Cachexia in Pancreatic Cancer: New Insights to Impact Quality of Life and Survival
Abstract
1. Introduction
2. Methods
3. Diagnosis of Pancreatic Cancer and Cancer Cachexia
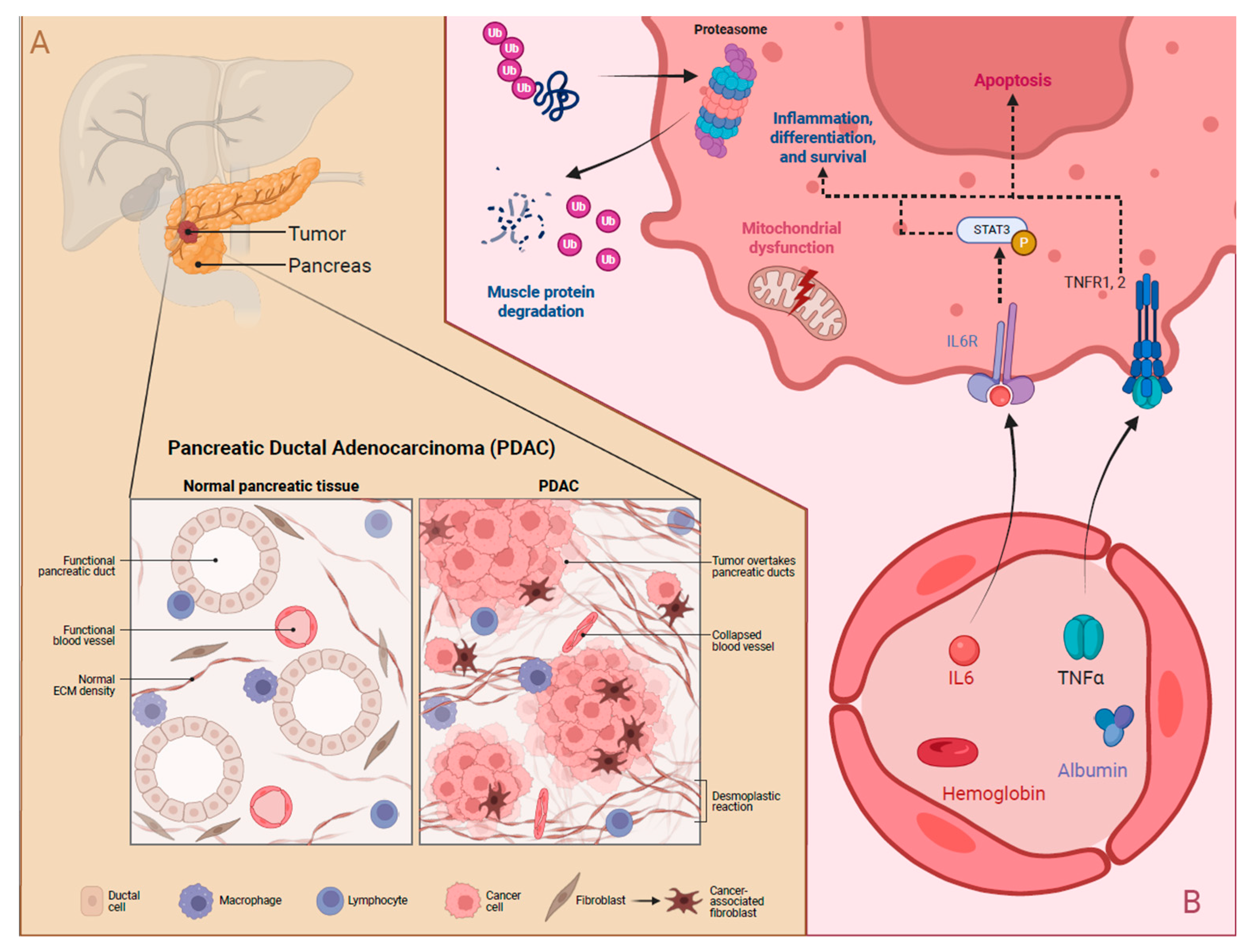
4. Mechanisms of Cachexia and Potential Biomarkers
4.1. Mechanisms of Cachexia
4.2. Potential Biomarkers in Pancreatic Cancer Cachexia
5. Symptoms Associated with Cachexia Due to Pancreatic Cancer
5.1. Symptoms of Cachexia in Patients with Pancreatic Cancer
5.2. Current Symptomatic Treatment of Cancer Cachexia
6. Impact of Cachexia on Pancreatic Cancer
7. Treatments for Cachexia and Improvement of Quality of Life in Pancreatic Cancer
7.1. Pharmacological Interventions
7.2. Nutritional Interventions
7.3. Non-Pharmacological Interventions
8. Future Directions and Potential Interventions
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Nikšić, M.; Matz, M.; Valkov, M.; Marcos-Gragera, R.; Stiller, C.; Rosso, S.; Coleman, M.P.; Allemani, C. World-wide trends in net survival from pancreatic cancer by morphological sub-type: An analysis of 1,258,329 adults diagnosed in 58 countries during 2000–2014 (CONCORD-3). Cancer Epidemiol. 2022, 80, 102196. [Google Scholar] [CrossRef] [PubMed]
- La Salvia, A.; Persano, I.; Parlagreco, E.; Audisio, A.; Cani, M.; Brizzi, M.P. Pancreatic adenocarcinoma and pancreatic high-grade neuroendocrine carcinoma: Two sides of the moon. Med. Oncol. 2022, 39, 168. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Pancreatic Neuroendocrine Tumors (Islet Cell Tumors) Treatment (PDQ®)–Health Professional Version. Available online: www.cancer.gov/types/pancreatic/hp/pnet-treatment-pdq (accessed on 5 November 2024).
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 major types of gastrointestinal cancer. Gastroenterology 2020, 159, 335–349.e315. [Google Scholar] [CrossRef]
- Verma, M. Pancreatic cancer epidemiology. Technol. Cancer Res. Treat. 2005, 4, 295–301. [Google Scholar] [CrossRef]
- Afghani, E.; Klein, A.P. Pancreatic adenocarcinoma: Trends in epidemiology, risk factors, and outcomes. Hematol. Clin. N. Am. 2022, 36, 879–895. [Google Scholar] [CrossRef]
- Grinstead, C.; Yoon, S.L. Geriatric Nutritional Risk Index (GNRI) and survival in pancreatic cancer: A retrospective study. Nutrients 2025, 17, 509. [Google Scholar] [CrossRef]
- Grinstead, C.; George, T.; Han, B.; Yoon, S.L. Associations of overall survival with Geriatric Nutritional Risk Index in patients with advanced pancreatic cancer. Nutrients 2022, 14, 3800. [Google Scholar] [CrossRef]
- Yoon, S.L.; Kim, J.A.; Kelly, D.L.; Lyon, D.; George, T.J., Jr. Predicting unintentional weight loss in patients with gastrointestinal cancer. J. Cachexia Sarcopenia Muscle 2019, 10, 526–535. [Google Scholar] [CrossRef]
