Directional Effects of Self-Regulation and Self-Efficacy Changes Within a Weight-Loss Treatment Focused on Exercise and Sweets Consumption: Accounting for Emotional Eating in Women with Obesity
Abstract
1. Introduction
- -
- There will be significant improvements in S-Reg, S-Eff, sweets intake, fruit/vegetable consumption, exercise, and weight. It was addressed as a research question whether those improvements will significantly differ by emotional eating group (high or low).
- -
- Another research question was whether paths from early changes in S-Reg leading to (→) longer-term changes in S-Eff → behavioral (i.e., exercise and sweets intake) changes → weight losses will be significant, and/or whether similar paths initiating from S-Eff → S-Reg changes will be significant.
- -
- A final research question was whether the above paths would be significantly affected by emotional eating level.
- -
- It was expected that a reduction of sweets intake would account for a greater portion of the explained variance in weight loss over six and twelve months than an increase of fruit/vegetable consumption and/or exercise.
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Self-Regulation
2.2.2. Self-Efficacy
2.2.3. Exercise
2.2.4. Eating Behaviors
2.2.5. Emotional Eating Propensity
2.2.6. Body Composition
2.3. Procedure
2.4. Data Analyses
3. Results
3.1. Changes in Study Variable Scores
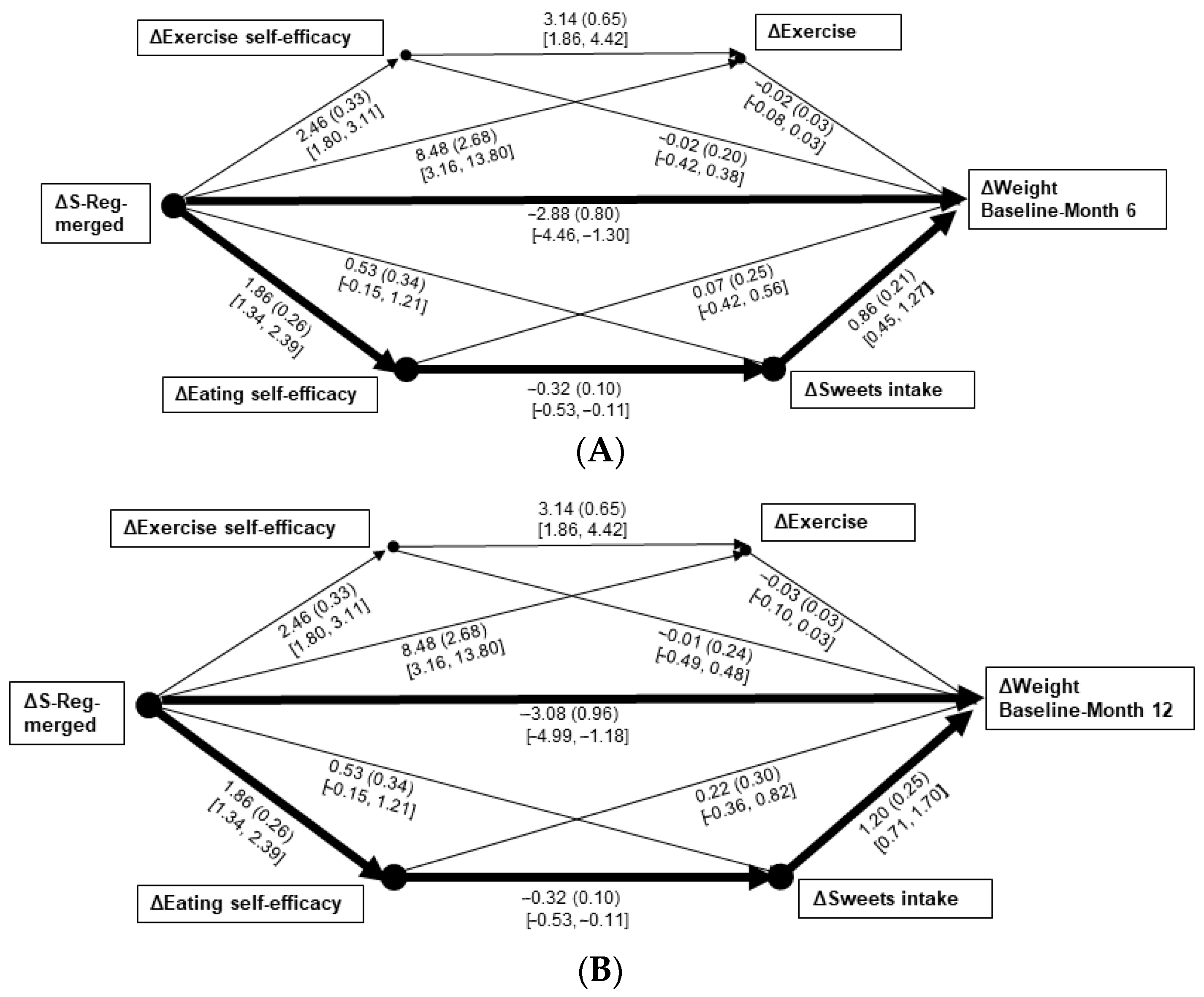
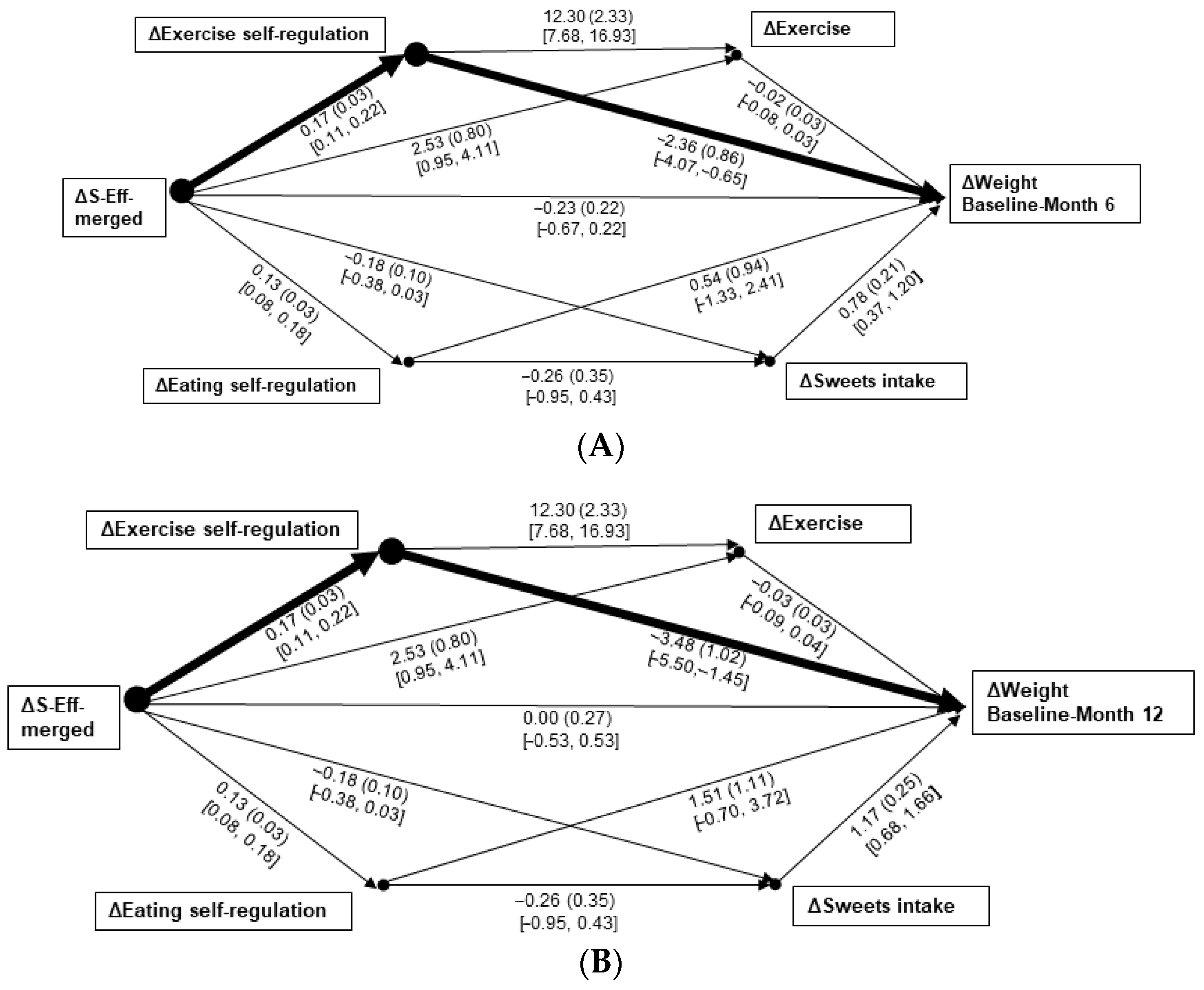
3.2. Regression and Path Analyses
3.3. Sensitivity Analyses: Prediction of Weight Changes via Behavioral Changes
3.4. Post Hoc Analyses
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2021 US Obesity Forecasting Collaborators. National-level and state-level prevalence of overweight and obesity among children, adolescents, and adults in the USA, 1990–2021, and forecasts up to 2050. Lancet 2024, 404, 2278–2298. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H.; World Obesity Federation. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef]
- Koenders, P.G.; van Strien, T. Emotional eating, rather than lifestyle behavior, drives weight gain in a prospective study in 1562 employees. J. Occup. Environ. Med. 2011, 53, 1287–1293. [Google Scholar] [CrossRef]
- Smith, J.M.; Serier, K.N.; Belon, K.E.; Sebastian, R.M.; Smith, J.E. Evaluation of the relationships between dietary restraint, emotional eating, and intuitive eating moderated by sex. Appetite 2020, 155, 104817. [Google Scholar] [CrossRef]
