Nutritional Vulnerability and Functional Decline in End-Stage Heart Failure and Chronic Respiratory Disease: Utility of the CONUT Score in a Palliative Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Design, Setting and Participants
2.2. Data Collection
2.3. Study Variables
2.4. Statistical Analyses
3. Results
3.1. Characteristics of the Study Population
3.2. Clinical and Symptom Profile
3.3. Nutritional Status and Biochemical Markers
3.4. Predictors of Malnutrition
3.4.1. Binary Logistic Regression for Moderate/Severe Malnutrition (CONUT ≥ 5)
3.4.2. Ordinal Logistic Regression for Nutritional Severity (Three-Level CONUT)
3.5. Mortality Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| Barthel | Barthel Index |
| CONUT | Controlling Nutritional Status |
| COPD | Chronic Obstructive Pulmonary Disease |
| CFS | Clinical Frailty Scale |
| CRD | Chronic Respiratory Disease |
| CPAP | continuous positive airway pressure |
| CI | confidence interval |
| CRP | C-reactive protein |
| HF | Heart Failure |
| ILD | Diffuse Interstitial Lung Disease |
| IQR | interquartile range |
| LVEF | Left Ventricular Ejection Fraction |
| mMRC | Modified Medical Research Council |
| NECPAL | Necesidades Paliativas (Palliative Care Needs) |
| NYHA | New York Heart Association |
| OR | odds ratio |
| PH | Pulmonary Hypertension |
| PPS | Palliative Performance Scale |
| Ref. | reference category |
| SD | standard deviation |
References
- Harrison, K.L.; Kotwal, A.A.; Smith, A.K. Palliative Care for Patients with Noncancer Illnesses. JAMA J. Am. Med. Assoc. 2020, 324, 1404–1405. [Google Scholar] [CrossRef]
- Kelley, A.S.; Morrison, R.S. Palliative Care for the Seriously Ill. N. Engl. J. Med. 2015, 373, 747–755. [Google Scholar] [CrossRef]
- Jang, H.; Lee, K.; Kim, S.; Kim, S. Unmet needs in palliative care for patients with common non-cancer diseases: A cross-sectional study. BMC Palliat. Care 2022, 21, 151. [Google Scholar] [CrossRef] [PubMed]
- Van Beers, M.; Rutten, M.P.M.H.; van de Bool, C.; Boland, M.; Kremers, S.P.J.; Franssen, F.M.E.; van Helvoort, A.; Gosker, H.R.; Wouters, E.F.; Schols, A.M.W.J. Clinical outcome and cost-effectiveness of a 1-year nutritional intervention programme in COPD patients with low muscle mass: The randomized controlled NUTRAIN trial. Clin. Nutr. 2020, 39, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Lennie, T.A.; Moser, D.K.; Biddle, M.J.; Welsh, D.; Bruckner, G.G.; Thomas, D.T.; Rayens, M.K.; Bailey, A.L. Nutrition intervention to decrease symptoms in patients with advanced heart failure. Res. Nurs. Health 2013, 36, 120–145. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition–A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 207–217. [Google Scholar] [CrossRef]
