Combined Effects of Diet Quality Scores and Frailty on All-Cause Mortality and Life Expectancy in Middle-Aged and Older Adults
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Dietary Assessment
2.3. Frailty
2.4. Covariates
2.5. Ascertainment of Mortality and Life Expectancy
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
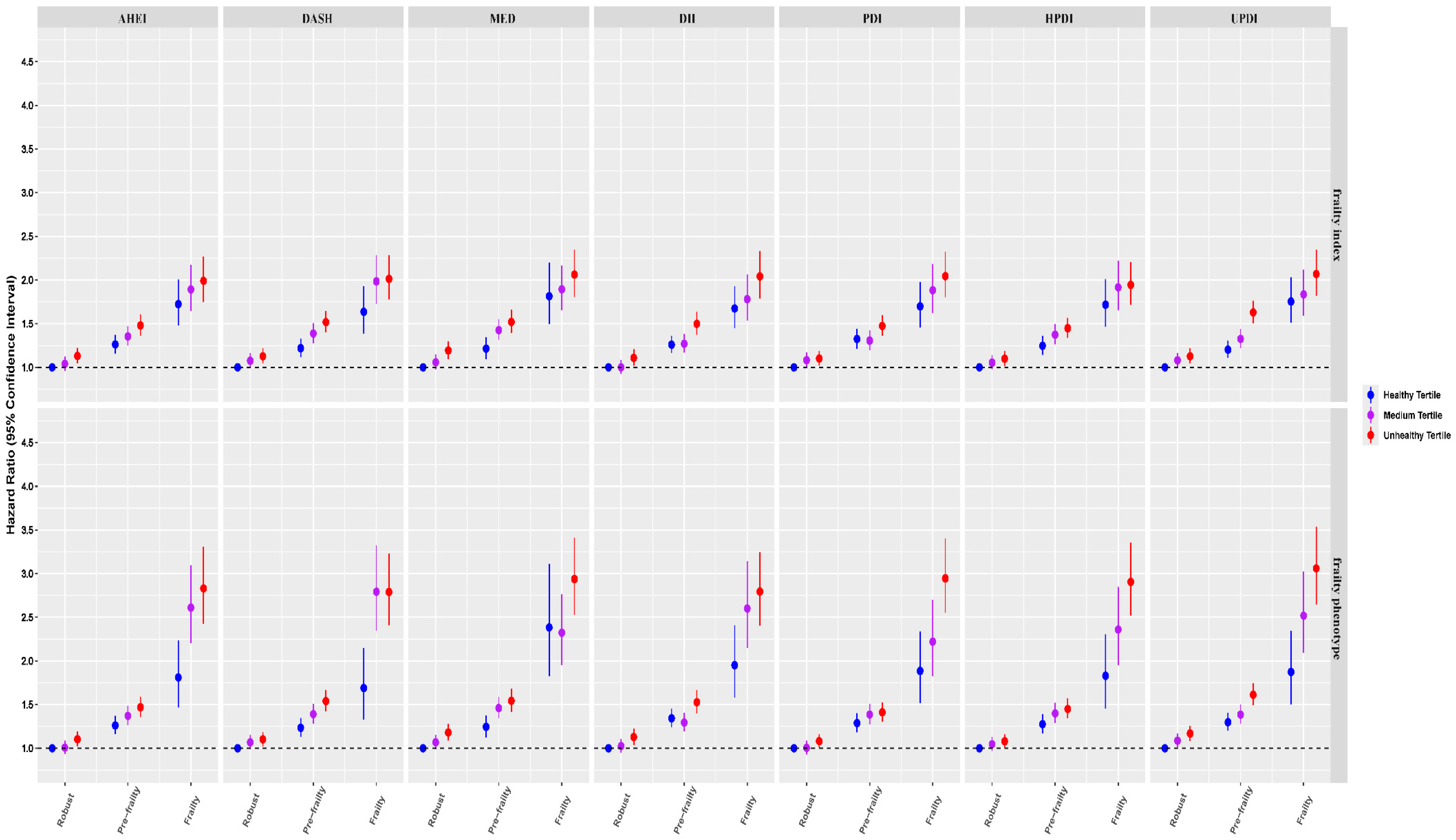
3.2. Joint Analysis Between Diet Quality Scores, Frailty, and All-Cause Mortality
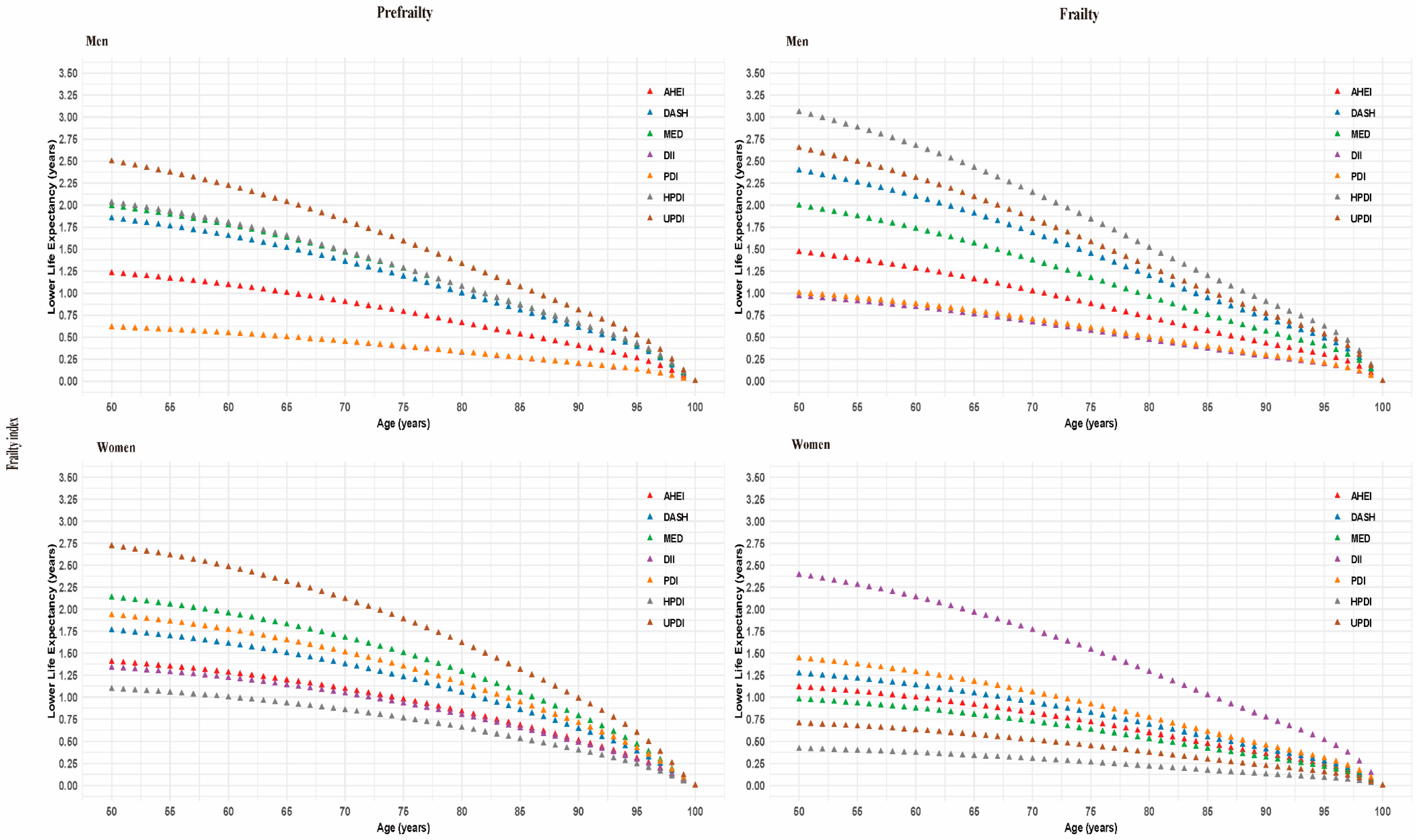
3.3. Life Expectancy
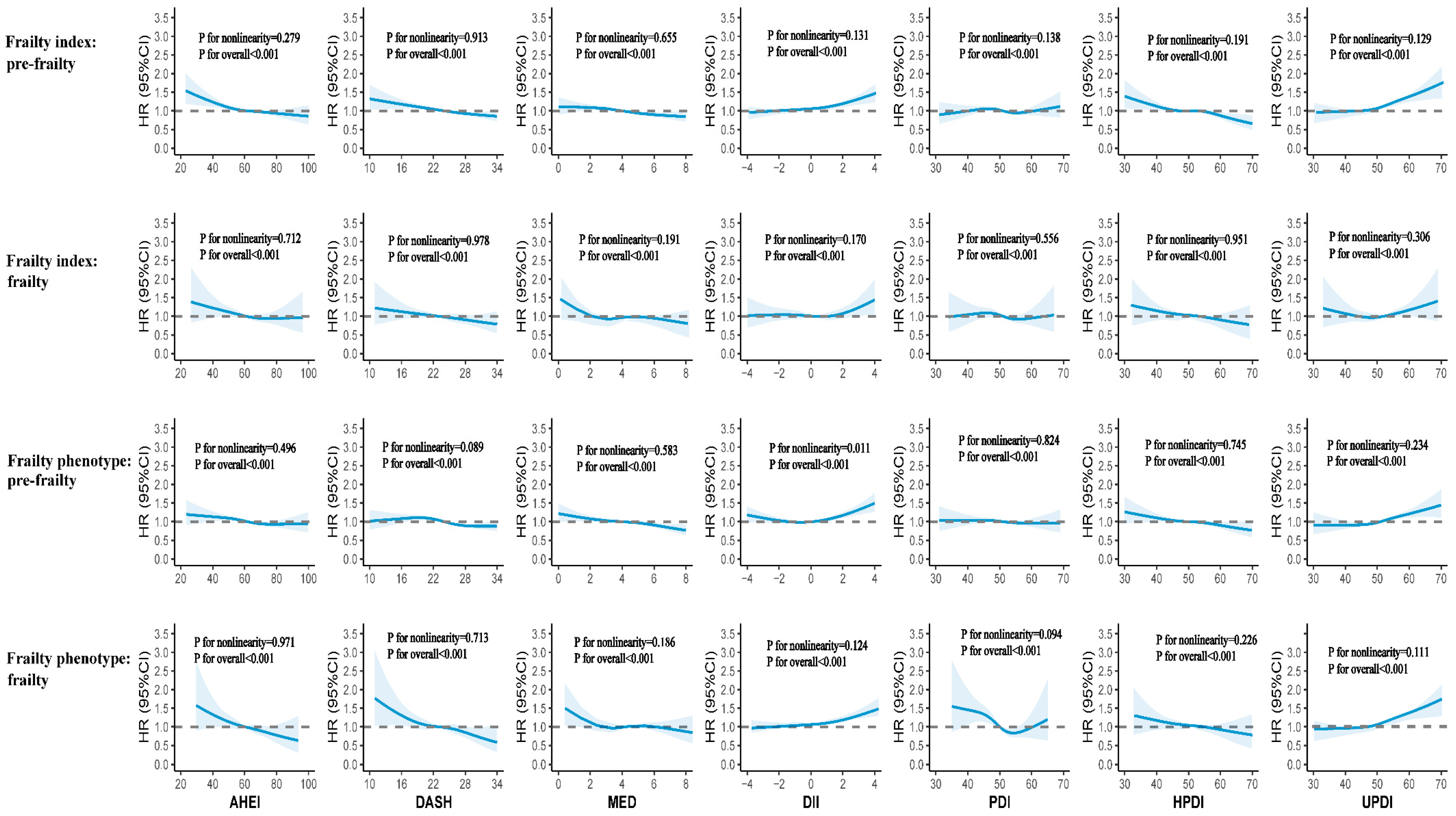
3.4. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Report on Ageing and Health 2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 2021, 50, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, S.; Vella-Azzopardi, R.; Beckwée, D.; Habbig, A.K.; Scafoglieri, A.; Jansen, B.; Bautmans, I.; Gerontopole Brussels Study Group. Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1163.e1–1163.e17. