Sweet Taste Adaptation to Sugars, Sucralose, and Their Blends: A Human and Rodent Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Study 1: Human Psychophysics Study Methods
2.1.1. Participants
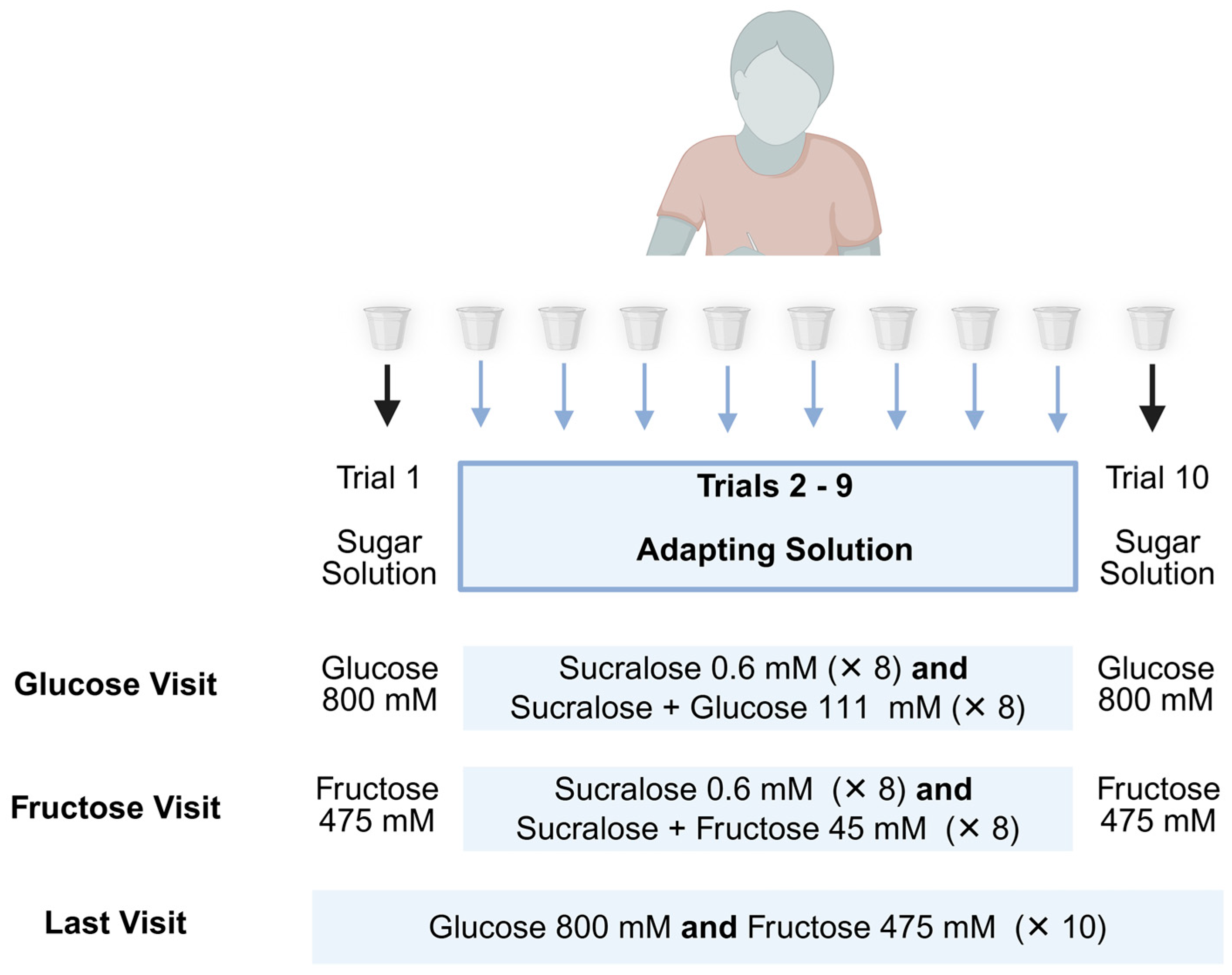
2.1.2. Procedure
2.1.3. Sensory Assessments Using the General Labeled Magnitude Scale
2.1.4. Sensory Stimuli
2.1.5. Taste Intensity Test
2.1.6. Sweet Taste Adaptation
2.2. Study 2: In Vivo Calcium Imaging During Sweet Adaptation in a Mouse Model
2.2.1. Mouse Preparation and Housing
2.2.2. Imaging Process & Tastants
2.3. Data Analysis
2.3.1. Study 1: Psychophysics Data Analysis
2.3.2. Study 2: Molecular Data Analysis
3. Results
3.1. Study 1: Human Psychophysics Study Results
3.1.1. Subject Characteristics
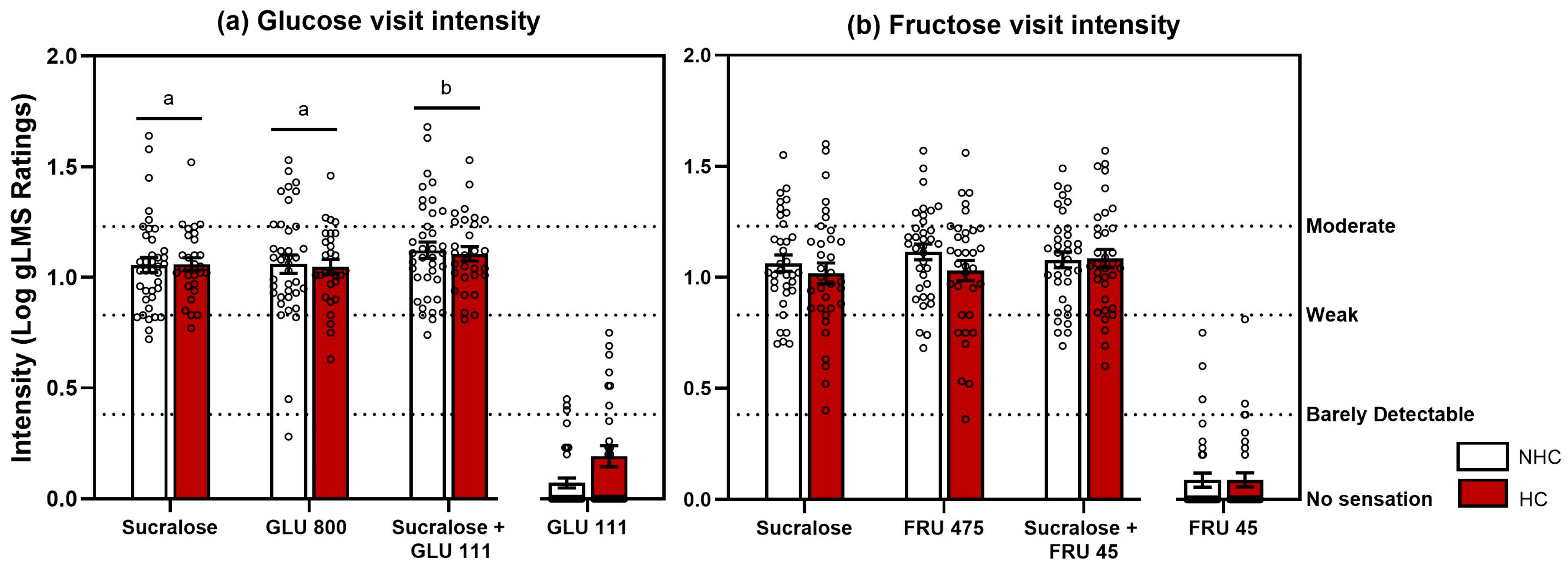
3.1.2. Taste Intensity Test Results
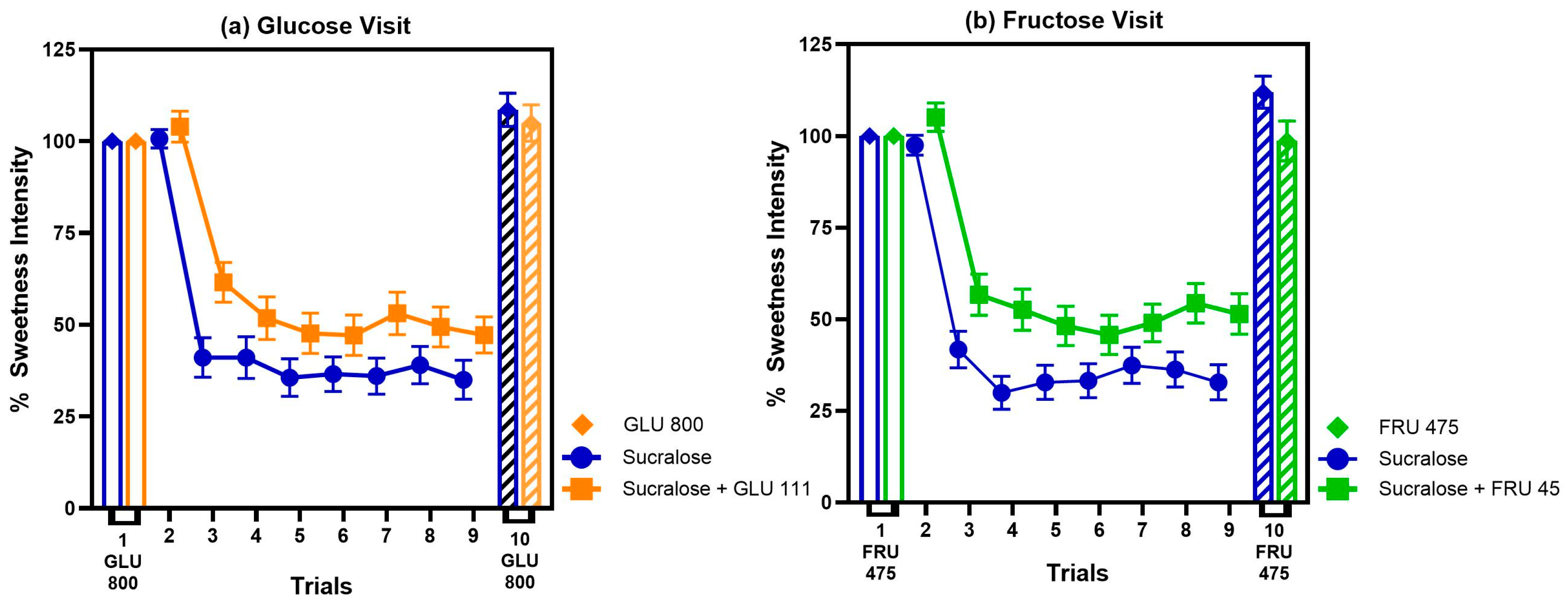
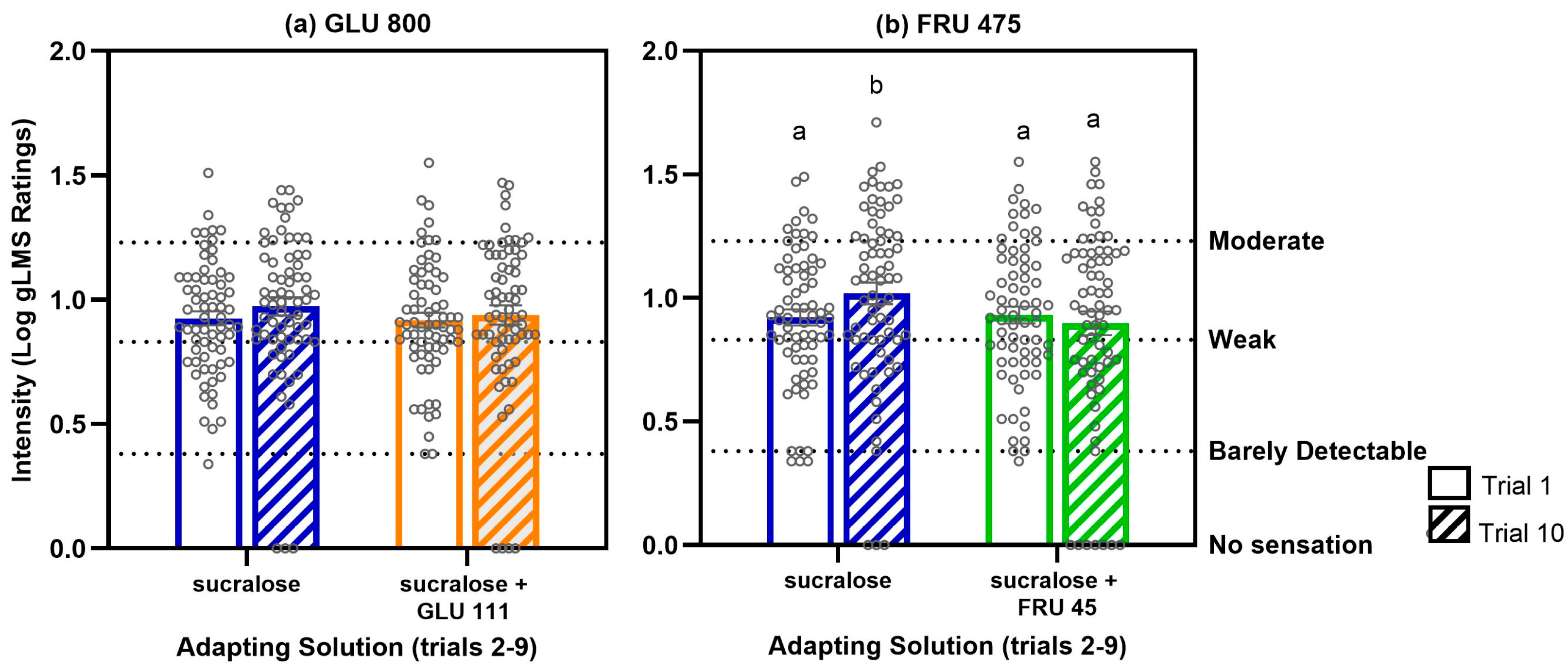
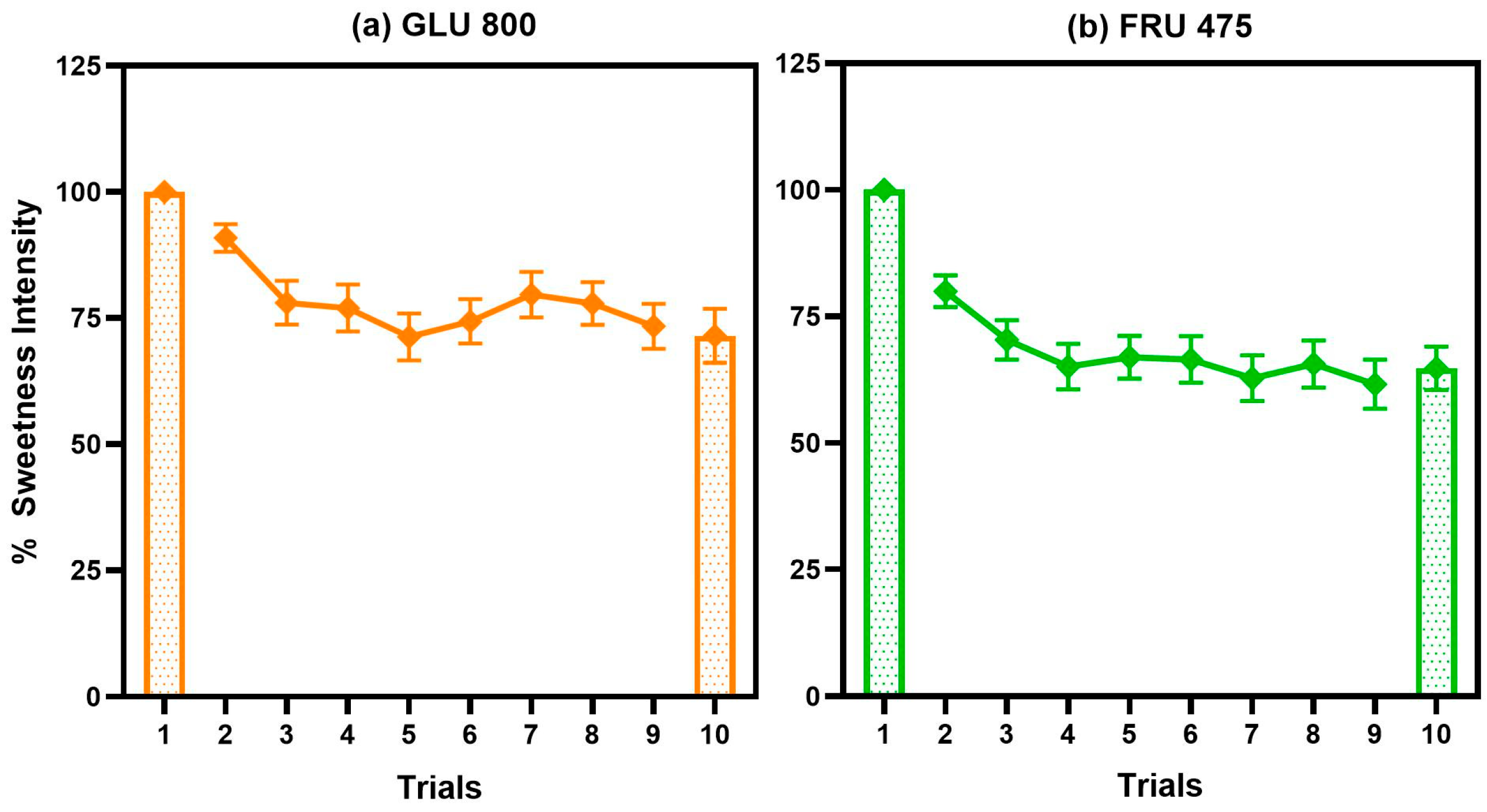
3.1.3. Sweet Taste Adaptation Results
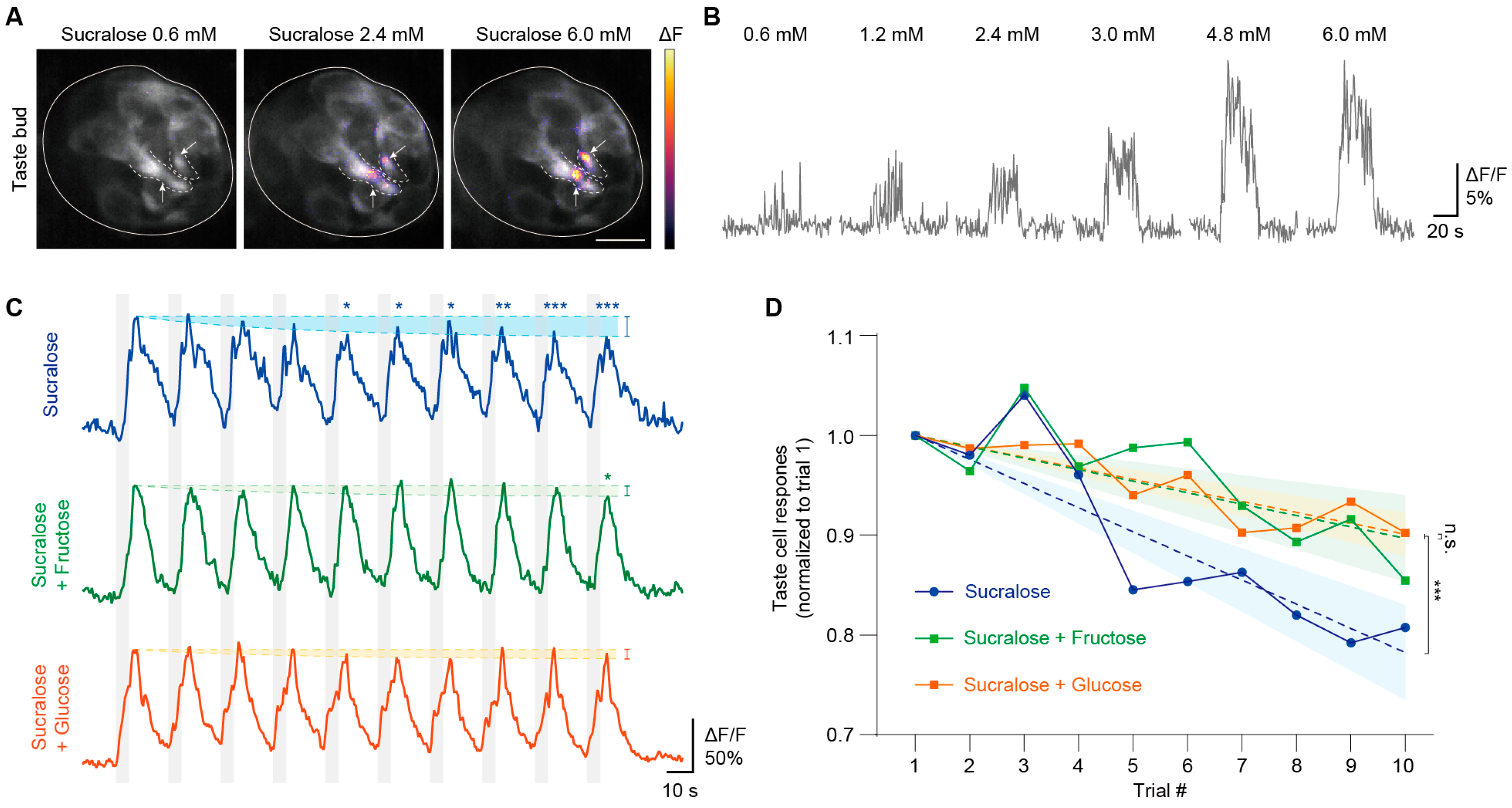
3.1.4. In Vivo Imaging of Taste Buds When Adapting to Sucralose and Its Mixtures with Sugars
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LCS | Low-Calorie Sweetener |
| HC | Habitual Low-Calorie Sweetener Consumers |
| NHC | Non-Habitual Low-Calorie Sweetener Consumers |
| GLU | Glucose |
| FRU | Fructose |
| GCPR | G-Coupled protein receptors |
| gLMS | General Labeled Magnitude Scale |
References
- Juen, Z.; Lu, Z.; Yu, R.; Chang, A.N.; Wang, B.; Fitzpatrick, A.W.P.; Zuker, C.S. The Structure of Human Sweetness. Cell 2025, 88, 4141–4153.e18. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Vigues, S.; Hobbs, J.R.; Conn, G.L.; Munger, S.D. Distinct Contributions of T1R2 and T1R3 Taste Receptor Subunits to the Detection of Sweet Stimuli. Curr. Biol. 2005, 15, 1948–1952. