Undernourished and Undertreated: The Role of Nutritional Care in Geriatric Hospital Outcomes
Abstract
1. Introduction
2. Materials and Methods
- For men: Ideal weight (kg) = 0.75 × height (cm) − 62.5
- For women: Ideal weight (kg) = 0.60 × height (cm) − 40.
Statistical Analyses
3. Results
3.1. Baseline Characteristics of Patients
3.2. Features of Nutrition Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| AN | Artificial Nutrition |
| BMI | Body Mass Index |
| CRP | C reactive Protein |
| EN | Enteral Nutrition |
| GNRI | Geriatric Nutritional Risk Index |
| LOS | Length of Hospital Stay |
| MNT | Medical Nutritional Therapy |
| MUST | Malnutrition Universal Screening Tool |
| PN | Parenteral Nutrition |
References
- OECD. Health on the glance 2023. In OECD Indicators; OECD: Paris, France, 2023; Available online: https://www.oecd.org/en/publications/health-at-a-glance-2023_7a7afb35-en.html (accessed on 1 June 2025).
- Prince, M.J.; Wu, F.; Guo, Y.; Gutierrez, R.L.M.; O’Donnell, M.; Sullivan, R.; Yusuf, S. The burden of disease in older people and implications for health policy and practice. Lancet 2015, 385, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Liotta, G.; Gilardi, F.; Orlando, S.; Rocco, G.; Proietti, M.G.; Asta, F.; De Sario, P.; Michelozzi, P.; Mancinelli, S.; Palombi, L.; et al. Cost of hospital care for the older adults according to their level of frailty. A cohort study in the Lazio region, Italy. PLoS ONE 2019, 14, e0217829. [Google Scholar] [CrossRef] [PubMed]
- WHO. Malnutrition. 2025. Available online: https://www.who.int/news-room/questions-and-answers/item/malnutrition (accessed on 3 March 2025).
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults-Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef]
- Dent, E.; Wright, O.R.L.; Woo, J.; Hoogendijk, E.O. Malnutrition in older adults. Lancet 2023, 401, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Pirlich, M.; Schütz, T.; Kemps, M.; Minko, N.; Lübke, H.J.; Rossnagel, K.; Willich, S.N.; Lochs, H. Social risk factors for hospital malnutrition. Nutrition 2005, 21, 295–300. [Google Scholar] [CrossRef]
- Orlandoni, P.; Jukic Peladic, N.; Cola, C.; Venturini, C.; Costantini, A.; Giorgini, N.; Basile, R.; Sparvoli, D.; David, S. Hospital acquired malnutrition in orally fed geriatric patients: What’s the role of a hospital dietetics and food service? Prog. Nutr. 2018, 20, 225–231. [Google Scholar] [CrossRef]
- Schuetz, P.; Seres, D.; Lobo, D.N.; Gomes, F.; Kaegi-Braun, N.; Stanga, Z. Management of disease-related malnutrition for patients being treated in hospital. Lancet 2021, 398, 1927–1938. [Google Scholar] [CrossRef]
- Ruiz-Rosso, R.; Moreno-Cámara, S.; Gutiérrez-Sánchez, B.; da-Silva-Domingues, H.; Del-Pino-Casado, R.; Palomino-Moral, P.Á. Factors Influencing Nutritional Status in Hospitalized Individuals Aged 70 and Above. Nutrients 2024, 16, 645. [Google Scholar] [CrossRef]
- Cass, A.R.; Charlton, K.E. Prevalence of hospital-acquired malnutrition and modifiable determinants of nutritional deterioration during inpatient admissions: A systematic review of the evidence. J. Hum. Nutr. Diet. 2022, 35, 1043–1058. [Google Scholar] [CrossRef]
- Souza, T.T.; Sturion, C.J.; Faintuch, J. Is the skeleton still in the hospital closet? A review of hospital malnutrition emphasizing health economic aspects. Clin. Nutr. 2015, 34, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.F.; de Jesus, J.D.S.; Britto, V.N.M.; de Jesus, S.A.; Santos, G.S.; de Oliveira, C.C. GLIM criteria to identify malnutrition in patients in hospital settings: A systematic review. JPEN J. Parenter. Enteral Nutr. 2023, 47, 70–709. [Google Scholar] [CrossRef]
- Pablo, A.M.; Izaga, M.A.; Alday, L.A. Assessment of nutritional status on hospital admission: Nutritional scores. Eur. J. Clin. Nutr. 2003, 57, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Lo Buglio, A.; Quiete, S.; Vendemiale, G. Malnutrition in Hospitalized Old Patients: Screening and Diagnosis, Clinical Outcomes, and Management. Nutrients 2022, 14, 910. [Google Scholar] [CrossRef] [PubMed]
