Modulation of Human Immune Cells by Propyl-Propane Thiosulfonate (PTSO) Inhibits Colorectal Tumor Progression in a Humanized Mouse Model
Abstract
1. Introduction
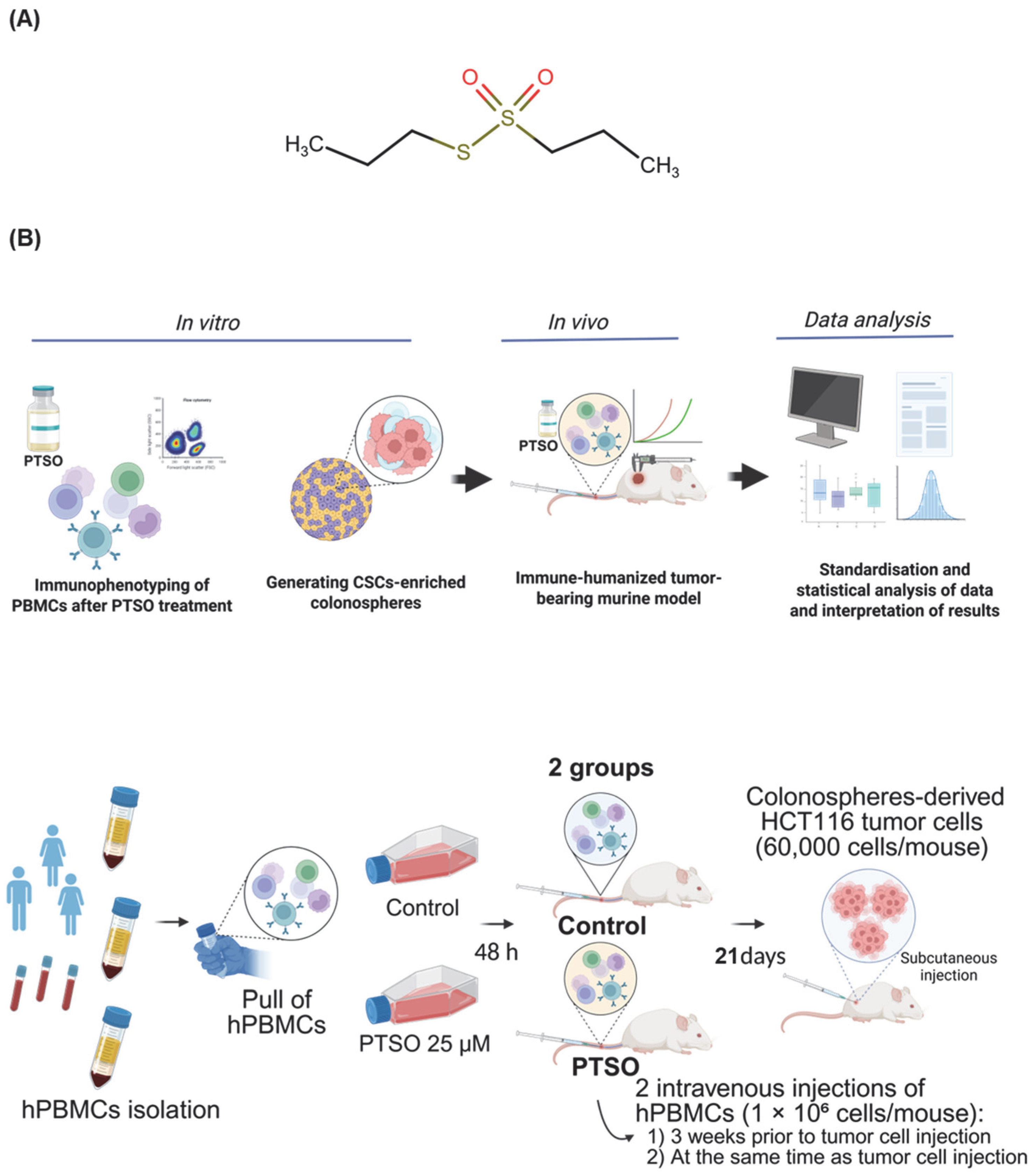
2. Materials and Methods
2.1. Reagents and Treatment
2.2. Generating CSCs-Enriched Colonospheres
2.3. Isolation and Culture of Human PBMCs
2.4. Immunophenotyping of PBMCs
2.5. Immune-Humanized Tumor-Bearing Murine Model
2.6. Immune Profiling of Blood and Tumor Samples
2.7. RNA Extraction and Analysis of Gene Expression
2.8. Histological Studies
2.9. Statistical Analysis
3. Results
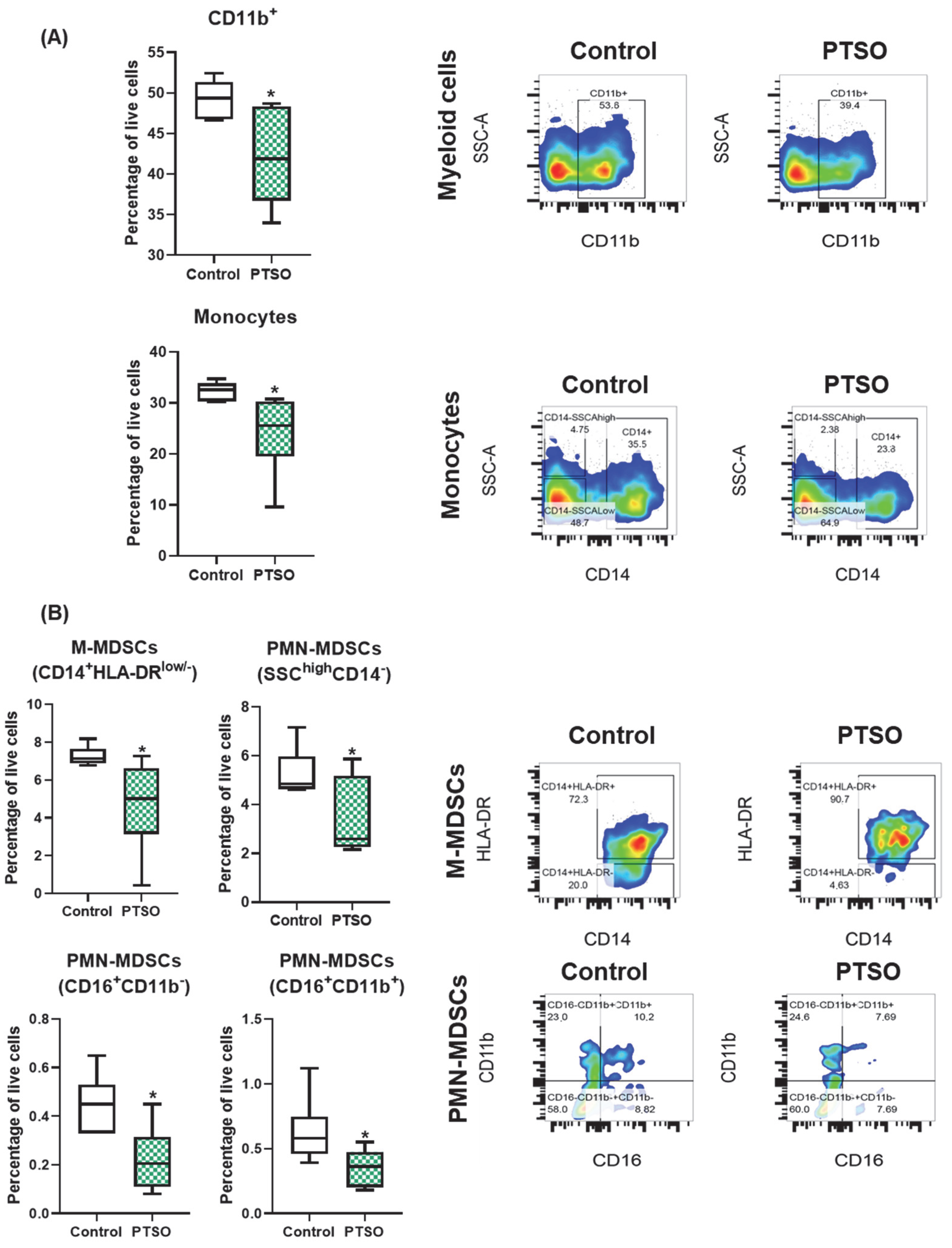
3.1. PTSO Reduces the Levels of Immunosuppressive Myeloid Populations In Vitro
3.2. PTSO Promotes Cytotoxic T Cell Expansion In Vitro
3.3. Immunomodulatory Activity of PTSO Impairs Tumor Growth in a CRC Xenograft Mouse Model
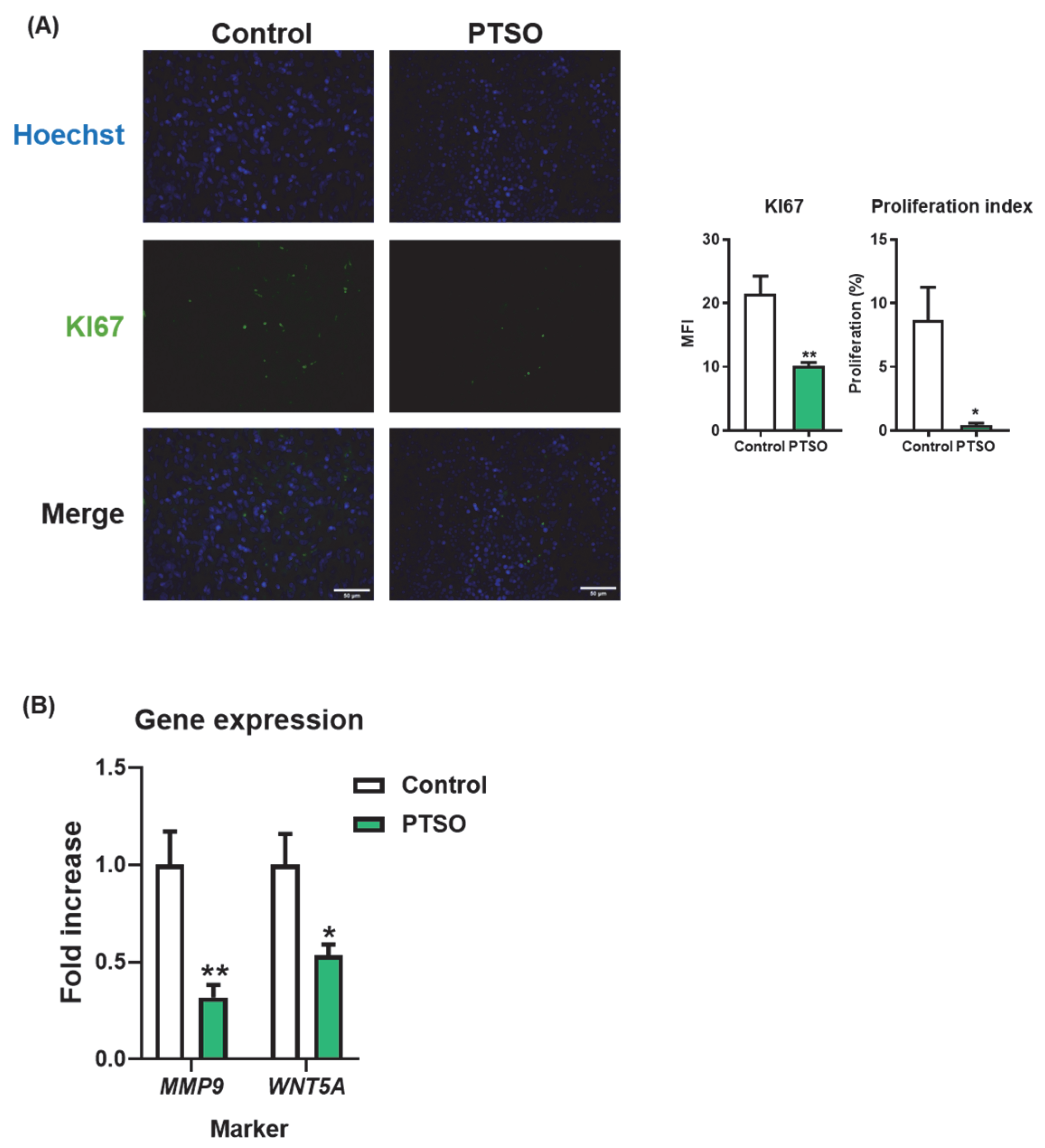
3.4. PTSO Modulates Tumor Cell Proliferation and Invasive Potential
3.5. PTSO-Treated hPBMCs Enhance Tumor Immune Infiltration and Deplete the Intratumoral Myeloid Suppressor Cells
4. Discussion
4.1. Impact of PTSO on MDSCs and Their Immunosuppressive Signaling in CRC
4.2. The Role of Anti-Tumor Effector Immune Cells and Their Modulation by PTSO
4.3. Cancer Stem Cells, Proliferation, and Invasion
4.4. Structure–Activity Relationship of PTSO
4.5. Translational Implications
4.6. Study Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARG1 | Arginase-1 |
| CRC | Colorectal Cancer |
| CSCs | Cancer stem cells |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl sulfoxide |
| hPBMCs | Human peripheral blood mononuclear cells |
| ICIs | Immune checkpoint inhibitors (ICIs) |
| IFNG | Interferon gamma |
| iNOS | Inducible nitric oxide synthase |
| MDSCs | Myeloid-derived suppressor cells |
| MFI | Mean fluorescence intensity |
| M-MDSCs | Monocytic myeloid-derived suppressor cells |
| MRI | Magnetic Resonance Imaging |
| MSS | Microsatellite stability |
| NK | Natural Killer |
| NSG | NOD scid gamma |
| pMMR | Proficient mismatch repair |
| PMN-MDSCs | Polymorphonuclear Myeloid-derived suppressor cells |
| PTSO | Propyl-Propane Thiosulfonate |
| Tc | T cytotoxic |
| Th | T helper |
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- Bai, J.; Chen, H.; Bai, X. Relationship between microsatellite status and immune microenvironment of colorectal cancer and its application to diagnosis and treatment. J. Clin. Lab. Anal. 2021, 35, e23810. [Google Scholar] [CrossRef]