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Guthrie, B.; Kim, S.K.; Balanda, S.; Kubicek, J.; Murtaza, B.; Khan, N.A.; Khakbaz, P.; Su, J.; Goddard, W.A. Steviol Rebaudiosides Bind to Four Different Sites of the Human Sweet Taste Receptor (T1R2/T1R3) Complex Explaining Confusing Experiments. Commun. Chem. 2024, 7, 236. [Google Scholar] [CrossRef]
- Servant, G.; Tachdjian, C.; Tang, X.Q.; Werner, S.; Zhang, F.; Li, X.; Kamdar, P.; Petrovic, G.; Ditschun, T.; Java, A.; et al. Positive Allosteric Modulators of the Human Sweet Taste Receptor Enhance Sweet Taste. Proc. Natl. Acad. Sci. USA 2010, 107, 4746–4751. [Google Scholar] [CrossRef]
- Kawasaki, M.; Kidera, Y.; Goda, R.; Taketani, C.; Ide, M.; Fujii, W.; Nakagita, T.; Misaka, T. Distinct Potency of Compounds Targeting the T1R3 Subunit in Modulating the Response of Human Sweet and Umami Taste Receptors. Sci. Rep. 2025, 15, 27167. [Google Scholar] [CrossRef]
- Wark, B.; Lundstrom, B.N.; Fairhall, A. Sensory Adaptation. Curr. Opin. Neurobiol. 2007, 17, 423–429. [Google Scholar] [CrossRef]
- Low, Y.Q.; Lacy, K.; Keast, R. The Role of Sweet Taste in Satiation and Satiety. Nutrients 2014, 6, 3431–3450. [Google Scholar] [CrossRef]
- Dubose, C.N.; Meiselman, H.L. Note: Individual Differences in Taste Adaptation. Chem. Senses 1979, 4, 177–181. [Google Scholar] [CrossRef]
- Theunissen, M.J.M.; Kroeze, J.H.A.; Schifferstein, H.N.J. Method of Stimulation, Mouth Movements, Concentration, and Viscosity: Effects on the Degree of Taste Adaptation. Percept. Psychophys. 2000, 62, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Green, B.G.; Nachtigal, D. Temperature Affects Human Sweet Taste via At Least Two Mechanisms. Chem. Senses 2015, 40, 391–399. [Google Scholar] [CrossRef]