- Duarte, R.R.P.; Gonzalez, M.C.; Oliveira, J.F.; Goulart, M.R.; Castro, I. Is there an association between the nutritional and functional parameters and congestive heart failure severity? Clin. Nutr. 2021, 40, 3354–3359. [Google Scholar] [CrossRef]
- Wleklik, M.; Uchmanowicz, I.; Jankowska-Polańska, B.; Andreae, C.; Regulska-Ilow, B. The Role of Nutritional Status in Elderly Patients with Heart Failure. J. Nutr. Health Aging 2018, 22, 581–588. [Google Scholar] [CrossRef]
- Pagnesi, M.; Serafini, L.; Chiarito, M.; Stolfo, D.; Baldetti, L.; Inciardi, R.M.; Tomasoni, D.; Adamo, M.; Lombardi, C.M.; Sammartino, A.M.; et al. Impact of malnutrition in patients with severe heart failure. Eur. J. Heart Fail. 2024, 26, 1585–1593. [Google Scholar] [CrossRef]
- Carime, N.A.; Cottenet, J.; Clerfond, G.; Eschalier, R.; Quilliot, D.; Eicher, J.-C.; Joly, B. Impact of nutritional status on heart failure mortality: A retrospective cohort study. Nutr. J. 2022, 21, 2. [Google Scholar] [CrossRef]
- Sze, S.; Pellicori, P.; Zhang, J.; Clark, A.L. Malnutrition, congestion and mortality in ambulatory patients with heart failure. Heart 2019, 105, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Gattermann Pereira, T.; Lima, J.; Silva, F.M. Undernutrition is associated with mortality, exacerbation, and poorer quality of life in patients with chronic obstructive pulmonary disease: A systematic review with meta-analysis of observational studies. J. Parenter. Enter. Nutr. 2022, 46, 977–996. [Google Scholar] [CrossRef] [PubMed]
- Kaluźniak-Szymanowska, A.; Krzymińska-Siemaszko, R.; Deskur-śmielecka, E.; Lewandowicz, M.; Kaczmarek, B.; Wieczorowska-Tobis, K. Malnutrition, sarcopenia, and malnutrition-sarcopenia syndrome in older adults with COPD. Nutrients 2022, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Gea, J.; Sancho-Muñoz, A.; Chalela, R. Nutritional status and muscle dysfunction in chronic respiratory diseases: Stable phase versus acute exacerbations. J. Thorac. Dis. 2018, 10, S1332–S1334. [Google Scholar] [CrossRef]
- Itoh, M.; Tsuji, T.; Nemoto, K.; Nakamura, H.; Aoshiba, K. Undernutrition in patients with COPD and its treatment. Nutrients 2013, 5, 1316–1335. [Google Scholar] [CrossRef]
- Hersberger, L.; Dietz, A.; Bürgler, H.; Bargetzi, A.; Bargetzi, L.; Kägi-Braun, N.; Tribolet, P.; Gomes, F.; Hoess, C.; Pavlicek, V.; et al. Individualized Nutritional Support for Hospitalized Patients with Chronic Heart Failure. J. Am. Coll. Cardiol. 2021, 77, 2307–2319. [Google Scholar] [CrossRef]
- Sze, S.; Pellicori, P.; Kazmi, S.; Rigby, A.; Cleland, J.G.; Wong, K.; Clark, A.L. Prevalence and Prognostic Significance of Malnutrition Using 3 Scoring Systems Among Outpatients with Heart Failure: A Comparison with Body Mass Index. JACC Heart Fail. 2018, 6, 476–486. [Google Scholar] [CrossRef]
