In Vitro Suppression Effects of Ephedra przewalskii Stapf-Derived Natural Compounds on SARS-CoV-2
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Virus and Cells
2.3. Extraction Procedures
2.3.1. Separation of Complex Compounds from E. przewalskii
2.3.2. Dissolution of Powders Obtained from Fractions 1–7
2.4. Extraction, Separation, and Identification of E. przewalskii Antiviral Components
2.4.1. Identification of Antiviral-Active Fractions
2.4.2. Identification of Antiviral-Active Compounds in the Active Fractions
2.5. Cytotoxicity Assay
2.6. Replication of SARS-CoV-2
2.7. Viral-Particle Inactivation Assay
2.8. Statistical Analysis
3. Results
3.1. Separation of E. przewalskii
3.2. Antiviral Effects of the E. przewalskii Fractions and Their Cytotoxicity to Vero/TMPRSS2 Cells
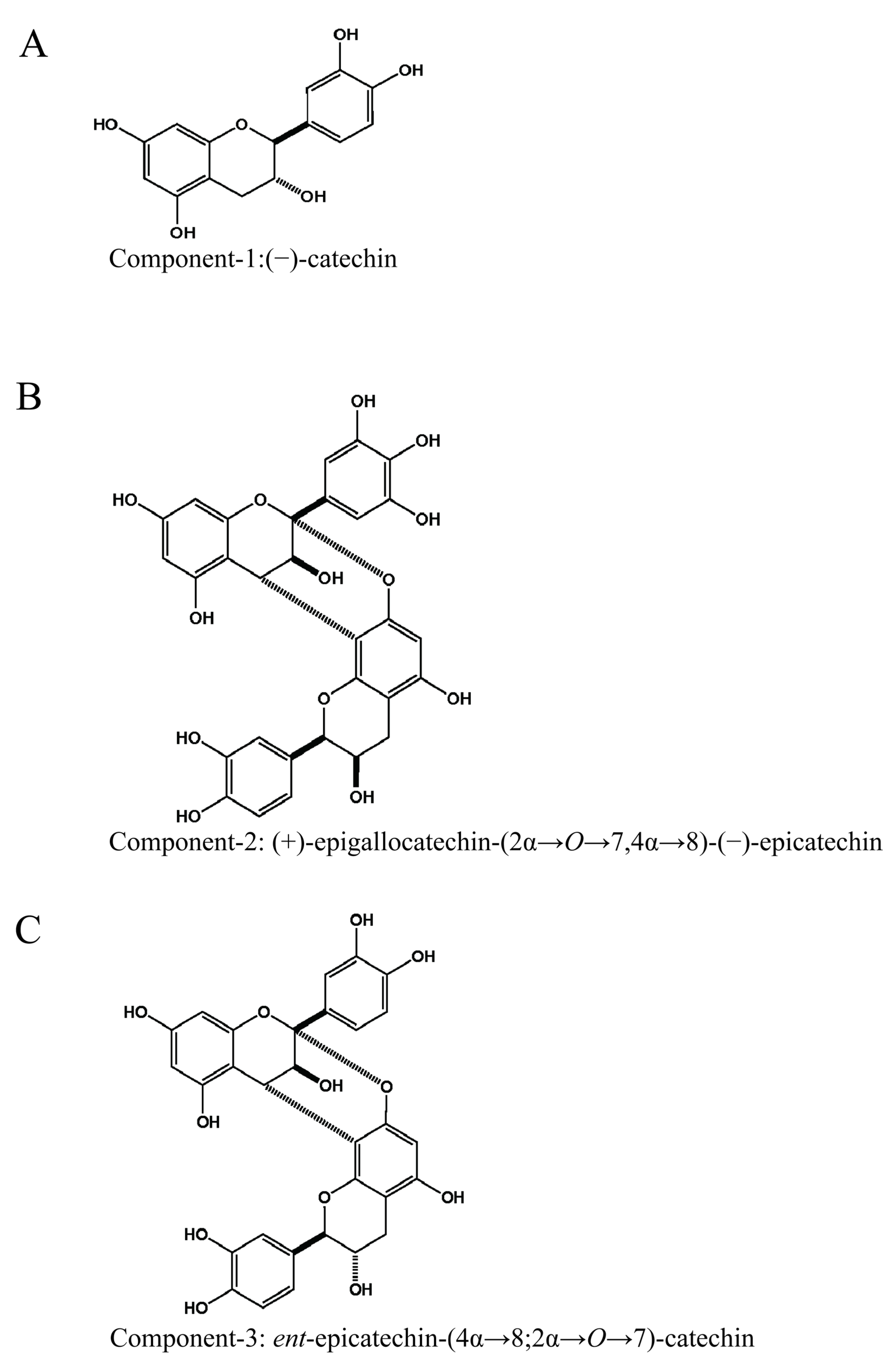
3.3. Structures of Isolated Compounds
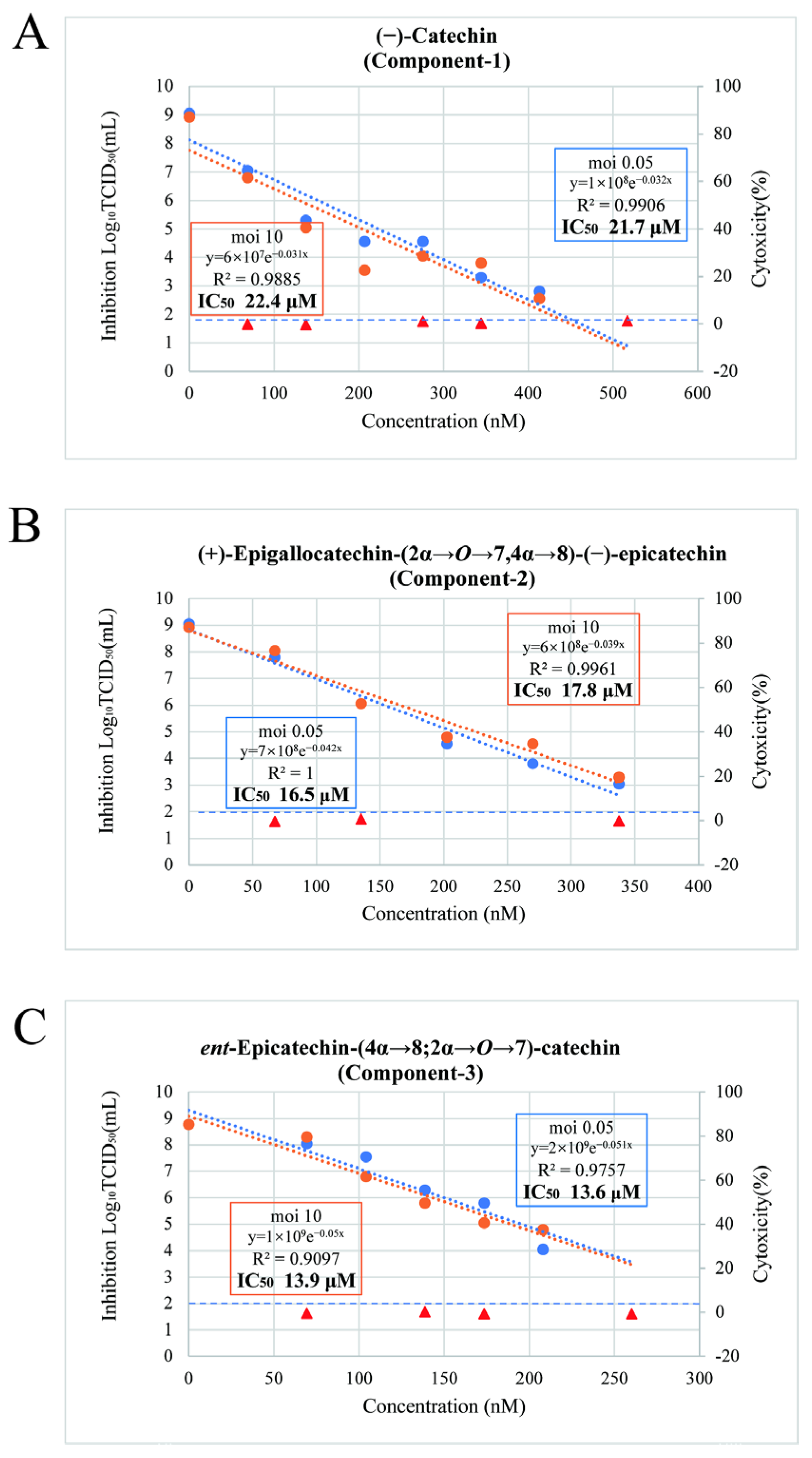
3.4. Antiviral Effects of Component-1, -2, and -3
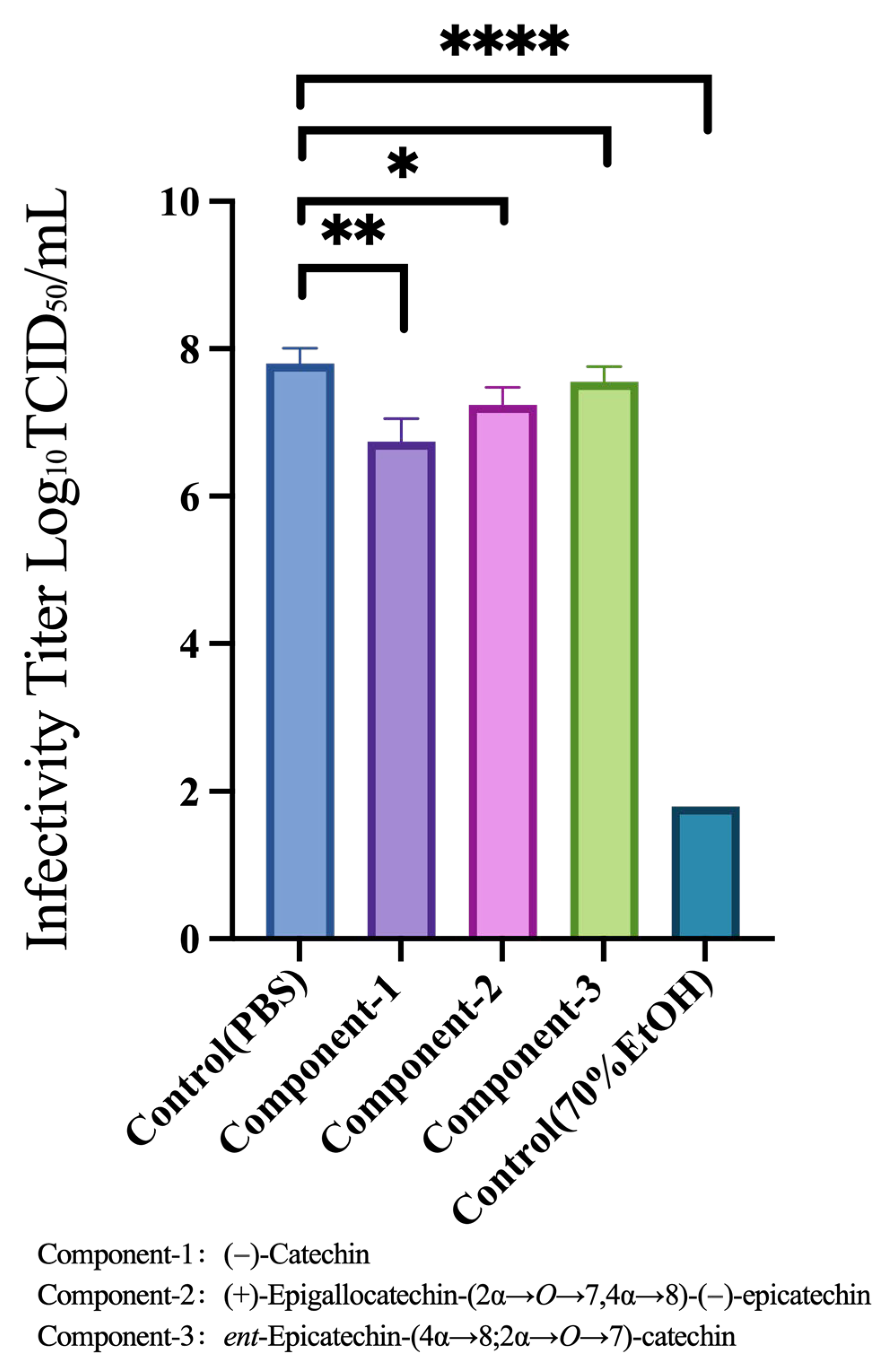
