surgery: Preliminary Results. Cureus 2025, 15, e48871. [Google Scholar] [CrossRef]
- Stuart, P.S.; Hicks, D.C. Nutritional Management for Patients with Nausea and Vomiting and Gastroparesis or Dumping Syndrome. In Nausea and Vomiting: Diagnosis and Treatment; Koch, K.L., Hasler, W.L., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 153–164. ISBN 978-3-319-34076-0. [Google Scholar]
- Chesser, H.; Abdulhussein, F.; Huang, A.; Lee, J.Y.; Gitelman, S.E. Continuous Glucose Monitoring to Diagnose Hypoglycemia Due to Late Dumping Syndrome in Children After Gastric Surgeries. J. Endocr. Soc. 2021, 5, bvaa197. [Google Scholar] [CrossRef] [PubMed]
- Hamamah, S.; Hajnal, A.; Covasa, M. Influence of Bariatric Surgery on Gut Microbiota Composition and Its Implication on Brain and Peripheral Targets. Nutrients 2024, 16, 1071. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Remely, M.; Hippe, B.; Geretschlaeger, I.; Stegmayer, S.; Hoefinger, I.; Haslberger, A. Increased gut microbiota diversity and abundance of Faecalibacterium prausnitzii and Akkermansia after fasting: A pilot study. Wien. Klin. Wochenschr. 2015, 127, 394–398. [Google Scholar] [CrossRef]
- Le Barz, M.; Anhê, F.F.; Varin, T.V.; Desjardins, Y.; Levy, E.; Roy, D.; Urdaci, M.C.; Marette, A. Probiotics as Complementary Treatment for Metabolic Disorders. Diabetes Metab. J. 2015, 39, 291–303. [Google Scholar] [CrossRef]
- Tan, N.; Gwee, K.A.; Tack, J.; Zhang, M.; Li, Y.; Chen, M.; Xiao, Y. Herbal medicine in the treatment of functional gastrointestinal disorders: A systematic review with meta-analysis. J. Gastroenterol. Hepatol. 2020, 35, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R.; Shepherd, S.J. Personal view: Food for thought—Western lifestyle and susceptibility to Crohn’s disease. The FODMAP hypothesis. Aliment. Pharmacol. Ther. 2005, 21, 1399–1409. [Google Scholar] [CrossRef]
- Sikaroudi, M.K.; Soltani, S.; Ghoreishy, S.M.; Ebrahimi, Z.; Shidfar, F.; Dehnad, A. Effects of a low FODMAP diet on the symptom management of patients with irritable bowel syndrome: A systematic umbrella review with the meta-analysis of clinical trials. Food Funct. 2024, 15, 5195–5208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Su, Q. Low-FODMAP Diet for Irritable Bowel Syndrome: Insights from Microbiome. Nutrients 2025, 17, 544. [Google Scholar] [CrossRef]
- Dumping Syndrome Treatment & Management: Medical Care, Surgical Care, Diet. 2023. Available online: https://emedicine.medscape.com/article/173594-treatment (accessed on 22 May 2025).
- Poljo, A.; Pentsch, A.; Raab, S.; Klugsberger, B.; Shamiyeh, A. Incidence of Dumping Syndrome after Sleeve Gastrectomy, Roux-en-Y Gastric Bypass and One-Anastomosis Gastric Bypass. J. Metab. Bariatr. Surg. 2021, 10, 23–31. [Google Scholar] [CrossRef]
- Chaves, Y.S.; Destefani, A.C. Pathophysiology, diagnosis and treatmentof dumping syndrome and its relation to bariatric surgery. ABCD Arq. Bras. Cir. Dig. 2016, 29 (Suppl S1), 116–119. [Google Scholar] [CrossRef] [PubMed]
- Ukleja, A. Dumping syndrome: Pathophysiology and treatment. Nutr. Clin. Pract. 2005, 20, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Carpentieri, G.B.; Gonçalves, S.E.A.B.; Mourad, W.M.; Pinto, L.G.C.; Zanella, M.T. Hypoglycemia post bariatric surgery: Drugs with different mechanisms of action to treat a unique disorder. Arch. Endocrinol. Metab. 2023, 67, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Cadegiani, F.A.; Silva, O.S. Acarbose promotes remission of both early and late dumping syndromes in post-bariatric patients. Diabetes Metab. Syndr. Obes. Targets Ther. 2016, 9, 443–446. [Google Scholar] [CrossRef]
