Effects of Cistanche deserticola Y.C. Ma Supplementation on Muscle Strength and Recovery: A Randomized Controlled Trial
Abstract
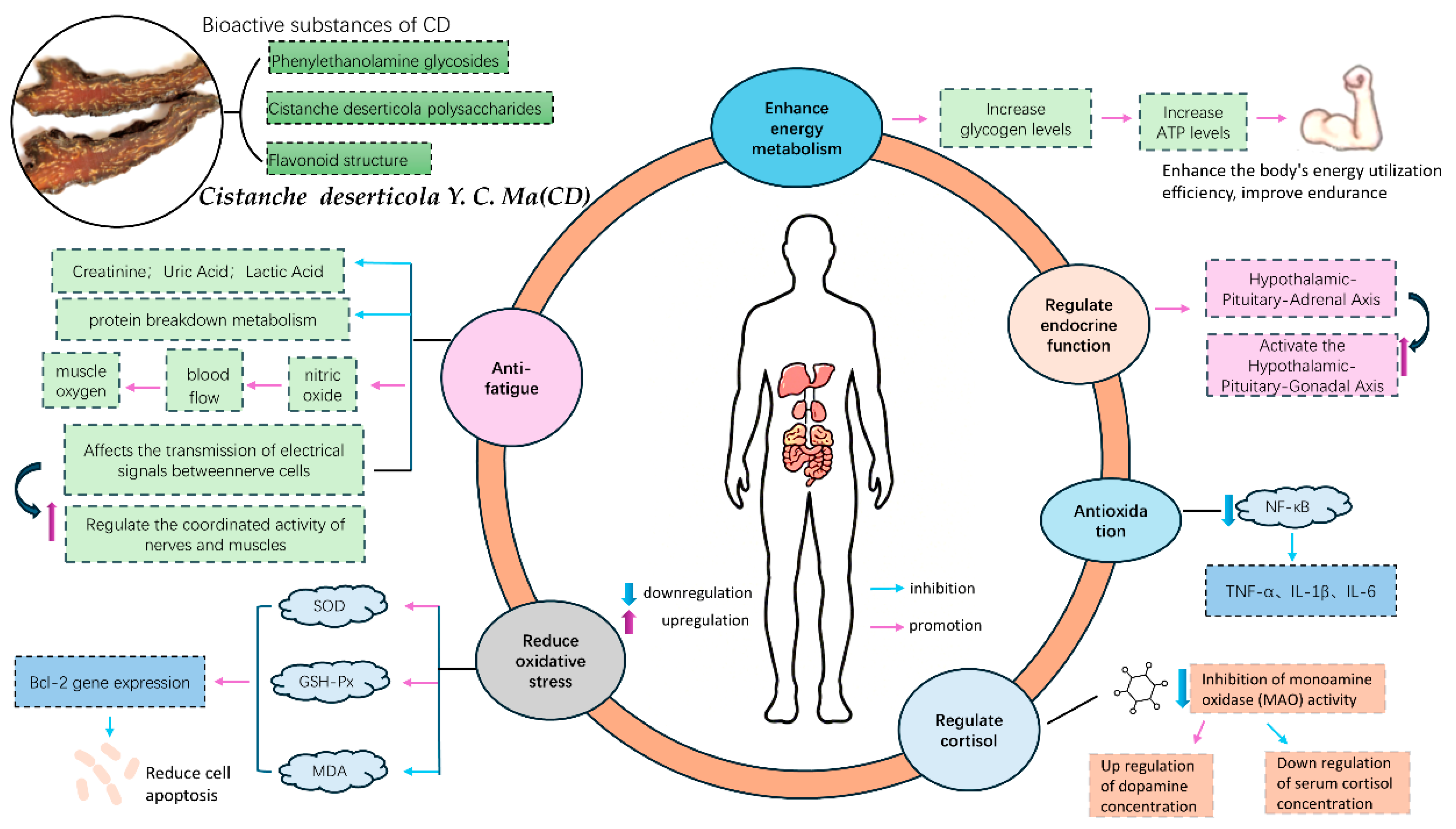
1. Introduction
2. Materials and Methods
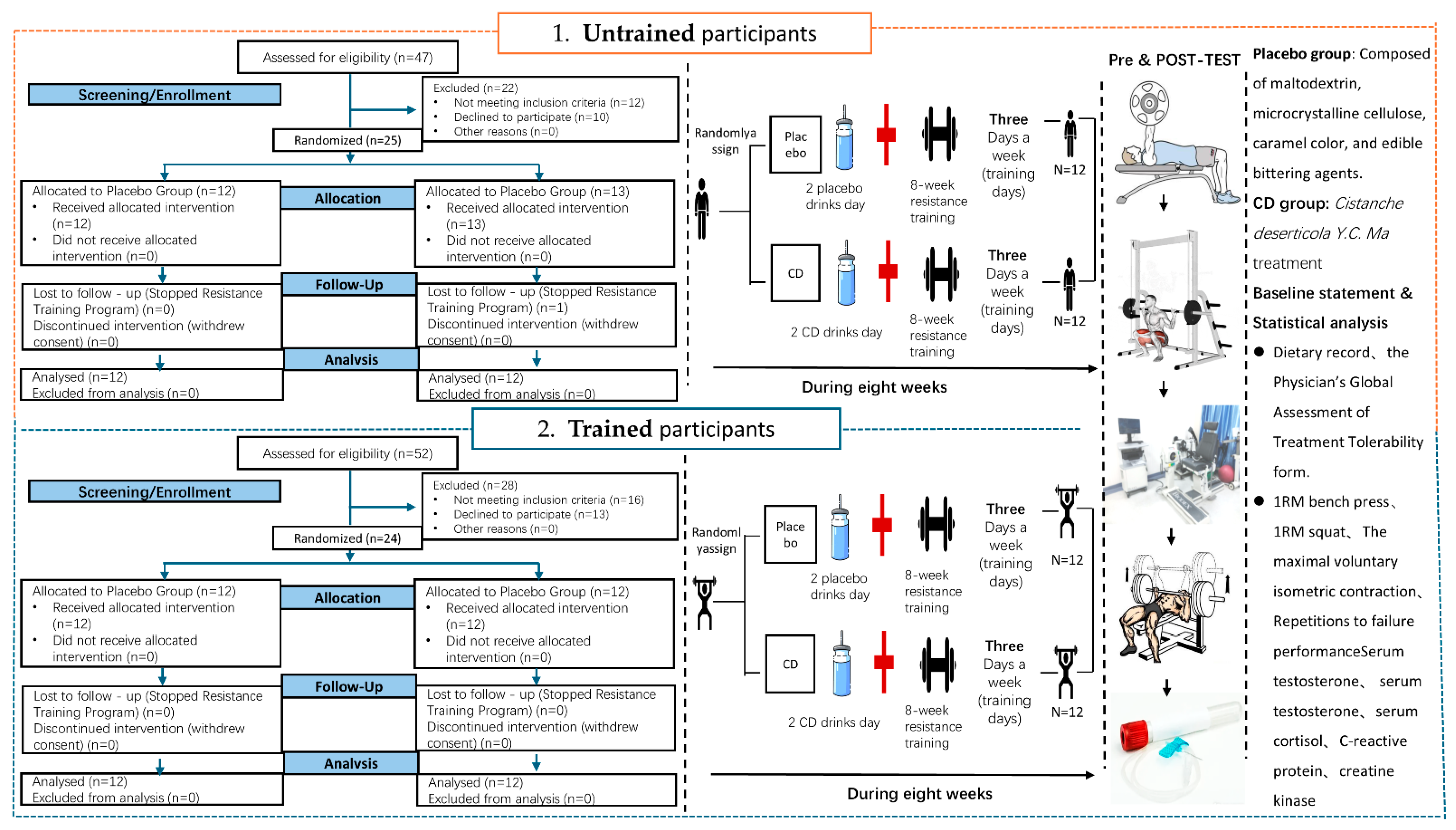
2.1. Participants
2.2. Ethics Approval
2.3. Study Design
2.4. Supplementation Preparation and Management
2.5. Training Intervention Program
2.6. Daily Dietary Intake Recording
2.7. Baseline Statement
2.7.1. Bench Press/Deep Squat 1RM
2.7.2. MVIC Test for the Quadriceps
2.7.3. Repetitions to Failure (RTF) Performance
2.7.4. Muscle Recovery
2.8. Tolerability
2.9. Statistical Analysis
3. Results
3.1. Dietary Intake Records
3.2. Changes in Untrained Participants After Supplementation with Training Supplements
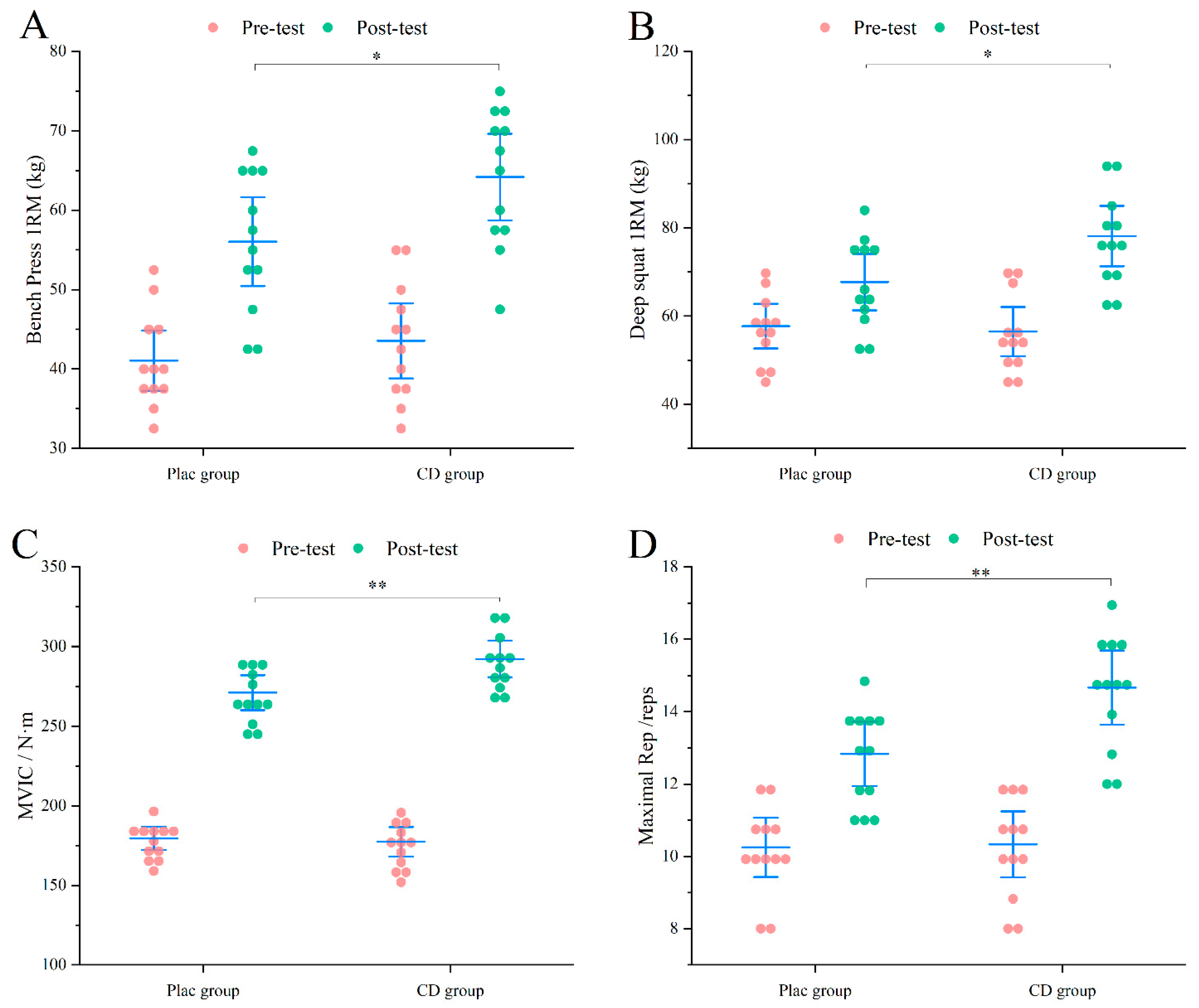
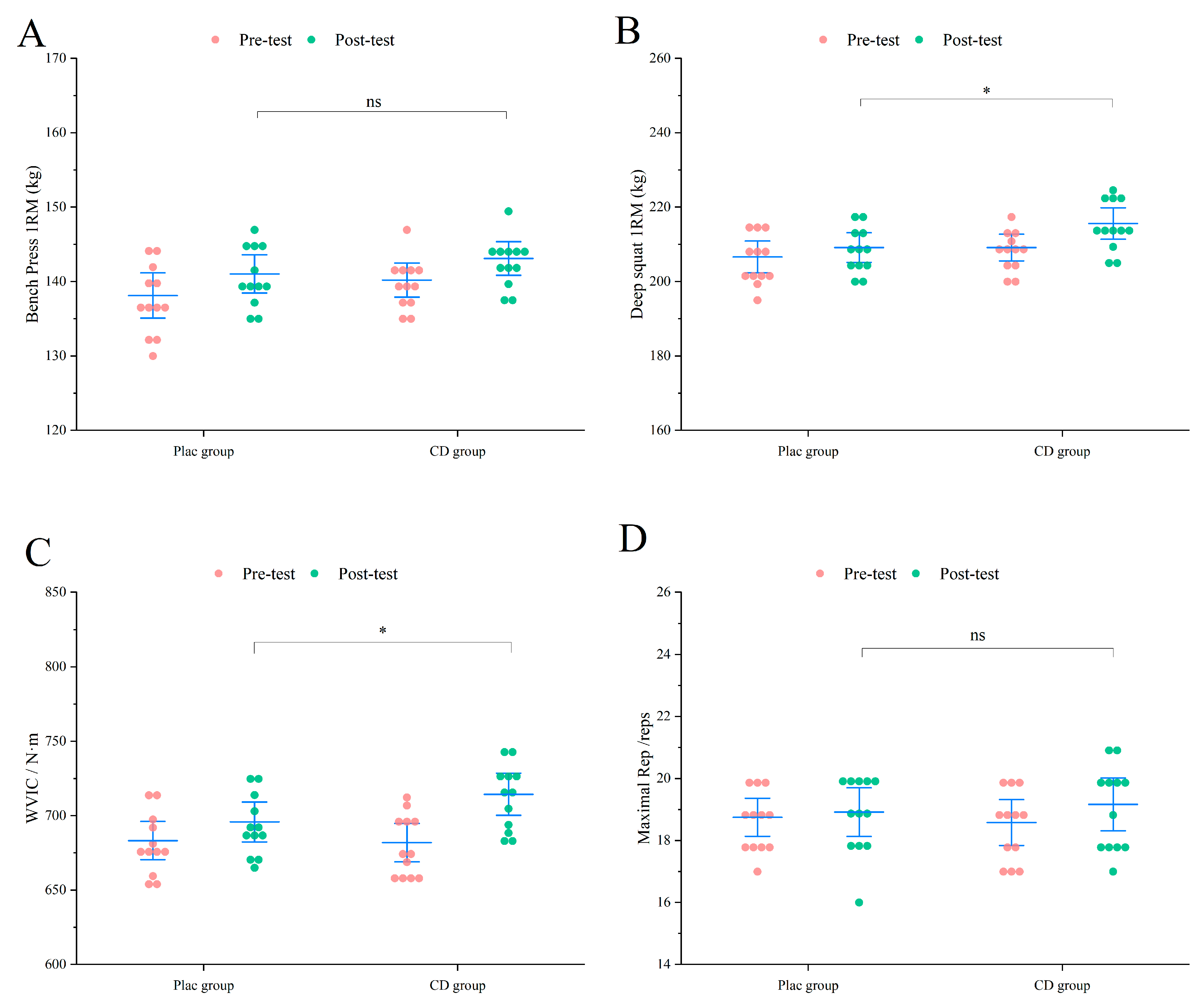
3.2.1. Muscle Strength
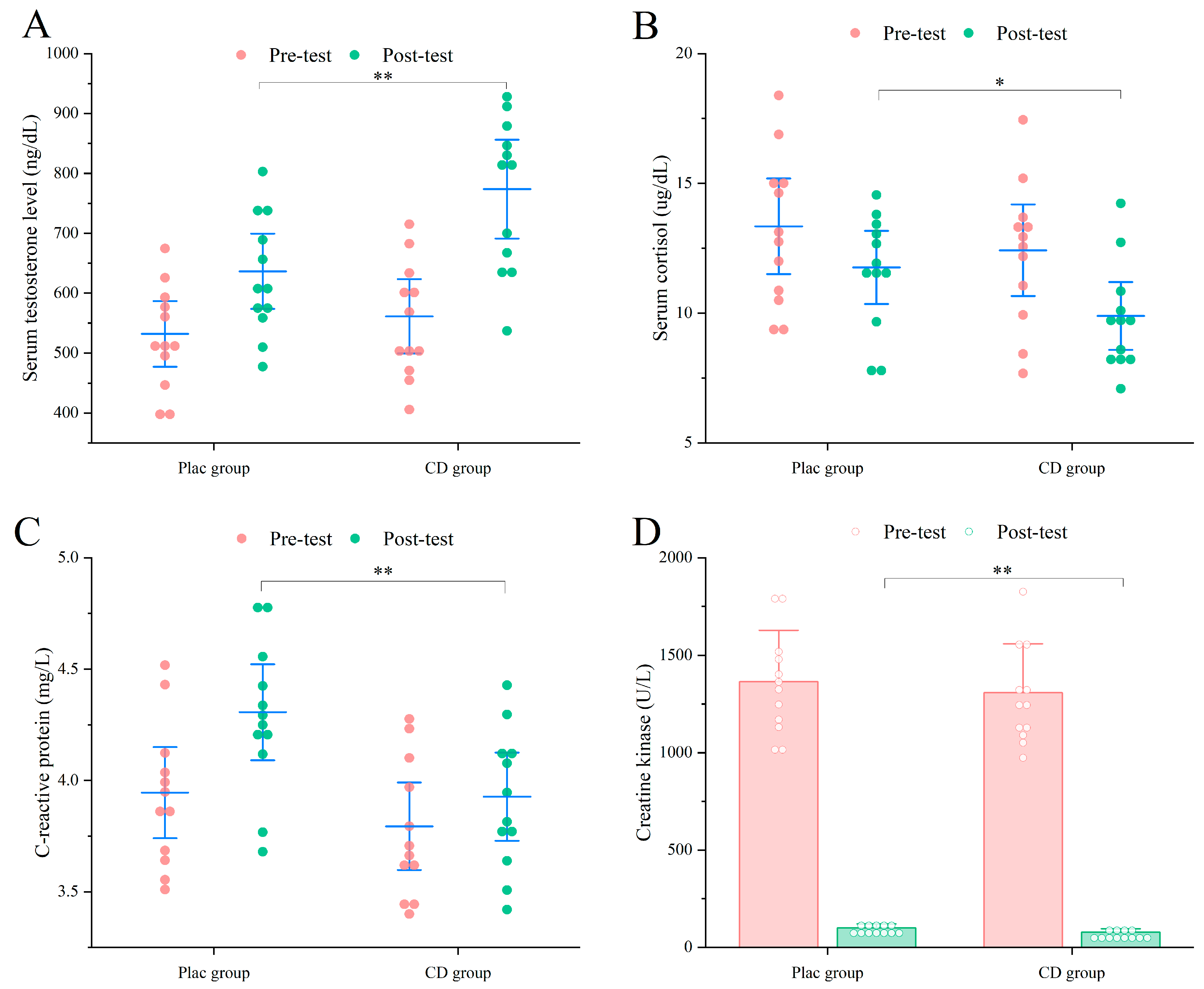
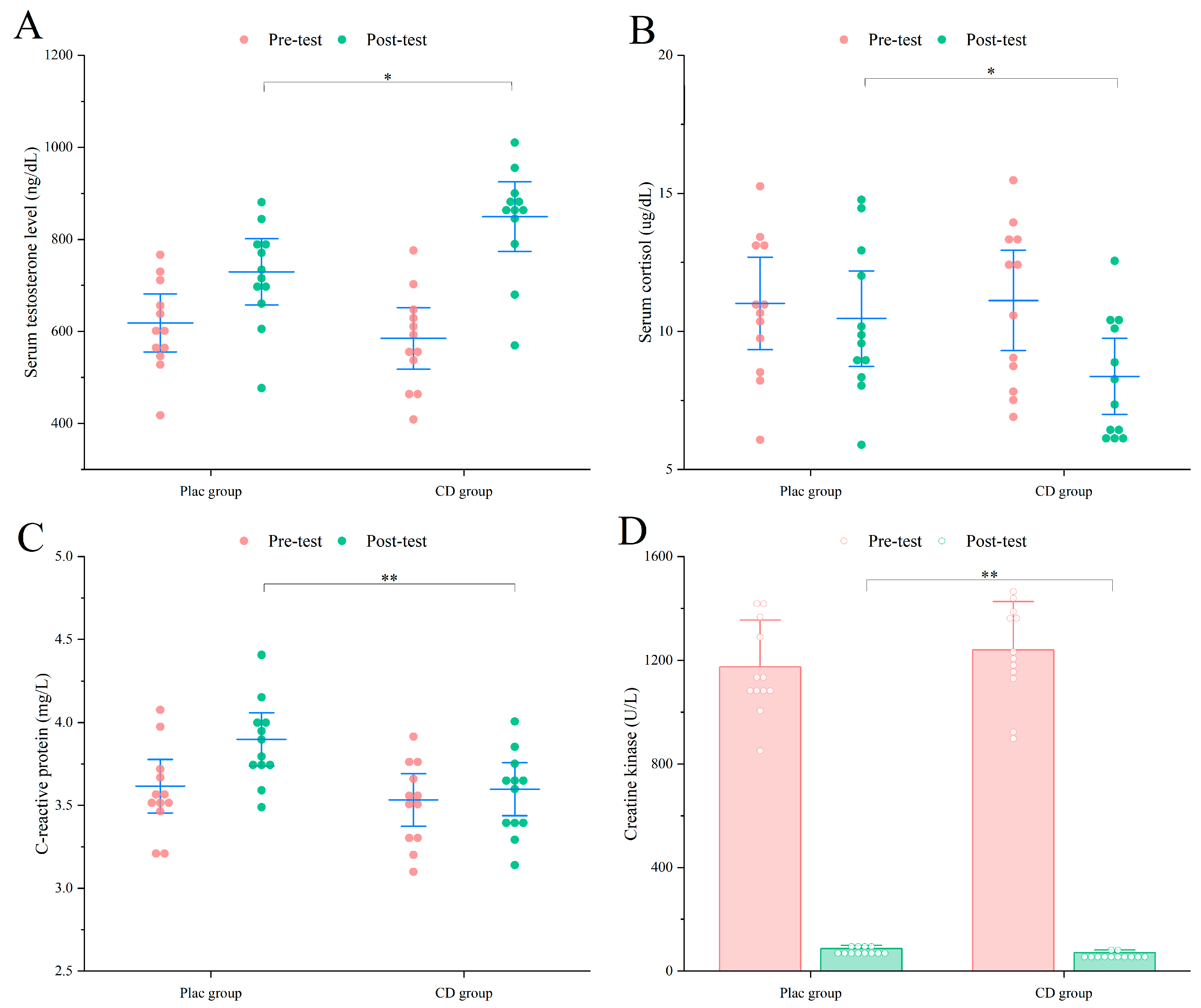
3.2.2. Muscle Recovery
3.3. Changes in Trained Participants After Supplementation with Training Supplements
3.3.1. Muscle Strength
3.3.2. Muscle Recovery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, N.; Wang, H.; Hao, H.; Rahman, F.U.; Zhang, Y. Research progress on polysaccharide components of Cistanche deserticola as potential pharmaceutical agents. Eur. J. Med. Chem. 2023, 245, 114892. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Xie, W. Cistanche deserticola Y.C. Ma, “Desert ginseng”: A review. Am. J. Chin. Med. 2012, 40, 1123–1141. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Zheng, D.; Wen, L.; Hou, Q.; He, S.; Zhang, H.; Gong, Y.; Li, M.; Hu, J.; Yang, J. Advance in Cistanche deserticola Y.C. Ma. polysaccharides: Isolation, structural characterization, bioactivities and application: A review. Int. J. Biol. Macromol. 2024, 278, 134786. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, Y.; Chen, J.; Yu, M.; Zhao, Q.; Jing, B.; Yang, N.; Ma, X.; Wang, Y. Chemical composition, pharmacological effects, and parasitic mechanisms of Cistanche deserticola: An update. Phytomedicine 2024, 132, 155808. [Google Scholar] [CrossRef]
- Cai, R.-L.; Yang, M.-H.; Shi, Y.; Chen, J.; Li, Y.-C.; Qi, Y. Antifatigue activity of phenylethanoid-rich extract from Cistanche deserticola. Phytother. Res. 2010, 24, 313–315. [Google Scholar] [CrossRef]
- Inada, Y.; Tohda, C.; Yang, X. Effects of Cistanche tubulosa Wight Extract on Locomotive Syndrome: A Placebo-Controlled, Randomized, Double-Blind Study. Nutrients 2021, 13, 264. [Google Scholar] [CrossRef] [PubMed]