- Hewson, L.; Tarrega, A. Sensory Adaptation. In Time-Dependent Measures of Perception in Sensory Evaluation; Wiley: Hoboken, NJ, USA, 2017; pp. 67–87. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Cahn, H.; Lindley, M.G. Multiple Receptor Sites Mediate Sweetness: Evidence from Cross Adaptation. Pharmacol. Biochem. Behav. 1981, 15, 377–388. [Google Scholar] [CrossRef]
- Rodriguez, Y.A.; Roebber, J.K.; Dvoryanchikov, G.; Makhoul, V.; Roper, S.D.; Chaudhari, N. “Tripartite Synapses” in Taste Buds: A Role for Type I Glial-like Taste Cells. J. Neurosci. 2021, 41, 9860–9871. [Google Scholar] [CrossRef]
- Park, G.Y.; Lee, G.; Yoon, J.; Han, J.; Choi, P.; Kim, M.; Lee, S.; Park, C.; Wu, Z.; Li, Y.; et al. Glia-like Taste Cells Mediate an Intercellular Mode of Peripheral Sweet Adaptation. Cell 2025, 188, 141–156.e16. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Booth, B.J.; Carr, B.T.; Losee, M.L.; Sattely-Miller, E.A.; Graham, B.G. Investigation of Synergism in Binary Mixtures of Sweeteners. Brain Res. Bull. 1995, 38, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, S.S.; Sattely-Miller, E.A.; Graham, B.G.; Zervakis, J.; Butchko, H.H.; Stargel, W.W. Effect of Repeated Presentation on Sweetness Intensity of Binary and Ternary Mixtures of Sweeteners. Chem. Senses 2003, 28, 219–229. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Sattely-Miller, E.A.; Bishay, I.E. Time to Maximum Sweetness Intensity of Binary and Ternary Blends of Sweeteners. Food Qual. Prefer. 2007, 18, 405–415. [Google Scholar] [CrossRef]
