Differential Plasma Carotenoid Profiles in Hypertensive Disorders of Pregnancy
Abstract
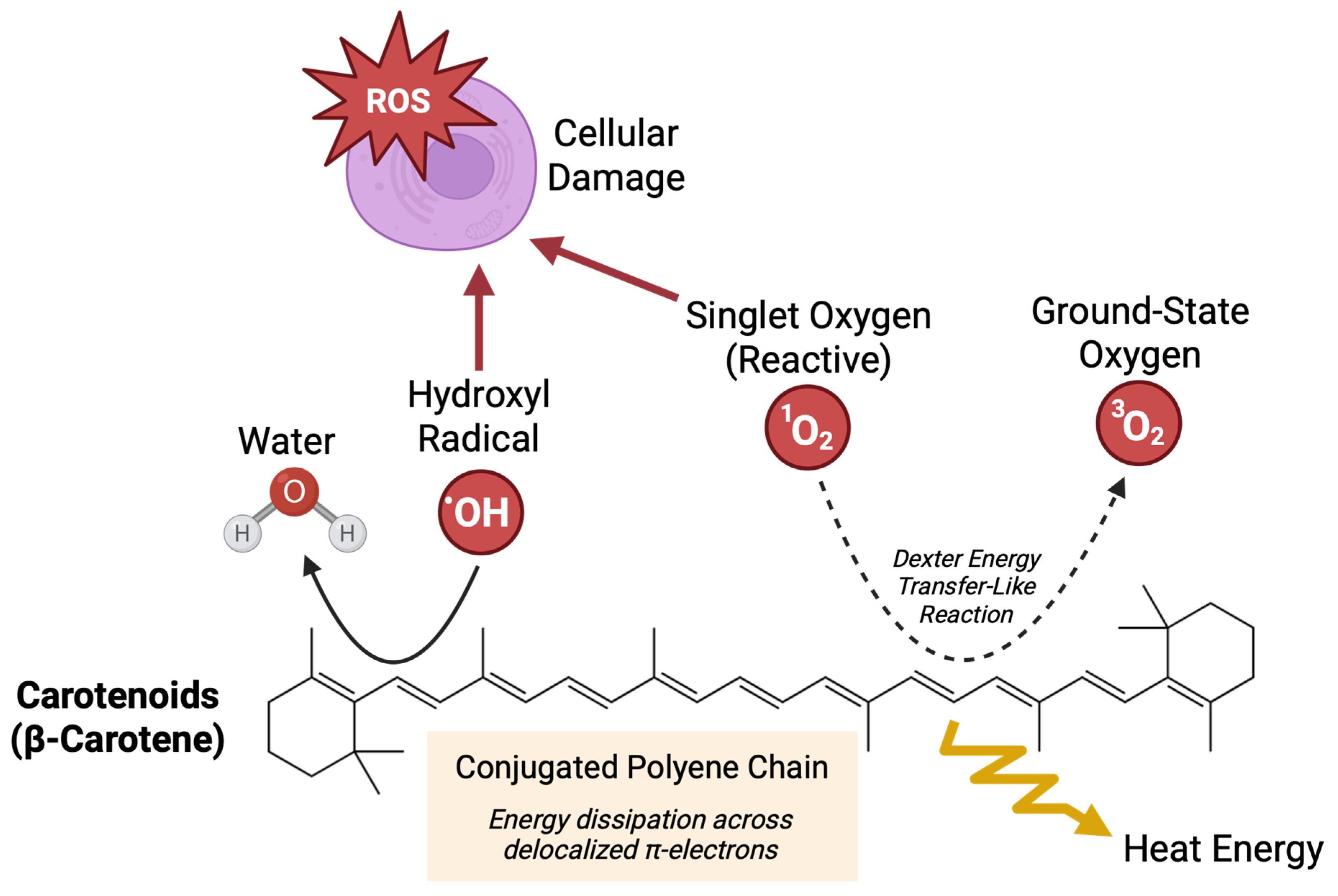
1. Introduction
2. Materials and Methods
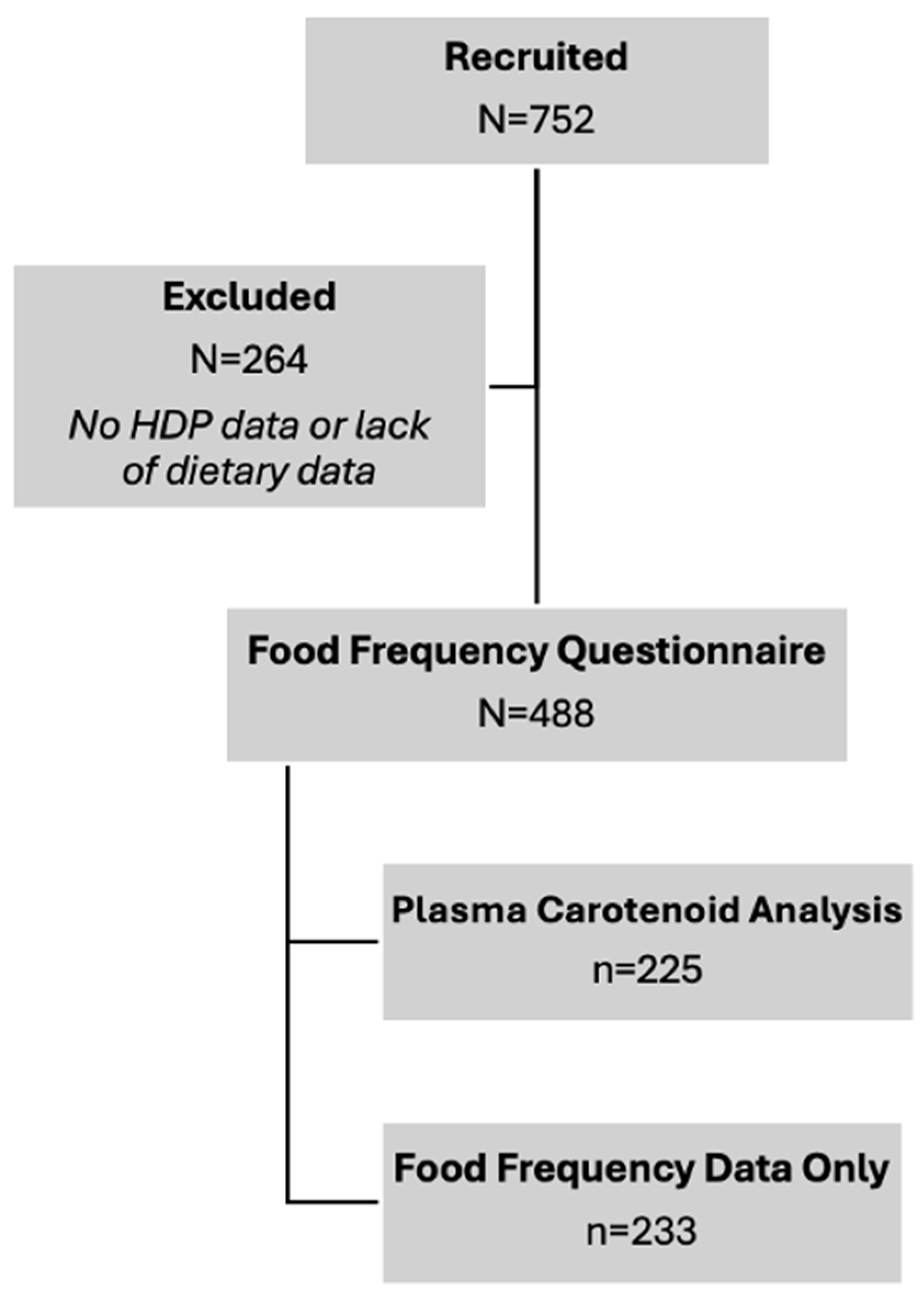
2.1. Participant Enrollment
2.2. Dietary Questionnaires
2.3. Clinical Data Collection
2.4. Blood Sample Collection
2.5. Carotenoid Analysis
2.6. Statistical Analysis
3. Results
3.1. Maternal Demographics
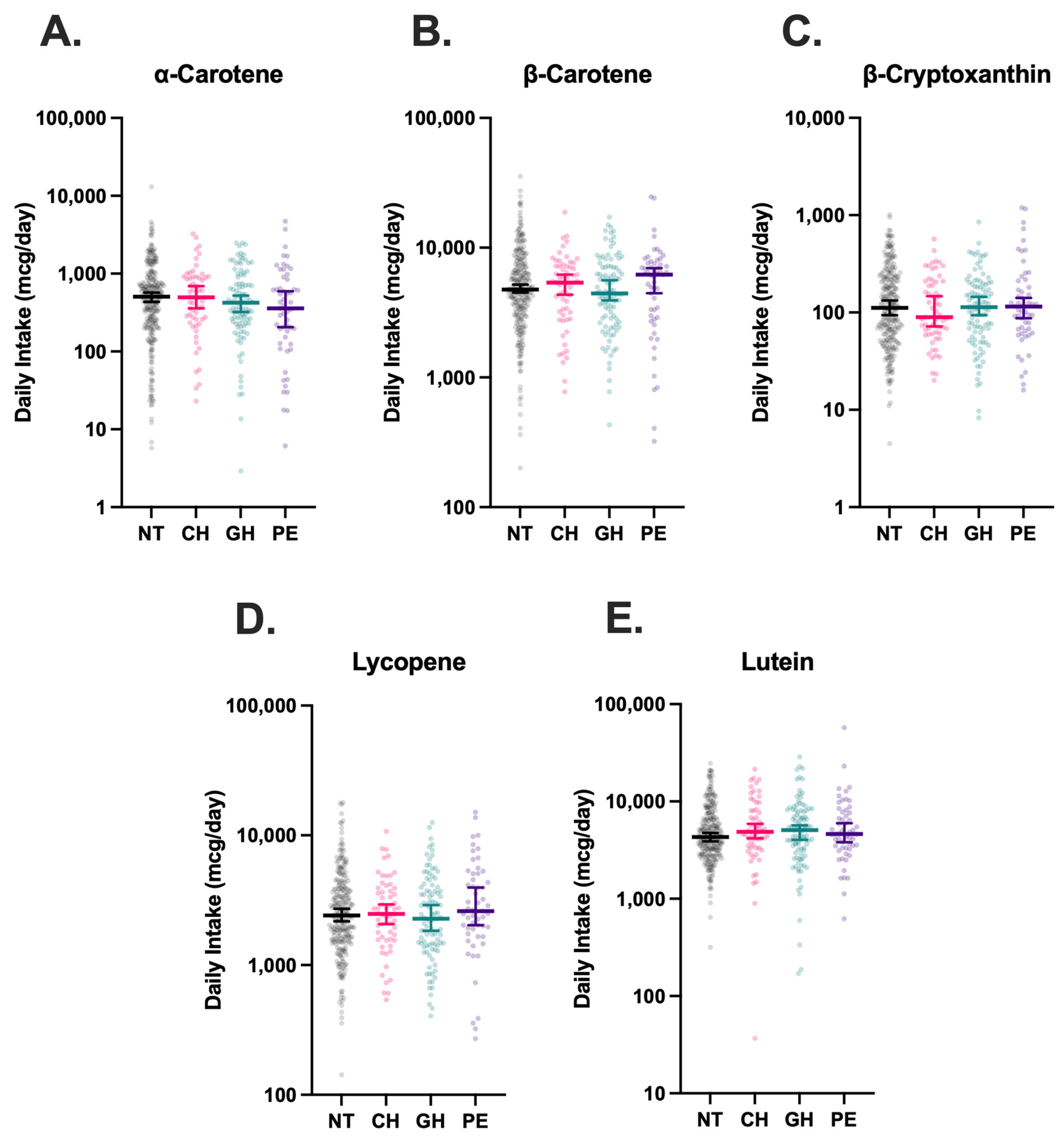
3.2. Dietary Carotenoid Intake
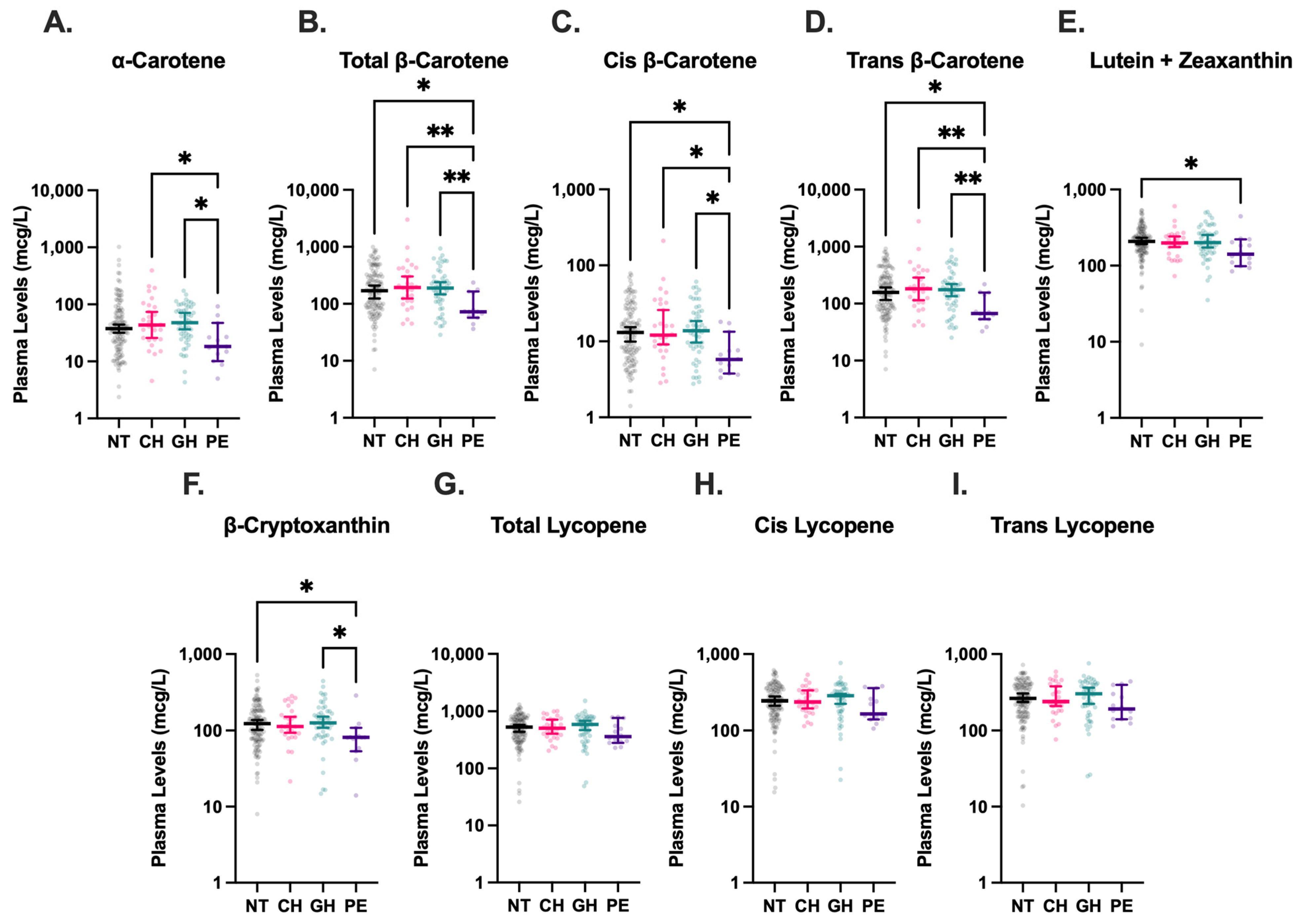
3.3. Plasma Carotenoid Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HDP | Hypertensive disorders of pregnancy |
| ROS | Reactive oxygen species |
| GH | Gestational hypertension |
| CH | Chronic hypertension |
| PE | Preeclampsia |
| NT | Normotension |
| HELLP | Hemolysis, elevated liver enzymes, low platelet count |
| FFQ | Food frequency questionnaire |
References
- Cameron, N.A.; Everitt, I.; Seegmiller, L.E.; Yee, L.M.; Grobman, W.A.; Khan, S.S. Trends in the Incidence of New–Onset Hypertensive Disorders of Pregnancy Among Rural and Urban Areas in the United States, 2007 to 2019. J. Am. Heart Assoc. 2022, 11, e023791. [Google Scholar] [CrossRef]
- Hladunewich, M.; Karumanchi, S.A.; Lafayette, R. Pathophysiology of the Clinical Manifestations of Preeclampsia. Clin. J. Am. Soc. Nephrol. 2007, 2, 543–549. [Google Scholar] [CrossRef]
