Bioinformatics-Guided Network Pharmacology Exploration of Taraxacum Officinale’s Renoprotective Effects Against Cisplatin-Induced Nephrotoxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
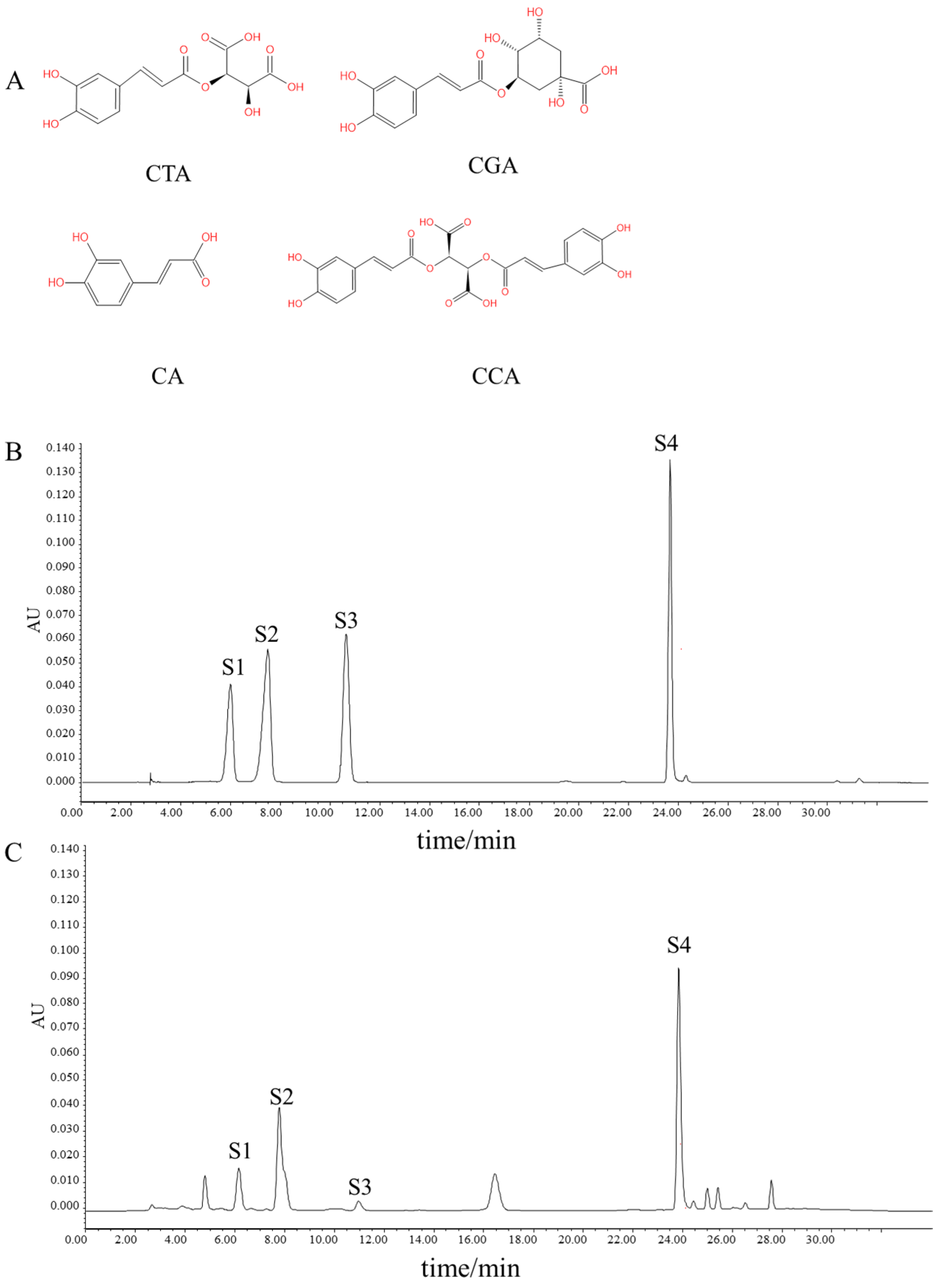
2.2. Preparation of TRWE and HPLC Analysis
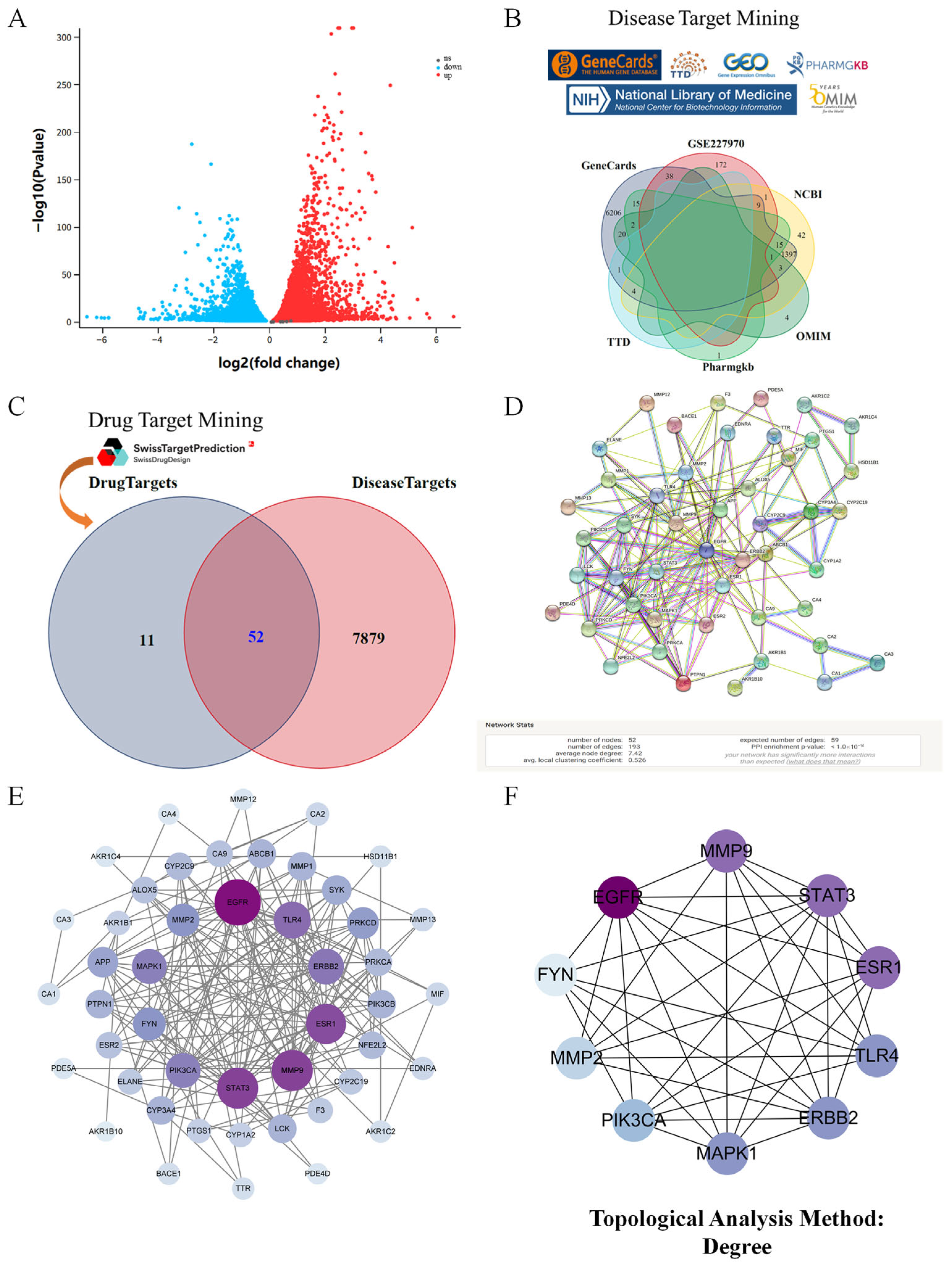
2.3. Acquisition of the Targets of Kidney Injury and Phenolic Compounds in Taraxacum
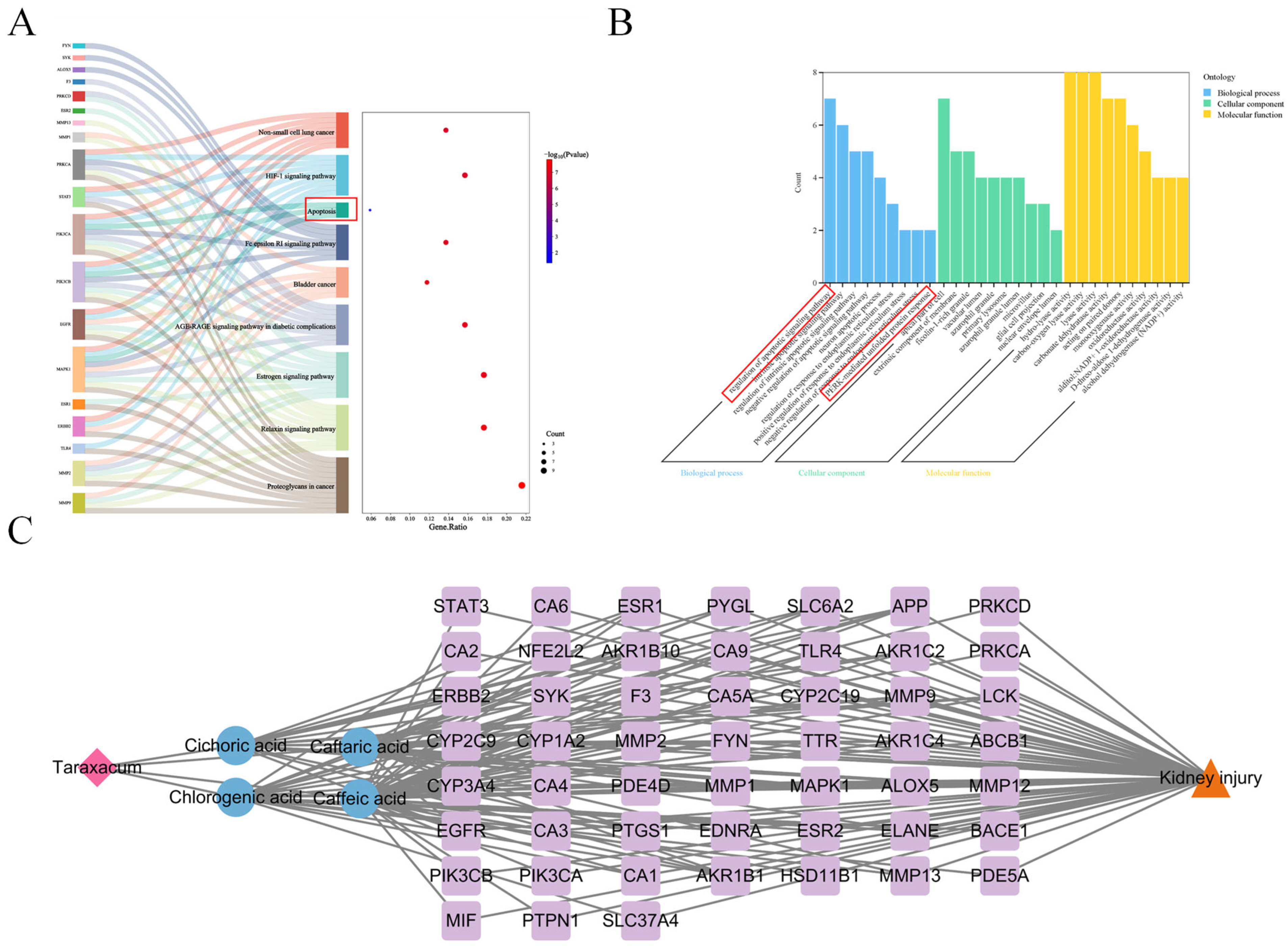
2.4. Network Construction and Enrichment Analysis
2.5. Molecular Docking
2.6. Animals and Experimental Design
2.7. Assessment of Biochemical Parameters and H&E Staining
2.8. Apoptotic Cell Assay and Immunofluorescence (IF) Staining
2.9. Western Blotting Analysis
2.10. Statistical Analysis
3. Results
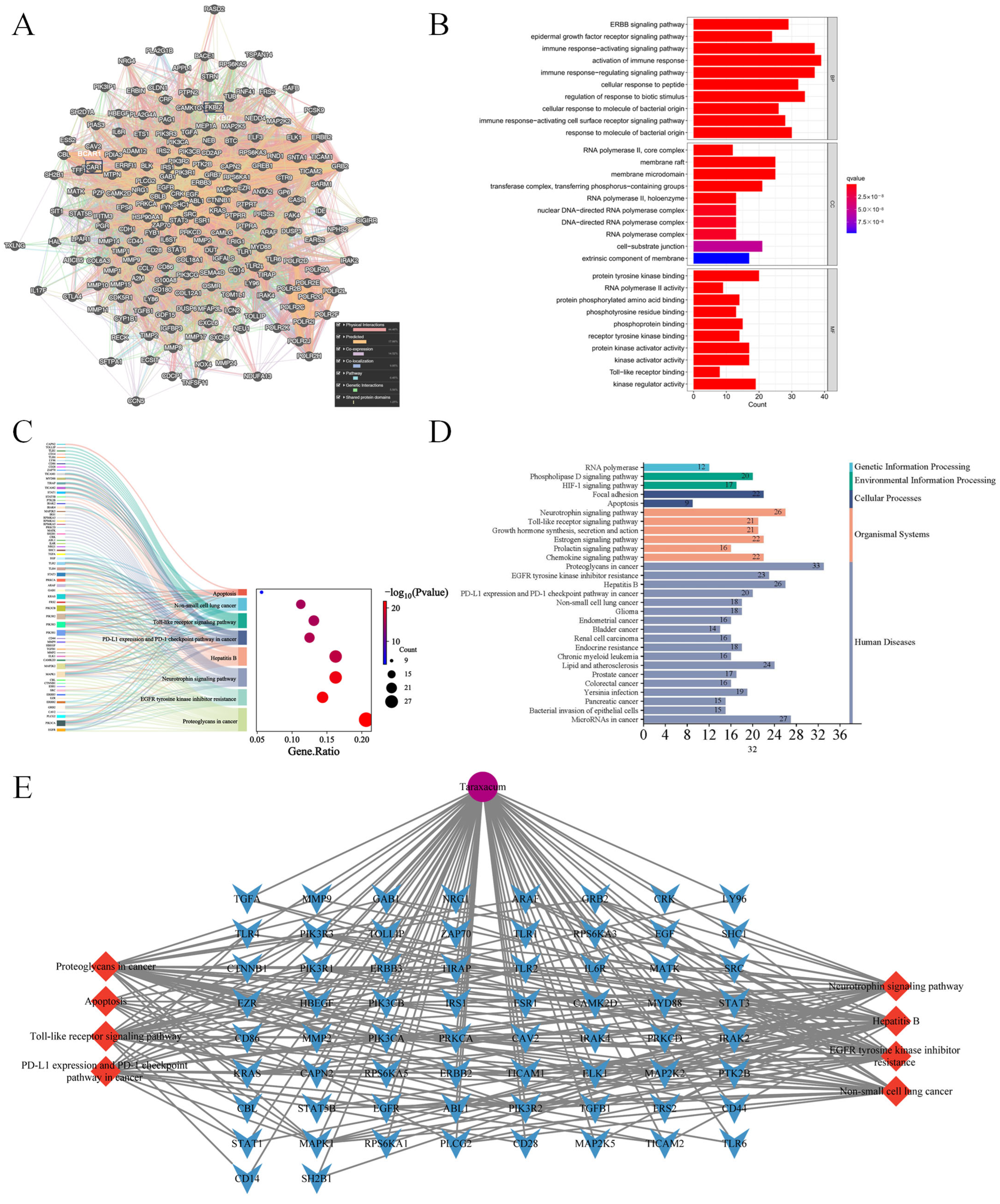
3.1. Network Pharmacology Revealed the Target Characteristics of Taraxacum for Kidney Injury Through GO Enrichment Analysis and KEGG Enrichment Analysis
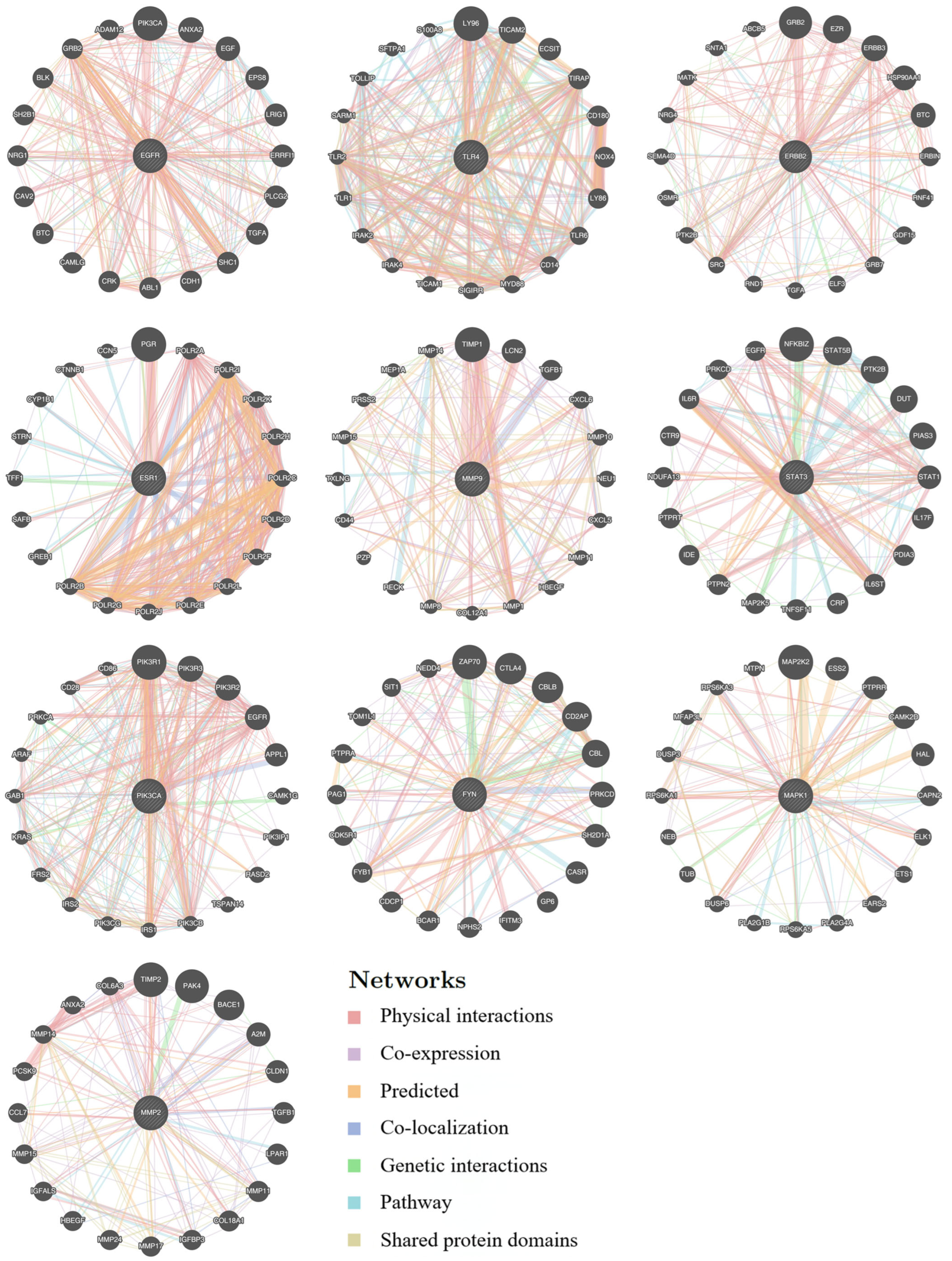
3.2. Functional Network Analysis of Top 10 Hub Targets Underlying Taraxacum’s Anti-Kidney Injury Effects Using GeneMANIA
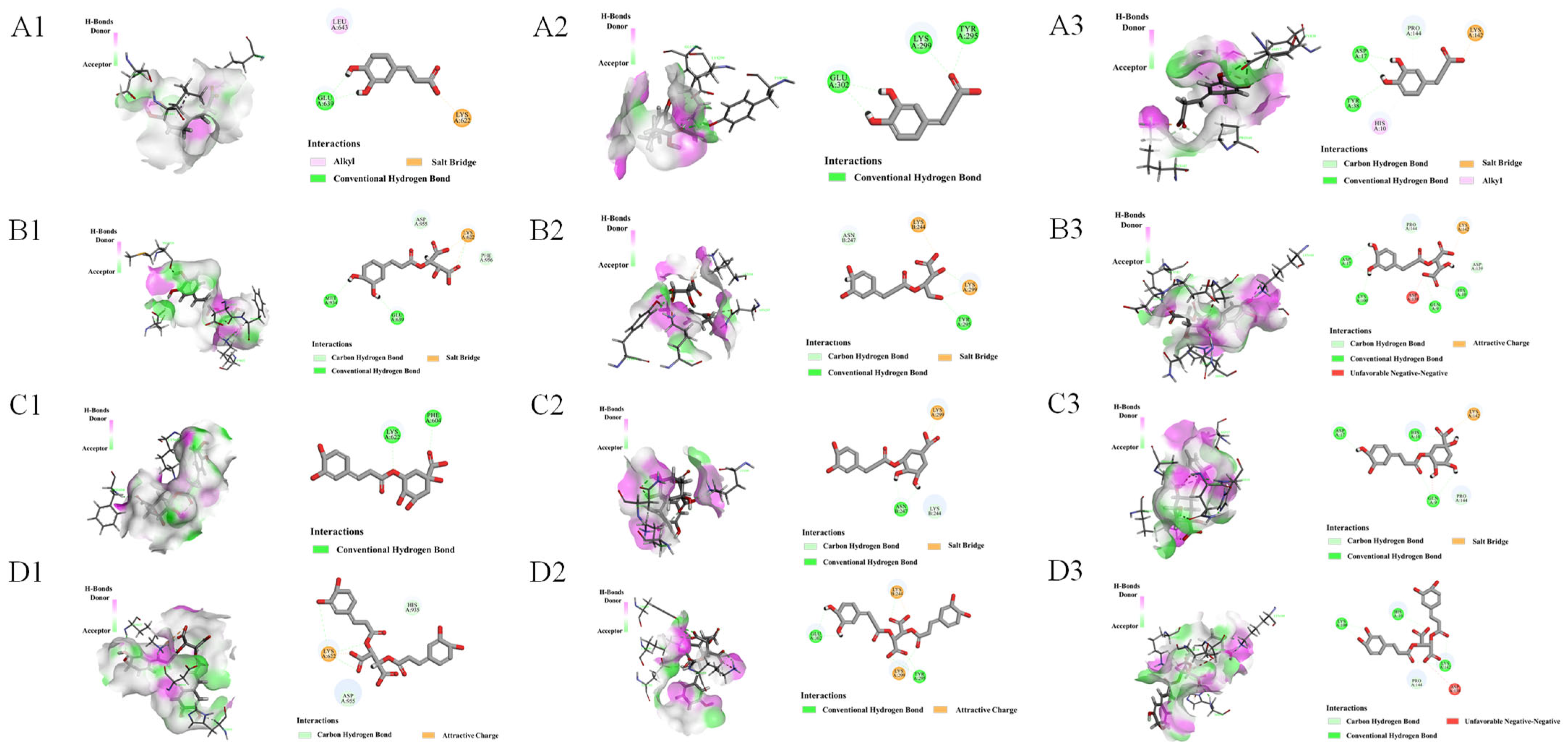
3.3. Molecular Docking of Active Compounds with Target Proteins
3.4. TRWE Ameliorated CP-Induced Oxidative Stress Injury
3.5. TRWE Reversed CP-Induced Kidney Dysfunction in Mice
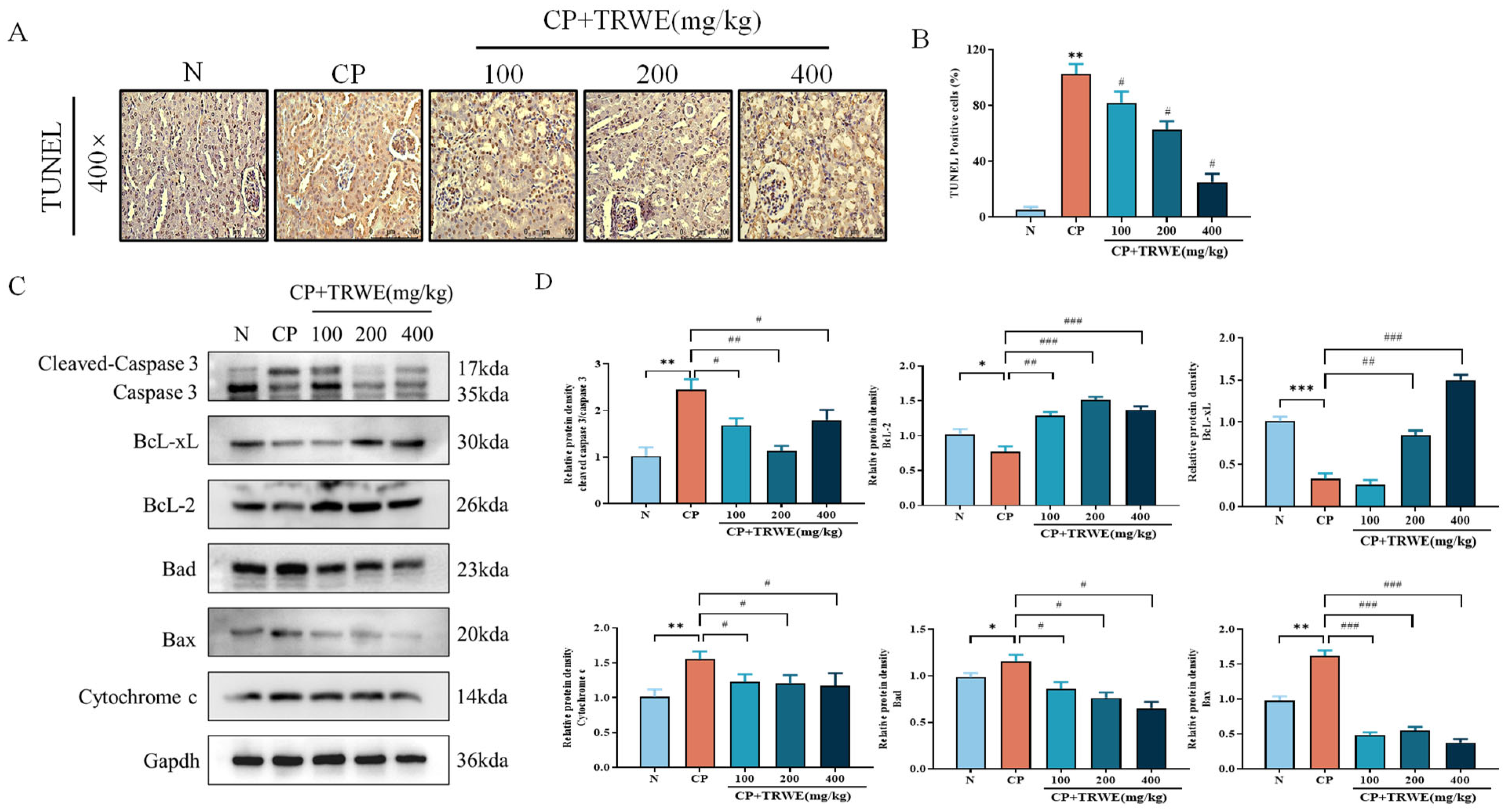
3.6. TRWE Inhibited CP-Induced Apoptosis in Kidney Tissues
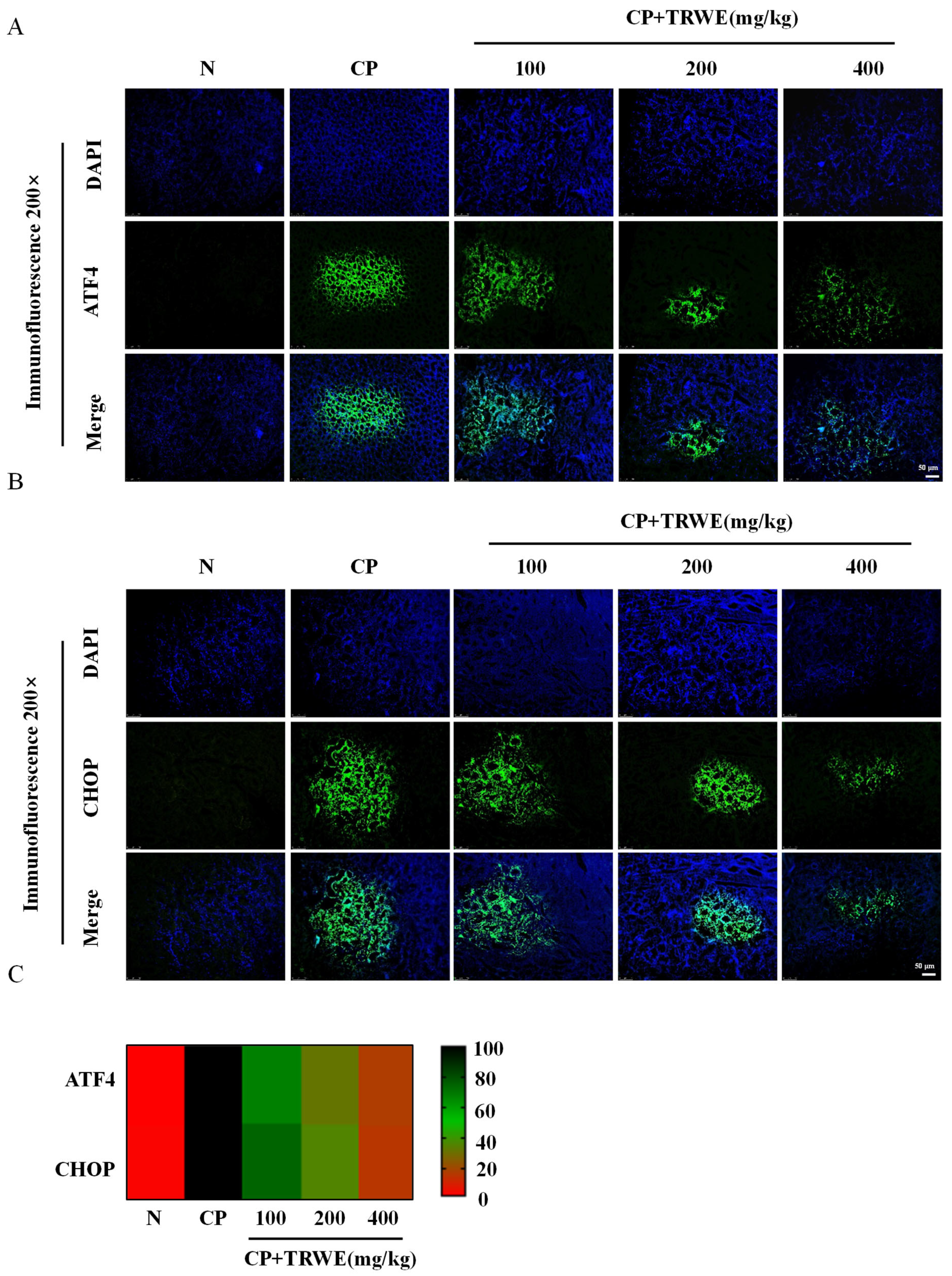
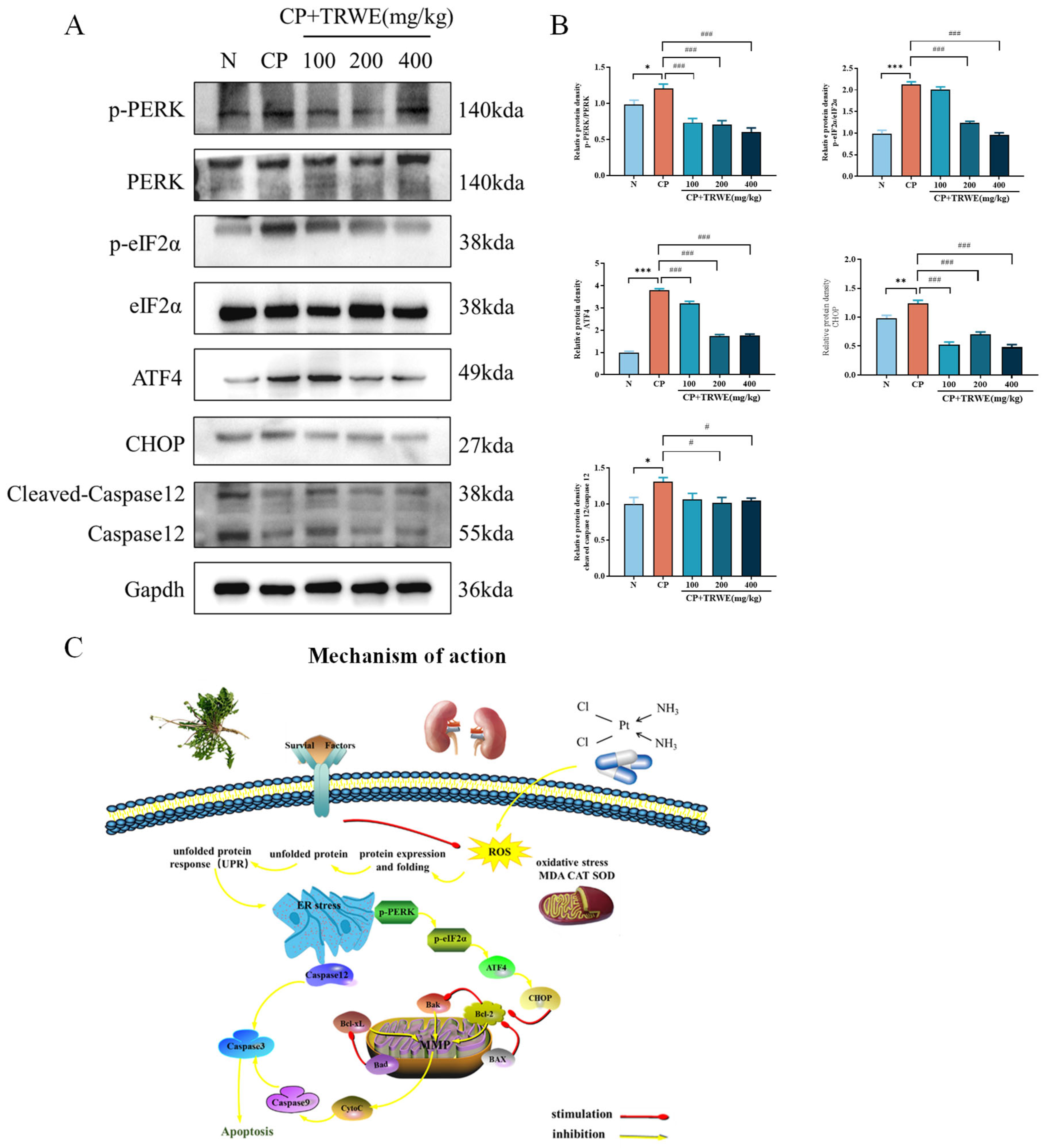
3.7. TRWE Reduced CP-Induced ERS Response
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CP | cisplatin |
| ER | endoplasmic reticulum |
| UPR | unfolded protein response |
| TRWE | water extract from Taraxacum roots |
| BUN | blood urea nitrogen |
| CRE | creatinine |
| MDA | malondialdehyde |
| SOD | superoxide dismutase |
| CAT | catalase |
| ERS | endoplasmic reticulum stress |
| PERK | protein kinase R-like ER kinase |
| eIF2α | eukaryotic initiation factor 2α |
| ATF4 | activating transcription factor 4 |
| CHOP | Children’s Hospital of Philadelphia |
| CTA | caftaric acid |
| CGA | chlorogenic acid |
| CA | caffeic acid |
| CCA | cichoric acid |
| AKI | acute kidney injury |
| ROS | reactive oxygen species |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
References
- Tang, C.; Livingston, M.J.; Safirstein, R.; Dong, Z. Cisplatin nephrotoxicity: New insights and therapeutic implications. Nat. Rev. Nephrol. 2023, 19, 53–72. [Google Scholar] [CrossRef]