- Bornstein, B.L.; Wiet, S.G.; Pombo, M. Sweetness Adaptation of Some Carbohydrate and High Potency Sweeteners. J. Food Sci. 1993, 58, 595–598. [Google Scholar] [CrossRef]
- Myers, E.A.; Passaro, E.M.; Hedrick, V.E. The Reproducibility and Comparative Validity of a Non-Nutritive Sweetener Food Frequency Questionnaire. Nutrients 2018, 10, 334. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Mennella, J.A. Effects of Cigarette Smoking and Family History of Alcoholism on Sweet Taste Perception and Food Cravings in Women. Alcohol. Clin. Exp. Res. 2007, 31, 1891–1899. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Bradley, D.; Eagon, J.C.; Sullivan, S.; Abumrad, N.A.; Klein, S. Changes in Taste Perception and Eating Behavior after Bariatric Surgery-Induced Weight Loss in Women. Obesity 2014, 22, E13–E20. [Google Scholar] [CrossRef] [PubMed]
- Wasalathanthri, S.; Hettiarachchi, P.; Prathapan, S. Sweet Taste Sensitivity in Pre-Diabetics, Diabetics and Normoglycemic Controls: A Comparative Cross Sectional Study. BMC Endocr. Disord. 2014, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L.; Shaman, P.; Dann, M. Development of the University of Pennsylvania Smell Identification Test: A Standardized Microencapsulated Test of Olfactory Function. Physiol. Behav. 1984, 32, 489–502. [Google Scholar] [CrossRef]
- Bartoshuk, L.M.; Duffy, V.B.; Green, B.G.; Hoffman, H.J.; Ko, C.W.; Lucchina, L.A.; Marks, L.E.; Snyder, D.J.; Weiffenbach, J.M. Valid Across-Group Comparisons with Labeled Scales: The GLMS versus Magnitude Matching. Physiol. Behav. 2004, 82, 109–114. [Google Scholar] [CrossRef]