- Dombrowski, S.U.; Knittle, K.; Avenell, A.; Araújo-Soares, V.; Sniehotta, F.F. Long term maintenance of weight loss with non-surgical interventions in obese adults: Systematic review and meta-analyses of randomised controlled trials. BMJ 2014, 348, g2646. [Google Scholar] [CrossRef]
- MacLean, P.S.; Wing, R.R.; Davidson, T.; Epstein, L.; Goodpaster, B.; Hall, K.D.; Levin, B.E.; Perri, M.G.; Rolls, B.J.; Rosenbaum, M.; et al. NIH working group report: Innovative research to improve maintenance of weight loss. Obesity 2015, 23, 7–15. [Google Scholar] [CrossRef]
- Altieri, M.S.; Irish, W.; Pories, W.J.; Shah, A.; DeMaria, E.J. Examining the rates of obesity and bariatric surgery in the United States. Obes. Surg. 2021, 31, 4754–4760. [Google Scholar] [CrossRef]
- Berning, P.; Adhikari, R.; Schroer, A.E.; Jelwan, Y.A.; Razavi, A.C.; Blaha, M.J.; Dzaye, O. Longitudinal analysis of obesity drug use and public awareness. JAMA Netw. Open 2025, 8, e2457232. [Google Scholar] [CrossRef]
- Betancourt-Núñez, A.; Torres-Castillo, N.; Martínez-López, E.; De Loera-Rodríguez, C.O.; Durán-Barajas, E.; Márquez-Sandoval, F.; Bernal-Orozco, M.F.; Garaulet, M.; Vizmanos, B. Emotional eating and dietary patterns: Reflecting food choices in people with and without abdominal obesity. Nutrients 2022, 14, 1371. [Google Scholar] [CrossRef]
- Aljadani, H.M.; Patterson, A.; Sibbritt, D.; Hutchesson, M.J.; Jensen, M.E.; Collins, C.E. Diet quality, measured by fruit and vegetable intake, predicts weight change in young women. J. Obes. 2013, 2013, 525161. [Google Scholar] [CrossRef]
- Annesi, J.J. Moderation of psychological factors in the relationship of increased fruit and vegetable intake with reductions in other food groups and weight in obese women. Minerva Psichiatr. 2018, 59, 1–9. [Google Scholar] [CrossRef]
- Sperandei, S.; Vieira, M.C.; Reis, A.C. Adherence to physical activity in an unsupervised setting: Explanatory variables for high attrition rates among fitness center members. J. Sci. Med. Sport 2016, 19, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Annesi, J.J.; Unruh, J.L. Effects of The Coach Approach intervention on drop-out rates among adults initiating exercise programs at nine YMCAs over three years. Percept. Mot. Skills 2007, 104, 459–466. [Google Scholar] [CrossRef]
- Donnelly, J.E.; Blair, S.N.; Jakicic, J.M.; Manore, M.M.; Rankin, J.W.; Smith, B.K.; American College of Sports Medicine. American College of Sports Medicine position stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sports Exerc. 2009, 41, 459–471. [Google Scholar] [CrossRef]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Mâsse, L.C.; Tilert, T.; McDowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef]
