Lactiplantibacillus plantarum as a Psychobiotic Strategy Targeting Parkinson’s Disease: A Review and Mechanistic Insights
Abstract
1. Introduction
1.1. Overview of Parkinson’s Disease: Epidemiology, Clinical Features, and Current Therapeutic Challenges
1.2. Emergence of the Microbiota–Gut–Brain Axis and the Therapeutic Potential of Lactiplantibacillus plantarum on Parkinson’s Disease
2. Preclinical Evidence Supporting the Functional Role of Lactiplantibacillus plantarum in Parkinson’s Disease
2.1. Preclinical Animal Models for Elucidating Microbiota-Gut–Brain Axis Mechanisms in Parkinson’s Disease
2.2. Multifaceted Neuroprotective Effects of Lactiplantibacillus plantarum in Rodent Models of Parkinson’s Disease
3. Strain-Specific Clinical Efficacy of Lactiplantibacillus plantarum PS128 in Parkinson’s Disease
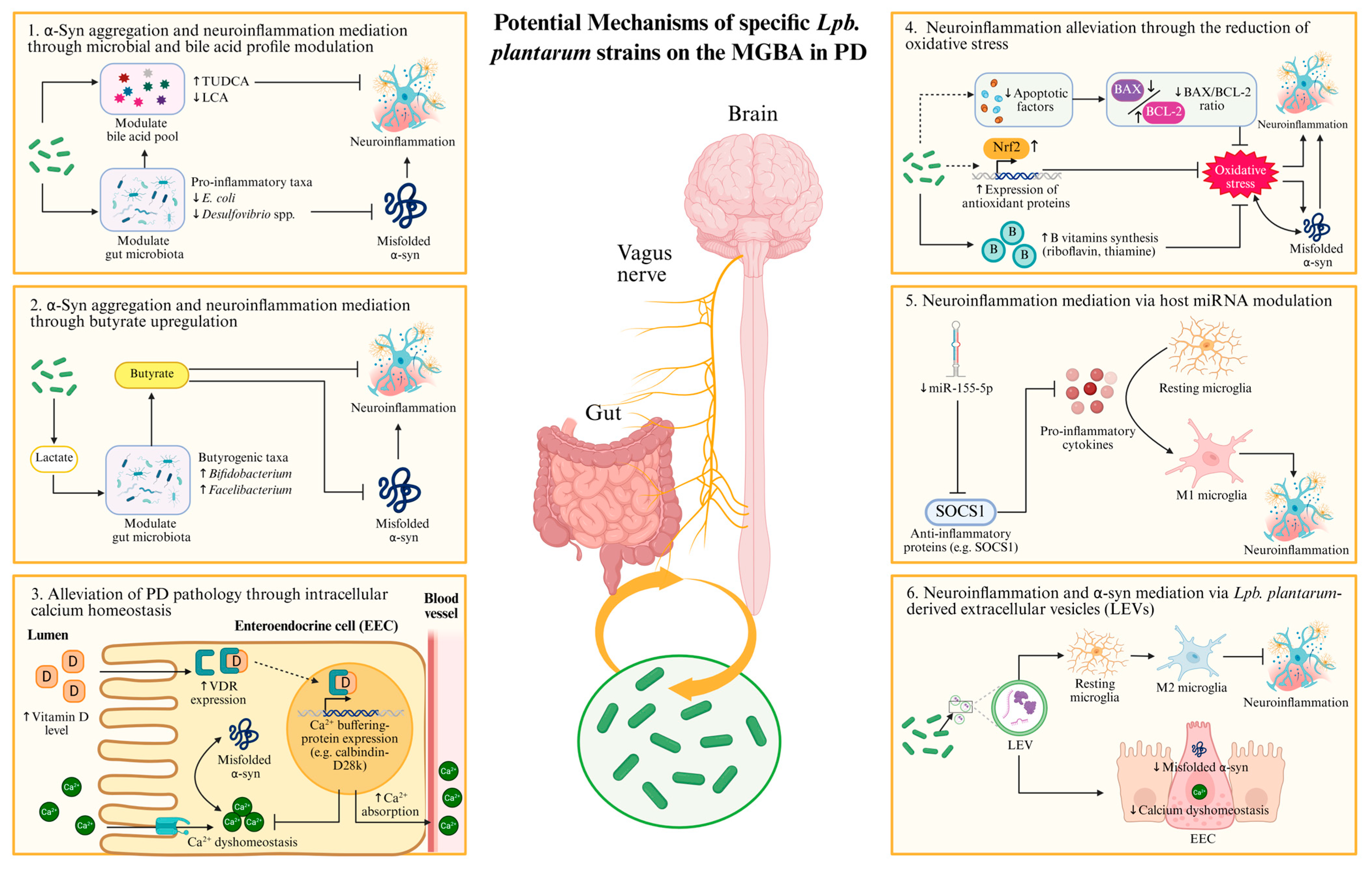
4. Possible Molecular Mechanisms of Lactiplantibacillus plantarum on the Hallmark Pathologies of Parkinson’s Disease
4.1. Gut-Origin Hypothesis of α-Synuclein Pathology and Its Implications for Parkinson’s Disease Intervention
4.2. Mechanisms of Lactiplantibacillus plantarum Against Parkinson’s Disease
4.2.1. Modulation of Gut Microbiota and Bile Acid Signaling by Lactiplantibacillus plantarum: Implications for α-Synuclein Pathology
4.2.2. Butyrate-Mediated Modulation of α-Synuclein Aggregation and the Role of Lactiplantibacillus plantarum in Butyrogenesis
4.2.3. Probiotic Modulation of Intracellular Calcium Homeostasis as a Strategy to Alleviate α-Synuclein Pathology in Parkinson’s Disease
4.2.4. Lactiplantibacillus plantarum-Mediated Regulation of Oxidative Stress in Parkinson’s Disease
4.2.5. Involvement of MicroRNAs in Lactiplantibacillus plantarum-Mediated Attenuation of Parkinsonian Pathology
5. Lactiplantibacillus plantarum-Derived Extracellular Vesicles: A Novel Mediator in Microbiota-Gut–Brain Axis and α-Synuclein Pathology
6. Future Challenges and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HT | 5-hydroxytryptamine |
| 6-OHDA | 6-hydroxydopamine |
| BA | Bile acid |
| BBB | Blood–brain barrier |
| Bcl-2 | B-cell lymphoma 2 |
| BDNF | Brain-derived neurotrophic factor |
| BSH | Bile salt hydrolase |
| CNS | Central nervous system |
| DA | Dopamine |
| DOPAC | 3,4-dihydroxyphenylacetic acid |
| EEC | Enteroendocrine cells |
| ENS | Enteric nervous system |
| EV | Extracellular vesicles |
| GFAP | Glial fibrillary acidic protein |
| GI | Gastrointestinal |
| GSH | Glutathione peroxide |
| Iba1 | Ionized calcium-binding adaptor molecule 1 |
| IFN-γ | Interferon-gamma |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| iNOS | Inducible nitric oxide synthase |
| LCA | Lithocholic acid |
| L-DOPA | Levodopa |
| LEVs | Lactiplantibacillus plantarum-derived EVs |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDA | Malondialdehyde |
| MGBA | Microbiota-gut–brain axis |
| miRNA | Micro RNAs |
| MPTP | N-methyl-4-phenyl-l,2,3,6-tetrahydropyridine |
| NA | Noradrenaline |
| NBT | Narrow beam test |
| NGF | Nerve growth factor |
| NLRP3 | NLR family pyrin domain containing 3 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| OFT | Open field test |
| PD | Parkinson’s disease |
| PDQ39 | 39-item Parkinson’s Disease Questionnaire |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PGI-C | Patient Global Impression of Change |
| ROS | Reactive oxygen species |
| RTR | Rotarod test |
| SCFA | Short-chain fatty acid |
| SNpc | Substantia nigra pars compacta |
| SOCS1 | Suppressor of cytokine signaling 1 |
| SOD | Superoxide dismutase |
| STR | Striatum |
| TH | Tyrosine hydroxylase |
| TNF-α | Tumor necrosis factor-alpha |
| TUDCA | Tauroursodeoxycholic acid |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| PT | Pole test |
| ZO-1 | Zonula occludens-1 |
| α-syn | Alpha-synuclein |
| β-PSD | Power spectral density of beta oscillations |
References
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef]
- Luo, Y.; Qiao, L.; Li, M.; Wen, X.; Zhang, W.; Li, X. Global, regional, national epidemiology and trends of Parkinson’s disease from 1990 to 2021: Findings from the Global Burden of Disease Study 2021. Front. Aging Neurosci. 2024, 16, 1498756. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hamilton, J.L.; Kopil, C.; Beck, J.C.; Tanner, C.M.; Albin, R.L.; Ray Dorsey, E.; Dahodwala, N.; Cintina, I.; Hogan, P.; et al. Current and projected future economic burden of Parkinson’s disease in the U.S. NPJ Park. Dis. 2020, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Pavelka, L.; Rawal, R.; Sapienza, S.; Klucken, J.; Pauly, C.; Satagopam, V.; Kruger, R. Genetically stratified Parkinson’s disease with freezing of gait is related to specific pattern of cognitive impairment and non-motor dominant endophenotype. Front. Aging Neurosci. 2024, 16, 1479572. [Google Scholar] [CrossRef]
- Pena-Zelayeta, L.; Delgado-Minjares, K.M.; Villegas-Rojas, M.M.; Leon-Arcia, K.; Santiago-Balmaseda, A.; Andrade-Guerrero, J.; Perez-Segura, I.; Ortega-Robles, E.; Soto-Rojas, L.O.; Arias-Carrion, O. Redefining Non-Motor Symptoms in Parkinson’s Disease. J. Pers. Med. 2025, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.; Lim, J.; Choi, H.J. Recent advances in the pathology of prodromal non-motor symptoms olfactory deficit and depression in Parkinson’s disease: Clues to early diagnosis and effective treatment. Arch. Pharm. Res. 2021, 44, 588–604. [Google Scholar] [CrossRef]
- Goldman, J.G.; Postuma, R. Premotor and nonmotor features of Parkinson’s disease. Curr. Opin. Neurol. 2014, 27, 434–441. [Google Scholar] [CrossRef]
- Kumaresan, M.; Khan, S. Spectrum of Non-Motor Symptoms in Parkinson’s Disease. Cureus 2021, 13, e13275. [Google Scholar] [CrossRef]
- Surmeier, D.J. Determinants of dopaminergic neuron loss in Parkinson’s disease. FEBS J. 2018, 285, 3657–3668. [Google Scholar] [CrossRef]
