Exploring the Anti-Inflammatory Potential of a Mediterranean-Style Ketogenic Diet in Women with Lipedema
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Groups
2.2. Body Composition and Anthropometric Parameters Measurements
2.3. Blood Samples
2.4. Serum IL-6 and hs-CRP Determination
2.5. Dietary Intake Assessment of Baseline Diets
2.6. Dietary Intervention
2.7. Dietary Inflammatory Index Assessment
2.8. Statistical Analysis
3. Results
3.1. Characteristics of Study Group
3.2. Body Composition and Anthropometric Parameters
3.3. Dietary Intake Assessment
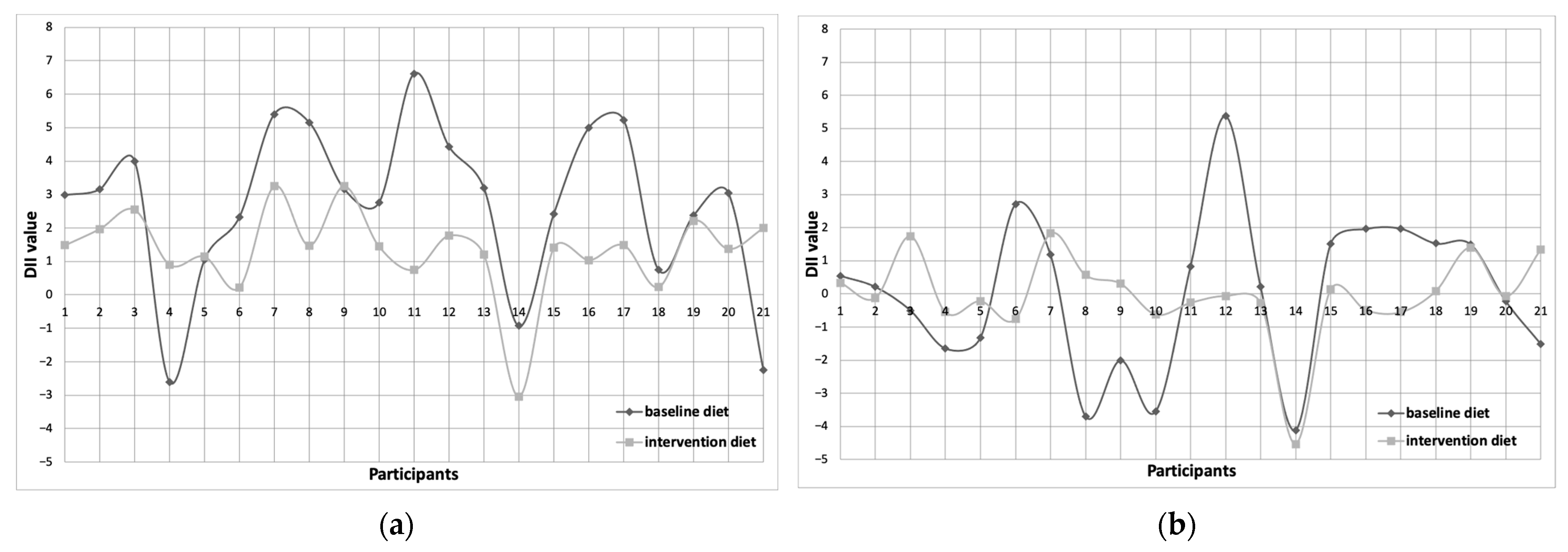
3.4. Dietary Inflammatory Index (DII) Assessment
3.5. Systemic Inflammation Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faerber, G.; Cornely, M.; Daubert, C.; Erbacher, G.; Fink, J.; Hirsch, T.; Mendoza, E.; Miller, A.; Rabe, E.; Rapprich, S.; et al. S2k guideline lipedema. J. Dtsch. Dermatol. Ges. 2024, 22, 1303–1315. [Google Scholar] [CrossRef]
- Forner-Cordero, I.; Forner-Cordero, A.; Szolnoky, G. Update in the management of lipedema. Int. Angiol. 2021, 40, 345–357. [Google Scholar] [CrossRef]
- AL-Ghadban, S.; Cromer, W.; Allen, M.; Ussery, C.; Badowski, M.; Harris, D.; Herbst, K.L. Dilated Blood and Lymphatic Microvessels, Angiogenesis, Increased Macrophages, and Adipocyte Hypertrophy in Lipedema Thigh Skin and Fat Tissue. J. Obes. 2019, 2019, 8747461. [Google Scholar] [CrossRef]
- Aksoy, H.; Karadag, A.S.; Wollina, U. Cause and management of lipedema-associated pain. Dermatol. Ther. 2021, 34, e14364. [Google Scholar] [CrossRef] [PubMed]
- Kruppa, P.; Georgiou, I.; Biermann, N.; Prantl, L.; Klein-Weigel, P.; Ghods, M. Lipedema—Pathogenesis, Diagnosis, and Treatment Options. Dtsch. Ärzteblatt Int. 2020, 117, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Buso, G.; Depairon, M.; Tomson, D.; Raffoul, W.; Vettor, R.; Mazzolai, L. Lipedema: A Call to Action! Obesity 2019, 27, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Keith, L.; Seo, C.A.; Rowsemitt, C.; Pfeffer, M.; Wahi, M.; Staggs, M.; Dudek, J.; Gower, B.; Carmody, M. Ketogenic diet as a potential intervention for lipedema. Med. Hypotheses 2021, 146, 110435. [Google Scholar] [CrossRef]
- Nankam, P.A.N.; Cornely, M.; Klöting, N.; Blüher, M. Is subcutaneous adipose tissue expansion in people living with lipedema healthier and reflected by circulating parameters? Front. Endocrinol. 2022, 13, 1000094. [Google Scholar] [CrossRef]