- Ritz, P.; Vaurs, C.; Bertrand, M.; Anduze, Y.; Guillaume, E.; Hanaire, H. Usefulness of acarbose and dietary modifications to limit glycemic variability following Roux-en-Y gastric bypass as assessed by continuous glucose monitoring. Diabetes Technol. Ther. 2012, 14, 736–740. [Google Scholar] [CrossRef]
- Gilliaux, Q.; Bertrand, C.; Hanon, F.; Donckier, J.E. Preoperative treatment of benign insulinoma: Diazoxide or somatostatin analogues? Acta Chir. Belg. 2022, 122, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Mesmar, B.; Kristan, M.; Satyarengga, M.; Lamos, E.M.; Malek, R.; Munir, K.M. The use of diazoxide in the management of spontaneous hypoglycemia in patients with ESRD. CEN Case Rep. 2020, 9, 271–277. [Google Scholar] [CrossRef]
- Thondam, S.K.; Nair, S.; Wile, D.; Gill, G.V. Diazoxide for the treatment of hypoglycaemic dumping syndrome. QJM Mon. J. Assoc. Physicians 2013, 106, 855–858. [Google Scholar] [CrossRef]
- Mejia-Otero, J.D.; Grishman, E.K.; Patni, N. Diazoxide for the Treatment of Hypoglycemia Resulting from Dumping Syndrome in a Child. J. Endocr. Soc. 2019, 3, 1357–1360. [Google Scholar] [CrossRef]
- Sato, D.; Morino, K.; Ohashi, N.; Ueda, E.; Ikeda, K.; Yamamoto, H.; Ugi, S.; Yamamoto, H.; Araki, S.; Maegawa, H. Octreotide improves early dumping syndrome potentially through incretins: A case report. Endocr. J. 2013, 60, 847–853. [Google Scholar] [CrossRef]
- Mohammadi, A.; Sulaiman, R.A.; Grossman, A.B. Pasireotide and octreotide in the treatment of severe late dumping syndrome. Clin. Case Rep. 2017, 5, 1608–1611. [Google Scholar] [CrossRef]
- Tack, J.; Aberle, J.; Arts, J.; Laville, M.; Oppert, J.-M.; Bender, G.; Bhoyrul, S.; McLaughlin, T.; Yoshikawa, T.; Vella, A.; et al. Safety and efficacy of pasireotide in dumping syndrome-results from a phase 2, multicentre study. Aliment. Pharmacol. Ther. 2018, 47, 1661–1672. [Google Scholar] [CrossRef]
- Guo, X.; Tang, R.; Yang, S.; Lu, Y.; Luo, J.; Liu, Z. Rutin and Its Combination with Inulin Attenuate Gut Dysbiosis, the Inflammatory Status and Endoplasmic Reticulum Stress in Paneth Cells of Obese Mice Induced by High-Fat Diet. Front. Microbiol. 2018, 9, 2651. [Google Scholar] [CrossRef]
- Rubino, D.M.; Greenway, F.L.; Khalid, U.; O’Neil, P.M.; Rosenstock, J.; Sørrig, R.; Wadden, T.A.; Wizert, A.; Garvey, W.T. Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults with Overweight or Obesity Without Diabetes. JAMA 2022, 327, 138–150. [Google Scholar] [CrossRef]
- Chiappetta, S.; Stier, C. A case report: Liraglutide as a novel treatment option in late dumping syndrome. Medicine 2017, 96, e6348. [Google Scholar] [CrossRef]
- Shaghouli, A.A.; Ballani, R.; Mesbah, N. Management of Late Dumping Syndrome Induced Hypoglycemia With GLP-1R Agonist. J. Endocr. Soc. 2021, 5, A416. [Google Scholar] [CrossRef]
- Moreira, R.O.; Moreira, R.B.M.; Machado, N.A.M.; Gonçalves, T.B.; Coutinho, W.F. Post-prandial hypoglycemia after bariatric surgery: Pharmacological treatment with verapamil and acarbose. Obes. Surg. 2008, 18, 1618–1621. [Google Scholar] [CrossRef] [PubMed]
- Øhrstrøm, C.C.; Worm, D.; Højager, A.; Andersen, D.; Holst, J.J.; Kielgast, U.L.; Hansen, D.L. Postprandial hypoglycaemia after Roux-en-Y gastric bypass and the effects of acarbose, sitagliptin, verapamil, liraglutide and pasireotide. Diabetes Obes. Metab. 2019, 21, 2142–2151. [Google Scholar] [CrossRef]
