Dose–Response Effect of Watermelon Consumption on Ambulatory Blood Pressure in Adults with Elevated Blood Pressure: A Randomized Controlled Pilot Trial
Abstract
1. Introduction
2. Materials and Methods
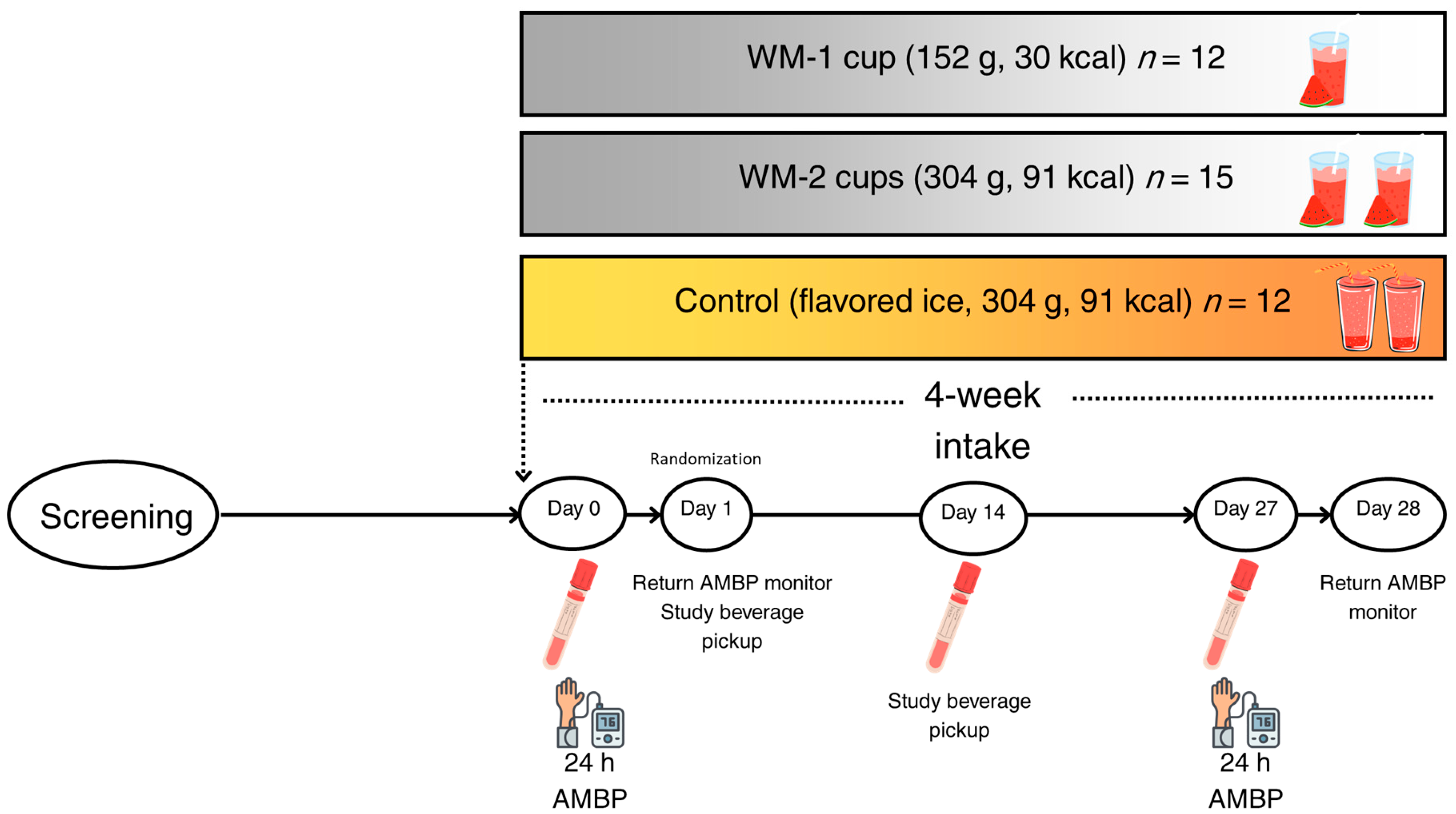
2.1. Study Design and Clinical Trial
2.2. Study Interventions
2.3. Ambulatory Blood Pressure Assessment
2.4. Analysis of Glucose, Insulin, and Lipid Profile in Plasma
2.5. Quantification of Select Amino Acids in Watermelon Flesh and Plasma
Chemicals and Materials
2.6. Plasma NO Concentrations
2.7. Statistical Analysis
3. Results
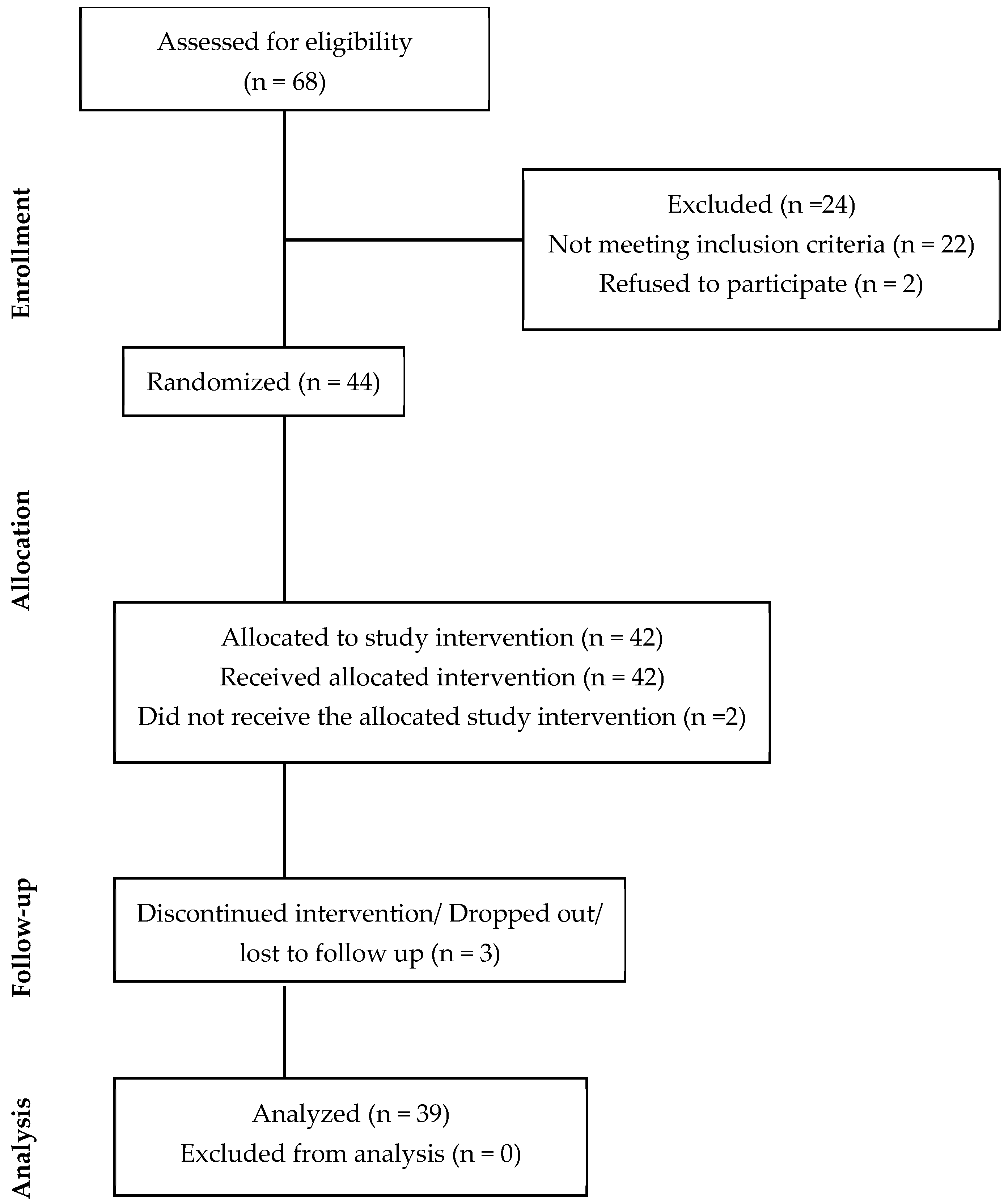
3.1. Participant Enrollment, Demographics, and Baseline Characteristics
3.2. L-Citrulline and L-Arginine Content in Study Beverages
3.3. Assessment of Ambulatory Blood Pressure
3.4. Plasma Analyses
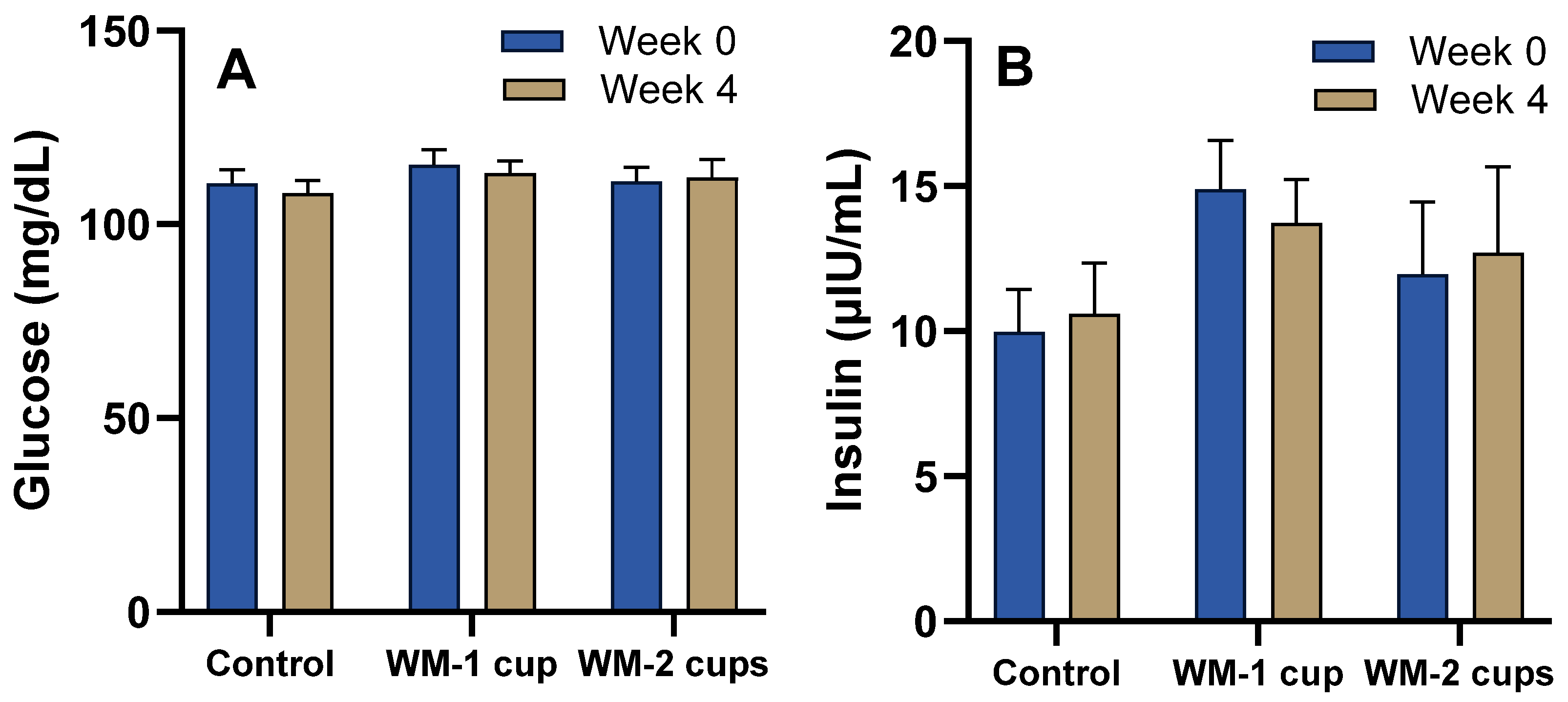
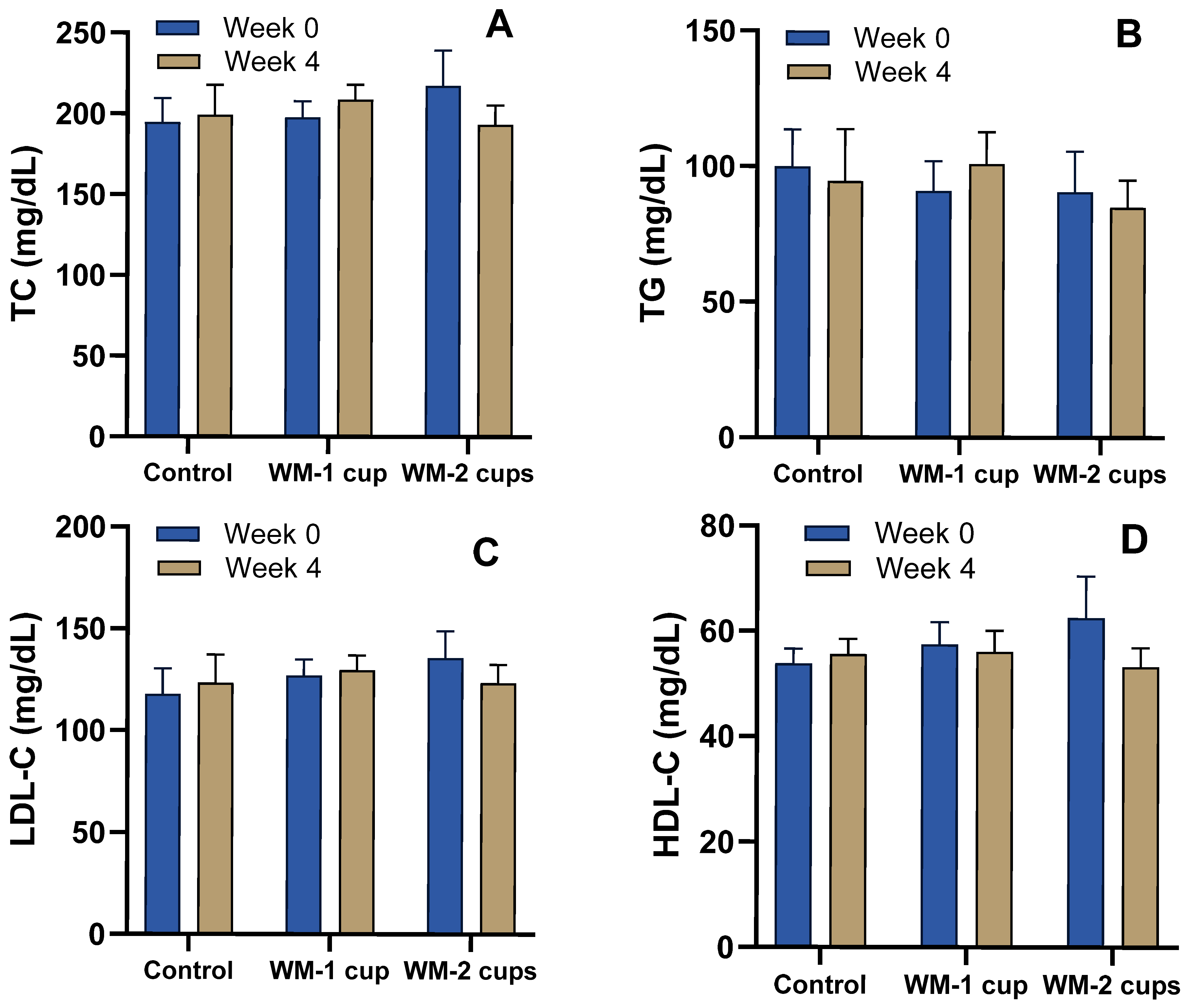
3.4.1. Glucose, Insulin, and Lipid Profile
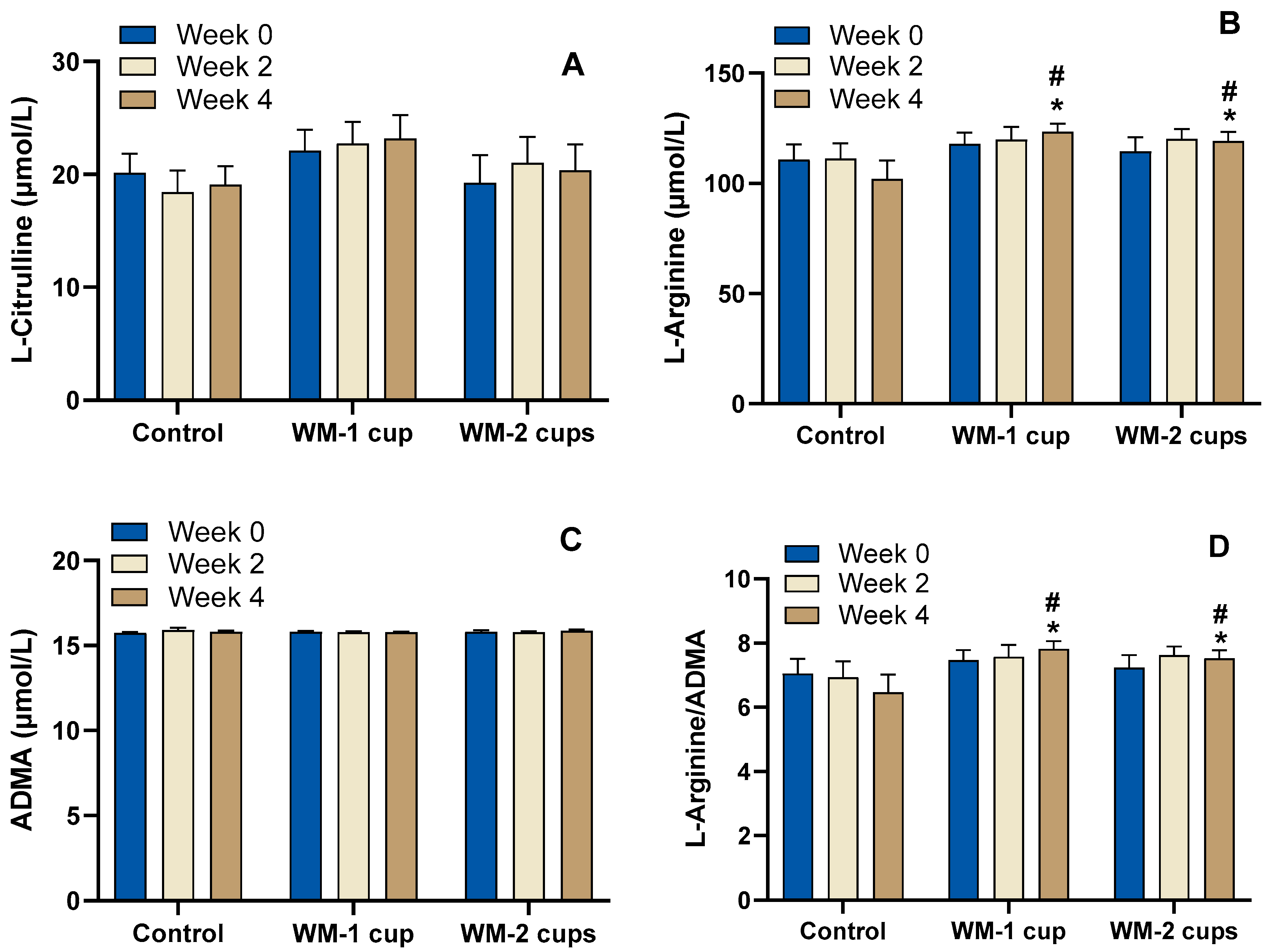
3.4.2. Watermelon-Specific Amino Acids, ADMA, and L-Arginine/ADMA Ratio
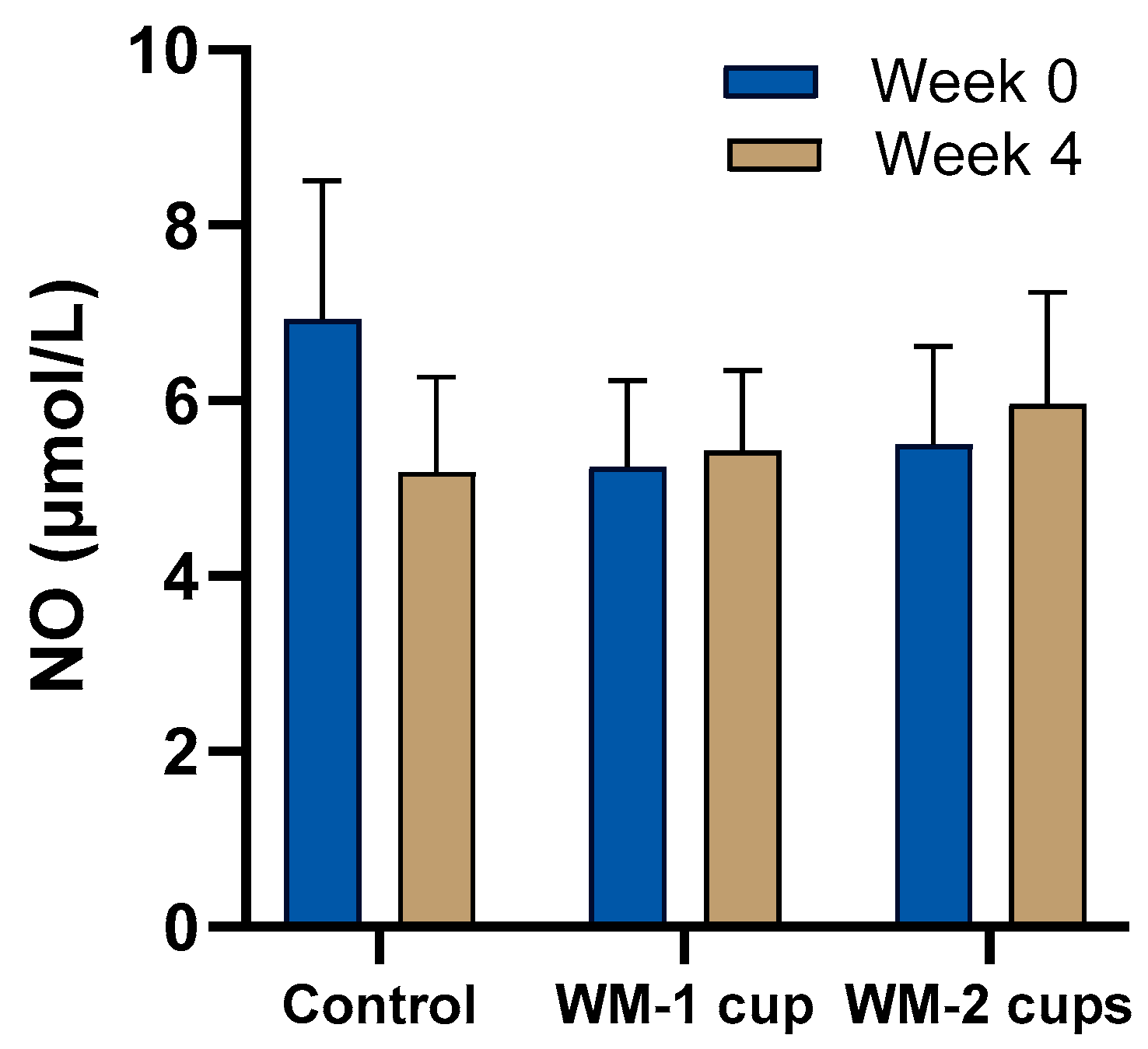
3.4.3. Nitric Oxide (NO)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | African American |
| ADMA | Asymmetric Dimethylarginine |
| AMBP | Ambulatory Blood Pressure |
| ANCOVA | Analysis of Covariance |
| ANOVA | Analysis of Variance |
| AS | Asian |
| ASL | Arginosuccinate Lyase |
| ASS | Arginosuccinate Synthase |
| BMI | Body Mass Index |
| BP | Blood Pressure |
| CAU | Caucasian |
| CNR | Center for Nutrition Research |
| CONSORT | Consolidated Standards of Reporting Trials |
| CVD | Cardiovascular Disease |
| EDTA | Ethylenediaminetetraacetic Acid |
| eNOS | Endothelial Nitric Oxide Synthase |
| ESI | Electrospray Ionization |
| HDL-C | High-Density Lipoprotein Cholesterol |
| HIS | Hispanic |
| HPLC | High-Performance Liquid Chromatography |
| IDACO | International Database of Ambulatory Blood Pressure Monitoring and Cardiovascular Outcomes |