- Iskander, A.; Yan, L.J. Cisplatin-Induced Kidney Toxicity: Potential Roles of Major NAD(+)-Dependent Enzymes and Plant-Derived Natural Products. Biomolecules 2022, 12, 1078. [Google Scholar] [CrossRef]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of cisplatin-induced acute kidney injury. Biomed. Res. Int. 2014, 2014, 967826. [Google Scholar] [CrossRef]
- Sung, C.Y.W.; Hayase, N.; Yuen, P.S.T.; Lee, J.; Fernandez, K.; Hu, X.; Cheng, H.; Star, R.A.; Warchol, M.E.; Cunningham, L.L. Macrophage depletion protects against cisplatin-induced ototoxicity and nephrotoxicity. Sci. Adv. 2024, 10, eadk9878. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.T.; Lai, W.Y.; Yeh, C.L.; Tseng, Y.T.; Peck, K.; Yang, P.C.; Lin, E.P. AptBCis1, An Aptamer-Cisplatin Conjugate, Is Effective in Lung Cancer Leptomeningeal Carcinomatosis. ACS Nano 2024, 18, 27905–27916. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.J.; Hou, J.G.; Liu, Y.; Zhang, R.B.; Jiang, S.; Ren, S.; Wang, Y.P.; Shen, Q.; Li, W.; Li, X.D.; et al. Supplementation of Saponins from Leaves of Panax quinquefolius Mitigates Cisplatin-Evoked Cardiotoxicity via Inhibiting Oxidative Stress-Associated Inflammation and Apoptosis in Mice. Antioxidants 2019, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kaufman, R.J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 2016, 529, 326–335. [Google Scholar] [CrossRef]
- Bi, Y.Y.; Chen, Q.; Yang, M.Y.; Xing, L.; Jiang, H.L. Nanoparticles targeting mutant p53 overcome chemoresistance and tumor recurrence in non-small cell lung cancer. Nat. Commun. 2024, 15, 2759. [Google Scholar] [CrossRef]
- Puthalakath, H.; O’Reilly, L.A.; Gunn, P.; Lee, L.; Kelly, P.N.; Huntington, N.D.; Hughes, P.D.; Michalak, E.M.; McKimm-Breschkin, J.; Motoyama, N.; et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 2007, 129, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.L.; Hung, J.H.; Huang, W. Association of the Hepatitis B Virus Large Surface Protein with Viral Infectivity and Endoplasmic Reticulum Stress-mediated Liver Carcinogenesis. Cells 2020, 9, 2052. [Google Scholar] [CrossRef]
- Wei, X.M.; Jiang, S.; Li, S.S.; Sun, Y.S.; Wang, S.H.; Liu, W.C.; Wang, Z.; Wang, Y.P.; Zhang, R.; Li, W. Endoplasmic Reticulum Stress-Activated PERK-eIF2α-ATF4 Signaling Pathway is Involved in the Ameliorative Effects of Ginseng Polysaccharides against Cisplatin-Induced Nephrotoxicity in Mice. ACS Omega 2021, 6, 8958–8966. [Google Scholar] [CrossRef]