- Fan, A.; Wang, B.; Wang, X.; Nie, Y.; Fan, D.; Zhao, X.; Lu, Y. Immunotherapy in colorectal cancer: Current achievements and future perspective. Int. J. Biol. Sci. 2021, 17, 3837–3849. [Google Scholar] [CrossRef]
- Flugel, C.L.; Majzner, R.G.; Krenciute, G.; Dotti, G.; Riddell, S.R.; Wagner, D.L.; Abou-El-Enein, M. Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat. Rev. Clin. Oncol. 2023, 20, 49–62. [Google Scholar] [CrossRef]
- Barnestein, R.; Galland, L.; Kalfeist, L.; Ghiringhelli, F.; Ladoire, S.; Limagne, E. Immunosuppressive tumor microenvironment modulation by chemotherapies and targeted therapies to enhance immunotherapy effectiveness. Oncoimmunology 2022, 11, 2120676. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [CrossRef]
- Allegrezza, M.J.; Rutkowski, M.R.; Stephen, T.L.; Svoronos, N.; Perales-Puchalt, A.; Nguyen, J.M.; Payne, K.K.; Singhal, S.; Eruslanov, E.B.; Tchou, J.; et al. Trametinib Drives T-cell-Dependent Control of KRAS-Mutated Tumors by Inhibiting Pathological Myelopoiesis. Cancer Res. 2016, 76, 6253–6265. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, K.; Tian, J.; Xia, X.; Ma, J.; Tang, X.; Xu, H.; Wang, S. Granulocytic Myeloid-Derived Suppressor Cells Promote the Stemness of Colorectal Cancer Cells through Exosomal S100A9. Adv. Sci. 2019, 6, 1901278. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Paul, S.; Manna, A.; Majumder, C.; Pal, K.; Casarcia, N.; Mondal, A.; Banerjee, S.; Nelson, V.K.; Ghosh, S.; et al. Phenolic Phytochemicals for Prevention and Treatment of Colorectal Cancer: A Critical Evaluation of In Vivo Studies. Cancers 2023, 15, 993. [Google Scholar] [CrossRef]
- Elattar, M.M.; Darwish, R.S.; Hammoda, H.M.; Dawood, H.M. An ethnopharmacological, phytochemical, and pharmacological overview of onion (Allium cepa L.). J. Ethnopharmacol. 2024, 324, 117779. [Google Scholar] [CrossRef]
- Guillamon, E.; Mut-Salud, N.; Rodriguez-Sojo, M.J.; Ruiz-Malagon, A.J.; Cuberos-Escobar, A.; Martinez-Ferez, A.; Rodriguez-Nogales, A.; Galvez, J.; Banos, A. In Vitro Antitumor and Anti-Inflammatory Activities of Allium-Derived Compounds Propyl Propane Thiosulfonate (PTSO) and Propyl Propane Thiosulfinate (PTS). Nutrients 2023, 15, 1363. [Google Scholar] [CrossRef]