| IRB | Institutional Review Board |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| MRM | Multiple-Reaction Monitoring |
| NO | Nitric Oxide |
| PTFE | Polytetrafluoroethylene |
| RM-ANOVA | Repeated Measures Analysis of Variance |
| RM-GLM | Repeated Measures General Linear Model |
| SD | Standard Deviation |
| SEM | Standard Error of the Mean |
| SPSS | Statistical Package for the Social Sciences |
| TC | Total Cholesterol |
| TG | Triglycerides |
| UHPLC-QQQ-MS/MS | Ultra-High-Performance Liquid Chromatography-Triple Quadrupole Tandem Mass Spectrometry |
| WM | Watermelon |
References
- Srivastava, A.; Mirza, T.M.; Vaqar, S.; Sharan, S. Prehypertension; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- McGuire, H.L.; Svetkey, L.P.; Harsha, D.W.; Elmer, P.J.; Appel, L.J.; Ard, J.D. Comprehensive Lifestyle Modification and Blood Pressure Control: A Review of the PREMIER Trial. J. Clin. Hypertens. 2004, 6, 383–390. [Google Scholar] [CrossRef]
- Vázquez-Manjarrez, N.; Vázquez-Manjarrez, N.; Vázquez-Manjarrez, N.; Ulaszewska, M.; Garcia-Aloy, M.; Garcia-Aloy, M.; Mattivi, F.; Mattivi, F.; Praticò, G.; Dragsted, L.O.; et al. Biomarkers of Intake for Tropical Fruits. Genes Nutr. 2020, 15, 1–21. [Google Scholar] [CrossRef]
- Allerton, T.D.; Proctor, D.N.; Stephens, J.M.; Dugas, T.R.; Spielmann, G.; Irving, B.A. L-Citrulline Supplementation: Impact on Cardiometabolic Health. Nutrients 2018, 10, 921. [Google Scholar] [CrossRef]
- Figueroa, A.; Trivino, J.A.; Sanchez-Gonzalez, M.A.; Vicil, F. Oral L-Citrulline Supplementation Attenuates Blood Pressure Response to Cold Pressor Test in Young Men. Am. J. Hypertens. 2010, 23, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Orea-Tejeda, A.; José orozco-Gutiérrez, J.; Castillo-Martínez, L.; Keirns-Davies, C.; Montaño-Hernández, P.; Vázquez-Díaz, O.; Valdespino-Trejo, A.; Infante, O.; Martínez-Memije, R. The Effect of L-Arginine and Citrulline on Endothelial Function in Patients in Heart Failure with Preserved Ejection Fraction. Cardiol. J. 2010, 17, 464–470. [Google Scholar] [PubMed]