- Romo-Romo, A.; Aguilar-Salinas, C.A.; Brito-Córdova, G.X.; Gómez-Díaz, R.A.; Almeda-Valdes, P. Sucralose Decreases Insulin Sensitivity in Healthy Subjects: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2018, 108, 485–491. [Google Scholar] [CrossRef]
- De Graaf, C.; Frijters, J.E.R.; Van Trijp, H.C.M. Taste Interaction between Glucose and Fructose Assessed by Functional Measurement. Percept. Psychophys. 1987, 41, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Breslin, P.A.S.; Beauchamp, G.K.; Pugh, E.N. Monogeusia for Fructose, Glucose, Sucrose, and Maltose. Percept. Psychophys. 1996, 58, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Choi, M. Comprehensive Functional Screening of Taste Sensation in Vivo. bioRxiv 2018, 371682. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Pnevmatikakis, E.A.; Giovannucci, A. NoRMCorre: An Online Algorithm for Piecewise Rigid Motion Correction of Calcium Imaging Data. J. Neurosci. Methods 2017, 291, 83–94. [Google Scholar] [CrossRef]
- Thurman, S.T.; Guizar-Sicairos, M.; Fienup, J.R. Efficient Subpixel Image Registration Algorithms. Opt. Lett. 2008, 33, 156–158. [Google Scholar] [CrossRef]
- Breslin, P.A.S.; Izumi, A.; Tharp, A.; Ohkuri, T.; Yokoo, Y.; Flammer, L.J.; Rawson, N.E.; Margolskee, R.F. Evidence That Human Oral Glucose Detection Involves a Sweet Taste Pathway and a Glucose Transporter Pathway. PLoS ONE 2021, 16, e0256989. [Google Scholar] [CrossRef]
- von Molitor, E.; Riedel, K.; Krohn, M.; Hafner, M.; Rudolf, R.; Cesetti, T. Sweet Taste Is Complex: Signaling Cascades and Circuits Involved in Sweet Sensation. Front. Hum. Neurosci. 2021, 15, 667709. [Google Scholar] [CrossRef]
- Ayya, N.; Lawless, H.T. Quantitative and Qualitative Evaluation of High-Intensity Sweeteners and Sweetener Mixtures. Chem. Senses 1992, 17, 245–259. [Google Scholar] [CrossRef]
