Development and Validation of a Universal Eating Monitor (UEM) for Distinguishing the Intake of Multiple Foods and Macronutrients
Abstract
1. Introduction
2. Methods
2.1. The Structure of Feeding Table
2.2. Position Preference Experiment
2.2.1. Participants
2.2.2. Procedure
2.2.3. Breakfast
2.2.4. Lunch—Using the Feeding Table
2.3. Repeatability Test
2.3.1. Procedure
2.3.2. Standardized Test Meals
2.4. Statistical Analysis
2.5. The Usage of Generative Artificial Intelligence
3. Results
3.1. Macronutrient and Energy Intake Was Unaffected by Position of the Foods
3.2. The Feeding Table Demonstrated Good Repeatability in Microstructure
3.3. Standard Meal Repeatability
4. Discussion
4.1. Restriction 1: Awareness of the Monitoring or Not
4.2. Restriction 2: Emptying Your Plate
4.3. Restriction 3: Fast Overnight, Fixed Breakfast or Adherence to Lifestyle Habits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeomans, M.R. Measuring Appetite and Food Intake. In Methods in Consumer Research; Woodhead Publishing: Sawston, UK, 2018; Volume 2, pp. 119–149. [Google Scholar] [CrossRef]
- Bajunaid, R.; Niu, C.; Hambly, C.; Liu, Z.; Yamada, Y.; Aleman-Mateo, H.; Anderson, L.J.; Arab, L.; Baddou, I.; Bandini, L.; et al. Predictive equation derived from 6497 doubly labelled water measurements enables the detection of erroneous self-reported energy intake. Nat. Food 2025, 6, 58–71. [Google Scholar] [CrossRef]
- Manton, S.; Magerowski, G.; Patriarca, L.; Alonso-Alonso, M. The “Smart Dining Table”: Automatic Behavioral Tracking of a Meal with a Multi-Touch-Computer. Front. Psychol. 2016, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Konstantakopoulos, F.S.; Georga, E.I.; Fotiadis, D.I. A Review of Image-Based Food Recognition and Volume Estimation Artificial Intelligence Systems. IEEE Rev. Biomed. Eng. 2024, 17, 136–152. [Google Scholar] [CrossRef] [PubMed]
- Lo, F.P.-W.; Qiu, J.; Wang, Z.; Chen, J.; Xiao, B.; Yuan, W.; Giannarou, S.; Frost, G.; Lo, B. Dietary Assessment With Multimodal ChatGPT: A Systematic Analysis. IEEE J. Biomed. Health Inform. 2024, 28, 7577–7587. [Google Scholar] [CrossRef] [PubMed]
- Allirot, X.; Saulais, L.; Disse, E.; Roth, H.; Cazal, C.; Laville, M. Validation of a buffet meal design in an experimental restaurant. Appetite 2012, 58, 889–897. [Google Scholar] [CrossRef]
- Kissileff, H.R.; Klingsberg, G.; Van Itallie, T.B. Universal eating monitor for continuous recording of solid or liquid consumption in man. Am. J. Physiol. Integr. Comp. Physiol. 1980, 238, R14–R22. [Google Scholar] [CrossRef]
- Thomas, J.M.; Dourish, C.T.; Higgs, S. Effects of awareness that food intake is being measured by a universal eating monitor on the consumption of a pasta lunch and a cookie snack in healthy female volunteers. Appetite 2015, 92, 247–251. [Google Scholar] [CrossRef]
- Thomas, J.M.; Dourish, C.T.; Tomlinson, J.; Hassan-Smith, Z.; Hansen, P.C.; Higgs, S. The 5-HT2C receptor agonist meta-chlorophenylpiperazine (mCPP) reduces palatable food consumption and BOLD fMRI responses to food images in healthy female volunteers. Psychopharmacology 2018, 235, 257–267. [Google Scholar] [CrossRef]
- Dovey, T.M.; Clark-Carter, D.; Boyland, E.J.; Halford, J.C. A guide to analysing Universal Eating Monitor data: Assessing the impact of different analysis techniques. Physiol. Behav. 2009, 96, 78–84. [Google Scholar] [CrossRef]
- Kissileff, H.R. The Universal Eating Monitor (UEM): Objective assessment of food intake behavior in the laboratory setting. Int. J. Obes. 2022, 46, 1114–1121. [Google Scholar] [CrossRef]
- Thomas, D.M.; Paynter, J.; Peterson, C.M.; Heymsfield, S.B.; Nduati, A.; Apolzan, J.W.; Martin, C.K. A new universal dynamic model to describe eating rate and cumulative intake curves. Am. J. Clin. Nutr. 2017, 105, 323–331. [Google Scholar] [CrossRef][Green Version]
- Martin, C.K.; Williamson, D.A.; Geiselman, P.J.; Walden, H.; Smeets, M.; Morales, S.; Redmann, S., Jr. Consistency of food intake over four eating sessions in the laboratory. Eat. Behav. 2005, 6, 365–372. [Google Scholar] [CrossRef]
- Hubel, R.; Laessle, R.; Lehrke, S.; Jass, J. Laboratory measurement of cumulative food intake in humans: Results on reliability. Appetite 2006, 46, 57–62. [Google Scholar] [CrossRef]
