Lepidium meyenii Walpers Promotes the Regeneration of Salivary Gland and Prevents Xerostomia After Irradiation Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lepidium Meyenii Walpers Extraction (LMWE)
2.2.1. Purchase and Configuration of LMWE
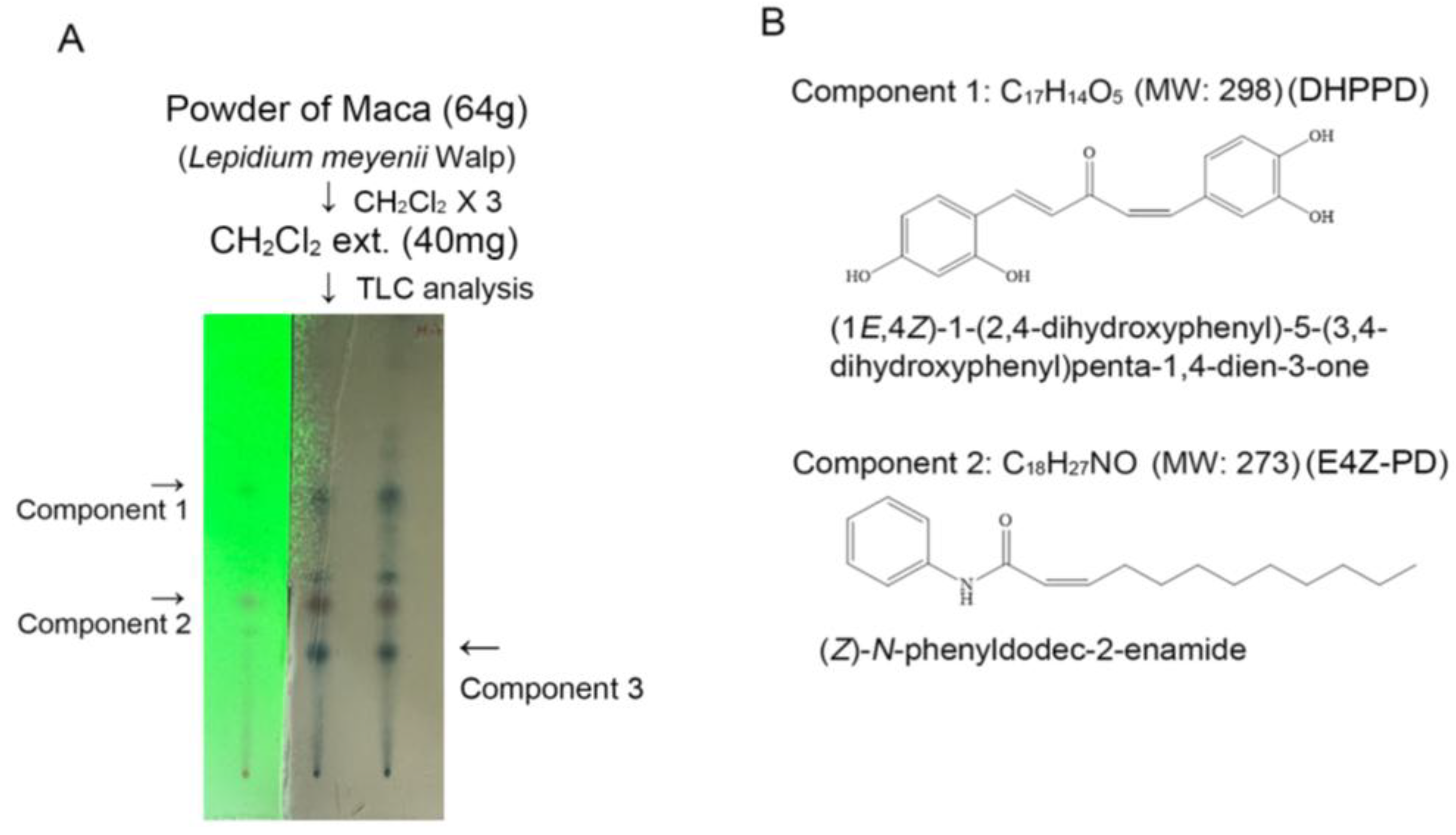
2.2.2. Analysis and Purification of Macaene Derivatives and Macamides
2.3. Animal Study
2.3.1. Ethical Approval
2.3.2. Induction of Salivary Gland Damage in Mice Through γ-Ray Radiation
2.3.3. Animal Grouping and Treatment
2.3.4. Morphological Analysis
2.3.5. Analysis of Salivary Lag Time, Saliva Secretion Volume, and Salivary Amylase
2.3.6. Biochemical Measurements
2.4. In Vitro Study
2.4.1. Cultivation of Par-C10 Cells and Salivary Organoid Preparation
2.4.2. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
2.4.3. Cellular Senescence Assay
2.4.4. Transepithelial Electrical Resistance (TEER) Assay for Cellular Barrier Capacity
2.4.5. Seahorse XFp Platform for Bioenergetic Analysis
2.4.6. Mitochondrial Potential Assay
2.4.7. Identifying the Markers of Salivary Gland Organoids
2.5. Statistical Analyses
3. Results
3.1. LMWE Primarily Contains (Z)-N-Phenyldodec-2-enamide and (1E,4Z)-1-(2,4-Dihydroxyphenyl)-5-(3,4-dihydroxyphenyl) Penta-1,4-dien-3-one
3.2. Administration of LMWE Did Not Affect the Biochemical Characteristics in C57BL/6 Mice
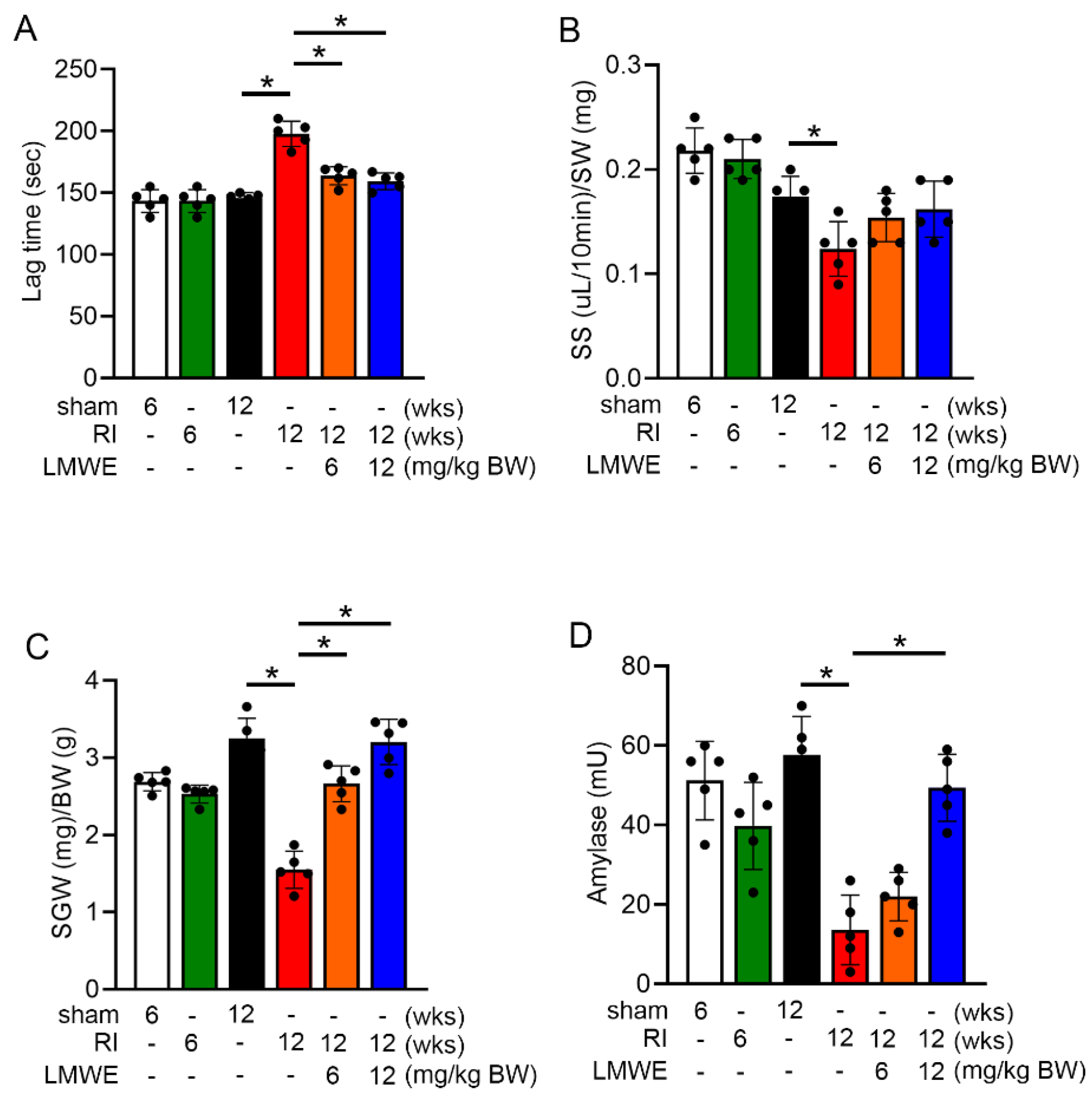
3.3. LMWE Reversed Reduction in Saliva Secretion and Prolonged Secretion Lag Time in Radiation-Exposed C57BL/6 Mice
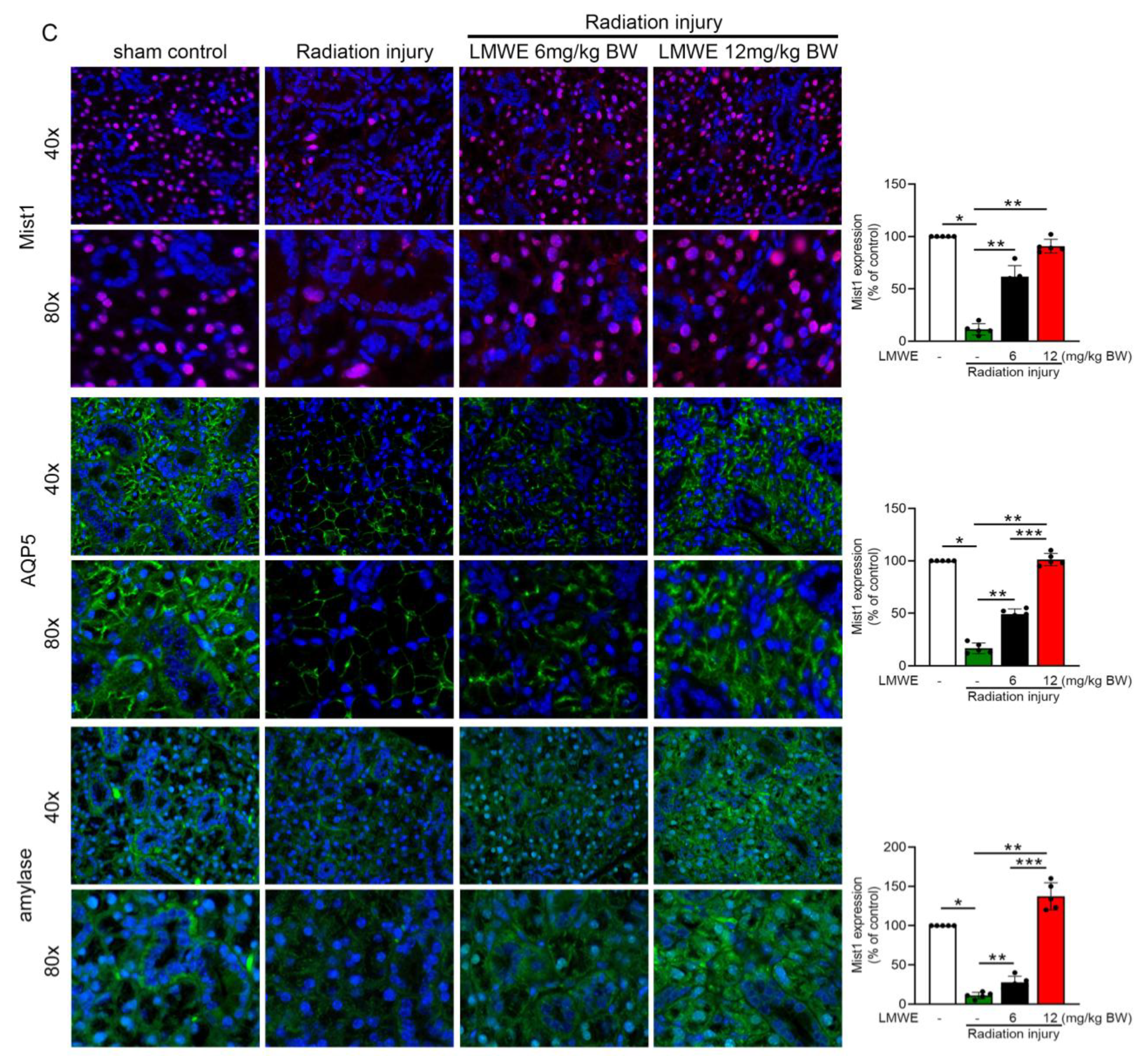
3.4. LMWE Protected Acinar Cells in Radiation-Exposed C57BL/6 Mice
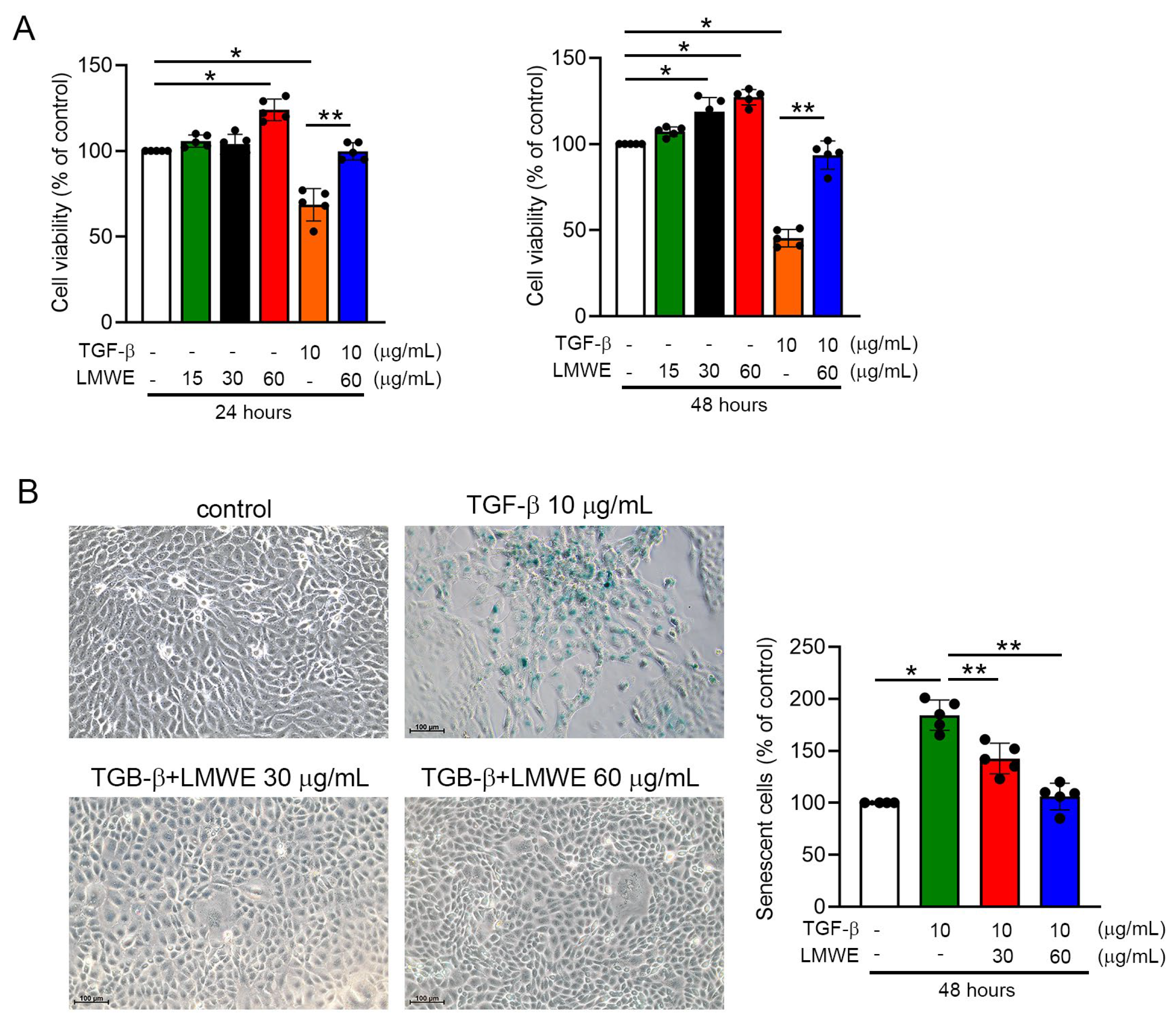
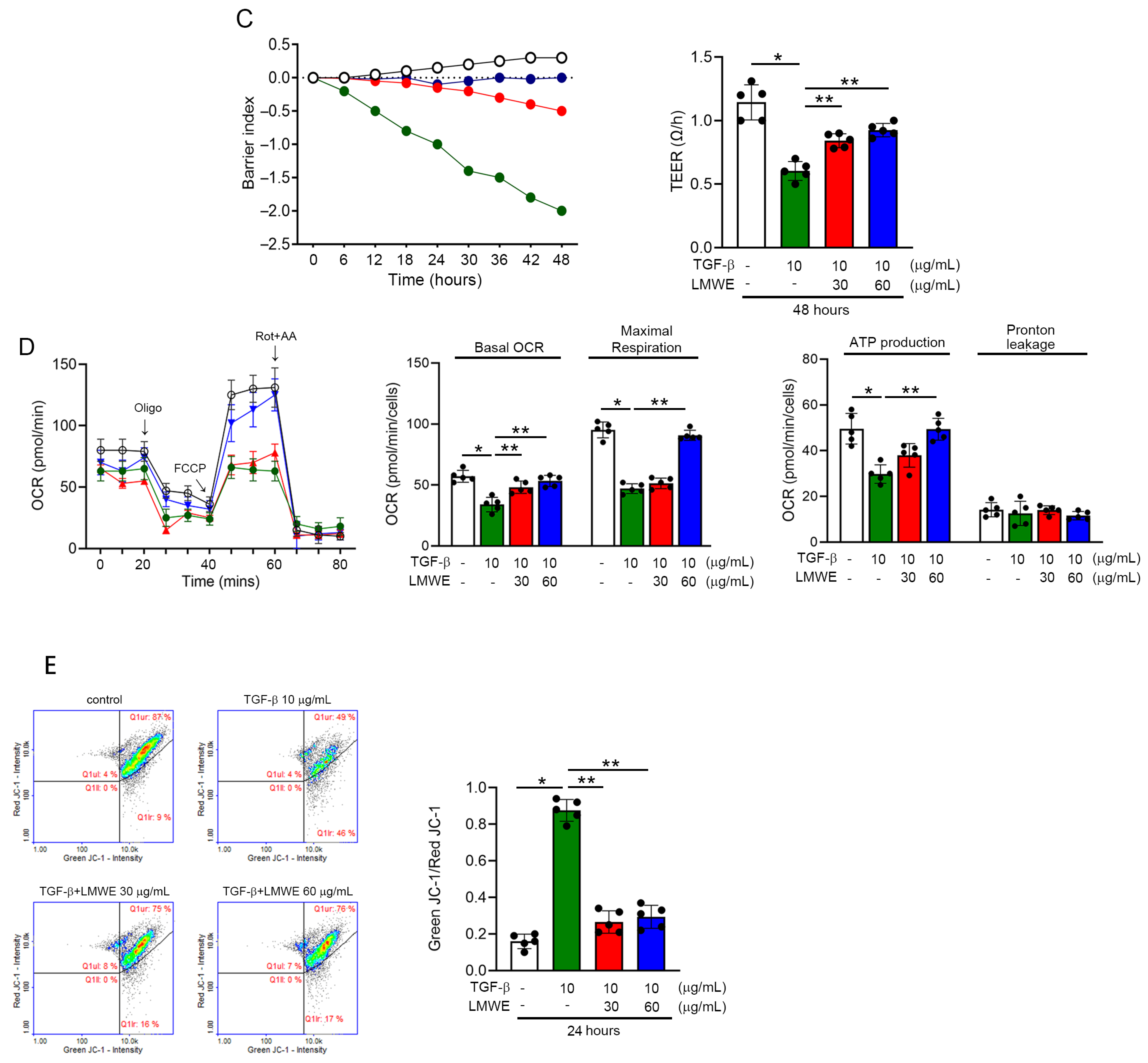
3.5. LMWE Survives Mitochondrial Membrane Potential and Function to Prevent Par-C10 Cell Function in TGF-β-Stimulated Situation
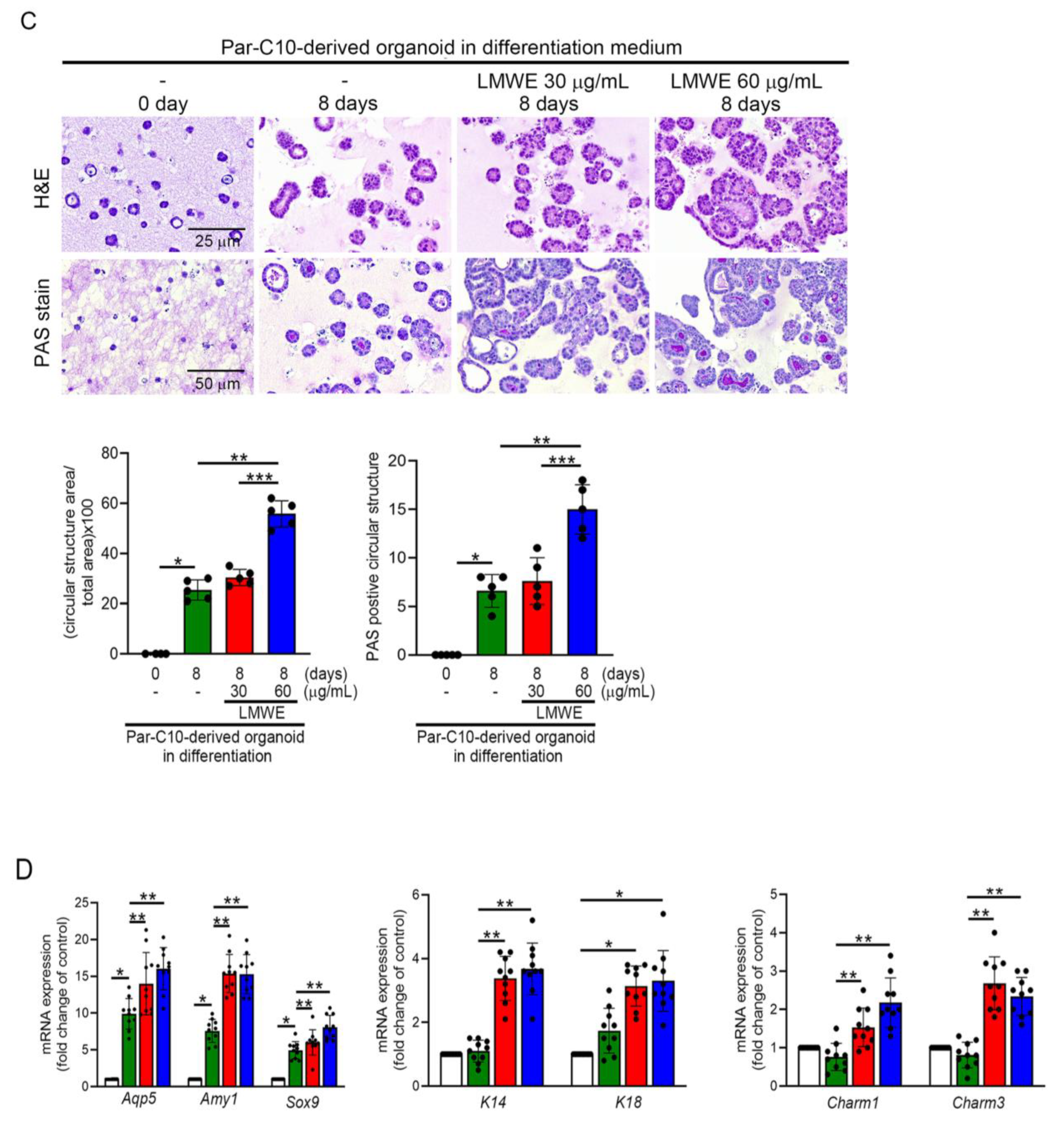
3.6. LMWE Accelerates the Differentiation of Par-C10 Cell-Derived Organoids
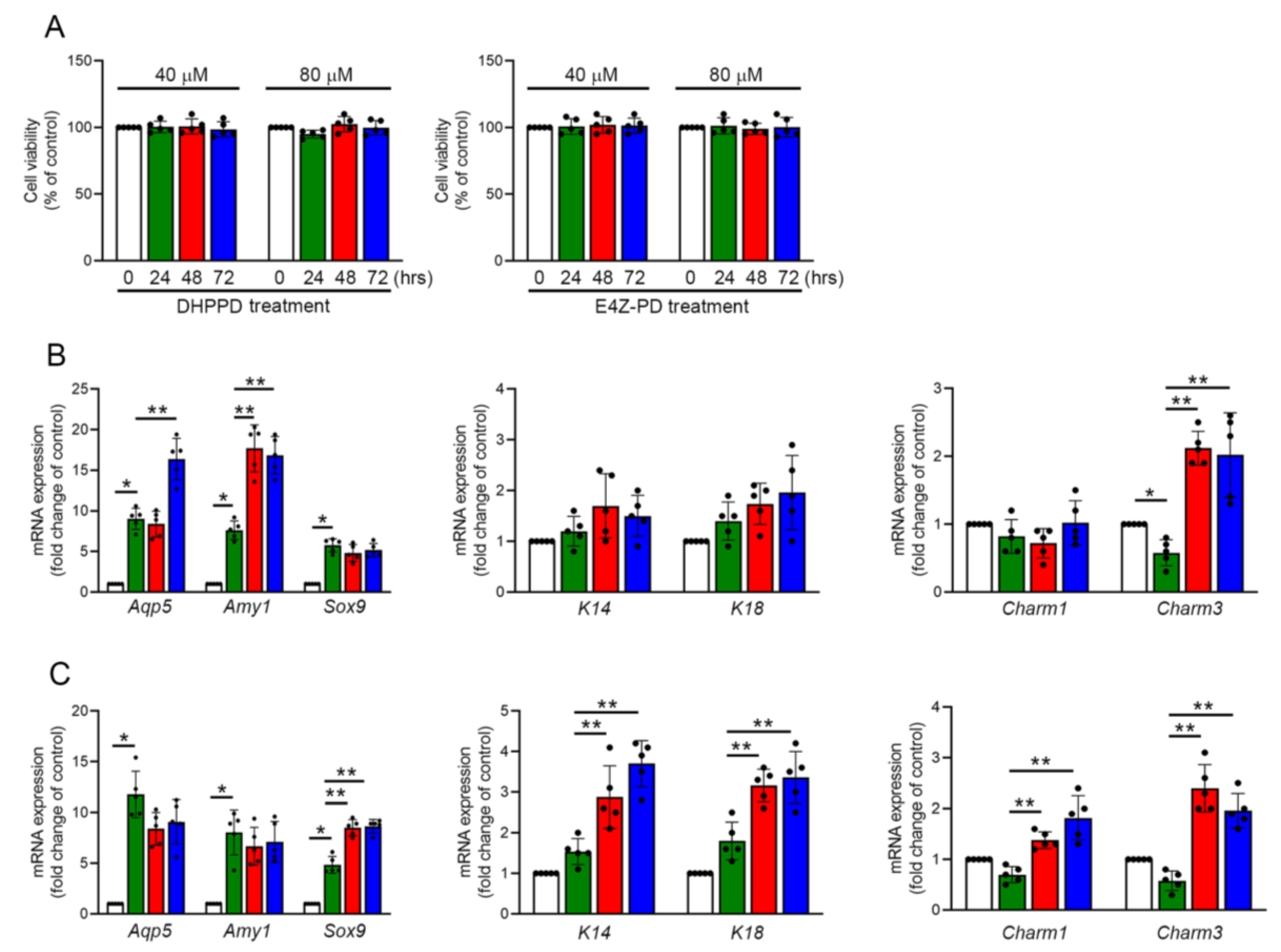
3.7. DHPPD and E4Z-PD Contained in LMWE Exhibit Different Effects on the Differentiation of Praa-C10 Cell-Derived Organoids
4. Discussion
5. Conclusions
6. Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BUN | Blood urea nitrogen |
| CRP | C-reactive protein |
| DHPPD | (1E,4Z)-1-(2,4-dihydroxyphenyl)-5-(3,4-dihydroxyphenyl) penta-1,4-dien-3-one |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl sulfoxide |
| E4Z-PD | (Z)-N-phenyldodec-2-enamide |
| EGF | Epidermal growth factor |
| FBS | Fetal bovine serum |
| FGF-2 | Fibroblast growth factor 2 |
| H&E | Hematoxylin and eosin |
| IHC | Immunohistochemistry |
| IF | Immunofluorescence |
| ITS-G | Insulin–transferrin–selenium supplement |
| JC-1 | 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide |
| LDH | Lactate dehydrogenase |
| LMWE | Lepidium meyenii extract |
| OCR | Oxygen consumption rate |
| Par-C10 | Rat parotid acinar cell line |
| PAS | Periodic acid–Schiff |
| RT-qPCR | Reverse transcription quantitative polymerase chain reaction |
| TGF-β1 | Transforming growth factor beta 1 |
| TEER | Transepithelial electrical resistance |
References
- Napenas, J.J.; Brennan, M.T.; Fox, P.C. Diagnosis and treatment of xerostomia (dry mouth). Odontology 2009, 97, 76–83. [Google Scholar] [CrossRef]