- Papadia, C.; Osowska, S.; Cynober, L.; Forbes, A. Citrulline in Health and Disease. Review on Human Studies. Clin. Nutr. 2018, 37, 1823–1828. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B.; Freeman, M.; Zhang, X.; Sandhu, A.; Edirisinghe, I. Watermelon and L-Citrulline in Cardio-Metabolic Health: Review of the Evidence 2000–2020. Curr. Atheroscler. Rep. 2021, 23, 1–24. [Google Scholar] [CrossRef]
- Cynober, L.A. Plasma Amino Acid Levels with a Note on Membrane Transport: Characteristics, Regulation, and Metabolic Significance. Nutrition 2002, 18, 761–766. [Google Scholar] [CrossRef]
- Böger, R.H.; Bode-Böger, S.M. The Clinical Pharmacology of L-Arginine. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 79–99. [Google Scholar] [CrossRef]
- Morris, S.M. Arginine Metabolism Revisited. J. Nutr. 2016, 146, 2579S–2586S. [Google Scholar] [CrossRef]
- Morris, S.M. Enzymes of Arginine Metabolism. J. Nutr. 2004, 134, 2743S–2747S. [Google Scholar] [CrossRef] [PubMed]
- Curis, E.; Nicolis, I.; Moinard, C.; Osowska, S.; Zerrouk, N.; Bénazeth, S.; Cynober, L. Almost All about Citrulline in Mammals. Amino Acids 2005, 29, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric Oxide Synthases: Regulation and Function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Brunetti-Pierri, N.; Erez, A.; Shchelochkov, O.; Craigen, W.; Lee, B. Systemic Hypertension in Two Patients with ASL Deficiency: A Result of Nitric Oxide Deficiency? Mol. Genet. Metab. 2009, 98, 195–197. [Google Scholar] [CrossRef]
- Erez, A.; Nagamani, S.C.S.; Shchelochkov, O.A.; Premkumar, M.H.; Campeau, P.M.; Chen, Y.; Garg, H.K.; Li, L.; Mian, A.; Bertin, T.K.; et al. Requirement of Argininosuccinate Lyase for Systemic Nitric Oxide Production. Nat. Med. 2011, 17, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Wong, A.; Kalfon, R. Effects of Watermelon Supplementation on Aortic Hemodynamic Responses to the Cold Pressor Test in Obese Hypertensive Adults. Am. J. Hypertens. 2014, 27, 899–906. [Google Scholar] [CrossRef]