- Tan, V.W.K.; Wee, M.S.M.; Tomic, O.; Forde, C.G. Temporal Sweetness and Side Tastes Profiles of 16 Sweeteners Using Temporal Check-All-That-Apply (TCATA). Food Res. Int. 2019, 121, 39–47. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Zhao, X.; Liu, B. Current Advances and Future Aspects of Sweetener Synergy: Properties, Evaluation Methods and Molecular Mechanisms. Appl. Sci. 2022, 12, 5096. [Google Scholar] [CrossRef]
- Mora, M.; Wijaya, F.; Jiang, G.; Gibney, P.; Dando, R. Sensory Profiling of Natural Sweeteners and Sucrose–Sweetener Binary Mixtures. J. Food Sci. 2023, 88, 2984–2995. [Google Scholar] [CrossRef]
- Ventura, E.E.; Davis, J.N.; Goran, M.I. Sugar Content of Popular Sweetened Beverages Based on Objective Laboratory Analysis: Focus on Fructose Content. Obesity 2011, 19, 868–874. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Pecore, S.D.; Booth, B.J.; Losee, M.L.; Carr, B.T.; Sattely-Miller, E.; Graham, B.G.; Warwick, Z.S. Adaptation of Sweeteners in Water and in Tannic Acid Solutions. Physiol. Behav. 1994, 55, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Mcburney, D.H. Gustatory Cross Adaptation between Sweet-Tasting Compounds. Percept. Psychophys. 1972, 11, 225–227. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Rother, K.I. Trends in the Consumption of Low-Calorie Sweeteners. Physiol. Behav. 2016, 164, 446–450. [Google Scholar] [CrossRef]
- Primo, M.J.; Fonseca-Rodrigues, D.; Almeida, A.; Teixeira, P.M.; Pinto-Ribeiro, F. Sucrose Preference Test: A Systematic Review of Protocols for the Assessment of Anhedonia in Rodents. Eur. Neuropsychopharmacol. 2023, 77, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Verharen, J.P.H.; de Jong, J.W.; Zhu, Y.; Lammel, S. A Computational Analysis of Mouse Behavior in the Sucrose Preference Test. Nat. Commun. 2023, 14, 2419. [Google Scholar] [CrossRef]
