Precision Nutrition and Gut–Brain Axis Modulation in the Prevention of Neurodegenerative Diseases
Abstract
1. Introduction
2. Methods
3. Pathophysiological Mechanisms Linking the Gut Microbiota and Neurodegeneration
4. Dietary Interventions as Modulators of Gut–Brain Axis Mechanisms
4.1. Probiotics and Psychobiotics
4.2. Prebiotics and Dietary Fibers
4.3. Polyphenols and Antioxidant
4.4. Omega-3 Fatty Acids
5. Neurodegenerative Diseases and the Role of the Microbiota
5.1. Gut Microbiome Dysbiosis and Its Association with Dementia and Alzheimer’s Disease
| Nature of Dysbiosis | Associated Changes in AD | Potential Mechanisms of Impact on the Central Nervous System | References |
|---|---|---|---|
| Deficiency of neuroprotective taxa | ↓ Firmicutes, Bifidobacterium ↑ Bacteroidetes | SCFAs deficiency, activation of neuroinflammation, neurotransmitter dysregulation, disruption of the gut–brain axis | [144] |
| Increase in pro-inflammatory bacteria | ↓ Lactobacillus, Bifidobacterium, Ruminococcus ↑ Escherichia, Enterococcus | Decreased SCFA production, increased lipopolysaccharide levels, immune dysregulation, development of neuroinflammation | [147] |
| Increase in pro-inflammatory bacteria | ↓Firmicutes/Bacteroidetes, Bifidobacterium ↑ Pseudomonadota, Synergistetes, Christensenellaceae | Disrupted metabolic pathways, suppressed SCFA degradation, and sugar metabolism dysregulation exacerbate oxidative stress and promote neuronal damage | [148] |
| Neuro-associated dysmetabolic imbalance | ↑ Blautia, Enterobacteriaceae, Enterobacteriales, Gammaproteobacteria, Bacilli | Neurotransmitter dysregulation, modulation of neuronal excitability | [149] |
| Pro-inflammatory dysbiosis with amyloid-associated microbial profile | ↓ Megamonas, Serratia, Leptotrichia, Clostridium (Clostridiaceae) ↑ Victivallis, Enterococcus, Mitsuokella, Clostridium (Erysipelotrichaceae) | Disruption of the gut–brain axis through microbial translocation and systemic inflammation | [150] |
| Increase in pro-inflammatory bacteria | ↓Bifidobacterium spp., Firmicutes, Actinobacteria↑ Akkermansia, Enterobacteria, Bacteroidetes, Bacillus cereus, Prevotella, Clostridium IV | Decreased SCFA production, neurotransmitter imbalance, BBB disruption, enhanced oxidative stress | [151] |
| Increased β-diversity | ↓ Bacteroides, Lachnospira, Ruminiclostridium_9 ↑ Prevotella | Reduced production of protective metabolites, enhanced systemic and neuroinflammation, progressive microbiome imbalance | [152] |
| Decrease in butyrate-producing bacteria and growth of opportunistic flora | ↓ Clostridiaceae, Lachnospiraceae ↑ Escherichia-Shigella, Bacteroides, Holdemanella, Romboutsia, Megamonas | Reduced SCFA production, activation of inflammatory processes, impaired synthesis and metabolism of neuroactive compounds | [153] |
5.2. The Microbiota and Parkinson’s Disease: From the Gut to the Brain
5.3. Multiple Sclerosis and Microbiota-Mediated Autoimmune Mechanism
6. Precision Nutrition and Personalized Strategies
6.1. The Concept of Precision Nutrition
6.2. Biomarkers for Nutrition Personalization
6.3. Nutritional Interventions and Chrononutrition
7. Limitations and Future Challenges
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| MS | Multiple sclerosis |
| WHO | World Health Organization |
| CNS | Central nervous system |
| HADS | Hospital Anxiety and Depression Scale |
| ISAPP | International Scientific Association for Probiotics and Prebiotics |
| EFSA | European Food Safety Authority |
| RRMS | Relapsing-remitting multiple sclerosis |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| GABA | γ-aminobutyric acid |
| ATP | Adenosine triphosphate |
| HPA axis | Hypothalamic–pituitary–adrenal system |
| GC | Glucocorticoid |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor-α |
| TJ | Tight junctions |
| TLRs | Toll-like receptors |
| NF-κB | Nuclear factor kappa B |
| EEPs | Enteroendocrine peptides |
| SCFAs | Short-chain fatty acids |
| BBB | Blood–brain barrier |
| TMAO | Trimethylamine-N-oxide |
| CSF | Cerebrospinal fluid |
| Aβ | β-amyloid |
| IFN-γ | Interferon-γ |
| IL-17 | Interleukin-17 |
| FOS | Fructo-oligosaccharides |
| GOS | Galactooligosaccharides |
| BDNF | Brain-derived neurotrophic factor |
| Treg | T-regulatory lymphocytes |
| PUFA | Polyunsaturated fatty acids |
| EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| CLA | Conjugated linoleic acid |
| CALA | Conjugated α-linolenic acid |
| SNCA | α-synuclein |
| MAPT | Microtubule-associated protein tau |
| LRRK2 | Leucine-rich repeat kinase 2 |
| SOD1 | Superoxide dismutase 1 |
| PARK7/DJ-1 | Parkinson’s disease 7 |
| PSEN1 | Presenilin 1 |
| POCT | Point-of-care tests |
| MMSE | Mini-Mental State Examination |
| AI | Artificial intelligence |
| TRF | Time-restricted eating |
| TRE | Time-restricted eating |
| IF | Intermittent fasting |
| FFAR | Free fatty acid receptors |
References
- Bhatti, G.K.; Reddy, A.P.; Reddy, P.H.; Bhatti, J.S. Lifestyle modifications and nutritional interventions in aging-associated cognitive decline and Alzheimer’s disease. Front. Aging Neurosci. 2020, 11, 369. [Google Scholar] [CrossRef]
- Bianchi, V.E.; Herrera, P.F.; Laura, R. Effect of nutrition on neurodegenerative diseases. A systematic review. Nutr. Neurosci. 2021, 24, 810–834. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Qin, W.; Zhu, M.; Jia, J. Model-based projection of dementia prevalence in China and worldwide: 2020–2050. J. Alzheimer’s Dis. 2021, 82, 1823–1831. [Google Scholar] [CrossRef]
- Hughes, T.F. Promotion of cognitive health through cognitive activity in the aging population. Aging Health 2010, 6, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Kurkinen, M.; Fułek, M.; Fułek, K.; Beszłej, J.A.; Kurpas, D.; Leszek, J. The amyloid cascade hypothesis in Alzheimer’s disease: Should we change our thinking? Biomolecules 2023, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Gardener, H.; Caunca, M.R. Mediterranean diet in preventing neurodegenerative diseases. Curr. Nutr. Rep. 2018, 7, 10–20. [Google Scholar] [CrossRef]
- Liu, X.; Etxeberria, U.; Ruiz-Canela, M. Nutrition and neurodegenerative diseases: Insights and perspectives on prevention strategies. Front. Nutr. 2023, 10, 1272338. [Google Scholar] [CrossRef]
- Grodzicki, W.; Dziendzikowska, K. The role of selected bioactive compounds in the prevention of Alzheimer’s disease. Antioxidants 2020, 9, 229. [Google Scholar] [CrossRef]
- Mohd Sairazi, N.S.; Sirajudeen, K.N.S. Natural products and their bioactive compounds: Neuroprotective potentials against neurodegenerative diseases. Evid.-Based Complement. Altern. Med. 2020, 2020, 6565396. [Google Scholar] [CrossRef]
- Samanta, S.; Chakraborty, S.; Bagchi, D. Pathogenesis of neurodegenerative diseases and the protective role of natural bioactive components. J. Am. Nutr. Assoc. 2024, 43, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Conti, V.; Zarrella, A.; Donnarumma, D.; Pagano, A.; Mazza, I.; De Stefano, A.; Gallo, F.; Di Landri, V.; De Pascale, D.; Manzo, V.; et al. Natural Health Products in the Prevention and Management of Alzheimer’s Disease: A Systematic Review of Randomized Clinical Trials. Appl. Sci. 2025, 15, 3513. [Google Scholar] [CrossRef]
- Mohajeri, M.H.; La Fata, G.; Steinert, R.E.; Weber, P. Relationship between the gut microbiome and brain function. Nutr. Rev. 2018, 76, 481–496. [Google Scholar] [CrossRef]
- Montagnani, M.; Bottalico, L.; Potenza, M.A.; Charitos, I.A.; Topi, S.; Colella, M.; Santacroce, L. The crosstalk between gut microbiota and nervous system: A bidirectional interaction between microorganisms and metabolome. Int. J. Mol. Sci. 2023, 24, 10322. [Google Scholar] [CrossRef]
- Alkasir, R.; Li, J.; Li, X.; Jin, M.; Zhu, B. Human gut microbiota: The links with dementia development. Protein Cell 2017, 8, 90–102. [Google Scholar] [CrossRef]
- Tang, W.; Meng, Z.; Li, N.; Liu, Y.; Li, L.; Chen, D.; Yang, Y. Roles of gut microbiota in the regulation of hippocampal plasticity, inflammation, and hippocampus-dependent behaviors. Front. Cell. Infect. Microbiol. 2021, 10, 611014. [Google Scholar] [CrossRef]
- Damiani, F.; Cornuti, S.; Tognini, P. The gut-brain connection: Exploring the influence of the gut microbiota on neuroplasticity and neurodevelopmental disorders. Neuropharmacology 2023, 231, 109491. [Google Scholar] [CrossRef]
- Kurhaluk, N.; Kamiński, P.; Bilski, R.; Kołodziejska, R.; Woźniak, A.; Tkaczenko, H. Role of Antioxidants in Modulating the Microbiota–Gut–Brain Axis and Their Impact on Neurodegenerative Diseases. Int. J. Mol. Sci. 2025, 26, 3658. [Google Scholar] [CrossRef]
- Kirk, D.; Catal, C.; Tekinerdogan, B. Precision nutrition: A systematic literature review. Comput. Biol. Med. 2021, 133, 104365. [Google Scholar] [CrossRef]
- Milošević, M.; Arsić, A.; Cvetković, Z.; Vučić, V. Memorable food: Fighting age-related neurodegeneration by precision nutrition. Front. Nutr. 2021, 8, 688086. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, D.; Dinesh, S.; Sharma, S.; Sathisha, G.J. Gut-Brain Axis Modulation of Metabolic Disorders: Exploring the Intertwined Neurohumoral Pathways and Therapeutic Prospects. Neurochem. Res. 2024, 49, 847–871. [Google Scholar] [CrossRef]
- Hwang, Y.K.; Oh, J.S. Interaction of the Vagus Nerve and Serotonin in the Gut–Brain Axis. Int. J. Mol. Sci. 2025, 26, 1160. [Google Scholar] [CrossRef]