- Kimbara, Y.; Shimada, Y.; Kuboyama, T.; Tohda, C. Cistanche tubulosa (Schenk) Wight Extract Enhances Hindlimb Performance and Attenuates Myosin Heavy Chain IId/IIx Expression in Cast-Immobilized Mice. Evid.-Based Complement. Altern. Med. Ecam 2019, 2019, 9283171. [Google Scholar] [CrossRef]
- Liu, K.; Li, J.; Hao, W.; Li, J.; Khan, I.; Liang, Y.; Wang, H.; Li, X.; Zhang, C. Lactiplantibacillus plantarum LZU-J-Q21 enhanced the functional metabolic profile and bioactivity of Cistanche deserticola. Food Chem. X 2024, 24, 101941. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Ju, B.; Liu, J.; Wen, L.; Zhao, Y.; Yang, J.; Hu, J. Phenylethanol Glycosides from Cistanche tubulosa Modulate the Gut Microbiota and Cecal Metabolites to Ameliorate Diabetic Nephropathy Induced by Streptozotocin Combined with High-Fat Diet in Rats. J. Med. Food 2025, 28, 219–231. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Shen, X.; Liu, Y. The use of traditional Chinese medicines in relieving exercise-induced fatigue. Front. Pharmacol. 2022, 13, 969827. [Google Scholar] [CrossRef]
- Luo, C.; Xu, X.; Wei, X.; Feng, W.; Huang, H.; Liu, H.; Xu, R.; Lin, J.; Han, L.; Zhang, D. Natural medicines for the treatment of fatigue: Bioactive components, pharmacology, and mechanisms. Pharmacol. Res. 2019, 148, 104409. [Google Scholar] [CrossRef]
- Lan, T.; Yu, Q. Cistanches deserticola PhG-RE through Inhibiting ERS Apoptosis Mechanism to Protect Myocardial Cell Apoptosis from H2O2-Induced Endoplasmic Reticulum Stress. Evid.-Based Complement. Altern. Med. Ecam 2020, 2020, 8219296. [Google Scholar] [CrossRef]
- Takaya, K.; Asou, T.; Kishi, K. Cistanche deserticola Polysaccharide Reduces Inflammation and Aging Phenotypes in the Dermal Fibroblasts through the Activation of the NRF2/HO-1 Pathway. Int. J. Mol. Sci. 2023, 24, 15704. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lin, H.; Gu, L.; Gao, J.; Tzeng, C.M. Herba Cistanche (Rou Cong-Rong): One of the Best Pharmaceutical Gifts of Traditional Chinese Medicine. Front. Pharmacol. 2016, 7, 41. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, H.; Xu, W.; Liu, C.; Huang, L. Cistanche Species Mitogenomes Suggest Diversity and Complexity in Lamiales-Order Mitogenomes. Genes 2022, 13, 1791. [Google Scholar] [CrossRef]
- Bai, R.; Fan, J.; Wang, Y.; Wang, Y.; Li, X.; Hu, F. Protective effect of Cistanche deserticola on gentamicin-induced nephrotoxicity in rats. Chin. Herb. Med. 2023, 15, 102–109. [Google Scholar] [CrossRef]