- Zhu, L.; Myhill, L.J.; Andersen-Civil, A.I.S.; Thamsborg, S.M.; Blanchard, A.; Williams, A.R. Garlic-Derived Organosulfur Compounds Regulate Metabolic and Immune Pathways in Macrophages and Attenuate Intestinal Inflammation in Mice. Mol. Nutr. Food Res. 2022, 66, e2101004. [Google Scholar] [CrossRef]
- Schafer, G.; Kaschula, C.H. The immunomodulation and anti-inflammatory effects of garlic organosulfur compounds in cancer chemoprevention. Anti-Cancer Agents Med. Chem. 2014, 14, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Amani, M.; Shokati, E.; Entezami, K.; Khorrami, S.; Jazayeri, M.H.; Safari, E. The immunomodulatory effects of low molecular weight garlic protein in crosstalk between peripheral blood mononuclear cells and colon cancer cells. Process Biochem. 2021, 108, 161–168. [Google Scholar] [CrossRef]
- Xu, J.; Yu, Y.; Zhang, Y.; Dai, H.; Yang, Q.; Wang, B.; Ma, Q.; Chen, Y.; Xu, F.; Shi, X.; et al. Oral administration of garlic-derived nanoparticles improves cancer immunotherapy by inducing intestinal IFNgamma-producing gammadelta T cells. Nat. Nanotechnol. 2024, 19, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.J.; Rao, Q.R.; Jiang, X.Q.; Ye, N.; Li, N.; Du, H.L.; Zhang, S.J.; Ye, H.Y.; Wu, W.S.; Zhao, M. Exploring the Immunomodulatory Properties of Red Onion (Allium cepa L.) Skin: Isolation, Structural Elucidation, and Bioactivity Study of Novel Onion Chalcones Targeting the A(2A) Adenosine Receptor. J. Agric. Food Chem. 2023, 71, 17046–17055. [Google Scholar] [CrossRef] [PubMed]
- Falcón Piñeiro, A.; Garrido Garrido, D.; Baños Arjona, A. PTS and PTSO, two organosulfur compounds from onion by-products as a novel solution for plant disease and pest management. Chem. Biol. Technol. Agric. 2023, 10, 76. [Google Scholar] [CrossRef]
- Vezza, T.; Algieri, F.; Garrido-Mesa, J.; Utrilla, M.P.; Rodriguez-Cabezas, M.E.; Banos, A.; Guillamon, E.; Garcia, F.; Rodriguez-Nogales, A.; Galvez, J. The Immunomodulatory Properties of Propyl-Propane Thiosulfonate Contribute to its Intestinal Anti-Inflammatory Effect in Experimental Colitis. Mol. Nutr. Food Res. 2019, 63, e1800653. [Google Scholar] [CrossRef]
- Jimenez, G.; Hackenberg, M.; Catalina, P.; Boulaiz, H.; Grinan-Lison, C.; Garcia, M.A.; Peran, M.; Lopez-Ruiz, E.; Ramirez, A.; Morata-Tarifa, C.; et al. Mesenchymal stem cell’s secretome promotes selective enrichment of cancer stem-like cells with specific cytogenetic profile. Cancer Lett. 2018, 429, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Mao, Z.; Wang, W.; Ma, J.; Tian, J.; Wang, S.; Yin, K. Netrin-1 Promotes the Immunosuppressive Activity of MDSCs in Colorectal Cancer. Cancer Immunol. Res. 2023, 11, 600–613. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Xu, Y.; Chang, J.; Chang, W.; Lv, Y.; Zheng, P.; Zhang, Z.; Li, Z.; Lin, Q.; Tang, W.; et al. The immune phenotypes and different immune escape mechanisms in colorectal cancer. Front. Immunol. 2022, 13, 968089. [Google Scholar] [CrossRef]
- Liang, R.; Ding, D.; Li, Y.; Lan, T.; Ryabtseva, S.; Huang, S.; Ren, J.; Huang, H.; Wei, B. HDACi combination therapy with IDO1i remodels the tumor microenvironment and boosts antitumor efficacy in colorectal cancer with microsatellite stability. J. Nanobiotechnol. 2024, 22, 753. [Google Scholar] [CrossRef]
- Iglesias-Escudero, M.; Arias-Gonzalez, N.; Martinez-Caceres, E. Regulatory cells and the effect of cancer immunotherapy. Mol. Cancer 2023, 22, 26. [Google Scholar] [CrossRef]
- Zhai, J.; Chen, H.; Wong, C.C.; Peng, Y.; Gou, H.; Zhang, J.; Pan, Y.; Chen, D.; Lin, Y.; Wang, S.; et al. ALKBH5 Drives Immune Suppression Via Targeting AXIN2 to Promote Colorectal Cancer and Is a Target for Boosting Immunotherapy. Gastroenterology 2023, 165, 445–462. [Google Scholar] [CrossRef]
- Bao, Y.; Zhai, J.; Chen, H.; Wong, C.C.; Liang, C.; Ding, Y.; Huang, D.; Gou, H.; Chen, D.; Pan, Y.; et al. Targeting m(6)A reader YTHDF1 augments antitumour immunity and boosts anti-PD-1 efficacy in colorectal cancer. Gut 2023, 72, 1497–1509. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Horlad, H.; Shiraishi, D.; Tsuboki, J.; Kudo, R.; Ikeda, T.; Nohara, T.; Takeya, M.; Komohara, Y. Onionin A, a sulfur-containing compound isolated from onions, impairs tumor development and lung metastasis by inhibiting the protumoral and immunosuppressive functions of myeloid cells. Mol. Nutr. Food Res. 2016, 60, 2467–2480. [Google Scholar] [CrossRef]
- Limagne, E.; Euvrard, R.; Thibaudin, M.; Rebe, C.; Derangere, V.; Chevriaux, A.; Boidot, R.; Vegran, F.; Bonnefoy, N.; Vincent, J.; et al. Accumulation of MDSC and Th17 Cells in Patients with Metastatic Colorectal Cancer Predicts the Efficacy of a FOLFOX-Bevacizumab Drug Treatment Regimen. Cancer Res. 2016, 76, 5241–5252. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.K.; Sinha, P.; Clements, V.K.; Rodriguez, P.; Ostrand-Rosenberg, S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010, 70, 68–77. [Google Scholar] [CrossRef]