- Espinoza, J. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [Google Scholar] [CrossRef]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative Stress and Hypertension. Circ. Res. 2021, 128, 993–1020. [Google Scholar] [CrossRef]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The Roles of Cellular Reactive Oxygen Species, Oxidative Stress and Antioxidants in Pregnancy Outcomes. Int. J. Biochem. Cell Biol. 2010, 42, 1634–1650. [Google Scholar] [CrossRef]
- Bendhack, L.M. Contribution of Oxidative Stress to Endothelial Dysfunction in Hypertension. Front. Physiol. 2012, 3, 441. [Google Scholar] [CrossRef]
- Myatt, L.; Cui, X. Oxidative Stress in the Placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef]
- Staff, A.C.; Fjeldstad, H.E.; Fosheim, I.K.; Moe, K.; Turowski, G.; Johnsen, G.M.; Alnaes-Katjavivi, P.; Sugulle, M. Failure of Physiological Transformation and Spiral Artery Atherosis: Their Roles in Preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S895–S906. [Google Scholar] [CrossRef]
- Gordon, M.H. Significance of Dietary Antioxidants for Health. Int. J. Mol. Sci. 2011, 13, 173–179. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, Inflammation, and Oxidative Stress—Implications of Cellular Signaling Pathways and Relation to Chronic Disease Prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Crupi, P.; Faienza, M.F.; Naeem, M.Y.; Corbo, F.; Clodoveo, M.L.; Muraglia, M. Overview of the Potential Beneficial Effects of Carotenoids on Consumer Health and Well-Being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef]
- Desmarchelier, C.; Borel, P. Overview of Carotenoid Bioavailability Determinants: From Dietary Factors to Host Genetic Variations. Trends Food Sci. Technol. 2017, 69, 270–280. [Google Scholar] [CrossRef]