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G. Frailty as a Predictor of Future Falls Among Community-Dwelling Older People: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2015, 16, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Tom, S.E.; Adachi, J.D.; Anderson, F.A., Jr.; Boonen, S.; Chapurlat, R.D.; Compston, J.E.; Cooper, C.; Gehlbach, S.H.; Greenspan, S.L.; Hooven, F.H.; et al. Frailty and fracture, disability, and falls: A multiple country study from the global longitudinal study of osteoporosis in women. J. Am. Geriatr. Soc. 2013, 61, 327–334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanlon, P.; Nicholl, B.I.; Jani, B.D.; Lee, D.; McQueenie, R.; Mair, F.S. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: A prospective analysis of 493 737 UK Biobank participants. Lancet Public Health 2018, 3, e323–e332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, Q.; Yu, D.; Fan, J.; Yu, C.; Guo, Y.; Pei, P.; Yang, L.; Chen, Y.; Du, H.; Yang, X.; et al. Healthy lifestyle and life expectancy at age 30 years in the Chinese population: An observational study. Lancet Public Health 2022, 7, e994–e1004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Pan, A.; Wang, D.D.; Liu, X.; Dhana, K.; Franco, O.H.; Kaptoge, S.; Di Angelantonio, E.; Stampfer, M.; Willett, W.C.; et al. Impact of Healthy Lifestyle Factors on Life Expectancies in the US Population. Circulation 2018, 138, 345–355, Erratum in: Circulation 2018, 138, e75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mathers, J.C. Impact of nutrition on the ageing process. Br. J. Nutr. 2015, 113, S18–S22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Jacques, P.F.; Tucker, K.L. Are dietary patterns useful for understanding the role of diet in chronic disease? Am. J. Clin. Nutr. 2001, 73, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Wirt, A.; Collins, C.E. Diet quality—What is it and does it matter? Public Health Nutr. 2009, 12, 2473–2492. [Google Scholar] [CrossRef] [PubMed]
- US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; US Department of Agriculture and US Department of Health and Human Services: Washington, DC, USA, 2020. Available online: http://DietaryGuidelines.gov (accessed on 16 May 2025).