- Pedersen, A.M.L.; Sorensen, C.E.; Proctor, G.B.; Carpenter, G.H.; Ekstrom, J. Salivary secretion in health and disease. J. Oral Rehabil. 2018, 45, 730–746. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.J.; McGurk, M. Incidence of salivary gland neoplasms in a defined UK population. Br. J. Oral Maxillofac. Surg. 2013, 51, 399–403. [Google Scholar] [CrossRef]
- Jensen, S.B.; Pedersen, A.M.; Vissink, A.; Andersen, E.; Brown, C.G.; Davies, A.N.; Dutilh, J.; Fulton, J.S.; Jankovic, L.; Lopes, N.N.; et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: Prevalence, severity and impact on quality of life. Support. Care Cancer 2010, 18, 1039–1060. [Google Scholar] [CrossRef]
- Stewart, C.M.; Berg, K.M.; Cha, S.; Reeves, W.H. Salivary dysfunction and quality of life in Sjogren syndrome: A critical oral-systemic connection. J. Am. Dent. Assoc. 2008, 139, 291–299, quiz 358-299. [Google Scholar] [CrossRef]
- Perez, P.; Kwon, Y.J.; Alliende, C.; Leyton, L.; Aguilera, S.; Molina, C.; Labra, C.; Julio, M.; Leyton, C.; Gonzalez, M.J. Increased acinar damage of salivary glands of patients with Sjogren’s syndrome is paralleled by simultaneous imbalance of matrix metalloproteinase 3/tissue inhibitor of metalloproteinases 1 and matrix metalloproteinase 9/tissue inhibitor of metalloproteinases 1 ratios. Arthritis Rheum. 2005, 52, 2751–2760. [Google Scholar] [PubMed]
- Yamauchi, Y.; Matsuno, T.; Omata, K.; Satoh, T. Relationship between hyposalivation and oxidative stress in aging mice. J. Clin. Biochem. Nutr. 2017, 61, 40–46. [Google Scholar] [CrossRef]
- Moon, M.Y.; Kim, H.J.; Kim, J.G.; Lee, J.Y.; Kim, J.; Kim, S.C.; Choi, I.G.; Kim, P.H.; Park, J.B. Small GTPase Rap1 regulates cell migration through regulation of small GTPase RhoA activity in response to transforming growth factor-beta1. J. Cell Physiol. 2013, 228, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Guneri, P.; Alpoz, E.; Epstein, J.B.; Cankaya, H.; Ates, M. In vitro antimicrobial effects of commercially available mouth-wetting agents. Spec. Care Dent. 2011, 31, 123–128. [Google Scholar]
- Takesh, T.; Ho, J.; Firmalino, M.V.; Islip, D.; Anbarani, A.; Wilder-Smith, P. Effects of a Novel Formulation on Oral Biofilm, pH Buffering, and Gingival Health in Patients with Dry Mouth. Int. J. Dent. 2018, 2018, 2748274. [Google Scholar] [CrossRef]
- Takakura, A.C.; Moreira, T.S.; Laitano, S.C.; De Luca Junior, L.A.; Renzi, A.; Menani, J.V. Central muscarinic receptors signal pilocarpine-induced salivation. J. Dent. Res. 2003, 82, 993–997. [Google Scholar] [CrossRef]
- Cheng, C.Q.; Xu, H.; Liu, L.; Wang, R.N.; Liu, Y.T.; Li, J.; Zhou, X.K. Efficacy and safety of pilocarpine for radiation-induced xerostomia in patients with head and neck cancer: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2016, 147, 236–243. [Google Scholar] [CrossRef]
- Chitapanarux, I.; Kamnerdsupaphon, P.; Tharavichitkul, E.; Sumitsawan, Y.; Sittitrai, P.; Pattarasakulchai, T.; Lorvidhaya, V.; Sukthomya, V.; Pukanhaphan, N.; Traisatit, P. Effect of oral pilocarpine on post-irradiation xerostomia in head and neck cancer patients: A single-center, single-blind clinical trial. J. Med. Assoc. Thai 2008, 91, 1410–1415. [Google Scholar]
- Kanat, O.; Evrensel, T.; Baran, I.; Coskun, H.; Zarifoglu, M.; Turan, O.F.; Kurt, E.; Demiray, M.; Gonullu, G.; Manavoglu, O. Protective effect of amifostine against toxicity of paclitaxel and carboplatin in non-small cell lung cancer: A single center randomized study. Med. Oncol. 2003, 20, 237–245. [Google Scholar] [CrossRef]