- Grainger, E.M.; Webb, M.Z.; Simpson, C.M.; Chitchumroonchokchai, C.; Riedl, K.; Moran, N.E.; Clinton, S.K. Assessment of Dietary Carotenoid Intake and Biologic Measurement of Exposure in Humans. Methods Enzymol. 2022, 674, 255–295. [Google Scholar]
- Abbasian, F.; Alavi, M.S.; Roohbakhsh, A. Dietary Carotenoids to Improve Hypertension. Heliyon 2023, 9, e19399. [Google Scholar] [CrossRef]
- Williams, M. Plasma Carotenoids, Retinol, Tocopherols, and Lipoproteins in Preeclamptic and Normotensive Pregnant Zimbabwean Women. Am. J. Hypertens. 2003, 16, 665–672. [Google Scholar] [CrossRef]
- Lai, J.S.; Yuan, W.L.; Ong, C.N.; Tan, K.H.; Yap, F.; Chong, Y.S.; Gluckman, P.D.; Godfrey, K.M.; Lee, Y.S.; Chan, J.K.Y.; et al. Perinatal Plasma Carotenoid and Vitamin E Concentrations with Maternal Blood Pressure during and after Pregnancy. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2811–2821. [Google Scholar] [CrossRef]
- Palan, P.R.; Mikhail, M.S.; Romney, S.L. Placental and Serum Levels of Carotenoids in Preeclampsia. Obstet. Gynecol. 2001, 98, 459–462. [Google Scholar] [CrossRef]
- Baer, H.J.; Blum, R.E.; Rockett, H.R.; Leppert, J.; Gardner, J.D.; Suitor, C.W.; Colditz, G.A. Use of a Food Frequency Questionnaire in American Indian and Caucasian Pregnant Women: A Validation Study. BMC Public Health 2005, 5, 135. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef]
- World Health Organization. Micronutrient Survey Manual, 1st ed.; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-001269-1. [Google Scholar]
- Thoene, M.; Anderson-Berry, A.; Van Ormer, M.; Furtado, J.; Soliman, G.A.; Goldner, W.; Hanson, C. Quantification of Lutein + Zeaxanthin Presence in Human Placenta and Correlations with Blood Levels and Maternal Dietary Intake. Nutrients 2019, 11, 134. [Google Scholar] [CrossRef]