- Trost, S.G.; Owen, N.; Bauman, A.E.; Sallis, J.F.; Brown, W. Correlates of adults’ participation in physical activity: Review and update. Med. Sci. Sports Exerc. 2002, 34, 1996–2001. [Google Scholar] [CrossRef] [PubMed]
- Annesi, J.J.; Sevene, P.G. Short- and long-term weight loss among women is unrelated to completed exercise within an obesity intervention focused on self-regulation. Perm. J. 2023, 27, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.M.; Coutinho, S.R.; Silva, M.N.; Mata, J.; Vieira, P.N.; Minderico, C.S.; Melanson, K.J.; Baptista, F.; Sardinha, L.B.; Teixeira, P.J. The effect of physical activity on weight loss is mediated by eating self-regulation. Patient Educ. Couns. 2010, 79, 320–326. [Google Scholar] [CrossRef]
- Arent, S.M.; Walker, A.J.; Arent, M.A. The Effects of Exercise on Anxiety and Depression. In Handbook of Sport Psychology, 4th ed.; Tennenbaum, G., Eklund, R.C., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 872–890. [Google Scholar]
- Carraça, E.V.; Encantado, J.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; van Baak, M.; Dicker, D.; Ermolao, A.; Farpour-Lambert, N.; et al. Effect of exercise training on psychological outcomes in adults with overweight or obesity: A systematic review and meta-analysis. Obes. Rev. 2021, 22 (Suppl. S4), e13261. [Google Scholar] [CrossRef]
- Annesi, J.J. Behavioral weight loss and maintenance: A 25-year research program informing innovative programming. Perm. J. 2002, 26, 98–117. [Google Scholar] [CrossRef]
- Caudwell, P.; Hopkins, M.; King, N.A.; Stubbs, R.J.; Blundell, J.E. Exercise alone is not enough: Weight loss also needs a healthy (Mediterranean) diet? Public Health Nutr. 2009, 12, 1663–1666. [Google Scholar] [CrossRef]
- Mann, T.; Tomiyama, A.J.; Westling, E.; Lew, A.M.; Samuels, B.; Chatman, J. Medicare’s search for effective obesity treatments: Diets are not the answer. Am. Psychol. 2007, 62, 220–233. [Google Scholar] [CrossRef]
- Baranowski, T.; Cerin, E.; Baranowski, J. Steps in the design, development and formative evaluation of obesity prevention-related behavior change trials. Int. J. Behav. Nutr. Phys. Act. 2009, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Rothman, A.J. “Is there nothing more practical than a good theory?”: Why innovations and advances in health behavior change will arise if interventions are used to test and refine theory. Int. J. Behav. Nutr. Phys. Act. 2004, 1, 11. [Google Scholar] [CrossRef]