- Lo Buglio, A.; Bellanti, F.; Carmignano, D.F.P.; Serviddio, G.; Vendemiale, G. Association between Controlling Nutritional Status (CONUT) Score and Body Composition, Inflammation and Frailty in Hospitalized Elderly Patients. Nutrients 2024, 16, 576. [Google Scholar] [CrossRef]
- Miano, N.; Di Marco, M.; Alaimo, S.; Coppolino, G.; L’Episcopo, G.; Leggio, S.; Scicali, R.; Piro, S.; Purrello, F.; Di Pino, A. Controlling Nutritional Status (CONUT) Score as a Potential Prognostic Indicator of In-Hospital Mortality, Sepsis and Length of Stay in an Internal Medicine Department. Nutrients 2023, 15, 1554. [Google Scholar] [CrossRef]
- Lo Buglio, A.; Scioscia, G.; Bellanti, F.; Tondo, P.; Soccio, P.; Natale, M.P.; Lacedonia, D.; Vendemiale, G. Controlling Nutritional Status Score as a Predictor for Chronic Obstructive Pulmonary Disease Exacerbation Risk in Elderly Patients. Metabolites 2023, 13, 1123. [Google Scholar] [CrossRef]
- Narumi, T.; Arimoto, T.; Funayama, A.; Kadowaki, S.; Otaki, Y.; Nishiyama, S.; Takahashi, H.; Shishido, T.; Miyashita, T.; Miyamoto, T.; et al. The prognostic importance of objective nutritional indexes in patients with chronic heart failure. J. Cardiol. 2013, 62, 307–313. [Google Scholar] [CrossRef]
- Shirakabe, A.; Hata, N.; Kobayashi, N.; Okazaki, H.; Matsushita, M.; Shibata, Y.; Nishigoori, S.; Uchiyama, S.; Asai, K. The prognostic impact of malnutrition in patients with severely decompensated acute heart failure, as assessed using the Prognostic Nutritional Index (PNI) and Controlling Nutritional Status (CONUT) score. Heart Vessel. 2018, 33, 134–144. [Google Scholar] [CrossRef] [PubMed]
- De Ulíbarri, J.I.; González-Madroño, A.; de Villar, N.G.P.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for Controlling Nutritional Status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38. [Google Scholar]
- Huang, X.W.; Luo, J.J.; Baldinger, B. The controlling nutritional status score and clinical outcomes in patients with heart failure: Pool analysis of observational studies. Front. Cardiovasc. Med. 2022, 9, 961141. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Ni, W.; Yuan, X.; Zhang, H.; Li, P.; Xu, J.; Zhao, Z. Sarcopenia in heart failure: A systematic review and meta-analysis. ESC Heart Fail. 2021, 8, 1007–1017. [Google Scholar] [CrossRef]
- Wawrzeńczyk, A.; Anaszewicz, M.; Wawrzeńczyk, A.; Budzyński, J. Clinical significance of nutritional status in patients with chronic heart failure—A systematic review. Heart Fail. Rev. 2019, 24, 671–700. [Google Scholar] [CrossRef]
- Horwich, T.B.; Kalantar-Zadeh, K.; MacLellan, R.W.; Fonarow, G.C. Albumin levels predict survival in patients with systolic heart failure. Am. Heart J. 2008, 155, 883–889. [Google Scholar] [CrossRef]
- Liu, M.; Chan, C.-P.; Yan, B.P.; Zhang, Q.; Lam, Y.-Y.; Li, R.-J.; Sanderson, J.E.; Coats, A.J.S.; Sun, J.-P.; Yip, G.W.-K.; et al. Albumin levels predict survival in patients with heart failure and preserved ejection fraction. Eur. J. Heart Fail. 2012, 14, 39–44. [Google Scholar] [CrossRef]