- Figueroa, A.; Wong, A.; Hooshmand, S.; Sanchez-Gonzalez, M.A. Effects of Watermelon Supplementation on Arterial Stiffness and Wave Reflection Amplitude in Postmenopausal Women. Menopause 2013, 20, 573–577. [Google Scholar] [CrossRef]
- Figueroa, A.; Sanchez-Gonzalez, M.A.; Perkins-Veazie, P.M.; Arjmandi, B.H. Effects of Watermelon Supplementation on Aortic Blood Pressure and Wave Reflection in Individuals with Prehypertension: A Pilot Study. Am. J. Hypertens. 2011, 24, 40–44. [Google Scholar] [CrossRef]
- Figueroa, A.; Sanchez-Gonzalez, M.A.; Wong, A.; Arjmandi, B.H. Watermelon Extract Supplementation Reduces Ankle Blood Pressure and Carotid Augmentation Index in Obese Adults with Prehypertension or Hypertension. Am. J. Hypertens. 2012, 25, 640–643. [Google Scholar] [CrossRef]
- Fan, J.; Park, E.; Zhang, L.; Edirisinghe, I.; Burton-Freeman, B.; Sandhu, A.K. Pharmacokinetic Parameters of Watermelon (Rind, Flesh, and Seeds) Bioactive Components in Human Plasma: A Pilot Study to Investigate the Relationship to Endothelial Function. J. Agric. Food Chem. 2020, 68, 7393–7403. [Google Scholar] [CrossRef]
- Bailey, S.J.; Blackwell, J.R.; Williams, E.; Vanhatalo, A.; Wylie, L.J.; Winyard, P.G.; Jones, A.M. Two Weeks of Watermelon Juice Supplementation Improves Nitric Oxide Bioavailability but Not Endurance Exercise Performance in Humans. Nitric Oxide 2016, 59, 10–20. [Google Scholar] [CrossRef]
- Fujie, S.; Iemitsu, K.; Inoue, K.; Ogawa, T.; Nakashima, A.; Suzuki, K.; Iemitsu, M. Wild Watermelon-Extracted Juice Ingestion Reduces Peripheral Arterial Stiffness with an Increase in Nitric Oxide Production: A Randomized Crossover Pilot Study. Nutrients 2022, 14, 5199. [Google Scholar] [CrossRef] [PubMed]