- Tsukumo, S.I.; Yasutomo, K. Regulation of CD8(+) T Cells and Antitumor Immunity by Notch Signaling. Front. Immunol. 2018, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Lu, C.; Payne, D.; Paschall, A.V.; Klement, J.D.; Redd, P.S.; Ibrahim, M.L.; Yang, D.; Han, Q.; Liu, Z.; et al. Autocrine IL6-Mediated Activation of the STAT3-DNMT Axis Silences the TNFalpha-RIP1 Necroptosis Pathway to Sustain Survival and Accumulation of Myeloid-Derived Suppressor Cells. Cancer Res. 2020, 80, 3145–3156. [Google Scholar] [CrossRef]
- Karakasheva, T.A.; Waldron, T.J.; Eruslanov, E.; Kim, S.B.; Lee, J.S.; O’Brien, S.; Hicks, P.D.; Basu, D.; Singhal, S.; Malavasi, F.; et al. CD38-Expressing Myeloid-Derived Suppressor Cells Promote Tumor Growth in a Murine Model of Esophageal Cancer. Cancer Res. 2015, 75, 4074–4085. [Google Scholar] [CrossRef]
- Holmgaard, R.B.; Zamarin, D.; Li, Y.; Gasmi, B.; Munn, D.H.; Allison, J.P.; Merghoub, T.; Wolchok, J.D. Tumor-Expressed IDO Recruits and Activates MDSCs in a Treg-Dependent Manner. Cell Rep. 2015, 13, 412–424. [Google Scholar] [CrossRef]
- Nikoo, S.; Bozorgmehr, M.; Namdar Ahmadabad, H.; Hassan, Z.M.; Moazzeni, S.M.; Pourpak, Z.; Ghazanfari, T. The 14kDa protein molecule isolated from garlic suppresses indoleamine 2, 3-dioxygenase metabolites in mononuclear cells in vitro. Iran. J. Allergy Asthma Immunol. 2008, 7, 203–208. [Google Scholar]
- Scodeller, P.; Simon-Gracia, L.; Kopanchuk, S.; Tobi, A.; Kilk, K.; Saalik, P.; Kurm, K.; Squadrito, M.L.; Kotamraju, V.R.; Rinken, A.; et al. Precision Targeting of Tumor Macrophages with a CD206 Binding Peptide. Sci. Rep. 2017, 7, 14655. [Google Scholar] [CrossRef]
- Luo, H.; Ikenaga, N.; Nakata, K.; Higashijima, N.; Zhong, P.; Kubo, A.; Wu, C.; Tsutsumi, C.; Shimada, Y.; Hayashi, M.; et al. Tumor-associated neutrophils upregulate Nectin2 expression, creating the immunosuppressive microenvironment in pancreatic ductal adenocarcinoma. J. Exp. Clin. Cancer Res. 2024, 43, 258. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.J.; He, Y.S.; Guo, T.; Tao, J.; Wei, Z.Y.; Zhang, J.L.; Bao, C.; Chen, J.H. ADAR-mediated RNA editing regulates PVR immune checkpoint in colorectal cancer. Biochem. Biophys. Res. Commun. 2024, 695, 149373. [Google Scholar] [CrossRef]
