Fractions from Sea Buckthorn Seeds and Their Bioactive Ingredients as Modulators of Human Blood Platelet Response In Vitro: The Role of Thermal Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
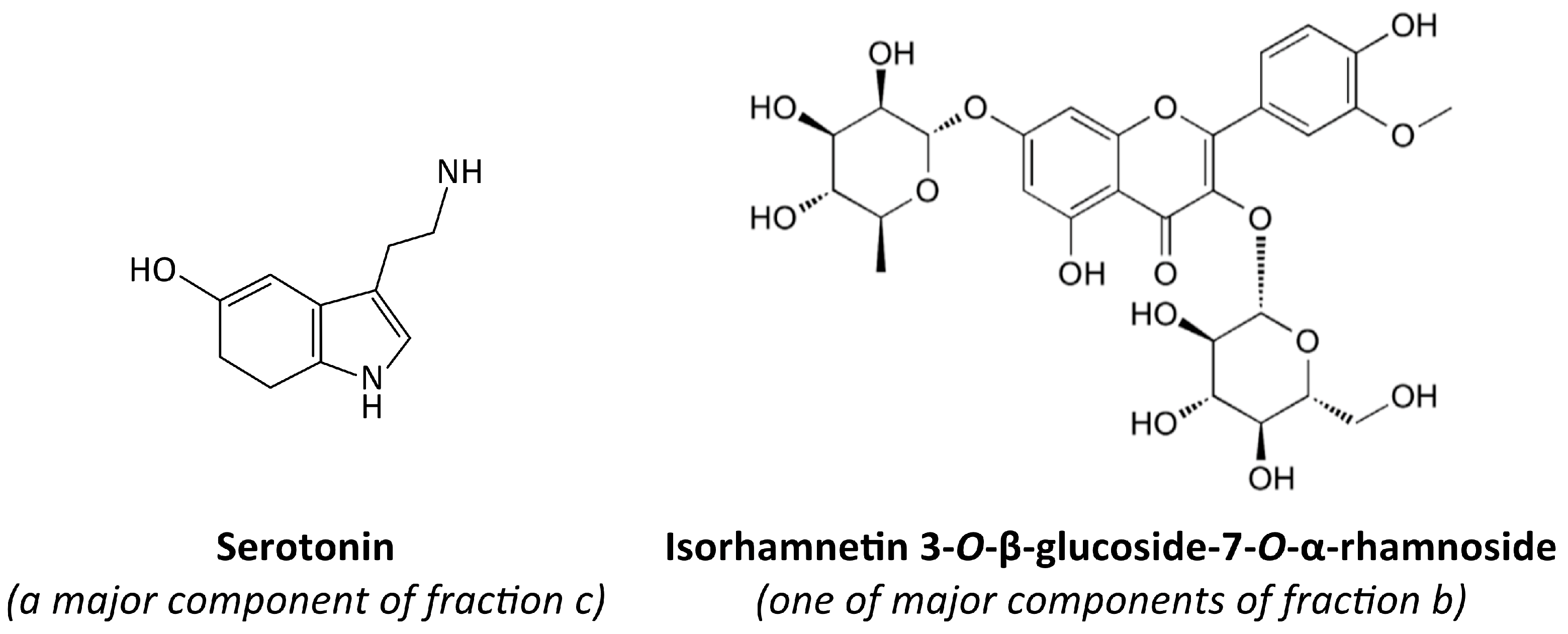
2.3. Preparation of Fractions b, c, d, and g, and Their Chemical Characteristics
2.4. Preparation of Stock Solutions of the Fractions and Tested Chemical Compounds for Bioassays
2.5. Blood Samples and Isolation of Blood Platelets
2.6. Total Thrombus-Formation Analysis System (T-TAS; PL-Chip)
2.7. Flow Cytometry (Measurement of Blood Platelet Activation in Whole Blood)
2.8. Blood Platelet Adhesion (Using Washed Blood Platelets)
2.9. COX-1 Activity
2.10. Data Analysis
3. Results
3.1. Measurement of the Exposition of P-Selectin and the Active Form of GPIIb/IIIa on the Surface of Platelets (In Whole Blood)
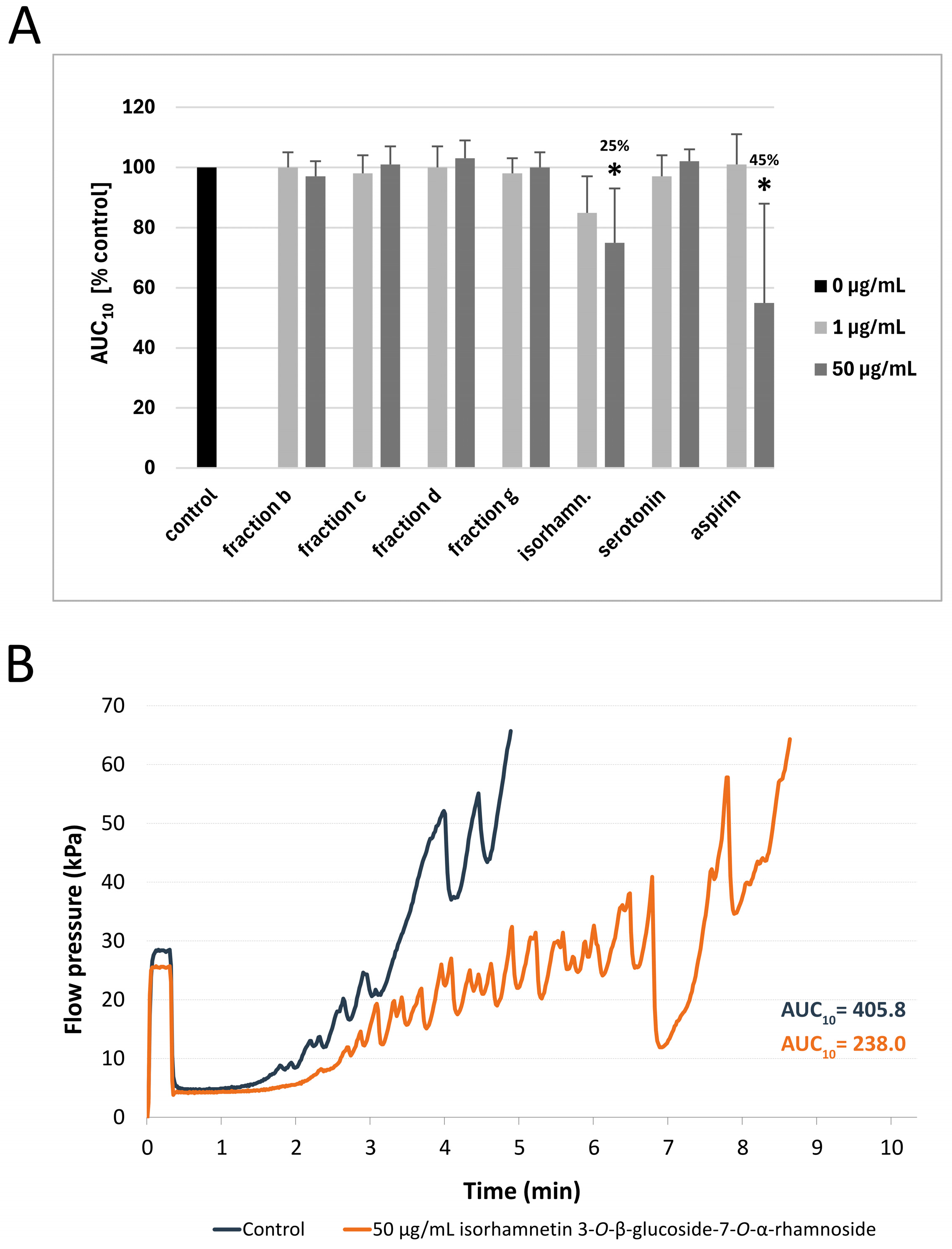
3.2. Measurement of Thrombus Formation with T-TAS (PL-Chip) in Whole Blood
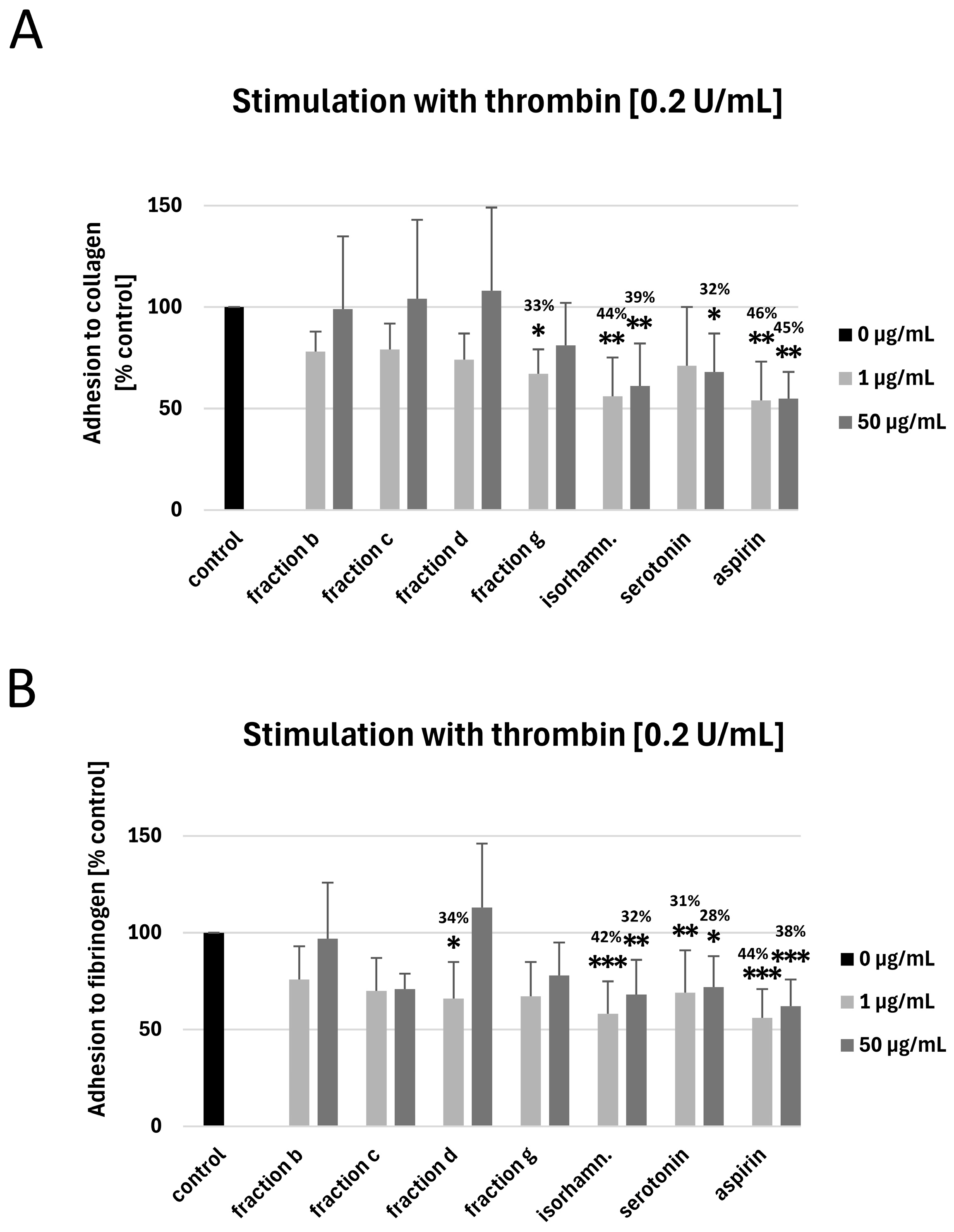
3.3. Measurement of Blood Platelet Adhesion to Collagen and Fibrinogen
3.4. Measurement of COX-1 Activity
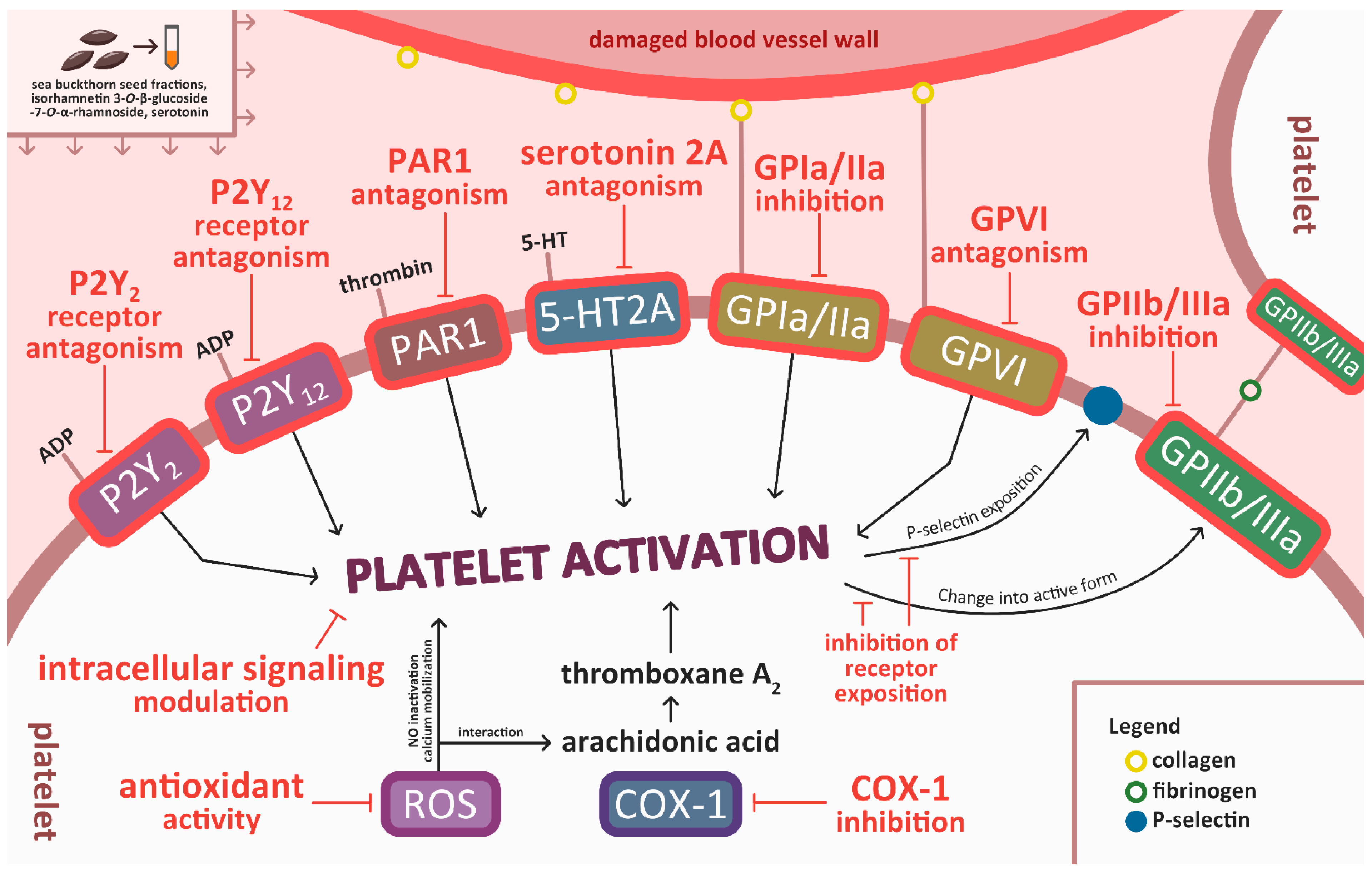
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Taylor, R.M.; Haslam, R.L.; Herbert, J.; Whatnall, M.C.; Trijsburg, L.; de Vries, J.H.M.; Josefsson, M.S.; Koochek, A.; Nowicka, P.; Neuman, N.; et al. Diet Quality and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Cohort Studies. Nutr. Diet. 2024, 81, 35–50. [Google Scholar] [CrossRef]
- Heneghan, C.; Kiely, M.; Lyons, J.; Lucey, A. The Effect of Berry-Based Food Interventions on Markers of Cardiovascular and Metabolic Health: A Systematic Review of Randomized Controlled Trials. Mol. Nutr. Food Res. 2018, 62, 1700645. [Google Scholar] [CrossRef]
- Ed Nignpense, B.; Chinkwo, K.A.; Blanchard, C.L.; Santhakumar, A.B. Polyphenols: Modulators of Platelet Function and Platelet Microparticle Generation? Int. J. Mol. Sci. 2019, 21, 146. [Google Scholar] [CrossRef]