3.5. Viral-Particle Inactivation Effects of Component-1, Component-2, and Component-3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPE | Cytopathic effect |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | Dimethylsulfoxide |
| HPLC | High-performance liquid chromatography |
| IC50 | Half-maximal inhibitory concentration |
| JCRB | Japanese Collection of Research Bioresources |
| LDH | Lactate dehydrogenase |
| MOI | Multiplicity of infection |
| PBS | Phosphate-buffered saline |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| TCID50 | 50% tissue culture infective dose |
| TGEV | Transmissible gastroenteritis virus |
References
- World Health Organization. COVID-19 Cases|WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 14 January 2025).
- Kitani, Y.; Zhu, S.; Omote, T.; Tanaka, K.; Batkhuu, J.; Sanchir, C.; Fushimi, H.; Mikage, M.; Komatsu, K. Molecular Analysis and Chemical Evaluation of Ephedra Plants in Mongolia. Biol. Pharm. Bull. 2009, 32, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Wu, W.; Li, K. A Message Efficient Intersection Control Algorithm for Intelligent Transportation in Smart Cities. Future Gener. Comput. Syst. 2017, 76, 339–349. [Google Scholar] [CrossRef]
- Medical Herbs Study Center. Хoнин Зээргэнэ|Эмийн Ургамал Судлалын Төв. Available online: https://urgamal.com/product/ephedra-przewalskii-stapf.html (accessed on 14 January 2025).
- Kakimoto, M.; Nomura, T.; Nazmul, T.; Yamamoto, A.; Sasaki, H.; Higashiura, A.; Ito, M.; Ohge, H.; Mikage, M.; Ogawa, K.O.; et al. In Vitro Anti-Severe Acute Respiratory Syndrome Coronavirus 2 Effect of Ephedra Przewalskii Stapf Extract. J. Ethnopharmacol. 2024, 319, 117341. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Yang, L.; Li, H.; An, Q.; Liu, Y.; Cheng, J.; Hou, F.; Guo, L.; Zhang, D. Exploration of Chemical Components and Metabolite Synthesis Pathways in Eight Ephedra Species Based on HS-GC-MS and UPLC-Q-TOF-MS. Front. Plant Sci. 2024, 15, 1421008. [Google Scholar] [CrossRef] [PubMed]