- Koukourakis, M.I. Amifostine in clinical oncology: Current use and future applications. Anticancer. Drugs 2002, 13, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Antonadou, D.; Pepelassi, M.; Synodinou, M.; Puglisi, M.; Throuvalas, N. Prophylactic use of amifostine to prevent radiochemotherapy-induced mucositis and xerostomia in head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Damron, T.A.; Spadaro, J.A.; Tamurian, R.M.; Damron, L.A. Sparing of radiation-induced damage to the physis: Fractionation alone compared to amifostine pretreatment. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Bardet, E.; Martin, L.; Calais, G.; Alfonsi, M.; Feham, N.E.; Tuchais, C.; Boisselier, P.; Dessard-Diana, B.; Seng, S.H.; Garaud, P.; et al. Subcutaneous compared with intravenous administration of amifostine in patients with head and neck cancer receiving radiotherapy: Final results of the GORTEC2000-02 phase III randomized trial. J. Clin. Oncol. 2011, 29, 127–133. [Google Scholar] [CrossRef]
- Brizel, D.M.; Wasserman, T.H.; Henke, M.; Strnad, V.; Rudat, V.; Monnier, A.; Eschwege, F.; Zhang, J.; Russell, L.; Oster, W.; et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J. Clin. Oncol. 2000, 18, 3339–3345. [Google Scholar] [CrossRef]
- Park, B.; Noh, H.; Choi, D.J. Herbal Medicine for Xerostomia in Cancer Patients: A Systematic Review of Randomized Controlled Trials. Integr. Cancer Ther. 2018, 17, 179–191. [Google Scholar] [CrossRef]
- Lim, R.J.; Nik Nabil, W.N.; Chan, S.Y.; Wong, Y.F.; Han, L.X.; Gong, J.Y.; Ho, K.L.; Shew, Y.S.; Xu, L. Effects of herbal medicine for xerostomia in head and neck cancer patients: An observational study in a tertiary cancer hospital. Support. Care Cancer 2019, 27, 3491–3498. [Google Scholar] [CrossRef]
- Han, G.; Ko, S.J.; Kim, J.; Oh, J.Y.; Park, J.W.; Kim, J. A randomized, double-blind, placebo-controlled trial of a traditional herbal formula, Yukmijihwang-tang in elderly subjects with xerostomia. J. Ethnopharmacol. 2016, 182, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Nik Nabil, W.N.; Lim, R.J.; Chan, S.Y.; Lai, N.M.; Liew, A.C. A systematic review on Chinese herbal treatment for radiotherapy-induced xerostomia in head and neck cancer patients. Complement. Ther. Clin. Pract. 2018, 30, 6–13. [Google Scholar] [CrossRef]
- Sardellitti, L.; Bortone, A.; Filigheddu, E.; Serralutzu, F.; Milia, E.P. Xerostomia: From Pharmacological Treatments to Traditional Medicine-An Overview on the Possible Clinical Management and Prevention Using Systemic Approaches. Curr. Oncol. 2023, 30, 4412–4426. [Google Scholar] [CrossRef]
- Kontogiannopoulos, K.N.; Kapourani, A.; Gkougkourelas, I.; Anagnostaki, M.E.; Tsalikis, L.; Assimopoulou, A.N.; Barmpalexis, P. A Review of the Role of Natural Products as Treatment Approaches for Xerostomia. Pharmaceuticals 2023, 16, 1136. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, H.W.; You, S.; Ha, K.T. The use of maca (Lepidium meyenii) to improve semen quality: A systematic review. Maturitas 2016, 92, 64–69. [Google Scholar] [CrossRef]