- Diab, A.; Dastmalchi, L.N.; Gulati, M.; Michos, E.D. A Heart-Healthy Diet for Cardiovascular Disease Prevention: Where Are We Now? Vasc. Health Risk Manag. 2023, 19, 237–253. [Google Scholar] [CrossRef]
- Wang, T.; Nova, A.; Cassidy, S.; Livingstone, K.M.; Fazia, T.; Mitra, S.; Kroeger, C.M.; Masedunskas, A.; Bernardinelli, L.; Willett, W.C.; et al. Association of Healthy Predominantly Plant-Based Diet with Reduced Cardiovascular Disease Incidence and Mortality and Development of Novel Heart-Protective Diet Index. Nutrients 2025, 17, 2675. [Google Scholar] [CrossRef]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Esrafil, M.; Akter, S.; Alam, M.J.; Alim, M.A.; Reza, M.S.A.; Zubair, M.A.; Joy, P.R.; Jahan, M.; Khatun, M. Development and Quality Evaluation of Novel Biscuits by Utilizing Fruits and Vegetables Seed. Food Res. 2024, 8, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Miolla, R.; Ottomano Palmisano, G.; Roma, R.; Caponio, F.; Difonzo, G.; De Boni, A. Functional Foods Acceptability: A Consumers’ Survey on Bread Enriched with Oenological By-Products. Foods 2023, 12, 2014. [Google Scholar] [CrossRef] [PubMed]
- Agirbas, H.E.T.; Yavuz-Duzgun, M.; Ozcelik, B. Valorization of Fruit Seed Flours: Rheological Characteristics of Composite Dough and Cake Quality. J. Food Meas. Charact. 2022, 16, 3117–3129. [Google Scholar] [CrossRef]
- Ali, A.; Islam, A.; Pal, T.K. The Effect of Microwave Roasting on the Antioxidant Properties of the Bangladeshi Groundnut Cultivar. Acta Sci. Pol. Technol. Aliment. 2016, 15, 429–438. [Google Scholar] [CrossRef]
- Yoon, S.; Jeong, H.; Jo, S.M.; Hong, S.J.; Park, H.; Ban, Y.; Youn, M.Y.; Shin, E.-C. Physicochemical and Chemosensory Properties of Pomegranate (Punica granatum L.) Seeds Under Various Oven-Roasting Conditions. Food Chem. 2024, 446, 138907. [Google Scholar] [CrossRef]