- Lundanes, J.; Nes, V.F.; Aakervik, O.; Ryan, L.; Hansson, P.; Rokstad, A.M.; Martins, C.; Nymo, S. Changes in Cytokines and Fibrotic Growth Factors after Low-Carbohydrate or Low-Fat Low-Energy Diets in Females with Lipedema. Curr. Dev. Nutr. 2025, 9, 104571. [Google Scholar] [CrossRef]
- Wolf, S.; Deuel, J.W.; Hollmén, M.; Felmerer, G.; Kim, B.-S.; Vasella, M.; Grünherz, L.; Giovanoli, P.; Lindenblatt, N.; Gousopoulos, E. A Distinct Cytokine Profile and Stromal Vascular Fraction Metabolic Status without Significant Changes in the Lipid Composition Characterizes Lipedema. Int. J. Mol. Sci. 2021, 22, 3313. [Google Scholar] [CrossRef]
- Verde, L.; Camajani, E.; Annunziata, G.; Sojat, A.; Marina, L.V.; Colao, A.; Caprio, M.; Muscogiuri, G.; Barrea, L. Ketogenic Diet: A Nutritional Therapeutic Tool for Lipedema? Curr. Obes. Rep. 2023, 12, 529–543. [Google Scholar] [CrossRef]
- Jeziorek, M.; Szuba, A.; Kujawa, K.; Regulska-Ilow, B. The Effect of a Low-Carbohydrate, High-Fat Diet versus Moderate-Carbohydrate and Fat Diet on Body Composition in Patients with Lipedema. DMSO 2022, 15, 2545–2561. [Google Scholar] [CrossRef]
- Jeziorek, M.; Chachaj, A.; Sowicz, M.; Adaszyńska, A.; Truszyński, A.; Putek, J.; Kujawa, K.; Szuba, A. The Benefits of Low-Carbohydrate, High-Fat (LCHF) Diet on Body Composition, Leg Volume, and Pain in Women with Lipedema. J. Obes. 2023, 2023, 5826630. [Google Scholar] [CrossRef]
- Guo, Q.; Li, F.; Duan, Y.; Wen, C.; Wang, W.; Zhang, L.; Huang, R.; Yin, Y. Oxidative stress, nutritional antioxidants and beyond. Sci. China Life Sci. 2020, 63, 866–874. [Google Scholar] [CrossRef]
- Myette-Côté, É.; St-Pierre, V.; Beaulieu, S.; Castellano, C.-A.; Fortier, M.; Plourde, M.; Bocti, C.; Fulop, T.; Cunnane, S.C. The effect of a 6-month ketogenic medium-chain triglyceride supplement on plasma cardiometabolic and inflammatory markers in mild cognitive impairment. Prostaglandins Leukot. Essent. Fat. Acids 2021, 169, 102236. [Google Scholar] [CrossRef] [PubMed]
- Palermo, A.; Li, S.; Ten Hoeve, J.; Chellappa, A.; Morris, A.; Dillon, B.; Ma, F.; Wang, Y.; Cao, E.; Shabane, B.; et al. A ketogenic diet can mitigate SARS-CoV-2 induced systemic reprogramming and inflammation. Commun. Biol. 2023, 6, 1115. [Google Scholar] [CrossRef]