- Bandura, A. Social Foundations of Thought and Action: A Social Cognitive Theory; Prentice Hall: Englewood Cliffs, NJ, USA, 1986. [Google Scholar]
- Baumeister, R.F.; Tice, D.M.; Vohs, K.D. The strength model of self-regulation: Conclusions from the second decade of willpower research. Perspect. Psychol. Sci. 2018, 13, 141–145. [Google Scholar] [CrossRef]
- Bandura, A. Self-Efficacy: The Exercise of Control; Freeman: New York, NY, USA, 1997. [Google Scholar]
- Mata, J.; Silva, M.N.; Vieira, P.N.; Carraça, E.V.; Andrade, A.M.; Coutinho, S.R.; Sardinha, L.B.; Teixeira, P.J. Motivational “spill-over” during weight control: Increased self-determination and exercise intrinsic motivation predict eating self-regulation. Health Psychol. 2009, 28, 709–716. [Google Scholar] [CrossRef]
- Oaten, M.; Cheng, K. Longitudinal gains in self-regulation from regular physical exercise. Br. J. Health Psychol. 2006, 11 Pt 4, 717–733. [Google Scholar] [CrossRef]
- Palmeira, A.L.; Sánchez-Oliva, D.; Encantado, J.; Marques, M.M.; Santos, I.; Duarte, C.; Matos, M.; Larsen, S.C.; Horgan, G.; Teixeira, P.J.; et al. Motivational and self-efficacy reciprocal effects during a 12-month weight regain prevention program. Br. J. Health Psychol. 2023, 28, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.S.; Paiva, A.L.; Mauriello, L.; Prochaska, J.O.; Redding, C.; Velicer, W.F. Coaction in multiple behavior change interventions: Consistency across multiple studies on weight management and obesity prevention. Health Psychol. 2014, 33, 475–480. [Google Scholar] [CrossRef]
- Anderson, E.S.; Wojcik, J.R.; Winett, R.A.; Williams, D.M. Social-cognitive determinants of physical activity: The influence of social support, self-efficacy, outcome expectations, and self-regulation among participants in a church-based health promotion study. Health Psychol. 2006, 25, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.S.; Winett, R.A.; Wojcik, J.R. Self-regulation, self-efficacy, outcome expectations, and social support: Social cognitive theory and nutrition behavior. Ann. Behav. Med. 2007, 34, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.J.; Carraça, E.V.; Marques, M.M.; Rutter, H.; Oppert, J.M.; De Bourdeaudhuij, I.; Lakerveld, J.; Brug, J. Successful behavior change in obesity interventions in adults: A systematic review of self-regulation mediators. BMC Med. 2015, 13, 84. [Google Scholar] [CrossRef]