- Galvan, A.; Devergnas, A.; Wichmann, T. Alterations in neuronal activity in basal ganglia-thalamocortical circuits in the parkinsonian state. Front. Neuroanat. 2015, 9, 5. [Google Scholar] [CrossRef]
- Salvade, M.; DiLuca, M.; Gardoni, F. An update on drug repurposing in Parkinson’s disease: Preclinical and clinical considerations. Biomed. Pharmacother. 2025, 183, 117862. [Google Scholar] [CrossRef]
- Stoker, T.B.; Greenland, J.C. (Eds.) Parkinson’s Disease: Pathogenesis and Clinical Aspects; Codon Publications: Brisbane, AU, USA, 2018. [Google Scholar] [CrossRef]
- Lee, H.; Elkamhawy, A.; Rakhalskaya, P.; Lu, Q.; Nada, H.; Quan, G.; Lee, K. Small Molecules in Parkinson’s Disease Therapy: From Dopamine Pathways to New Emerging Targets. Pharmaceuticals 2024, 17, 1688. [Google Scholar] [CrossRef]
- Fabbri, M.; Ferreira, J.J.; Rascol, O. COMT Inhibitors in the Management of Parkinson’s Disease. CNS Drugs 2022, 36, 261–282. [Google Scholar] [CrossRef]
- Choi, J.; Horner, K.A. Dopamine Agonists. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Zheng, C.; Zhang, F. New insights into pathogenesis of l-DOPA-induced dyskinesia. Neurotoxicology 2021, 86, 104–113. [Google Scholar] [CrossRef]
- Binda, S.; Tremblay, A.; Iqbal, U.H.; Kassem, O.; Le Barz, M.; Thomas, V.; Bronner, S.; Perrot, T.; Ismail, N.; Parker, J.A. Psychobiotics and the Microbiota-Gut-Brain Axis: Where Do We Go from Here? Microorganisms 2024, 12, 634. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, B.; Guo, J. Parkinson’s disease and gut microbiota: From clinical to mechanistic and therapeutic studies. Transl. Neurodegener. 2023, 12, 59. [Google Scholar] [CrossRef]
- Yang, D.; Zhao, D.; Ali Shah, S.Z.; Wu, W.; Lai, M.; Zhang, X.; Li, J.; Guan, Z.; Zhao, H.; Li, W.; et al. The Role of the Gut Microbiota in the Pathogenesis of Parkinson’s Disease. Front. Neurol. 2019, 10, 1155. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef]
- Zhu, X.; Han, Y.; Du, J.; Liu, R.; Jin, K.; Yi, W. Microbiota-gut-brain axis and the central nervous system. Oncotarget 2017, 8, 53829–53838. [Google Scholar] [CrossRef]
- Ashique, S.; Mohanto, S.; Ahmed, M.G.; Mishra, N.; Garg, A.; Chellappan, D.K.; Omara, T.; Iqbal, S.; Kahwa, I. Gut-brain axis: A cutting-edge approach to target neurological disorders and potential synbiotic application. Heliyon 2024, 10, e34092. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef]
- Abou Izzeddine, N.; Ahmad, K.; Bacha, C.; Jabbour, M.; Najjar, M.; Salhab, S.; Ghadieh, H.E.; Kanaan, A.; Azar, S.; Khattar, Z.A.; et al. The microbial guardians: Unveiling the role of gut microbiota in shaping neurodegenerative disease. IBRO Neurosci. Rep. 2025, 19, 17–37. [Google Scholar] [CrossRef]
- Dhyani, P.; Goyal, C.; Dhull, S.B.; Chauhan, A.K.; Singh Saharan, B.; Harshita; Duhan, J.S.; Goksen, G. Psychobiotics for Mitigation of Neuro-Degenerative Diseases: Recent Advancements. Mol. Nutr. Food Res. 2024, 68, e2300461. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Dziedzic, A.; Maciak, K.; Blizniewska-Kowalska, K.; Galecka, M.; Kobierecka, W.; Saluk, J. The Power of Psychobiotics in Depression: A Modern Approach through the Microbiota-Gut-Brain Axis: A Literature Review. Nutrients 2024, 16, 1054. [Google Scholar] [CrossRef]
- Kyei-Baffour, V.O.; Vijaya, A.K.; Burokas, A.; Daliri, E.B. Psychobiotics and the gut-brain axis: Advances in metabolite quantification and their implications for mental health. Crit. Rev. Food Sci. Nutr. 2025, 1–20. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef]
- Chatsirisakul, O.; Leenabanchong, N.; Siripaopradit, Y.; Chang, C.W.; Buhngamongkol, P.; Pongpirul, K. Strain-Specific Therapeutic Potential of Lactiplantibacillus plantarum: A Systematic Scoping Review. Nutrients 2025, 17, 1165. [Google Scholar] [CrossRef]
- Beltran-Velasco, A.I.; Reiriz, M.; Uceda, S.; Echeverry-Alzate, V. Lactiplantibacillus (Lactobacillus) plantarum as a Complementary Treatment to Improve Symptomatology in Neurodegenerative Disease: A Systematic Review of Open Access Literature. Int. J. Mol. Sci. 2024, 25, 3010. [Google Scholar] [CrossRef]
- Qi, Y.; Dong, Y.; Chen, J.; Xie, S.; Ma, X.; Yu, X.; Yu, Y.; Wang, Y. Lactiplantibacillus plantarum SG5 inhibits neuroinflammation in MPTP-induced PD mice through GLP-1/PGC-1alpha pathway. Exp. Neurol. 2025, 383, 115001. [Google Scholar] [CrossRef]
- Lee, Y.Z.; Cheng, S.H.; Chang, M.Y.; Lin, Y.F.; Wu, C.C.; Tsai, Y.C. Neuroprotective Effects of Lactobacillus plantarum PS128 in a Mouse Model of Parkinson’s Disease: The Role of Gut Microbiota and MicroRNAs. Int. J. Mol. Sci. 2023, 24, 6794. [Google Scholar] [CrossRef]
- Chu, C.; Yu, L.; Li, Y.; Guo, H.; Zhai, Q.; Chen, W.; Tian, F. Lactobacillus plantarum CCFM405 against Rotenone-Induced Parkinson’s Disease Mice via Regulating Gut Microbiota and Branched-Chain Amino Acids Biosynthesis. Nutrients 2023, 15, 1737. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Z.; Zhao, L.; Zhao, Y.; Yang, G.; Wang, C.; Gao, L.; Niu, C.; Li, S. Lactobacillus plantarum DP189 Reduces alpha-SYN Aggravation in MPTP-Induced Parkinson’s Disease Mice via Regulating Oxidative Damage, Inflammation, and Gut Microbiota Disorder. J. Agric. Food Chem. 2022, 70, 1163–1173. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Jiang, Y.; Zhao, Z.; Shen, Y.; Zhang, J.; Zhao, L. Neuroprotective effect of Lactobacillus plantarum DP189 on MPTP-induced Parkinson’s disease model mice. J. Funct. Foods 2021, 85, 104635. [Google Scholar] [CrossRef]
- Wong, H.H.; Chou, C.Y.C.; Watt, A.J.; Sjostrom, P.J. Comparing mouse and human brains. eLife 2023, 12, e90017. [Google Scholar] [CrossRef]
- Barker, R.A.; Bjorklund, A. Animal Models of Parkinson’s Disease: Are They Useful or Not? J. Park. Dis. 2020, 10, 1335–1342. [Google Scholar] [CrossRef]
- Gonzales, N.M.; Seo, J.; Hernandez Cordero, A.I.; St Pierre, C.L.; Gregory, J.S.; Distler, M.G.; Abney, M.; Canzar, S.; Lionikas, A.; Palmer, A.A. Genome wide association analysis in a mouse advanced intercross line. Nat. Commun. 2018, 9, 5162. [Google Scholar] [CrossRef]