- Domblides, C.; Crampton, S.; Liu, H.; Bartleson, J.M.; Nguyen, A.; Champagne, C.; Landy, E.E.; Spiker, L.; Proffitt, C.; Bhattarai, S.; et al. Human NLRC4 expression promotes cancer survival and associates with type I interferon signaling and immune infiltration. J. Clin. Investig. 2024, 134, e166085. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Alves, N.; Dalmasso, G.; Nikitina, D.; Vaysse, A.; Ruez, R.; Ledoux, L.; Pedron, T.; Bergsten, E.; Boulard, O.; Autier, L.; et al. The colibactin-producing Escherichia coli alters the tumor microenvironment to immunosuppressive lipid overload facilitating colorectal cancer progression and chemoresistance. Gut Microbes 2024, 16, 2320291. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, D.; Li, Y.; Qi, L.; Si, W.; Bo, Y.; Chen, X.; Ye, Z.; Fan, H.; Liu, B.; et al. Spatiotemporal single-cell analysis decodes cellular dynamics underlying different responses to immunotherapy in colorectal cancer. Cancer Cell 2024, 42, 1268–1285.e7. [Google Scholar] [CrossRef] [PubMed]
- Nersesian, S.; Schwartz, S.L.; Grantham, S.R.; MacLean, L.K.; Lee, S.N.; Pugh-Toole, M.; Boudreau, J.E. NK cell infiltration is associated with improved overall survival in solid cancers: A systematic review and meta-analysis. Transl. Oncol. 2021, 14, 100930. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gogenur, I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Mohammad Hassan, Z.; Mostafaie, A.; Zare Mehrjardi, N.; Ghazanfari, T. Purif ied Protein Fraction of Garlic Extract Modulates Cellular Immune Response against Breast Transplanted Tumors in BALB/c Mice Model. Cell J. 2013, 15, 65–75. [Google Scholar]
- Park, S.Y.; Pylaeva, E.; Bhuria, V.; Gambardella, A.R.; Schiavoni, G.; Mougiakakos, D.; Kim, S.H.; Jablonska, J. Harnessing myeloid cells in cancer. Mol. Cancer 2025, 24, 69. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh, E.; Orangi, M.; Mohammadi, H.; Babaie, F.; Baradaran, B. Myeloid-derived suppressor cells: Important contributors to tumor progression and metastasis. J. Cell Physiol. 2018, 233, 3024–3036. [Google Scholar] [CrossRef]
- Abbaszadegan, M.R.; Bagheri, V.; Razavi, M.S.; Momtazi, A.A.; Sahebkar, A.; Gholamin, M. Isolation, identification, and characterization of cancer stem cells: A review. J. Cell Physiol. 2017, 232, 2008–2018. [Google Scholar] [CrossRef]