- Guan, M.; Hendifar, A.E.; Shinde, A.M. Pancreatic cancer cachexia: Current concepts and clinical management. In Frailty and Sarcopenia—Onset, Development and Clinical Challenges; Dionyssiotis, Y., Ed.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar][Green Version]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Hendifar, A.E.; Petzel, M.Q.B.; Zimmers, T.A.; Denlinger, C.S.; Matrisian, L.M.; Picozzi, V.J.; Rahib, L. Pancreas cancer-associated weight loss. Oncologist 2019, 24, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Pekarek, L.; Fraile-Martinez, O.; Garcia-Montero, C.; Saez, M.A.; Barquero-Pozanco, I.; Del Hierro-Marlasca, L.; de Castro Martinez, P.; Romero-Bazán, A.; Alvarez-Mon, M.A.; Monserrat, J.; et al. Clinical applications of classical and novel biological markers of pancreatic cancer. Cancers 2022, 14, 1866. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Jin, K.; Deng, S.; Cheng, H.; Fan, Z.; Gong, Y.; Qian, Y.; Huang, Q.; Ni, Q.; Liu, C.; et al. Roles of CA19-9 in pancreatic cancer: Biomarker, predictor and promoter. Biochim. Biophys. Acta (BBA) Rev. Cancer 2021, 1875, 188409. [Google Scholar] [CrossRef]
- Liu, C.; Deng, S.; Jin, K.; Gong, Y.; Cheng, H.; Fan, Z.; Qian, Y.; Huang, Q.; Ni, Q.; Luo, G.; et al. Lewis antigen-negative pancreatic cancer: An aggressive subgroup. Int. J. Oncol. 2020, 56, 900–908. [Google Scholar] [CrossRef]
- Hou, Y.C.; Chen, C.Y.; Huang, C.J.; Wang, C.J.; Chao, Y.J.; Chiang, N.J.; Wang, H.C.; Tung, H.L.; Liu, H.C.; Shan, Y.S. The differential clinical impacts of cachexia and sarcopenia on the prognosis of advanced pancreatic cancer. Cancers 2022, 14, 3137. [Google Scholar] [CrossRef]
- Vigano, A.A.; Morais, J.A.; Ciutto, L.; Rosenthall, L.; di Tomasso, J.; Khan, S.; Olders, H.; Borod, M.; Kilgour, R.D. Use of routinely available clinical, nutritional, and functional criteria to classify cachexia in advanced cancer patients. Clin. Nutr. 2017, 36, 1378–1390. [Google Scholar] [CrossRef]
- Regel, I.; Mayerle, J. Nutrient Scavenging From Muscle Cells: A survival strategy of pancreatic cancer cells ends in cachexia. Gastroenterology 2022, 163, 1161–1163. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Ahmad, K.; Shaikh, S.; You, H.J.; Lee, E.Y.; Ali, S.; Lee, E.J.; Choi, I. Molecular mechanisms and current treatment options for cancer cachexia. Cancers 2022, 14, 2107. [Google Scholar] [CrossRef]
- Yakovenko, A.; Cameron, M.; Trevino, J.G. Molecular therapeutic strategies targeting pancreatic cancer induced cachexia. World J. Gastrointest. Surg. 2018, 10, 95–106. [Google Scholar] [CrossRef]
- Brouwer, T.P.; de Vries, N.L.; Abdelaal, T.; Krog, R.T.; Li, Z.; Ruano, D.; Fariña, A.; Lelieveldt, B.P.F.; Morreau, H.; Bonsing, B.A.; et al. Local and systemic immune profiles of human pancreatic ductal adenocarcinoma revealed by single-cell mass cytometry. J. Immunother. Cancer 2022, 10, e004638. [Google Scholar] [CrossRef]
- Stark, G.R.; Darnell, J.E., Jr. The JAK-STAT pathway at twenty. Immunity 2012, 36, 503–514. [Google Scholar] [CrossRef]