- Al-Bader, I.; Khoursheed, M.; Al Sharaf, K.; Mouzannar, D.A.; Ashraf, A.; Fingerhut, A. Revisional Laparoscopic Gastric Pouch Resizing for Inadequate Weight Loss After Roux-en-Y Gastric Bypass. Obes. Surg. 2015, 25, 1103–1108. [Google Scholar] [CrossRef]
- Shah, K.; Gislason, H. Roux-en-Y Gastric Bypass Reversal: A Novel Technique With Functional Reversal—Case Series. Obes. Surg. 2020, 30, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Pucher, P.H.; Lord, A.C.; Sodergren, M.H.; Ahmed, A.R.; Darzi, A.; Purkayastha, S. Reversal to normal anatomy after failed gastric bypass: Systematic review of indications, techniques, and outcomes. Surg. Obes. Relat. Dis. 2016, 12, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Clancy, T.E.; Moore Jr, F.D.; Zinner, M.J. Post-gastric bypass hyperinsulinism with nesidioblastosis: Subtotal or total pancreatectomy may be needed to prevent recurrent hypoglycemia. J. Gastrointest. Surg. 2006, 8, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Mala, T. Postprandial hyperinsulinemic hypoglycemia after gastric bypass surgical treatment. Surg. Obes. Relat. Dis. 2014, 10, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Ritz, P.; Vaurs, C.; Barigou, M.; Hanaire, H. Hypoglycaemia after gastric bypass: Mechanisms and treatment. Diabetes Obes. Metab. 2016, 18, 217–223. [Google Scholar] [CrossRef]
- Gribsholt, S.B.; Pedersen, A.M.; Svensson, E.; Thomsen, R.W.; Richelsen, B. Prevalence of Self-reported Symptoms After Gastric Bypass Surgery for Obesity. JAMA Surg. 2016, 151, 504–511. [Google Scholar] [CrossRef]
- Laurenius, A.; Hedberg, S.; Olbers, T. Possible relation between partial small bowel obstruction and severe postprandial reactive hypoglycemia after Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2019, 15, 1024–1028. [Google Scholar] [CrossRef]
- Baud, G.; Daoudi, M.; Hubert, T.; Raverdy, V.; Pigeyre, M.; Hervieux, E.; Devienne, M.; Ghunaim, M.; Bonner, C.; Quenon, A.; et al. Bile Diversion in Roux-en-Y Gastric Bypass Modulates Sodium-Dependent Glucose Intestinal Uptake. Cell Metab. 2016, 23, 547–553. [Google Scholar] [CrossRef]
- Ahmad, A.F.; Caparrós-Martín, J.A.; Lee, S.; O’Gara, F.; Yeap, B.B.; Green, D.J.; Ballal, M.; Ward, N.C.; Dwivedi, G. Gut Microbiome and Associated Metabolites Following Bariatric Surgery and Comparison to Healthy Controls. Microorganisms 2023, 11, 1126. [Google Scholar] [CrossRef]
- Juárez-Fernández, M.; Román-Sagüillo, S.; Porras, D.; García-Mediavilla, M.V.; Linares, P.; Ballesteros-Pomar, M.D.; Urioste-Fondo, A.; Álvarez-Cuenllas, B.; González-Gallego, J.; Sánchez-Campos, S.; et al. Long-Term Effects of Bariatric Surgery on Gut Microbiota Composition and Faecal Metabolome Related to Obesity Remission. Nutrients 2021, 13, 2519. [Google Scholar] [CrossRef]
- Kim, Y.; Son, D.; Kim, B.K.; Kim, K.H.; Seo, K.W.; Jung, K.; Park, S.J.; Lim, S.; Kim, J.H. Association between the Blautia/Bacteroides Ratio and Altered Body Mass Index after Bariatric Surgery. Endocrinol. Metab. 2022, 37, 701–702. [Google Scholar] [CrossRef] [PubMed]
- Martin, O.A.; Grant-Beurmann, S.; Orellana, E.R.; Hajnal, A.; Fraser, C.M. Changes in the Gut Microbiota Following Bariatric Surgery Are Associated with Increased Alcohol Intake in a Female Rat Model. Alcohol Alcohol. 2021, 56, 605–613. [Google Scholar] [CrossRef]
- Coimbra, V.O.R.; Crovesy, L.; Ribeiro-Alves, M.; Faller, A.L.K.; Mattos, F.; Rosado, E.L. Gut Microbiota Profile in Adults Undergoing Bariatric Surgery: A Systematic Review. Nutrients 2022, 14, 4979. [Google Scholar] [CrossRef] [PubMed]