- Wu, H.; Kelley, C.J.; Pino-Figueroa, A.; Vu, H.D.; Maher, T.J. Macamides and their synthetic analogs: Evaluation of in vitro FAAH inhibition. Bioorg Med. Chem. 2013, 21, 5188–5197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tian, Y.; Yan, L.; Zhang, G.; Wang, X.; Zeng, Y.; Zhang, J.; Ma, X.; Tan, Y.; Long, N.; et al. Genome of Plant Maca (Lepidium meyenii) Illuminates Genomic Basis for High-Altitude Adaptation in the Central Andes. Mol. Plant 2016, 9, 1066–1077. [Google Scholar] [CrossRef]
- Patel, S.S.; Raghuwanshi, R.; Masood, M.; Acharya, A.; Jain, S.K. Medicinal plants with acetylcholinesterase inhibitory activity. Rev. Neurosci. 2018, 29, 491–529. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, F.; Jikyo, T.; Takeda, R.; Ogata, M. Lepidium meyenii (Maca) enhances the serum levels of luteinising hormone in female rats. J. Ethnopharmacol. 2014, 151, 897–902. [Google Scholar] [CrossRef]
- Vecera, R.; Orolin, J.; Skottova, N.; Kazdova, L.; Oliyarnik, O.; Ulrichova, J.; Simanek, V. The influence of maca (Lepidium meyenii) on antioxidant status, lipid and glucose metabolism in rat. Plant Foods Hum. Nutr. 2007, 62, 59–63. [Google Scholar] [CrossRef]
- Gonzales, G.F.; Valerio, L.G., Jr. Medicinal plants from Peru: A review of plants as potential agents against cancer. Anticancer Agents Med. Chem. 2006, 6, 429–444. [Google Scholar] [CrossRef]
- Barberis, C.; Pribish, J.; Tserlin, E.; Gross, A.; Czekaj, M.; Barrague, M.; Erdman, P.; Maniar, S.; Jiang, J.; Fire, L.; et al. Discovery of N-substituted 7-azaindoles as Pan-PIM kinases inhibitors—Lead optimization—Part III. Bioorg. Med. Chem. Lett. 2019, 29, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.L.; He, K.; Kim, C.H.; Rogers, L.; Shao, Y.; Huang, Z.Y.; Lu, Y.; Yan, S.J.; Qien, L.C.; Zheng, Q.Y. Effect of a lipidic extract from Lepidium meyenii on sexual behavior in mice and rats. Urology 2000, 55, 598–602. [Google Scholar] [CrossRef] [PubMed]
- McKay, D. Nutrients and botanicals for erectile dysfunction: Examining the evidence. Altern. Med. Rev. 2004, 9, 4–16. [Google Scholar]
- McCollom, M.M.; Villinski, J.R.; McPhail, K.L.; Craker, L.E.; Gafner, S. Analysis of macamides in samples of Maca (Lepidium meyenii) by HPLC-UV-MS/MS. Phytochem. Anal. 2005, 16, 463–469. [Google Scholar] [CrossRef]
- Sandoval, M.; Okuhama, N.N.; Angeles, F.M.; Melchor, V.V.; Condezo, L.A.; Lao, J.; Miller, M.J.S. Antioxidant activity of the cruciferous vegetable Maca (Lepidium meyenii). Food Chem. 2002, 79, 7. [Google Scholar] [CrossRef]
- Ulloa Del Carpio, N.; Alvarado-Corella, D.; Quinones-Laveriano, D.M.; Araya-Sibaja, A.; Vega-Baudrit, J.; Monagas-Juan, M.; Navarro-Hoyos, M.; Villar-Lopez, M. Exploring the chemical and pharmacological variability of Lepidium meyenii: A comprehensive review of the effects of maca. Front. Pharmacol. 2024, 15, 1360422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yun, J.S.; Han, D.; Yook, J.I.; Kim, H.S.; Cho, E.S. TGF-beta Pathway in Salivary Gland Fibrosis. Int. J. Mol. Sci. 2020, 21, 9138. [Google Scholar] [CrossRef]
- Cicero, A.F.; Bandieri, E.; Arletti, R. Lepidium meyenii Walp. improves sexual behaviour in male rats independently from its action on spontaneous locomotor activity. J. Ethnopharmacol. 2001, 75, 225–229. [Google Scholar] [CrossRef]
- Cicero, A.F.; Piacente, S.; Plaza, A.; Sala, E.; Arletti, R.; Pizza, C. Hexanic Maca extract improves rat sexual performance more effectively than methanolic and chloroformic Maca extracts. Andrologia 2002, 34, 177–179. [Google Scholar] [CrossRef]