- Bhanpuri, N.H.; Hallberg, S.J.; Williams, P.T.; McKenzie, A.L.; Ballard, K.D.; Campbell, W.W.; McCarter, J.P.; Phinney, S.D.; Volek, J.S. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at 1 year: An open label, non-randomized, controlled study. Cardiovasc. Diabetol. 2018, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Cannataro, R.; Michelini, S.; Ricolfi, L.; Caroleo, M.C.; Gallelli, L.; De Sarro, G.; Onorato, A.; Cione, E. Management of Lipedema with Ketogenic Diet: 22-Month Follow-Up. Life 2021, 11, 1402. [Google Scholar] [CrossRef]
- Wirth, M.D.; Burch, J.; Shivappa, N.; Violanti, J.M.; Burchfiel, C.M.; Fekedulegn, D.; Andrew, M.E.; Hartley, T.A.P.; Miller, D.B.; Mnatsakanova, A.; et al. Association of a Dietary Inflammatory Index With Inflammatory Indices and Metabolic Syndrome Among Police Officers. J. Occup. Environ. Med. 2014, 56, 986–989. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Bonaccio, M.; Hebert, J.R.; Di Castelnuovo, A.; Costanzo, S.; Ruggiero, E.; Pounis, G.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; et al. Association of proinflammatory diet with low-grade inflammation: Results from the Moli-sani study. Nutrition 2018, 54, 182–188. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Brorson, H.; Svensson, B.; Ohlin, K. Volume Measurements and Follow-Up. In Lymphedema; Greene, A.K., Slavin, S.A., Brorson, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 115–122. [Google Scholar] [CrossRef]
- Dehghan, M.; Ilow, R.; Zatonska, K.; Szuba, A.; Zhang, X.; Mente, A.; Regulska-Ilow, B. Development, reproducibility and validity of the food frequency questionnaire in the Poland arm of the Prospective Urban and Rural Epidemiological (PURE) study. J. Hum. Nutr. Diet. 2012, 25, 225–232. [Google Scholar] [CrossRef]
- Kunachowicz, H.; Nadolna, J.; Przygoda, B.; Iwanow, K. Tabele Składu i Wartości Odżywczej Żywności [Food Composition Tables]; PZWL: Warsaw, Poland, 2005. (In Polish) [Google Scholar]
- Szponar, L.; Wolnicka, K.; Rychlik, E. Album of Photographs of Food Products and Dishes; The Food and Nutrition Institute: Warsaw, Poland, 2000. [Google Scholar]
- Jeziorek, M.; Szuba, A.; Kujawa, K.; Regulska-Ilow, B. Comparison of Actual and Predicted Resting Metabolic Rate in Women with Lipedema. Lymphat. Res. Biol. 2023. ahead of print. [Google Scholar] [CrossRef]
- United Nations University; World Health Organization. Human Energy Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation: Rome, 17–24 October 2001; Food & Agriculture Organization of the United Nations: Rome, Italy, 2004; Volume 1. [Google Scholar]
- US Department of Agriculture, Agricultural Research Service. FoodData Central. 2019. Available online: https://Fdc.nal.usda.gov (accessed on 5 January 2021).