- Wu, A.; Dvoryanchikov, G.; Pereira, E.; Chaudhari, N.; Roper, S.D. Breadth of Tuning in Taste Afferent Neurons Varies with Stimulus Strength. Nat. Commun. 2015, 6, 8171. [Google Scholar] [CrossRef]
- Tordoff, M.G. Taste Solution Preferences of C57BL/6J and 129X1/SvJ Mice: Influence of Age, Sex, and Diet. Chem. Senses 2007, 32, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Barretto, R.P.J.; Gillis-Smith, S.; Chandrashekar, J.; Yarmolinsky, D.A.; Schnitzer, M.J.; Ryba, N.J.P.; Zuker, C.S. The Neural Representation of Taste Quality at the Periphery. Nature 2015, 517, 373–376. [Google Scholar] [CrossRef] [PubMed]

| Solution | Abbreviation | Glucose Visit | Fructose Visit | Last Visit | ||
|---|---|---|---|---|---|---|
| Taste Intensity | Adaptation | Taste Intensity | Adaptation | Adaptation | ||
| Sucralose 0.6 mM | SCL | + | + | + | + | - |
| Glucose 111 mM | GLU 111 | + | - | - | - | - |
| Glucose 800 mM | GLU 800 | + | + | - | - | + |
| Sucralose blend G | SCL + GLU 111 | + | + | - | - | - |
| Fructose 45 mM | FRU 45 | - | - | + | - | - |
| Fructose 475 mM | FRU 475 | - | - | + | + | + |
| Sucralose blend F | SCL + FRU 45 | - | - | + | + | - |
| Glucose Visit (n = 67) | Fructose Visit (n = 69) | Last Visit (n = 60) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NHC (n = 38) | HC (n = 29) | p-Value | NHC (n = 35) | HC (n = 34) | p-Value | NHC (n = 31) | HC (n = 29) | p-Value | |
| Sex, n | |||||||||
| Male | 16 | 13 | 0.8 | 15 | 17 | 0.5 | 13 | 15 | 0.6 |
| Female | 22 | 16 | 20 | 17 | 18 | 14 | |||
| Race, n | |||||||||