- Valentova, K.; Buckiova, D.; Kren, V.; Peknicova, J.; Ulrichova, J.; Simanek, V. The in vitro biological activity of Lepidium meyenii extracts. Cell Biol. Toxicol. 2006, 22, 91–99. [Google Scholar] [CrossRef]
- Tang, W.; Jin, L.; Xie, L.; Huang, J.; Wang, N.; Chu, B.; Dai, X.; Liu, Y.; Wang, R.; Zhang, Y. Structural Characterization and Antifatigue Effect In Vivo of Maca (Lepidium meyenii Walp) Polysaccharide. J. Food Sci. 2017, 82, 757–764. [Google Scholar] [CrossRef]
- Beharry, S.; Heinrich, M. Is the hype around the reproductive health claims of maca (Lepidium meyenii Walp.) justified? J. Ethnopharmacol. 2018, 211, 126–170. [Google Scholar] [CrossRef]
- Varghese, J.J.; Schmale, I.L.; Mickelsen, D.; Hansen, M.E.; Newlands, S.D.; Benoit, D.S.W.; Korshunov, V.A.; Ovitt, C.E. Localized Delivery of Amifostine Enhances Salivary Gland Radioprotection. J. Dent. Res. 2018, 97, 1252–1259. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, J.M.; Choi, M.E.; Kim, S.K.; Kim, Y.M.; Choi, J.S. Adipose-derived mesenchymal stem cells regenerate radioiodine-induced salivary gland damage in a murine model. Sci. Rep. 2019, 9, 15752. [Google Scholar] [CrossRef]
- Quissell, D.O.; Barzen, K.A.; Redman, R.S.; Camden, J.M.; Turner, J.T. Development and characterization of SV40 immortalized rat parotid acinar cell lines. In Vitro Cell Dev. Biol. Anim. 1998, 34, 58–67. [Google Scholar] [CrossRef]
- McCall, A.D.; Nelson, J.W.; Leigh, N.J.; Duffey, M.E.; Lei, P.; Andreadis, S.T.; Baker, O.J. Growth factors polymerized within fibrin hydrogel promote amylase production in parotid cells. Tissue Eng. Part A 2013, 19, 2215–2225. [Google Scholar] [CrossRef]
- Sui, Y.; Zhang, S.; Li, Y.; Zhang, X.; Hu, W.; Feng, Y.; Xiong, J.; Zhang, Y.; Wei, S. Generation of functional salivary gland tissue from human submandibular gland stem/progenitor cells. Stem Cell Res. Ther. 2020, 11, 127. [Google Scholar] [CrossRef]
- Van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar]
- Bandyopadhyay, D.; Gatza, C.; Donehower, L.A.; Medrano, E.E. Analysis of cellular senescence in culture in vivo: The senescence-associated beta-galactosidase assay. Curr. Protoc. Cell Biol. 2005, 27, 18.9.1–18.9.9. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Desousa, B.R.; Kim, K.K.; Jones, A.E.; Ball, A.B.; Hsieh, W.Y.; Swain, P.; Morrow, D.H.; Brownstein, A.J.; Ferrick, D.A.; Shirihai, O.S.; et al. Calculation of ATP production rates using the Seahorse XF Analyzer. EMBO Rep. 2023, 24, e56380. [Google Scholar] [CrossRef]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the Mitochondrial Membrane Potential Using the Cationic JC-1 Dye as a Sensitive Fluorescent Probe. Bio Protoc. 2019, 9, e3128. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Dording, C.M.; Fisher, L.; Papakostas, G.; Farabaugh, A.; Sonawalla, S.; Fava, M.; Mischoulon, D. A double-blind, randomized, pilot dose-finding study of maca root (L. meyenii) for the management of SSRI-induced sexual dysfunction. CNS Neurosci. Ther. 2008, 14, 182–191. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Kozumbo, W.J. The hormetic dose-response mechanism: Nrf2 activation. Pharmacol. Res. 2021, 167, 105526. [Google Scholar] [CrossRef]
- Yu, Z.; Jin, W.; Dong, X.; Ao, M.; Liu, H.; Yu, L. Safety evaluation and protective effects of ethanolic extract from maca (Lepidium meyenii Walp.) against corticosterone and H(2)O(2) induced neurotoxicity. Regul. Toxicol. Pharmacol. 2020, 111, 104570. [Google Scholar] [CrossRef]