- Lefler, J.E.; MarElia-Bennett, C.B.; Thies, K.A.; Hildreth, B.E., 3rd; Sharma, S.M.; Pitarresi, J.R.; Han, L.; Everett, C.; Koivisto, C.; Cuitino, M.C.; et al. STAT3 in tumor fibroblasts promotes an immunosuppressive microenvironment in pancreatic cancer. Life Sci. Alliance 2022, 5, e202201460. [Google Scholar] [CrossRef]
- Tsukinaga, S.; Kajihara, M.; Takakura, K.; Ito, Z.; Kanai, T.; Saito, K.; Takami, S.; Kobayashi, H.; Matsumoto, Y.; Odahara, S.; et al. Prognostic significance of plasma interleukin-6/-8 in pancreatic cancer patients receiving chemoimmunotherapy. World J. Gastroenterol. 2015, 21, 11168–11178. [Google Scholar] [CrossRef] [PubMed]
- Mitsunaga, S.; Ikeda, M.; Shimizu, S.; Ohno, I.; Furuse, J.; Inagaki, M.; Higashi, S.; Kato, H.; Terao, K.; Ochiai, A. Serum levels of IL-6 and IL-1β can predict the efficacy of gemcitabine in patients with advanced pancreatic cancer. Br. J. Cancer 2013, 108, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Pais, A.; Ferreira, R.; Oliveira, P.A.; Duarte, J.A. Sarcopenia versus cancer cachexia: The muscle wasting continuum in healthy and diseased aging. Biogerontology 2021, 22, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Kobayashi, T.; Chayahara, N.; Imamura, Y.; Toyoda, M.; Kiyota, N.; Mukohara, T.; Nishiumi, S.; Azuma, T.; Yoshida, M.; et al. Metabolomics evaluation of serum markers for cachexia and their intra-day variation in patients with advanced pancreatic cancer. PLoS ONE 2014, 9, e113259. [Google Scholar] [CrossRef]
- Zhang, Y.; Dos Santos, M.; Huang, H.; Chen, K.; Iyengar, P.; Infante, R.; Polanco, P.M.; Brekken, R.A.; Cai, C.; Caijgas, A.; et al. A molecular pathway for cancer cachexia-induced muscle atrophy revealed at single-nucleus resolution. Cell Rep. 2024, 43, 114587. [Google Scholar] [CrossRef]
- Gicquel, T.; Marchiano, F.; Reyes-Castellanos, G.; Audebert, S.; Camoin, L.; Habermann, B.H.; Giannesini, B.; Carrier, A. Integrative study of skeletal muscle mitochondrial dysfunction in a murine pancreatic cancer-induced cachexia model. eLife 2024, 13, RP93312. [Google Scholar] [CrossRef]
- Martin, A.; Freyssenet, D. Phenotypic features of cancer cachexia-related loss of skeletal muscle mass and function: Lessons from human and animal studies. J. Cachexia Sarcopenia Muscle 2021, 12, 252–273. [Google Scholar] [CrossRef]
- Poulia, K.A.; Antoniadou, D.; Sarantis, P.; Karamouzis, M.V. Pancreatic cancer prognosis, malnutrition risk, and quality of life: A cross-sectional study. Nutrients 2022, 14, 442. [Google Scholar] [CrossRef] [PubMed]
- Kordes, M.; Larsson, L.; Engstrand, L.; Löhr, J.M. Pancreatic cancer cachexia: Three dimensions of a complex syndrome. Br. J. Cancer 2021, 124, 1623–1636. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, A.J.; Chu, L.C.; Deig, C.R.; Fishman, E.K.; Hwang, W.L.; Maitra, A.; Marks, D.L.; Mehta, A.; Nabavizadeh, N.; Simeone, D.M.; et al. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. CA Cancer J. Clin. 2020, 70, 375–403. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Guo, Y.; Du, J.; Gu, J.; Kong, L.; Tao, B.; Li, J.; Fu, D. The intricate crosstalk between insulin and pancreatic ductal adenocarcinoma: A review from clinical to molecular. Front. Cell Dev. Biol. 2022, 10, 844028. [Google Scholar] [CrossRef]