- He, S.; Ru, Q.; Chen, L.; Xu, G.; Wu, Y. Advances in animal models of Parkinson’s disease. Brain Res. Bull. 2024, 215, 111024. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, X.; Ye, Y.; Yan, X.; Cheng, Y.; Zhao, L.; Chen, F.; Ling, Z. Gut Microbiota: A Novel Therapeutic Target for Parkinson’s Disease. Front. Immunol. 2022, 13, 937555. [Google Scholar] [CrossRef] [PubMed]
- Duty, S.; Jenner, P. Animal models of Parkinson’s disease: A source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 2011, 164, 1357–1391. [Google Scholar] [CrossRef]
- Giunti, S.; Andersen, N.; Rayes, D.; De Rosa, M.J. Drug discovery: Insights from the invertebrate Caenorhabditis elegans. Pharmacol. Res. Perspect. 2021, 9, e00721. [Google Scholar] [CrossRef] [PubMed]
- Pandey, U.B.; Nichols, C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef]
- Segalat, L. Invertebrate animal models of diseases as screening tools in drug discovery. ACS Chem. Biol. 2007, 2, 231–236. [Google Scholar] [CrossRef]
- Chia, S.J.; Tan, E.K.; Chao, Y.X. Historical Perspective: Models of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 2464. [Google Scholar] [CrossRef]
- Cooper, J.F.; Van Raamsdonk, J.M. Modeling Parkinson’s Disease in C. elegans. J. Park. Dis. 2018, 8, 17–32. [Google Scholar] [CrossRef]
- Shukla, A.K.; Pragya, P.; Chaouhan, H.S.; Patel, D.K.; Abdin, M.Z.; Kar Chowdhuri, D. Mutation in Drosophila methuselah resists paraquat induced Parkinson-like phenotypes. Neurobiol. Aging 2014, 35, 2419.e1–2419.e16. [Google Scholar] [CrossRef]
- Menegola, E.; Battistoni, M.; Metruccio, F.; Di Renzo, F. Advantages and disadvantages of the use of Xenopus laevis embryos and zebra fish as alternative methods to assess teratogens. Curr. Opin. Toxicol. 2023, 34, 100387. [Google Scholar] [CrossRef]
- Chia, K.; Klingseisen, A.; Sieger, D.; Priller, J. Zebrafish as a model organism for neurodegenerative disease. Front. Mol. Neurosci. 2022, 15, 940484. [Google Scholar] [CrossRef]
- Bailone, R.L.; Fukushima, H.C.S.; Ventura Fernandes, B.H.; De Aguiar, L.K.; Correa, T.; Janke, H.; Grejo Setti, P.; Roca, R.O.; Borra, R.C. Zebrafish as an alternative animal model in human and animal vaccination research. Lab. Anim. Res. 2020, 36, 13. [Google Scholar] [CrossRef]
- Vaz, R.; Hofmeister, W.; Lindstrand, A. Zebrafish Models of Neurodevelopmental Disorders: Limitations and Benefits of Current Tools and Techniques. Int. J. Mol. Sci. 2019, 20, 1296. [Google Scholar] [CrossRef] [PubMed]
- Sheta, R.; Berard, M.; Musiol, D.; Martinez-Drudis, L.; Oueslati, A. Behavioral analysis of motor and non-motor impairment in rodent models of Parkinson’s disease. Front. Aging Neurosci. 2024, 16, 1464706. [Google Scholar] [CrossRef]
- Guimaraes, R.P.; Resende, M.C.S.; Tavares, M.M.; Belardinelli de Azevedo, C.; Ruiz, M.C.M.; Mortari, M.R. Construct, Face, and Predictive Validity of Parkinson’s Disease Rodent Models. Int. J. Mol. Sci. 2024, 25, 8971. [Google Scholar] [CrossRef]
- Day, J.O.; Mullin, S. The Genetics of Parkinson’s Disease and Implications for Clinical Practice. Genes 2021, 12, 1006. [Google Scholar] [CrossRef]
- Yamakado, H.; Takahashi, R. Experimental Animal Models of Prodromal Parkinson’s Disease. J. Park. Dis. 2024, 14, S369–S379. [Google Scholar] [CrossRef]
- Dawson, T.M.; Ko, H.S.; Dawson, V.L. Genetic animal models of Parkinson’s disease. Neuron 2010, 66, 646–661. [Google Scholar] [CrossRef]
- Zeng, X.S.; Geng, W.S.; Jia, J.J. Neurotoxin-Induced Animal Models of Parkinson Disease: Pathogenic Mechanism and Assessment. ASN Neuro 2018, 10, 1759091418777438. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Ibarra-Gutierrez, M.T.; Serrano-Garcia, N.; Orozco-Ibarra, M. Rotenone-Induced Model of Parkinson’s Disease: Beyond Mitochondrial Complex I Inhibition. Mol. Neurobiol. 2023, 60, 1929–1948. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.E.; Stringer, A.; Bobrovskaya, L. Rotenone induces gastrointestinal pathology and microbiota alterations in a rat model of Parkinson’s disease. Neurotoxicology 2018, 65, 174–185. [Google Scholar] [CrossRef]
- Niederberger, E.; Wilken-Schmitz, A.; Manderscheid, C.; Schreiber, Y.; Gurke, R.; Tegeder, I. Non-Reproducibility of Oral Rotenone as a Model for Parkinson’s Disease in Mice. Int. J. Mol. Sci. 2022, 23, 2658. [Google Scholar] [CrossRef]
- Mustapha, M.; Mat Taib, C.N. MPTP-induced mouse model of Parkinson’s disease: A promising direction of therapeutic strategies. Bosn. J. Basic. Med. Sci. 2021, 21, 422–433. [Google Scholar] [CrossRef]
- Wichmann, T.; Nelson, A.; Torres, E.R.S.; Svenningsson, P.; Marongiu, R. Leveraging animal models to understand non-motor symptoms of Parkinson’s disease. Neurobiol. Dis. 2025, 208, 106848. [Google Scholar] [CrossRef]
- Liao, J.F.; Cheng, Y.F.; You, S.T.; Kuo, W.C.; Huang, C.W.; Chiou, J.J.; Hsu, C.C.; Hsieh-Li, H.M.; Wang, S.; Tsai, Y.C. Lactobacillus plantarum PS128 alleviates neurodegenerative progression in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse models of Parkinson’s disease. Brain Behav. Immun. 2020, 90, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Teran, M.D.M.; Perez Visnuk, D.; Savoy de Giori, G.; de Moreno de LeBlanc, A.; LeBlanc, J.G. Neuroprotective effect of thiamine-producing lactic acid bacteria in a murine Parkinsonian model. Food Funct. 2022, 13, 8056–8067. [Google Scholar] [CrossRef]
- Ma, Y.F.; Lin, Y.A.; Huang, C.L.; Hsu, C.C.; Wang, S.; Yeh, S.R.; Tsai, Y.C. Lactiplantibacillus plantarum PS128 Alleviates Exaggerated Cortical Beta Oscillations and Motor Deficits in the 6-Hydroxydopamine Rat Model of Parkinson’s Disease. Probiotics Antimicrob. Proteins 2023, 15, 312–325. [Google Scholar] [CrossRef]