- Chung, F.; Rubio, J.; Gonzales, C.; Gasco, M.; Gonzales, G.F. Dose-response effects of Lepidium meyenii (Maca) aqueous extract on testicular function and weight of different organs in adult rats. J. Ethnopharmacol. 2005, 98, 143–147. [Google Scholar] [CrossRef]
- Shin, D.; Jeon, S.H.; Piao, J.; Park, H.J.; Tian, W.J.; Moon, D.G.; Ahn, S.T.; Jeon, K.H.; Zhu, G.Q.; Park, I.; et al. Efficacy and Safety of Maca (Lepidium meyenii) in Patients with Symptoms of Late-Onset Hypogonadism: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. World J. Mens Health 2023, 41, 692–700. [Google Scholar] [CrossRef]
- Xiao, A.; He, H.Y.; Chen, Q.; Ma, S.W. Drug-induced Liver Injury Due to Lepidium meyenii (Maca) Medicinal Liquor. Chin. Med. J. 2017, 130, 3005–3006. [Google Scholar] [CrossRef]
- Gonzales, G.F.; Gonzales, C.; Gonzales-Castaneda, C. Lepidium meyenii (Maca): A plant from the highlands of Peru--from tradition to science. Forsch. Komplementmed. 2009, 16, 373–380. [Google Scholar] [CrossRef]
- Rubio, J.; Qiong, W.; Liu, X.; Jiang, Z.; Dang, H.; Chen, S.L.; Gonzales, G.F. Aqueous Extract of Black Maca (Lepidium meyenii) on Memory Impairment Induced by Ovariectomy in Mice. Evid. Based Complement. Altern. Med. 2011, 2011, 253958. [Google Scholar] [CrossRef]
- Sato, M.; Hayashi, Y.; Yoshida, H.; Yanagawa, T.; Yura, Y.; Nitta, T. Search for specific markers of neoplastic epithelial duct and myoepithelial cell lines established from human salivary gland and characterization of their growth in vitro. Cancer 1984, 54, 2959–2967. [Google Scholar] [CrossRef]
- Joraku, A.; Sullivan, C.A.; Yoo, J.J.; Atala, A. Tissue engineering of functional salivary gland tissue. Laryngoscope 2005, 115, 244–248. [Google Scholar] [CrossRef]
- Nanduri, L.S.; Maimets, M.; Pringle, S.A.; van der Zwaag, M.; van Os, R.P.; Coppes, R.P. Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother. Oncol. 2011, 99, 367–372. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.H.; Moon, Y.W.; Hwang, S.; Kim, D.; Jo, S.H.; Oh, S.B.; Kim, J.S.; Jahng, J.W.; Lee, J.H.; et al. Histamine H1 receptor induces cytosolic calcium increase and aquaporin translocation in human salivary gland cells. J. Pharmacol. Exp. Ther. 2009, 330, 403–412. [Google Scholar] [CrossRef]
- Royce, L.S.; Kibbey, M.C.; Mertz, P.; Kleinman, H.K.; Baum, B.J. Human neoplastic submandibular intercalated duct cells express an acinar phenotype when cultured on a basement membrane matrix. Differentiation 1993, 52, 247–255. [Google Scholar] [CrossRef]
- Maria, O.M.; Maria, O.; Liu, Y.; Komarova, S.V.; Tran, S.D. Matrigel improves functional properties of human submandibular salivary gland cell line. Int. J. Biochem. Cell Biol. 2011, 43, 622–631. [Google Scholar] [CrossRef]
- Vasquez, M.M.; Mustafa, S.B.; Choudary, A.; Seidner, S.R.; Castro, R. Regulation of epithelial Na+ channel (ENaC) in the salivary cell line SMG-C6. Exp. Biol. Med. 2009, 234, 522–531. [Google Scholar] [CrossRef]
- Aure, M.H.; Roed, A.; Galtung, H.K. Intracellular Ca2+ responses and cell volume regulation upon cholinergic and purinergic stimulation in an immortalized salivary cell line. Eur. J. Oral Sci. 2010, 118, 237–244. [Google Scholar] [CrossRef]
- Liu, X.B.; Sun, X.; Mork, A.C.; Dodds, M.W.; Martinez, J.R.; Zhang, G.H. Characterization of the calcium signaling system in the submandibular cell line SMG-C6. Proc. Soc. Exp. Biol. Med. 2000, 225, 211–220. [Google Scholar] [CrossRef]