- Jing, X.; Han, J.; Zhang, J.; Chen, Y.; Yuan, J.; Wang, J.; Neo, S.; Li, S.; Yu, X.; Wu, J. Long non-coding RNA MEG3 promotes cisplatin-induced nephrotoxicity through regulating AKT/TSC/mTOR-mediated autophagy. Int. J. Biol. Sci. 2021, 17, 3968–3980. [Google Scholar] [CrossRef]
- Wang, J.Q.; Liu, X.X.; Zhang, J.J.; Jiang, C.; Zheng, S.W.; Wang, Z.; Li, D.Y.; Li, W.; Shi, D.F. Amelioration of Cisplatin-Induced kidney injury by Arabinogalactan based on network pharmacology and molecular docking. J. Funct. Food. 2023, 104, 105504. [Google Scholar] [CrossRef]
- Doğan, D.; Meydan, İ.; Kömüroğlu, A.U. Protective Effect of Silymarin and Gallic Acid against Cisplatin-Induced Nephrotoxicity and Hepatotoxicity. Int. J. Clin. Pract. 2022, 2022, 6541026. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Poirrier, P.; Chamy, R.; Prüfer, D.; Schulze-Gronover, C.; Jorquera, L.; Ruiz, G. Taraxacum officinale and related species-An ethnopharmacological review and its potential as a commercial medicinal plant. J. Ethnopharmacol. 2015, 169, 244–262. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef]
- Qu, J.; Ke, F.; Yang, X.; Wang, Y.; Xu, H.; Li, Q.; Bi, K. Induction of P-glycoprotein expression by dandelion in tumor and heart tissues: Impact on the anti-tumor activity and cardiotoxicity of doxorubicin. Phytomedicine 2022, 104, 154275. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Zhang, X.; Song, H.; Zhang, Y. Dandelion (Taraxacum Genus): A Review of Chemical Constituents and Pharmacological Effects. Molecules 2023, 28, 5022. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Deng, X.; Yu, X.; Wang, W.; Yan, W.; Zhao, X.; Wang, X.; Bai, C.; Wang, Z.; Han, L. Taraxacum: A Review of Ethnopharmacology, Phytochemistry and Pharmacological Activity. Am. J. Chin. Med. 2024, 52, 183–215. [Google Scholar] [CrossRef]
- Kamal, F.Z.; Lefter, R.; Mihai, C.T.; Farah, H.; Ciobica, A.; Ali, A.; Radu, I.; Mavroudis, I.; Ech-Chahad, A. Chemical Composition, Antioxidant and Antiproliferative Activities of Taraxacum officinale Essential Oil. Molecules 2022, 27, 6477. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, D.; Wang, B.; Zhao, W.; Zhang, T.; Sutcharitchan, C.; Li, S. Network pharmacology: Advancing the application of large language models in traditional Chinese medicine research. Sci. Tradit. Chin. Med. 2025, 3, 113–123. [Google Scholar] [CrossRef]
- Liu, H.; Feng, X.; Wang, D.; Liu, L.; Liu, Y.; Liu, B.; Zhu, L.; Zhang, C.; Yang, W. Mechanism of Sishen Pills-Tongxie Yaofang in the treatment of ulcerative colitis based on network pharmacology and experimental verification. Sci. Tradit. Chin. Med. 2024, 2, 224–236. [Google Scholar] [CrossRef]