- Volino-Souza, M.; Oliveira, G.V.; Vargas, R.; Tavares, A.C.; Conte-Junior, C.A.; Alvares, T.d.S. Effect of Microencapsulated Watermelon (Citrullus lanatus) Intake on Plasma Amino Acids and Glycemic Response in Healthy Adults. Food Biosci. 2022, 46, 101553. [Google Scholar] [CrossRef]
- Anstey, D.E.; Colantonio, L.D.; Yano, Y.; Booth, J.N.; Muntner, P. The Importance of Using 24-Hour and Nighttime Blood Pressure for the Identification of White Coat Hypertension: Data from the Jackson Heart Study. J. Clin. Hypertens. 2018, 20, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Booth, J.N.; Muntner, P.; Abdalla, M.; Diaz, K.M.; Viera, A.J.; Reynolds, K.; Schwartz, J.E.; Shimbo, D. Differences in Night-Time and Daytime Ambulatory Blood Pressure When Diurnal Periods Are Defined by Self-Report, Fixed-Times, and Actigraphy: Improving the Detection of Hypertension Study. J. Hypertens. 2016, 34, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Thijs, L.; Hansen, T.W.; Kikuya, M.; Björklund-Bodegård, K.; Lie, Y.; Dolan, E.; Tikhonoff, V.; Seidlerová, J.; Kuznetsova, T.; Stolarz, K.; et al. The International Database of Ambulatory Blood Pressure in Relation to Cardiovascular Outcome (IDACO): Protocol and Research Perspectives. Blood Press. Monit. 2007, 12, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Huart, J.; Persu, A.; Lengelé, J.P.; Krzesinski, J.M.; Jouret, F.; Stergiou, G.S. Pathophysiology of the Nondipping Blood Pressure Pattern. Hypertension 2023, 80, 719–729. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Davis, A.; Collins, J.K. Watermelon: From Dessert to Functional Food. Isr. J. Plant. Sci. 2012, 60, 395–402. [Google Scholar] [CrossRef]
- Lum, T.; Connolly, M.; Marx, A.; Beidler, J.; Hooshmand, S.; Kern, M.; Liu, C.; Hong, M.Y. Effects of Fresh Watermelon Consumption on the Acute Satiety Response and Cardiometabolic Risk Factors in Overweight and Obese Adults. Nutrients 2019, 11, 595. [Google Scholar] [CrossRef]