- Yoo, W.; Choi, H.; Son, Y.H.; Lee, J.; Jo, S.; Jung, D.; Kim, Y.J.; Koh, S.S.; Yang, Y.R.; Kwon, E.S.; et al. Pancreatic cancer induces muscle wasting by promoting the release of pancreatic adenocarcinoma upregulated factor. Exp. Mol. Med. 2021, 53, 432–445. [Google Scholar] [CrossRef]
- Gooden, H.M.; White, K.J. Pancreatic cancer and supportive care--pancreatic exocrine insufficiency negatively impacts on quality of life. Support. Care Cancer 2013, 21, 1835–1841. [Google Scholar] [CrossRef]
- Sikkens, E.C.; Cahen, D.L.; de Wit, J.; Looman, C.W.; van Eijck, C.; Bruno, M.J. A prospective assessment of the natural course of the exocrine pancreatic function in patients with a pancreatic head tumor. J. Clin. Gastroenterol. 2014, 48, e43–e46. [Google Scholar] [CrossRef]
- Gilliland, T.M.; Villafane-Ferriol, N.; Shah, K.P.; Shah, R.M.; Tran Cao, H.S.; Massarweh, N.N.; Silberfein, E.J.; Choi, E.A.; Hsu, C.; McElhany, A.L.; et al. Nutritional and metabolic derangements in pancreatic cancer and pancreatic resection. Nutrients 2017, 9, 243. [Google Scholar] [CrossRef]
- Cangemi, D.J.; Kuo, B. Practical Perspectives in the Treatment of Nausea and Vomiting. J. Clin. Gastroenterol. 2019, 53, 170–178. [Google Scholar] [CrossRef]
- Michalski, C.W.; Oti, F.E.; Erkan, M.; Sauliunaite, D.; Bergmann, F.; Pacher, P.; Batkai, S.; Müller, M.W.; Giese, N.A.; Friess, H.; et al. Cannabinoids in pancreatic cancer: Correlation with survival and pain. Int. J. Cancer 2008, 122, 742–750. [Google Scholar] [CrossRef]
- Sharafi, G.; He, H.; Nikfarjam, M. Potential use of cannabinoids for the treatment of pancreatic cancer. J. Pancreat. Cancer 2019, 5, 1–7. [Google Scholar] [CrossRef]
- Torres, P.J.; Fletcher, E.M.; Gibbons, S.M.; Bouvet, M.; Doran, K.S.; Kelley, S.T. Characterization of the salivary microbiome in patients with pancreatic cancer. PeerJ 2015, 3, e1373. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef]
- Pötgens, S.A.; Brossel, H.; Sboarina, M.; Catry, E.; Cani, P.D.; Neyrinck, A.M.; Delzenne, N.M.; Bindels, L.B. Klebsiella oxytoca expands in cancer cachexia and acts as a gut pathobiont contributing to intestinal dysfunction. Sci. Rep. 2018, 8, 12321. [Google Scholar] [CrossRef]
- Permuth, J.B.; Park, M.A.; Chen, D.T.; Basinski, T.; Powers, B.D.; Gwede, C.K.; Dezsi, K.B.; Gomez, M.; Vyas, S.L.; Biachi, T.; et al. Leveraging real-world data to predict cancer cachexia stage, quality of life, and survival in a racially and ethnically diverse multi-institutional cohort of treatment-naïve patients with pancreatic ductal adenocarcinoma. Front. Oncol. 2024, 14, 1362244. [Google Scholar] [CrossRef] [PubMed]