- Hyuga, M.; Uchiyama, N.; Yoshimura, M.; Amakura, Y.; Hyuga, S.; Uema, M.; Tsuji, G.; Nishi, A.; Odaguchi, H.; Demizu, Y.; et al. Molecular Mechanism of Anti-SARS-CoV-2 Activity of Ephedra Herb Macromolecule Condensed-Tannin Contained in Ephedrine Alkaloids-Free Ephedra Herb Extract. Chem. Pharm. Bull. 2025, 73, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Tamura, K.; Jamsransuren, D.; Matsuda, S.; Ogawa, H. Severe Acute Respiratory Syndrome Coronavirus-2 Inactivation Activity of the Polyphenol-Rich Tea Leaf Extract with Concentrated Theaflavins and Other Virucidal Catechins. Molecules 2021, 26, 4803. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xie, D.-Y. Docking Characterization and in Vitro Inhibitory Activity of Flavan-3-Ols and Dimeric Proanthocyanidins Against the Main Protease Activity of SARS-Cov-2. Front. Plant Sci. 2020, 11, 601316. [Google Scholar] [CrossRef] [PubMed]
- The World Flora Online Ephedra Przewalskii Stapf. Available online: https://www.worldfloraonline.org/taxon/wfo-0000794461 (accessed on 14 January 2025).