- Gu, X.; Hao, D.; Xiao, P. Research progress of Chinese herbal medicine compounds and their bioactivities: Fruitful 2020. Chin. Herb. Med. 2022, 14, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Qu, J.; Ke, F.; Liu, Z.; Yang, X.; Li, X.; Xu, H.; Li, Q.; Bi, K. Uncovering the mechanisms of dandelion against triple-negative breast cancer using a combined network pharmacology, molecular pharmacology and metabolomics approach. Phytomedicine 2022, 99, 153986. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, Y.; Bai, Y.Y.; Zhang, J.T.; Wang, L.Q.; Ren, S.; Li, X.D.; Hu, J.N.; Li, W. Platycodin D Improves Early Atherosclerosis in Type 2 Diabetes Mellitus by Regulating Endothelial Inflammation and Apoptosis. Am. J. Chin. Med. 2025, 53, 1887–1912. [Google Scholar] [CrossRef]
- Zhou, B.; Qian, Z.; Li, Q.; Gao, Y.; Li, M. Assessment of pulmonary infectious disease treatment with Mongolian medicine formulae based on data mining, network pharmacology and molecular docking. Chin. Herb. Med. 2022, 14, 432–448. [Google Scholar] [CrossRef] [PubMed]
- Rajadnya, R.; Sharma, N.; Mahajan, A.; Ulhe, A.; Patil, R.; Hegde, M.; Mali, A. Novel systems biology experimental pipeline reveals matairesinol’s antimetastatic potential in prostate cancer: An integrated approach of network pharmacology, bioinformatics, and experimental validation. Brief. Bioinform. 2024, 25, bbae466. [Google Scholar] [CrossRef]
- Xu, W.; Wu, J.; Yang, D.; Chen, Y.; Wu, X.; Wen, R.; Yan, L.; Li, C.; Yu, H. Anti-acute gastric ulcer resistance of Aurantii Fructus Immaturus juice processing Atractylodis Macrocephalae Rhizoma by regulating PTGS2, MAPK1, and KDR targets based on metabolomics and integrated network pharmacology analysis. Sci. Tradit. Chin. Med. 2024, 2, 121–137. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Park, S.; Saravanakumar, K.; Sathiyaseelan, A.; Wang, M.H. Ethyl Acetate Fraction of Helianthus tuberosus L. Induces Anti-Diabetic, and Wound-Healing Activities in Insulin-Resistant Human Liver Cancer and Mouse Fibroblast Cells. Antioxidants 2021, 10, 99. [Google Scholar] [CrossRef]
- Duan, Y.Y.; Mi, X.J.; Su, W.Y.; Tang, S.; Jiang, S.; Wang, Z.; Zhao, L.C.; Li, W. Trilobatin, an Active Dihydrochalcone from Lithocarpus polystachyus, Prevents Cisplatin-Induced Nephrotoxicity via Mitogen-Activated Protein Kinase Pathway-Mediated Apoptosis in Mice. ACS Omega 2022, 7, 37401–37409. [Google Scholar] [CrossRef]