- Visavadiya, N.P.; Rossiter, H.B.; Khamoui, A.V. Distinct glycolytic pathway regulation in liver, tumour and skeletal muscle of mice with cancer cachexia. Cell Biochem. Funct. 2021, 39, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, H.; Hu, L.; Liu, Y.; Duan, Y.; Cui, R.; Tian, W. The Warburg effect in human pancreatic cancer cells triggers cachexia in athymic mice carrying the cancer cells. BMC Cancer 2018, 18, 360. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.H.; Wolfe, R.R. Fatty acid and glycerol kinetics in septic patients and in patients with gastrointestinal cancer. The response to glucose infusion and parenteral feeding. Ann. Surg. 1987, 205, 368–376. [Google Scholar] [CrossRef]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef]
- Groarke, J.D.; Crawford, J.; Collins, S.M.; Lubaczewski, S.L.; Breen, D.M.; Harrington, M.A.; Jacobs, I.; Qiu, R.; Revkin, J.; Rossulek, M.I.; et al. Phase 2 study of the efficacy and safety of ponsegromab in patients with cancer cachexia: PROACC-1 study design. J. Cachexia Sarcopenia Muscle 2024, 15, 1054–1061. [Google Scholar] [CrossRef]
- Werner, K.; Küllenberg de Gaudry, D.; Taylor, L.A.; Keck, T.; Unger, C.; Hopt, U.T.; Massing, U. Dietary supplementation with n-3-fatty acids in patients with pancreatic cancer and cachexia: Marine phospholipids versus fish oil—A randomized controlled double-blind trial. Lipids Health Dis. 2017, 16, 104. [Google Scholar] [CrossRef] [PubMed]
- Moss, C.L. Comparison of the histochemical and contractile properties of human triceps surae. Med. Biol. Eng. Comput. 1992, 30, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.; Kraft, K.; Gärtner, S.; Mayerle, J.; Simon, P.; Weber, E.; Schütte, K.; Stieler, J.; Koula-Jenik, H.; Holzhauer, P.; et al. L-Carnitine-supplementation in advanced pancreatic cancer (CARPAN)—A randomized multicentre trial. Nutr. J. 2012, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Krüger, J.; Meffert, P.J.; Vogt, L.J.; Gärtner, S.; Steveling, A.; Kraft, M.; Mayerle, J.; Lerch, M.M.; Aghdassi, A.A. Early parenteral nutrition in patients with biliopancreatic mass lesions, a prospective, randomized intervention trial. PLoS ONE 2016, 11, e0166513. [Google Scholar] [CrossRef]
- Pelzer, U.; Arnold, D.; Gövercin, M.; Stieler, J.; Doerken, B.; Riess, H.; Oettle, H. Parenteral nutrition support for patients with pancreatic cancer. Results of a phase II study. BMC Cancer 2010, 10, 86. [Google Scholar] [CrossRef]
- Gordon, J.N.; Trebble, T.M.; Ellis, R.D.; Duncan, H.D.; Johns, T.; Goggin, P.M. Thalidomide in the treatment of cancer cachexia: A randomised placebo controlled trial. Gut 2005, 54, 540–545. [Google Scholar] [CrossRef]
- Thong, M.S.Y.; van Noorden, C.J.F.; Steindorf, K.; Arndt, V. Cancer-related fatigue: Causes and current treatment options. Curr. Treat. Options Oncol. 2020, 21, 17. [Google Scholar] [CrossRef]
- Grande, A.J.; Silva, V.; Sawaris Neto, L.; Teixeira Basmage, J.P.; Peccin, M.S.; Maddocks, M. Exercise for cancer cachexia in adults. Cochrane Database Syst. Rev. 2021, 3, Cd010804. [Google Scholar] [CrossRef]