- Keller, H.H.; Vesnaver, E.; Davidson, B.; Allard, J.; Laporte, M.; Bernier, P.; Payette, H.; Jeejeebhoy, K.; Duerksen, D.; Gramlich, L.; et al. Providing quality nutrition care in acute care hospitals: Perspectives of nutrition care personnel. J. Hum. Nutr. Diet. 2014, 27, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Huang, Y.; Banks, M.D.; Sowa, P.M.; Bauer, J.D. A Cost-Consequence Analysis of Nutritional Interventions Used in Hospital Settings for Older Adults with or at Risk of Malnutrition. Healthcare 2024, 12, 1041. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Pichard, C.; Lochs, H.; Pirlich, M. Prognostic impact of disease-related malnutrition. Clin. Nutr. 2008, 27, 5–15. [Google Scholar] [CrossRef]
- Liu, L.; Bopp, M.M.; Roberson, P.K.; Sullivan, D.H. Undernutrition and risk of mortality in elderly patients within 1 year of hospital discharge. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M741-6. [Google Scholar] [CrossRef]
- Robinson, D.; Walker, R.; Adams, S.C.; Walker, E.; Adams, S.C.; Allen, K.; Arnold, M.A.; Bechtold, M.; Bridges, K.; Wolke, S. American Society for Parenteral and Enteral Nutrition (ASPEN) Definition of Terms, Style, and Conventions Used in ASPEN Board of Directors–Approved Documents. 2018. Available online: https://nutritioncare.org/wp-content/uploads/2024/12/ASPEN-Definition-of-Terms-Style-and-Conventions-Used-in-ASPEN-Board-of-Directors%E2%80%93Approved-Documents.pdf (accessed on 15 February 2025).
- Bustacchini, S.; Abbatecola, A.M.; Bonfigli, A.R.; Chiatti, C.; Corsonello, A.; Di Stefano, G.; Galeazzi, R.; Fabbietti, P.; Lisa, R.; Guffanti, E.E. Report-Age study group. The Report-AGE project: A permanent epidemiological observatory to identify clinical and biological markers of health outcomes in elderly hospitalized patients in Italy. Aging Clin. Exp. Res. 2015, 27, 893–901. [Google Scholar] [CrossRef]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut Off Points. In StatPearls; StatPearls Publishing: Petersburg, FL, USA, 2025. [Google Scholar]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef]
- Bond, A.; Mccay, K.; Lal, S. Artificial intelligence & clinical nutrition: What the future might have in store. Clin. Nutr. ESPEN 2023, 57, 542–549. [Google Scholar] [CrossRef]
- Hartford, A.M.; Li, W.; Qureshi, D. Use of Feeding Tubes Among Hospitalized Older Adults With Dementia. JAMA Netw. Open 2025, 3, e2460780. [Google Scholar] [CrossRef]
- Veronese, N.; Cella, A.; Cruz-Jentoft, A.J. Enteral tube feeding and mortality in hospitalized older patients: A multicenter longitudinal study. Clin. Nutr. 2020, 39, 1608–1612. [Google Scholar] [CrossRef]
- Plotnikov, G.; Levy, Y.; Trotzky, D.; Nassar, A.; Bushkar, Y.; Derazne, E.; Kagansky, D.; Sharfman, M.; Kagansky, N. Characteristics of older adults receiving enteral feeding at a geriatric medical center. BMC Geriatr. 2024, 24, 628. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Wang, M.; Sun, N.X.; Liu, Y.Y.; Yin, T.F.; Chen, C. Screening and application of nutritional support in elderly hospitalized patients of a tertiary care hospital in China. PLoS ONE 2019, 14, e0213076. [Google Scholar] [CrossRef] [PubMed]
- Vishnupriya, K.; Wright, S.M.; Harris, C.M. An examination of the most expensive adult hospitalizations in America. Hosp. Pract. 2022, 50, 340–345. [Google Scholar] [CrossRef]
- Druml, C.; Ballmer, P.E.; Druml, W.; Oehmichen, F.; Shenkin, A.; Singer, P.; Soeters, P.; Weimann, A.; Bischoff, S.C. ESPEN guideline on ethical aspects of artificial nutrition and hydration. Clin. Nutr. 2016, 35, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Hicks, M.A.; Popowicz, P.; Lopez, P.P. Central Line Management. In StatPearls; StatPearls Publishing: Petersburg, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539811/ (accessed on 20 April 2025).