- Laessle, R.; Geiermann, L. Reliability of laboratory measurement of human food intake. Appetite 2012, 58, 249–251. [Google Scholar] [CrossRef]
- Laessle, R.G.; Lehrke, S.; Dückers, S. Laboratory eating behavior in obesity. Appetite 2007, 49, 399–404. [Google Scholar] [CrossRef]
- Schulz, S.; Laessle, R. Stress-induced laboratory eating behavior in obese women with binge eating disorder. Appetite 2012, 58, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Laessle, R.G.; Uhl, H.; Lindel, B. Parental influences on eating behavior in obese and nonobese preadolescents. Int. J. Eat. Disord. 2001, 30, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Laessle, R.G.; Lehrke, S. Differences in laboratory eating behaviour between overweight boys and girls before treatment. Eat. Weight. Disord. 2012, 17, e137–e139. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.A. Dietary Guidelines for Americans, 2020–2025. Workplace Health Saf. 2021, 69, 395. [Google Scholar] [CrossRef]
- Chinese Nutrition Society. Dietary Guidelines for Chinese Residents; People’s Medical Publishing House: Beijing, China, 2022. [Google Scholar]
- Public Health England WG; Food Standards Scotland; Food Standards Agency in Northern Ireland. Protecting and Improving the Nation’s Health: Eatwell Guide. 2016. Available online: https://www.foodstandards.gov.scot/consumers/healthy-eating/eatwell (accessed on 5 August 2025).
- Yuan, M.; Li, Q.; Yang, C.; Zhi, L.; Zhuang, W.; Xu, X.S.; Tao, F. Waist-to-Height Ratio Is a Stronger Mediator in the Association between DASH Diet and Hypertension: Potential Micro/Macro Nutrients Intake Pathways. Nutrients 2023, 15, 2189. [Google Scholar] [CrossRef]
- Li, C.-J.; Norstedt, G.; Hu, Z.-G.; Yu, P.; Li, D.-Q.; Li, J.; Yu, Q.; Sederholm, M.; Yu, D.-M. Effects of a Macro-Nutrient Preload on Type 2 Diabetic Patients. Front. Endocrinol. 2015, 6, 139. [Google Scholar] [CrossRef]
- Shi, Z.; Li, X.; Shuai, Y.; Lu, Y.; Liu, Q. The development of wearable technologies and their potential for measuring nutrient intake: Towards precision nutrition. Nutr. Bull. 2022, 47, 388–406. [Google Scholar] [CrossRef]
- Bell, B.M.; Alam, R.; Alshurafa, N.; Thomaz, E.; Mondol, A.S.; de la Haye, K.; Stankovic, J.A.; Lach, J.; Spruijt-Metz, D. Automatic, wearable-based, in-field eating detection approaches for public health research: A scoping review. NPJ Digit. Med. 2020, 3, 38. [Google Scholar] [CrossRef]
- Hong, W.; Lee, W.G. Wearable sensors for continuous oral cavity and dietary monitoring toward personalized healthcare and digital medicine. Analyst 2021, 145, 7796–7808. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Sazonov, E. A Novel Wearable Device for Food Intake and Physical Activity Recognition. Sensors 2016, 16, 1067. [Google Scholar] [CrossRef]
- Farooq, M.; Sazonov, E. Accelerometer-Based Detection of Food Intake in Free-Living Individuals. IEEE Sens. J. 2018, 18, 3752–3758. [Google Scholar] [CrossRef]
- Mannoor, M.S.; Tao, H.; Clayton, J.D.; Sengupta, A.; Kaplan, D.L.; Naik, R.R.; Verma, N.; Omenetto, F.G.; McAlpine, M.C. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun. 2012, 3, 763. [Google Scholar] [CrossRef]
- Tseng, P.; Napier, B.; Garbarini, L.; Kaplan, D.L.; Omenetto, F.G. Functional, RF-Trilayer Sensors for Tooth-Mounted, Wireless Monitoring of the Oral Cavity and Food Consumption. Adv. Mater. 2018, 30, e1703257. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, A.; Lee, S.; Cooray, N.F.; Lee, S.; Mori, M.; Matsuhisa, N.; Jin, H.; Yoda, L.; Yokota, T.; Itoh, A.; et al. Inflammation-free, gas-permeable, lightweight, stretchable on-skin electronics with nanomeshes. Nat. Nanotechnol. 2017, 12, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Nørnberg, T.; Houlby, L.; Jørgensen, L.; He, C.; Pérez-Cueto, F. Do we know how much we put on the plate? Assessment of the accuracy of self-estimated versus weighed vegetables and whole grain portions using an Intelligent Buffet at the FoodScape Lab. Appetite 2014, 81, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Annabel, L. Merrill BKW: Energy Value of Foods—Basis and Derivation; United States Department of Agriculture: Washington, DC, USA, 1955; p. 74.