- Oracz, J.; Prejzner, M.; Grzelczyk, J.; Kowalska, G.; Żyżelewicz, D. Bioactive Compounds, Antioxidant Activity and Sensory Properties of Northern Red Oak (Quercus rubra L., Syn. Q. Borealis F. Michx) Seeds Affected by Roasting Conditions. Molecules 2023, 28, 2299. [Google Scholar] [CrossRef]
- Ali, A.; Singh, T.; Kumar, R.R.; T., V.; Kundu, A.; Singh, S.P.; Meena, M.C.; Satyavathi, C.T.; Praveen, S.; Goswami, S. Effect of Thermal Treatments on the Matrix Components, Inherent Glycemic Potential, and Bioaccessibility of Phenolics and Micronutrients in Pearl Millet Rotis. Food Funct. 2023, 14, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Suri, K.; Singh, B.; Kaur, A.; Yadav, M.P.; Singh, N. Influence of Microwave Roasting on Chemical Composition, Oxidative Stability and Fatty Acid Composition of Flaxseed (Linum usitatissimum L.) Oil. Food Chem. 2020, 326, 126974. [Google Scholar] [CrossRef] [PubMed]
- Skalski, B.; Rywaniak, J.; Szustka, A.; Żuchowski, J.; Stochmal, A.; Olas, B. Anti-Platelet Properties of Phenolic and Nonpolar Fractions Isolated from Various Organs of Elaeagnus rhamnoides (L.) A. Nelson in Whole Blood. Int. J. Mol. Sci. 2021, 22, 3282. [Google Scholar] [CrossRef] [PubMed]
- Skalski, B.; Rywaniak, J.; Żuchowski, J.; Stochmal, A.; Olas, B. The Changes of Blood Platelet Reactivity in the Presence of Elaeagnus rhamnoides (L.) A. Nelson Leaves and Twig Extract in Whole Blood. Biomed. Pharmacother. 2023, 162, 114594. [Google Scholar] [CrossRef]
- Pang, X.; Zhao, J.; Zhang, W.; Zhuang, X.; Wang, J.; Xu, R.; Xu, Z.; Qu, W. Antihypertensive Effect of Total Flavones Extracted from Seed Residues of Hippophae rhamnoides L. in Sucrose-Fed Rats. J. Ethnopharmacol. 2008, 117, 325–331. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Q.; Pang, Z.R.; Pan, M.R.; Zhang, W. Flavonoid-Enriched Extract from Hippophae rhamnoides Seed Reduces High Fat Diet Induced Obesity, Hypertriglyceridemia, and Hepatic Triglyceride Accumulation in C57BL/6 Mice. Pharm. Biol. 2017, 55, 1207. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Zhu, D.; Zhu, X.; Pang, X.; Qu, W. Hypolipidaemic and Hypoglycaemic Effects of Total Flavonoids from Seed Residues of Hippophae rhamnoides L. in Mice Fed a High-Fat Diet. J. Sci. Food Agric. 2011, 91, 1446–1451. [Google Scholar] [CrossRef]
- Kopčeková, J.; Mrázová, J.; Fatrcová-Šramková, K.; Habánová, M.; Gažarová, M.; Lenártová, P. Benefits of Sea Buckthorn Juice Consumption in Women of Productive Age with Hypercholesterolemia. Rocz. Panstw. Zakl. Hig. 2023, 74, 187–193. [Google Scholar] [CrossRef]
- Li, G.; Chu, M.; Tong, Y.; Liang, Y.; Wang, S.; Ma, C.; Wang, Z.; Zhou, W. Protective Effects of Hippophae rhamnoides L. Phenylpropanoids on Doxorubicin-Induced Cardiotoxicity in Zebrafish. Molecules 2022, 27, 8858. [Google Scholar] [CrossRef]
- Suchal, K.; Bhatia, J.; Malik, S.; Malhotra, R.K.; Gamad, N.; Goyal, S.; Nag, T.C.; Arya, D.S.; Ojha, S. Seabuckthorn Pulp Oil Protects against Myocardial Ischemia–Reperfusion Injury in Rats through Activation of Akt/ENOS. Front. Pharmacol. 2016, 7, 155. [Google Scholar] [CrossRef]
- Sławińska, N.; Żuchowski, J.; Stochmal, A.; Olas, B. Extract from Sea Buckthorn Seeds—A Phytochemical, Antioxidant, and Hemostasis Study; Effect of Thermal Processing on Its Chemical Content and Biological Activity In Vitro. Nutrients 2023, 15, 686. [Google Scholar] [CrossRef]
- Sławińska, N.; Żuchowski, J.; Stochmal, A.; Olas, B. Anti-Platelet Activity of Sea Buckthorn Seeds and Its Relationship with Thermal Processing. Foods 2024, 13, 2400. [Google Scholar] [CrossRef]
- Sławińska, N.; Żuchowski, J.; Stochmal, A.; Olas, B. Comparative Phytochemical, Antioxidant, and Hemostatic Studies of Fractions from Raw and Roasted Sea Buckthorn Seeds in Vitro. Sci. Rep. 2024, 14, 21175. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5202, Serotonin. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Serotonin (accessed on 20 August 2025).