- Massa, N.M.L.; Silva, A.S.; Toscano, L.T.; Silva, J.D.G.R.; Persuhn, D.C.; Gonçalves, M.D.C.R. Watermelon Extract Reduces Blood Pressure but Does Not Change Sympathovagal Balance in Prehypertensive and Hypertensive Subjects. Blood Press. 2016, 25, 244–248. [Google Scholar] [CrossRef]
- Alshahrani, S.H.; Ramaiah, P.; Dheyab, A.S.; Rudiansyah, M.; Qasim, Q.A.; Altalbawy, F.M.A.; Obaid, R.F.; Almulla, A.F.; Ramírez-Coronel, A.A.; Gabr, G.A.; et al. The Effect of Watermelon Supplementation on Blood Pressure: A Meta-Analysis of Randomised Clinical Trials. J. Herb. Med. 2023, 41, 100726. [Google Scholar] [CrossRef]
- Ellis, A.C.; Mehta, T.; Nagabooshanam, V.A.; Dudenbostel, T.; Locher, J.L.; Crowe-White, K.M. Daily 100% Watermelon Juice Consumption and Vascular Function among Postmenopausal Women: A Randomized Controlled Trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2959–2968. [Google Scholar] [CrossRef] [PubMed]
- Smeets, E.T.H.C.; Mensink, R.P.; Joris, P.J. Effects of L-Citrulline Supplementation and Watermelon Consumption on Longer-Term and Postprandial Vascular Function and Cardiometabolic Risk Markers: A Meta-Analysis of Randomised Controlled Trials in Adults. Br. J. Nutr. 2022, 128, 1758–1770. [Google Scholar] [CrossRef] [PubMed]
- Daughtry, J.; Rasmussen, C.; Rosas, M.; Zhang, L.; Lu, S.; Hooshmand, S.; Liu, C.; Kern, M.; Hong, M.Y. Blenderized Watermelon Consumption Decreases Body Mass Index, Body Mass Index Percentile, Body Fat and HbA1c in Children with Overweight or Obesity. Pediatr. Obes. 2023, 18, e13038. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.R.; Iii, C.L.W.; Fish, W.W.; Wehner, T.C.; King, S.; Perkins-Veazie, P. L-Citrulline Levels in Watermelon Cultigens Tested in Two Environments. HortScience 2011, 46, 1572–1575. [Google Scholar] [CrossRef]
- USDA National Nutrient Database for Standard Reference, Legacy. Version Current: April 2018. Available online: https://fdc.nal.usda.gov/food-details/167765/nutrients (accessed on 24 March 2025).