- Chowdhury, E.A.; Richardson, J.D.; Tsintzas, K.; Thompson, D.; Betts, J.A. Carbohydrate-rich breakfast attenuates glycaemic, insulinaemic and ghrelin response to ad libitum lunch relative to morning fasting in lean adults. Br. J. Nutr. 2015, 114, 98–107. [Google Scholar] [CrossRef]
- Pearce, A.L.; Brick, T.R. Validation of computational models to characterize cumulative intake curves from video-coded meals. Front. Nutr. 2023, 10, 1088053. [Google Scholar] [CrossRef]
- Uddén, J.; Björntorp, P.; Arner, P.; Barkeling, B.; Meurling, L.; Rössner, S. Effects of glucocorticoids on leptin levels and eating behaviour in women. J. Intern. Med. 2003, 253, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Rössner, S.; Barkeling, B.; Erlanson-Albertsson, C.; Larsson, P.; Whlin-Boll, E. Intravenous enterostatin does not affect single meal food intake in man. Appetite 1995, 24, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Wansink, B.; Painter, J.E.; North, J. Bottomless bowls: Why visual cues of portion size may influence intake. Obes. Res. 2005, 13, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Fay, S.H.; Ferriday, D.; Hinton, E.C.; Shakeshaft, N.G.; Rogers, P.J.; Brunstrom, J.M. What determines real-world meal size? Evidence for pre-meal planning. Appetite 2011, 56, 284–289. [Google Scholar] [CrossRef]