- Żuchowski, J.; Pecio, Ł.; Marciniak, B.; Kontek, R.; Stochmal, A. Unusual Isovalerylated Flavonoids from the Fruit of Sea Buckthorn (Elaeagnus rhamnoides) Grown in Sokółka, Poland. Phytochemistry 2019, 163, 178–186. [Google Scholar] [CrossRef]
- Lis, B.; Rolnik, A.; Jedrejek, D.; Soluch, A.; Stochmal, A.; Olas, B. Dandelion (Taraxacum officinale L.) Root Components Exhibit Anti-Oxidative and Antiplatelet Action in an in Vitro Study. J. Funct. Foods 2019, 59, 16–24. [Google Scholar] [CrossRef]
- Walkowiak, B.; Kęsy, A.; Michalec, L. Microplate Reader—A Convenient Tool in Studies of Blood Coagulation. Thromb. Res. 1997, 87, 95–103. [Google Scholar] [CrossRef]
- Hosokawa, K.; Ohnishi, T.; Kondo, T.; Fukasawa, M.; Koide, T.; Maruyama, I.; Tanaka, K.A. A Novel Automated Microchip Flow-Chamber System to Quantitatively Evaluate Thrombus Formation and Antithrombotic Agents Under Blood Flow Conditions. J. Thromb. Haemost. 2011, 9, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Morel, A.; Rywaniak, J.; Bijak, M.; Miller, E.; Niwald, M.; Saluk, J. Flow Cytometric Analysis Reveals the High Levels of Platelet Activation Parameters in Circulation of Multiple Sclerosis Patients. Mol. Cell. Biochem. 2017, 430, 69. [Google Scholar] [CrossRef] [PubMed]
- Rolnik, A.; Skalski, B.; Stochmal, A.; Olas, B. Preparations from Selected Cucurbit Vegetables as Antiplatelet Agents. Sci. Rep. 2021, 11, 22694. [Google Scholar] [CrossRef] [PubMed]
- Bellavite, P.; Andrioli, G.; Guzzo, P.; Arigliano, P.; Chirumbolo, S.; Manzato, F.; Santonastaso, C. A Colorimetric Method for the Measurement of Platelet Adhesion in Microtiter Plates. Anal. Biochem. 1994, 216, 444–450. [Google Scholar] [CrossRef]
- Kannan, M.; Ahmad, F.; Saxena, R. Platelet Activation Markers in Evaluation of Thrombotic Risk Factors in Various Clinical Settings. Blood Rev. 2019, 37, 100583. [Google Scholar] [CrossRef]
- Gremmel, T.; Frelinger, A.; Michelson, A. Platelet Physiology. Semin. Thromb. Hemost. 2016, 42, 191–204. [Google Scholar] [CrossRef]
- Tomaiuolo, M.; Brass, L.F.; Stalker, T.J. Regulation of Platelet Activation and Coagulation and Its Role in Vascular Injury and Arterial Thrombosis. Interv. Cardiol. Clin. 2017, 6, 1–12. [Google Scholar] [CrossRef]
- Rubenstein, D.A.; Yin, W. Platelet-Activation Mechanisms and Vascular Remodeling. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2018; Volume 8, pp. 1117–1156. [Google Scholar]
- Tummala, R.; Rai, M.P. Glycoprotein IIb/IIIa Inhibitors; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Murphy, E.; Curneen, J.M.G.; McEvoy, J.W. Aspirin in the Modern Era of Cardiovascular Disease Prevention. Methodist Debakey Cardiovasc. J. 2021, 17, 36–47. [Google Scholar] [CrossRef]
- Dawson, L.P.; Chen, D.; Dagan, M.; Bloom, J.; Taylor, A.; Duffy, S.J.; Shaw, J.; Lefkovits, J.; Stub, D. Assessment of Pretreatment with Oral P2Y12 Inhibitors and Cardiovascular and Bleeding Outcomes in Patients with Non-ST Elevation Acute Coronary Syndromes. JAMA Netw. Open 2021, 4, e2134322. [Google Scholar] [CrossRef] [PubMed]
- Franchi, F.; Angiolillo, D.J. Novel Antiplatelet Agents in Acute Coronary Syndrome. Nat. Rev. Cardiol. 2015, 12, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L. Mechanisms of Platelet Activation: Need for New Strategies to Protect against Platelet-Mediated Atherothrombosis. Thromb. Haemost. 2009, 102, 248–257. [Google Scholar] [CrossRef]
- Xu, X.R.; Carrim, N.; Neves, M.A.D.; McKeown, T.; Stratton, T.W.; Coelho, R.M.P.; Lei, X.; Chen, P.; Xu, J.; Dai, X.; et al. Platelets and Platelet Adhesion Molecules: Novel Mechanisms of Thrombosis and Anti-Thrombotic Therapies. Thromb. J. 2016, 14, 29. [Google Scholar] [CrossRef]
- Quatromoni, N.; Tuteja, S.; Kolansky, D.M.; Matthai, W.H.; Giri, J. Novel Anti-Platelet Agents in Acute Coronary Syndrome: Mechanisms of Action and Opportunities to Tailor Therapy. Curr. Atheroscler. Rep. 2015, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Soriano Jerez, E.M.; Gibbins, J.M.; Hughes, C.E. Targeting Platelet Inhibition Receptors for Novel Therapies: PECAM-1 and G6b-B. Platelets 2021, 32, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.C.J.; Truss, N.J.; Ali, F.Y.; Dhanji, A.A.; Vojnovic, I.; Zain, Z.N.M.; Bishop-Bailey, D.; Paul-Clark, M.J.; Tucker, A.T.; Mitchell, J.A.; et al. Aspirin and the In Vitro Linear Relationship Between Thromboxane A2-Mediated Platelet Aggregation and Platelet Production of Thromboxane A2. J. Thromb. Haemost. 2008, 6, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Freitas, M.; Tomé, S.M.; Silva, A.M.S.; Laufer, S.; Lima, J.L.F.C.; Fernandes, E. Flavonoids Inhibit COX-1 and COX-2 Enzymes and Cytokine/Chemokine Production in Human Whole Blood. Inflammation 2015, 38, 858–870. [Google Scholar] [CrossRef]