- Sanlier, N.; Baltacı, S. Therapeutic Applications of Ketogenic Diets in Lipedema: A Narrative Review of Current Evidence. Curr. Obes. Rep. 2025, 14, 49. [Google Scholar] [CrossRef]
- Poojari, A.; Dev, K.; Rabiee, A. Lipedema: Insights into Morphology, Pathophysiology, and Challenges. Biomedicines 2022, 10, 3081. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I. Dietary polyphenols suppress chronic inflammation by modulation of multiple inflammation-associated cell signaling pathways. J. Nutr. Biochem. 2021, 93, 108634. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, M.; Liang, J.; He, G.; Chen, N. Ketogenic Diet Benefits to Weight Loss, Glycemic Control, and Lipid Profiles in Overweight Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trails. Int. J. Environ. Res. Public Health 2022, 19, 10429. [Google Scholar] [CrossRef]
- Oteng, A.B.; Kersten, S. Mechanisms of Action of trans Fatty Acids. Adv. Nutr. 2020, 11, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Cinelli, G.; Romano, L.; Zomparelli, S.; De Santis, G.L.; Nocerino, P.; Bigioni, G.; Arsini, L.; Cenname, G.; Pujia, A.; et al. Potential Effects of a Modified Mediterranean Diet on Body Composition in Lipoedema. Nutrients 2021, 13, 358. [Google Scholar] [CrossRef] [PubMed]
- Naude, C.E.; Brand, A.; Schoonees, A.; Nguyen, K.A.; Chaplin, M.; Volmink, J. Low-carbohydrate versus balanced-carbohydrate diets for reducing weight and cardiovascular risk. Cochrane Database Syst. Rev. 2022, 2022, CD013334. [Google Scholar] [CrossRef]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. J. Parenter. Enteral. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef]
- Alesi, S.; Villani, A.; Mantzioris, E.; Takele, W.W.; Cowan, S.; Moran, L.J.; Mousa, A. Anti-Inflammatory Diets in Fertility: An Evidence Review. Nutrients 2022, 14, 3914. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef]
- Kim, J.; Cho, H.J.; Kim, Z.; Youn, H.J.; Cho, J.; Min, J.W.; Kim, Y.S.; Lee, J.E. Intakes of saturated and unsaturated fat and circulating levels of inflammatory markers among breast cancer survivors. Sci. Rep. 2025, 15, 9481. [Google Scholar] [CrossRef]
- Lenighan, Y.M.; McNulty, B.A.; Roche, H.M. Dietary fat composition: Replacement of saturated fatty acids with PUFA as a public health strategy, with an emphasis on α-linolenic acid. Proc. Nutr. Soc. 2019, 78, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, C.D.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef] [PubMed]

| Group | Nutrients and Foods |
|---|---|