- Lorig, F.; Kießl, G.R.R.; Laessle, R.G. Stress-related cortisol response and laboratory eating behavior in obese women. Eat. Weight. Disord.–Stud. Anorex. Bulim. Obes. 2016, 21, 237–243. [Google Scholar] [CrossRef]
- Melanson, K.J.; Matsumoto, C.N.; Greene, G.W. Eating pace instruction is effective in slowing eating rate in women with overweight and obesity. Eat. Behav. 2023, 48, 101701. [Google Scholar] [CrossRef]
- Sayer, R.D.; Amankwaah, A.F.; Tamer, G.G., Jr.; Chen, N.; Wright, A.J.; Tregellas, J.R.; Cornier, M.A.; Kareken, D.A.; Talavage, T.M.; McCrory, M.A.; et al. Effects of Dietary Protein and Fiber at Breakfast on Appetite, ad Libitum Energy Intake at Lunch, and Neural Responses to Visual Food Stimuli in Overweight Adults. Nutrients 2016, 8, 21. [Google Scholar] [CrossRef]
- Liu, Y.; Mei, H.; Xue, L.; Cheng, C.; Wu, Y.; Zou, C.; Yu, Y.; Gao, L.; Zhang, H.; Gao, X.; et al. Testing the carbohydrate-insulin model: Short-term metabolic responses to consumption of meals with varying glycemic index in healthy adults. Cell Metab. 2025, 37, 606–615.e603. [Google Scholar] [CrossRef]