- McConnell, C.; Thoene, M.; Van Ormer, M.; Furtado, J.D.; Korade, Z.; Genaro-Mattos, T.C.; Hanson, C.; Anderson-Berry, A. Plasma Concentrations and Maternal-Umbilical Cord Plasma Ratios of the Six Most Prevalent Carotenoids across Five Groups of Birth Gestational Age. Antioxidants 2021, 10, 1409. [Google Scholar] [CrossRef]
- Gibson, E.M.; Geske, J.A.; Khandalavala, B.N. Temporal Trends of Obesity Among Nebraska Adults: EMR Data Shows a More Rapid Increase than Projected. J. Prim. Care Community Health 2024, 15, 21501319241301236. [Google Scholar] [CrossRef]
- Levin, G.; Mokady, S. Antioxidant Activity of 9-Cis Compared to All-Trans Beta-Carotene In Vitro. Free Radic. Biol. Med. 1994, 17, 77–82. [Google Scholar] [CrossRef]
- Erdman, J.W.; Thatcher, A.J.; Hofmann, N.E.; Lederman, J.D.; Block, S.S.; Lee, C.M.; Mokady, S. All-Trans β-Carotene Is Absorbed Preferentially to 9-Cis β-Carotene, but the Latter Accumulates in the Tissues of Domestic Ferrets (Mustela Putorius Puro). J. Nutr. 1998, 128, 2009–2013. [Google Scholar] [CrossRef]
- Mikhail, M.S.; Anyaegbunam, A.; Garfinkel, D.; Palan, P.R.; Basu, J.; Romney, S.L. Preeclampsia and Antioxidant Nutrients: Decreased Plasma Levels of Reduced Ascorbic Acid, Alpha-Tocopherol, and Beta-Carotene in Women with Preeclampsia. Am. J. Obstet. Gynecol. 1994, 171, 150–157. [Google Scholar] [CrossRef]