- Napoles-Medina, A.Y.; Aguilar-Uscanga, B.R.; Solis-Pacheco, J.R.; Tejeda-Martinez, A.R.; Ramirez-Jirano, L.J.; Urmeneta-Ortiz, M.F.; Chaparro-Huerta, V.; Flores-Soto, M.E. Oral Administration of Lactobacillus Inhibits the Permeability of Blood-Brain and Gut Barriers in a Parkinsonism Model. Behav. Neurol. 2023, 2023, 6686037. [Google Scholar] [CrossRef]
- Shahin, N.N.; Ahmed-Farid, O.A.; Sakr, E.A.E.; Kamel, E.A.; Mohamed, M.M. Oral Supplements of Combined Lactobacillus plantarum and Asparagus officinalis Modulate Gut Microbiota and Alleviate High-Fat Diet-Induced Cognitive Deficits and Neurodegeneration in Rats. Probiotics Antimicrob. Proteins 2025. [Google Scholar] [CrossRef] [PubMed]
- Ghalandari, N.; Assarzadegan, F.; Habibi, S.A.H.; Esmaily, H.; Malekpour, H. Efficacy of Probiotics in Improving Motor Function and Alleviating Constipation in Parkinson’s Disease: A Randomized Controlled Trial. Iran. J. Pharm. Res. 2023, 22, e137840. [Google Scholar] [CrossRef]
- Barichella, M.; Pacchetti, C.; Bolliri, C.; Cassani, E.; Iorio, L.; Pusani, C.; Pinelli, G.; Privitera, G.; Cesari, I.; Faierman, S.A.; et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology 2016, 87, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.S.; Chang, H.C.; Weng, Y.H.; Chen, C.C.; Kuo, Y.S.; Tsai, Y.C. The Add-On Effect of Lactobacillus plantarum PS128 in Patients with Parkinson’s Disease: A Pilot Study. Front. Nutr. 2021, 8, 650053. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Di Lazzaro, G.; Ghiglieri, V. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: From overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 2023, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Lashuel, H.A.; Overk, C.R.; Oueslati, A.; Masliah, E. The many faces of alpha-synuclein: From structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 2013, 14, 38–48. [Google Scholar] [CrossRef]
- Stefanis, L. alpha-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef]
- Vishwanathan Padmaja, M.; Jayaraman, M.; Srinivasan, A.V.; Srikumari Srisailapathy, C.R.; Ramesh, A. The SNCA (A53T, A30P, E46K) and LRRK2 (G2019S) mutations are rare cause of Parkinson’s disease in South Indian patients. Park. Relat. Disord. 2012, 18, 801–802. [Google Scholar] [CrossRef]
- Jia, F.; Fellner, A.; Kumar, K.R. Monogenic Parkinson’s Disease: Genotype, Phenotype, Pathophysiology, and Genetic Testing. Genes 2022, 13, 471. [Google Scholar] [CrossRef]
- Pickrell, A.M.; Youle, R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015, 85, 257–273. [Google Scholar] [CrossRef]
- Rakovic, A.; Grunewald, A.; Kottwitz, J.; Bruggemann, N.; Pramstaller, P.P.; Lohmann, K.; Klein, C. Mutations in PINK1 and Parkin impair ubiquitination of Mitofusins in human fibroblasts. PLoS ONE 2011, 6, e16746. [Google Scholar] [CrossRef]
- Ariga, H.; Takahashi-Niki, K.; Kato, I.; Maita, H.; Niki, T.; Iguchi-Ariga, S.M. Neuroprotective function of DJ-1 in Parkinson’s disease. Oxid. Med. Cell Longev. 2013, 2013, 683920. [Google Scholar] [CrossRef]
- Shendelman, S.; Jonason, A.; Martinat, C.; Leete, T.; Abeliovich, A. DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol. 2004, 2, e362. [Google Scholar] [CrossRef] [PubMed]
- Madureira, M.; Connor-Robson, N.; Wade-Martins, R. “LRRK2: Autophagy and Lysosomal Activity”. Front. Neurosci. 2020, 14, 498. [Google Scholar] [CrossRef]
- Wallings, R.; Manzoni, C.; Bandopadhyay, R. Cellular processes associated with LRRK2 function and dysfunction. FEBS J. 2015, 282, 2806–2826. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Scheper, W.; Hoozemans, J.J. The unfolded protein response in neurodegenerative diseases: A neuropathological perspective. Acta Neuropathol. 2015, 130, 315–331. [Google Scholar] [CrossRef]
- Kroemer, G.; Marino, G.; Levine, B. Autophagy and the integrated stress response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef]
- Wong, Y.C.; Krainc, D. alpha-synuclein toxicity in neurodegeneration: Mechanism and therapeutic strategies. Nat. Med. 2017, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kwon, S.H.; Kam, T.I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.H.; Kim, W.R.; Kook, M.; Foss, C.A.; et al. Transneuronal Propagation of Pathologic alpha-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103, 627–641.e7. [Google Scholar] [CrossRef] [PubMed]
- Visanji, N.P.; Brooks, P.L.; Hazrati, L.N.; Lang, A.E. The prion hypothesis in Parkinson’s disease: Braak to the future. Acta Neuropathol. Commun. 2013, 1, 2. [Google Scholar] [CrossRef]
- Vascellari, S.; Orru, C.D.; Groveman, B.R.; Parveen, S.; Fenu, G.; Pisano, G.; Piga, G.; Serra, G.; Oppo, V.; Murgia, D.; et al. alpha-Synuclein seeding activity in duodenum biopsies from Parkinson’s disease patients. PLoS Pathog. 2023, 19, e1011456. [Google Scholar] [CrossRef]
- Kurnik, M.; Gil, K.; Gajda, M.; Thor, P.; Bugajski, A. Neuropathic alterations of the myenteric plexus neurons following subacute intraperitoneal administration of salsolinol. Folia Histochem. Cytobiol. 2015, 53, 49–61. [Google Scholar] [CrossRef]
- Chandra, R.; Hiniker, A.; Kuo, Y.M.; Nussbaum, R.L.; Liddle, R.A. alpha-Synuclein in gut endocrine cells and its implications for Parkinson’s disease. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Yang, R.; Gao, G.; Yang, H. The Pathological Mechanism Between the Intestine and Brain in the Early Stage of Parkinson’s Disease. Front. Aging Neurosci. 2022, 14, 861035. [Google Scholar] [CrossRef]
- Seddik, H.A.; Bendali, F.; Gancel, F.; Fliss, I.; Spano, G.; Drider, D. Lactobacillus plantarum and Its Probiotic and Food Potentialities. Probiotics Antimicrob. Proteins 2017, 9, 111–122. [Google Scholar] [CrossRef]
- Kleerebezem, M.; Boekhorst, J.; van Kranenburg, R.; Molenaar, D.; Kuipers, O.P.; Leer, R.; Tarchini, R.; Peters, S.A.; Sandbrink, H.M.; Fiers, M.W.; et al. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 2003, 100, 1990–1995. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Baldia, A.; Tiwari, S.K. Plantaricin LD1 Inhibits the Growth and Biofilm Formation of Staphylococcus aureus in Milk. J. Explor. Res. Pharmacol. 2024, 9, 1–7. [Google Scholar] [CrossRef]