- Nakamori, S.; Takahashi, J.; Hyuga, S.; Yang, J.; Takemoto, H.; Maruyama, T.; Oshima, N.; Uchiyama, N.; Amakura, Y.; Hyuga, M.; et al. Analgesic Effects of Ephedra Herb Extract, Ephedrine Alkaloids–Free Ephedra Herb Extract, Ephedrine, and Pseudoephedrine on Formalin-Induced Pain. Biol. Pharm. Bull. 2019, 42, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Kakimoto, M.; Nomura, T.; Nazmul, T.; Kitagawa, H.; Kanno, K.; Ogawa-Ochiai, K.; Ohge, H.; Ito, M.; Sakaguchi, T. In Vitro Suppression of SARS-CoV-2 Infection by Existing Kampo Formulas and Crude Constituent Drugs Used for Treatment of Common Cold Respiratory Symptoms. Front. Pharmacol. 2022, 13, 804103. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yamano, Y.; Kawakami, S.; Al-Hamoud, G.A.; Sugimoto, S.; Otsuka, H.; Matsunami, K. New ψ-Santonin Derivatives from Crossostephium Chinense and Their Anti-Proliferative Activities against Leishmania Major and Human Cancer Cells A549. Molecules 2023, 28, 8108. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, H.; Sakar, M.K.; Burger, J.F.W.; Engelshowe, R.; Ferreira, D. A-Type Proanthocyanidins from Prunus Spinosa. Phytochemistry 1991, 30, 2041–2047. [Google Scholar] [CrossRef]
- Zang, X.; Shang, M.; Xu, F.; Liang, J.; Wang, X.; Mikage, M.; Cai, S. A-Type Proanthocyanidins from the Stems of Ephedra Sinica (Ephedraceae) and Their Antimicrobial Activities. Molecules 2013, 18, 5172–5189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jayaprakasam, B.; Seeram, N.P.; Olson, L.K.; DeWitt, D.; Nair, M.G. Insulin Secretion and Cyclooxygenase Enzyme Inhibition by Cabernet Sauvignon Grape Skin Compounds. J. Agric. Food Chem. 2004, 52, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Nazmul, T.; Yoshimoto, R.; Higashiura, A.; Oda, K.; Sakaguchi, T. Ethanol