- Cohen, J.M.; Kramer, M.S.; Platt, R.W.; Basso, O.; Evans, R.W.; Kahn, S.R. The Association between Maternal Antioxidant Levels in Midpregnancy and Preeclampsia. Am. J. Obstet. Gynecol. 2015, 213, 695.e1–695.e13. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T. Carotenoids and Markers of Oxidative Stress in Human Observational Studies and Intervention Trials: Implications for Chronic Diseases. Antioxidants 2019, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Jeyaseelan, S.; Guleria, R. Trial of Lycopene to Prevent Pre-eclampsia in Healthy Primigravidas: Results Show Some Adverse Effects. J. Obstet. Gynaecol. Res. 2009, 35, 477–482. [Google Scholar] [CrossRef]
- Kang, T.; Liu, Y.; Chen, X.; Huang, X.; Cao, Y.; Dou, W.; Duan, D.; Bo, Y.; Traore, S.S.; Zhao, X.; et al. Dietary Carotenoid Intake and Risk of Developing Preeclampsia: A Hospital-Based Case–Control Study. BMC Pregnancy Childbirth 2022, 22, 427. [Google Scholar] [CrossRef] [PubMed]
- Rocha, H.R.; Coelho, M.C.; Gomes, A.M.; Pintado, M.E. Carotenoids Diet: Digestion, Gut Microbiota Modulation, and Inflammatory Diseases. Nutrients 2023, 15, 2265. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Y.; Lv, Y.; Ding, H. Dissecting the Roles of Lipids in Preeclampsia. Metabolites 2022, 12, 590. [Google Scholar] [CrossRef] [PubMed]
- Tyrmi, J.S.; Kaartokallio, T.; Lokki, A.I.; Jääskeläinen, T.; Kortelainen, E.; Ruotsalainen, S.; Karjalainen, J.; Ripatti, S.; Kivioja, A.; Laisk, T.; et al. Genetic Risk Factors Associated With Preeclampsia and Hypertensive Disorders of Pregnancy. JAMA Cardiol. 2023, 8, 674. [Google Scholar] [CrossRef]