- Te Morenga, L.; Mallard, S.; Mann, J. Dietary sugars and body weight: Systematic review and meta-analysis of randomized controlled trials and cohort studies. BMJ 2012, 346, e7492. [Google Scholar] [CrossRef] [PubMed]
- Arnow, B.; Kenardy, J.; Agras, W.S. The Emotional Eating Scale: The development of a measure to assess coping with negative affect by eating. Int. J. Eat. Disord. 1995, 18, 79–90. [Google Scholar] [CrossRef]
- Eldredge, K.L.; Agras, W.S. Weight and shape overconcern and emotional eating in binge eating disorder. Int. J. Eat. Disord. 1996, 19, 73–82. [Google Scholar] [CrossRef]
- Green, G.C.; Buckroyd, J. Disordered eating cognitions and behaviours among slimming organization competition winners. J. Hum. Nutr. Diet. 2008, 21, 31–38. [Google Scholar] [CrossRef]
- Waller, G.; Osman, S. Emotional eating and eating psychopathology among non-eating-disordered women. Int. J. Eat. Disord. 1998, 23, 419–424. [Google Scholar] [CrossRef]
- Annesi, J.J.; Marti, C.N. Path analysis of exercise treatment-induced changes in psychological factors leading to weight loss. Psychol. Health 2011, 26, 1081–1098. [Google Scholar] [CrossRef]
- Abraham, C.; Michie, S. A taxonomy of behavior change techniques used in interventions. Health Psychol. 2008, 27, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Ashford, S.; Sniehotta, F.F.; Dombrowski, S.U.; Bishop, A.; French, D.P. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: The CALO-RE taxonomy. Psychol. Health 2011, 26, 1479–1498. [Google Scholar] [CrossRef]
- Marcus, B.H.; Selby, V.C.; Niaura, R.S.; Rossi, J.S. Self-efficacy and the stages of exercise behavior change. Res. Q. Exerc. Sport. 1992, 63, 60–66. [Google Scholar] [CrossRef]
- Clark, M.M.; Abrams, D.B.; Niaura, R.S.; Eaton, C.A.; Rossi, J.S. Self-efficacy in weight management. J. Consult. Clin. Psychol. 1991, 59, 739–744. [Google Scholar] [CrossRef]
- Godin, G. The Godin-Shephard Leisure-Time Physical Activity Questionnaire. Health Fit. J. Can. 2011, 4, 18–22. [Google Scholar]
- Amireault, S.; Godin, G. The Godin-Shephard Leisure Time Physical Activity Questionnaire: Validity evidence supporting its use for classifying healthy adults into active and insufficiently active categories. Percept. Mot. Skil. 2015, 120, 604–622. [Google Scholar] [CrossRef] [PubMed]
- Amireault, S.; Godin, G.; Lacombe, J.; Sabiston, C.M. Validation of the Godin-Shephard Leisure-Time Physical Activity Questionnaire classification coding system using accelerometer assessment among breast cancer survivors. J. Cancer Surviv. 2015, 9, 532–540. [Google Scholar] [CrossRef]