- Hu, J.N.; Yang, J.Y.; Jiang, S.; Zhang, J.; Liu, Z.; Hou, J.G.; Gong, X.J.; Wang, Y.P.; Wang, Z.; Li, W. Panax quinquefolium saponins protect against cisplatin evoked intestinal injury via ROS-mediated multiple mechanisms. Phytomedicine 2021, 82, 153446. [Google Scholar] [CrossRef]
- Zhang, J.J.; Zhou, Y.D.; Liu, Y.B.; Wang, J.Q.; Li, K.K.; Gong, X.J.; Lin, X.H.; Wang, Y.P.; Wang, Z.; Li, W. Protective Effect of 20(R)-Ginsenoside Rg3 Against Cisplatin-Induced Renal Toxicity via PI3K/AKT and NF-kappa B Signaling Pathways Based on the Premise of Ensuring Anticancer Effect. Am. J. Chin. Med. 2021, 49, 1739–1756. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.J.; Hou, J.G.; Wang, Z.; Han, Y.; Ren, S.; Hu, J.N.; Chen, C.; Li, W. The protective effects of maltol on cisplatin-induced nephrotoxicity through the AMPK-mediated PI3K/Akt and p53 signaling pathways. Sci. Rep. 2018, 8, 15922. [Google Scholar] [CrossRef]
- Sha, J.Y.; Li, J.H.; Zhou, Y.D.; Yang, J.Y.; Liu, W.; Jiang, S.; Wang, Y.P.; Zhang, R.; Di, P.; Li, W. The p53/p21/p16 and PI3K/Akt signaling pathways are involved in the ameliorative effects of maltol on D-galactose-induced liver and kidney aging and injury. Phytother. Res. 2021, 35, 4411–4424. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, M.X.; Li, H.M.; Sun, J.; Huang, L.Q.; Yuan, Y. Potential benefits of Rehmanniae Radix after ancient rice-steaming process in promotion of antioxidant activity in rats’ health. Food Sci. Nutr. 2023, 11, 5532–5542. [Google Scholar] [CrossRef]
- Li, Y.; Hou, J.G.; Liu, Z.; Gong, X.J.; Hu, J.N.; Wang, Y.P.; Liu, W.C.; Lin, X.H.; Wang, Z.; Li, W. Alleviative effects of 20(R)-Rg3 on HFD/STZ-induced diabetic nephropathy via MAPK/NF-κB signaling pathways in C57BL/6 mice. J. Ethnopharmacol. 2021, 267, 113500. [Google Scholar] [CrossRef]
- Nguepi Tsopmejio, I.S.; Zhang, J.T.; Wang, Z.; Tian, Z.F.; Zhu, H.Y.; Zhang, J.; Ren, S.; Liu, S.; Liu, J.H.; Hu, J.N.; et al. Comparative study of ginsenoside Rg2, 20(S)-protopanaxatriol, and AFG from ginseng on aging-related kidney injury in SAMP8 mice. J. Ethnopharmacol. 2025, 348, 119807. [Google Scholar] [CrossRef]