- Sarıkaya, B.; Aktaç, Ş; Çetinkaya, E. Assessing nutrition risk and malnutrition rates in patients with chronic obstructive pulmonary disease: A cross-sectional study. Nutr. Clin. Pract. 2025, 40, 880–892. [Google Scholar] [CrossRef]
- Shen, Q.; Zhou, S.; Song, M.; Ouyang, X.; Tan, Y.; Peng, Y.; Zhou, Z.; Peng, H. Prevalence and prognostic value of malnutrition in patients with IPF using three scoring systems. Respir. Med. 2024, 233, 107774. [Google Scholar] [CrossRef]
- Tramontano, A.; Palange, P. Nutritional State and COPD: Effects on Dyspnoea and Exercise Tolerance. Nutrients 2023, 15, 1786. [Google Scholar] [CrossRef] [PubMed]
- Faverio, P.; Bocchino, M.; Caminati, A.; Fumagalli, A.; Gasbarra, M.; Iovino, P.; Petruzzi, A.; Scalfi, L.; Sebastiani, A.; Stanziola, A.A.; et al. Nutrition in patients with idiopathic pulmonary fibrosis: Critical issues analysis and future research directions. Nutrients 2020, 12, 1131. [Google Scholar] [CrossRef]
- Aniwidyaningsih, W.; Varraso, R.; Cano, N.; Pison, C. Impact of nutritional status on body functioning in chronic obstructive pulmonary disease and how to intervene. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Cabré, M.; Ferreiro, C.; Arus, M.; Roca, M.; Palomera, E.; Serra-Prat, M. Evaluation of CONUT for Clinical Malnutrition Detection and Short-Term Prognostic Assessment in Hospitalized Elderly People. J. Nutr. Health Aging 2015, 19, 729–733. [Google Scholar] [CrossRef]
- Özkan, A.E.; Koca, N.; Tekeli, A.H. Assessment of nutritional status and clinical outcomes: A comprehensive retrospective analysis of critically ill patients. Medicine 2023, 102, E36018. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pombo, A.; Rodríguez-Carnero, G.; Castro, A.I.; Cantón-Blanco, A.; Seoane, L.M.; Casanueva, F.F.; Crujeiras, A.B.; Martínez-Olmos, M.A. Relevance of nutritional assessment and treatment to counteract cardiac cachexia and sarcopenia in chronic heart failure. Clin. Nutr. 2021, 40, 5141–5155. [Google Scholar] [CrossRef]
| General Characteristics | All Patients (N = 80) | Cardiologic Disease (N = 41) | Respiratory Disease (N = 39) | p Value |
|---|---|---|---|---|
| Age (years), mean ± SD | 77.8 ± 6.8 | 77.1 ± 7.6 | 78.5 ± 5.9 | 0.382 |
| Sex (male), n (%) | 52 (65%) | 27 (66%) | 25 (64%) | 0.870 |
| Origin, n (%) | 0.751 * | |||
| Home | 74 (92%) | 38 (93%) | 36 (92%) | |
| Nursing home | 3 (4%) | 2 (5%) | 1 (3%) | |
| Intermediate Care Facility | 3 (4%) | 1 (2%) | 2 (5%) | |
| Dementia, n (%) | 4 (5%) | 3 (7%) | 1 (3%) | 0.616 * |
| Dependence (Barthel Index), n (%) | 0.925 * | |||
| Independence—100 | 23 (29%) | 12 (29%) | 11 (28%) | |
| Mild dependence—>60 | 42 (53%) | 22 (54%) | 20 (51%) | |
| Moderate dependence—40–55 | 7 (9%) | 4 (10%) | 3 (8%) | |