- Oude Griep, L.M.; Wang, H.; Chan, Q. Empirically-derived dietary patterns, diet quality scores, and markers of inflammation and endothelial dysfunction. Curr. Nutr. Rep. 2013, 2, 97–104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jayanama, K.; Theou, O.; Godin, J.; Cahill, L.; Shivappa, N.; Hébert, J.R.; Wirth, M.D.; Park, Y.M.; Fung, T.T.; Rockwood, K. Relationship between diet quality scores and the risk of frailty and mortality in adults across a wide age spectrum. BMC Med. 2021, 19, 64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sotos-Prieto, M.; Bhupathiraju, S.N.; Mattei, J.; Fung, T.T.; Li, Y.; Pan, A.; Willett, W.C.; Rimm, E.B.; Hu, F.B. Association of Changes in Diet Quality with Total and Cause-Specific Mortality. N. Engl. J. Med. 2017, 377, 143–153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tessier, A.J.; Wang, F.; Korat, A.A.; Eliassen, A.H.; Chavarro, J.; Grodstein, F.; Li, J.; Liang, L.; Willett, W.C.; Sun, Q.; et al. Optimal dietary patterns for healthy aging. Nat. Med. 2025, 31, 1644–1652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Collins, R. What makes UK Biobank special? Lancet 2012, 379, 1173–1174. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, B.; Young, H.; Crowe, F.L.; Benson, V.S.; Spencer, E.A.; Key, T.J.; Appleby, P.N.; Beral, V. Development and evaluation of the Oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies. Public Health Nutr. 2011, 14, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.; Littlejohns, T.J.; Sudlow, C.; Doherty, N.; Adamska, L.; Sprosen, T.; Collins, R.; Allen, N.E. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants with Those of the General Population. Am. J. Epidemiol. 2017, 186, 1026–1034. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greenwood, D.C.; Hardie, L.J.; Frost, G.S.; Alwan, N.A.; Bradbury, K.E.; Carter, M.; Elliott, P.; Evans, C.E.L.; Ford, H.E.; Hancock, N.; et al. Validation of the Oxford WebQ Online 24-Hour Dietary Questionnaire Using Biomarkers. Am. J. Epidemiol. 2019, 188, 1858–1867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heianza, Y.; Zhou, T.; Sun, D.; Hu, F.B.; Qi, L. Healthful plant-based dietary patterns, genetic risk of obesity, and cardiovascular risk in the UK biobank study. Clin. Nutr. 2021, 40, 4694–4701. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petermann-Rocha, F.; Hanlon, P.; Gray, S.R.; Welsh, P.; Gill, J.M.R.; Foster, H.; Katikireddi, S.V.; Lyall, D.; Mackay, D.F.; O’Donnell, C.A.; et al. Comparison of two different frailty measurements and risk of hospitalisation or death from COVID-19: Findings from UK Biobank. BMC Med. 2020, 18, 355. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Foster, H.M.E.; Celis-Morales, C.A.; Nicholl, B.I.; Petermann-Rocha, F.; Pell, J.P.; Gill, J.M.R.; O’Donnell, C.A.; Mair, F.S. The effect of socioeconomic deprivation on the association between an extended measurement of unhealthy lifestyle factors and health outcomes: A prospective analysis of the UK Biobank cohort. Lancet Public Health 2018, 3, e576–e585. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, Y.V.; Khunti, K.K.; Zaccardi, F.; Rowlands, A.V.; Yates, T.; Gillies, C.L.; Davies, M.J.; Dhalwani, N.N. Physical activity, multimorbidity, and life expectancy: A UK Biobank longitudinal study. BMC Med. 2019, 17, 108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, H.; Xue, Q.; Wang, X.; Li, X.; Franco, O.H.; Li, Y.; Heianza, Y.; Manson, J.E.; Qi, L. Adding salt to foods and hazard of premature mortality. Eur. Heart J. 2022, 43, 2878–2888. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Single-Year Life Tables, UK: 1980–2020. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/singleyearlifetablesuk1980to2018/singleyearlifetablesuk (accessed on 12 March 2024).