- Yoon, S.L.; Grundmann, O.; Williams, J.J.; Wu, S.S.; Leeuwenburgh, C.; Huo, Z.; George, T.J., Jr. Differential response to targeted acupuncture by gender in patients with gastrointestinal cancer cachexia: Secondary analysis of a randomized controlled trial. Acupunct. Med. 2020, 38, 53–60. [Google Scholar] [CrossRef]
- Grundmann, O.; Yoon, S.L.; Williams, J.J.; Gordan, L.; George, T.J., Jr. Augmentation of cancer cachexia components with targeted acupuncture in patients with gastrointestinal cancers: A randomized controlled pilot study. Integr. Cancer Ther. 2019, 18, 1534735418823269. [Google Scholar] [CrossRef]
- Uster, A.; Ruehlin, M.; Mey, S.; Gisi, D.; Knols, R.; Imoberdorf, R.; Pless, M.; Ballmer, P.E. Effects of nutrition and physical exercise intervention in palliative cancer patients: A randomized controlled trial. Clin. Nutr. 2018, 37, 1202–1209. [Google Scholar] [CrossRef]
- Avancini, A.; Trestini, I.; Tregnago, D.; Cavallo, A.; Bragato, M.; Bonaiuto, C.; Lanza, M.; Milella, M.; Pilotto, S. Multidisciplinary lifestyle intervention to manage pancreatic cancer-related cachexia: A case report. Future Sci. OA 2020, 7, Fso659. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Davidson, W.; Ash, S.; Capra, S.; Bauer, J. Weight stabilisation is associated with improved survival duration and quality of life in unresectable pancreatic cancer. Clin. Nutr. 2004, 23, 239–247. [Google Scholar] [CrossRef]
- Daly, L.; Dolan, R.; Power, D.; Ní Bhuachalla, É.; Sim, W.; Fallon, M.; Cushen, S.; Simmons, C.; McMillan, D.C.; Laird, B.J.; et al. The relationship between the BMI-adjusted weight loss grading system and quality of life in patients with incurable cancer. J. Cachexia Sarcopenia Muscle 2020, 11, 160–168. [Google Scholar] [CrossRef]
- Toth, M.J.; Callahan, D.M.; Miller, M.S.; Tourville, T.W.; Hackett, S.B.; Couch, M.E.; Dittus, K. Skeletal muscle fiber size and fiber type distribution in human cancer: Effects of weight loss and relationship to physical function. Clin. Nutr. 2016, 35, 1359–1365. [Google Scholar] [CrossRef]
- Han, B.; Zhao, S.; Trevino, J.; Grossman, S.; Lenz, H.-J.; Hoang, B.X. Abstract 382: Pancreatic cancer-derived organoids alter muscle fiber type and increased energy consumption leading to cachexia. Cancer Res. 2024, 84 (Suppl. 6), 382. [Google Scholar] [CrossRef]
- Youn, S.E.; Jiang, F.; Won, H.Y.; Hong, D.E.; Kang, T.H.; Park, Y.Y.; Koh, S.S. PAUF Induces migration of human pancreatic cancer cells exclusively via the TLR4/MyD88/NF-κB signaling pathway. Int. J. Mol. Sci. 2022, 23, 11414. [Google Scholar] [CrossRef]
- Brieger, A.; Rink, L.; Haase, H. Differential regulation of TLR-dependent MyD88 and TRIF signaling pathways by free zinc ions. J. Immunol. 2013, 191, 1808–1817. [Google Scholar] [CrossRef]
- Feuerecker, B.; Biechl, P.; Veltkamp, C.; Saur, D.; Eisenreich, W. Metabolic response of pancreatic carcinoma cells under treatment with dichloroacetate. Metabolites 2021, 11, 350. [Google Scholar] [CrossRef]