- Martiniakova, M.; Penzes, N.; Biro, R.; Sarocka, A.; Kovacova, V.; Mondockova, V.; Ciernikova, S.; Omelka, R. Sea Buckthorn and Its Flavonoids Isorhamnetin, Quercetin, and Kaempferol Favorably Influence Bone and Breast Tissue Health. Front. Pharmacol. 2024, 15, 1462823. [Google Scholar] [CrossRef]
- Rodríguez, L.; Badimon, L.; Méndez, D.; Padró, T.; Vilahur, G.; Peña, E.; Carrasco, B.; Vogel, H.; Palomo, I.; Fuentes, E. Antiplatelet Activity of Isorhamnetin via Mitochondrial Regulation. Antioxidants 2021, 10, 666. [Google Scholar] [CrossRef]
- Tan, P.; Hao, Y.; Liu, Y. Effects of the Main Monomer Ingredients of Ginkgo Biloba Extract on Phosphodiesterase 3 Activity of Platelet. Chin. J. Clin. Physicians 2013, 7, 11569–11573. [Google Scholar]
- Kwon, S.U.; Lee, H.Y.; Xin, M.; Ji, S.J.; Cho, H.K.; Kim, D.S.; Kim, D.K.; Lee, Y.M. Antithrombotic Activity of Vitis Labrusca Extract on Rat Platelet Aggregation. Blood Coagul. Fibrinolysis 2016, 27, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Yang, J.; Bao, C.; Lu, F.; Wu, Q.; Wu, Z.; Lv, H.; Zhou, Y.; Liu, Y.; Zhu, N.; et al. Isorhamnetin: What Is the In Vitro Evidence for Its Antitumor Potential and Beyond? Front. Pharmacol. 2024, 15, 1309178. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, M.; Zhao, J.; Lu, M.; Hao, J.; Guan, X.; Li, C. Anti-Atherosclerotic Effect of Sea Buckthorn (Hippophae rhamnoides Linn) and Its Molecular Mechanism. J. Funct. Foods 2024, 117, 106248. [Google Scholar] [CrossRef]
- Dudau, M.; Vilceanu, A.C.; Codrici, E.; Mihai, S.; Popescu, I.D.; Albulescu, L.; Tarcomnicu, I.; Moise, G.; Ceafalan, L.C.; Hinescu, M.E.; et al. Sea-Buckthorn Seed Oil Induces Proliferation of Both Normal and Dysplastic Keratinocytes in Basal Conditions and Under UVA Irradiation. J. Pers. Med. 2021, 11, 278. [Google Scholar] [CrossRef]
- Marciniak, B.; Kontek, R.; Żuchowski, J.; Stochmal, A. Novel Bioactive Properties of Low-Polarity Fractions from Sea-Buckthorn Extracts (Elaeagnus rhamnoides (L.) A. Nelson)—(In Vitro). Biomed. Pharmacother. 2021, 135, 111141. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xu, Z.; Liao, X. Bioactive Compounds, Health Benefits and Functional Food Products of Sea Buckthorn: A Review. Crit. Rev. Food Sci. Nutr. 2021, 62, 6761–6782. [Google Scholar] [CrossRef]
- Fan, J.; Ding, X.; Gu, W. Radical-Scavenging Proanthocyanidins from Sea Buckthorn Seed. Food Chem. 2007, 102, 168–177. [Google Scholar] [CrossRef]
- Guo, R.; Guo, X.; Li, T.; Fu, X.; Liu, R.H. Comparative Assessment of Phytochemical Profiles, Antioxidant and Antiproliferative Activities of Sea Buckthorn (Hippophaë rhamnoides L.) Berries. Food Chem. 2017, 221, 997–1003. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Liu, P. Active Components from Sea Buckthorn (Hippophae rhamnoides L.) Regulate Hepatic Stellate Cell Activation and Liver Fibrogenesis. J. Agric. Food Chem. 2018, 66, 12257–12264. [Google Scholar] [CrossRef]
- Chand, N.; Naz, S.; Irfan, M.; Khan, R.U.; Ur Rehman, Z. Effect of Sea Buckthorn (Hippophae rhamnoides L.) Seed Supplementation on Egg Quality and Cholesterol of Rhode Island Red×Fayoumi Laying Hens. Korean J. Food Sci. Anim. Resour. 2018, 38, 468–475. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Huo, Y.; Zhou, F.; Wu, W.; Lu, F.; Yang, X.; Guo, X.; Chen, P.; Deng, Q.; et al. Protective Effect of Proanthocyanidins from Sea Buckthorn (Hippophae rhamnoides L.) Seed Against Visible Light-Induced Retinal Degeneration In Vivo. Nutrients 2016, 8, 245. [Google Scholar] [CrossRef]