| Nutrients | energy value, protein, carbohydrates, fiber, total fat, saturated fatty acids, cholesterol, monounsaturated fatty acids, polyunsaturated fatty acids, omega-3 fatty acids, omega-6 fatty acids, alcohol, vitamin A and β-carotene, vitamins D, E, thiamine, riboflavin, niacin, vitamins B6, B12, C, and folic acid, zinc, magnesium, iron |
| Plant bioactives | anthocyanins, eugenol, flavan-3-ols, flavones, flavonols, flavanones, isoflavones, caffeine |
| Products with anti-inflammatory properties | onion, garlic, ginger, turmeric, pepper, rosemary, saffron, thyme + oregano black + green tea |
| Parameter | Lipedema Group (n = 24) Mean ± SD/Me (Q1, Q3) | Overweight/Obesity Group (n = 24) Mean ± SD/Me (Q1, Q3) | ||||
|---|---|---|---|---|---|---|
| Before | After | p-Values | Before | After | p-Values | |
| Weight [kg] | 86.1 ± 17.8 | 74.1 ± 12.9 | <0.0001 | 90.5 (81.7, 97.5) | 77.1 (69.2, 86.5) | <0.0001 |
| BMI [kg/m2] | 31.6 ± 6.4 | 27.3 ± 5.1 | <0.0001 | 33.9 (30.5, 36.3) | 28.0 (26.1, 33.1) | <0.0001 |
| PBF [%] | 39.9 (35.7, 42.8) | 33.8 (27.8, 37.4) | <0.001 | 40.5 (36.8, 43.6) | 33.2 (28.9, 39.3) | <0.001 |
| MBF [kg] | 33.6 (28.4, 39.2) | 25.2 (18.8, 30.2) | <0.001 | 36.7 (29.3, 43.1) | 25.5 (20.0, 34.5) | <0.001 |
| VFL | 14.0 (8.5, 16.5) | 9.5 (4.0, 12.0) | <0.001 | 10.5 (8.0, 13.5) | 8.0 (6.0, 10.0) | <0.001 |
| Waist [cm] | 98.0 ± 12.9 | 85.2 ± 11.3 | <0.0001 | 106.7 ± 10.6 | 94.4 ± 12.1 | <0.0001 |
| Hips [cm] | 115.1 ± 12.0 | 105.4 ± 9.2 | <0.0001 | 115.0 (109.5, 119.8) | 104.8 (101.0, 108.3) | <0.0001 |
| WHR | 0.84 (0.78, 0.91) | 0.79 (0.74, 0.86) | 0.0001 | 0.93 ± 0.1 | 0.9 ± 0.1 | 0.006 |
| Left thigh [cm] | 65.1 ± 7.5 | 59.1 ± 5.9 | <0.0001 | 64.9 ± 5.6 | 59.2 ± 5.3 | <0.0001 |
| Right thigh [cm] | 65.1 ± 7.3 | 58.9 ± 6.0 | <0.0001 | 64.9 ± 5.8 | 59.1 ± 5.4 | <0.0001 |
| Left calf [cm] | 44.7 ± 5.2 | 40.7 ± 3.9 | <0.0001 | 42.1 ± 3.9 | 39.5 ± 3.7 | <0.0001 |
| Right calf [cm] | 44.5 ± 5.3 | 40.8 ± 4.3 | <0.0001 | 42.4 ± 4.1 | 39.9 ± 4.0 | <0.000 |
| Left ankle [cm] | 25.0 ± 2.3 | 23.7 ± 2.1 | <0.0001 | 23.0 (22.3, 24.8) | 23.0 (22.3, 24.5) | NS |
| Right ankle [cm] | 24.0 (23.0, 26.0) | 23.0 (22.3, 24.8) | 0.0001 | 23.9 ± 2.5 | 23.7 ± 2.4 | NS |
| Parameter | Lipedema (n = 21) | Overweight/Obesity (n = 24) | ||
|---|---|---|---|---|
| Baseline Diet Me (Q1, Q3) | Intervention Diet Me (Q1, Q3) | Baseline Diet Me (Q1, Q3) | Intervention Diet Me (Q1, Q3) | |
| Energy [kcal] | 1513.8 (641.5–2421.0) | 1670.6 (1424.7–2022.0) | 1592.5 (626.8–2515.9) | 1687.5 (1401.0–1866.0) |
| Total protein [g] | 74.1 (34.0–153.9) | 86.7 (65.1–113.4) | 69.9 (30.4–109.1) | 88.9 (79.9–102.2) |
| Total carbohydrates [g] | 175.5 (20.0–346.7) | 29.6 (23.1–85.0) | 182.4 (68.2–310.6) | 30.4 (24.8–37.7) |