Susceptibility of SARS-CoV-2 and Other Enveloped Viruses. Biocontrol Sci. 2021, 26, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Nazmul, T.; Lawal-Ayinde, B.M.; Morita, T.; Yoshimoto, R.; Higashiura, A.; Yamamoto, A.; Nomura, T.; Nakano, Y.; Hirayama, M.; Kurokawa, H.; et al. Capture and Neutralization of SARS-CoV-2 and Influenza Virus by Algae-derived Lectins with High-mannose and Core Fucose Specificities. Microbiol. Immunol. 2023, 67, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Zanwar, A.A.; Badole, S.L.; Shende, P.S.; Hegde, M.V.; Bodhankar, S.L. Chapter 21—Antioxidant Role of Catechin in Health and Disease. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 267–271. ISBN 978-0-12-398456-2. [Google Scholar]
- Liang, W.; He, L.; Ning, P.; Lin, J.; Li, H.; Lin, Z.; Kang, K.; Zhang, Y. (+)-Catechin Inhibition of Transmissible Gastroenteritis Coronavirus in Swine Testicular Cells Is Involved Its Antioxidation. Res. Vet. Sci. 2015, 103, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-F.; Bai, L.-P.; Huang, W.; Li, X.-Z.; Zhao, S.-S.; Zhong, N.-S.; Jiang, Z.-H. Comparison of in Vitro Antiviral Activity of Tea Polyphenols against Influenza A and B Viruses and Structure–Activity Relationship Analysis. Fitoterapia 2014, 93, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Mantani, N.; Andoh, T.; Kawamata, H.; Terasawa, K.; Ochiai, H. Inhibitory Effect of Ephedrae Herba, an Oriental Traditional Medicine, on the Growth of Influenza A/PR/8 Virus in MDCK Cells. Antivir. Res. 1999, 44, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Uema, M.; Hyuga, M.; Yonemitsu, K.; Hyuga, S.; Amakura, Y.; Uchiyama, N.; Mizoguchi, K.; Odaguchi, H.; Goda, Y. Antiviral Effect of Ephedrine Alkaloids-Free Ephedra Herb Extract against SARS-CoV-2 In Vitro. Microorganisms 2023, 11, 534. [Google Scholar] [CrossRef] [PubMed]