- Moesgaard, L.; Beck, M.M.; Christiansen, L.; Aagaard, P.; Lundbye-Jensen, J. Effects of Periodization on Strength and Muscle Hypertrophy in Volume-Equated Resistance Training Programs: A Systematic Review and Meta-analysis. Sports Med. 2022, 52, 1647–1666. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Grgic, J.; Ogborn, D.; Krieger, J.W. Strength and Hypertrophy Adaptations Between Low- vs. High-Load Resistance Training: A Systematic Review and Meta-analysis. J. Strength Cond. Res. 2017, 31, 3508–3523. [Google Scholar] [CrossRef] [PubMed]
- Spiering, B.A.; Clark, B.C.; Schoenfeld, B.J.; Foulis, S.A.; Pasiakos, S.M. Maximizing Strength: The Stimuli and Mediators of Strength Gains and Their Application to Training and Rehabilitation. J. Strength Cond. Res. 2023, 37, 919–929. [Google Scholar] [CrossRef]
- Bjornsen, T.; Wernbom, M.; Paulsen, G.; Berntsen, S.; Brankovic, R.; Stålesen, H.; Sundnes, J.; Raastad, T. Frequent blood flow restricted training not to failure and to failure induces similar gains in myonuclei and muscle mass. Scand. J. Med. Sci. Sports 2021, 31, 1420–1439. [Google Scholar] [CrossRef]
- Chen, F.; He, X.; Zhou, M.; Cheng, P.; Ming, F.; Huang, Y. Study on the anti-fatigue and anti-oxidant effects of herbs Schnabelia tetradonta in mice and rats. Pharmacol. Clin. Chin. Mater. Medica 2015, 31, 100–102. [Google Scholar]
- Luo, X.; Liu, W.; Wang, J.; Du, J.; Feng, F. Effects of the combination of sea cucumber peptide and Cistanche deserticola on hormone regulation and testicular anti-oxidative damage in acute-exercising mice. J. Zhejiang Univ. Agric. Life Sci. 2023, 49, 105–116,140. [Google Scholar]
- Zhou, H.; Cao, J.; Lin, Q. Effects of Herba Cistanches on Testosterone Content, Substance Metabolism and Exercise Capacity in Rats After Exercise Training. Chin. Pharm. J. 2012, 47, 1035–1038. [Google Scholar]
- Lin, W.Y.; Yao, C.; Cheng, J.; Kao, S.T.; Tsai, F.J.; Liu, H.P. Molecular pathways related to the longevity promotion and cognitive improvement of Cistanche tubulosa in Drosophila. Phytomedicine 2017, 26, 37–44. [Google Scholar] [CrossRef]
- Wen, S.Y.; Ng, S.C.; Noriega, L.; Chen, T.J.; Chen, C.J.; Lee, S.D.; Huang, C.Y.; Kuo, W.W. Echinacoside promotes collagen synthesis and survival via activation of IGF-1 signaling to alleviate UVB-induced dermal fibroblast photoaging. Biofactors 2025, 51, e2152. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Li, Y.; Guo, L.; Xu, P.; Du, H.; Lin, N.; Xu, Y. The role of Cistanches Herba and its ingredients in improving reproductive outcomes: A comprehensive review. Phytomedicine 2024, 129, 155681. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, J.; Li, Y.; Jiang, L.; Ouyang, Y.; Li, Y.; Yang, L.; Zhao, X.; Huang, L.; Xiang, H.; et al. Cistanche deserticola polysaccharide induces melanogenesis in melanocytes and reduces oxidative stress via activating NRF2/HO-1 pathway. J. Cell. Mol. Med. 2020, 24, 4023–4035. [Google Scholar] [CrossRef]