- Castro, R.; Barlow-Walden, L.; Woodson, T.; Kerecman, J.D.; Zhang, G.H.; Martinez, J.R. Ion transport in an immortalized rat submandibular cell line SMG-C6. Proc. Soc. Exp. Biol. Med. 2000, 225, 39–48. [Google Scholar] [CrossRef]
- Baker, O.J.; Schulz, D.J.; Camden, J.M.; Liao, Z.; Peterson, T.S.; Seye, C.I.; Petris, M.J.; Weisman, G.A. Rat parotid gland cell differentiation in three-dimensional culture. Tissue Eng. Part C Methods 2010, 16, 1135–1144. [Google Scholar] [CrossRef]
- Borghese, E. The development in vitro of the submandibular and sublingual glands of Mus musculus. J. Anat. 1950, 84, 287–302. [Google Scholar]
- Patel, V.N.; Rebustini, I.T.; Hoffman, M.P. Salivary gland branching morphogenesis. Differentiation 2006, 74, 349–364. [Google Scholar] [CrossRef]
- Barrows, C.M.L.; Wu, D.; Farach-Carson, M.C.; Young, S. Building a Functional Salivary Gland for Cell-Based Therapy: More than Secretory Epithelial Acini. Tissue Eng. Part A 2020, 26, 1332–1348. [Google Scholar] [CrossRef]
- Gervais, E.M.; Sequeira, S.J.; Wang, W.; Abraham, S.; Kim, J.H.; Leonard, D.; DeSantis, K.A.; Larsen, M. Par-1b is required for morphogenesis and differentiation of myoepithelial cells during salivary gland development. Organogenesis 2016, 12, 194–216. [Google Scholar] [CrossRef]
- Aure, M.H.; Symonds, J.M.; Mays, J.W.; Hoffman, M.P. Epithelial Cell Lineage and Signaling in Murine Salivary Glands. J. Dent. Res. 2019, 98, 1186–1194. [Google Scholar] [CrossRef]
- Song, E.C.; Min, S.; Oyelakin, A.; Smalley, K.; Bard, J.E.; Liao, L.; Xu, J.; Romano, R.A. Genetic and scRNA-seq Analysis Reveals Distinct Cell Populations that Contribute to Salivary Gland Development and Maintenance. Sci. Rep. 2018, 8, 14043. [Google Scholar] [CrossRef]
- Emmerson, E.; May, A.J.; Nathan, S.; Cruz-Pacheco, N.; Lizama, C.O.; Maliskova, L.; Zovein, A.C.; Shen, Y.; Muench, M.O.; Knox, S.M. SOX2 regulates acinar cell development in the salivary gland. eLife 2017, 6, e26620. [Google Scholar] [PubMed]
- Athwal, H.K.; Murphy, G.; Tibbs, E., 3rd; Cornett, A.; Hill, E.; Yeoh, K.; Berenstein, E.; Hoffman, M.P.; Lombaert, I.M.A. Sox10 Regulates Plasticity of Epithelial Progenitors toward Secretory Units of Exocrine Glands. Stem Cell Rep. 2019, 12, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Diaz-de-Valdes, L.; Conejeros, C.; Martinez, R.; Niklander, S. Interventions for the treatment of xerostomia: A randomized controlled clinical trial. J. Clin. Exp. Dent. 2021, 13, e104–e111. [Google Scholar] [CrossRef]
- Connolly, E.L.; Sim, M.; Travica, N.; Marx, W.; Beasy, G.; Lynch, G.S.; Bondonno, C.P.; Lewis, J.R.; Hodgson, J.M.; Blekkenhorst, L.C. Glucosinolates From Cruciferous Vegetables and Their Potential Role in Chronic Disease: Investigating the Preclinical and Clinical Evidence. Front. Pharmacol. 2021, 12, 767975. [Google Scholar] [CrossRef]
- Nicoara, C.; Fezza, F.; Maccarrone, M. FAAH Modulators from Natural Sources: A Collection of New Potential Drugs. Cells 2025, 14, 551. [Google Scholar] [CrossRef]


| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Aqp5 | 5’-ATT GGC TTG TCT GTC ACA CTG G -3’ | 5’-AAT AGG CCC TAC CCA GAA GAC -3’ |
| Amy1 | 5’-TCT GGG TGG TGA AGC AGT GT-3’ | 5’-AAG GGC TCT GTC AGA AGG CA -3’ |