- Shin, C.; Kim, Y.-K. Chapter 3—The interactions between gut and brain in psychiatric and neurological disorders. In The Complex Interplay Between Gut-Brain, Gut-Liver, and Liver-Brain Axes; Stasi, C., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 49–65. [Google Scholar] [CrossRef]
- Bonaz, B.; Sinniger, V.; Pellissier, S. Therapeutic potential of vagus nerve stimulation for inflammatory bowel diseases. Front. Neurosci. 2021, 15, 650971. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M. Gut bacteria and neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef]
- Yang, X.; Lou, J.; Shan, W.; Ding, J.; Jin, Z.; Hu, Y.; Du, Q.; Liao, Q.; Xie, R.; Xu, J. Pathophysiologic role of neurotransmitters in digestive diseases. Front. Physiol. 2021, 12, 567650. [Google Scholar] [CrossRef]
- Yan, M.; Man, S.; Sun, B.; Ma, L.; Guo, L.; Huang, L.; Gao, W. Gut liver brain axis in diseases: The implications for therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 443. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef]
- Tsigos, C.; Chrousos, G.P. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus nerve as modulator of the brain–gut axis in psychiatric and inflammatory disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef]
- Huo, R.; Zeng, B.; Zeng, L.; Cheng, K.; Li, B.; Luo, Y.; Wang, H.; Zhou, C.; Fang, L.; Li, W.; et al. Microbiota modulate anxiety-like behavior and endocrine abnormalities in hypothalamic-pituitary-adrenal axis. Front. Cell. Infect. Microbiol. 2017, 7, 489. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Y.; Zhang, Q.; Song, Y.; Wang, L.; Zhu, Z. Interactions between intestinal microbiota and neural mitochondria: A new perspective on communicating pathway from gut to brain. Front. Microbiol. 2022, 13, 798917. [Google Scholar] [CrossRef]
- Pellegrini, C.; Fornai, M.; D’Antongiovanni, V.; Antonioli, L.; Bernardini, N.; Derkinderen, P. The intestinal barrier in disorders of the central nervous system. Lancet Gastroenterol. Hepatol. 2023, 8, 66–80. [Google Scholar] [CrossRef]
- Stadlbauer, V.; Engertsberger, L.; Komarova, I.; Feldbacher, N.; Leber, B.; Pichler, G.; Fink, N.; Scarpatetti, M.; Schippinger, W.; Schmidt, R.; et al. Dysbiosis, gut barrier dysfunction and inflammation in dementia: A pilot study. BMC Geriatr. 2020, 20, 248. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.; Li, J.; Zhao, Y.; Jiang, H.; Luo, S.; He, G. Intestinal changes in permeability, tight junction and mucin synthesis in a mouse model of Alzheimer’s disease. Int. J. Mol. Med. 2023, 52, 113. [Google Scholar] [CrossRef]
- Aho, V.T.; Houser, M.C.; Pereira, P.A.; Chang, J.; Rudi, K.; Paulin, L.; Hertzberg, V.; Auvinen, P.; Tansey, M.G.; Scheperjans, F. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 2021, 16, 6. [Google Scholar] [CrossRef]
- De Magistris, L.; Familiari, V.; Pascotto, A.; Sapone, A.; Frolli, A.; Iardino, P.; Carteni, M.; De Rosa, M.; Francavilla, R.; Riegler, G.; et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 418–424. [Google Scholar] [CrossRef]
- Trzeciak, P.; Herbet, M. Role of the intestinal microbiome, intestinal barrier and psychobiotics in depression. Nutrients 2021, 13, 927. [Google Scholar] [CrossRef]
- Camara-Lemarroy, C.R.; Metz, L.; Meddings, J.B.; Sharkey, K.A.; Wee Yong, V. The intestinal barrier in multiple sclerosis: Implications for pathophysiology and therapeutics. Brain 2018, 141, 1900–1916. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Pongkorpsakol, P.; Xiong, Z.; Li, L.; Jiang, X.; Zhao, H.; Yuan, D.; Zhang, C.; Guo, Y.; et al. Relief effects of icariin on inflammation-induced decrease of tight junctions in intestinal epithelial cells. Front. Pharmacol. 2022, 13, 903762. [Google Scholar] [CrossRef]
- Rios-Arce, N.D.; Collins, F.L.; Schepper, J.D.; Steury, M.D.; Raehtz, S.; Mallin, H.; Schoenherr, D.T.; Parameswaran, N.; McCabe, L.R. Epithelial barrier function in gut-bone signaling. In Understanding the Gut-Bone Signaling Axis: Mechanisms and Therapeutic Implications; Springer: Cham, Switzerland, 2017; Volume 1033, pp. 151–183. [Google Scholar] [CrossRef]
- Zhao, M.A.; Chu, J.; Feng, S.; Guo, C.; Xue, B.; He, K.; Li, L. Immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: A review. Biomed. Pharmacother. 2023, 164, 114985. [Google Scholar] [CrossRef]
- Watnick, P.I.; Jugder, B.E. Microbial control of intestinal homeostasis via enteroendocrine cell innate immune signaling. Trends Microbiol. 2020, 28, 141–149. [Google Scholar] [CrossRef]
- Caballero, I.; Boyd, J.; Almiñana, C.; Sánchez-López, J.A.; Basatvat, S.; Montazeri, M.; Lay, N.M.; Elliot, S.; Spiller, D.G.; White, M.R.H.; et al. Understanding the dynamics of Toll-like Receptor 5 response to flagellin and its regulation by estradiol. Sci. Rep. 2017, 7, 40981. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Maguire, M.; Maguire, G. Gut dysbiosis, leaky gut, and intestinal epithelial proliferation in neurological disorders: Towards the development of a new therapeutic using amino acids, prebiotics, probiotics, and postbiotics. Rev. Neurosci. 2019, 30, 179–201. [Google Scholar] [CrossRef]
- Mahbub, N.U.; Islam, M.M.; Hong, S.T.; Chung, H.J. Dysbiosis of the gut microbiota and its effect on α-synuclein and prion protein misfolding: Consequences for neurodegeneration. Front. Cell. Infect. Microbiol. 2024, 14, 1348279. [Google Scholar] [CrossRef]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef]
- Block, M.L.; Hong, J.S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 508738. [Google Scholar] [CrossRef]
- Liu, X.F.; Shao, J.H.; Liao, Y.T.; Wang, L.N.; Jia, Y.; Dong, P.J.; Liu, Z.Z.; He, D.D.; Li, C.; Zhang, X. Regulation of short-chain fatty acids in the immune system. Front. Immunol. 2023, 14, 1186892. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef]
- Li, M.; Van Esch, B.C.; Henricks, P.A.; Folkerts, G.; Garssen, J. The anti-inflammatory effects of short chain fatty acids on lipopolysaccharide-or tumor necrosis factor α-stimulated endothelial cells via activation of GPR41/43 and inhibition of HDACs. Front. Pharmacol. 2018, 9, 533. [Google Scholar] [CrossRef]
- Bonnet, U.; Bingmann, D.; Wiemann, M. Intracellular pH modulates spontaneous and epileptiform bioelectric activity of hippocampal CA3-neurones. Eur. Neuropsychopharmacol. 2000, 10, 97–103. [Google Scholar] [CrossRef]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoorj, A.; Keyvani, H.; et al. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed. Pharmacother. 2021, 139, 111661. [Google Scholar] [CrossRef]
- Ryue, J.; Lin, L.; Liu, Y.; Lu, W.; McCartney, D.; Dhar, B.R. Comparative effects of GAC addition on methane productivity and microbial community in mesophilic and thermophilic anaerobic digestion of food waste. Biochem. Eng. J. 2019, 146, 79–87. [Google Scholar] [CrossRef]
- Pentassuglia, S.; Agostino, V.; Tommasim, T. EAB—Electroactive bioflm: A biotechnological resource. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elsevier: Oxford, UK, 2018; pp. 110–123. [Google Scholar] [CrossRef]
- Fock, E.; Parnova, R. Mechanisms of blood–brain barrier protection by microbiota-derived short-chain fatty acids. Cells 2023, 12, 657. [Google Scholar] [CrossRef]
- Majumdar, A.; Siva Venkatesh, I.P.; Basu, A. Short-chain fatty acids in the microbiota–gut–brain axis: Role in neurodegenerative disorders and viral infections. ACS Chem. Neurosci. 2023, 14, 1045–1062. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan metabolism and gut-brain homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, R.; Bajpai, V.K.; Baek, K.H. Production of GABA (γ-aminobutyric acid) by microorganisms: A review. Braz. J. Microbiol. 2012, 43, 1230–1241. [Google Scholar] [CrossRef]
- Kabała, K.; Janicka, M. Relationship between the GABA pathway and signaling of other regulatory molecules. Int. J. Mol. Sci. 2024, 25, 10749. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.J.; Kosek, V.; Beltrán, D.; Tomás-Barberán, F.A.; Hajslova, J. Production of new microbially conjugated bile acids by human gut microbiota. Biomolecules 2022, 12, 687. [Google Scholar] [CrossRef]
- Engevik, K.A.; Hazzard, A.; Puckett, B.; Hoch, K.M.; Haidacher, S.J.; Haag, A.M.; Spinler, J.K.; Versalovic, J.; Engevik, M.A.; Horvath, T.D. Phylogenetically diverse bacterial species produce histamine. Syst. Appl. Microbiol. 2024, 47, 126539. [Google Scholar] [CrossRef]
- Carthy, E.; Ellender, T. Histamine, neuroinflammation and neurodevelopment: A review. Front. Neurosci. 2021, 15, 680214. [Google Scholar] [CrossRef] [PubMed]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio 2015, 6, 10–1128. [Google Scholar] [CrossRef]
- Praveenraj, S.S.; Sonali, S.; Anand, N.; Tousif, H.A.; Vichitra, C.; Kalyan, M.; Kanna, P.V.; Chandana, K.A.; Shasthara, P.; Mahalakshmi, A.M.; et al. The role of a gut microbial-derived metabolite, trimethylamine N-oxide (TMAO), in neurological disorders. Mol. Neurobiol. 2022, 59, 6684–6700. [Google Scholar] [CrossRef]
- Grimaldi, L.; Cavallaro, R.A.; De Angelis, D.; Fuso, A.; Sancesario, G. Vitamin K properties in stroke and Alzheimer’s disease: A janus bifrons in protection and prevention. Molecules 2025, 30, 1027. [Google Scholar] [CrossRef]
- Smith, A.D.; Smith, S.M.; De Jager, C.A.; Whitbread, P.; Johnston, C.; Agacinski, G.; Oulhaj, A.; Bradley, K.M.; Jacoby, R.; Refsum, H. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: A randomized controlled trial. PLoS ONE 2010, 5, e12244. [Google Scholar] [CrossRef]