- Stekhoven, D.J.; Bühlmann, P. MissForest--non-parametric missing value imputation for mixed-type data. Bioinformatics 2012, 28, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lang, P.O.; Michel, J.P.; Zekry, D. Frailty syndrome: A transitional state in a dynamic process. Gerontology 2009, 55, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-López, L.; Maseda, A.; de Labra, C.; Regueiro-Folgueira, L.; Rodríguez-Villamil, J.L.; Millán-Calenti, J.C. Nutritional determinants of frailty in older adults: A systematic review. BMC Geriatr. 2017, 17, 108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Angulo, J.; El Assar, M.; Rodríguez-Mañas, L. Frailty and sarcopenia as the basis for the phenotypic manifestation of chronic diseases in older adults. Mol. Asp. Med. 2016, 50, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Jung, H.; Shin, J.; Kim, D.H. Assessment of Frailty Index at 66 Years of Age and Association With Age-Related Diseases, Disability, and Death Over 10 Years in Korea. JAMA Netw. Open 2023, 6, e2248995. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, X.; Li, H.; Leng, S.X. Inflammation and immune system alterations in frailty. Clin. Geriatr. Med. 2011, 27, 79–87. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hébert, J.R.; Shivappa, N.; Wirth, M.D.; Hussey, J.R.; Hurley, T.G. Perspective: The Dietary Inflammatory Index (DII)-Lessons Learned, Improvements Made, and Future Directions. Adv. Nutr. 2019, 10, 185–195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teissier, T.; Boulanger, E.; Cox, L.S. Interconnections between Inflammageing and Immunosenescence during Ageing. Cells 2022, 11, 359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tamura, Y.; Omura, T.; Toyoshima, K.; Araki, A. Nutrition Management in Older Adults with Diabetes: A Review on the Importance of Shifting Prevention Strategies from Metabolic Syndrome to Frailty. Nutrients 2020, 12, 3367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults-Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mutz, J.; Roscoe, C.J.; Lewis, C.M. Exploring health in the UK Biobank: Associations with sociodemographic characteristics, psychosocial factors, lifestyle and environmental exposures. BMC Med. 2021, 19, 240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arias, E. United States life tables, 2008. Natl. Vital Stat. Rep. 2012, 61, 1–63. [Google Scholar] [PubMed]
- Woloshin, S.; Schwartz, L.M.; Welch, H.G. The risk of death by age, sex, and smoking status in the United States: Putting health risks in context. J. Natl. Cancer Inst. 2008, 100, 845–853. [Google Scholar] [CrossRef]
- McCullough, M.L.; Feskanich, D.; Stampfer, M.J.; Giovannucci, E.L.; Rimm, E.B.; Hu, F.B.; Spiegelman, D.; Hunter, D.J.; Colditz, G.A.; Willett, W.C. Diet quality and major chronic disease risk in men and women: Moving toward improved dietary guidance. Am. J. Clin. Nutr. 2002, 76, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R., 3rd; Simons-Morton, D.G.; et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Chiuve, S.E.; McCullough, M.L.; Rexrode, K.M.; Logroscino, G.; Hu, F.B. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch. Intern. Med. 2008, 168, 713–720, Erratum in: Arch. Intern. Med. 2008, 168, 1276. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Lista, J.; Alcala-Diaz, J.F.; Torres-Peña, J.D.; Quintana-Navarro, G.M.; Fuentes, F.; Garcia-Rios, A.; Ortiz-Morales, A.M.; Gonzalez-Requero, A.I.; Perez-Caballero, A.I.; Yubero-Serrano, E.M.; et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): A randomised controlled trial. Lancet 2022, 399, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, K.M.; Milte, C.; Bowe, S.J.; Duckham, R.L.; Ward, J.; Keske, M.A.; McEvoy, M.; Brayner, B.; Abbott, G. Associations between three diet quality indices, genetic risk and body composition: A prospective cohort study. Clin. Nutr. 2022, 41, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, Y.; Lin, F.; Li, Y.; Wang, Y.; Chen, X.; Meng, F.; Ye, Q.; Cai, G. Association of pro-inflammatory diet with increased risk of all-cause dementia and Alzheimer’s dementia: A prospective study of 166,377 UK Biobank participants. BMC Med. 2023, 21, 266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Satija, A.; Bhupathiraju, S.N.; Spiegelman, D.; Chiuve, S.E.; Manson, J.E.; Willett, W.; Rexrode, K.M.; Rimm, E.B.; Hu, F.B. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J. Am. Coll. Cardiol. 2017, 70, 411–422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tresserra-Rimbau, A.; Thompson, A.S.; Bondonno, N.; Jennings, A.; Kühn, T.; Cassidy, A. Plant-Based Dietary Patterns and Parkinson’s Disease: A Prospective Analysis of the UK Biobank. Mov. Disord. 2023, 38, 1994–2004. [Google Scholar] [CrossRef] [PubMed]

| Total | Frailty Index | Frailty Phenotype | |||||
|---|---|---|---|---|---|---|---|
| Robust | Prefrailty | Frailty | Robust | Prefrailty | Frailty | ||
| (N = 151,628) | (N = 93,414) | (N = 51,393) | (N = 6821) | (N = 96,697) | (N = 51,849) | (N = 3082) | |
| Age at recruitment, years, mean (SD) | 57.8 (6.7) | 57.3 (6.7) | 58.4 (6.6) | 58.8 (6.5) | 57.6 (6.7) | 58.0 (6.7) | 58.5 (6.5) |
| Sex, n (%) | |||||||
| female | 81,886 (54.0%) | 49,032 (52.5%) | 28,841 (56.1%) | 4013 (58.8%) | 50,401 (52.1%) | 29,506 (56.9%) | 1979 (64.2%) |
| male | 69,742 (46.0%) | 44,382 (47.5%) | 22,552 (43.9%) | 2808 (41.2%) | 46,296 (47.9%) | 22,343 (43.1%) | 1103 (35.8%) |
| Body mass index (BMI), kg/m2, n (%) | |||||||
| <25.0 | 56,271 (37.1%) | 39,051 (41.8%) | 16,003 (31.1%) | 1217 (17.8%) | 40,612 (42.0%) | 15,183 (29.3%) | 476 (15.4%) |
| 25.0–29.9 | 64,154 (42.3%) | 39,836 (42.6%) | 21,767 (42.4%) | 2551 (37.4%) | 41,309 (42.7%) | 21,901 (42.2%) | 944 (30.6%) |
| ≥30.0 | 31,203 (20.6%) | 14,527 (15.6%) | 13,623 (26.5%) | 3053 (44.8%) | 14,776 (15.3%) | 14,765 (28.5%) | 1662 (53.9%) |
| Ethnicity, n (%) | |||||||
| white | 146,918 (96.9%) | 90,690 (97.1%) | 49,688 (96.7%) | 6540 (95.9%) | 94,381 (97.6%) | 49,660 (95.8%) | 2877 (93.3%) |
| other | 4710 (3.1%) | 2724 (2.9%) | 1705 (3.3%) | 281 (4.1%) | 2316 (2.4%) | 2189 (4.2%) | 205 (6.7%) |
| Education, n (%) | |||||||
| college or university degree | 65,108 (42.9%) | 43,283 (46.3%) | 19,891 (38.7%) | 1934 (28.4%) | 44,134 (45.6%) | 20,205 (39.0%) | 769 (25.0%) |
| secondary school | 56,596 (37.3%) | 34,051 (36.5%) | 19,833 (38.6%) | 2712 (39.8%) | 35,202 (36.4%) | 20,110 (38.8%) | 1284 (41.7%) |
| primary school | 13,327 (8.8%) | 6428 (6.9%) | 5643 (11.0%) | 1256 (18.4%) | 7065 (7.3%) | 5616 (10.8%) | 646 (21.0%) |
| professional qualification | 16,597 (10.9%) | 9652 (10.3%) | 6026 (11.7%) | 919 (13.5%) | 10,296 (10.6%) | 5918 (11.4%) | 383 (12.4%) |
| Employment, n (%) | |||||||
| employed | 85,250 (56.2%) | 55,911 (59.9%) | 26,727 (52.0%) | 2612 (38.3%) | 56,014 (57.9%) | 28,116 (54.2%) | 1120 (36.3%) |
| other | 66,378 (43.8%) | 37,503 (40.1%) | 24,666 (48.0%) | 4209 (61.7%) | 40,683 (42.1%) | 23,733 (45.8%) | 1962 (63.7%) |
| Household income, £/year, n (%) | |||||||
| less than 18,000 | 25,890 (17.1%) | 12,250 (13.1%) | 10,983 (21.4%) | 2657 (39.0%) | 13,772 (14.2%) | 10,794 (20.8%) | 1324 (43.0%) |
| 18,000 to 30,999 | 37,849 (25.0%) | 22,145 (23.7%) | 13,866 (27.0%) | 1838 (26.9%) | 23,369 (24.2%) | 13,685 (26.4%) | 795 (25.8%) |
| 31,000 to 51,999 | 41,422 (27.3%) | 26,365 (28.2%) | 13,665 (26.6%) | 1392 (20.4%) | 27,044 (28.0%) | 13,813 (26.6%) | 565 (18.3%) |