- Gao, L.; Zhao, S.; Lu, X.; He, N.; Zhu, H.; Dou, J.; Liu, W. Comparative Transcriptome Analysis Reveals Key Genes Potentially Related to Soluble Sugar and Organic Acid Accumulation in Watermelon. PLoS ONE 2018, 13, e0190096. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Collins, J.K.; Davis, A.R.; Roberts, W. Carotenoid Content of 50 Watermelon Cultivars. J. Agric. Food Chem. 2006, 54, 2593–2597. [Google Scholar] [CrossRef]
- Assefa, A.D.; Hur, O.S.; Ro, N.Y.; Lee, J.E.; Hwang, A.J.; Kim, B.S.; Rhee, J.H.; Yi, J.Y.; Kim, J.H.; Lee, H.S.; et al. Fruit Morphology, Citrulline, and Arginine Levels in Diverse Watermelon (Citrullus lanatus) Germplasm Collections Article. Plants 2020, 9, 1054. [Google Scholar] [CrossRef]
- Orozco-Gutiérrez, J.J.; Castillo-Martínez, L.; Orea-Tejeda, A.; Vázquez-Díaz, O.; Valdespino-Trejo, A.; Narváez-David, R.; Keirns-Davis, C.; Carrasco-Ortiz, O.; Navarro-Navarro, A.; Sánchez-Santillán, R. Effect of L-Arginine or L-Citrulline Oral Supplementation on Blood Pressure and Right Ventricular Function in Heart Failure Patients with Preserved Ejection Fraction. Cardiol. J. 2010, 17, 612–618. [Google Scholar]
- Böger, R.H.; Maas, R.; Schulze, F.; Schwedhelm, E. Asymmetric Dimethylarginine (ADMA) as a Prospective Marker of Cardiovascular Disease and Mortality-An Update on Patient Populations with a Wide Range of Cardiovascular Risk. Pharmacol. Res. 2009, 60, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.K.; Wu, G.; Perkins-Veazie, P.; Spears, K.; Claypool, P.L.; Baker, R.A.; Clevidence, B.A. Watermelon Consumption Increases Plasma Arginine Concentrations in Adults. Nutrition 2007, 23, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Shanely, R.A.; Zwetsloot, J.J.; Jurrissen, T.J.; Hannan, L.C.; Zwetsloot, K.A.; Needle, A.R.; Bishop, A.E.; Wu, G.; Perkins-Veazie, P. Daily Watermelon Consumption Decreases Plasma SVCAM-1 Levels in Overweight and Obese Postmenopausal Women. Nutr. Res. 2020, 76, 9–19. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Angulo, J.; Santos-Ruiz, M.; Ruiz de Adana, J.C.; Pindado, M.L.; Sánchez-Ferrer, A.; Hernández, A.; Rodríguez-Mañas, L. Asymmetric Dimethylarginine (ADMA) Elevation and Arginase up-Regulation Contribute to Endothelial Dysfunction Related to Insulin Resistance in Rats and Morbidly Obese Humans. J. Physiol. 2016, 594, 3045–3060. [Google Scholar] [CrossRef]
- Wierzchowska-McNew, R.A.; Engelen, M.P.; Thaden, J.J.; ten Have, G.A.; Deutz, N.E. Obesity- and Sex-Related Metabolism of Arginine and Nitric Oxide in Adults. Am. J. Clin. Nutr. 2022, 116, 1610–1620. [Google Scholar] [CrossRef] [PubMed]
- Holguin, F.; Comhair, S.A.A.; Hazen, S.L.; Powers, R.W.; Khatri, S.S.; Bleecker, E.R.; Busse, W.W.; Calhoun, W.J.; Castro, M.; Fitzpatrick, A.M.; et al. An Association between L-Arginine/Asymmetric Dimethyl Arginine Balance, Obesity, and the Age of Asthma Onset Phenotype. Am. J. Respir. Crit. Care Med. 2013, 187, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Glyn, M.C.; Anderssohn, M.; Lüneburg, N.; Van Rooyen, J.M.; Schutte, R.; Huisman, H.W.; Fourie, C.M.T.; Smith, W.; Malan, L.; Malan, N.T.; et al. Ethnicity-Specific Differences in L-Arginine Status in South African Men. J. Hum. Hypertens. 2012, 26, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.C.; Deutz, N.E.; Ten Have, G.A.; Thaden, J.J.; Engelen, M.P. Aging in Older Adults Induces Changes in Arginine and Glutamine Kinetics, Partly Independent of The Presence of Comorbidities. Clin. Nutr. ESPEN 2023, 54, 474. [Google Scholar] [CrossRef]
| Nutrients | Control (304 g) | WM-1 Cup (152 g) | WM-2 Cups (304 g) |
|---|---|---|---|
| Energy (kcal) | ~91 | ~30 | ~91 |
| Carbohydrate (g) | 18.85 | 7.70 | 23 |
| Total sugar (g) | 18.85 | 6.32 | 18.85 |