- Ford, N.D.; Cox, S.; Ko, J.Y.; Ouyang, L.; Romero, L.; Colarusso, T.; Ferre, C.D.; Kroelinger, C.D.; Hayes, D.K.; Barfield, W.D. Hypertensive Disorders in Pregnancy and Mortality at Delivery Hospitalization—United States, 2017–2019. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 585–591. [Google Scholar] [CrossRef]
- Harrell, F.E.; Lee, K.L.; Mark, D.B. Multivariable Prognostic Models: Issues in Developing Models, Evaluating Assumptions and Adequacy, and Measuring and Reducing Errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Goldstein, S.A.; Savu, A.; Islam, S.; Ward, C.C.; Krasuski, R.A.; Grotegut, C.A.; Newby, L.K.; Hornberger, L.K.; Windram, J.; Kaul, P. Risk Factors and Outcomes Associated With Hypertensive Disorders of Pregnancy in Maternal Congenital Heart Disease. JACC Adv. 2022, 1, 100036. [Google Scholar] [CrossRef]
- Zhou, L.; Tian, Y.; Su, Z.; Sun, J.-Y.; Sun, W. Risk Factors and Prediction Model for New-Onset Hypertensive Disorders of Pregnancy: A Retrospective Cohort Study. Front. Cardiovasc. Med. 2024, 11, 1272779. [Google Scholar] [CrossRef] [PubMed]
| NT (n = 270) | CH (n = 61) | GH (n = 102) | PE (n = 55) | p-Value | |
|---|---|---|---|---|---|
| Age (years; median, inner quartiles) | 30 (25–33) | 30 (25–33) | 30 (25–34) | 28 (23–34) | 0.7000 |
| Body Mass Index (kg/m2; median, inner quartiles) | 27.3 (23.4–31.8) | 26.8 (22.4–33.2) | 28.0 (22.9–32.4) | 34.2 (25.7–38.8) | 0.0007 |
| Race (N, %) | 0.295 | ||||
| White | 180 (66.9%) | 46 (75.4%) | 73 (71.6%) | 37 (67.3%) | |
| African American | 40 (14.9%) | 6 (9.8%) | 9 (8.8%) | 8 (14.6%) | |
| Hispanic | 22 (8.2%) | 2 (3.3%) | 7 (6.7%) | 3 (5.5%) | |
| Asian or Pacific Islander | 6 (2.2%) | 3 (4.9%) | 1 (1.0%) | 2 (3.6%) | |