| 52,000 to 100,000 | 34,892 (23.0%) | 24,027 (25.7%) | 10,114 (19.7%) | 751 (11.0%) | 24,160 (25.0%) | 10,414 (20.1%) | 318 (10.3%) |
| greater than 100,000 | 11,575 (7.6%) | 8627 (9.2%) | 2765 (5.4%) | 183 (2.7%) | 8352 (8.6%) | 3143 (6.1%) | 80 (2.6%) |
| Townsend deprivation index, n (%) | |||||||
| first quartile | 37,934 (25.0%) | 24,624 (26.4%) | 12,136 (23.6%) | 1174 (17.2%) | 25,850 (26.7%) | 11,640 (22.4%) | 444 (14.4%) |
| second quartile | 37,882 (25.0%) | 23,952 (25.6%) | 12,513 (24.3%) | 1417 (20.8%) | 24,908 (25.8%) | 12,392 (23.9%) | 582 (18.9%) |
| third quartile | 37,906 (25.0%) | 23,354 (25.0%) | 12,938 (25.2%) | 1614 (23.7%) | 24,039 (24.9%) | 13,129 (25.3%) | 738 (23.9%) |
| fourth quartile | 37,906 (25.0%) | 21,484 (23.0%) | 13,806 (26.9%) | 2616 (38.4%) | 21,900 (22.6%) | 14,688 (28.3%) | 1318 (42.8%) |
| Smoking status, n (%) | |||||||
| never | 85,091 (56.1%) | 55,644 (59.6%) | 26,465 (51.5%) | 2982 (43.7%) | 55,658 (57.6%) | 28,005 (54.0%) | 1428 (46.3%) |
| previous | 55,795 (36.8%) | 31,898 (34.1%) | 20,856 (40.6%) | 3041 (44.6%) | 34,918 (36.1%) | 19,614 (37.8%) | 1263 (41.0%) |
| current | 10,742 (7.1%) | 5872 (6.3%) | 4072 (7.9%) | 798 (11.7%) | 6121 (6.3%) | 4230 (8.2%) | 391 (12.7%) |
| Alcohol drinking frequency, n (%) | |||||||
| ≥3 times/week | 76,319 (50.3%) | 49,856 (53.4%) | 24,177 (47.0%) | 2286 (33.5%) | 52,772 (54.6%) | 22,774 (43.9%) | 773 (25.1%) |
| <3 times/week | 66,389 (43.8%) | 39,070 (41.8%) | 23,687 (46.1%) | 3632 (53.2%) | 39,512 (40.9%) | 25,119 (48.4%) | 1758 (57.0%) |
| never | 8920 (5.9%) | 4488 (4.8%) | 3529 (6.9%) | 903 (13.2%) | 4413 (4.6%) | 3956 (7.6%) | 551 (17.9%) |
| Sleep duration, n (%) | |||||||
| 7–8 h/day | 107,921 (71.2%) | 70,894 (75.9%) | 33,670 (65.5%) | 3357 (49.2%) | 71,742 (74.2%) | 34,715 (67.0%) | 1464 (47.5%) |
| <7h/day | 33,649 (22.2%) | 17,298 (18.5%) | 13,766 (26.8%) | 2585 (37.9%) | 19,394 (20.1%) | 13,138 (25.3%) | 1117 (36.2%) |
| >8h/day | 10,058 (6.6%) | 5222 (5.6%) | 3957 (7.7%) | 879 (12.9%) | 5561 (5.8%) | 3996 (7.7%) | 501 (16.3%) |
| Physical activity, n (%) | |||||||
| high | 59,729 (39.4%) | 38,445 (41.2%) | 19,025 (37.0%) | 2259 (33.1%) | 39,854 (41.2%) | 19,267 (37.2%) | 608 (19.7%) |
| moderate | 60,258 (39.7%) | 37,284 (39.9%) | 20,575 (40.0%) | 2399 (35.2%) | 39,446 (40.8%) | 19,936 (38.5%) | 876 (28.4%) |
| low | 31,641 (20.9%) | 17,685 (18.9%) | 11,793 (22.9%) | 2163 (31.7%) | 17,397 (18.0%) | 12,646 (24.4%) | 1598 (51.8%) |
| Energy, kcal, mean (SD) | 2030 (524) | 2030 (516) | 2040 (533) | 2020 (567) | 2050 (516) | 2000 (534) | 1920 (565) |
| AHEI, n (%) | |||||||
| unhealthy tertile | 50,543 (33.3%) | 30,429 (32.6%) | 17,609 (34.3%) | 2505 (36.7%) | 31,712 (32.8%) | 17,646 (34.0%) | 1185 (38.4%) |
| medium tertile | 50,542 (33.3%) | 31,287 (33.5%) | 17,024 (33.1%) | 2231 (32.7%) | 32,293 (33.4%) | 17,259 (33.3%) | 990 (32.1%) |
| healthy tertile | 50,543 (33.3%) | 31,698 (33.9%) | 16,760 (32.6%) | 2085 (30.6%) | 32,692 (33.8%) | 16,944 (32.7%) | 907 (29.4%) |
| DASH, n (%) | |||||||
| unhealthy tertile | 54,277 (35.8%) | 32,168 (34.4%) | 19,237 (37.4%) | 2872 (42.1%) | 33,482 (34.6%) | 19,395 (37.4%) | 1400 (45.4%) |
| medium tertile | 49,349 (32.5%) | 30,744 (32.9%) | 16,447 (32.0%) | 2158 (31.6%) | 31,797 (32.9%) | 16,614 (32.0%) | 938 (30.4%) |
| healthy tertile | 48,002 (31.7%) | 30,502 (32.7%) | 15,709 (30.6%) | 1791 (26.3%) | 31,418 (32.5%) | 15,840 (30.6%) | 744 (24.1%) |
| MED, n (%) | |||||||
| unhealthy tertile | 53,299 (35.2%) | 31,433 (33.6%) | 18,951 (36.9%) | 2915 (42.7%) | 32,250 (33.4%) | 19,551 (37.7%) | 1498 (48.6%) |
| medium tertile | 64,070 (42.3%) | 39,768 (42.6%) | 21,509 (41.9%) | 2793 (40.9%) | 41,306 (42.7%) | 21,614 (41.7%) | 1150 (37.3%) |