- Li, W.; Yan, M.H.; Liu, Y.; Liu, Z.; Wang, Z.; Chen, C.; Zhang, J.; Sun, Y.S. Ginsenoside Rg5 Ameliorates Cisplatin-Induced Nephrotoxicity in Mice through Inhibition of Inflammation, Oxidative Stress, and Apoptosis. Nutrients 2016, 8, 566. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.N.; Leng, J.; Shen, Q.; Liu, Y.; Li, X.D.; Wang, S.H.; Li, H.P.; Wang, Z.; Wang, Y.P.; Li, W. Platycodin D suppresses cisplatin-induced cytotoxicity by suppressing ROS-mediated oxidative damage, apoptosis, and inflammation in HEK-293 cells. J. Biochem. Mol. Toxicol. 2021, 35, e22624. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.W.; Xie, L.Y.; Qi, M.H.; Ren, S.; Wang, Y.Q.; Hu, J.N.; Wang, Z.; Tang, S.; Zhang, J.T.; Li, W. Platycodin D Ameliorates Cognitive Impairment in Type 2 Diabetes Mellitus Mice via Regulating PI3K/Akt/GSK3β Signaling Pathway. J. Agric. Food Chem. 2024, 72, 12516–12528. [Google Scholar] [CrossRef]
- Li, R.Y.; Zhang, W.Z.; Yan, X.T.; Hou, J.G.; Wang, Z.; Ding, C.B.; Liu, W.C.; Zheng, Y.N.; Chen, C.; Li, Y.R.; et al. Arginyl-fructosyl-glucose, a Major Maillard Reaction Product of Red Ginseng, Attenuates Cisplatin-Induced Acute Kidney Injury by Regulating Nuclear Factor κB and Phosphatidylinositol 3-Kinase/Protein Kinase B Signaling Pathways. J. Agric. Food Chem. 2019, 67, 5754–5763. [Google Scholar] [CrossRef]
- Liu, Z.; Qu, C.Y.; Li, J.X.; Wang, Y.F.; Li, W.; Wang, C.Z.; Wang, D.S.; Song, J.; Sun, G.Z.; Yuan, C.S. Hypoglycemic and Hypolipidemic Effects of Malonyl Ginsenosides from American Ginseng (Panax quinquefolius L.) on Type 2 Diabetic Mice. ACS Omega 2021, 6, 33652–33664. [Google Scholar] [CrossRef]
- Xing, J.J.; Mi, X.J.; Hou, J.G.; Cai, E.B.; Zheng, S.W.; Wang, S.H.; Wang, Z.; Chen, C.; Li, W. Maltol mitigates cisplatin-evoked cardiotoxicity via inhibiting the PI3K/Akt signaling pathway in rodents in vivo and in vitro. Phytother. Res. 2022, 36, 1724–1735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, D.; Zhou, W.; Wang, L.; Wang, B.; Zhang, T.; Li, S. Network pharmacology: Towards the artificial intelligence-based precision traditional Chinese medicine. Brief. Bioinform. 2024, 25, bbad518. [Google Scholar] [CrossRef]
- Yao, Y.X.; Xu, Y.L.; Liu, B.W.; Yang, H.; Li, S.Y.; Zhazo, L.L.; Liu, T. Identification and verification of effective components of Huanghuai for dysfunctional uterine bleeding based on network pharmacology and molecular docking. Chin. Herb. Med. 2021, 13, 177–188. [Google Scholar] [CrossRef] [PubMed]
- González-Nicolás, M.Á.; González-Guerrero, C.; Goicoechea, M.; Boscá, L.; Valiño-Rivas, L.; Lázaro, A. Biomarkers in contrast-induced acute kidney injury: Towards a new perspective. Int. J. Mol. Sci. 2024, 25, 3438. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, Y.; Ramírez, V.; Robledo-Márquez, K.; García-Rojas, N.; Rojas-Morales, P.; Arango, N.; Pedraza-Chaverri, J.; Medina-Campos, O.N.; Pérez-Rojas, J.M.; Flores-Ramírez, R.; et al. Stenocereus huastecorum-fruit juice concentrate protects against cisplatin-induced nephrotoxicity by nitric oxide pathway activity and antioxidant and antiapoptotic effects. Food Res. Int. 2022, 160, 111337. [Google Scholar] [CrossRef]