- Chudzik, A.; Orzyłowska, A.; Rola, R.; Stanisz, G.J. Probiotics, prebiotics and postbiotics on mitigation of depression symptoms: Modulation of the brain–gut–microbiome axis. Biomolecules 2021, 11, 1000. [Google Scholar] [CrossRef]
- Naomi, R.; Embong, H.; Othman, F.; Ghazi, H.F.; Maruthey, N.; Bahari, H. Probiotics for Alzheimer’s disease: A systematic review. Nutrients 2021, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Kim, N.; Yun, M.; Oh, Y.J.; Choi, H.J. Mind-altering with the gut: Modulation of the gut-brain axis with probiotics. J. Microbiol. 2018, 56, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Del Toro-Barbosa, M.; Hurtado-Romero, A.; Garcia-Amezquita, L.E.; García-Cayuela, T. Psychobiotics: Mechanisms of action, evaluation methods and effectiveness in applications with food products. Nutrients 2020, 12, 3896. [Google Scholar] [CrossRef]
- Williams, N.T. Probiotics. Am. J. Health-Syst. Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef]
- De Oliveira, F.L.; Salgaço, M.K.; de Oliveira, M.T.; Mesa, V.; Sartoratto, A.; Peregrino, A.M.; Ramos, W.S.; Sivieri, K. Exploring the potential of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 as promising psychobiotics using SHIME. Nutrients 2023, 15, 1521. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Violle, N.; Bisson, J.F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2011, 2, 256–261. [Google Scholar] [CrossRef]
- Knezevic, E.; Nenic, K.; Milanovic, V.; Knezevic, N.N. The role of cortisol in chronic stress, neurodegenerative diseases, and psychological disorders. Cells 2023, 12, 2726. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W. Psychobiotics and the manipulation of bacteria–gut–brain signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, X.; Zhang, Y.; Hu, A.; Zhou, Q.; Yue, X.; Liu, Z.; Li, M. The Role of Probiotics in Modulating the Gut Microbiome in Alzheimer’s Disease: A Review. Foods 2025, 14, 1531. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Sugahara, H.; Shimada, K.; Mitsuyama, E.; Kuhara, T.; Yasuoka, A.; Kondo, T.; Abe, K.; Xiao, J.Z. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci. Rep. 2017, 7, 13510. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kuhara, T.; Oki, M.; Xiao, J.Z. Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: A randomised, double-blind, placebo-controlled trial. Benef. Microbes 2019, 10, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Asl, Z.R.; Sepehri, G.; Salami, M. Probiotic treatment improves the impaired spatial cognitive performance and restores synaptic plasticity in an animal model of Alzheimer’s disease. Behav. Brain Res. 2019, 376, 112183. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Heidari-Soureshjani, R.; Mirhosseini, N.; Kouchaki, E.; Bahmani, F.; Aghadavod, E.; Tajabadi-Ebrahimi, M.; Asemi, Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin. Nutr. 2019, 38, 2569–2575. [Google Scholar] [CrossRef]
- Agahi, A.; Hamidi, G.A.; Daneshvar, R.; Hamdieh, M.; Soheili, M.; Alinaghipour, A.; Taba, S.M.E.; Salami, M. Does severity of Alzheimer’s disease contribute to its responsiveness to modifying gut microbiota? A double blind clinical trial. Front. Neurol. 2018, 9, 662. [Google Scholar] [CrossRef] [PubMed]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: A randomized, double-blind and controlled trial. Front. Aging Neurosci. 2016, 8, 229544. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Giannos, P.; Ispoglou, T.; Witard, O.C.; Isanejad, M. Dietary fiber intake is associated with cognitive function in older adults: Data from the national health and nutrition examination survey. Am. J. Med. 2022, 135, e257–e262. [Google Scholar] [CrossRef]
- Azuma, N.; Mawatari, T.; Saito, Y.; Tsukamoto, M.; Sampei, M.; Iwama, Y. Effect of continuous ingestion of bifidobacteria and dietary fiber on improvement in cognitive function: A randomized, double-blind, placebo-controlled trial. Nutrients 2023, 15, 4175. [Google Scholar] [CrossRef]
- La Torre, D.; Verbeke, K.; Dalile, B. Dietary fibre and the gut–brain axis: Microbiota-dependent and independent mechanisms of action. Gut Microbiome 2021, 2, e3. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Savignac, H.M.; Corona, G.; Mills, H.; Chen, L.; Spencer, J.P.; Tzortzis, G.; Burnet, P.W. Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-D-aspartate receptor subunits and D-serine. Neurochem. Int. 2013, 63, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, E.; Barranco, A.; Ramírez, M.; Gruart, A.; Delgado-García, J.M.; Martínez-Lara, E.; Blanco, S.; Martin, M.J.; Castanys, E.; Buck, R.; et al. Effects of a human milk oligosaccharide, 2′-fucosyllactose, on hippocampal long-term potentiation and learning capabilities in rodents. J. Nutr. Biochem. 2015, 26, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Cowen, P.J.; Harmer, C.J.; Tzortzis, G.; Errington, S.; Burnet, P.W. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology 2015, 232, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Baião, R.; Capitão, L.P.; Higgins, C.; Browning, M.; Harmer, C.J.; Burnet, P.W. Multispecies probiotic administration reduces emotional salience and improves mood in subjects with moderate depression: A randomised, double-blind, placebo-controlled study. Psychol. Med. 2023, 53, 3437–3447. [Google Scholar] [CrossRef]
- Johnstone, N.; Kadosh, K.C. A Randomised Controlled Trial of the effects of Galacto-Oligosaccharides on the gut brain-axis of young females. Brain Behav. Immun. 2025, 129, 573–584. [Google Scholar] [CrossRef]
- Johnstone, N.; Dart, S.; Knytl, P.; Nauta, A.; Hart, K.; Cohen Kadosh, K. Nutrient intake and gut microbial genera changes after a 4-week placebo controlled galacto-oligosaccharides intervention in young females. Nutrients 2021, 13, 4384. [Google Scholar] [CrossRef] [PubMed]
- Looijesteijn, E.; Schoemaker, M.H.; Van Den Belt, M.; Hester, E.R.; Kortman, G.A.; Viskaal-van Dongen, M.; Nauta, A. A double-blind intervention trial in healthy women demonstrates the beneficial impact on Bifidobacterium with low dosages of prebiotic galacto-oligosaccharides. Front. Nutr. 2024, 11, 1440319. [Google Scholar] [CrossRef]
- Láng, L.; McArthur, S.; Lazar, A.S.; Pourtau, L.; Gaudout, D.; Pontifex, M.G.; Müller, M.; Vauzour, D. Dietary (Poly) phenols and the Gut–Brain Axis in Ageing. Nutrients 2024, 16, 1500. [Google Scholar] [CrossRef]
- Baldi, S.; Tristán Asensi, M.; Pallecchi, M.; Sofi, F.; Bartolucci, G.; Amedei, A. Interplay between lignans and gut microbiota: Nutritional, functional and methodological aspects. Molecules 2023, 28, 343. [Google Scholar] [CrossRef]
- Reddy, V.P.; Aryal, P.; Robinson, S.; Rafiu, R.; Obrenovich, M.; Perry, G. Polyphenols in Alzheimer’s disease and in the gut–brain axis. Microorganisms 2020, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.; Almeida, L.M.; Dinis, T.C. Dietary polyphenols: A novel strategy to modulate microbiota-gut-brain axis. Trends Food Sci. Technol. 2018, 78, 224–233. [Google Scholar] [CrossRef]
- Braune, A.; Blaut, M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes 2016, 7, 216–234. [Google Scholar] [CrossRef]
- Williamson, G.; Clifford, M.N. Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols. Biochem. Pharmacol. 2017, 139, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Verzelloni, E.; Pellacani, C.; Tagliazucchi, D.; Tagliaferri, S.; Calani, L.; Costa, L.G.; Brighenti, F.; Borges, G.; Crozier, A.; Conte, A.; et al. Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol. Nutr. Food Res. 2011, 55 (Suppl. S1), S35–S43. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, T.; Liu, Z.; Danzengquzhen; Cisangzhuoma; Ma, J.; Li, X.; Huang, X.; Li, B. The neuromodulatory effects of flavonoids and gut Microbiota through the gut-brain axis. Front. Cell. Infect. Microbiol. 2023, 13, 1197646. [Google Scholar] [CrossRef] [PubMed]
- Zinkow, A.; Grodzicki, W.; Czerwińska, M.; Dziendzikowska, K. Molecular Mechanisms Linking Omega-3 Fatty Acids and the Gut–Brain Axis. Molecules 2024, 30, 71. [Google Scholar] [CrossRef]
- Guixà-González, R.; Javanainen, M.; Gómez-Soler, M.; Cordobilla, B.; Domingo, J.C.; Sanz, F.; Pastor, M.; Ciruela, F.; Martinez-Seara, H.; Selent, J. Membrane omega-3 fatty acids modulate the oligomerisation kinetics of adenosine A2A and dopamine D2 receptors. Sci. Rep. 2016, 6, 19839. [Google Scholar] [CrossRef]
- Bazan, N.G.; Musto, A.E.; Knott, E.J. Endogenous signaling by omega-3 docosahexaenoic acid-derived mediators sustains homeostatic synaptic and circuitry integrity. Mol. Neurobiol. 2011, 44, 216–222. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of omega-3 fatty acids on the gut microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Zou, B.; Zhao, D.; Zhou, S.; Kang, J.X.; Wang, B. Insight into the effects of Omega-3 fatty acids on gut microbiota: Impact of a balanced tissue Omega-6/Omega-3 ratio. Front. Nutr. 2025, 12, 1575323. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, M.M.; El Aidy, S.; Crispie, F.; O’Sullivan, O.; Cotter, P.; Stanton, C.; Kelly, P.; Cryan, J.F.; Dinan, T.G. N-3 polyunsaturated fatty acids (PUFAs) reverse the impact of early-life stress on the gut microbiota. PLoS ONE 2015, 10, e0139721. [Google Scholar] [CrossRef]