- Liang, D.; Liu, H.; Jin, R.; Feng, R.; Wang, J.; Qin, C.; Zhang, R.; Chen, Y.; Zhang, J.; Teng, J.; et al. Escherichia coli triggers alpha-synuclein pathology in the LRRK2 transgenic mouse model of PD. Gut Microbes 2023, 15, 2276296. [Google Scholar] [CrossRef] [PubMed]
- Murros, K.E.; Huynh, V.A.; Takala, T.M.; Saris, P.E.J. Desulfovibrio Bacteria Are Associated with Parkinson’s Disease. Front. Cell Infect. Microbiol. 2021, 11, 652617. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Mao, Y.; Wei, L.; Zhao, A.; Chen, L.; Zhang, F.; Cui, X.; Pan, M.H.; Wang, B. Lactiplantibacillus plantarum JS19-assisted fermented goat milk alleviates d-galactose-induced aging by modulating oxidative stress and intestinal microbiota in mice. J. Dairy Sci. 2024, 107, 7564–7577. [Google Scholar] [CrossRef]
- Ruiz, M.J.; Garcia, M.D.; Canalejo, L.M.M.; Kruger, A.; Padola, N.L.; Etcheverria, A.I. Antimicrobial activity of Lactiplantibacillus plantarum against shiga toxin-producing Escherichia coli. J. Appl. Microbiol. 2023, 134, lxad202. [Google Scholar] [CrossRef]
- Prete, R.; Long, S.L.; Gallardo, A.L.; Gahan, C.G.; Corsetti, A.; Joyce, S.A. Beneficial bile acid metabolism from Lactobacillus plantarum of food origin. Sci. Rep. 2020, 10, 1165. [Google Scholar] [CrossRef]
- Lambert, J.M.; Bongers, R.S.; de Vos, W.M.; Kleerebezem, M. Functional analysis of four bile salt hydrolase and penicillin acylase family members in Lactobacillus plantarum WCFS1. Appl. Environ. Microbiol. 2008, 74, 4719–4726. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Huang, X.; Wang, D.; Yu, D.; Hou, S.; Cui, H.; Song, L. Correction: Roles of bile acids signaling in neuromodulation under physiological and pathological conditions. Cell Biosci. 2023, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Sun, L.; Gonzalez, F.J. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 2022, 30, 289–300. [Google Scholar] [CrossRef]
- Kaur, H.; Swadia, D.; Sinha, S. Bile Acids as Modulators of alpha-Synuclein Aggregation: Implications for Parkinson’s Therapy. ACS Chem. Neurosci. 2024, 15, 4055–4065. [Google Scholar] [CrossRef]
- Cuevas, E.; Burks, S.; Raymick, J.; Robinson, B.; Gomez-Crisostomo, N.P.; Escudero-Lourdes, C.; Lopez, A.G.G.; Chigurupati, S.; Hanig, J.; Ferguson, S.A.; et al. Tauroursodeoxycholic acid (TUDCA) is neuroprotective in a chronic mouse model of Parkinson’s disease. Nutr. Neurosci. 2022, 25, 1374–1391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, R.; Ma, Y.; Ma, Y.; Feng, H.; Ding, X.; Zhang, Q.; Li, Y.; Shan, J.; Bian, H.; et al. Lactiplantibacillus plantarum ATCC8014 Alleviates Postmenopausal Hypercholesterolemia in Mice by Remodeling Intestinal Microbiota to Increase Secondary Bile Acid Excretion. J. Agric. Food Chem. 2024, 72, 6236–6249. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, X.; Ma, Y.; Fan, Y.; Zhang, Y.; Nan, B.; Li, X.; Wang, Y.; Liu, J. Prevention of High-Fat-Diet-Induced Dyslipidemia by Lactobacillus plantarum LP104 through Mediating Bile Acid Enterohepatic Axis Circulation and Intestinal Flora. J. Agric. Food Chem. 2023, 71, 7334–7347. [Google Scholar] [CrossRef]
- Geng, T.; He, F.; Su, S.; Sun, K.; Zhao, L.; Zhao, Y.; Bao, N.; Pan, L.; Sun, H. Probiotics Lactobacillus rhamnosus GG ATCC53103 and Lactobacillus plantarum JL01 induce cytokine alterations by the production of TCDA, DHA, and succinic and palmitic acids, and enhance immunity of weaned piglets. Res. Vet. Sci. 2021, 137, 56–67. [Google Scholar] [CrossRef]
- Lin, S.; Yang, X.; Long, Y.; Zhong, H.; Wang, P.; Yuan, P.; Zhang, X.; Che, L.; Feng, B.; Li, J.; et al. Dietary supplementation with Lactobacillus plantarum modified gut microbiota, bile acid profile and glucose homoeostasis in weaning piglets. Br. J. Nutr. 2020, 124, 797–808. [Google Scholar] [CrossRef]
- Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.; Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life 2024, 14, 559. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef]
- Shi, J.; Mao, W.; Song, Y.; Wang, Y.; Zhang, L.; Xu, Y.; Gu, H.; Yao, S.; Yao, Y.; Liu, Z.; et al. Butyrate alleviates food allergy by improving intestinal barrier integrity through suppressing oxidative stress-mediated Notch signaling. Imeta 2025, 4, e70024. [Google Scholar] [CrossRef] [PubMed]
- Bayazid, A.B.; Kim, J.G.; Azam, S.; Jeong, S.A.; Kim, D.H.; Park, C.W.; Lim, B.O. Sodium butyrate ameliorates neurotoxicity and exerts anti-inflammatory effects in high fat diet-fed mice. Food Chem. Toxicol. 2022, 159, 112743. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Lv, L.; Wu, W.; Li, Y.; Shi, D.; Fang, D.; Guo, F.; Jiang, H.; Yan, R.; Ye, W.; et al. Butyrate Protects Mice Against Methionine-Choline-Deficient Diet-Induced Non-alcoholic Steatohepatitis by Improving Gut Barrier Function, Attenuating Inflammation and Reducing Endotoxin Levels. Front. Microbiol. 2018, 9, 1967. [Google Scholar] [CrossRef]
- Cao, X.; Di, Y.; Tian, Y.J.; Huang, X.B.; Zhou, Y.; Zhang, D.M.; Song, Y. Sodium butyrate inhibits activation of ROS/NF-kappaB/NLRP3 signaling pathway and angiogenesis in human retinal microvascular endothelial cells. Int. Ophthalmol. 2025, 45, 108. [Google Scholar] [CrossRef]
- Khan, S.; Jena, G. Sodium butyrate, a HDAC inhibitor ameliorates eNOS, iNOS and TGF-β1-induced fibrogenesis, apoptosis and DNA damage in the kidney of juvenile diabetic rats. Food Chem. Toxicol. 2014, 73, 127–139. [Google Scholar] [CrossRef]