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A. Society of Critical Care Medicine; American Society for Parenteral and Enteral Nutrition. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J. Parenter. Enter. Nutr. 2016, 40, 159–211, Erratum in J. Parenter. Enter. Nutr. 2016, 40, 1200. https://doi.org/10.1177/0148607116670155. [Google Scholar] [CrossRef]
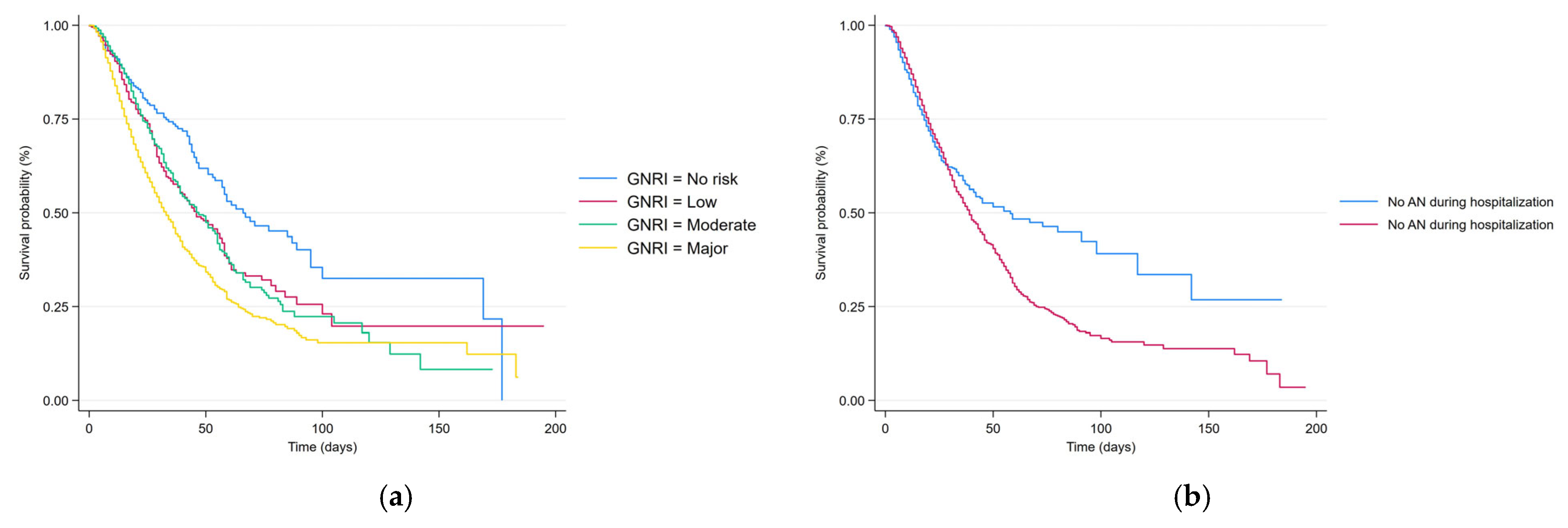
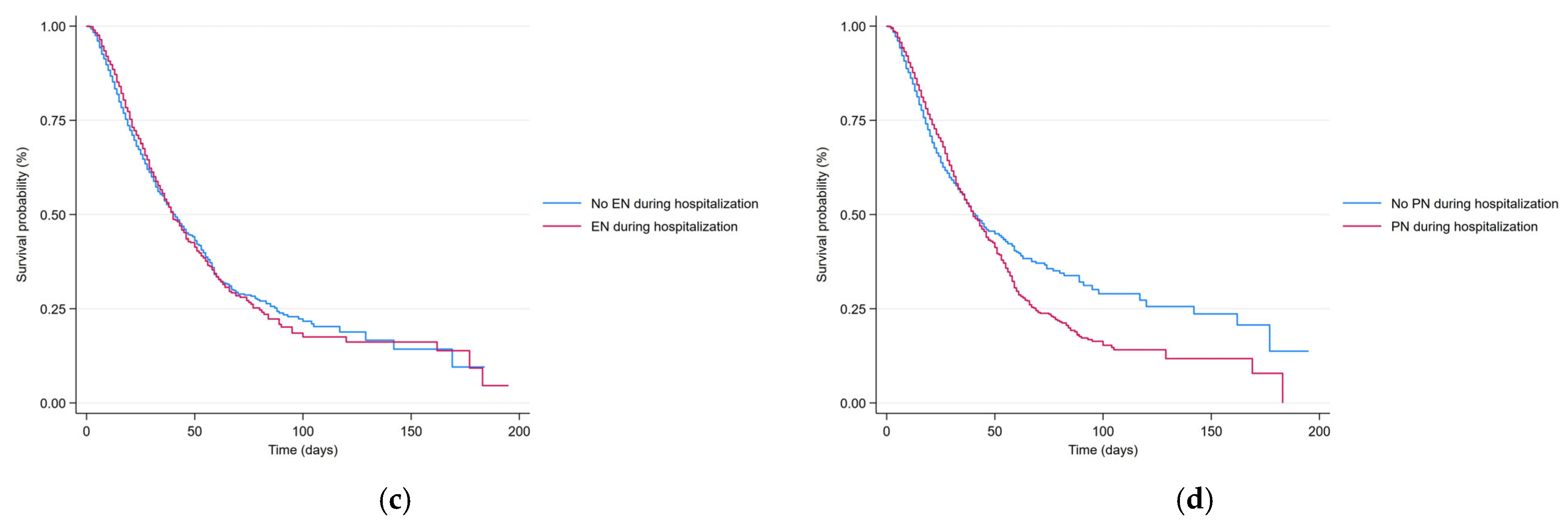
| Total | Survivor Group | Non-Survivor Group | p | |
|---|---|---|---|---|
| N = 4687 (100.0%) | N = 3008 (64.2%) | N = 1679 (35.8%) | ||
| Gender * | <0.001 | |||
| F | 2865 (61.1%) | 1921 (63.9%) | 944 (56.2%) | |
| M | 1822 (38.9%) | 1087 (36.1%) | 735 (43.8%) | |
| Age ^ | 88 (84–92) | 87 (83–91) | 89 (85–92) | <0.001 |
| Pluripathology * | 3690 (78.7%) | 2327 (77.4%) | 1363 (81.2%) | 0.002 |
| Pressure ulcers * | 1957 (41.8%) | 1186 (39.4%) | 771 (45.9%) | <0.001 |
| Laboratory parameters | ||||
| Albumin (mg/L) ^ | 3 (2.6–3.4) | 3 (2.6–3.5) | 2.9 (2.5–3.4) | <0.001 |
| Prealbumin ^ | 11.8 (7.9–16.2) | 12 (8.2–16.3) | 11.25 (7.2–15.7) | 0.023 |
| Total proteins ^ | 5.9 (5.3–6.5) | 5.9 (5.4–6.5) | 5.9 (5.2–6.5) | 0.289 |
| CRP ^ | 5.56 (1.98–11.15) | 5.16 (1.77–10.31) | 6.34 (2.42–12.72) | <0.001 |
| Diseases | ||||
| Other diseases * | 1067 (22.8%) | 701 (23.3%) | 366 (21.8%) | 0.238 |
| Neurological disease * | 2877 (61.4%) | 1876 (62.4%) | 1001 (59.6%) | 0.064 |
| Lung disease * | 826 (17.6%) | 488 (16.2%) | 338 (20.1%) | 0.001 |
| Kidney disease * | 1035 (22.1%) | 566 (18.8%) | 469 (27.9%) | <0.001 |
| Gastrointestinal disease * | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Cancer * | 573 (12.2%) | 365 (12.1%) | 208 (12.4%) | 0.799 |
| Infection * | 29 (0.6%) | 17 (0.6%) | 12 (0.7%) | 0.531 |
| Malnutrition * | 18 (0.4%) | 14 (0.5%) | 4 (0.2%) | 0.228 |
| Cardiovascular disease+ | 2400 (51.2%) | 1493 (49.6%) | 907 (54%) | 0.004 |
| Diabetes mellitus II * | 1003 (21.4%) | 638 (21.2%) | 365 (21.7%) | 0.672 |
| Reason for hospitalization * | <0.001 | |||