- Jacobs, D.R.; Ainsworth, B.E.; Hartman, T.J.; Leon, A.S. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med. Sci. Sports Exerc. 1993, 25, 81–91. [Google Scholar] [CrossRef]
- Miller, D.J.; Freedson, P.S.; Kline, G.M. Comparison of activity levels using Caltrac accelerometer and five questionnaires. Med. Sci. Sports Exerc. 1994, 26, 376–382. [Google Scholar] [CrossRef]
- Pereira, M.A.; FitzGerald, S.J.; Gregg, E.W.; Joswiak, M.L.; Ryan, W.J.; Suminski, R.R.; Utter, A.C.; Zmuda, J.M. A collection of physical activity questionnaires for health-related research. Med. Sci. Sports Exerc. 1997, 29 (Suppl. S6), S1–S205. [Google Scholar] [PubMed]
- Sikes, E.M.; Richardson, E.V.; Cederberg, K.J.; Sasaki, J.E.; Sandroff, B.M.; Motl, R.W. Use of the Godin Leisure-Time Exercise Questionnaire in multiple sclerosis research: A comprehensive narrative review. Disabil. Rehabil. 2019, 41, 1243–1267. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. MyPlate and Historical Food Pyramid Resources; National Agricultural Library: Washington, DC, USA, 2017.
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th ed.; U.S. Department of Health and Human Services and U.S. Department of Agriculture: Washington, DC, USA. Available online: https://www.dietaryguidelines.gov/about-dietary-guidelines/previous-editions/2015-dietary-guidelines (accessed on 23 September 2025).
- Block, G.; Hartman, A.M.; Dresser, C.M.; Carroll, M.D.; Gannon, J.; Gardner, L. A data-based approach to diet questionnaire design and testing. Am. J. Epidemiol. 1986, 124, 453–469. [Google Scholar] [CrossRef]
- Mares-Perlman, J.A.; Klein, B.E.; Klein, R.; Ritter, L.L.; Fisher, M.R.; Freudenheim, J.L. A diet history questionnaire ranks nutrient intakes in middle-aged and older men and women similarly to multiple food records. J. Nutr. 1993, 123, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Annesi, J.J. Relationship of emotional eating and mood changes through self-regulation within three behavioral treatments for obesity. Psychol. Rep. 2019, 122, 1689–1706. [Google Scholar] [CrossRef]
- Warner, L.M.; Schwarzer, R. Self-Efficacy and Health. In Handbook of Concepts in Health, Health Behavior and Environmental Health; Liamputtong, P., Ed.; Springer Nature: New York, NY, USA, 2024; pp. 1–26. [Google Scholar]
- U.S. Department of Health and Human Services/Office of Disease Prevention and Health Promotion. Physical Activity Guidelines for Americans. Available online: https://odphp.health.gov/our-work/nutrition-physical-activity/physical-activity-guidelines (accessed on 15 August 2025).