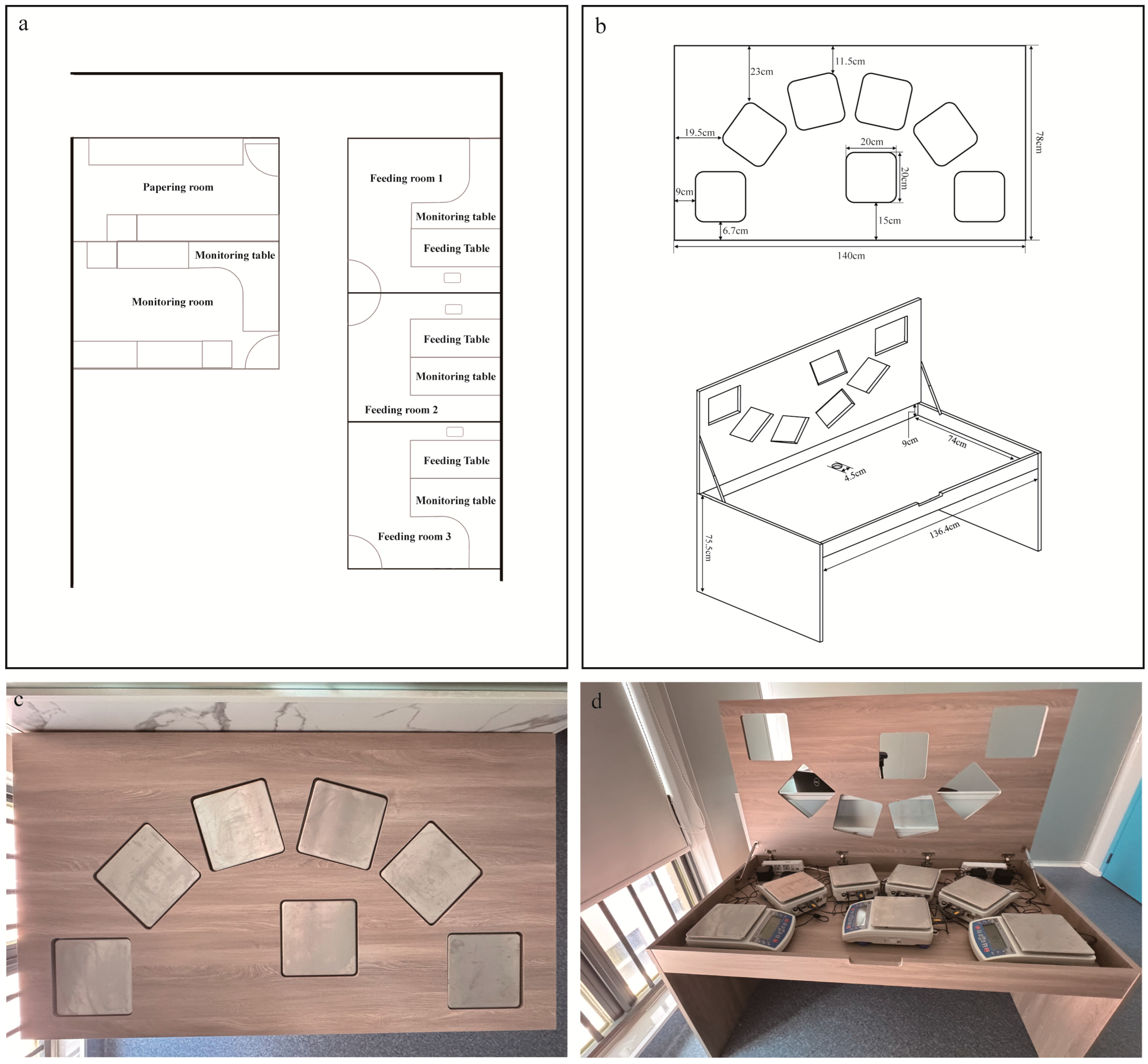

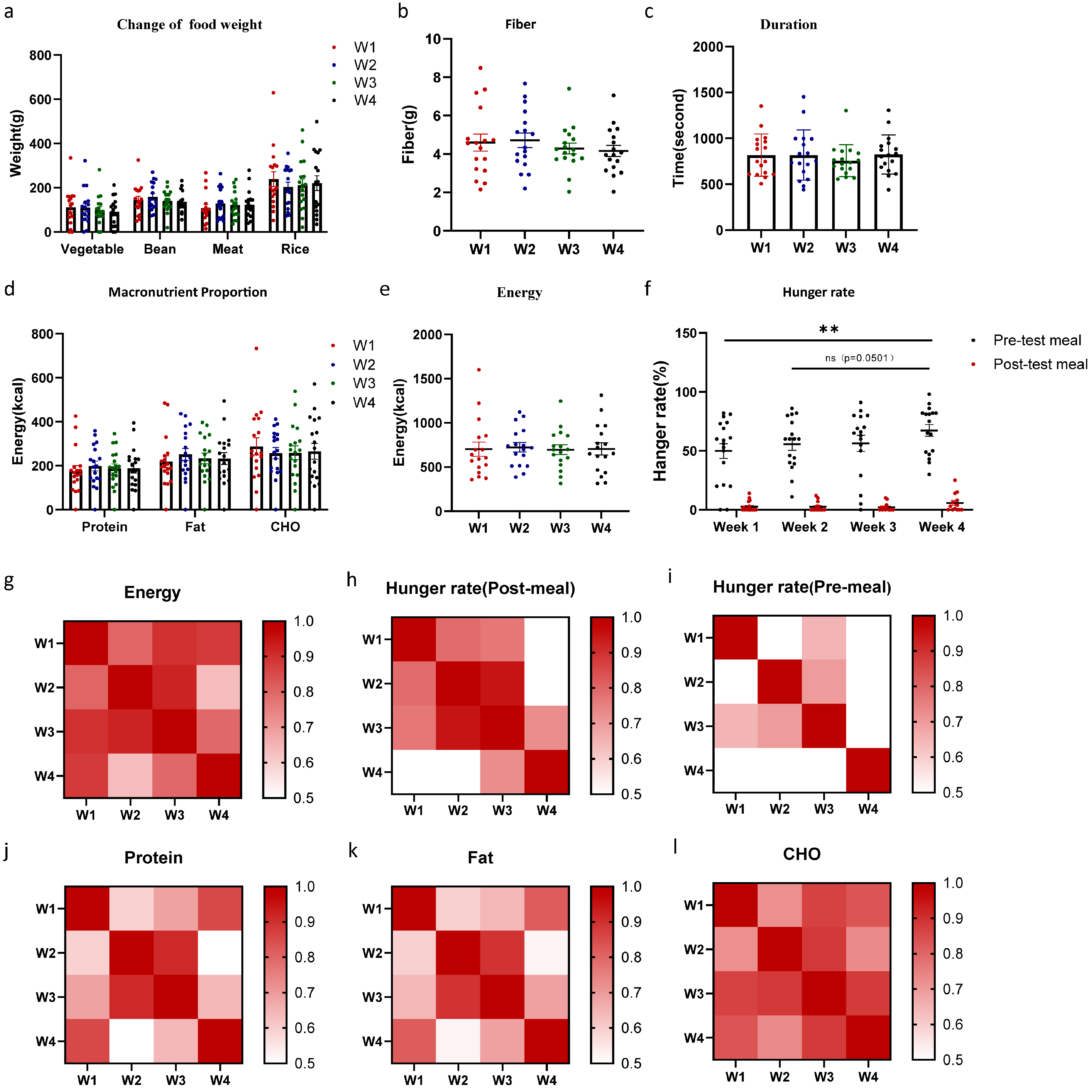

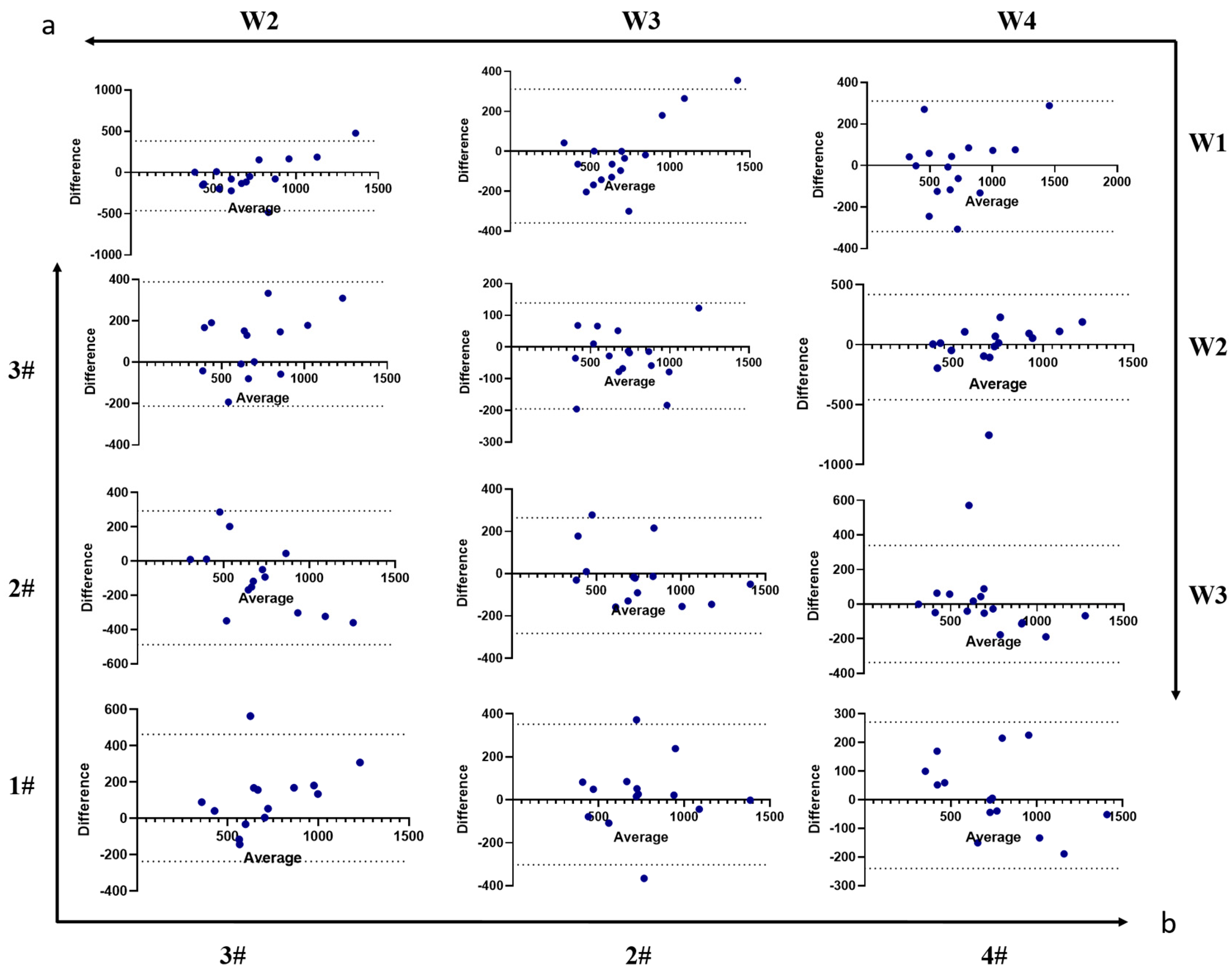

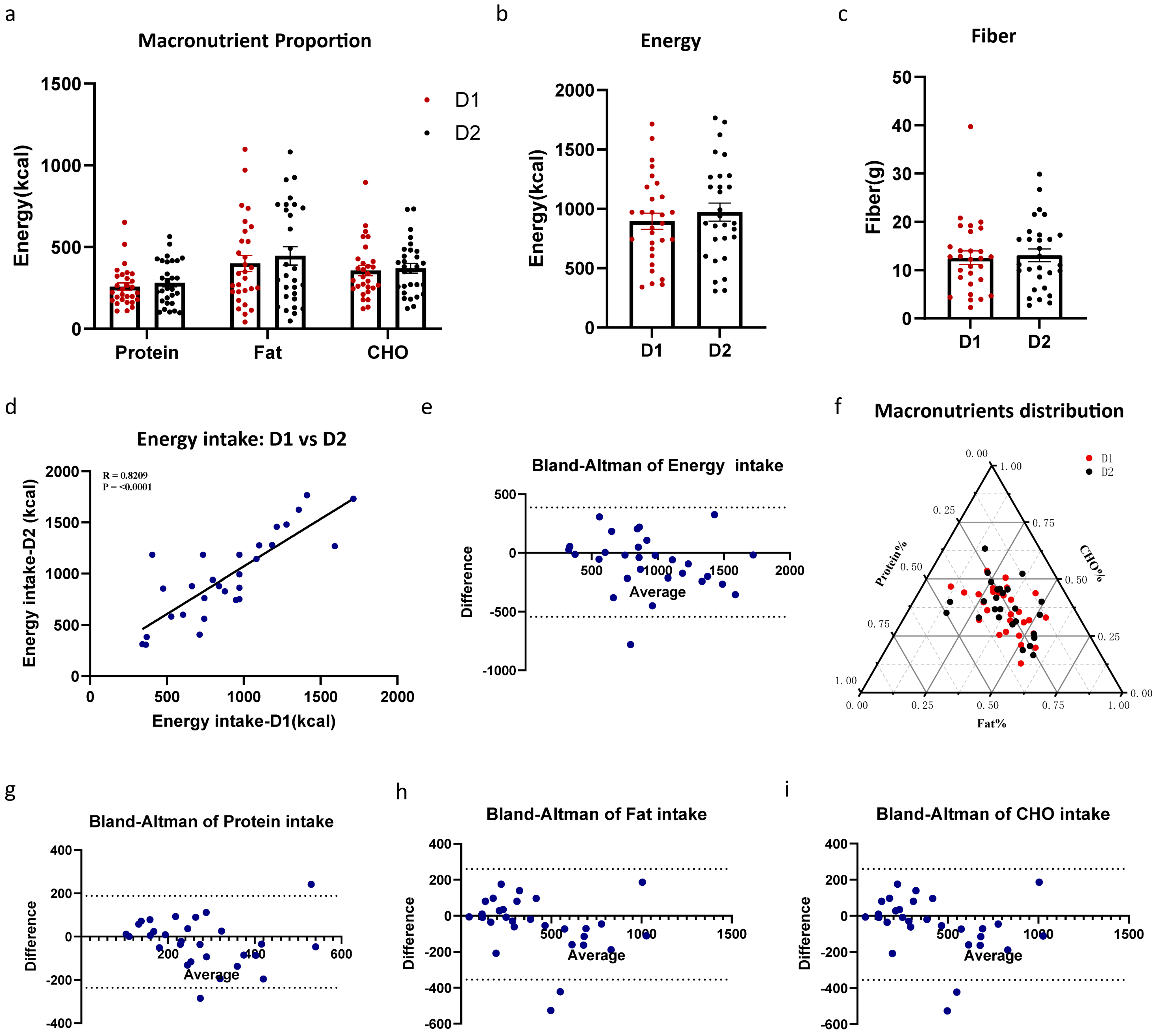
| Number | Age | Sex (M:F) | Height (cm) | Weight (kg) | BMI (kg/m2) | Handedness (R:L) | |
|---|---|---|---|---|---|---|---|
| Position preference | 18 | 29 ± 5.66 [21, 36] | 10:8 | 168.45 ± 11.29 [148, 188.6] | 66.37 ± 10.16 [49, 82.7] | 24.67 ± 3.78 [18.22, 30.75] | 14:4 |
| Standard testing meal | 31 | 26.26 ± 4.26 [22, 40] | 15:16 | 168.60 ± 8.85 [154, 185] | 59.41 ± 8.07 [45.4, 75] | 20.81 ± 1.61 [18.07, 23.55] | 31:0 |
| Food (n = 4) | Protein (g/100 g) | Fat (g/100 g) | CHO (g/100 g) | Fiber (g/100 g) | Energy (kJ) |
|---|---|---|---|---|---|
| Pork | 28.4 | 15.4 | 0 | 0 | 992 |
| Long bean | 5.86 | 4.95 | 7.50 | 1.66 | 393 |
| Green Vegetable | 1.44 | 1.74 | 3.2 | 1.34 | 130 |
| Rice | 2.6 | 0.3 | 25.9 | 0.3 | 485 |
| Food (n = 12) | Protein (g/100 g) | Fat (g/100 g) | CHO (g/100 g) | Fiber (g/100 g) | Water (g/100 g) | Energy (kJ) |
|---|---|---|---|---|---|---|