| Artificial Nutrition * | 54 (1.2%) | 47 (1.6%) | 7 (0.4%) | |
| Aspiration pneumonia * | 230 (4.9%) | 146 (4.9%) | 84 (5%) | |
| Cancer * | 190 (4.1%) | 125 (4.2%) | 65 (3.9%) | |
| Cardiovascular disease * | 343 (7.3%) | 191 (6.3%) | 152 (9.1%) | |
| Electrolytic alteration * | 142 (3%) | 95 (3.2%) | 47 (2.8%) | |
| Gastrointestinal disease * | 512 (10.9%) | 384 (12.8%) | 128 (7.6%) | |
| Infection * | 493 (10.5%) | 337 (11.2%) | 156 (9.3%) | |
| Kidney disease * | 137 (2.9%) | 82 (2.7%) | 55 (3.3%) | |
| Lung disease * | 613 (13.1%) | 351 (11.7%) | 262 (15.6%) | |
| Malnutrition * | 289 (6.2%) | 203 (6.7%) | 86 (5.1%) | |
| Metabolic alteration * | 22 (0.5%) | 13 (0.4%) | 9 (0.5%) | |
| Neuropsychiatric disease * | 1163 (24.8%) | 710 (23.6%) | 453 (27%) | |
| Other * | 499 (10.6%) | 324 (10.8%) | 175 (10.4%) | |
| BMI ^ | 21.8 (19.1–24.6) | 22 (19.4–24.7) | 21.4 (18.8–24.2) | <0.001 |
| GNRI ^ | 82.10 (74.07–90.38) | 83.39 (75.14–90.84) | 80.41 (71.78–89.35) | <0.001 |
| GNRI Risk * | 370 (7.9%) | 258 (8.6%) | 112 (6.7%) | <0.001 |
| No risk * | 645 (13.8%) | 424 (14.1%) | 221 (13.2%) | |
| Low * | 1333 (28.4%) | 927 (30.8%) | 406 (24.2%) | |
| Moderate * | 2339 (49.9%) | 1399 (46.5%) | 940 (56%) | |
| Major * | ||||
| Nutrition before the nutritional consultation * | ||||
| <0.001 | ||||
| By mouth * | 1601 (34.2%) | 1138 (37.8%) | 463 (27.6%) | |
| EN * | 312 (6.7%) | 230 (7.6%) | 82 (4.9%) | |
| Hydration * | 2182 (46.6%) | 1256 (41.8%) | 926 (55.2%) | |
| Liquid diet * | 83 (1.8%) | 62 (2.1%) | 21 (1.3%) | |
| PN * | 155 (3.3%) | 99 (3.3%) | 56 (3.3%) | |
| Missing * | 354 (7.6%) | 223 (7.4%) | 131 (7.8%) | |
| Length of hospitalization ^ | 10 (5–22) | 10 (5–21) | 11 (5–24) | 0.197 |
| Total | Survivor Group | Non-Survivor Group | p | |
|---|---|---|---|---|
| N = 4687 (100. 0%) | N = 3008 (64.2%) | N = 1679 (35.8%) | ||
| Days from hospitalization to nutritional consultation ^ | 4 (2–7) | 4 (2–7) | 4 (2–8) | 0.007 |
| Nutrition therapy first visit * | <0.001 | |||