| Fiber [g] | 17.2 (2.5–31.2) | 8.6 (6.7–24.5) | 14.9 (5.8–24.1) | 8.7 (6.7–20.6) |
| Fat [g] | 58.7 (32.0–119.4) | 136.6 (102.4–154.2) | 63.0 (26–95.4) | 133.4 (70.6–147.2) |
| SFA [g] | 22.8 (8.2–50.4) | 38.1 (23.8–52.3) | 25.3 (8.5–38.4) | 36.8 (22.7–52.3) |
| MUFA [g] | 22.2 (9.6–44.4) | 58.0 (38.0–69.1) | 23.2 (9.6–37.2) | 56.3 (44.0–78.5) |
| PUFA [g] | 9.5 (5.9–16.5) | 25.0 (14.8–39.6) | 10.7 (3.1–15.2) | 26.5 (12.9–47.1) |
| Total n-3 [g] | 1.4 (0.6–3.5) | 4.0 (0.8–8.7) | 1.5 (0.6–2.4) | 4.6 (2.5–5.8) |
| Total n-6 [g] | 7.8 (3.8–13) | 10.2 (7.9–17.6) | 8.8 (2.5–13.3) | 12.4 (7.5–20.6) |
| Cholesterol [mg] | 261.8 (97.8–728.2) | 608.7 (469.4–798.8) | 288.3 (183.7–655.6) | 622.1 (301.6–801.4) |
| Thiamine [mg] | 1.0 (0.5–1.7) | 0.8 (0.5–6.3) | 0.9 (0.3–1.6) | 0.8 (0.5–1.2) |
| Riboflavin [mg] | 1.5 (0.7–2.9) | 2.1 (1.3–4.1) | 1.6 (0.6–2.6) | 1.9 (1.3–4.8) |
| Niacin [mg] | 19.2 (6.0–41.7) | 18.8 (13.2–27.1) | 19.3 (9.1–30.2) | 18.7 (9.2–31.1) |
| Vitamin B6 [mg] | 1.6 (0.6–3.2) | 1.7 (1.1–2.7) | 1.5 (0.6–2.2) | 1.9 (1.4–3.4) |
| Folate [µg] | 252.8 (148.6–486.6) | 327.6 (217.1–479.7) | 243.0 (129.0–383.7) | 320.9 (190.0–416.0) |
| Vitamin B12 [µg] | 3.3 (0.7–8.3) | 8.0 (4.1–17.0) | 3.5 (1.0–6.4) | 6.7 (4.2–21.2) |
| Vitamin C [mg] | 97 (31.1–284.4) | 118.6 (83.1–220.2) | 107.8 (31.8–204.6) | 128.5 (71.1–205.8) |
| Vitamin A [µg] | 1108.3 (528.6–3130.1) | 1234.3 (699.8–1954.4) | 1035.5 (583.4–2190.3) | 1178.1 (673.1–3657.1) |
| β-Carotene [µg] | 3609.0 (1082.8–8504.9) | 3151.3 (1868.6–7696) | 2935.4 (1822.8–8947.5) | 3492.0 (2016.9–6820.3) |
| Vitamin D [µg] | 2.0 (0.5–5.5) | 9.1 (4.5–18.7) | 1.9 (1.2–3.6) | 8.5 (5–10.8) |
| Vitamin E [mg] | 8.9 (5.7–17.3) | 16.4 (13.4–28.7) | 9.6 (3.7–14.0) | 19.7 (11.9–30.0) |
| Zinc [mg] | 9.2 (4.6–15.8) | 8.4 (6.6–12.1) | 8.3 (3.6–13.5) | 9.1 (6.5–17.4) |
| Magnesium [mg] | 314.9 (126.4–579.7) | 223.4 (153.6–766.9) | 325.4 (94.8–493.3) | 259.7 (178.8–414.6) |
| Manganese [mg] | 5.1 (2.4–9.0) | 1.2 (0.7–5.4) | 4.2 (2.2–8.1) | 1.5 (0.7–2.6) |
| Copper [mg] | 1.3 (0.5–2.4) | 0.9 (0.7–1.6) | 1.3 (0.3–1.8) | 1.0 (0.7–1.5) |
| Iron [mg] | 10.4 (6.0–17.6) | 9.7 (7.3–15) | 10.7 (4.6–14.7) | 9.2 (6.5–13.0) |
| Lipedema (n = 21) | Overweight/Obesity (n = 24) | Differences Between Before and After Intervention | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline Diet Me (Q1, Q3) | Interventional Diet Me (Q1, Q3) | p-Value | Baseline Diet Me (Q1, Q3) | Interventional Diet Me (Q1, Q3) | p-Value | Lipedema | Overweight/Obesity | p-Value | |
| DII/day | 3.04 (−2.60–6.61) | 1.45 (−3.0–3.26) | 0.008 | 4.00 (−1.27–7.09) | 1.27 (−0.91–3.05) | <0.001 | −1.50 (−2.13–−0.48) | −2.65 (−3.89–−1.19) | NS |