- Mapuskar, K.A.; Pulliam, C.F.; Tomanek-Chalkley, A.; Rastogi, P.; Wen, H.; Dayal, S.; Griffin, B.R.; Zepeda-Orozco, D.; Sindler, A.L.; Anderson, C.M.; et al. The antioxidant and anti-inflammatory activities of avasopasem manganese in age-associated, cisplatin-induced renal injury. Redox Biol. 2024, 70, 103022. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Shen, J.D.; Li, Y.Y.; Huang, Q. Antidepressant effects of the water extract from Taraxacum officinale leaves and roots in mice. Pharm. Biol. 2014, 52, 1028–1032. [Google Scholar] [CrossRef]
- Chang, X.; Tian, M.; Zhang, Q.; Liu, F.; Gao, J.; Li, S.; Liu, H.; Hou, X.; Li, L.; Li, C.; et al. Grape seed proanthocyanidin extract ameliorates cisplatin-induced testicular apoptosis via PI3K/Akt/mTOR and endoplasmic reticulum stress pathways in rats. J. Food Biochem. 2021, 45, e13825. [Google Scholar] [CrossRef]
- Merighi, A.; Lossi, L. Endoplasmic Reticulum Stress Signaling and Neuronal Cell Death. Int. J. Mol. Sci. 2022, 23, 15186. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Z.; Rui, X.; Wang, Y.; Wang, Y.; Zhou, Y.; Chen, R.; Chen, Y.; Wang, Y.; Li, S.; et al. GSDMD enhances cisplatin-induced apoptosis by promoting the phosphorylation of eIF2α and activating the ER-stress response. Cell Death Discov. 2022, 8, 114. [Google Scholar] [CrossRef]
- Chen, H.; Miao, Y.; Bian, A.; Ye, J.; Wang, J.; Cong, X.; Jian, S.; Yi, Z.; Liang, L.; Sun, Z.; et al. A novel small-molecule activator of unfolded protein response suppresses castration-resistant prostate cancer growth. Cancer Lett. 2022, 532, 215580. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Fan, X.; Chen, S.; Deng, L.; Jiang, L.; Yang, S.; Dong, Z. Geraniol-Mediated Suppression of Endoplasmic Reticulum Stress Protects against Cerebral Ischemia-Reperfusion Injury via the PERK-ATF4-CHOP Pathway. Int. J. Mol. Sci. 2023, 24, 544. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xiao, W.; Li, Z.; Li, X.; Chuang, P.Y.; Jim, B.; Zhang, W.; Wei, C.; Wang, N.; Jia, W.; et al. Erratum: RTN1 mediates progression of kidney disease by inducing ER stress. Nat. Commun. 2015, 6, 8710. [Google Scholar] [CrossRef] [PubMed]