| Diet for Dysphagia * | 635 (13.5%) | 503 (16.7%) | 132 (7.9%) | |
| Diet for a specific pathology * | 115 (2.5%) | 98 (3.3%) | 17 (1%) | |
| EN * | 1030 (22%) | 659 (21.9%) | 371 (22.1%) | |
| Free diet * | 116 (2.5%) | 102 (3.4%) | 14 (0.8%) | |
| Hydration * | 709 (15.1%) | 356 (11.8%) | 353 (21%) | |
| Liquid diet * | 26 (0.6%) | 18 (0.6%) | 8 (0.5%) | |
| Oral Supplements * | 232 (4.9%) | 195 (6.5%) | 37 (2.2%) | |
| Other * | 50 (1.1%) | 30 (1%) | 20 (1.2%) | |
| PN * | 1774 (37.8%) | 1047 (34.8%) | 727 (43.3%) | |
| EN during hospitalization * | 1385 (29.5%) | 886 (29.5%) | 499 (29.7%) | 0.849 |
| PN during hospitalization * | 2221 (47.4%) | 1270 (42.2%) | 951 (56.6%) | <0.001 |
| N. nutritional consultations * | <0.001 | |||
| 1 | 3217 (68.6%) | 2124 (70.6%) | 1093 (65.1%) | |
| 2 | 991 (21.1%) | 619 (20.6%) | 372 (22.2%) | |
| 3 | 479 (10.2%) | 265 (8.8%) | 214 (12.7%) | |
| Time between the nutritional consultation and the end of hospitalization ^ | 16 (10–29) | 15 (10–28) | 18 (10–31) | 0.014 |
| Complications | ||||
| Aspiration pneumonia * | 18 (0.4%) | 6 (0.2%) | 12 (0.7%) | 0.006 |
| Electrolytic alteration * | 311 (6.6%) | 148 (4.9%) | 163 (9.7%) | <0.001 |
| Glycemic alteration * | 48 (1%) | 21 (0.7%) | 27 (1.6%) | 0.003 |
| Diarrhea * | 90 (1.9%) | 50 (1.7%) | 40 (2.4%) | 0.085 |
| Bronchial secretion * | 20 (0.4%) | 8 (0.3%) | 12 (0.7%) | 0.024 |
| Dislocation VC and PN * | 14 (0.3%) | 10 (0.3%) | 4 (0.2%) | 0.571 |
| NGT dislocation * | 178 (3.8%) | 117 (3.9%) | 61 (3.6%) | 0.660 |
| Total complications * | <0.001 | |||
| 0 | 3893 (83.1%) | 2588 (86%) | 1305 (77.7%) | |
| 1 | 628 (13.4%) | 328 (10.9%) | 300 (17.9%) | |
| 2 | 144 (3.1%) | 83 (2.8%) | 61 (3.6%) | |
| 3 | 21 (0.4%) | 9 (0.3%) | 12 (0.7%) | |
| 4 | 1 (0%) | 0 (0%) | 1 (0.1%) |
| MODEL 1 | MODEL 2 | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| GNRI, ref. No risk | ||||
| Low | 1.28 (1.02–1.61) | 0.033 | 1.31 (1.05–1.65) | 0.019 |
| Moderate | 1.25 (1.01–1.55) | 0.038 | 1.28 (1.04–1.58) | 0.023 |