- Hernández-Reyes, A.; Cámara-Martos, F.; Vidal, Á.; Molina-Luque, R.; Moreno-Rojas, R. Effects of self-weighing during weight loss treatment: A 6-month randomized controlled trial. Front. Psychol. 2020, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- White, I.R.; Horton, N.J.; Carpenter, J.; Pocock, S.J. Strategy for intention to treat data in randomized trials with missing outcome data. BMJ 2011, 342, d40. [Google Scholar] [CrossRef]
- McLachlan, G.J.; Krishnan, T. The EM Algorithm and Extensions; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Little, R.J.; Rubin, D.B. Statistical Analysis with Missing Data, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Cohen, J.; Cohen, P.; West, S.G.; Aiken, L.S. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences, 3rd ed.; Lawrence Erlbaum Publishers: Mahwah, NJ, USA, 2003. [Google Scholar]
- Verma, J.P. Repeated Measures Design for Empirical Researchers; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach, 3rd ed.; Guilford: New York, NY, USA, 2022. [Google Scholar]
- Des Jarlais, D.C.; Lyles, C.; Crepaz, N.; TREND Group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: The TREND statement. Am. J. Public Health 2004, 94, 361–366. [Google Scholar] [CrossRef]
- Williamson, D.A.; Bray, G.A.; Ryan, D.H. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity 2015, 23, 2319–2320. [Google Scholar] [CrossRef]
- Hu, F.B. Resolved: There is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes. Rev. 2013, 14, 606–619. [Google Scholar] [CrossRef]
- Dansinger, M.L.; Gleason, J.A.; Griffith, J.L.; Selker, H.P.; Schaefer, E.J. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: A randomized trial. JAMA 2005, 293, 43–53. [Google Scholar] [CrossRef]
- Freire, R. Scientific evidence of diets for weight loss: Different macronutrient composition, intermittent fasting, and popular diets. Nutrition 2020, 69, 110549. [Google Scholar] [CrossRef]
- Swinburn, B.; Sacks, G.; Ravussin, E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am. J. Clin. Nutr. 2009, 90, 1453–1456. [Google Scholar] [CrossRef]
- Johns, D.J.; Hartmann-Boyce, J.; Jebb, S.A.; Aveyard, P.; Behavioural Weight Management Review Group. Diet or exercise interventions vs combined behavioral weight management programs: A systematic review and meta-analysis of direct comparisons. J. Acad. Nutr. Diet. 2014, 114, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Dakanalis, A.; Mentzelou, M.; Papadopoulou, S.K.; Papandreou, D.; Spanoudaki, M.; Vasios, G.K.; Pavlidou, E.; Mantzorou, M.; Giaginis, C. The association of emotional eating with overweight/obesity, depression, anxiety/stress, and dietary patterns: A review of the current clinical evidence. Nutrients 2023, 15, 1173. [Google Scholar] [CrossRef] [PubMed]
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22 (Suppl. S7), S176–S185. [Google Scholar] [PubMed]
| Baseline | Month 3 | Month 6 | Month 12 | Group × Time Contrasts | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grouping | M | SD | M | SD | M | SD | M | SD | F(1, 104) | p | η2partial |
| Self-regulation-merged | |||||||||||
| Low emotional eating | 1.90 | 0.42 | 2.57 | 0.29 | |||||||
| High emotional eating | 1.81 | 0.43 | 2.52 | 0.36 | |||||||
| Aggregated data | 1.86 | 0.43 | 2.55 | 0.32 | 0.18 | 0.672 | 0.00 | ||||
| Self-efficacy-merged | |||||||||||
| Low emotional eating | 5.16 | 1.28 | 6.15 | 1.19 | |||||||
| High emotional eating | 4.41 | 1.61 | 6.19 | 1.64 | |||||||
| Aggregated data | 4.78 | 1.50 | 6.17 | 1.56 | 6.15 | 0.015 | 0.06 | ||||
| Exercise self-efficacy | |||||||||||
| Low emotional eating | 5.28 | 1.69 | 6.71 | 2.05 | |||||||
| High emotional eating | 5.01 | 2.00 | 7.01 | 2.09 | |||||||
| Aggregated data | 5.14 | 1.85 | 6.86 | 2.06 | 1.73 | 0.191 | 0.02 | ||||
| Exercise self-regulation | |||||||||||