| Sox9 | 5’-ATC TTC AAG GCG CTG CAA GCG -3’ | 5’-ACT TTC CCA GCT TGC ACG TCT G -3’ |
| Krt14 | 5’-CAT CGT CTC AAT TCT CCT CTG GC -3’ | 5’-AAG CCT GAG CAG CAT CTA GA G -3’ |
| Krt18 | 5’-GAA CAT CAA GGT CAA GCT TGA G -3’ | 5’-GAA CTC TGG TAT CAT TGG TCT C -3’ |
| Charm1 | 5’-CTT CAT CCT CAC CTG GAC ACC -3’ | 5’-GTT GCA CAG TGC ATA GCA CAT GG -3’ |
| Charm3 | 5’-GCT GTG CTA TAT CAA CAG CAC CG -3’ | 5’-ACC GAC TGT CTC TGC TGG TAC -3’ |
| GAPDH | 5’-TGC CCC CTC TGC TGA TGC C-3’ | 5’-CCT CCG ACG CCT GCT TCA CCA C-3’ |
| Sham Control | Radiation Injury | Radiation Injury LMWE 6mg/kg BW | Radiation Injury LMWE 12mg/kg BW | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline n = 10 | 6 Weeks n = 10 | 12 Weeks n = 5 | Baseline n = 10 | 6 Weeks n = 10 | 12 Weeks n = 5 | Baseline n = 10 | 6 Weeks n = 10 | 12 Weeks n = 5 | Baseline n = 10 | 6 Weeks n = 10 | 12 Weeks n = 5 | |
| BW (g) | 18.8 ± 1.0 | 22.0 ± 0.9 | 25.1 ± 0.9 | 18.7 ± 1.8 | 21.9 ± 0.9 | 25.5 ± 0.8 | 18.4 ± 0.6 | 21.8 ± 1.1 | 25.6 ± 0.7 | 18.2 ± 1.1 | 21.9 ± 0.8 | 25.8 ± 0.3 |
| BUN (mg/dL) | 25.5 ± 1.5 | 27.6 ± 1.4 | 27.6 ± 2.3 | 24.4 ± 1.8 | 26.8 ± 1.6 | 27.6 ± 1.6 | 25.9 ± 2.1 | 29.4 ± 2.5 | 26.7 ± 2.0 | 27.5 ± 1.8 | 26.7 ± 1.9 | 25.7 ± 1.5 |
| Cr (mg/dL) | 0.5 ± 0.2 | 0.6 ± 0.1 | 0.7 ± 0.2 | 0.5 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.4 ± 0.1 | 0.7 ± 0.2 | 0.5 ± 0.3 | 0.6 ± 0.2 | 0.5 ± 0.1 |
| ALT (IU/L) | 29.3 ± 1.9 | 29.7 ± 3.8 | 28.0 ± 2.8 | 27.5 ± 1.3 | 30.5 ± 2.4 | 25.8 ± 1.7 | 29.8 ± 2.9 | 29.9 ± 2.7 | 31.7.0 ± 2.6 | 30.1 ± 1.9 | 29.8 ± 2.0 | 29.4 ± 1.9 |
| AST (IU/L) | 38.5 ± 1.3 | 39.5 ± 3.7 | 39.8 ± 4.3 | 35.7 ± 3.8 | 36.2 ± 5.4 | 37.6 ± 3.4 | 36.2 ± 4.2 | 37.3 ± 5.5 | 36.6 ± 4.3 | 36.2 ± 5.5 | 32.9 ± 5.8 | 38.5 ± 2.7 |
| LDH (IU/L) | 86.6 ± 27.9 | 88.9 ± 35.7 | 92.7 ± 19.7 | 90.8 ± 27.7 | 198.4 ± 62.7 | 96.2 ± 31.5 | 93.2 ± 32.6 | 123.5 ± 56.1 | 99.7 ± 42.0 | 87.1 ± 29.5 | 135.7 ± 43.1 | 93.7 ± 37.2 |
| CRP (mg/dL) | 30.3 ± 0.9 | 28.9 ± 6.7 | 33.7 ± 9.5 | 39.2 ± 9.5 | 39.8 ± 5.7 | 27.5 ± 10.5 | 22.7 ± 9.9 | 34.6 ± 16.7 | 36.7 ± 8.9 | 32.5 ± 8.5 | 42.5 ± 9.7 | 37.0 ± 9.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-T.; Lin, Y.-C.; Cheng, M.-J.; Shih, C.-M.; Tsai, C.-S.; Lai, Z.-H.; Wu, C.-Y.; Liu, C.-W.; Lin, F.-Y.; Lin, Y.-W. Lepidium meyenii Walpers Promotes the Regeneration of Salivary Gland and Prevents Xerostomia After Irradiation Injury. Nutrients 2025, 17, 3033. https://doi.org/10.3390/nu17193033
Tsai Y-T, Lin Y-C, Cheng M-J, Shih C-M, Tsai C-S, Lai Z-H, Wu C-Y, Liu C-W, Lin F-Y, Lin Y-W. Lepidium meyenii Walpers Promotes the Regeneration of Salivary Gland and Prevents Xerostomia After Irradiation Injury. Nutrients. 2025; 17(19):3033. https://doi.org/10.3390/nu17193033
Chicago/Turabian StyleTsai, Yi-Ting, Yuan-Chuan Lin, Ming-Jen Cheng, Chun-Ming Shih, Chien-Sung Tsai, Ze-Hao Lai, Ching-Yi Wu, Chen-Wei Liu, Feng-Yen Lin, and Yi-Wen Lin. 2025. "Lepidium meyenii Walpers Promotes the Regeneration of Salivary Gland and Prevents Xerostomia After Irradiation Injury" Nutrients 17, no. 19: 3033. https://doi.org/10.3390/nu17193033
APA StyleTsai, Y.-T., Lin, Y.-C., Cheng, M.-J., Shih, C.-M., Tsai, C.-S., Lai, Z.-H., Wu, C.-Y., Liu, C.-W., Lin, F.-Y., & Lin, Y.-W. (2025). Lepidium meyenii Walpers Promotes the Regeneration of Salivary Gland and Prevents Xerostomia After Irradiation Injury. Nutrients, 17(19), 3033. https://doi.org/10.3390/nu17193033