- Stathopoulos, G.P.; Syrigos, K.; Aravantinos, G.; Polyzos, A.; Papakotoulas, P.; Fountzilas, G.; Potamianou, A.; Ziras, N.; Boukovinas, J.; Varthalitis, J.; et al. A multicenter phase III trial comparing irinotecan-gemcitabine (IG) with gemcitabine (G) monotherapy as first-line treatment in patients with locally advanced or metastatic pancreatic cancer. Br. J. Cancer 2006, 95, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Oberstein, P.; Saif, M. First-line treatment for advanced pancreatic cancer. JOP J. Pancreas 2011, 12, 5. [Google Scholar] [CrossRef]
- Cortez, N.E.; Pathak, S.; Rodriguez Lanzi, C.; Hong, B.V.; Crone, R.; Sule, R.; Wang, F.; Chen, S.; Gomes, A.V.; Baar, K.; et al. A ketogenic diet in combination with gemcitabine mitigates pancreatic cancer-associated cachexia in male and female KPC mice. Int. J. Mol. Sci. 2023, 24, 10753. [Google Scholar] [CrossRef]
- Ueno, M.; Sugimori, K.; Taguri, M.; Ohkawa, S.; Kobayashi, S.; Miwa, H.; Kaneko, T.; Morimoto, M.; Yamanaka, T. Randomized Phase II study of gemcitabine monotherapy vs. gemcitabine with an EPA-enriched oral supplement in advanced pancreatic cancer. Nutr. Cancer 2022, 74, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.; Clarke, L.; Goldberg, A.; Bishop, K.S. Pancreatic cancer cachexia: The role of nutritional interventions. Healthcare 2019, 7, 89. [Google Scholar] [CrossRef]
- Smith, H.J.; Mukerji, P.; Tisdale, M.J. Attenuation of proteasome-induced proteolysis in skeletal muscle by {beta}-hydroxy-{beta}-methylbutyrate in cancer-induced muscle loss. Cancer Res. 2005, 65, 277–283. [Google Scholar] [CrossRef]
- Birhanu, G.; Javar, H.A.; Seyedjafari, E.; Zandi-Karimi, A. Nanotechnology for delivery of gemcitabine to treat pancreatic cancer. Biomed. Pharmacother. 2017, 88, 635–643. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.; Ma, P.; Jiang, Y.; Cheng, K.; Yu, Y.; Jiang, N.; Miao, H.; Tang, Q.; Liu, F.; et al. Drug conjugate-based anticancer therapy—Current status and perspectives. Cancer Lett. 2023, 552, 215969. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Li, S. Polymeric micelles: Nanocarriers for cancer-targeted drug delivery. AAPS PharmSciTech 2014, 15, 862–871. [Google Scholar] [CrossRef]
- Paolino, D.; Cosco, D.; Racanicchi, L.; Trapasso, E.; Celia, C.; Iannone, M.; Puxeddu, E.; Costante, G.; Filetti, S.; Russo, D.; et al. Gemcitabine-loaded PEGylated unilamellar liposomes vs GEMZAR: Biodistribution, pharmacokinetic features and in vivo antitumor activity. J. Control Release 2010, 144, 144–150. [Google Scholar] [CrossRef]
- Narasimhan, A.; Jengelley, D.H.A.; Huot, J.R.; Umberger, T.S.; Doud, E.H.; Mosley, A.L.; Wang, M.; Zhong, X.; Counts, B.R.; Rupert, J.E.; et al. Gemcitabine plus nab-paclitaxel preserves skeletal and cardiac mass and function in a murine model of pancreatic cancer cachexia. bioRxiv 2023. [Google Scholar] [CrossRef]
 by Fearon et al. [13] and
by Fearon et al. [13] and  Vigano et al. [19]; CRP: C-Reactive Protein, WBC: White Blood Cell Count, aPG-SGA: abridged Patient-Generated Subjective Global Assessment.