- Steffen, K.J.; Sorgen, A.A.; Fodor, A.A.; Carroll, I.M.; Crosby, R.D.; Mitchell, J.E.; Bond, D.S.; Heinberg, L.J. Early changes in the gut microbiota are associated with weight outcomes over 2 years following metabolic and bariatric surgery. Obesity 2024, 32, 1985–1997. [Google Scholar] [CrossRef]
- Wagner, N.R.F.; Zaparolli, M.R.; Cruz, M.R.R.; Schieferdecker, M.E.M.; Campos, A.C.L. Postoperative changes in intestinal microbiota and use of probiotics in roux-en-y gastric bypass and sleeve vertical gastrectomy: An integrative review. ABCD Arq. Bras. Cir. Dig. 2018, 31, e1400. [Google Scholar] [CrossRef]
- Furet, J.-P.; Kong, L.-C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.-L.; Mariat, D.; Corthier, G.; Doré, J.; Henegar, C.; et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef]
- Custers, E.; van der Burgh, Y.G.R.; Vreeken, D.; Schuren, F.; van den Broek, T.J.; Verschuren, L.; de Blaauw, I.; Bouwens, M.; Kleemann, R.; Kiliaan, A.J.; et al. Gastrointestinal complaints after Roux-en-Y gastric bypass surgery. Impact of microbiota and its metabolites. Heliyon 2024, 10, e39899. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, A.K.; Paz-Cruz, E.; Ruiz-Pozo, V.A.; Cadena-Ullauri, S.; Tamayo-Trujillo, R.; Guevara-Ramírez, P.; Zambrano-Villacres, R.; Simancas-Racines, D. Microbiota dynamics preceding bariatric surgery as obesity treatment: A comprehensive review. Front. Nutr. 2024, 11, 1393182. [Google Scholar] [CrossRef] [PubMed]
- ULKER, İ.; YILDIRAN, H. The effects of bariatric surgery on gut microbiota in patients with obesity: A review of the literature. Biosci. Microbiota Food Health 2019, 38, 3–9. [Google Scholar] [CrossRef]
- Long-Term Effects of Bariatric Surgery on Gut Microbiota. Available online: https://encyclopedia.pub/entry/15831 (accessed on 23 May 2025).
- Almheiri, R.T.; Hajjar, B.; Alkhaaldi, S.M.I.; Rabeh, N.; Aljoudi, S.; Abd-Elrahman, K.S.; Hamdan, H. Beyond weight loss: Exploring the neurological ramifications of altered gut microbiota post-bariatric surgery. J. Transl. Med. 2025, 23, 223. [Google Scholar] [CrossRef]
- Yi, C.; Huang, S.; Zhang, W.; Guo, L.; Xia, T.; Huang, F.; Yan, Y.; Li, H.; Yu, B. Synergistic interactions between gut microbiota and short chain fatty acids: Pioneering therapeutic frontiers in chronic disease management. Microb. Pathog. 2025, 199, 107231. [Google Scholar] [CrossRef]
- Mabey, J.G.; Chaston, J.M.; Castro, D.G.; Adams, T.D.; Hunt, S.C.; Davidson, L.E. Gut microbiota differs a decade after bariatric surgery relative to a nonsurgical comparison group. Surg. Obes. Relat. Dis. 2020, 16, 1304–1311. [Google Scholar] [CrossRef]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.-Z.; et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 2018, 174, 1388–1405.e21. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756. [Google Scholar] [CrossRef]
- Mafe, A.N.; Edo, G.I.; Majeed, O.S.; Gaaz, T.S.; Akpoghelie, P.O.; Isoje, E.F.; Igbuku, U.A.; Owheruo, J.O.; Opiti, R.A.; Garba, Y.; et al. A review on probiotics and dietary bioactives: Insights on metabolic well-being, gut microbiota, and inflammatory responses. Food Chem. Adv. 2025, 6, 100919. [Google Scholar] [CrossRef]