| Phytochemical | Target | Phytochemical | Target | Phytochemical | Target | Phytochemical | Target |
|---|---|---|---|---|---|---|---|
| Caffeic acid | MAPK1 | Caftaric acid | MAPK1 | Cichoric acid | MAPK1 | Chlorogenic acid | ABCB1 |
| TLR4 | MMP9 | MMP9 | MMP2 | ||||
| MMP9 | TTR | TTR | EDNRA | ||||
| TTR | EGFR | EGFR | ELANE | ||||
| EGFR | MMP2 | MMP2 | APP | ||||
| PIK3CA | MMP1 | MMP1 | SLC37A4 | ||||
| STAT3 | ELANE | APP | PRKCD | ||||
| NFE2L2 | APP | ERBB2 | PRKCA | ||||
| F3 | ERBB2 | PTGS1 | AKR1B1 | ||||
| MMP2 | PTGS1 | AKR1B1 | MMP12 | ||||
| MMP1 | AKR1B1 | ESR1 | CA9 | ||||
| ELANE | ESR1 | MMP12 | CA2 | ||||
| MIF | MMP12 | FYN | PDE4D | ||||
| APP | FYN | PYGL | MMP13 | ||||
| ERBB2 | MMP13 | SLC6A2 | BACE1 | ||||
| CYP2C9 | PYGL | AKR1C2 | PDE5A | ||||
| CYP3A4 | SLC6A2 | LCK | PYGL | ||||
| PTGS1 | AKR1C2 | AKR1C4 | CA1 | ||||
| AKR1B1 | LCK | AKR1B10 | AKR1B10 | ||||
| ESR1 | AKR1C4 | ||||||
| CA9 | AKR1B10 | ||||||
| CYP2C19 | |||||||
| FYN | |||||||
| ALOX5 | |||||||
| PIK3CB | |||||||
| PTPN1 | |||||||
| CA2 | |||||||
| SYK | |||||||
| CYP1A2 | |||||||
| CA4 | |||||||
| ESR2 | |||||||
| HSD11B1 | |||||||
| SLC6A2 | |||||||
| AKR1C2 | |||||||
| LCK | |||||||
| AKR1C4 | |||||||
| CA3 | |||||||
| CA1 | |||||||
| CA6 | |||||||
| AKR1B10 | |||||||
| CA5A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, R.; Tang, S.; Gao, X.; Qi, S.; Ren, S.; Wang, Z.; Li, X.; Li, W. Bioinformatics-Guided Network Pharmacology Exploration of Taraxacum Officinale’s Renoprotective Effects Against Cisplatin-Induced Nephrotoxicity. Nutrients 2025, 17, 3092. https://doi.org/10.3390/nu17193092
Hu R, Tang S, Gao X, Qi S, Ren S, Wang Z, Li X, Li W. Bioinformatics-Guided Network Pharmacology Exploration of Taraxacum Officinale’s Renoprotective Effects Against Cisplatin-Induced Nephrotoxicity. Nutrients. 2025; 17(19):3092. https://doi.org/10.3390/nu17193092
Chicago/Turabian StyleHu, Ruiyi, Shan Tang, Xufei Gao, Simin Qi, Shen Ren, Zi Wang, Xindian Li, and Wei Li. 2025. "Bioinformatics-Guided Network Pharmacology Exploration of Taraxacum Officinale’s Renoprotective Effects Against Cisplatin-Induced Nephrotoxicity" Nutrients 17, no. 19: 3092. https://doi.org/10.3390/nu17193092
APA StyleHu, R., Tang, S., Gao, X., Qi, S., Ren, S., Wang, Z., Li, X., & Li, W. (2025). Bioinformatics-Guided Network Pharmacology Exploration of Taraxacum Officinale’s Renoprotective Effects Against Cisplatin-Induced Nephrotoxicity. Nutrients, 17(19), 3092. https://doi.org/10.3390/nu17193092