- Pinchaud, K.; Hafeez, Z.; Auger, S.; Chatel, J.M.; Chadi, S.; Langella, P.; Paoli, J.; Dary-Mourot, A.; Maguin-Gaté, K.; Olivier, J.L. Impact of Dietary Arachidonic Acid on Gut Microbiota Composition and Gut–Brain Axis in Male BALB/C Mice. Nutrients 2022, 14, 5338. [Google Scholar] [CrossRef]
- Salsinha, A.S.; Araújo-Rodrigues, H.; Dias, C.; Cima, A.; Rodríguez-Alcalá, L.M.; Relvas, J.B.; Pintado, M. Omega-3 and conjugated fatty acids impact on human microbiota modulation using an in vitro fecal fermentation model. Clin. Nutr. 2025, 49, 102–117. [Google Scholar] [CrossRef]
- Jayapala, H.P.; Lim, S.Y. N-3 polyunsaturated fatty acids and gut microbiota. Comb. Chem. High Throughput Screen. 2023, 26, 892–905. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.C.; Oriach, C.S.; Murphy, K.; Moloney, G.M.; Cryan, J.F.; Dinan, T.G.; Poss, R.P.; Stanton, C. Deficiency of essential dietary n-3 PUFA disrupts the caecal microbiome and metabolome in mice. Br. J. Nutr. 2017, 118, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, H.; Pédrono, F.; Boulier-Monthéan, N.; Catheline, D.; Rioux, V.; Legrand, P. Comparative effects of well-balanced diets enriched in α-linolenic or linoleic acids on LC-PUFA metabolism in rat tissues. Prostaglandins, Leukot. Essent. Fat. Acids 2013, 88, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 2022, 100, 233–241. [Google Scholar] [CrossRef]
- Schaub, A.C.; Schneider, E.; Vazquez-Castellanos, J.F.; Schweinfurth, N.; Kettelhack, C.; Doll, J.P.; Yamanbaeva, G.; Mählmann, L.; Brand, S.; Beglinger, C.; et al. Clinical, gut microbial and neural effects of a probiotic add-on therapy in depressed patients: A randomized controlled trial. Transl. Psychiatry 2022, 12, 227. [Google Scholar] [CrossRef]
- Sanborn, V.; Azcarate-Peril, M.A.; Updegraff, J.; Manderino, L.; Gunstad, J. Randomized clinical trial examining the impact of Lactobacillus rhamnosus GG probiotic supplementation on cognitive functioning in middle-aged and older adults. Neuropsychiatr. Dis. Treat. 2020, 16, 2765–2777. [Google Scholar] [CrossRef]
- Lan, Y.; Lu, J.; Qiao, G.; Mao, X.; Zhao, J.; Wang, G.; Tian, P.; Chen, W. Bifidobacterium breve CCFM1025 improves sleep quality via regulating the activity of the HPA axis: A randomized clinical trial. Nutrients 2023, 15, 4700. [Google Scholar] [CrossRef] [PubMed]
- Elhossiny, R.M.; Elshahawy, H.H.; Mohamed, H.M.; Abdelmageed, R.I. Assessment of probiotic strain Lactobacillus acidophilus LB supplementation as adjunctive management of attention-deficit hyperactivity disorder in children and adolescents: A randomized controlled clinical trial. BMC Psychiatry 2023, 23, 823. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gonzalez, C.; Cardona, D.; Rueda-Ruzafa, L.; Rodriguez-Arrastia, M.; Ropero-Padilla, C.; Roman, P. Cognitive and Emotional Effect of a Multi-species Probiotic Containing Lactobacillus rhamnosus and Bifidobacterium lactis in Healthy Older Adults: A Double-Blind Randomized Placebo-Controlled Crossover Trial. In Probiotics and Antimicrobial Proteins; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–13. [Google Scholar] [CrossRef]
- Ho, Y.T.; Tsai, Y.C.; Kuo, T.B.; Yang, C.C. Effects of Lactobacillus plantarum PS128 on depressive symptoms and sleep quality in self-reported insomniacs: A randomized, double-blind, placebo-controlled pilot trial. Nutrients 2021, 13, 2820. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: A pilot study in patients with irritable bowel syndrome. Gastroenterology 2017, 153, 448–459. [Google Scholar] [CrossRef]
- Prata-Martins, D.; Nobre, C.; Almeida-Antunes, N.; Azevedo, P.; Sousa, S.S.; Crego, A.; Cryan, J.; Sampaio, A.; Carbia, C.; López-Caneda, E. Assessing the impact of binge drinking and a prebiotic intervention on the gut–brain axis in young adults: Protocol for a randomised controlled trial. BMJ Open 2025, 15, e095932. [Google Scholar] [CrossRef]
- Colombo, J.; Carlson, S.E.; Algarín, C.; Reyes, S.; Chichlowski, M.; Harris, C.L.; Wampler, J.L.; Peirano, P.; Berseth, C.L. Developmental effects on sleep–wake patterns in infants receiving a cow’s milk-based infant formula with an added prebiotic blend: A Randomized Controlled Trial. Pediatr. Res. 2021, 89, 1222–1231. [Google Scholar] [CrossRef]
- Gillies, N.A.; Wilson, B.C.; Miller, J.R.; Roy, N.C.; Scholey, A.; Braakhuis, A.J. Effects of a flavonoid-rich blackcurrant beverage on markers of the gut-brain axis in healthy females: Secondary findings from a 4-week randomized crossover control trial. Curr. Dev. Nutr. 2024, 8, 102158. [Google Scholar] [CrossRef]
- Kamarunzaman, N.N.Z.; Le Sayec, M.; Li, Y.; Wu, H.; Mesnage, R.; Dalrymple, K.; Halket, J.; Caldwell, A.; Borsini, A.; Pariante, C.; et al. Effects of cranberry (poly) phenols on mental health in university students: The CRANMOOD randomized controlled trial. Proc. Nutr. Soc. 2024, 83, E279. [Google Scholar] [CrossRef]
- de la Torre-Aguilar, M.J.; Gomez-Fernandez, A.; Flores-Rojas, K.; Martin-Borreguero, P.; Mesa, M.D.; Perez-Navero, J.L.; Olivares, M.; Gil, A.; Gil-Campos, M. Docosahexaenoic and eicosapentaenoic intervention modifies plasma and erythrocyte omega-3 fatty acid profiles but not the clinical course of children with autism spectrum disorder: A randomized control trial. Front. Nutr. 2022, 9, 790250. [Google Scholar] [CrossRef]
- Liu, S.; Gao, J.; Zhu, M.; Liu, K.; Zhang, H.L. Gut microbiota and dysbiosis in Alzheimer’s disease: Implications for pathogenesis and treatment. Mol. Neurobiol. 2020, 57, 5026–5043. [Google Scholar] [CrossRef]
- Solanki, R.; Karande, A.; Ranganathan, P. Emerging role of gut microbiota dysbiosis in neuroinflammation and neurodegeneration. Front. Neurol. 2023, 14, 1149618. [Google Scholar] [CrossRef] [PubMed]
- Frost, B.; Diamond, M.I. Prion-like mechanisms in neurodegenerative diseases. Nat. Rev. Neurosci. 2010, 11, 155–159. [Google Scholar] [CrossRef]
- Hasegawa, M. Molecular mechanisms in the pathogenesis of Alzheimer’s disease and tauopathies-prion-like seeded aggregation and phosphorylation. Biomolecules 2016, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Asti, A.; Gioglio, L. Can a bacterial endotoxin be a key factor in the kinetics of amyloid fibril formation? J. Alzheimer’s Dis. 2014, 39, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, Y.K.; Yuk, D.Y.; Choi, D.Y.; Ban, S.B.; Oh, K.W.; Hong, J.T. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J. Neuroinflammation 2008, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Bhattacharjee, S.; Pogue, A.I.; Lukiw, W.J. The gastrointestinal tract microbiome and potential link to Alzheimer’s disease. Front. Neurol. 2014, 5, 43. [Google Scholar] [CrossRef]
- Hill, J.M.; Clement, C.; Pogue, A.I.; Bhattacharjee, S.; Zhao, Y.; Lukiw, W.J. Pathogenic microbes, the microbiome, and Alzheimer’s disease (AD). Front. Aging Neurosci. 2014, 6, 97417. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fang, L.; Chen, S.; Zhou, H.; Fan, Y.; Lin, L.; Li, J.; Xu, J.; Chen, Y.; Ma, Y.; et al. Gut microbiome alterations precede cerebral amyloidosis and microglial pathology in a mouse model of Alzheimer’s disease. BioMed Res. Int. 2020, 2020, 8456596. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Xiayu, X.; Shi, C.; Chen, W.; Song, N.; Fu, X.; Zhou, R.; Xu, Y.-F.; Huang, L.; et al. Altered gut microbiota in a mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 60, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef] [PubMed]
- Verhaar, B.J.; Hendriksen, H.M.; de Leeuw, F.A.; Doorduijn, A.S.; van Leeuwenstijn, M.; Teunissen, C.E.; Barkhof, F.; Scheltens, P.; Kraaij, R.; van Duijn, C.M.; et al. Gut microbiota composition is related to AD pathology. Front. Immunol. 2022, 12, 794519. [Google Scholar] [CrossRef]
- Kaiyrlykyzy, A.; Kozhakhmetov, S.; Babenko, D.; Zholdasbekova, G.; Alzhanova, D.; Olzhayev, F.; Baibulatova, A.; Kushugulova, A.R.; Askarova, S. Study of gut microbiota alterations in Alzheimer’s dementia patients from Kazakhstan. Sci. Rep. 2022, 12, 15115. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Li, X.H.; Liu, P.; Li, J.; Liu, L. The relationship between Alzheimer’s disease and intestinal microflora structure and inflammatory factors. Front. Aging Neurosci. 2022, 14, 972982. [Google Scholar] [CrossRef] [PubMed]
- Kozhakhmetov, S.; Kaiyrlykyzy, A.; Jarmukhanov, Z.; Vinogradova, E.; Zholdasbekova, G.; Alzhanova, D.; Kunz, J.; Kushugulova, A.; Askarova, S. Inflammatory manifestations associated with gut dysbiosis in Alzheimer’s Disease. Int. J. Alzheimer’s Dis. 2024, 2024, 9741811. [Google Scholar] [CrossRef]
- Zhuang, Z.; Yang, R.; Wang, W.; Qi, L.; Huang, T. Associations between gut microbiota and Alzheimer’s disease, major depressive disorder, and schizophrenia. J. Neuroinflammation 2020, 17, 288. [Google Scholar] [CrossRef]
- Jung, J.H.; Kim, G.; Byun, M.S.; Lee, J.H.; Yi, D.; Park, H.; Lee, D.Y. KBASE Research Group Gut microbiome alterations in preclinical Alzheimer’s disease. PLoS ONE 2022, 17, e0278276. [Google Scholar] [CrossRef]
- Khedr, E.M.; Omeran, N.; Karam-Allah Ramadan, H.; Ahmed, G.K.; Abdelwarith, A.M. Alteration of gut microbiota in Alzheimer’s disease and their relation to the cognitive impairment. J. Alzheimer’s Dis. 2022, 88, 1103–1114. [Google Scholar] [CrossRef]
- Guo, M.; Peng, J.; Huang, X.; Xiao, L.; Huang, F.; Zuo, Z. Gut microbiome features of Chinese patients newly diagnosed with Alzheimer’s disease or mild cognitive impairment. J. Alzheimer’s Dis. 2021, 80, 299–310. [Google Scholar] [CrossRef]