| American Indian | - | 1 (1.6%) | - | - | |
| Other/Unknown | 21 (7.8%) | 3 (4.9%) | 12 (11.8%) | 5 (9.1%) | |
| Parity (N, %) | 0.024 | ||||
| Nulliparous | 31 (11.5%) | 4 (6.6%) | 11 (10.8%) | 14 (25.5%) | |
| Primiparous | 83 (30.7%) | 24 (39.3%) | 37 (36.3%) | 20 (36.4%) | |
| Multiparous | 156 (57.8%) | 33 (54.1%) | 54 (52.9%) | 21 (38.2%) | |
| Diabetes (N, %) | 34 (12.6%) | 4 (6.6%) | 10 (9.8%) | 11 (20.0%) | 0.135 |
| Smoking (N, %) | 0.297 | ||||
| Current | 28 (10.4%) | 5 (8.2%) | 7 (6.9%) | 5 (9.1%) | |
| Former | 32 (11.9%) | 5 (8.2%) | 17 (16.7%) | 12 (21.2%) | |
| Pregnancy Duration (weeks; median, inner quartiles) | 39.3 (38.4–40.2) | 39.4 (38.3–40.4) | 39.2 (38–40.3) | 37 (34.1–38.6) | 0.0001 |
| Carotenoid Supplementation (N, %) | 93.0% (251) | 85.3% (52) | 90.2% (92) | 83.6% (46) | 0.079 |
| CH (n = 61) | GH (n = 95) | PE (n = 49) | |
|---|---|---|---|
| α-carotene | 1.03 (0.81–1.32) | 0.94 (0.77–1.14) | 0.86 (0.66–1.13) |
| β-carotene | 0.96 (0.65–1.42) | 0.85 (0.62–1.17) | 0.78 (0.50–1.23) |
| β-cryptoxanthin | 0.91 (0.67–1.23) | 0.88 (0.68–1.16) | 0.99 (0.69–1.41) |
| Lycopene | 1.14 (0.71–1.83) | 1.02 (0.72–1.45) | 1.09 (0.74–1.62) |
| Lutein | 0.95 (0.67–1.36) | 0.84 (0.60–1.17) | 1.03 (0.64–1.67) |
| CH (n = 28) | GH (n = 49) | PE (n = 11) | |
|---|---|---|---|
| Lutein + Zeaxanthin | 0.68 (0.29–1.62) | 1.31 (0.60–2.86) | 0.67 (0.18–2.46) |
| β-cryptoxanthin | 0.94 (0.49–1.81) | 1.17 (0.63–2.16) | 0.63 (0.27–1.48) |
| Trans Lycopene | 1.07 (0.57–2.00) | 1.34 (0.71–2.52) | 0.83 (0.34–2.01) |
| Cis Lycopene | 1.19 (0.65–2.17) | 1.35 (0.68–2.67) | 1.06 (0.41–2.74) |