| Total fat (g) | 0 | 2 | 4 |
| Added sugar (g) | 18.85 | 0 | 0 |
| Saturated fat (g) | 0 | 0.02 | 0.5 |
| Protein (g) | 0 | 1.85 | 2 |
| Total fiber (g) | 0 | 0.41 | 1 |
| Soluble fiber (g) | 0 | 0.10 | 0.30 |
| Characteristics 1 | Control (n = 12) | WM-1 Cup (n = 15) | WM-2 Cups (n = 12) |
|---|---|---|---|
| Age, years | 35 ± 12 | 41 ± 15 | 43 ± 14 |
| Sex, F/M, n | 15/8 | 7/8 | 9/14 |
| AS:HIS:CAU:AA | 6:0:1:5 | 2:1:5:7 | 0:2:5:5 |
| BMI (kg/m2) | 28.4 ± 1.5 | 31.9 ± 1.3 | 30.6 ± 2.1 |
| Systolic blood pressure, mm Hg | 127.1 ± 2.2 | 127.1 ± 2.8 | 123.3 ± 2.2 |
| Diastolic blood pressure, mm Hg | 85.1 ± 1.8 | 80.7 ± 1.9 | 79.5 ± 1.6 |
| Fasting blood glucose—Capillary mg/dL | 103.3 ± 3.2 | 109.9 ± 2.9 | 103.6 ± 2.3 |
| Samples | L-Citrulline | L-Arginine |
|---|---|---|
| WM flesh (mg/g) | 4.85 ± 0.3 | 0.54 ± 0.0 |
| WM-1 cup beverage (mg/152 g) | 737.8 ± 34.8 | 82.0 ± 5.8 |
| WM-2 cups beverage (mg/304 g) | 1475.6 ± 69.7 | 164.0 ± 11.6 |
| Control beverage (mg/304 g) | ND | ND |
| AMBP Data (Average—mm Hg) | Control (n = 12) | Control (n = 12) | WM-1 Cup (n = 15) | WM-1 Cup (n = 15) | WM-2 Cups (n = 12) | WM-2 Cups (n = 12) |
|---|---|---|---|---|---|---|
| Systolic | Diastolic | Systolic | Diastolic | Systolic | Diastolic | |
| Week 0 (Baseline) | ||||||
| 24 h BP | 131.9 ± 3.2 | 82.3 ± 2.5 | 131.2 ± 3.0 | 79.1 ± 2.3 | 128.2 ± 3.1 | 76.8 ± 2.4 |
| Daytime BP | 134.3 ± 3.6 | 86.0 ± 2.9 | 133.9 ± 2.2 | 82.5 ± 2.3 | 130.1 ± 3.1 | 78.3 ± 2.8 |
| Nighttime BP | 127.8 ± 4.4 | 75.4 ± 3.0 | 127.3 ± 4.7 | 74.5 ± 3.2 | 121.8 ± 4.6 | 70.6 ± 3.0 |
| Morning surge | −0.4 ± 2.1 | 2.0 ± 1.7 | 8.4 ± 2.9 | 9.3 ± 4.0 | 2.0 ± 2.5 | 4.2 ± 2.5 |
| Week 4 | ||||||
| 24 h BP | 130.2 ± 3.9 | 79.5 ± 3.0 | 130.1 ± 3.2 | 78.3 ± 2.1 | 124.9 ± 3.9 | 76.1 ± 2.5 |
| Daytime BP | 130.0 ± 4.5 | 81.0 ± 3.2 | 131.7 ± 3.1 | 81.7 ± 2.6 | 127.5 ± 4.1 | 78.4 ± 2.6 |
| Nighttime BP | 123.6 ± 3.1 | 75.3 ± 2.8 | 120.0 ± 3.5 | 70.5 ± 2.8 | 122.7 ± 5.3 | 73.3 ± 3.2 |
| Morning surge | 3.5 ± 3.5 | 1.6 ± 2.3 | 5.8 ± 2.3 | 7.1 ± 5.5 | 5.7 ± 2.2 | 7.0 ± 2.5 |
| Change (Δ) Week 4–Week 0 | ||||||
| 24-h BP | −1.8 ± 2.9 | −2.8 ± 2.0 | −1.1 ± 2.8 | −0.8 ± 3.7 | −3.2 ± 1.9 | −0.8 ± 1.6 |
| Daytime BP | −4.3 ± 4.5 | −5.0 ± 2.8 | −2.2 ± 2.9 | −0.8 ± 2.6 | −3.2 ± 2.0 | −0.2 ± 1.6 |
| Nighttime BP | −6.4 ± 3.9 | −2.1 ± 2.3 | −7.3 ± 4.2 | −0.2 ± 4.5 | 2.3 ± 2.6 | 3.5 ± 2.0 |
| Morning surge | −2.3 ± 4.3 | −1.6 ± 3.1 | −0.2 ± 4.5 | −3.1 ± 5.5 | 3.4 ± 3.4 | 2.4 ± 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, K.; Liao, H.; Edirisinghe, I.; Burton-Freeman, B.; Sandhu, A.K. Dose–Response Effect of Watermelon Consumption on Ambulatory Blood Pressure in Adults with Elevated Blood Pressure: A Randomized Controlled Pilot Trial. Nutrients 2025, 17, 3073. https://doi.org/10.3390/nu17193073
Singh K, Liao H, Edirisinghe I, Burton-Freeman B, Sandhu AK. Dose–Response Effect of Watermelon Consumption on Ambulatory Blood Pressure in Adults with Elevated Blood Pressure: A Randomized Controlled Pilot Trial. Nutrients. 2025; 17(19):3073. https://doi.org/10.3390/nu17193073
Chicago/Turabian StyleSingh, Kanishka, Huiling Liao, Indika Edirisinghe, Britt Burton-Freeman, and Amandeep K. Sandhu. 2025. "Dose–Response Effect of Watermelon Consumption on Ambulatory Blood Pressure in Adults with Elevated Blood Pressure: A Randomized Controlled Pilot Trial" Nutrients 17, no. 19: 3073. https://doi.org/10.3390/nu17193073
APA StyleSingh, K., Liao, H., Edirisinghe, I., Burton-Freeman, B., & Sandhu, A. K. (2025). Dose–Response Effect of Watermelon Consumption on Ambulatory Blood Pressure in Adults with Elevated Blood Pressure: A Randomized Controlled Pilot Trial. Nutrients, 17(19), 3073. https://doi.org/10.3390/nu17193073