| Pork (fat) | 2.4 | 88.6 | 0 | 0 | 8.8 | 3319 |
| Pork (lean and fat) | 36.8 | 54.5 | 3.1 | 0.8 | 39.9 | 1959.5 |
| Chicken breast | 24.6 | 1.9 | 0.6 | 0 | 71.7 | 499 |
| Tofu | 6.6 | 5.3 | 3.4 | — | 83.8 | 3512 |
| Rice | 2.6 | 0.3 | 25.9 | 0.3 | 70.9 | 486 |
| Broccoli | 3.5 | 0.6 | 3.7 | 1.6 | 91.6 | 111 |
| Mushroom | 38.7 | 3.3 | 31.6 | 17.2 | 9.2 | 1162 |
| Apple | 0.4 | 0.2 | 13.7 | 1.7 | 86.1 | 227 |
| Banana | 1.4 | 0.2 | 22 | 1.2 | 75.8 | 389 |
| Cheese cake | 5.3 | 24.2 | 23.4 | — | — | 1384 |
| Butter | 1.4 | 98 | 0 | 0 | 0.5 | 3715 |
| Water | 0 | 0 | 0 | 0 | 100 | 0 |
| Coke | 0 | 0 | 10.6 | 0 | — | 180 |
| Food | Protein | Fat | CHO | Energy |
|---|---|---|---|---|
| ICCs | 0.90 a | 0.90 a | 0.93 a | 0.94 a |
| R value across 4 sessions | ||||
| W1 vs. W2 | 0.59 | 0.60 | 0.72 | 0.88 |
| W1 vs. W3 | 0.69 | 0.65 | 0.87 | 0.67 |
| W1 vs. W4 | 0.69 | 0.82 | 0.83 | 0.84 |
| W2 vs. W3 | 0.91 | 0.89 | 0.88 | 0.83 |
| W2 vs. W4 | 0.49 | 0.52 | 0.73 | 0.74 |
| W3 vs. W4 | 0.64 | 0.69 | 0.88 | 0.73 |
| Food | Protein | Fat | CHO | Energy |
|---|---|---|---|---|
| ICCs | 0.90 a | 0.89 a | 0.93 a | 0.95 a |
| R value across 4 sessions | ||||