| DII/1000 kcal | 0.22 (−4.12–5.37) | −0.06 (−4.54–1.83) | NS | 1.17 (−3.41–4.89) | −0.68 (−1.95–1.33) | <0.001 | −0.21 (−5.43–4.27) | −1.32 (−5.50–3.44) | 0.044 |
| Parameter | Lipedema Group Me (Q1, Q3) | Overweight/Obesity Group Me (Q1, Q3) | Differences Between Before and After Intervention | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before Me (Q1, Q3) | After Me (Q1, Q3) | p-Value | Before Me (Q1, Q3) | After Me (Q1, Q3) | p-Value | Lipedema Me (Q1, Q3) | Overweight/Obesity Me (Q1, Q3) | p-Value | |
| hs-CRP [mg/dL] | 3.44 (2.00–5.55) | 2.99 (1.84–4.73) | 0.016 | 3.76 (3.22–4.37) | 2.46 (2.60–4.02) | 0.001 | −0.39 (−0.89–0.26) | −0.56 (−1.64–−0.04) | NS |
| IL-6 [pg/mL] | 8.93 (7.22–10.5) | 8.54 (6.48–9.42) | 0.034 | 9.11 (7.62–10.6) | 7.62 (7.56–9.63) | 0.014 | −0.33 (−1.38–−0.28) | −0.95 (−1.91–0.13) | NS |
| DII | Lipedema Group | Overweight/Obesity Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Before/Baseline Diet | After/Intervention Diet | Before/Baseline Diet | After/Intervention Diet | |||||
| hs-CRP | IL-6 | hs-CRP | IL-6 | hs-CRP | IL-6 | hs-CRP | IL-6 | |
| DII/day | r = 0.34 | r = 0.50 | r = 0.55 | r = 0.17 | r = 0.18 | r = 0.32 | r = 0.41 | r = 0.22 |
| p = 0.106 | p = 0.013 | p = 0.005 | p = 0.440 | p = 0.393 | p = 0.131 | p = 0.044 | p = 0.297 | |
| DII/1000 kcal | r = 0.08 | r = 0.27 | r = 0.41 | r = −0.01 | r = −0.07 | r = 0.04 | r = 0.44 | r = 0.39 |
| p = 0.713 | p = 0.207 | p = 0.047 | p = 0.961 | p = 0.712 | p = 0.864 | p = 0.031 | p = 0.063 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeziorek, M.; Chachaj, A.; Szuba, A.; Różańska, D.; Prescha, A. Exploring the Anti-Inflammatory Potential of a Mediterranean-Style Ketogenic Diet in Women with Lipedema. Nutrients 2025, 17, 3014. https://doi.org/10.3390/nu17183014
Jeziorek M, Chachaj A, Szuba A, Różańska D, Prescha A. Exploring the Anti-Inflammatory Potential of a Mediterranean-Style Ketogenic Diet in Women with Lipedema. Nutrients. 2025; 17(18):3014. https://doi.org/10.3390/nu17183014
Chicago/Turabian StyleJeziorek, Małgorzata, Angelika Chachaj, Andrzej Szuba, Dorota Różańska, and Anna Prescha. 2025. "Exploring the Anti-Inflammatory Potential of a Mediterranean-Style Ketogenic Diet in Women with Lipedema" Nutrients 17, no. 18: 3014. https://doi.org/10.3390/nu17183014
APA StyleJeziorek, M., Chachaj, A., Szuba, A., Różańska, D., & Prescha, A. (2025). Exploring the Anti-Inflammatory Potential of a Mediterranean-Style Ketogenic Diet in Women with Lipedema. Nutrients, 17(18), 3014. https://doi.org/10.3390/nu17183014