- Wanapaisan, P.; Chuansangeam, M.; Nopnipa, S.; Mathuranyanon, R.; Nonthabenjawan, N.; Ngamsombat, C.; Thientunyakit, T.; Muangpaisan, W. Association between gut microbiota with mild cognitive impairment and Alzheimer’s disease in a Thai population. Neurodegener. Dis. 2023, 22, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Issilbayeva, A.; Kaiyrlykyzy, A.; Vinogradova, E.; Jarmukhanov, Z.; Kozhakhmetov, S.; Kassenova, A.; Nurgaziyev, M.; Mukhanbetzhanov, N.; Alzhanova, D.; Zholdasbekova, G.; et al. Oral microbiome stamp in Alzheimer’s disease. Pathogens 2024, 13, 195. [Google Scholar] [CrossRef]
- Marzouk, N.H.; Rashwan, H.H.; El-Hadidi, M.; Ramadan, R.; Mysara, M. Proinflammatory and GABA eating bacteria in Parkinson’s disease gut microbiome from a meta-analysis prospective. npj Park. Dis. 2025, 11, 145. [Google Scholar] [CrossRef]
- Fernández-Espejo, E. Microorganisms associated with increased risk of Parkinson’s disease. Neurología (Engl. Ed.) 2023, 38, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Hopfner, F.; Künstner, A.; Müller, S.H.; Künzel, S.; Zeuner, K.E.; Margraf, N.G.; Deuschl, G.; Baines, J.F.; Kuhlenbäumer, G. Gut microbiota in Parkinson disease in a northern German cohort. Brain Res. 2017, 1667, 41–45. [Google Scholar] [CrossRef]
- Qian, Y.; Yang, X.; Xu, S.; Wu, C.; Song, Y.; Qin, N.; Chen, S.D.; Xiao, Q. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav. Immun. 2018, 70, 194–202. [Google Scholar] [CrossRef]
- Nishiwaki, H.; Ueyama, J.; Ito, M.; Hamaguchi, T.; Takimoto, K.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Mori, H.; Kurokawa, K.; et al. Meta-analysis of shotgun sequencing of gut microbiota in Parkinson’s disease. npj Park. Dis. 2024, 10, 106. [Google Scholar] [CrossRef]
- Petrov, V.A.; Saltykova, I.V.; Zhukova, I.A.; Alifirova, V.M.; Zhukova, N.G.; Dorofeeva, Y.B.; Tyakht, A.V.; Kovarsky, B.A.; Alekseev, D.G.; Kostryukova, E.S.; et al. Analysis of gut microbiota in patients with Parkinson’s disease. Bull. Exp. Biol. Med. 2017, 162, 734–737. [Google Scholar] [CrossRef]
- Zapała, B.; Stefura, T.; Wójcik-Pędziwiatr, M.; Kabut, R.; Bałajewicz-Nowak, M.; Milewicz, T.; Dudek, A.; Stój, A.; Rudzińska-Bar, M. Differences in the composition of gut microbiota between patients with parkinson’s disease and healthy controls: A cohort study. J. Clin. Med. 2021, 10, 5698. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, F.; Cao, J.; Ding, W.; Yan, S.; Shi, W.; Wen, S.; Yao, L. Alterations of gut microbiota and metabolome with Parkinson’s disease. Microb. Pathog. 2021, 160, 105187. [Google Scholar] [CrossRef] [PubMed]
- Pavan, S.; Gorthi, S.P.; Prabhu, A.N.; Das, B.; Mutreja, A.; Vasudevan, K.; Vignesh, S.; Thandavarayan, R.; Ballal, M. Dysbiosis of the beneficial gut bacteria in patients with Parkinson’s disease from India. Ann. Indian Acad. Neurol. 2023, 26, 908–916. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Mulak, A.; Bonaz, B. Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 2015, 21, 10609. [Google Scholar] [CrossRef] [PubMed]
- Lücking, C.B.; Brice, A. Alpha-synuclein and Parkinson’s disease. Cell. Mol. Life Sci. CMLS 2000, 57, 1894–1908. [Google Scholar] [CrossRef]
- Lei, Q.; Wu, T.; Wu, J.; Hu, X.; Guan, Y.; Wang, Y.; Yan, J.; Shi, G. Roles of α-synuclein in gastrointestinal microbiome dysbiosis-related Parkinson’s disease progression. Mol. Med. Rep. 2021, 24, 734. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pardo, P.; Dodiya, H.B.; Engen, P.A.; Naqib, A.; Forsyth, C.B.; Green, S.J.; Garssen, J.; Keshavarzian, A.; Kraneveld, A.D. Gut bacterial composition in a mouse model of Parkinson’s disease. Benef. Microbes 2018, 9, 799–814. [Google Scholar] [CrossRef]
- Radisavljevic, N.; Cirstea, M.; Bauer, K.; Lo, C.; Metcalfe-Roach, A.; Bozorgmehr, T.; Bar-Yoseph, H.; Brett Finlay, B. Effects of Gut Microbiota alterations on motor, gastrointestinal, and behavioral phenotype in a mouse model of Parkinson’s disease. J. Park. Dis. 2022, 12, 1479–1495. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Hu, X.; Wang, T.; Liang, S.; Duan, Y.; Jin, F.; Qin, B. Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci. China Life Sci. 2017, 60, 1223–1233. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, C.C.; Chiang, H.L.; Liou, J.M.; Chang, C.M.; Lu, T.P.; Chuang, E.Y.; Tai, Y.C.; Cheng, C.; Lin, H.Y.; et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflammation 2019, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Vascellari, S.; Palmas, V.; Melis, M.; Pisanu, S.; Cusano, R.; Uva, P.; Perra, D.; Madau, V.; Sarchioto, M.; Oppo, V.; et al. Gut microbiota and metabolome alterations associated with Parkinson’s disease. Msystems 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- Gerhardt, S.; Mohajeri, M.H. Changes of colonic bacterial composition in Parkinson’s disease and other neurodegenerative diseases. Nutrients 2018, 10, 708. [Google Scholar] [CrossRef]
- Pietrucci, D.; Cerroni, R.; Unida, V.; Farcomeni, A.; Pierantozzi, M.; Mercuri, N.B.; Biocca, S.; Stefani, A.; Desideri, A. Dysbiosis of gut microbiota in a selected population of Parkinson’s patients. Park. Relat. Disord. 2019, 65, 124–130. [Google Scholar] [CrossRef]
- Wu, G.F.; Alvarez, E. The immunopathophysiology of multiple sclerosis. Neurol. Clin. 2011, 29, 257–278. [Google Scholar] [CrossRef]
- van Noort, J.M.; Baker, D.; Kipp, M.; Amor, S. The pathogenesis of multiple sclerosis: A series of unfortunate events. Clin. Exp. Immunol. 2023, 214, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Moser, T.; Akgün, K.; Proschmann, U.; Sellner, J.; Ziemssen, T. The role of TH17 cells in multiple sclerosis: Therapeutic implications. Autoimmun. Rev. 2020, 19, 102647. [Google Scholar] [CrossRef] [PubMed]
- Jadidi-Niaragh, F.; Mirshafiey, A. Th17 cell, the new player of neuroinflammatory process in multiple sclerosis. Scand. J. Immunol. 2011, 74, 1–13. [Google Scholar] [CrossRef]
- Rostami, A.; Ciric, B. Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination. J. Neurol. Sci. 2013, 333, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Y.; Zhu, M.; Liu, K.; Zhang, H.L. Gut flora in multiple sclerosis: Implications for pathogenesis and treatment. Neural Regen. Res. 2024, 19, 1480–1488. [Google Scholar] [CrossRef]
- Schepici, G.; Silvestro, S.; Bramanti, P.; Mazzon, E. The gut microbiota in multiple sclerosis: An overview of clinical trials. Cell Transplant. 2019, 28, 1507–1527. [Google Scholar] [CrossRef]
- Melbye, P.; Olsson, A.; Hansen, T.H.; Søndergaard, H.B.; Bang Oturai, A. Short-chain fatty acids and gut microbiota in multiple sclerosis. Acta Neurol. Scand. 2019, 139, 208–219. [Google Scholar] [CrossRef]
- Sittipo, P.; Choi, J.; Lee, S.; Lee, Y.K. The function of gut microbiota in immune-related neurological disorders: A review. J. Neuroinflammation 2022, 19, 154. [Google Scholar] [CrossRef]
- Gandy, K.A.O.; Zhang, J.; Nagarkatti, P.; Nagarkatti, M. The role of gut microbiota in shaping the relapse-remitting and chronic-progressive forms of multiple sclerosis in mouse models. Sci. Rep. 2019, 9, 6923. [Google Scholar] [CrossRef]
- Shahi, S.K.; Ghimire, S.; Lehman, P.; Mangalam, A.K. Obesity induced gut dysbiosis contributes to disease severity in an animal model of multiple sclerosis. Front. Immunol. 2022, 13, 966417. [Google Scholar] [CrossRef]
- Kadowaki, A.; Saga, R.; Lin, Y.; Sato, W.; Yamamura, T. Gut microbiota-dependent CCR9+ CD4+ T cells are altered in secondary progressive multiple sclerosis. Brain 2019, 142, 916–931. [Google Scholar] [CrossRef]
- Ordoñez-Rodriguez, A.; Roman, P.; Rueda-Ruzafa, L.; Campos-Rios, A.; Cardona, D. Changes in gut microbiota and multiple sclerosis: A systematic review. Int. J. Environ. Res. Public Health 2023, 20, 4624. [Google Scholar] [CrossRef]
- Cox, L.M.; Maghzi, A.H.; Liu, S.; Tankou, S.K.; Dhang, F.H.; Willocq, V.; Song, A.; Wasén, C.; Tauhid, S.; Chu, R.; et al. Gut microbiome in progressive multiple sclerosis. Ann. Neurol. 2021, 89, 1195–1211. [Google Scholar] [CrossRef] [PubMed]
- Boussamet, L.; Rajoka, M.S.R.; Berthelot, L. Microbiota, IgA and multiple sclerosis. Microorganisms 2022, 10, 617. [Google Scholar] [CrossRef]
- Kujawa, D.; Laczmanski, L.; Budrewicz, S.; Pokryszko-Dragan, A.; Podbielska, M. Targeting gut microbiota: New therapeutic opportunities in multiple sclerosis. Gut Microbes 2023, 15, 2274126. [Google Scholar] [CrossRef]
- Campagnoli, L.I.M.; Marchesi, N.; Varesi, A.; Morozzi, M.; Mascione, L.; Ricevuti, G.; Esposito, C.; Galeotti, N.; Pascale, A. New therapeutic avenues in multiple sclerosis: Is there a place for gut microbiota-based treatments? Pharmacol. Res. 2024, 209, 107456. [Google Scholar] [CrossRef]
- Altieri, C.; Speranza, B.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Gut-microbiota, and multiple sclerosis: Background, evidence, and perspectives. Nutrients 2023, 15, 942. [Google Scholar] [CrossRef] [PubMed]
- de Toro-Martín, J.; Arsenault, B.J.; Després, J.P.; Vohl, M.C. Precision nutrition: A review of personalized nutritional approaches for the prevention and management of metabolic syndrome. Nutrients 2017, 9, 913. [Google Scholar] [CrossRef] [PubMed]
- Singh, V. Current challenges and future implications of exploiting the omics data into nutrigenetics and nutrigenomics for personalized diagnosis and nutrition-based care. Nutrition 2023, 110, 112002. [Google Scholar] [CrossRef]