| Severe dependence—20–35 | 6 (7%) | 2 (5%) | 4 (10%) | |
| Total dependence—<20 | 2 (2%) | 1 (2%) | 1 (3%) | |
| Frailty—(CFS), n (%) | 0.663 * | |||
| CFS 1 | 0 (0%) | 0 (0%) | 0 (0%) | |
| CFS 2 | 2 (2%) | 2 (5%) | 0 (0%) | |
| CFS 3 | 18 (23%) | 10 (25%) | 8 (21%) | |
| CFS 4 | 22 (28%) | 9 (22%) | 13 (33%) | |
| CFS 5 | 15 (17%) | 7 (17%) | 7 (18%) | |
| CFS 6 | 18 (23%) | 9 (22%) | 9 (23%) | |
| CFS 7 | 5 (6%) | 3 (7%) | 2 (5%) | |
| CFS 8 | 1 (1%) | 1 (2%) | 0 (0%) | |
| CFS 9 | 0 (0%) | 0 (0%) | 0 (0%) | |
| NECPAL, n (%) | <0.001 | |||
| I (1–2) | 11 (14%) | 4 (10%) | 7 (18%) | |
| II (3–4) | 45 (56%) | 16 (39%) | 29 (74%) | |
| III (5–6) | 24 (30%) | 21 (51%) | 3 (8%) | |
| PPS (Palliative Performance Scale), n (%) | 0.490 * | |||
| >60 | 41 (51%) | 22 (54%) | 19 (49%) | |
| 30–50 | 35 (44%) | 16 (39%) | 19 (49%) | |
| 10–20 | 4 (5%) | 3 (7%) | 1 (3%) | |
| Mortality, n (%) | 52 (65%) | 30 (73%) | 22 (56%) | 0.116 |
| Clinical and Symptoms Characteristics | All Patients (N = 80) | Cardiologic Disease (N = 41) | Respiratory Disease (N = 39) | p Value |
|---|---|---|---|---|
| Cardiologic disease | ||||
| Cardiac disease, n (%) | ||||
| Ischemic | 8 (20%) | |||
| Idiopathic/Familial Dilated | 4 (10%) | |||
| Hypertrophic | 0 (0%) | |||
| Valvular | 15 (36%) | |||
| Amyloidosis | 2 (5%) | |||
| Transplant | 4 (10%) | |||
| Ischemic-valvular | 7 (17%) | |||
| Others | 1 (2%) | |||
| LVEF, n (%) | ||||
| Normal ((50–55%) | 11(27%) | |||
| Mildly (41–49%) | 5 (12%) | |||
| Reduced ((40%) | 8 (19%) | |||
| Severely reduced (<30%) | 17 (42%) | |||
| NYHA, n (%) | ||||
| I | 5 (12%) | |||
| II | 10 (24%) | |||
| III | 20 (49%) | |||
| IV | 6 (15%) | |||
| Cardiac treatment, n (%) | ||||
| Inotropes (Levosimendan or Dobutamine) | 10 (25%) | |||
| Hemodialysis (Peritoneal or conventional) | 3 (7%) | |||
| Inotropes + Peritoneal Dialysis | 3 (7%) | |||
| Diuretic | 23 (56%) | |||
| External ventricular drain | 2 (5%) | |||
| Respiratory disease | ||||
| Respiratory disease, n (%) | ||||
| Chronic Obstructive Pulmonary Disease | 15 (38%) | |||
| Diffuse Interstitial Lung Disease | 19 (49%) | |||
| Pulmonary Hypertension | 2 (5%) | |||
| Other | 3 (8%) | |||
| mMRC, n (%) | ||||
| 1 | 0 (0%) | |||
| 2 | 2 (5%) | |||
| 3 | 32 (82%) | |||
| 4 | 5 (13%) | |||
| Respiratory treatment, n (%) | ||||
| Home oxygen | 29 (%) | |||
| Home high-flow nasal cannula | 1 (%) | |||
| Nocturnal CPAP | 2 (%) | |||
| Bronchodilator therapy | 6 (%) | |||
| Other | 1 (%) | |||
| Symptoms | ||||
| Dyspnea, n (%) | <0.001 | |||
| At rest | 20 (25%) | 7 (17%) | 13 (33%) | |
| On effort | 38 (48%) | 13 (32%) | 25 (64%) | |
| No dyspnea | 22 (27%) | 21 (51%) | 1 (3%) | |
| Anorexia, n (%) | 38 (48%) | 24 (59%) | 14 (36%) | 0.043 |
| Asthenia/Fatigue, n (%) | 44 (55%) | 24 (58%) | 20 (51%) | 0.514 |
| Xerostomia, n (%) | 35 (44%) | 14 (34%) | 21 (54%) | 0.076 |