- Suchomel, T.J.; Nimphius, S.; Bellon, C.R.; Hornsby, W.G.; Stone, M.H. Training for Muscular Strength: Methods for Monitoring and Adjusting Training Intensity. Sports Med. 2021, 51, 2051–2066. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, H.; Yan, Y.; Yang, W.; Chen, S.; Song, G.; Li, X.; Gu, Y.; Yun, H.; Li, Y. Synergistic Effect of Rhodiola rosea and Caffeine Supplementation on the Improvement of Muscle Strength and Muscular Endurance: A Pilot Study for Rats, Resistance Exercise-Untrained and -Trained Volunteers. Nutrients 2023, 15, 582. [Google Scholar] [CrossRef]
- Tao, B.; Sun, H.; Li, H.; Xu, Z.; Xu, Y.; Chen, L.; Ma, C.; Zhang, X.; Yu, L.; Bao, S.; et al. Combined Effects of Rhodiola Rosea and Caffeine Supplementation on Straight Punch Explosive Power in Untrained and Trained Boxing Volunteers: A Synergistic Approach. Metabolites 2025, 15, 262. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.T.; Zheng, D.X.; Hou, Q.; Wen, L.M.; Wang, B.J.; Geng, R.Y.; Wang, Q.Q.; Dai, W.; Tian, L.Y.; He, S.Q.; et al. Optimization of Extraction Process, Structural Characterization, and Antioxidant and Hypoglycemic Activity Evaluation of Polysaccharides From the Medicinal and Edible Plant: Cistanche deserticola Ma. Phytochem. Anal. PCA 2025, 36, 1333–1350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, J.; Wang, W.; Li, Q.; Chen, Y.; Feng, W.; Zheng, D.; Zhao, T.; Mao, G.; Yang, L.; et al. Extraction, purification, characterization and antioxidant activities of polysaccharides from Cistanche tubulosa. Int. J. Biol. Macromol. 2016, 93, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Yang, X.; Li, Q.; Yang, Y.; Zhao, G.; Wang, B.; Wu, D. Immunostimulatory activity of water-extractable polysaccharides from Cistanche deserticola as a plant adjuvant in vitro and in vivo. PLoS ONE 2018, 13, e0191356. [Google Scholar] [CrossRef]
- Aube, D.; Wadhi, T.; Rauch, J.; Anand, A.; Barakat, C.; Pearson, J.; Bradshaw, J.; Zazzo, S.; Ugrinowitsch, C.; De Souza, E.O. Progressive Resistance Training Volume: Effects on Muscle Thickness, Mass, and Strength Adaptations in Resistance-Trained Individuals. J. Strength Cond. Res. 2022, 36, 600–607. [Google Scholar] [CrossRef]
- Brigatto, F.A.; Lima, L.E.M.; Germano, M.D.; Aoki, M.S.; Braz, T.V.; Lopes, C.R. High Resistance-Training Volume Enhances Muscle Thickness in Resistance-Trained Men. J. Strength Cond. Res. 2022, 36, 22–30. [Google Scholar] [CrossRef]