- Wagner, N.R.F.; Ramos, M.R.Z.; de Oliveira Carlos, L.; da Cruz, M.R.R.; Taconeli, C.A.; Filho, A.J.B.; Nassif, L.S.; Schieferdecker, M.E.M.; Campos, A.C.L. Effects of Probiotics Supplementation on Gastrointestinal Symptoms and SIBO after Roux-en-Y Gastric Bypass: A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. Obes. Surg. 2021, 31, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Melali, H.; Abdolahi, A.; Sheikhbahaei, E.; Vakili, K.; Mahmoudieh, M.; Keleidari, B.; Shahabi, S. Impact of Probiotics on Gastrointestinal Function and Metabolic Status After Roux-en-Y Gastric Bypass: A Double-Blind, Randomized Trial. Obes. Surg. 2024, 34, 2033–2041. [Google Scholar] [CrossRef]
- Koulas, S.G.; Stefanou, C.K.; Stefanou, S.K.; Tepelenis, K.; Zikos, N.; Tepetes, K.; Kapsoritakis, A. Gut Microbiota in Patients with Morbid Obesity Before and After Bariatric Surgery: A Ten-Year Review Study (2009–2019). Obes. Surg. 2021, 31, 317–326. [Google Scholar] [CrossRef]
- Karimi Behnagh, A.; Eghbali, M.; Abdolmaleki, F.; Abbasi, M.; Mottaghi, A. Pre- and Post-surgical Prevalence of Thiamine Deficiency in Patients Undergoing Bariatric Surgery: A Systematic Review and Meta-Analysis. Obes. Surg. 2024, 34, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.; Königsrainer, A. Malnutrition as a Complication of Bariatric Surgery—A Clear and Present Danger? Visc. Med. 2019, 35, 305–311. [Google Scholar] [CrossRef]
- Suárez-Sánchez, F.; Pérez-Ruiz, E.; Ramírez-Silva, C.I.; Molina-Ayala, M.A.; Rivera-Gutiérrez, S.; León-Solís, L.; García-Morales, L.; Rodríguez-González, A.; Martínez-Ortiz, C.; Meneses-Tapia, L.A.; et al. A species of Coprococcus is related to BMI in patients who underwent malabsorptive bariatric surgery and its abundance is modified by magnesium and thiamin intake. arXiv 2025. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, L.; Qiu, Y.; Ge, W. High-dose thiamine supplementation ameliorates obesity induced by a high-fat and high-fructose diet in mice by reshaping gut microbiota. Front. Nutr. 2025, 12, 1532581. [Google Scholar] [CrossRef]
- Lakhani, S.V.; Shah, H.N.; Alexander, K.; Finelli, F.C.; Kirkpatrick, J.R.; Koch, T.R. Small intestinal bacterial overgrowth and thiamine deficiency after Roux-en-Y gastric bypass surgery in obese patients. Nutr. Res. 2008, 28, 293–298. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano, R.; Rodríguez, D.; Duran, P.; Cano, C.; Rojas-Gómez, D.; Rivera-Porras, D.; Barboza-González, P.; Fuentes-Barría, H.; Angarita, L.; Boscan, A.; et al. Dumping Syndrome After Bariatric Surgery: Advanced Nutritional Perspectives and Integrated Pharmacological Management. Nutrients 2025, 17, 3123. https://doi.org/10.3390/nu17193123
Cano R, Rodríguez D, Duran P, Cano C, Rojas-Gómez D, Rivera-Porras D, Barboza-González P, Fuentes-Barría H, Angarita L, Boscan A, et al. Dumping Syndrome After Bariatric Surgery: Advanced Nutritional Perspectives and Integrated Pharmacological Management. Nutrients. 2025; 17(19):3123. https://doi.org/10.3390/nu17193123
Chicago/Turabian StyleCano, Raquel, Daniel Rodríguez, Pablo Duran, Clímaco Cano, Diana Rojas-Gómez, Diego Rivera-Porras, Paola Barboza-González, Héctor Fuentes-Barría, Lissé Angarita, Arturo Boscan, and et al. 2025. "Dumping Syndrome After Bariatric Surgery: Advanced Nutritional Perspectives and Integrated Pharmacological Management" Nutrients 17, no. 19: 3123. https://doi.org/10.3390/nu17193123
APA StyleCano, R., Rodríguez, D., Duran, P., Cano, C., Rojas-Gómez, D., Rivera-Porras, D., Barboza-González, P., Fuentes-Barría, H., Angarita, L., Boscan, A., & Bermúdez, V. (2025). Dumping Syndrome After Bariatric Surgery: Advanced Nutritional Perspectives and Integrated Pharmacological Management. Nutrients, 17(19), 3123. https://doi.org/10.3390/nu17193123