| Dysphagia, n (%) | 9 (11%) | 6 (15%) | 3 (8%) | 0.483 * |
| Pain, n (%) | 15 (19%) | 6 (15%) | 9 (23%) | 0.334 |
| Constipation, n (%) | 23 (29%) | 13 (32%) | 10 (26%) | 0.549 |
| Nutritional Characteristics | All Patients (N = 80) | Cardiologic Disease (N = 41) | Respiratory Disease (N = 39) | p Value | |
|---|---|---|---|---|---|
| Weight loss in the last 6 months, n (%) | 18 (23%) | 11 (27%) | 7 (18%) | 0.342 | |
| Nutritional follow-up, n (%) | 44 (55%) | 27 (66%) | 17 (44%) | 0.045 | |
| Protein supplementation, n (%) | 34 (42%) | 20 (49%) | 14 (36%) | 0.244 | |
| High adherence, n (%) | 31 (91%) | 14 (100%) | 17 (85%) | 0.251 * | |
| BMI(Kg/m2), median (IQR) | 23.56 (5.36) | 22.65 (5.36) | 25.13 (I8.87) | 0.045 | |
| BMI classification, n (%) | 0.052 | ||||
| Underweight—(<18.5) | 10 (13%) | 5 (12%) | 5 (13%) | ||
| Normal weight—(18.5–24.9) | 39 (49%) | 25 (61%) | 14 (36%) | ||
| Overweight—(25.0–29.9) | 20 (25%) | 9 (22%) | 11 (28%) | ||
| Obesity (>30.0) | 11 (13%) | 2 (5%) | 9 (24%) | ||
| CONUT score, n (%) | 0.002 * | ||||
| Normal (0–1) | 9 (11%) | 1 (2%) | 8 (21%) | ||
| Mild (2–4) | 49 (61%) | 22 (54%) | 27 (69%) | ||
| Moderate (5–8) | 19 (24%) | 15 (37%) | 4 (10%) | ||
| Severe (9–12) | 3 (4%) | 3 (7%) | 0 (0%) | ||
| Biochemical characteristics | N | ||||
| Total proteins (g/L), mean ± SD | 80 | 62.83 ± 8.9 | 65.98 ± 8.85 | 59.51 ± 7.77 | 0.001 |
| Albumin (g/L), median (IQR) | 80 | 38.00 (5) | 39.00 (6) | 36.00 (6) | 0.090 |
| Prealbumin (g/L), mean (SD) | 45 | 0.18 (0.07) | 0.21 (0.07) | 0.15 (0.07) | 0.006 |
| Total cholesterol mg/dL, median (IQR) | 80 | 156.00 (76) | 125.00 (59) | 175.00 (68) | 0.000 |
| Triglycerides (mg/dL), median (IQR) | 80 | 101.5 (56) | 101.00 (55) | 107.00 (48) | 0.461 |
| Phosphorus (mg/dL), median (IQR) | 64 | 3.45 (1.2) | 3.75 (1.1) | 3.20 (0.6) | 0.005 |
| Magnesium (mg/dL), mean (SD) | 77 | 2.05 (0.31) | 2.10 (0.38) | 1.99 (0.22) | 0.118 |
| Zinc (µg/dL), median (IQR) | 31 | 71.45 (14.21) | 68.00 (12.23) | 73.94 (15.33) | 0.257 |
| Calcium (mg/dL), median (IQR) | 78 | 8.70 (0.5) | 9.00 (0.8) | 8.60 (0.5) | 0.019 |
| Creatinine (mg/dL), median (IQR) | 80 | 1.15 (1.41) | 2.05 (1.75) | 0.81 (0.25) | 0.001 |
| Lymphocyte (×109/L), median (IQR) | 80 | 1.20 (1.00) | 0.82 (0.80) | 1.70 (1.10) | 0.000 |
| PCR (mg/dL), median (IQR) | 72 | 1.05 (2.77) | 1.07 (1.46) | 0.40 (5.50) | 0.312 |
| Variable | Normal/Mild (N = 58) | Moderate/Severe (N = 22) | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Demographics | ||||
| Sex, male, n (%) | 39 (67%) | 13 (51%) | Ref. | 0.601 * |
| Female, n (%) | 19 (33%) | 9 (41%) | 1.42 (0.51–3.94) | |
| Age, median (IQR) | 78.0 (8.0) | 78.5 (9.0) | 0.99 (0.93–1.06) | 0.871 ** |
| Clinical Variables, n (%) | 0.001 * | |||
| Respiratory | 35 (60%) | 4 (18%) | Ref. | |