| White | 23 | 22 | 0.3 | 23 | 26 | 0.2 | 18 | 23 | 0.08 |
| Asian | 11 | 3 | 10 | 3 | 10 | 2 | |||
| Black | 3 | 3 | 2 | 3 | 3 | 3 | |||
| Mixed race | 1 | 1 | - | 1 | - | 1 | |||
| Did not Specify | - | - | - | 1 | - | - | |||
| Age, years | 28.0 (21.0–34.0) | 31.0 (25.0–35.0) | 0.1 | 28.0 (21.0–36.0) | 31.5 (26.0–41.0) | 0.1 | 28.0 (21.0–37.0) | 32.0 (28.0–41.0) | 0.2 |
| Weight, kg | 73.4 (20.0) | 86.9 (17.3) | 0.005 | 73.3 (19.9) | 88.1 (15.9) | 0.001 | 74.1 (20.6) | 88.5 (17.1) | 0.005 |
| Height, m | 1.69 (0.08) | 1.70 (0.1) | 0.7 | 1.70 (0.08) | 1.72 (0.09) | 0.4 | 1.69 (0.09) | 1.71 (0.1) | 0.4 |
| BMI, kg/m2 | 25.6 (6.9) | 30.0 (5.9) | 0.007 | 25.4 (6.6) | 29.9 (5.8) | 0.003 | 25.8 (6.8) | 30.3 (6.0) | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okoye, S.I.; Kim, M.; Petty, S.; Choi, M.; Pepino, M.Y. Sweet Taste Adaptation to Sugars, Sucralose, and Their Blends: A Human and Rodent Perspective. Nutrients 2025, 17, 3075. https://doi.org/10.3390/nu17193075
Okoye SI, Kim M, Petty S, Choi M, Pepino MY. Sweet Taste Adaptation to Sugars, Sucralose, and Their Blends: A Human and Rodent Perspective. Nutrients. 2025; 17(19):3075. https://doi.org/10.3390/nu17193075
Chicago/Turabian StyleOkoye, Stephanie I., Minjae Kim, Sara Petty, Myunghwan Choi, and Marta Yanina Pepino. 2025. "Sweet Taste Adaptation to Sugars, Sucralose, and Their Blends: A Human and Rodent Perspective" Nutrients 17, no. 19: 3075. https://doi.org/10.3390/nu17193075
APA StyleOkoye, S. I., Kim, M., Petty, S., Choi, M., & Pepino, M. Y. (2025). Sweet Taste Adaptation to Sugars, Sucralose, and Their Blends: A Human and Rodent Perspective. Nutrients, 17(19), 3075. https://doi.org/10.3390/nu17193075