- Suomela, J.-P.; Ahotupa, M.; Yang, B.; Vasankari, T.; Kallio, H. Absorption of Flavonols Derived from Sea Buckthorn (Hippophaë rhamnoides L.) and Their Effect on Emerging Risk Factors for Cardiovascular Disease in Humans. J. Agric. Food Chem. 2006, 54, 7364–7369. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhang, J.; Zhao, A.; Zhang, Y.; Wang, P. Effects of Sea Buckthorn Puree on Risk Factors of Cardiovascular Disease in Hypercholesterolemia Population: A Double-Blind, Randomized, Placebo-Controlled Trial. Anim. Biotechnol. 2020, 33, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Sun, G.; Dong, X.; Wang, M.; Qin, M.; Yu, Y.; Sun, X. Isorhamnetin Attenuates Atherosclerosis by Inhibiting Macrophage Apoptosis via PI3K/AKT Activation and HO-1 Induction. PLoS ONE 2015, 10, e0120259. [Google Scholar] [CrossRef]
- Stochmal, A.; Rolnik, A.; Skalski, B.; Zuchowski, J.; Olas, B. Antiplatelet and Anticoagulant Activity of Isorhamnetin and Its Derivatives Isolated from Sea Buckthorn Berries, Measured in Whole Blood. Molecules 2022, 27, 4429. [Google Scholar] [CrossRef] [PubMed]
- Stainer, A.R.; Sasikumar, P.; Bye, A.P.; Unsworth, A.J.; Holbrook, L.M.; Tindall, M.; Lovegrove, J.A.; Gibbins, J.M. The Metabolites of the Dietary Flavonoid Quercetin Possess Potent Antithrombotic Activity, and Interact with Aspirin to Enhance Antiplatelet Effects. TH Open 2019, 3, e244–e258. [Google Scholar] [CrossRef] [PubMed]
- Kaikita, K.; Hosokawa, K.; Dahlen, J.R.; Tsujita, K. Total Thrombus-Formation Analysis System (T-TAS): Clinical Application of Quantitative Analysis of Thrombus Formation in Cardiovascular Disease. Thromb. Haemost. 2019, 119, 1554–1562. [Google Scholar] [CrossRef]
- Zaragozá, C.; Álvarez-Mon, M.Á.; Zaragozá, F.; Villaescusa, L. Flavonoids: Antiplatelet Effect as Inhibitors of COX-1. Molecules 2022, 27, 1146. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.A.; Rumbeiha, W.; Nair, M.G. Constituents in Easter Lily Flowers with Medicinal Activity. Life Sci. 2004, 76, 671–683. [Google Scholar] [CrossRef]
- Krisnamurti, G.C.; Bare, Y.; Amin, M.; Primiani, C.N. Combination of Curcumin from Curcuma longa and Procyanidin from Tamarindus indica in Inhibiting Cyclooxygenases for Primary Dysmenorrhea Therapy: In Silico Study. Biointerface Res. Appl. Chem. 2020, 11, 7460–7467. [Google Scholar] [CrossRef]
- Jayakumar, J.A.K.J.; Panicker, M.M. The Roles of Serotonin in Cell Adhesion and Migration, and Cytoskeletal Remodeling. Cell Adh. Migr. 2021, 15, 261–271. [Google Scholar] [CrossRef]
- Ramirez, J.E.M.; Alarabi, A.B.; Khasawneh, F.T.; Alshbool, F.Z. A Novel Antibody Targeting the Second Extracellular Loop of the Serotonin 5-HT2A Receptor Inhibits Platelet Function. Int. J. Mol. Sci. 2022, 23, 8794. [Google Scholar] [CrossRef]
- Duerschmied, D.; Canault, M.; Lievens, D.; Brill, A.; Cifuni, S.M.; Bader, M.; Wagner, D.D. Serotonin Stimulates Platelet Receptor Shedding by Tumor Necrosis Factor-Alpha-Converting Enzyme (ADAM17). J. Thromb. Haemost. 2009, 7, 1163–1171. [Google Scholar] [CrossRef]
- Kilic, F. The Coordinated Changes in Platelet Glycan Patterns with Blood Serotonin and Exosomes. Int. J. Mol. Sci. 2024, 25, 11940. [Google Scholar] [CrossRef]
- Takahashi, T.; Miyazawa, M. N-Caffeoyl Serotonin as Selective COX-2 Inhibitor. Bioorg. Med. Chem. Lett. 2012, 22, 2494–2496. [Google Scholar] [CrossRef]
- Zuo, X.; Li, Q.; Ya, F.; Ma, L.-J.; Tian, Z.; Zhao, M.; Fan, D.; Zhao, Y.; Mao, Y.-H.; Wan, J.-B.; et al. Ginsenosides Rb2 and Rd2 Isolated from Panax notoginseng Flowers Attenuate Platelet Function Through P2Y12-Mediated CAMP/PKA and PI3K/Akt/Erk1/2 Signaling. Food Funct. 2021, 12, 5793–5805. [Google Scholar] [CrossRef]
- Irfan, M.; Jeong, D.; Kwon, H.W.; Shin, J.H.; Park, S.J.; Kwak, D.; Kim, T.H.; Lee, D.H.; Park, H.J.; Rhee, M.H. Ginsenoside-Rp3 Inhibits Platelet Activation and Thrombus Formation by Regulating MAPK and Cyclic Nucleotide Signaling. Vasc. Pharmacol. 2018, 109, 45–55. [Google Scholar] [CrossRef]
- Ouyang, X.-L.; Mao, W.-H.; Wang, C.-G.; Pan, Y.-M.; Liang, D.; Wang, H.-S. Five 11α, 12α-Epoxy Pentacyclic Triterpenoid Saponins with Antithrombus Activities from Glechoma longituba. Fitoterapia 2019, 138, 104345. [Google Scholar] [CrossRef]
- Luo, Q.; Wei, G.; Wu, X.; Tang, K.; Xu, M.; Wu, Y.; Liu, Y.; Li, X.; Sun, Z.; Ju, W.; et al. Platycodin D Inhibits Platelet Function and Thrombus Formation Through Inducing Internalization of Platelet Glycoprotein Receptors. J. Transl. Med. 2018, 16, 311. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Tang, X.; Su, W.; Yang, C.; Pan, D.; Zhao, D.; Qi, B.; Li, X. Hemostatic Effect of 20(S)-Panaxadiol by Induced Platelet Aggregation Depending on Calcium Signaling Pathway. Biomed Res. Int. 2022, 2022, 8265898. [Google Scholar] [CrossRef]