| Specialty, Cardiology | 23 (40%) | 18 (82%) | 6.85 (2.06–22.78) | |
| NECPAL, n (%) | 0.013 * | |||
| NECPAL Stage I | 9 (15%) | 2 (9%) | 0.22 (0.04–1.26) | |
| NECPAL Stage II | 37 (64%) | 8 (36%) | 0.22 (0.07–0.66) | |
| NECPAL Stage III | 12 (21%) | 12 (55%) | Ref. | |
| PPS, n (%) | 0.001 * | |||
| PPS >60 | 34 (59%) | 7 (32%) | Ref. | |
| PPS 30–50 | 24 (41%) | 11 (50%) | 2.23 (0.75–6.53) | |
| PPS 10–20 | 0 (0%) | 4 (18%) | Not calculable ‡ | |
| Barthel, n (%) | 0.007 * | |||
| Barthel: Independent | 21 (36%) | 2 (9%) | Ref. | |
| Barthel: Mild dependence | 26 (45%) | 16 (73%) | 6.46 (1.33–31.37) | |
| Barthel: Moderate/Severe | 11 (19%) | 4 (18%) | 3.82 (0.62–23.52) | |
| LVEF, n (%) | 0.642 * | |||
| Normal (≥50–55) | 5 (22%) | 6 (33%) | Ref. | |
| Mildly (41–49%) | 2 (9%) | 3 (17%) | 1.25 (0.15–10.44) | |
| Reduced (≤40%) | 5 (22%) | 3 (17%) | 0.50 (0.08–3.20) | |
| Severely reduced (<30%) | 11 (48%) | 6 (33%) | 0.45 (0.10–2.11) | |
| Symptoms | ||||
| Pain (Yes) | 43 (74%) | 22 (100%) | Not calculable ‡ | 0.008 * |
| Dysphagia (Yes) | 6 (10%) | 3 (14%) | 1.37 (0.31–6.03) | 0.700 * |
| Anorexia (Yes) | 24 (41%) | 14 (64%) | 2.48 (0.89–6.91) | 0.086 * |
| Xerostomy (Yes) | 24 (41%) | 11 (50%) | 1.42 (0.52–3.85) | 0.615 * |
| BMI classification, n (%) | 0.403 * | |||
| Underweight—(<18.5) | 6 (10%) | 4 (18%) | 6.67 (0.60–74.34) | |
| Normal weight—(18.5–24.9) | 27 (47%) | 12 (54%) | 4.44 (0.51–38.74) | |
| Overweight—(25.0–29.9) | 15 (26%) | 5 (23%) | 3.33 (0.34–32.83) | |
| Obesity (>30.0) | 10 (17%) | 1 (5%) | Ref. | |
| Nutritional characteristics, n (%) | ||||
| Weight loss in the last 6 month | 14 (24%) | 4 (18%) | 0.70 (0.20–2.42) | 0.766 * |
| Nutritional follow-up (Yes) | 27 (47%) | 17 (77%) | 3.91 (1.29–11.86) | 0.022 * |
| Protein supplementation (Yes) | 21 (36%) | 13 (60%) | 2.55 (0.93–6.96) | 0.080 * |
| Biochemical characteristics | ||||
| Albumin, g/L (median) | 39.0 (5.0) | 30.5 (7.0) | 0.85 (0.77–0.94) † | 0.004 * |
| Total cholesterol, mg/dL | 165.0 (72.0) | 119.0 (37.0) | 0.98 (0.97–0.99) † | 0.001 * |
| Total protein, g/L | 64.0 (10.0) | 58.0 (12.0) | 0.94 (0.89–0.99) † | 0.029 * |
| Creatinine, mg/dL | 0.98 (1.10) | 1.42 (1.80) | 1.36 (0.99–1.87) † | 0.047 * |
| Lymphocytes, (×109/L) | 1.3 (1.0) | 0.9 (0.8) | 0.54 (0.31–0.93) † | 0.002 * |
| Predictor | OR (Exp(B)) | 95% CI | p-Value |
|---|---|---|---|
| Specialty (Cardiology vs. Pulmonology) | 9.07 | 2.33–35.25 | 0.001 |
| PPS (<50 vs. >60) | 4.24 | 1.20–14.94 | 0.025 |
| Nutritional follow-up (No vs. Yes) | 0.42 | 0.12–1.48 | 0.176 |
| Sex (Female vs. Male) | 1.47 | 0.45–4.82 | 0.524 |
| Age (per year) | 1.05 | 0.96–1.15 | 0.291 |
| Predictor | OR (Exp(B)) | 95% CI | p-Value |
|---|---|---|---|
| Specialty (Cardiology vs. Pulmonology) | 9.30 | 2.90–30.30 | <0.001 |
| PPS (<50 vs. >60) | 3.65 | 1.30–13.00 | 0.014 |
| Age (per year increase) | 1.06 | 0.99–1.14 | 0.100 |