| healthy tertile | 34,259 (22.6%) | 22,213 (23.8%) | 10,933 (21.3%) | 1113 (16.3%) | 23,141 (23.9%) | 10,684 (20.6%) | 434 (14.1%) |
| DII, n (%) | |||||||
| healthy tertile | 50,543 (33.3%) | 31,239 (33.4%) | 17,201 (33.5%) | 2103 (30.8%) | 33,553 (34.7%) | 16,242 (31.3%) | 748 (24.3%) |
| medium tertile | 50,542 (33.3%) | 31,511 (33.7%) | 16,987 (33.1%) | 2044 (30.0%) | 32,791 (33.9%) | 16,912 (32.6%) | 839 (27.2%) |
| unhealthy tertile | 50,543 (33.3%) | 30,664 (32.8%) | 17,205 (33.5%) | 2674 (39.2%) | 30,353 (31.4%) | 18,695 (36.1%) | 1495 (48.5%) |
| PDI, n (%) | |||||||
| unhealthy tertile | 59,537 (39.3%) | 36,406 (39.0%) | 20,409 (39.7%) | 2722 (39.9%) | 37,074 (38.3%) | 21,078 (40.7%) | 1385 (44.9%) |
| medium tertile | 45,210 (29.8%) | 28,190 (30.2%) | 15,028 (29.2%) | 1992 (29.2%) | 29,075 (30.1%) | 15,272 (29.5%) | 863 (28.0%) |
| healthy tertile | 46,881 (30.9%) | 28,818 (30.8%) | 15,956 (31.0%) | 2107 (30.9%) | 30,548 (31.6%) | 15,499 (29.9%) | 834 (27.1%) |
| HPDI, n (%) | |||||||
| unhealthy tertile | 55,751 (36.8%) | 33,120 (35.5%) | 19,710 (38.4%) | 2921 (42.8%) | 34,791 (36.0%) | 19,572 (37.7%) | 1388 (45.0%) |
| medium tertile | 46,252 (30.5%) | 28,673 (30.7%) | 15,595 (30.3%) | 1984 (29.1%) | 29,682 (30.7%) | 15,670 (30.2%) | 900 (29.2%) |
| healthy tertile | 49,625 (32.7%) | 31,621 (33.9%) | 16,088 (31.3%) | 1916 (28.1%) | 32,224 (33.3%) | 16,607 (32.0%) | 794 (25.8%) |
| UPDI, n (%) | |||||||
| healthy tertile | 53,591 (35.3%) | 33,718 (36.1%) | 17,835 (34.7%) | 2038 (29.9%) | 35,286 (36.5%) | 17,548 (33.8%) | 757 (24.6%) |
| medium tertile | 48,237 (31.8%) | 29,984 (32.1%) | 16,160 (31.4%) | 2093 (30.7%) | 31,033 (32.1%) | 16,297 (31.4%) | 907 (29.4%) |
| unhealthy tertile | 49,800 (32.8%) | 29,712 (31.8%) | 17,398 (33.9%) | 2690 (39.4%) | 30,378 (31.4%) | 18,004 (34.7%) | 1418 (46.0%) |
| Family history of CVD, n (%) | |||||||
| no | 36,339 (24.0%) | 24,673 (26.4%) | 10,605 (20.6%) | 1061 (15.6%) | 23,944 (24.8%) | 11,786 (22.7%) | 609 (19.8%) |
| yes | 115,289 (76.0%) | 68,741 (73.6%) | 40,788 (79.4%) | 5760 (84.4%) | 72,753 (75.2%) | 40,063 (77.3%) | 2473 (80.2%) |
| Family history of cancer, n (%) | |||||||
| no | 95,205 (62.8%) | 59,425 (63.6%) | 31,645 (61.6%) | 4135 (60.6%) | 60,942 (63.0%) | 32,344 (62.4%) | 1919 (62.3%) |
| yes | 56,423 (37.2%) | 33,989 (36.4%) | 19,748 (38.4%) | 2686 (39.4%) | 35,755 (37.0%) | 19,505 (37.6%) | 1163 (37.7%) |
| Family history of diabetes, n (%) | |||||||
| no | 119,765 (79.0%) | 75,375 (80.7%) | 39,535 (76.9%) | 4855 (71.2%) | 77,684 (80.3%) | 39,952 (77.1%) | 2129 (69.1%) |
| yes | 31,863 (21.0%) | 18,039 (19.3%) | 11,858 (23.1%) | 1966 (28.8%) | 19,013 (19.7%) | 11,897 (22.9%) | 953 (30.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Liu, H.; Chen, L.; Filippidis, F.T. Combined Effects of Diet Quality Scores and Frailty on All-Cause Mortality and Life Expectancy in Middle-Aged and Older Adults. Nutrients 2025, 17, 3115. https://doi.org/10.3390/nu17193115
Yang Y, Liu H, Chen L, Filippidis FT. Combined Effects of Diet Quality Scores and Frailty on All-Cause Mortality and Life Expectancy in Middle-Aged and Older Adults. Nutrients. 2025; 17(19):3115. https://doi.org/10.3390/nu17193115
Chicago/Turabian StyleYang, Yang, Huaicun Liu, Liangkai Chen, and Filippos T. Filippidis. 2025. "Combined Effects of Diet Quality Scores and Frailty on All-Cause Mortality and Life Expectancy in Middle-Aged and Older Adults" Nutrients 17, no. 19: 3115. https://doi.org/10.3390/nu17193115
APA StyleYang, Y., Liu, H., Chen, L., & Filippidis, F. T. (2025). Combined Effects of Diet Quality Scores and Frailty on All-Cause Mortality and Life Expectancy in Middle-Aged and Older Adults. Nutrients, 17(19), 3115. https://doi.org/10.3390/nu17193115