| Major | 1.89 (1.55–2.31) | <0.001 | 1.96 (1.61–2.39) | <0.001 |
| Days from hospitalization to nutritional consultation | 1.00 (1.00–1.00) | 0.678 | 1.00 (1.00–1.00) | 0.843 |
| EN during hospitalization | 0.96 (0.86–1.07) | 0.488 | 0.94 (0.84–1.04) | 0.230 |
| PN during hospitalization | 1.01 (0.91–1.11) | 0.860 | 1.00 (0.91–1.10) | 0.993 |
| AN during hospitalization | 1.14 (1.01–1.28) | 0.030 | 1.09 (0.97–1.23) | 0.137 |
| MODEL 1 | MODEL 2 | |||
|---|---|---|---|---|
| β (SE) | p | β (SE) | p | |
| GNRI, ref. No risk | ||||
| Low | −5.37 (1.42) | <0.001 | −5.69 (1.42) | <0.001 |
| Moderate | −7.29 (1.29) | <0.001 | −7.63 (1.29) | <0.001 |
| Major | −9.79 (1.24) | <0.001 | −10.29 (1.23) | <0.001 |
| Days from hospitalization to nutritional consultation | 0.01 (0.00) | 0.083 | 0.01 (0.00) | 0.112 |
| EN during hospitalization | 1.88 (0.73) | 0.010 | 1.98 (0.71) | 0.005 |
| PN during hospitalization | 6.84 (0.64) | <0.001 | 6.58 (0.64) | <0.001 |
| AN during hospitalization | 5.87 (0.70) | <0.001 | 5.67 (0.70) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlandoni, P.; Peladic, N.J.; Di Rosa, M.; Venturini, C.; Lattanzio, F. Undernourished and Undertreated: The Role of Nutritional Care in Geriatric Hospital Outcomes. Nutrients 2025, 17, 3021. https://doi.org/10.3390/nu17183021
Orlandoni P, Peladic NJ, Di Rosa M, Venturini C, Lattanzio F. Undernourished and Undertreated: The Role of Nutritional Care in Geriatric Hospital Outcomes. Nutrients. 2025; 17(18):3021. https://doi.org/10.3390/nu17183021
Chicago/Turabian StyleOrlandoni, Paolo, Nikolina Jukic Peladic, Mirko Di Rosa, Claudia Venturini, and Fabrizia Lattanzio. 2025. "Undernourished and Undertreated: The Role of Nutritional Care in Geriatric Hospital Outcomes" Nutrients 17, no. 18: 3021. https://doi.org/10.3390/nu17183021
APA StyleOrlandoni, P., Peladic, N. J., Di Rosa, M., Venturini, C., & Lattanzio, F. (2025). Undernourished and Undertreated: The Role of Nutritional Care in Geriatric Hospital Outcomes. Nutrients, 17(18), 3021. https://doi.org/10.3390/nu17183021