- Qiao, C.M.; Sun, M.F.; Jia, X.B.; Shi, Y.; Zhang, B.P.; Zhou, Z.L.; Zhao, L.P.; Cui, C.; Shen, Y.Q. Sodium butyrate causes alpha-synuclein degradation by an Atg5-dependent and PI3K/Akt/mTOR-related autophagy pathway. Exp. Cell Res. 2020, 387, 111772. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.; Liu, S.; Du, J.; Hu, X.; Xiong, J.; Fang, R.; Chen, W.; Sun, J. Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J. Neurol. Sci. 2017, 381, 176–181. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Qian, Y.; He, X.; Mo, C.; Yang, X.; Xiao, Q. Sodium butyrate attenuates rotenone-induced toxicity by activation of autophagy through epigenetically regulating PGC-1alpha expression in PC12 cells. Brain Res. 2022, 1776, 147749. [Google Scholar] [CrossRef]
- Aiello, A.; Pizzolongo, F.; De Luca, L.; Blaiotta, G.; Aponte, M.; Addeo, F.; Romano, R. Production of butyric acid by different strains of Lactobacillus plantarum (Lactiplantibacillus plantarum). Int. Dairy J. 2023, 140, 105589. [Google Scholar] [CrossRef]
- He, Y.; Mei, L.; Wang, L.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Lactiplantibacillus plantarum CCFM1019 attenuate polycystic ovary syndrome through butyrate dependent gut-brain mechanism. Food Funct. 2022, 13, 1380–1392. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Zhao, S.; Lau, R.; Zhong, Y.; Chen, M.H. Lactate cross-feeding between Bifidobacterium species and Megasphaera indica contributes to butyrate formation in the human colonic environment. Appl. Environ. Microbiol. 2024, 90, e0101923. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Gueimonde, M.; Duncan, S.H.; Flint, H.J.; de los Reyes-Gavilan, C.G. Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiol. Lett. 2015, 362, fnv176. [Google Scholar] [CrossRef]
- Wang, B.; Gong, L.; Zhou, Y.; Tang, L.; Zeng, Z.; Wang, Q.; Zou, P.; Yu, D.; Li, W. Probiotic Paenibacillus polymyxa 10 and Lactobacillus plantarum 16 enhance growth performance of broilers by improving the intestinal health. Anim. Nutr. 2021, 7, 829–840. [Google Scholar] [CrossRef]
- Mamelak, M. Parkinson’s Disease, the Dopaminergic Neuron and Gammahydroxybutyrate. Neurol. Ther. 2018, 7, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.N.; Sanchez-Padilla, J.; Chan, C.S.; Surmeier, D.J. Robust pacemaking in substantia nigra dopaminergic neurons. J. Neurosci. 2009, 29, 11011–11019. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M. Mitochondrial Dysfunction in Neurodegenerative Diseases. Cells 2025, 14, 276. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.; Nath, S.; Engelborghs, Y.; Pountney, D.L. Raised calcium and oxidative stress cooperatively promote alpha-synuclein aggregate formation. Neurochem. Int. 2013, 62, 703–711. [Google Scholar] [CrossRef]
- Dyrda-Terniuk, T.; Railean, V.; Florkiewicz, A.B.; Walczak-Skierska, J.; Kolankowski, M.; Rudnicka, J.; Białczak, D.; Pomastowski, P. The impact of Lactiplantibacillus plantarum on the cream composition: Insight into changes of vitamin D3 content and fatty acid composition. Int. Dairy J. 2025, 161, 106118. [Google Scholar] [CrossRef]
- Orme, R.; Bhangal, M.; Fricker, R. Vitamin D3 promotes dopamine neuron survival through upregulation of GDNF. Neuroreport 2014, 25, 153–154. [Google Scholar]
- Zhang, Y.; Ji, W.; Zhang, S.; Gao, N.; Xu, T.; Wang, X.; Zhang, M. Vitamin D Inhibits the Early Aggregation of alpha-Synuclein and Modulates Exocytosis Revealed by Electrochemical Measurements. Angew. Chem. Int. Ed. Engl. 2022, 61, e202111853. [Google Scholar] [CrossRef] [PubMed]
- Rcom-H’cheo-Gauthier, A.N.; Meedeniya, A.C.; Pountney, D.L. Calcipotriol inhibits alpha-synuclein aggregation in SH-SY5Y neuroblastoma cells by a Calbindin-D28k-dependent mechanism. J. Neurochem. 2017, 141, 263–274. [Google Scholar] [CrossRef]
- Vidyadhara, D.J.; Yarreiphang, H.; Abhilash, P.L.; Raju, T.R.; Alladi, P.A. Differential expression of calbindin in nigral dopaminergic neurons in two mice strains with differential susceptibility to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J. Chem. Neuroanat. 2016, 76, 82–89. [Google Scholar] [CrossRef]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Park. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [PubMed]
- Bock, H.J.; Lee, H.W.; Lee, N.K.; Paik, H.D. Probiotic Lactiplantibacillus plantarum KU210152 and its fermented soy milk attenuates oxidative stress in neuroblastoma cells. Food Res. Int. 2024, 177, 113868. [Google Scholar] [CrossRef]
- Choi, G.H.; Bock, H.J.; Lee, N.K.; Paik, H.D. Soy yogurt using Lactobacillus plantarum 200655 and fructooligosaccharides: Neuroprotective effects against oxidative stress. J. Food Sci. Technol. 2022, 59, 4870–4879. [Google Scholar] [CrossRef]
- Cheon, M.J.; Lee, N.K.; Paik, H.D. Neuroprotective Effects of Heat-Killed Lactobacillus plantarum 200655 Isolated from Kimchi Against Oxidative Stress. Probiotics Antimicrob. Proteins 2021, 13, 788–795. [Google Scholar] [CrossRef]
- Yim, N.H.; Gu, M.J.; Park, H.R.; Hwang, Y.H.; Ma, J.Y. Enhancement of neuroprotective activity of Sagunja-tang by fermentation with lactobacillus strains. BMC Complement. Altern. Med. 2018, 18, 312. [Google Scholar] [CrossRef]
- Wu, S.; Chen, Y.; Wei, F.; Chen, Z.; Fan, J.; Luo, Y.; Li, P.; Gu, Q. Lactiplantibacillus plantarum ZJ316 alleviates the oxidative stress and cellular apoptosis via modulating Nrf2/HO-1 signaling pathway. J. Funct. Foods 2024, 121, 106409. [Google Scholar] [CrossRef]
- Chen, Y.; Guan, W.; Zhang, N.; Wang, Y.; Tian, Y.; Sun, H.; Li, X.; Wang, Y.; Liu, J. Lactobacillus plantarum Lp2 improved LPS-induced liver injury through the TLR-4/MAPK/NFkappaB and Nrf2-HO-1/CYP2E1 pathways in mice. Food Nutr. Res. 2022, 66. [Google Scholar] [CrossRef]
- Aboulgheit, A.; Karbasiafshar, C.; Zhang, Z.; Sabra, M.; Shi, G.; Tucker, A.; Sodha, N.; Abid, M.R.; Sellke, F.W. Lactobacillus plantarum probiotic induces Nrf2-mediated antioxidant signaling and eNOS expression resulting in improvement of myocardial diastolic function. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H839–H849. [Google Scholar] [CrossRef]