- Singar, S.; Nagpal, R.; Arjmandi, B.H.; Akhavan, N.S. Personalized nutrition: Tailoring dietary recommendations through genetic insights. Nutrients 2024, 16, 2673. [Google Scholar] [CrossRef] [PubMed]
- García-Cañas, V.; Simó, C.; León, C.; Cifuentes, A. Advances in Nutrigenomics research: Novel and future analytical approaches to investigate the biological activity of natural compounds and food functions. J. Pharm. Biomed. Anal. 2010, 51, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Kussmann, M.; Krause, L.; Siffert, W. Nutrigenomics: Where are we with genetic and epigenetic markers for disposition and susceptibility? Nutr. Rev. 2010, 68 (Suppl. S1), S38–S47. [Google Scholar] [CrossRef]
- Afman, L.A.; Müller, M. Human nutrigenomics of gene regulation by dietary fatty acids. Prog. Lipid Res. 2012, 51, 63–70. [Google Scholar] [CrossRef]
- Masotti, A.; Da Sacco, L.; Bottazzo, G.F.; Alisi, A. Microarray technology: A promising tool in nutrigenomics. Crit. Rev. Food Sci. Nutr. 2010, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Hu, X.H.; Singh, A.K.; Solanki, M.K.; Vijayaraghavan, P.; Srivastav, R.; Joshi, N.K.; Kumari, M.; Singh, S.K.; Wang, Z.; et al. Precision nutrition-based strategy for management of human diseases and healthy aging: Current progress and challenges forward. Front. Nutr. 2024, 11, 1427608. [Google Scholar] [CrossRef]
- Mansour, S.; Alkhaaldi, S.M.; Sammanasunathan, A.F.; Ibrahim, S.; Farhat, J.; Al-Omari, B. Precision nutrition unveiled: Gene–nutrient interactions, microbiota dynamics, and lifestyle factors in obesity management. Nutrients 2024, 16, 581. [Google Scholar] [CrossRef]
- Larroya, A.; Pantoja, J.; Codoñer-Franch, P.; Cenit, M.C. Towards tailored gut microbiome-based and dietary interventions for promoting the development and maintenance of a healthy brain. Front. Pediatr. 2021, 9, 705859. [Google Scholar] [CrossRef]
- Lang, J.M.; Pan, C.; Cantor, R.M.; Tang, W.W.; Garcia-Garcia, J.C.; Kurtz, I.; Hazen, S.L.; Bergeron, N.; Krauss, R.M.; Lusis, A.J. Impact of individual traits, saturated fat, and protein source on the gut microbiome. MBio 2018, 9, 10–1128. [Google Scholar] [CrossRef]
- Tap, J.; Furet, J.P.; Bensaada, M.; Philippe, C.; Roth, H.; Rabot, S.; Lakhdari, O.; Lombard, V.; Henrissat, B.; Corthier, G.; et al. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environ. Microbiol. 2015, 17, 4954–4964. [Google Scholar] [CrossRef]
- Bashiardes, S.; Abdeen, S.K.; Elinav, E. Personalized nutrition: Are we there yet? J. Pediatr. Gastroenterol. Nutr. 2019, 69, 633–638. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet–microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Bäckhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef]
- Dominika, Ś.; Arjan, N.; Karyn, R.P.; Henryk, K. The study on the impact of glycated pea proteins on human intestinal bacteria. Int. J. Food Microbiol. 2011, 145, 267–272. [Google Scholar] [CrossRef]
- Pokushalov, E.; Ponomarenko, A.; Shrainer, E.; Kudlay, D.; Miller, R. Biomarker-Guided dietary supplementation: A narrative review of precision in personalized nutrition. Nutrients 2024, 16, 4033. [Google Scholar] [CrossRef]
- Lee, S.; Srinivasan, B.; Vemulapati, S.; Mehta, S.; Erickson, D. Personalized nutrition diagnostics at the point-of-need. Lab Chip 2016, 16, 2408–2417. [Google Scholar] [CrossRef] [PubMed]
- Bahous, R.H.; Cosín-Tomás, M.; Deng, L.; Leclerc, D.; Malysheva, O.; Ho, M.K.; Pallàs, M.; Kaliman, P.; Bedell, B.J.; Caudill, M.A.; et al. Early manifestations of brain aging in mice due to low dietary folate and mild MTHFR deficiency. Mol. Neurobiol. 2018, 56, 4175–4191. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Manis, M.; Long, J.; Wang, K.; Sullivan, P.M.; Remolina Serrano, J.; Hoyle, R.; Holtzman, D.M. Microglia drive APOE-dependent neurodegeneration in a tauopathy mouse model. J. Exp. Med. 2019, 216, 2546–2561. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.J.; Pervaiz, N.; Abbasi, A.A. The Parkinson Disease gene SNCA: Evolutionary and structural insights with pathological implication. Sci. Rep. 2016, 6, 24475. [Google Scholar] [CrossRef]
- Strang, K.H.; Golde, T.E.; Giasson, B.I. MAPT mutations, tauopathy, and mechanisms of neurodegeneration. Lab. Investig. 2019, 99, 912–928. [Google Scholar] [CrossRef]
- Tsika, E.; Moore, D.J. Mechanisms of LRRK2-mediated neurodegeneration. Curr. Neurol. Neurosci. Rep. 2012, 12, 251–260. [Google Scholar] [CrossRef]
- Ralph, G.S.; Radcliffe, P.A.; Day, D.M.; Carthy, J.M.; Leroux, M.A.; Lee, D.C.; Wong, L.F.; Bilsland, L.G.; Greensmith, L.; Kingsman, S.M.; et al. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat. Med. 2005, 11, 429–433. [Google Scholar] [CrossRef]
- Pap, D.; Veres-Székely, A.; Szebeni, B.; Vannay, Á. PARK7/DJ-1 as a therapeutic target in gut-brain axis diseases. Int. J. Mol. Sci. 2022, 23, 6626. [Google Scholar] [CrossRef]
- Yang, Y.; Bagyinszky, E.; An, S.S.A. Presenilin-1 (PSEN1) mutations: Clinical phenotypes beyond Alzheimer’s disease. Int. J. Mol. Sci. 2023, 24, 8417. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Romano, L.; Marrone, G.; Noce, A.; Pujia, A.; Perrone, M.A.; Aiello, V.; Colica, C.; De Lorenzo, A. Role of personalized nutrition in chronic-degenerative diseases. Nutrients 2019, 11, 1707. [Google Scholar] [CrossRef] [PubMed]
- De Olazarra, A.S.; Wang, S.X. Advances in point-of-care genetic testing for personalized medicine applications. Biomicrofluidics 2023, 17, 031501. [Google Scholar] [CrossRef] [PubMed]
- Acar, E.; Gürdeniz, G.; Khakimov, B.; Savorani, F.; Korndal, S.K.; Larsen, T.M.; Engelsen, S.B.; Dragsted, L.O. Biomarkers of individual foods, and separation of diets using untargeted LC–MS-based plasma metabolomics in a randomized controlled trial. Mol. Nutr. Food Res. 2019, 63, 1800215. [Google Scholar] [CrossRef]
- Popp, J.; Oikonomidi, A.; Tautvydaitė, D.; Dayon, L.; Bacher, M.; Migliavacca, E.; Henry, H.; Kirkland, R.; Severin, I.; Wojcik, J.; et al. Markers of neuroinflammation associated with Alzheimer’s disease pathology in older adults. Brain Behav. Immun. 2017, 62, 203–211. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, Z.; Shi, J.; An, Y.; Zhang, K.; Wang, Y.; Li, S.; Jin, L.; Ye, W.; Cui, M.; et al. Metabolomics in the development and progression of dementia: A systematic review. Front. Neurosci. 2019, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre-Arbogast, S.; Wagner, M.; Proust-Lima, C.; Samieri, C. Nutrition and metabolic profiles in the natural history of dementia: Recent insights from systems biology and life course epidemiology. Curr. Nutr. Rep. 2019, 8, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Dayon, L.; Wojcik, J.; Núñez Galindo, A.; Corthésy, J.; Cominetti, O.; Oikonomidi, A.; Henry, H.; Migliavacca, E.; Bowman, G.L.; Popp, J. Plasma proteomic profiles of cerebrospinal fluid-defined Alzheimer’s disease pathology in older adults. J. Alzheimer’s Dis. 2017, 60, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.; Sasaki, K.; Masuda, T.; Ataka, T.; Matsumoto, M.; Kitamura, M.; Nakamura, Y.; Matsubara, E. Machine learning models for dementia screening to classify brain amyloid positivity on positron emission tomography using blood markers and demographic characteristics: A retrospective observational study. Alzheimer’s Res. Ther. 2025, 17, 25. [Google Scholar] [CrossRef]
- Wasilewski, T.; Kamysz, W.; Gębicki, J. AI-assisted detection of biomarkers by sensors and biosensors for early diagnosis and monitoring. Biosensors 2024, 14, 356. [Google Scholar] [CrossRef] [PubMed]
- Samieri, C.; Yassine, H.N.; Melo van Lent, D.; Lefèvre-Arbogast, S.; van de Rest, O.; Bowman, G.L.; Scarmeas, N. Personalized nutrition for dementia prevention. Alzheimer’s Dement. 2022, 18, 1424–1437. [Google Scholar] [CrossRef]
- Castro-Mata, P.C.; Cueto-Manzano, A.M.; Vizmanos, B.; González-Ortiz, A.; Betancourt-Núñez, A.; Martín-del-Campo, F. Chrononutrition in Chronic Kidney Disease. Nutrients 2025, 17, 389. [Google Scholar] [CrossRef]
- Wilking, M.; Ndiaye, M.; Mukhtar, H.; Ahmad, N. Circadian rhythm connections to oxidative stress: Implications for human health. Antioxid. Redox Signal. 2013, 19, 192–208. [Google Scholar] [CrossRef]
- Huang, W.; Zong, J.; Zhang, Y.; Zhou, Y.; Zhang, L.; Wang, Y.; Shan, Z.; Xie, Q.; Li, M.; Pan, S.; et al. The Role of Circadian Rhythm in Neurological Diseases: A Translational Perspective. Aging Dis. 2024, 15, 1565–1587. [Google Scholar] [CrossRef]
- Sardon Puig, L.; Valera-Alberni, M.; Cantó, C.; Pillon, N.J. Circadian rhythms and mitochondria: Connecting the dots. Front. Genet. 2018, 9, 452. [Google Scholar] [CrossRef]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef]
- Gasmi, M.; Silvia Hardiany, N.; van der Merwe, M.; Martins, I.J.; Sharma, A.; Williams-Hooker, R. The influence of time-restricted eating/feeding on Alzheimer’s biomarkers and gut microbiota. Nutr. Neurosci. 2025, 28, 156–170. [Google Scholar] [CrossRef]
- Hernandez, A.R.; Watson, C.; Federico, Q.P.; Fletcher, R.; Brotgandel, A.; Buford, T.W.; Carter, C.S.; Burke, S.N. Twelve months of time-restricted feeding improves cognition and alters microbiome composition independent of macronutrient composition. Nutrients 2022, 14, 3977. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, M.; Ding, C.; Bao, B.; Li, H.; Ma, J.; Dong, W.; Gao, R.; Chen, X.; Chen, J.; et al. Time-restricted feeding mitigates Alzheimer’s disease-associated cognitive impairments via a B. pseudolongum-propionic acid-FFAR3 axis. iMeta 2025, 4, e70006. [Google Scholar] [CrossRef]