- Neves, R.P.; Vechin, F.C.; Teixeira, E.L.; da Silva, D.D.; Ugrinowitsch, C.; Roschel, H.; Aihara, A.Y.; Tricoli, V. Effect of different training frequencies on maximal strength performance and muscle hypertrophy in trained individuals-a within-subject design. PLoS ONE 2022, 17, e0276154. [Google Scholar] [CrossRef]
- Lozano, C.P.; Neubig, K.E.; Saha, S.; Broyles, S.T.; Apolzan, J.W.; Martin, C.K. Validity of the PortionSize application compared with that of MyFitnessPal for accurately estimating intake: A randomized crossover laboratory-based evaluation. Am. J. Clin. Nutr. 2024, 120, 419–430. [Google Scholar] [CrossRef]
- Fernandez Ortega, J.A.; Mendoza Romero, D.; Sarmento, H.; Prieto Mondragón, L. Bar Load-Velocity Profile of Full Squat and Bench Press Exercises in Young Recreational Athletes. Int. J. Environ. Res. Public Health 2022, 19, 6756. [Google Scholar] [CrossRef]
- Sato, S.; Yoshida, R.; Murakoshi, F.; Sasaki, Y.; Yahata, K.; Nosaka, K.; Nakamura, M. Effect of daily 3-s maximum voluntary isometric, concentric, or eccentric contraction on elbow flexor strength. Scand. J. Med. Sci. Sports 2022, 32, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J.; Schoenfeld, B.J.; Orazem, J.; Sabol, F. Effects of resistance training performed to repetition failure or non-failure on muscular strength and hypertrophy: A systematic review and meta-analysis. J. Sport Health Sci. 2022, 11, 202–211. [Google Scholar] [CrossRef]
- Bonilla, D.A.; Kreider, R.B.; Stout, J.R.; Forero, D.A.; Kerksick, C.M.; Roberts, M.D.; Rawson, E.S. Metabolic Basis of Creatine in Health and Disease: A Bioinformatics-Assisted Review. Nutrients 2021, 13, 1238. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Arribalzaga, S.; Gutiérrez-Abejón, E.; Azarbayjani, M.A.; Mielgo-Ayuso, J.; Roche, E. Omega-3 Fatty Acid Supplementation on Post-Exercise Inflammation, Muscle Damage, Oxidative Response, and Sports Performance in Physically Healthy Adults-A Systematic Review of Randomized Controlled Trials. Nutrients 2024, 16, 2044. [Google Scholar] [CrossRef]
- Moore, E.; Fuller, J.T.; Buckley, J.D.; Saunders, S.; Halson, S.L.; Broatch, J.R.; Bellenger, C.R. Impact of Cold-Water Immersion Compared with Passive Recovery Following a Single Bout of Strenuous Exercise on Athletic Performance in Physically Active Participants: A Systematic Review with Meta-analysis and Meta-regression. Sports Med. 2022, 52, 1667–1688. [Google Scholar] [CrossRef]
- Kaul, I.; Sawchak, S.; Correll, C.U.; Kakar, R.; Breier, A.; Zhu, H.; Miller, A.C.; Paul, S.M.; Brannan, S.K. Efficacy and safety of the muscarinic receptor agonist KarXT (xanomeline-trospium) in schizophrenia (EMERGENT-2) in the USA: Results from a randomised, double-blind, placebo-controlled, flexible-dose phase 3 trial. Lancet 2024, 403, 160–170. [Google Scholar] [CrossRef]