- Pothuraju, R.; Rachagani, S.; Krishn, S.R.; Chaudhary, S.; Nimmakayala, R.K.; Siddiqui, J.A.; Ganguly, K.; Lakshmanan, I.; Cox, J.L.; Mallya, K.; et al. Molecular implications of MUC5AC-CD44 axis in colorectal cancer progression and chemoresistance. Mol. Cancer 2020, 19, 37. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, C.; Zhu, Y.; Xie, M.; He, D.; Pan, Q.; Zhang, P.; Hua, D.; Wang, T.; Jin, L.; et al. TrpC5 regulates differentiation through the Ca2+/Wnt5a signalling pathway in colorectal cancer. Clin. Sci. 2017, 131, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xiao, X.; Wang, J.; Dasari, S.; Pepin, D.; Nephew, K.P.; Zamarin, D.; Mitra, A.K. Cancer associated fibroblasts serve as an ovarian cancer stem cell niche through noncanonical Wnt5a signaling. NPJ Precis. Oncol. 2024, 8, 7. [Google Scholar] [CrossRef]
- Sun, G.; Wu, L.; Sun, G.; Shi, X.; Cao, H.; Tang, W. WNT5a in Colorectal Cancer: Research Progress and Challenges. Cancer Manag. Res. 2021, 13, 2483–2498. [Google Scholar] [CrossRef] [PubMed]
- Kusmartsev, S. Metastasis-promoting functions of myeloid cells. Cancer Metastasis Rev. 2025, 44, 61. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, X.; Liu, X.; Cai, X.; Shen, T.; Pan, D.; Liang, R.; Ding, R.; Hu, R.; Dong, J.; et al. Single-cell sequencing reveals the immune microenvironment landscape related to anti-PD-1 resistance in metastatic colorectal cancer with high microsatellite instability. BMC Med. 2023, 21, 161. [Google Scholar] [CrossRef]
- Singh, N.; Gusain, A.; Nigam, M.; Mishra, A.P. The pharmacological and therapeutic versatility of Allium species: A comprehensive exploration of bioactive constituents and biological activities. Discov. Appl. Sci. 2025, 7, 349. [Google Scholar] [CrossRef]
- Abbasi, A.; Sanej, K.D.; Moradi, S.; Bazzaz, S.; Esmaeili, A.; Ghafourian, K.; Sabahi, S.; Lahouty, M.; Akrami, S.; Aslani, R.; et al. Bioactive Compounds and Biological Activities of Allium sativum L. In Bioactive Compounds in the Storage Organs of Plants; Reference Series in Phytochemistry; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–40. [Google Scholar]
- Hou, Y.; Lv, B.; Du, J.; Ye, M.; Jin, H.; Yi, Y.; Huang, Y. Sulfide regulation and catabolism in health and disease. Signal Transduct. Target. Ther. 2025, 10, 174. [Google Scholar] [CrossRef]
- Rogala, J.; Sieminska, I.; Baran, J.; Rubinkiewicz, M.; Zybaczynska, J.; Szczepanik, A.M.; Pach, R. Myeloid-Derived Suppressor Cells May Predict the Occurrence of Postoperative Complications in Colorectal Cancer Patients-a Pilot Study. J. Gastrointest. Surg. 2022, 26, 2354–2357. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhang, B.; Li, B.; Wu, H.; Jiang, M. Cold and hot tumors: From molecular mechanisms to targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 274. [Google Scholar] [CrossRef]