- Zeb, F.; Osaili, T.; Obaid, R.S.; Naja, F.; Radwan, H.; Cheikh Ismail, L.; Hasan, H.; Hashim, M.; Alam, I.; Sehar, B.; et al. Gut microbiota and time-restricted feeding/eating: A targeted biomarker and approach in precision nutrition. Nutrients 2023, 15, 259. [Google Scholar] [CrossRef]
- Ramos Meyers, G.; Samouda, H.; Bohn, T. Short chain fatty acid metabolism in relation to gut microbiota and genetic variability. Nutrients 2022, 14, 5361. [Google Scholar] [CrossRef]
- Xu, R.C.; Miao, W.T.; Xu, J.Y.; Xu, W.X.; Liu, M.R.; Ding, S.T.; Jian, Y.X.; Lei, Y.H.; Yan, N.; Liu, H.D. Neuroprotective effects of sodium butyrate and monomethyl fumarate treatment through GPR109A modulation and intestinal barrier restoration on PD mice. Nutrients 2022, 14, 4163. [Google Scholar] [CrossRef]
- Wang, X.; Cai, Z.; Wang, Q.; Wu, C.; Sun, Y.; Wang, Z.; Xu, X.; Xue, W.; Cao, Z.; Zhang, M.; et al. Bacteroides methylmalonyl-CoA mutase produces propionate that promotes intestinal goblet cell differentiation and homeostasis. Cell Host Microbe 2024, 32, 63–78. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. β-Hydroxybutyrate: A signaling metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Brocchi, A.; Rebelos, E.; Dardano, A.; Mantuano, M.; Daniele, G. Effects of intermittent fasting on brain metabolism. Nutrients 2022, 14, 1275. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Z.; Zuo, Z. Chronic intermittent fasting improves cognitive functions and brain structures in mice. PLoS ONE 2013, 8, e66069. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Manchanda, S.; Kaur, T.; Kumar, S.; Lakhanpal, D.; Lakhman, S.S.; Kaur, G. Middle age onset short-term intermittent fasting dietary restriction prevents brain function impairments in male Wistar rats. Biogerontology 2015, 16, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Ooi, T.C.; Meramat, A.; Rajab, N.F.; Shahar, S.; Ismail, I.S.; Azam, A.A.; Sharif, R. Intermittent fasting enhanced the cognitive function in older adults with mild cognitive impairment by inducing biochemical and metabolic changes: A 3-year progressive study. Nutrients 2020, 12, 2644. [Google Scholar] [CrossRef] [PubMed]
- Boujelbane, M.A.; Trabelsi, K.; Jahrami, H.A.; Masmoudi, L.; Ammar, A.; Khacharem, A.; Boukhris, O.; Puce, L.; Garbarino, S.; Scoditti, E.; et al. Time-restricted feeding and cognitive function in sedentary and physically active elderly individuals: Ramadan diurnal intermittent fasting as a model. Front. Nutr. 2022, 9, 1041216. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Latre, A.; Eckel-Mahan, K. Interdependence of nutrient metabolism and the circadian clock system: Importance for metabolic health. Mol. Metab. 2016, 5, 133–152. [Google Scholar] [CrossRef]
- Gubin, D.; Weinert, D.; Stefani, O.; Otsuka, K.; Borisenkov, M.; Cornelissen, G. Wearables in Chronomedicine and interpretation of circadian health. Diagnostics 2025, 15, 327. [Google Scholar] [CrossRef]
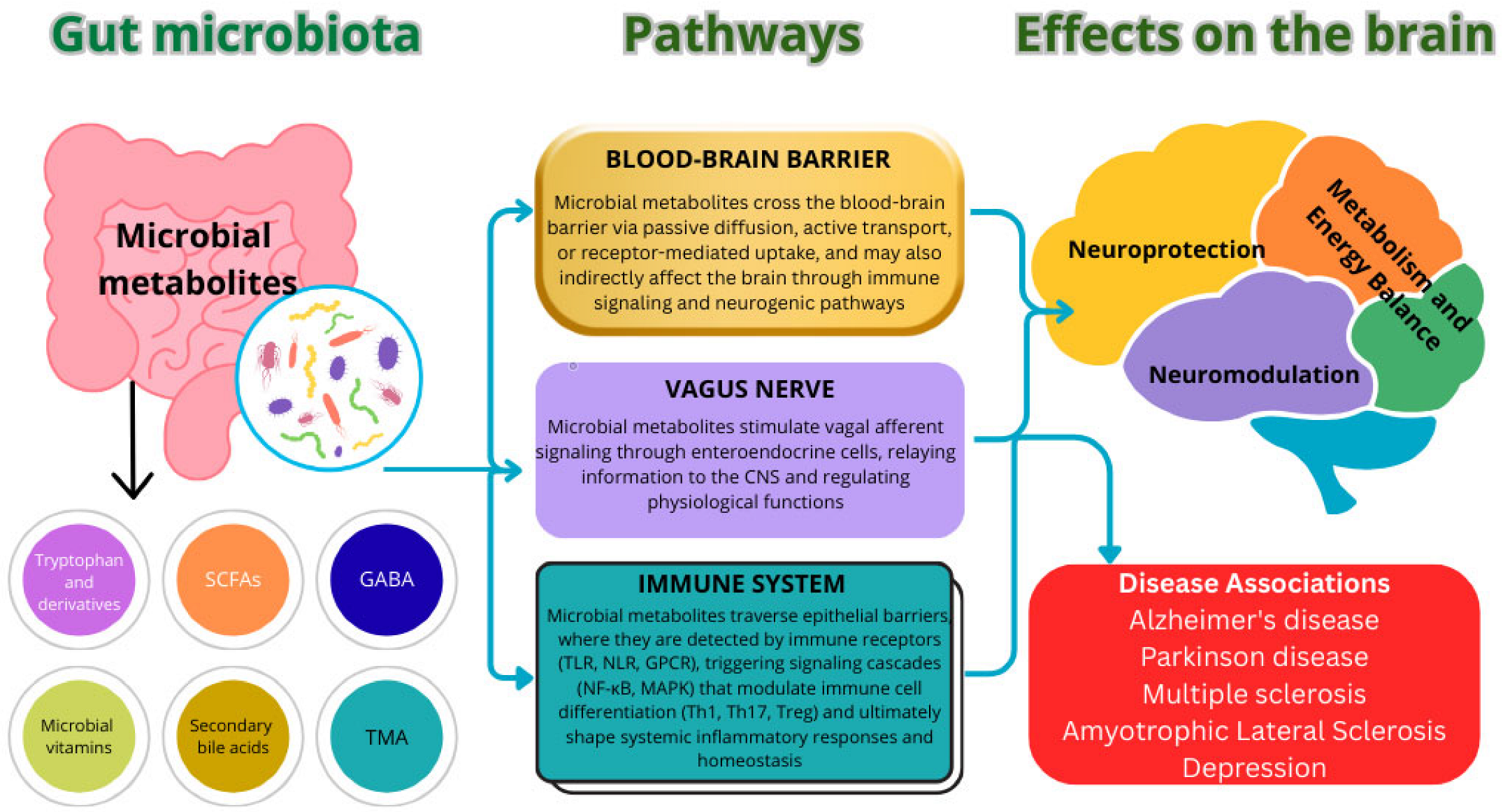
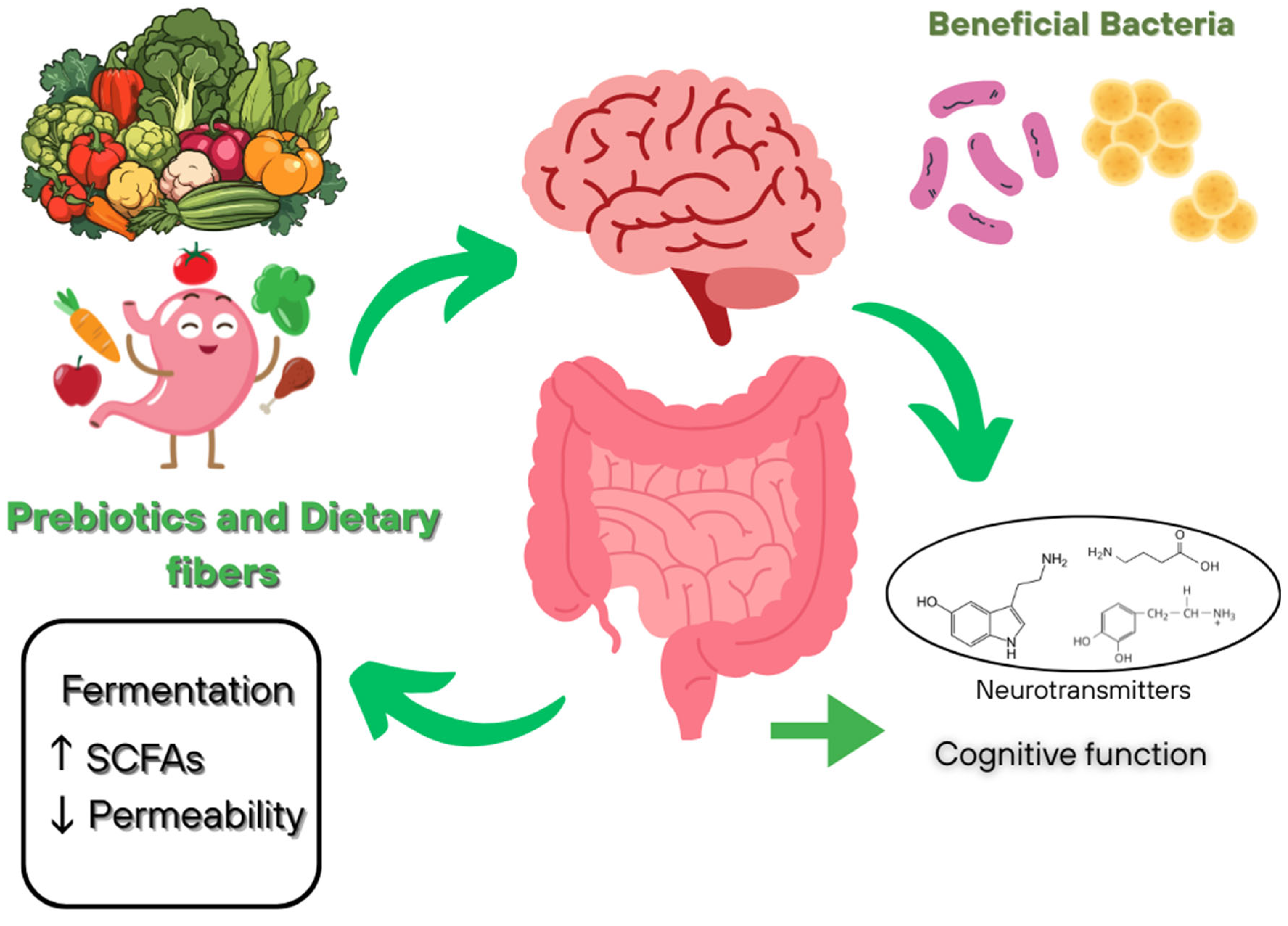
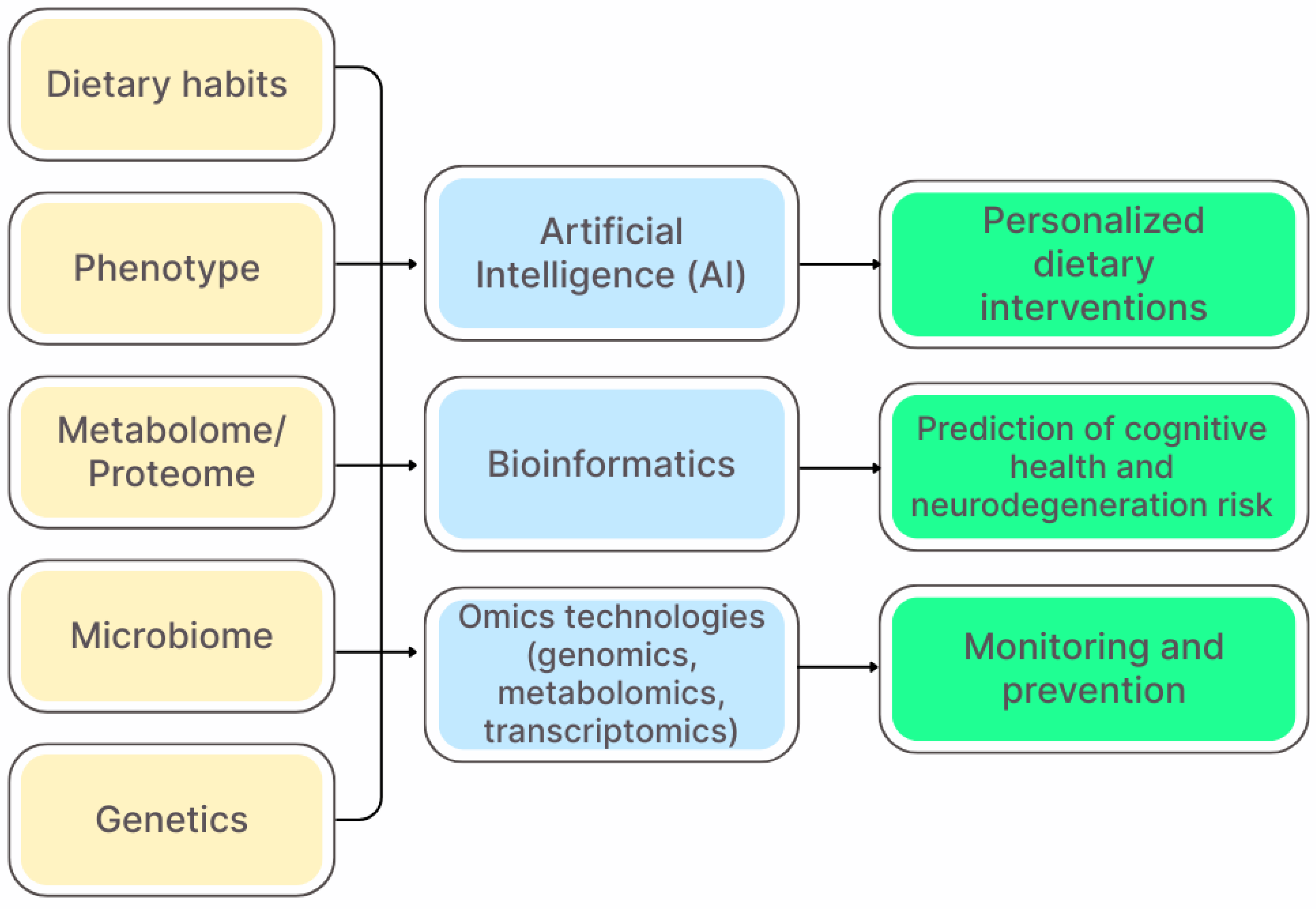
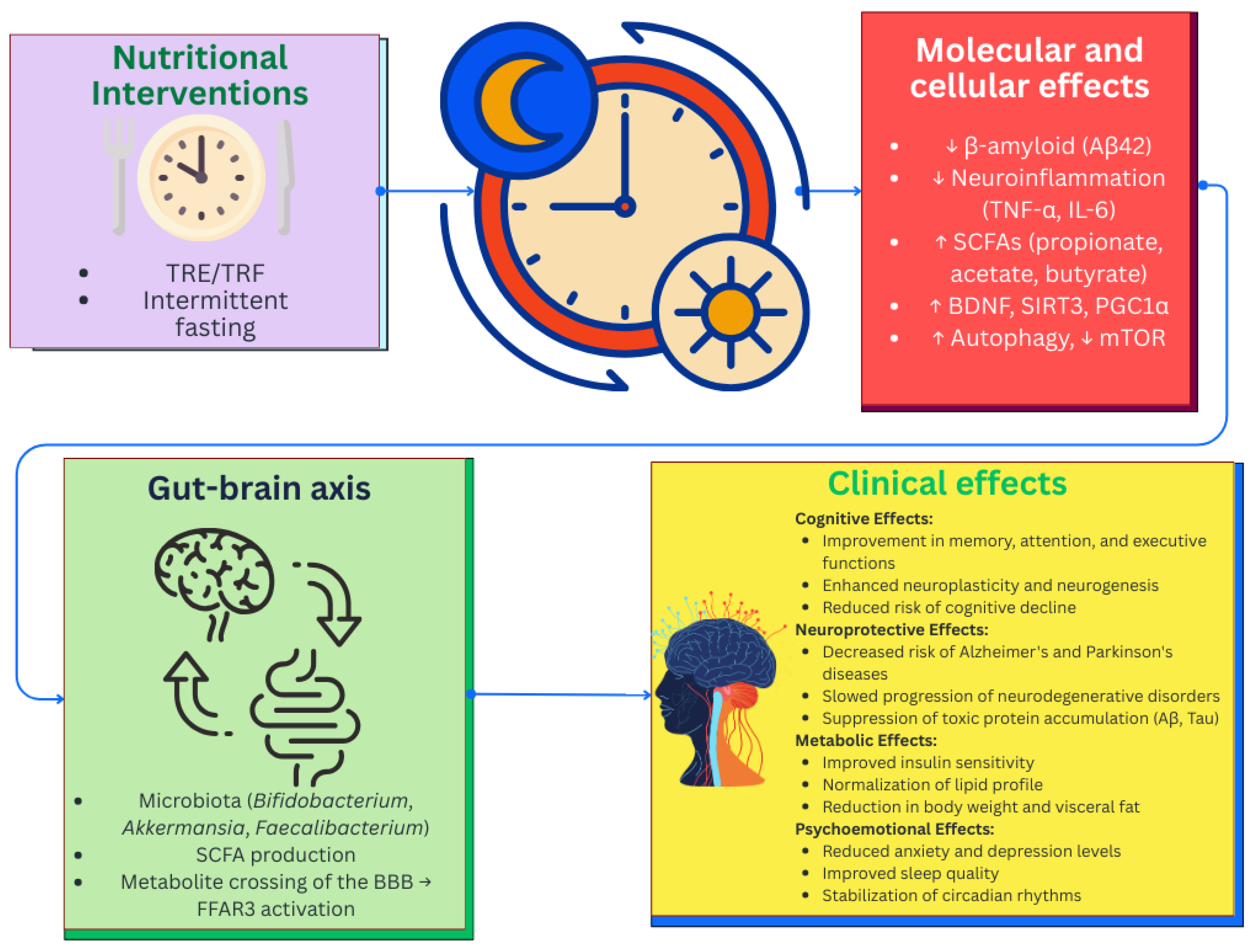
| Metabolite | Primary Producers | Pathways of Influence on the Brain | Effects | Association with Diseases | References |
|---|---|---|---|---|---|
| SCFAs | Clostridium, Sporanaerobacter, Streptococcus, Syntrophomonas | BBB, vagus nerve, immune modulation | Enhanced neurogenesis, anti-inflammatory activity | Alzheimer’s disease, autism spectrum disorders, depression | [59,60,61,62] |
| Tryptophan and its derivatives | Bifidobacterium, Lactobacillus, E. coli | Gut–brain axis, neurotransmitter synthesis | Mood modulation, reduction in neuroinflammation | Depression, anxiety, schizophrenia | [63,64] |
| GABA | Lactobacillus, Bifidobacterium | Vagus nerve, BBB | Anxiolytic effect, reduced neuronal excitability | Anxiety disorders, epilepsy | [65,66] |
| Secondary bile acids | Clostridium, Bacteroides | BBB, gut–brain axis signaling pathways | Neuroprotection, regulation of apoptosis | Parkinson’s disease, multiple sclerosis | [67] |
| Histamine | Lactobacillus, Enterococcus | Interaction with H1–H4 receptors, effects on cerebral vasculature | Maintenance of cognitive functions, anti-inflammatory effect | Migraine, neurodegenerative diseases | [68,69] |
| Category of Component | Key Strains/Compounds | Participants | Study Design | Cognitive Effects | References |
|---|---|---|---|---|---|
| Probiotics/Psychobiotics | Bifidobacterium breve CCFM1025 | Patients with major depressive disorder | Randomized placebo-controlled clinical trial | A statistically significant reduction in depressive symptoms according to the HDRS and MADRS scales, improvement in emotional state, and a decrease in the severity of psychiatric and gastrointestinal symptoms (as measured by BRPS and GSRS). | [121] |
| Probiotics/Psychobiotics | Streptococcus thermophilus NCIMB30438, Bifidobacterium breve NCIMB30441, Bifidobacterium longum NCIMB30435, Bifidobacterium infantis NCIMB30436, Lactobacillus acidophilus NCIMB30442, Lactobacillus plantarum NCIMB30437, Lactobacillus paracasei NCIMB30439, Lactobacillus delbrueckii subsp. Bulgaricus NCIMB30440 | Patients with a current depressive episode | Randomized, placebo-controlled clinical trial | A significant reduction in HAM-D depression scores in the probiotic group; improvement in depressive symptoms; and decreased putamen activation in response to neutral faces (according to neuroimaging data). | [122] |
| Probiotics/Psychobiotics | Lactobacillus rhamnosus GG | Middle-aged and older adults (aged 52 to 75 years) | Double-blind, placebo-controlled, randomized trial | A significant improvement in the overall cognitive score among participants with cognitive impairment. | [123] |
| Probiotics/Psychobiotics | Bifidobacterium breve CCFM1025 | Adults aged 18–65 with stress-induced insomnia | Double-blind, randomized, controlled trial | A significant improvement in sleep quality, a reduction in PSQI and AIS scores, enhanced subjective sleep quality, and a decrease in sleep disturbances. | [124] |
| Probiotics/Psychobiotics | Lactobacillus acidophilus LB | Children and adolescents aged 6–16 years with ADHD | Randomized controlled trial | A significant reduction in ADHD symptom severity compared with the control group according to the CPRS-R-L and CBCL measures, along with improvements in selective and sustained attention as assessed by the CPT. | [125] |
| Probiotics/Psychobiotics | Lactobacillus rhamnosus, Bifidobacterium lactis | Healthy older adults aged 55 and over | Double-blind, randomized, placebo-controlled crossover trial | Improvement in overall cognitive function, working and visuospatial memory, planning and problem-solving abilities, selective attention, cognitive flexibility, and inhibitory control, as well as a reduction in depressive symptoms and enhancement of sleep quality. | [126] |
| Probiotics/Psychobiotics | Lactobacillus plantarum PS128 | Adults aged 20–40 years with chronic insomnia | Randomized, double-blind, placebo-controlled pilot study | A reduction in depressive symptoms and fatigue, along with decreased cortical excitability | [127] |
| Probiotics/Psychobiotics | Bifidobacterium longum NCC3001 | Adults with mild to moderate anxiety and/or depressive symptoms | Randomized, double-blind, placebo-controlled pilot study | A reduction in depressive symptoms according to the HAD-D scale, an improvement in the physical component of quality of life, and decreased activation of the amygdala and fronto-limbic regions in response to threatening stimuli | [128] |
| Prebiotics | Galactooligosaccharides (GOS) | Healthy women aged 17–25 years | Randomized, double-blind, placebo-controlled trial | Neurochemical alterations and a transient increase in the abundance of Bifidobacterium within the gut microbiota | [99] |
| Prebiotics | Inulin | Students aged 18–23 years | Double-blind randomized controlled trial | Improvement in executive functions | [129] |
| Prebiotics | Polydextrose + GOS | Healthy full-term infants | Double-blind randomized controlled trial | Faster consolidation of daytime wakefulness | [130] |
| Polyphenols | Blackcurrant-based beverage 151 mg anthocyanins, 308 mg total polyphenols/day; also 150 mg of Pinus radiata extract (proanthocyanidins) and 200 mg L-theanine | Healthy women aged 18–45 years | Randomized double-blind placebo-controlled crossover trial | Improvement in working memory under multitasking conditions, along with reductions in tension/anxiety and irritability | [131] |
| Polyphenols | Cranberry drink: (~442 mg polyphenols) | Healthy students | Randomized double-blind placebo-controlled parallel-group trial | Improvement in short-term memory by week 12 | [132] |
| Omega-3 fatty acids | DHA, EPA | Children aged 2–6 years with Autism Spectrum Disorder | Double-blind, randomized, placebo-controlled trial | A reduction in emotional symptoms, behavioral problems, and impact on quality of life (within the autism spectrum range) | [133] |
| Microbiota Alterations | Associated Taxa | Functional Implication | Clinical/Pathogenetic Correlations | References |
|---|---|---|---|---|