Vigano et al. [19]; CRP: C-Reactive Protein, WBC: White Blood Cell Count, aPG-SGA: abridged Patient-Generated Subjective Global Assessment.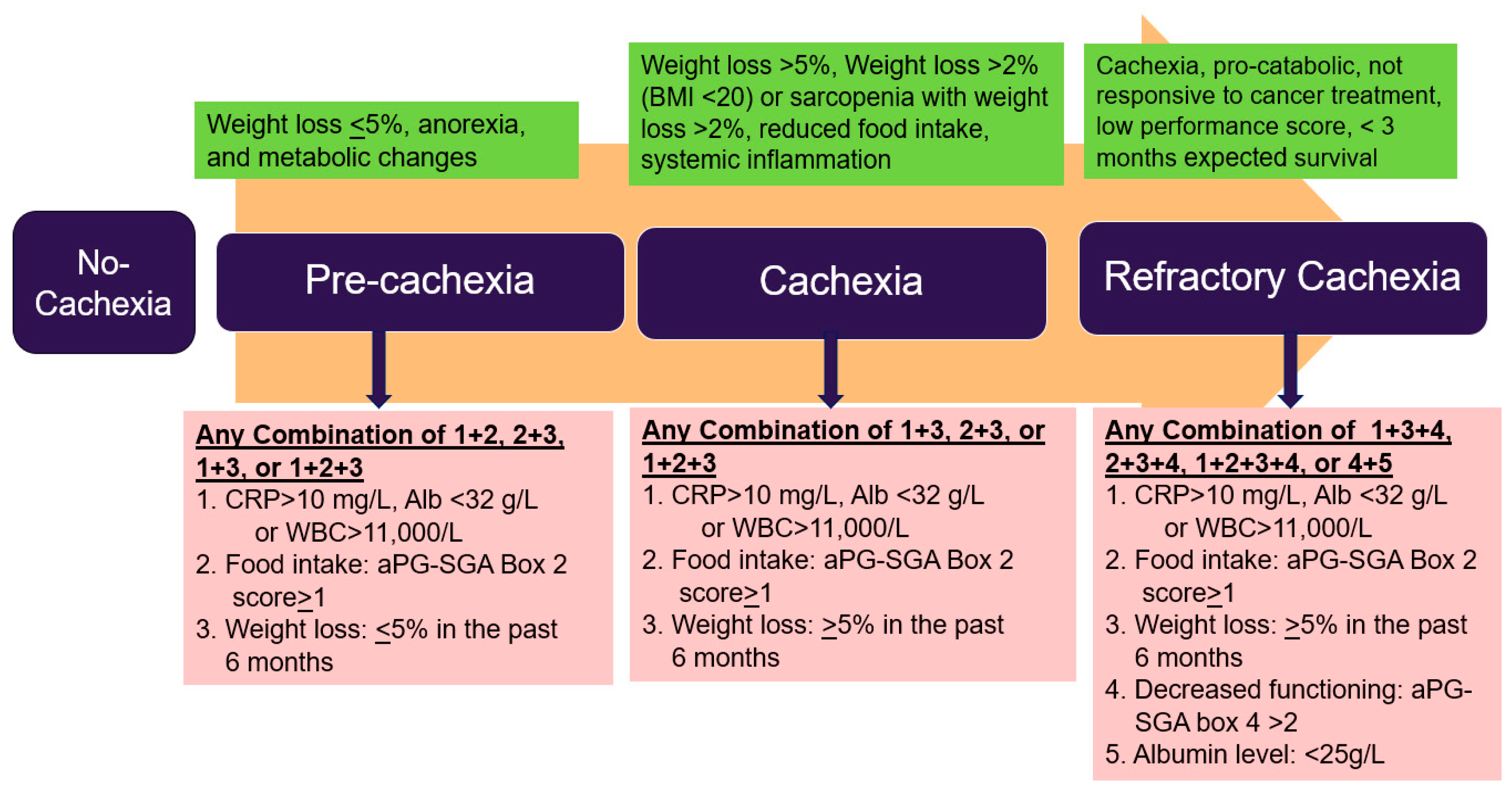
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, S.L.; Grundmann, O.; Rogers, S.; Schlaeger, J.M.; Han, B.; Agyare, E.; Wilkie, D.J. Cachexia in Pancreatic Cancer: New Insights to Impact Quality of Life and Survival. Nutrients 2025, 17, 3064. https://doi.org/10.3390/nu17193064
Yoon SL, Grundmann O, Rogers S, Schlaeger JM, Han B, Agyare E, Wilkie DJ. Cachexia in Pancreatic Cancer: New Insights to Impact Quality of Life and Survival. Nutrients. 2025; 17(19):3064. https://doi.org/10.3390/nu17193064
Chicago/Turabian StyleYoon, Saunjoo L., Oliver Grundmann, Sherise Rogers, Judith M. Schlaeger, Bo Han, Edward Agyare, and Diana J. Wilkie. 2025. "Cachexia in Pancreatic Cancer: New Insights to Impact Quality of Life and Survival" Nutrients 17, no. 19: 3064. https://doi.org/10.3390/nu17193064
APA StyleYoon, S. L., Grundmann, O., Rogers, S., Schlaeger, J. M., Han, B., Agyare, E., & Wilkie, D. J. (2025). Cachexia in Pancreatic Cancer: New Insights to Impact Quality of Life and Survival. Nutrients, 17(19), 3064. https://doi.org/10.3390/nu17193064