| Total Lycopene | 1.13 (0.61–2.12) | 1.35 (0.69–2.65) | 0.91 (0.36–2.32) |
| α-carotene | 1.14 (0.74–1.77) | 1.30 (0.94–1.81) | 0.65 (0.33–1.26) |
| Trans β-carotene | 1.22 (0.75–1.98) | 1.22 (0.84–1.76) | 0.35 (0.19–0.62) *** |
| Cis β-carotene | 1.10 (0.63–1.91) | 1.20 (0.82–1.76) | 0.42 (0.18–0.99) * |
| Total β-carotene | 1.21 (0.74–1.99) | 1.23 (0.85–1.78) | 0.35 (0.19–0.64) ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freel, C.I.; Scheffler, J.; Drakowski, R.A.; Lyden, E.; VanOrmer, M.; Thoene, M.K.; Mishra, P.K.; Hanson, C.K.; Anderson-Berry, A.L. Differential Plasma Carotenoid Profiles in Hypertensive Disorders of Pregnancy. Nutrients 2025, 17, 3104. https://doi.org/10.3390/nu17193104
Freel CI, Scheffler J, Drakowski RA, Lyden E, VanOrmer M, Thoene MK, Mishra PK, Hanson CK, Anderson-Berry AL. Differential Plasma Carotenoid Profiles in Hypertensive Disorders of Pregnancy. Nutrients. 2025; 17(19):3104. https://doi.org/10.3390/nu17193104
Chicago/Turabian StyleFreel, Colman I., Jonah Scheffler, Rebecca A. Drakowski, Elizabeth Lyden, Matthew VanOrmer, Melissa K. Thoene, Paras Kumar Mishra, Corrine K. Hanson, and Ann L. Anderson-Berry. 2025. "Differential Plasma Carotenoid Profiles in Hypertensive Disorders of Pregnancy" Nutrients 17, no. 19: 3104. https://doi.org/10.3390/nu17193104
APA StyleFreel, C. I., Scheffler, J., Drakowski, R. A., Lyden, E., VanOrmer, M., Thoene, M. K., Mishra, P. K., Hanson, C. K., & Anderson-Berry, A. L. (2025). Differential Plasma Carotenoid Profiles in Hypertensive Disorders of Pregnancy. Nutrients, 17(19), 3104. https://doi.org/10.3390/nu17193104