| 1# vs. 2# | 0.81 | 0.81 | 0.84 | 0.92 |
| 1# vs. 3# | 0.66 | 0.63 | 0.91 | 0.82 |
| 1# vs. 4# | 0.67 | 0.67 | 0.77 | 0.77 |
| 2# vs. 3# | 0.90 | 0.83 | 0.88 | 0.91 |
| 2# vs. 4# | 0.62 | 0.60 | 0.62 | 0.82 |
| 3# vs. 4# | 0.63 | 0.58 | 0.68 | 0.84 |
| Quadratic Model | LODE Model | |||
|---|---|---|---|---|
| R2 | SD | R2 | SD | |
| Energy | 0.97 | 0.03 | 0.99 a | 0.01 |
| Protein | 0.97 | 0.02 | 0.99 a | 0.01 |
| Fat | 0.97 | 0.03 | 0.98 a | 0.01 |
| CHO | 0.97 | 0.03 | 0.98 a | 0.02 |
| Fiber | 0.97 | 0.03 | 0.98 a | 0.01 |
| Correlation | Bland–Altman | |||||
|---|---|---|---|---|---|---|
| r | p | 95%CI | Bias | SD of Bias | 95%CI | |
| Protein | 0.58 | 0.001 | 0.27 to 0.78 | −7.26 | 126.7 | −255.6 to 241.1 |
| Fat | 0.86 | <0.0001 | 0.71 to 0.93 | −47.54 | 156.6 | −354.4 to 259.3 |
| CHO | 0.86 | <0.0001 | 0.71 to 0.93 | −14.39 | 90.29 | −191.4 to 162.6 |
| Energy | 0.83 | <0.0001 | 0.65 to 0.91 | −77.82 | 237.3 | −542.9 to 387.3 |
| Fiber | 0.65 | 0.0001 | 0.37 to 0.82 | −0.52 | 6.123 | −12.52 to 11.