| Nutritional follow-up (No vs. Yes) | 0.50 | 0.18–1.42 | 0.196 |
| Sex (Female vs. Male) | 1.32 | 0.51–3.58 | 0.580 |
| Variable | Group | Median Survival (Days) | 95% CI | Log-Rank p-Value |
|---|---|---|---|---|
| Specialty | Cardiology | 177 | 28–326 | 0.797 |
| Pulmonology | 220 | 146–294 | ||
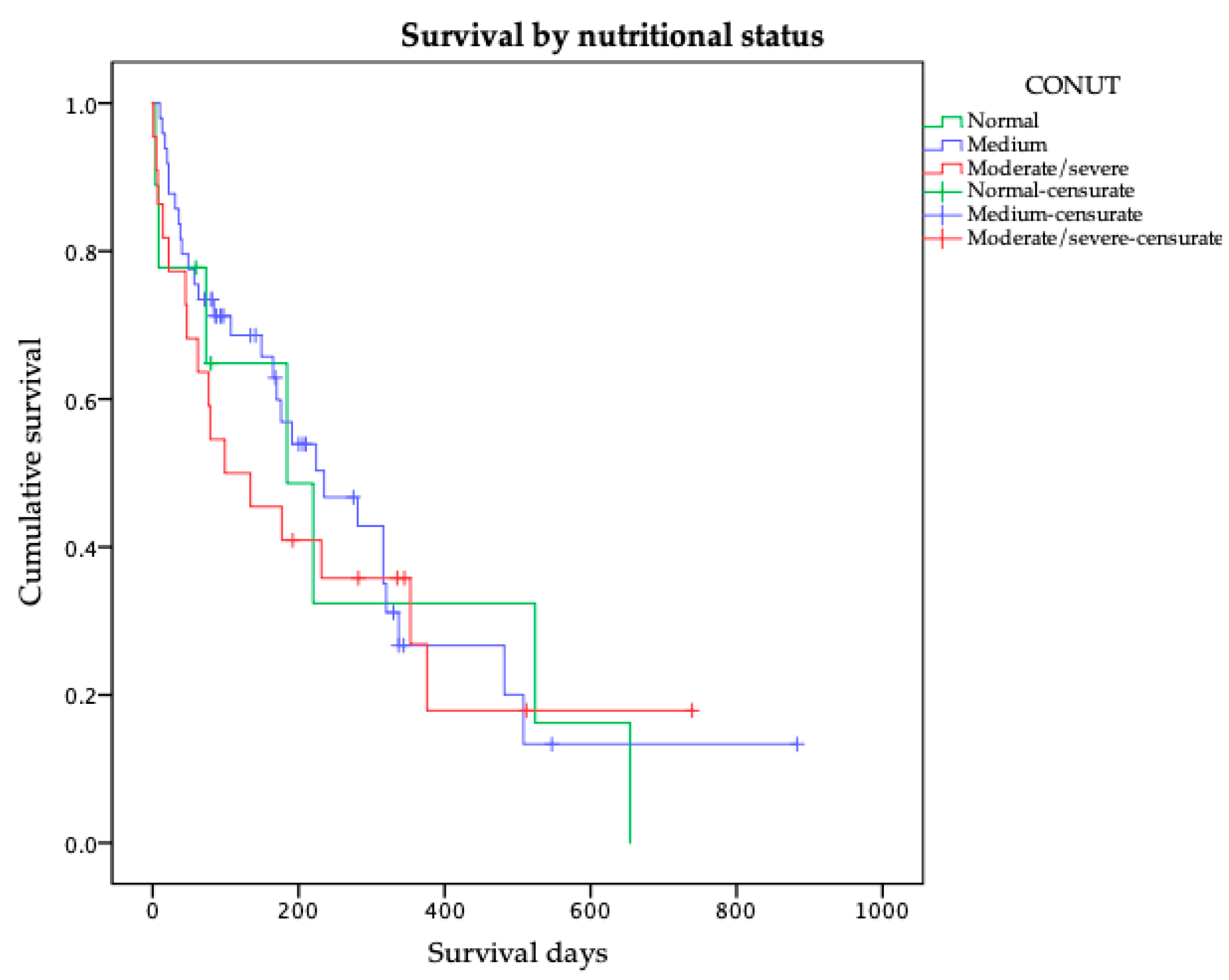
| CONUT (Score) | Normal/Mild (<5) | 224 | 157–291 | 0.635 |
| Moderate/Severe (≥5) | 99 | 0–214 | ||
| Nutritional Status (BMI) | Undernutrition | 170 | 133–207 | 0.590 |
| Normal weight | 177 | 27–327 | ||
| Overweight | 320 | 119–521 | ||
| Obesity | 235 | 7–463 | ||
| Nutritional Follow-up | No | 191 | 78–304 | 0.412 |
| Yes | 231 | 91–371 | ||
| Protein Supplements | No | 220 | 159–281 | 0.882 |
| Yes | 177 | 78–276 |
| Predictor | HR (Exp(B)) | 95% CI for HR | p-Value |
|---|---|---|---|
| Nutritional follow up (No vs. Yes) | 1.430 | 0.757–2.699 | 0.254 |
| Specialty (Cardiology vs. Respiratory) | 0.878 | 0.447–1.725 | 0.704 |
| CONUT–Normal vs. Moderate/Severe | 0.978 | 0.334–2.862 | 0.967 |
| CONUT—Mild vs. Moderate/Severe | 0.747 | 0.376–1.486 | 0.409 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellicé, M.; Ladino, A.; Treviño-García, K.B.; Suárez-Lombraña, A.; Arroyo-Huidobro, M.; Capdevila-Reniu, A.; Solari, B.D.; Sacanella, E.; Perez-Castejon, J.M.; Masanes, F. Nutritional Vulnerability and Functional Decline in End-Stage Heart Failure and Chronic Respiratory Disease: Utility of the CONUT Score in a Palliative Cohort. Nutrients 2025, 17, 3040. https://doi.org/10.3390/nu17193040
Pellicé M, Ladino A, Treviño-García KB, Suárez-Lombraña A, Arroyo-Huidobro M, Capdevila-Reniu A, Solari BD, Sacanella E, Perez-Castejon JM, Masanes F. Nutritional Vulnerability and Functional Decline in End-Stage Heart Failure and Chronic Respiratory Disease: Utility of the CONUT Score in a Palliative Cohort. Nutrients. 2025; 17(19):3040. https://doi.org/10.3390/nu17193040
Chicago/Turabian StylePellicé, Martina, Andrea Ladino, Karla Belén Treviño-García, Ana Suárez-Lombraña, Marta Arroyo-Huidobro, Aina Capdevila-Reniu, Bryan David Solari, Emilio Sacanella, Juan Manuel Perez-Castejon, and Ferran Masanes. 2025. "Nutritional Vulnerability and Functional Decline in End-Stage Heart Failure and Chronic Respiratory Disease: Utility of the CONUT Score in a Palliative Cohort" Nutrients 17, no. 19: 3040. https://doi.org/10.3390/nu17193040
APA StylePellicé, M., Ladino, A., Treviño-García, K. B., Suárez-Lombraña, A., Arroyo-Huidobro, M., Capdevila-Reniu, A., Solari, B. D., Sacanella, E., Perez-Castejon, J. M., & Masanes, F. (2025). Nutritional Vulnerability and Functional Decline in End-Stage Heart Failure and Chronic Respiratory Disease: Utility of the CONUT Score in a Palliative Cohort. Nutrients, 17(19), 3040. https://doi.org/10.3390/nu17193040