- Ziegenfuss, T.N.; Kedia, A.W.; Sandrock, J.E.; Raub, B.J.; Kerksick, C.M.; Lopez, H.L. Effects of an Aqueous Extract of Withania somnifera on Strength Training Adaptations and Recovery: The STAR Trial. Nutrients 2018, 10, 1807. [Google Scholar] [CrossRef]
- Tian, S.; Guo, L.; Song, Y.; Miao, J.; Peng, M.; Fang, X.; Bai, M.; Miao, M. Transcriptomic analysis the mechanisms of anti-osteoporosis of desert-living Cistanche herb in ovariectomized rats of postmenopausal osteoporosis. Funct. Integr. Genom. 2023, 23, 237. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, J.; Yu, J.; Li, Z.; Zhang, M.; Chen, Y.; Liu, F.; Jiang, D.; Guo, J.; Li, X.; et al. Mfn2 regulates calcium homeostasis and suppresses PASMCs proliferation via interaction with IP3R3 to mitigate pulmonary arterial hypertension. J. Transl. Med. 2025, 23, 366. [Google Scholar] [CrossRef] [PubMed]
- Wat, E.; Ng, C.F.; Koon, C.M.; Wong, E.C.; Tomlinson, B.; Lau, C.B. The protective effect of Herba Cistanches on statin-induced myotoxicity in vitro. J. Ethnopharmacol. 2016, 190, 68–73. [Google Scholar] [CrossRef]
- Xiong, W.T.; Gu, L.; Wang, C.; Sun, H.X.; Liu, X. Anti-hyperglycemic and hypolipidemic effects of Cistanche tubulosa in type 2 diabetic db/db mice. J. Ethnopharmacol. 2013, 150, 935–945. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, L.L.; Ding, S.Q.; Liu, J.J.; Dong, Y.H.; Li, Y.T.; Li, N.; Zhao, X.J.; Hu, C.L.; Jiang, Y.; et al. Anti-Osteoporotic Activity of an Edible Traditional Chinese Medicine Cistanche deserticola on Bone Metabolism of Ovariectomized Rats Through RANKL/RANK/TRAF6-Mediated Signaling Pathways. Front. Pharmacol. 2019, 10, 1412. [Google Scholar] [CrossRef]
- Zhu, K.; Meng, Z.; Tian, Y.; Gu, R.; Xu, Z.; Fang, H.; Liu, W.; Huang, W.; Ding, G.; Xiao, W. Hypoglycemic and hypolipidemic effects of total glycosides of Cistanche tubulosa in diet/streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2021, 276, 113991. [Google Scholar] [CrossRef]
- Kan, J.; Cheng, J.; Hu, C.; Chen, L.; Liu, S.; Venzon, D.; Murray, M.; Li, S.; Du, J. A Botanical Product Containing Cistanche and Ginkgo Extracts Potentially Improves Chronic Fatigue Syndrome Symptoms in Adults: A Randomized, Double-Blind, and Placebo-Controlled Study. Front. Nutr. 2021, 8, 658630. [Google Scholar] [CrossRef]