| Low emotional eating | 1.90 | 0.48 | 2.68 | 0.25 | |||||||
| High emotional eating | 1.72 | 0.51 | 2.60 | 0.41 | |||||||
| Aggregated data | 1.81 | 0.50 | 2.64 | 0.34 | 0.82 | 0.367 | 0.01 | ||||
| Eating self-efficacy | |||||||||||
| Low emotional eating | 5.04 | 1.24 | 6.35 | 1.46 | |||||||
| High emotional eating | 3.80 | 1.55 | 6.19 | 1.19 | |||||||
| Aggregated data | 4.41 | 1.53 | 6.27 | 1.33 | 11.30 | 0.001 | 0.10 | ||||
| Eating self-regulation | |||||||||||
| Low emotional eating | 1.90 | 0.45 | 2.60 | 0.28 | |||||||
| High emotional eating | 1.91 | 0.43 | 2.58 | 0.35 | |||||||
| Aggregated data | 1.90 | 0.44 | 2.59 | 0.32 | 0.09 | 0.764 | 0.00 | ||||
| Exercise (METs/week) | |||||||||||
| Low emotional eating | 10.60 | 6.71 | 33.66 | 14.58 | |||||||
| High emotional eating | 6.52 | 7.26 | 31.91 | 16.49 | |||||||
| Aggregated data | 8.52 | 7.26 | 32.77 | 15.53 | 0.59 | 0.443 | 0.01 | ||||
| Sweets (portions/day) | |||||||||||
| Low emotional eating | 1.63 | 1.09 | 1.12 | 0.81 | |||||||
| High emotional eating | 2.65 | 2.04 | 1.06 | 0.82 | |||||||
| Aggregated data | 2.15 | 1.71 | 1.08 | 0.81 | 13.68 | <0.001 | 0.12 | ||||
| Fruit/vegetable (portions/day) | |||||||||||
| Low emotional eating | 4.12 | 2.09 | 6.09 | 2.29 | |||||||
| High emotional eating | 3.76 | 1.81 | 6.57 | 1.97 | |||||||
| Aggregated data | 3.93 | 1.95 | 6.33 | 2.14 | 3.54 | 0.063 | 0.03 | ||||
| Weight (kg) | |||||||||||
| Low emotional eating | 95.19 | 11.54 | 89.50 | 11.66 | 90.37 | 11.51 | |||||
| High emotional eating | 94.55 | 11.74 | 88.62 | 11.78 | 88.30 | 12.39 | |||||
| Aggregated data | 94.86 | 11.59 | 89.05 | 11.68 | 89.32 | 11.95 | 2.66 | 0.106 | 0.03 | ||
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. ΔSelf-regulation-merged, baseline-Month 3 | --- | |||||||||
| 2. ΔSelf-efficacy-merged, baseline-Month 3 | 0.67 † | --- | ||||||||
| 3. ΔExercise self-efficacy, baseline-Month 6 | 0.59 † | 0.77 † | --- | |||||||
| 4. ΔExercise self-regulation, baseline-Month 6 | 0.79 † | 0.49 † | 0.58 † | --- | ||||||
| 5. ΔEating self-efficacy, baseline-Month 6 | 0.57 † | 0.73 † | 0.62 † | 0.50 † | --- | |||||
| 6. ΔEating self-regulation, baseline-Month | 0.78 † | 0.46 † | 0.50 † | 0.73 † | 0.49 † | --- | ||||
| 7. ΔExercise, baseline-Month 6 | 0.55 † | 0.50 † | 0.62 † | 0.59 † | 0.56 † | 0.52 † | --- | |||
| 8. ΔSweets intake, baseline-Month 6 | −0.02 | −0.22 * | −0.19 * | −0.07 | −0.25 * | −0.17 | −0.26 ** | --- | ||
| 9. ΔWeight, baseline-Month 6 | −0.45 † | −0.36 † | −0.36 † | −0.43 † | −0.35 † | −0.34 † | −0.40 † | 0.39 † | --- | |
| 10. ΔWeight, baseline-Month 12 | −0.38 † | −0.28 ** | −0.31 ** | −0.40 † | −0.29 ** | −0.27 ** | −0.37 † | 0.44 † | 0.92 † | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annesi, J.J. Directional Effects of Self-Regulation and Self-Efficacy Changes Within a Weight-Loss Treatment Focused on Exercise and Sweets Consumption: Accounting for Emotional Eating in Women with Obesity. Nutrients 2025, 17, 3048. https://doi.org/10.3390/nu17193048
Annesi JJ. Directional Effects of Self-Regulation and Self-Efficacy Changes Within a Weight-Loss Treatment Focused on Exercise and Sweets Consumption: Accounting for Emotional Eating in Women with Obesity. Nutrients. 2025; 17(19):3048. https://doi.org/10.3390/nu17193048
Chicago/Turabian StyleAnnesi, James J. 2025. "Directional Effects of Self-Regulation and Self-Efficacy Changes Within a Weight-Loss Treatment Focused on Exercise and Sweets Consumption: Accounting for Emotional Eating in Women with Obesity" Nutrients 17, no. 19: 3048. https://doi.org/10.3390/nu17193048
APA StyleAnnesi, J. J. (2025). Directional Effects of Self-Regulation and Self-Efficacy Changes Within a Weight-Loss Treatment Focused on Exercise and Sweets Consumption: Accounting for Emotional Eating in Women with Obesity. Nutrients, 17(19), 3048. https://doi.org/10.3390/nu17193048