- Vomund, S.; Schafer, A.; Parnham, M.J.; Brune, B.; von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Nam, T.J.; Choi, Y.H. Capsosiphon fulvescens Glycoproteins Enhance Probiotics-Induced Cognitive Improvement in Aged Rats. Nutrients 2020, 12, 837. [Google Scholar] [CrossRef] [PubMed]
- Teran, M.D.M.; de Moreno de LeBlanc, A.; Savoy de Giori, G.; LeBlanc, J.G. Thiamine-producing lactic acid bacteria and their potential use in the prevention of neurodegenerative diseases. Appl. Microbiol. Biotechnol. 2021, 105, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Levit, R.; Savoy de Giori, G.; de Moreno de LeBlanc, A.; LeBlanc, J.G. Protective effect of the riboflavin-overproducing strain Lactobacillus plantarum CRL2130 on intestinal mucositis in mice. Nutrition 2018, 54, 165–172. [Google Scholar] [CrossRef]
- Prieto-Paredes, R.; Landete, J.M.; Peiroten, A.; Curiel, J.A.; Langa, S. Polymerase chain reaction for molecular detection of the genes involved in the production of riboflavin in lactic acid bacteria. J. Microbiol. Methods 2023, 206, 106678. [Google Scholar] [CrossRef]
- Ratti, M.; Lampis, A.; Ghidini, M.; Salati, M.; Mirchev, M.B.; Valeri, N.; Hahne, J.C. MicroRNAs (miRNAs) and Long Non-Coding RNAs (lncRNAs) as New Tools for Cancer Therapy: First Steps from Bench to Bedside. Target. Oncol. 2020, 15, 261–278. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A brief review on the mechanisms of miRNA regulation. Genom. Proteom. Bioinform. 2009, 7, 147–154. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Recasens, A.; Perier, C.; Sue, C.M. Role of microRNAs in the Regulation of alpha-Synuclein Expression: A Systematic Review. Front. Mol. Neurosci. 2016, 9, 128. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, M.; Yan, R.; Liu, J.; Maddila, S.; Junn, E.; Mouradian, M.M. MicroRNA-7 Protects Against Neurodegeneration Induced by alpha-Synuclein Preformed Fibrils in the Mouse Brain. Neurotherapeutics 2021, 18, 2529–2540. [Google Scholar] [CrossRef]
- Choi, D.C.; Yoo, M.; Kabaria, S.; Junn, E. MicroRNA-7 facilitates the degradation of alpha-synuclein and its aggregates by promoting autophagy. Neurosci. Lett. 2018, 678, 118–123. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zhang, J.L.; Duan, Y.L.; Zhang, Q.S.; Li, G.F.; Zheng, D.L. MicroRNA-214 participates in the neuroprotective effect of Resveratrol via inhibiting alpha-synuclein expression in MPTP-induced Parkinson’s disease mouse. Biomed. Pharmacother. 2015, 74, 252–256. [Google Scholar] [CrossRef]
- Kabaria, S.; Choi, D.C.; Chaudhuri, A.D.; Mouradian, M.M.; Junn, E. Inhibition of miR-34b and miR-34c enhances alpha-synuclein expression in Parkinson’s disease. FEBS Lett. 2015, 589, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Cai, Y.; Chi, J.; Yang, Y.; Chen, Q.; Chen, L.; Zhang, J.; Ke, J.; Wu, Y.; He, X. Silencing miR-155-5p alleviates hippocampal damage in kainic acid-induced epileptic rats via the Dusp14/MAPK pathway. Brain Res. Bull. 2024, 217, 111057. [Google Scholar] [CrossRef]
- Rachmawati, E.; Sargowo, D.; Rohman, M.S.; Widodo, N.; Kalsum, U. miR-155-5p predictive role to decelerate foam cell atherosclerosis through CD36, VAV3, and SOCS1 pathway. Noncoding RNA Res. 2021, 6, 59–69. [Google Scholar] [CrossRef]
- Kurata, A.; Kiyohara, S.; Imai, T.; Yamasaki-Yashiki, S.; Zaima, N.; Moriyama, T.; Kishimoto, N.; Uegaki, K. Characterization of extracellular vesicles from Lactiplantibacillus plantarum. Sci. Rep. 2022, 12, 13330. [Google Scholar] [CrossRef]
- Liu, X.; Shen, L.; Wan, M.; Xie, H.; Wang, Z. Peripheral extracellular vesicles in neurodegeneration: Pathogenic influencers and therapeutic vehicles. J. Nanobiotechnol. 2024, 22, 170. [Google Scholar] [CrossRef]
- Huang-Doran, I.; Zhang, C.Y.; Vidal-Puig, A. Extracellular Vesicles: Novel Mediators of Cell Communication In Metabolic Disease. Trends Endocrinol. Metab. 2017, 28, 3–18. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, Z.; Hao, P.; Qiu, Y.; Zhao, M.; Zhou, G.; Zhang, C.; Kang, J.; Li, P. Biological Functions and Cross-Kingdom Host Gene Regulation of Small RNAs in Lactobacillus plantarum-Derived Extracellular Vesicles. Front. Microbiol. 2022, 13, 944361. [Google Scholar] [CrossRef]
- Gong, S.; Zeng, R.; Liu, L.; Wang, R.; Xue, M.; Dong, H.; Wu, Z.; Zhang, Y. Extracellular vesicles from a novel Lactiplantibacillus plantarum strain suppress inflammation and promote M2 macrophage polarization. Front. Immunol. 2024, 15, 1459213. [Google Scholar] [CrossRef]
- Kim, W.; Lee, E.J.; Bae, I.H.; Myoung, K.; Kim, S.T.; Park, P.J.; Lee, K.H.; Pham, A.V.Q.; Ko, J.; Oh, S.H.; et al. Lactobacillus plantarum-derived extracellular vesicles induce anti-inflammatory M2 macrophage polarization in vitro. J. Extracell. Vesicles 2020, 9, 1793514. [Google Scholar] [CrossRef]
- Cao, Q.; Luo, S.; Yao, W.; Qu, Y.; Wang, N.; Hong, J.; Murayama, S.; Zhang, Z.; Chen, J.; Hashimoto, K.; et al. Suppression of abnormal alpha-synuclein expression by activation of BDNF transcription ameliorates Parkinson’s disease-like pathology. Mol. Ther. Nucleic Acids 2022, 29, 1–15. [Google Scholar] [CrossRef]
- Choi, J.; Kim, Y.K.; Han, P.L. Extracellular Vesicles Derived from Lactobacillus plantarum Increase BDNF Expression in Cultured Hippocampal Neurons and Produce Antidepressant-like Effects in Mice. Exp. Neurobiol. 2019, 28, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Sun, J.; Zhao, M.; Hu, J.; Wang, X.; Du, G.; Chen, N.H. Overexpression of alpha-synuclein down-regulates BDNF expression. Cell Mol. Neurobiol. 2010, 30, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gao, Z.; Yang, Z.; Zhang, Y.; Chen, H.; Yang, X.; Fang, X.; Zhu, Y.; Zhang, J.; Ouyang, F.; et al. Lactobacillus plantarum-derived extracellular vesicles protect against ischemic brain injury via the microRNA-101a-3p/c-Fos/TGF-beta axis. Pharmacol. Res. 2022, 182, 106332. [Google Scholar] [CrossRef]