- Stalker, T.J.; Newman, D.K.; Ma, P.; Wannemacher, K.M.; Brass, L.F. Platelet Signaling. Handb. Exp. Pharmacol. 2012, 210, 59–85. [Google Scholar] [CrossRef]
- Fuentes, F.; Palomo, I.; Fuentes, E. Platelet Oxidative Stress as a Novel Target of Cardiovascular Risk in Frail Older People. Vasc. Pharmacol. 2017, 93–95, 14–19. [Google Scholar] [CrossRef]
- Olas, B. New Light on Changes in the Number and Function of Blood Platelets Stimulated by Cocoa and Its Products. Front. Pharmacol. 2024, 15, 1366076. [Google Scholar] [CrossRef]
- Johnson, W. Final Report on the Safety Assessment of Capsicum annuum Extract, Capsicum annuum Fruit Extract, Capsicum annuum Resin, Capsicum annuum Fruit Powder, Capsicum frutescens Fruit, Capsicum frutescens Fruit Extract, Capsicum frutescens Resin, and Capsaicin1. Int. J. Toxicol. 2007, 26, 3–106. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. S1), 230S–242S. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Xie, Y. Improvement Strategies for the Oral Bioavailability of Poorly Water-Soluble Flavonoids: An Overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef]
- Wang, H.; Cui, Y.; Fu, Q.; Deng, B.; Li, G.; Yang, J.; Wu, T.; Xie, Y. A Phospholipid Complex to Improve the Oral Bioavailability of Flavonoids. Drug Dev. Ind. Pharm. 2015, 41, 1693–1703. [Google Scholar] [CrossRef]
- Chen, Z.P.; Sun, J.; Chen, H.X.; Xiao, Y.Y.; Liu, D.; Chen, J.; Cai, H.; Cai, B.C. Comparative Pharmacokinetics and Bioavailability Studies of Quercetin, Kaempferol and Isorhamnetin After Oral Administration of Ginkgo biloba Extracts, Ginkgo biloba Extract Phospholipid Complexes and Ginkgo biloba Extract Solid Dispersions in Rats. Fitoterapia 2010, 81, 1045–1052. [Google Scholar] [CrossRef]
- Rassu, G.; Vlčková, H.K.; Giunchedi, P.; Dias, P.; Cossu, M.; Pourová, J.; Harčárová, P.; Lomozová, Z.; Nováková, L.; Gavini, E.; et al. A Water-Soluble Preparation for Intravenous Administration of Isorhamnetin and Its Pharmacokinetics in Rats. Chem. Biol. Interact. 2024, 396, 111064. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhang, L.; Cai, Z.; Wu, Y.; Chen, N.; Gu, C.; Chen, Y.; Lin, X.; Xia, X.; Liu, A. Pharmacokinetics Study of Isorhamnetin in Rat Plasma by a Sensitive Electrochemical Sensor Based on Reduced Graphene Oxide. RSC Adv. 2017, 7, 36728–36734. [Google Scholar] [CrossRef]
- He, Y.; Hu, Z.; Li, A.; Zhu, Z.; Yang, N.; Ying, Z.; He, J.; Wang, C.; Yin, S.; Cheng, S. Recent Advances in Biotransformation of Saponins. Molecules 2019, 24, 2365. [Google Scholar] [CrossRef] [PubMed]

| Concentration | Inhibition of COX-1 [%] | ||||||
|---|---|---|---|---|---|---|---|
| Fraction | Isorhamnetin 3-O-β-glucoside-7-O-α-rhamnoside | Serotonin | Aspirin | ||||
| b | c | d | g | ||||
| 1 μg/mL | 31 | 0 | −49 | −23 | −31 | −6 | −22 |
| 50 μg/mL | 61 | 87 | −10 | −45 | −1 | −66 | 91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sławińska, N.; Janko, L.; Żuchowski, J.; Olas, B. Fractions from Sea Buckthorn Seeds and Their Bioactive Ingredients as Modulators of Human Blood Platelet Response In Vitro: The Role of Thermal Processing. Nutrients 2025, 17, 3074. https://doi.org/10.3390/nu17193074
Sławińska N, Janko L, Żuchowski J, Olas B. Fractions from Sea Buckthorn Seeds and Their Bioactive Ingredients as Modulators of Human Blood Platelet Response In Vitro: The Role of Thermal Processing. Nutrients. 2025; 17(19):3074. https://doi.org/10.3390/nu17193074
Chicago/Turabian StyleSławińska, Natalia, Luiza Janko, Jerzy Żuchowski, and Beata Olas. 2025. "Fractions from Sea Buckthorn Seeds and Their Bioactive Ingredients as Modulators of Human Blood Platelet Response In Vitro: The Role of Thermal Processing" Nutrients 17, no. 19: 3074. https://doi.org/10.3390/nu17193074
APA StyleSławińska, N., Janko, L., Żuchowski, J., & Olas, B. (2025). Fractions from Sea Buckthorn Seeds and Their Bioactive Ingredients as Modulators of Human Blood Platelet Response In Vitro: The Role of Thermal Processing. Nutrients, 17(19), 3074. https://doi.org/10.3390/nu17193074