- Wang, F.J.; Li, R.Y.; Tu, P.F.; Chen, J.P.; Zeng, K.W.; Jiang, Y. Total Glycosides of Cistanche deserticola Promote Neurological Function Recovery by Inducing Neurovascular Regeneration via Nrf-2/Keap-1 Pathway in MCAO/R Rats. Front. Pharmacol. 2020, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Z.; Han, M.; Zhang, Y.; Muhammad, H.; Zhong, H.; Guan, R. Bioactive Components, Pharmacological Properties, and Applications of Cistanche deserticola Y.C. Ma: A Comprehensive Review. Nutrients 2025, 17, 1501. [Google Scholar] [CrossRef]
- Tao, J.; Zhong, X.; Lin, H.; Lai, Y.; Jian, Z.; Tao, A.; Jiang, G. The potential, challenges, and prospects of polysaccharides from the genus Cistanche as therapeutic agents for aging-related diseases: A review. Int. J. Biol. Macromol. 2025, 312, 144144. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.X.; Song, X.M.; Wang, L.C.; Lv, H.N.; Chen, J.F.; Liu, D.; Fu, G.; Zhao, M.B.; Jiang, Y.; Zeng, K.W.; et al. Highly selective inhibition of IMPDH2 provides the basis of antineuroinflammation therapy. Proc. Natl. Acad. Sci. USA 2017, 114, E5986–E5994. [Google Scholar] [CrossRef]
- Zhang, S.; Gong, F.; Liu, J.; Liu, T.; Yang, J.; Hu, J. A novel PHD2 inhibitor acteoside from Cistanche tubulosa induces skeletal muscle mitophagy to improve cancer-related fatigue. Biomed. Pharmacother. 2022, 150, 113004. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, Y.; Wang, Z.; Zeng, F.; Zhen, Q.; Zhao, H.; Li, J.; Ma, T.; Huang, C. Echinacoside from Cistanche tubulosa ameliorates alcohol-induced liver injury and oxidative stress by targeting Nrf2. FASEB J. 2023, 37, e22792. [Google Scholar] [CrossRef]
- Qiao, M.; Xue, T.; Zhu, Y.; Yang, J.; Hu, J. Polysaccharides from Cistanche deserticola mitigate inflammatory bowel disease via modulating intestinal microbiota and SRC/EGFR/PI3K/AKT signaling pathways. Int. J. Biol. Macromol. 2025, 308, 142452. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhang, Y.; Ren, H.; Zhang, Y.; Liu, X.; Pu, J.; Yu, J.; Yu, X.; Pei, X. Cistanche deserticola polysaccharides extracted from Cistanche deserticola Y.C. Ma promote the differentiation of mouse female germline stem cells in vitro. J. Ethnopharmacol. 2022, 296, 115495. [Google Scholar] [CrossRef]
- Zhou, S.; Feng, D.; Zhou, Y.; Duan, H.; Jiang, Y.; Yan, W. Analysis of the active ingredients and health applications of cistanche. Front. Nutr. 2023, 10, 1101182. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ning, Z. Echinacoside alleviates Ang II-induced cardiac fibrosis by enhancing the SIRT1/IL-11 pathway. Iran. J. Basic Med. Sci. 2025, 28, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Henselmans, M.; Bjørnsen, T.; Hedderman, R.; Vårvik, F.T. The Effect of Carbohydrate Intake on Strength and Resistance Training Performance: A Systematic Review. Nutrients 2022, 14, 856. [Google Scholar] [CrossRef] [PubMed]