- Al-Harrasi, A.; Behl, T.; Upadhyay, T.; Chigurupati, S.; Bhatt, S.; Sehgal, A.; Bhatia, S.; Singh, S.; Sharma, N.; Vijayabalan, S.; et al. Targeting Natural Products against SARS-CoV-2. Environ. Sci. Pollut. Res. 2022, 29, 42404–42432. [Google Scholar] [CrossRef] [PubMed]
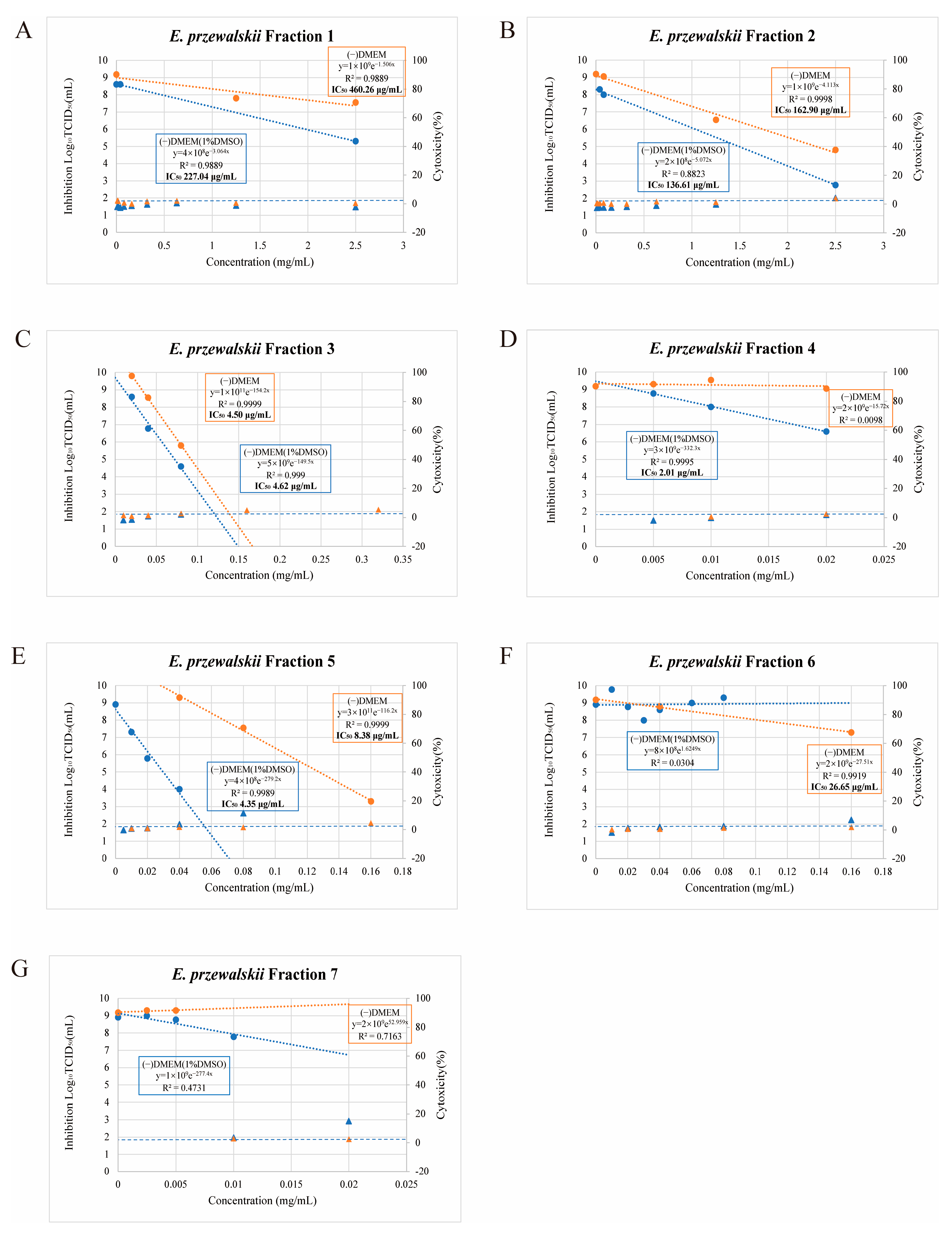
| Fraction | DMEM(−) (µg/mL) | DMEM with 1%DMSO (µg/mL) |
|---|---|---|
| 1 | 460.3 | 227 |
| 2 | 162.9 | 136.6 |
| 3 | 4.5 | 4.6 |
| 4 | not determined | 2 |
| 5 | 8.4 | 4.4 |
| 6 | 26.6 | not determined |
| 7 | not determined | not determined |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Elsayed, A.M.A.; Kakimoto, M.; Sugimoto, S.; Sakaguchi, T.; Ogawa-Ochiai, K. In Vitro Suppression Effects of Ephedra przewalskii Stapf-Derived Natural Compounds on SARS-CoV-2. Nutrients 2025, 17, 2958. https://doi.org/10.3390/nu17182958
Zhu X, Elsayed AMA, Kakimoto M, Sugimoto S, Sakaguchi T, Ogawa-Ochiai K. In Vitro Suppression Effects of Ephedra przewalskii Stapf-Derived Natural Compounds on SARS-CoV-2. Nutrients. 2025; 17(18):2958. https://doi.org/10.3390/nu17182958
Chicago/Turabian StyleZhu, Xiaolan, Abeer Mohamed Abdelfattah Elsayed, Masaki Kakimoto, Sachiko Sugimoto, Takemasa Sakaguchi, and Keiko Ogawa-Ochiai. 2025. "In Vitro Suppression Effects of Ephedra przewalskii Stapf-Derived Natural Compounds on SARS-CoV-2" Nutrients 17, no. 18: 2958. https://doi.org/10.3390/nu17182958
APA StyleZhu, X., Elsayed, A. M. A., Kakimoto, M., Sugimoto, S., Sakaguchi, T., & Ogawa-Ochiai, K. (2025). In Vitro Suppression Effects of Ephedra przewalskii Stapf-Derived Natural Compounds on SARS-CoV-2. Nutrients, 17(18), 2958. https://doi.org/10.3390/nu17182958