- Garcia-Nicolas, M.; Pastor-Belda, M.; Campillo, N.; Rodriguez-Sojo, M.J.; Ruiz-Malagon, A.J.; Hidalgo-Garcia, L.; Abad, P.; de la Torre, J.M.; Guillamon, E.; Banos, A.; et al. Analytical Platform for the Study of Metabolic Pathway of Propyl Propane Thiosulfonate (PTSO) from Allium spp. Foods 2023, 12, 823. Foods 2023, 12, 823. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, J.; Fan, Y.; Huang, L.; Cai, X. Understanding the cellular and molecular heterogeneity in colorectal cancer through the use of single-cell RNA sequencing. Transl. Oncol. 2025, 55, 102374. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Sojo, M.J.; Gbati, L.; Molina-Tijeras, J.A.; Ho-Plágaro, A.; Vezza, T.; López-Escánez, L.; Griñán-Lisón, C.; Marchal, J.A.; Baños, A.; Rodríguez-Sánchez, M.J.; et al. Modulation of Human Immune Cells by Propyl-Propane Thiosulfonate (PTSO) Inhibits Colorectal Tumor Progression in a Humanized Mouse Model. Nutrients 2025, 17, 2993. https://doi.org/10.3390/nu17182993
Rodríguez-Sojo MJ, Gbati L, Molina-Tijeras JA, Ho-Plágaro A, Vezza T, López-Escánez L, Griñán-Lisón C, Marchal JA, Baños A, Rodríguez-Sánchez MJ, et al. Modulation of Human Immune Cells by Propyl-Propane Thiosulfonate (PTSO) Inhibits Colorectal Tumor Progression in a Humanized Mouse Model. Nutrients. 2025; 17(18):2993. https://doi.org/10.3390/nu17182993
Chicago/Turabian StyleRodríguez-Sojo, María Jesús, Luckman Gbati, Jose Alberto Molina-Tijeras, Ailec Ho-Plágaro, Teresa Vezza, Laura López-Escánez, Carmen Griñán-Lisón, Juan Antonio Marchal, Alberto Baños, María José Rodríguez-Sánchez, and et al. 2025. "Modulation of Human Immune Cells by Propyl-Propane Thiosulfonate (PTSO) Inhibits Colorectal Tumor Progression in a Humanized Mouse Model" Nutrients 17, no. 18: 2993. https://doi.org/10.3390/nu17182993
APA StyleRodríguez-Sojo, M. J., Gbati, L., Molina-Tijeras, J. A., Ho-Plágaro, A., Vezza, T., López-Escánez, L., Griñán-Lisón, C., Marchal, J. A., Baños, A., Rodríguez-Sánchez, M. J., García-García, J., Ruiz-Malagón, A. J., Gálvez, J., Rodríguez-Cabezas, M. E., & Rodríguez-Nogales, A. (2025). Modulation of Human Immune Cells by Propyl-Propane Thiosulfonate (PTSO) Inhibits Colorectal Tumor Progression in a Humanized Mouse Model. Nutrients, 17(18), 2993. https://doi.org/10.3390/nu17182993