| Changes in β-Diversity and Bacterial Families | ↑ Lactobacillaceae, Barnesiellaceae, Enterococcaceae | Compromised intestinal barrier and increased systemic inflammation, promoting microglial activation and neurodegeneration | Associated with neurotransmitter imbalance and dysregulation of SCFA metabolism, collectively contributing to disease development and progression via gut–brain axis mechanisms | [157] |
| Increased α-Diversity | ↓ Lactobacillus, Sediminibacterium ↑ Clostridium IV, Aquabacterium, Holdemania, Sphingomonas, Clostridium XVIII, Butyricicoccus, Anaerotruncus | Reduced metabolic activity of pathways involved in the synthesis of vitamins and cofactors, alongside enhanced energy metabolism, potentially contributing to neuroinflammation | The identified alterations in gut microbiota composition in Parkinson’s disease are pathogenetically correlated with disease duration, cognitive decline, depressive symptoms, and the presence of motor complications | [158] |
| Increased α-Diversity | ↓ Roseburia intestinalis, Faecalibacterium prausnitzii ↑ Akkermansia muciniphila | Reduced production of SCFAs and polyamines due to impaired microbial synthesis of riboflavin and biotin | Thinning of the intestinal mucosa, increased epithelial permeability, enhanced neuroinflammation, and formation of pathological α-synuclein fibrils in the enteric nervous system | [159] |
| Reduced α-Diversity | ↓ Dorea, Bacteroides, Prevotella, Faecalibacterium, Bacteroides massiliensis, Stoquefichus massiliensis, Bacteroides coprocola, Blautia glucerasea, Dorea longicatena, Bacteroides dorei, Bacteroides plebeus, Prevotella copri, Coprococcus eutactus, Ruminococcus callidus↑ Christensenella, Catabacter, Lactobacillus, Oscillospira, Bifidobacterium, Christensenella minuta, Catabacter hongkongensis, Lactobacillus mucosae, Ruminococcus bromii, Papillibacter cinnamivorans | Dysbiotic shifts in bacterial taxa are associated with chronic intestinal inflammation, metabolic disturbances, and potentially accelerated neurodegenerative processes | The observed alterations in microbial composition may trigger localized intestinal inflammation, subsequently promoting α-synuclein aggregation and the formation of Lewy bodies | [160] |
| Increased α- and β-Diversity | ↓ Faecalibacterium, Blautia, Fusicatenibacter ↑ Bacteroides, Corynebacteria, Deltaproteobacteria, Butyricimonas, Robinsoniella, Flavonifractor | Enhanced degradation of the intestinal mucus layer, increased gut permeability, and the development of systemic inflammation | The observed gut microbiota alterations in Parkinson’s disease are associated with impaired intestinal barrier function, increased exposure to endotoxins and oxidative stress, and accumulation of α-synuclein in both the enteric and central nervous systems | [161] |
| Selective Dysbiosis with an Increase in Opportunistic Genera | ↓ Faecalibacterium ↑ Alistipes, Rikenellaceae, Bifidobacterium, Parabacteroides | Decreased concentrations of branched-chain (BCAA) and aromatic amino acids | Disrupted metabolic activity of the microbial community, potentially affecting immune regulation and promoting neurodegenerative progression due to a deficit of metabolites involved in neuromodulation and maintenance of neuronal function | [162] |
| Reduction in SCFA-Producing Taxa | ↓ Lachnospiraceae, Coriobacteriaceae, Faecalibacterium, Fusicatenibacter, Roseburia, Blautia ↑ Clostridia UCG014, Christensenella, Oscillospiraceae | Decreased SCFA synthesis and increased intestinal barrier permeability | The identified alterations in gut microbiota in Parkinson’s disease patients show clear pathogenetic correlations with key clinical symptoms, including functional constipation, neuroinflammation, metabolic disturbances, and progressive motor impairment | [163] |
| Reduction in SCFA-Producing Taxa | ↓ Blautia, Coprococccus, Roseburia, Faecalibacterium ↑ Proteobacteria | Decreased SCFA synthesis, increased intestinal barrier permeability, and immune activation | The observed gut microbiota alterations in Parkinson’s disease patients are associated with inflammatory processes, oxidative stress, and α-synuclein aggregation | [164] |
| Biomarkers | Function | Impact on Nutrition | Association with Neurodegeneration | Therapeutic and Preventive Significance | References |
|---|---|---|---|---|---|
| MTHFR | Methylenetetrahydrofolate reductase | Folate metabolism | Disruption of synaptic transmission, epigenetic dysregulation, and diminished neurotrophic support are key contributors to the early pathogenesis of neurodegenerative disorders | An adequate folate status can prevent early epigenetic and metabolic disturbances, thereby reducing the risk of cognitive impairment | [211] |
| APOE | Apolipoprotein E | Microgliosis and diet sensitivity | The APOE4 allele promotes neurodegeneration by mediating microglial activation and amplifying neuroinflammatory processes | It serves as a therapeutic target and highlights the potential for preventive interventions through the modulation of diet and inflammatory responses | [212] |
| SNCA | α-synuclein | Eating disorder | The formation of pathogenic protein aggregates and their interaction with synaptic components, along with structural abnormalities caused by mutations, are directly linked to the mechanisms of neurodegeneration in Parkinson’s disease | It represents a key target for therapeutic development aimed at inhibiting alpha-synuclein aggregation, and forms the basis for preventive approaches focused on early-stage intervention in populations at risk for neurodegenerative diseases, such as Parkinson’s disease | [213] |
| MAPT | Tau protein | Regulation of nutrient transport in neurons | Pathological hyperphosphorylation and aggregation of tau protein disrupt axonal transport and contribute to neuronal degeneration | Modulation of MAPT gene expression and suppression of pathological tau protein synthesis represent a promising approach for the therapy and prevention of tauopathies, including Alzheimer’s disease and frontotemporal dementia, aiming to slow neurodegenerative processes and preserve cognitive function | [214] |
| LRRK2 | Leucine-rich repeat kinase 2 | Eating disorder | Mutations in the LRRK2 gene disrupt key cellular processes, including mitochondrial dysfunction, impaired autophagy, cytoskeletal dysregulation, and altered synaptic transmission, ultimately driving the progressive degeneration of dopaminergic neurons | The potential for targeted inhibition of kinase and GTPase activity offers a promising strategy to slow neurodegenerative processes and reduce the risk of Parkinson’s disease, particularly in carriers of pathogenic mutations | [215] |
| SOD1 | Superoxide dismutase 1 | Oxidative stress, metabolic imbalance, and neuromuscular degeneration | Mutations in the SOD1 gene are a known cause of familial amyotrophic lateral sclerosis (ALS), leading to selective degeneration of motor neurons in the brain and spinal cord | The targeted suppression of mutant SOD1 gene expression holds therapeutic potential for slowing neurodegeneration, preserving motor neuron function, and improving nutritional status | [216] |
| PARK7/DJ-1 | Parkinson’s disease 7 | Regulation of metabolism, gut microbiota, and mucosal barrier function | The PARK7/DJ-1 protein exerts neuroprotective effects through its antioxidant activity, inhibition of α-synuclein aggregation, prevention of protein glycation, regulation of neuroinflammation, and maintenance of BBB integrity under conditions of systemic inflammation | Antioxidant activity, prevention of glycation and aggregation of neurotoxic proteins, modulation of inflammatory responses, and maintenance of BBB integrity | [217] |
| PSEN1 | Presenilin 1 | Eating disorder | Mutations in the PSEN1 gene contribute to the development of neuroinflammation by promoting the activation of proinflammatory cytokines and disrupting the regulation of Notch signaling, thereby exacerbating neurodegenerative processes | The ability to identify carriers at high risk of neurodegeneration at an early stage is crucial for implementing preventive interventions and developing targeted therapies | [218] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuigunov, D.; Sinyavskiy, Y.; Nurgozhin, T.; Zholdassova, Z.; Smagul, G.; Omarov, Y.; Dolmatova, O.; Yeshmanova, A.; Omarova, I. Precision Nutrition and Gut–Brain Axis Modulation in the Prevention of Neurodegenerative Diseases. Nutrients 2025, 17, 3068. https://doi.org/10.3390/nu17193068
Tuigunov D, Sinyavskiy Y, Nurgozhin T, Zholdassova Z, Smagul G, Omarov Y, Dolmatova O, Yeshmanova A, Omarova I. Precision Nutrition and Gut–Brain Axis Modulation in the Prevention of Neurodegenerative Diseases. Nutrients. 2025; 17(19):3068. https://doi.org/10.3390/nu17193068
Chicago/Turabian StyleTuigunov, Dilyar, Yuriy Sinyavskiy, Talgat Nurgozhin, Zhibek Zholdassova, Galiya Smagul, Yerzhan Omarov, Oksana Dolmatova, Ainur Yeshmanova, and Indira Omarova. 2025. "Precision Nutrition and Gut–Brain Axis Modulation in the Prevention of Neurodegenerative Diseases" Nutrients 17, no. 19: 3068. https://doi.org/10.3390/nu17193068
APA StyleTuigunov, D., Sinyavskiy, Y., Nurgozhin, T., Zholdassova, Z., Smagul, G., Omarov, Y., Dolmatova, O., Yeshmanova, A., & Omarova, I. (2025). Precision Nutrition and Gut–Brain Axis Modulation in the Prevention of Neurodegenerative Diseases. Nutrients, 17(19), 3068. https://doi.org/10.3390/nu17193068