- Melnik, B.C. Synergistic Effects of Milk-Derived Exosomes and Galactose on alpha-Synuclein Pathology in Parkinson’s Disease and Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 1059. [Google Scholar] [CrossRef] [PubMed]
- Amorim Neto, D.P.; Bosque, B.P.; Pereira de Godoy, J.V.; Rodrigues, P.V.; Meneses, D.D.; Tostes, K.; Costa Tonoli, C.C.; Faustino de Carvalho, H.; Gonzalez-Billault, C.; de Castro Fonseca, M. Akkermansia muciniphila induces mitochondrial calcium overload and alpha-synuclein aggregation in an enteroendocrine cell line. iScience 2022, 25, 103908. [Google Scholar] [CrossRef] [PubMed]

| Rotenone | MPTP | 6-OHDA | |
|---|---|---|---|
| Primary mechanism | Mitochondrial Complex I inhibition | Mitochondrial Complex I inhibition | Selective degeneration of catecholaminergic neurons |
| Site of action | Systemic (brain and gut) | SNpc | Nigrostriatal pathway (injected site) |
| Rodent species used | Rat and mouse | Mouse | Rat |
| Onset and duration | Chronic (weeks to months) | Acute/subacute (days-weeks) | Acute (1–2 weeks) |
| Lewy body presence | Yes | Absent | Absent |
| Motor deficit | Moderate-high | High | Very high |
| Non-motor symptom | High | High | Limited |
| MGBA relevance | High | Moderate | Limited |
| Model variability | High | Low | Low |
| Experimental cost | Hight | Moderate | Moderate-high |
| References | [60,62,63,64] | [60,65] | [42,60] |
| Treatment | Model | Dosage (CFU) | Period | Sample Size | Main Findings | Ref. |
|---|---|---|---|---|---|---|
| Lpb. plantarum PS128 | Rotenone | 1 × 109 | 6 weeks | 4 groups; n = 10 per group | Improved motor function [↓Walking time on NBT, ↑Retention time on RTR] Neuroprotection [↑TH+, ↓Iba1, ↑BDNF, ↑DA] Microbiota modulation [↑Bifidobacterium, ↓Ruminococcaceae_UCG_014, ↓Bacteroides, ↓Alistipes] | [35] |
| Lpb. plantarum CCFM405 | Rotenone | 1 × 109 | 8 weeks | 3 groups; n = 12 per group | Neuroprotection [↑TH+ in STR, ↑DA, ↑5-HT, ↓Microglia activation, ↓Astrocyte activation] Improved motor function [↓Time on PT, ↓Walking time on NBT, ↑Retention time on RTR, ↑Total walking distance in OFT] Reduced GI deficits [↑Colon length, ↑Fecal pellet size, ↑Intestinal lining thickness (↑ZO-1, ↑Occludin), ↑Goblet cell count] Reduced intestinal inflammation [↓IL-6, ↓TNF-α] Microbiota modulation [↑Bifidobacterium, ↑Faecalibaculum, ↑Turicibacter, ↓Alistipes, ↓Akkermansia, ↓Bilophila, ↓Ruminococcaceae_UCG_004, ↓Ruminococcaceae_UCG_009] Altered serum and fecal metabolite composition | [36] |
| Lpb. plantarum DP189 | MPTP | 2 × 108 | 2 weeks | 4 groups; n = 10 per group | Reduced α-syn aggregation Neuroprotection [↑SOD, ↑GSH-Px, ↑IL-10, ↓MDA, ↓ROS, ↓TNF-α, ↓IL-6, ↓IL-1β] Microbiota modulation [↑Prevotella, ↓Proteobacteria, ↓Actinobacteria] | [37] |
| Lpb. plantarum SG5 | MPTP | 1 × 109 | 5 weeks | 5 groups; n = 10 per group | Reduced α-syn aggregation Neuroprotection Improved motor function Microbiota modulation [↑Bacteroidetes, ↑Proteobacteria, ↓Desulfovibrio] | [34] |
| Lpb. plantarum PS128 | MPTP | 1 × 109 | 4 weeks | 4 groups; n = 18 per group | Neuroprotection [↑DA, ↑DOPAC, ↑TH+, ↑BDNF, ↑NGF, ↓Iba1, ↓GFAP, ↓TNF-α/IL-1β/IL-6] Improved motor function [↓Inversion time and descent time on PT, ↓Walking time on NBT, ↑Retention time on RTR] Microbiota modulation [↓Enterobacteriaceae] ↓Oxidative stress [↑GSH, ↑SOD] | [67] |
| Lpb. plantarum CRL1905 | MPTP | 8 ± 2 × 108 | 4 weeks | 5 groups; n = 6 per group | Neuroprotection [↑TH+, ↓IL-6, ↓TNF-α, ↓IFN-γ, ↓MCP-1] Improved motor function Increased thiamine production | [68] |
| Lpb. plantarum PS128 | 6-OHDA | 1.5 × 1010 | 12 weeks | L-dopa n = 7 DBS n = 6 PS128 n = 9 Saline n = 4 | Neuroprotection [↑DA, ↓turnover ratios of DA and NA, ↑TH+] Improved motor function [↓β-PSD, ↑Contralateral paw use, ↑Total walking distance in OFT] | [69] |
| Treatment | Dosage (CFU) | Period | Sample Size | Main Findings | Ref. |
|---|---|---|---|---|---|
| Lpb. plantarum, Lbs. casei, Lab. acidophilus, Lab. bulgaricus, B. infantis, B. longum, B. breve, S. thermophilus | 4.5 × 1011 | 8 weeks | 2 groups; Placebo (n = 13) Probiotics (n = 14) | Improved gastrointestinal outcomes [↑Number of defecations with a sense of complete evacuation per week, ↑Stool consistency, ↑Frequency of bowel movements] | [72] |
| S. thermophilus, E. faecium, Lbs. rhamnosus GG, Lab. acidophilus, Lpb. plantarum, Lbs. paracasei, Lab. bulgaricus, B. breve, B. lactis and prebiotic fibers | 2.5 × 1011 | 4 weeks | 2 groups; Placebo (n = 40) Probiotics (n = 80) | Improved gastrointestinal outcomes [↑Number of complete bowel movements, ↑Stool consistency, ↓Use of laxatives] | [73] |
| Lpb. plantarum PS128 | 6 × 1010 | 12 weeks | 1 group Probiotics (n = 25) | Improved motor function [OFF state (↓UPDRS-III motor scores, ↓akinesia subscores), ↓OFF period, ON state (↓UPDRS-III motor scores, ↓Total UPDRS scores), ↑ON period] Improved quality of life [Mobility, Activities of daily living, Stigma, Cognition, PGI-C] | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-L.; Deng, F.-S.; Tsai, Y.-C. Lactiplantibacillus plantarum as a Psychobiotic Strategy Targeting Parkinson’s Disease: A Review and Mechanistic Insights. Nutrients 2025, 17, 3047. https://doi.org/10.3390/nu17193047
Chen W-L, Deng F-S, Tsai Y-C. Lactiplantibacillus plantarum as a Psychobiotic Strategy Targeting Parkinson’s Disease: A Review and Mechanistic Insights. Nutrients. 2025; 17(19):3047. https://doi.org/10.3390/nu17193047
Chicago/Turabian StyleChen, Wu-Lin, Fu-Sheng Deng, and Ying-Chieh Tsai. 2025. "Lactiplantibacillus plantarum as a Psychobiotic Strategy Targeting Parkinson’s Disease: A Review and Mechanistic Insights" Nutrients 17, no. 19: 3047. https://doi.org/10.3390/nu17193047
APA StyleChen, W.-L., Deng, F.-S., & Tsai, Y.-C. (2025). Lactiplantibacillus plantarum as a Psychobiotic Strategy Targeting Parkinson’s Disease: A Review and Mechanistic Insights. Nutrients, 17(19), 3047. https://doi.org/10.3390/nu17193047