| Between-Group Comparison | ||||||
|---|---|---|---|---|---|---|
| Training Status | Variables | n | Baseline (Week 0) | Post (Week 8) | 95% CI | p-Value |
| Untrained Participants | Calorie Intake (kcals/day) | |||||
| CD | 12 | 2138 ± 611 | 2339 ± 623 | (−5.9, 144.1) | 0.76 | |
| PLAC | 12 | 2289 ± 465 | 2270 ± 504 | |||
| Carbohydrate Intake (g/day) | ||||||
| CD | 12 | 210 ± 90 | 215 ± 94 | (−16.6, 3.3) | 0.85 | |
| PLAC | 12 | 216 ± 78 | 222 ± 79 | |||
| Protein Intake (g/day) | ||||||
| CD | 12 | 96 ± 35 | 98 ± 40 | (3.7, 8.5) | 0.70 | |
| PLAC | 12 | 89 ± 30 | 92 ± 36 | |||
| Fat Intake (g/day) | ||||||
| CD | 12 | 76 ± 31 | 80 ± 35 | (0.1, 13.0) | 0.71 | |
| PLAC | 12 | 71 ± 29.92 | 75 ± 32 | |||
| Trained Participants | Calorie Intake (kcals/day) | |||||
| CD | 12 | 3382 ± 651 | 3578 ± 757 | (48.3, 72.6) | 0.84 | |
| PLAC | 12 | 3441 ± 691 | 3518 ± 738 | |||
| Carbohydrate Intake (g/day) | ||||||
| CD | 12 | 515 ± 108 | 557 ± 127 | (8.7, 20.1) | 0.77 | |
| PLAC | 12 | 520 ± 103 | 543 ± 118 | |||
| Protein Intake (g/day) | ||||||
| CD | 12 | 71 ± 14 | 73 ± 15 | (1.8, 3.3) | 0.79 | |
| PLAC | 12 | 68 ± 13 | 72 ± 14 | |||
| Fat Intake (g/day) | ||||||
| CD | 12 | 102 ± 18 | 109 ± 23 | (0.4, 1.0) | 0.90 | |
| PLAC | 12 | 103 ± 19 | 106 ± 22 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, B.; Lian, W.; Min, R.; Zhang, X.; Chen, L.; Hao, S.; Li, Z.; Ma, C.; Zhang, H.; Liu, C. Effects of Cistanche deserticola Y.C. Ma Supplementation on Muscle Strength and Recovery: A Randomized Controlled Trial. Nutrients 2025, 17, 2965. https://doi.org/10.3390/nu17182965
Tao B, Lian W, Min R, Zhang X, Chen L, Hao S, Li Z, Ma C, Zhang H, Liu C. Effects of Cistanche deserticola Y.C. Ma Supplementation on Muscle Strength and Recovery: A Randomized Controlled Trial. Nutrients. 2025; 17(18):2965. https://doi.org/10.3390/nu17182965
Chicago/Turabian StyleTao, Biaoxu, Weihao Lian, Rongrong Min, Xiaoyu Zhang, Liqi Chen, Sun Hao, Ze Li, Chengzhe Ma, Haojie Zhang, and Chang Liu. 2025. "Effects of Cistanche deserticola Y.C. Ma Supplementation on Muscle Strength and Recovery: A Randomized Controlled Trial" Nutrients 17, no. 18: 2965. https://doi.org/10.3390/nu17182965
APA StyleTao, B., Lian, W., Min, R., Zhang, X., Chen, L., Hao, S., Li, Z., Ma, C., Zhang, H., & Liu, C. (2025). Effects of Cistanche deserticola Y.C. Ma Supplementation on Muscle Strength and Recovery: A Randomized Controlled Trial. Nutrients, 17(18), 2965. https://doi.org/10.3390/nu17182965