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, L.; Liu, Y.; Mei, H.; Yu, Y.; Zhang, H.; Gao, L.; Jin, Z.; Wang, L.; Niu, C.; Speakman, J.R. Development and Validation of a Universal Eating Monitor (UEM) for Distinguishing the Intake of Multiple Foods and Macronutrients. Nutrients 2025, 17, 2929. https://doi.org/10.3390/nu17182929
Xue L, Liu Y, Mei H, Yu Y, Zhang H, Gao L, Jin Z, Wang L, Niu C, Speakman JR. Development and Validation of a Universal Eating Monitor (UEM) for Distinguishing the Intake of Multiple Foods and Macronutrients. Nutrients. 2025; 17(18):2929. https://doi.org/10.3390/nu17182929
Chicago/Turabian StyleXue, Li, Ying Liu, Huihui Mei, Ying Yu, Huanan Zhang, Lin Gao, Zengguang Jin, Lu Wang, Chaoqun Niu, and John R. Speakman. 2025. "Development and Validation of a Universal Eating Monitor (UEM) for Distinguishing the Intake of Multiple Foods and Macronutrients" Nutrients 17, no. 18: 2929. https://doi.org/10.3390/nu17182929
APA StyleXue, L., Liu, Y., Mei, H., Yu, Y., Zhang, H., Gao, L., Jin, Z., Wang, L., Niu, C., & Speakman, J. R. (2025). Development and Validation of a Universal Eating Monitor (UEM) for Distinguishing the Intake of Multiple Foods and Macronutrients. Nutrients, 17(18), 2929. https://doi.org/10.3390/nu17182929






