Perspective: Vitamin D Deficiency Relationship to Initiation of Diseases
Abstract
1. Introduction
2. Endocrine Role of 1,25(OH)2D
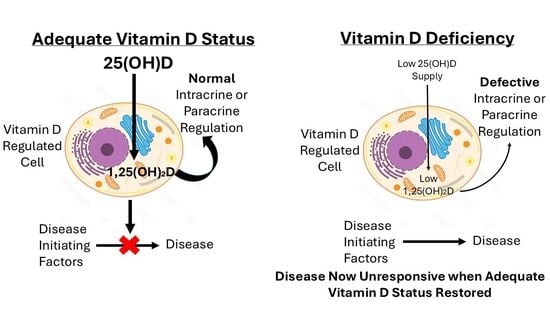
3. Vitamin D Deficiency and Disease
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Haddad, J.G.; Chyu, K.J. Competitive protein-binding radioassay for 25-hydroxycholecalciferol. J. Clin. Endocrinol. Metab. 1971, 33, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Arrebola, M.M.; Filella, X.; Albaladejo-Oton, M.D.; Giménez, N.; Serrano-Olmedo, M.G.; García-Martínez, R.J.; Bonet-Estruch, E.; Santamaría-González, M.; Pérez-Torrella, D.; Morell-García, D.; et al. Vitamin D Controversies in the Laboratory Medicine: A Review of Clinical Guidelines and Recommendations. EJIFCC 2024, 35, 223–243. [Google Scholar]
- Giustina, A.; Bilezikian, J.P.; Adler, R.A.; Banfi, G.; Bikle, D.D.; Binkley, N.C.; Bollerslev, J.; Bouillon, R.; Brandi, M.L.; Casanueva, F.F.; et al. Consensus Statement on Vitamin D Status Assessment and Supplementation: Whys, Whens, and Hows. Endocr. Rev. 2024, 45, 625–654. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.L.; Santana, K.V.; Ribeiro, H. The Effect of Sunlight Exposure on Vitamin D Status in Countries of Low and High Latitudes: A Systematic Literature Review. Curr. Nutr. Rep. 2023, 12, 1–13. [Google Scholar] [CrossRef]
- Mellanby, E. The part played by an ‘accessory factor’ in the production of experimental rickets. J. Physiol. 1918, 52, xi–xii. [Google Scholar]
- Mellanby, E. A further demonstration of the part played by accessory food factors in the aetiology of rickets. J. Physiol. 1918, 52, liii–liv. [Google Scholar]
- McCollum, E.V.; Simmonds, N.; Becker, J.E.; Shipley, P.G. Studies on experimental rickets. XXI. An experimental demonstration of the existence of a vitamin which promotes calcium deposition. J. Biol. Chem. 1922, 53, 293–312. [Google Scholar] [CrossRef]
- Huldschinsky, K. Heilung von Rachitis durch kϋnstliche Hohensonne. Deutsche Med. Wochenschr. 1919, 45, 712–713. [Google Scholar] [CrossRef]
- Chick, H.; Dalyell, E.J.; Hume, E.M.; Mackay, H.M.M.; Smith, H.H.; Wimberger, H. Studies of rickets in Vienna, 1919–1922. Med. Hist. 1976, 20, 41–51. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Guo, J.; Lovegrove, J.A.; Givens, D.I. A Narrative Review of The Role of Foods as Dietary Sources of Vitamin D of Ethnic Minority Populations with Darker Skin: The Underestimated Challenge. Nutrients 2019, 11, 81. [Google Scholar] [CrossRef]
- Kimlin, M.; Sun, J.; Sinclair, C.; Heward, S.; Hill, J.; Dunstone, K.; Brodie, A. Are the current Australian sun exposure guidelines effective in maintaining adequate levels of 25-hydroxyvitamin D? J. Steroid Biochem. Mol. Biol. 2016, 155, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: A pooled analysis of 7.9 million participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef]
- Harvey, N.C.; Ward, K.A.; Agnusdei, D.; Binkley, N.; Biver, E.; Campusano, C.; Cavalier, E.; Clark, P.; Diaz-Curiel, M.; Fuleihan, G.H.; et al. Optimisation of vitamin D status in global populations. Osteoporos. Int. 2024, 35, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Sparks, A.M.; Johnston, S.E.; Handel, I.; Pilkington, J.G.; Berry, J.; Pemberton, J.M.; Nussey, D.H.; Mellanby, R.J. Vitamin D status is heritable and under environment-dependent selection in the wild. Mol. Ecol. 2022, 31, 4607–4621. [Google Scholar] [CrossRef]
- Smith, B.B.; Van Saun, R.J. Seasonal changes in serum calcium, phosphorus, and vitamin D concentrations in llamas and alpacas. Am. J. Vet. Res. 2001, 62, 1187–1193. [Google Scholar] [CrossRef]
- Risco, D.; Gonçalves, P.; Bravo, M.; García-Jiménez, W.; Cerrato, R.; Hermoso de Mendoza, J.; Fernández-Llario, P. Seasonal and dietary effects on Vitamin D deficiencies detected in wild boar from mid-western Spain. J. Anim. Physiol. Anim. Nutr. 2019, 103, 668–674. [Google Scholar] [CrossRef]
- Waters, W.R.; Nonnecke, B.J.; Gibbs, S.E.; Yabsley, M.J.; Schmitt, S.M.; Cosgrove, M.K.; Palmer, M.V.; Thacker, T.C.; Olsen, S.C.; Horst, R.L.; et al. Serum 25-hydroxyvitamin D concentrations in captive and free-ranging, white-tailed deer (Odocoileus virginianus). Int. J. Vitam. Nutr. Res. 2009, 79, 180–187. [Google Scholar] [CrossRef]
- Mäkitaipale, J.; Hietanen, P.; Grönthal, T. Low 25-hydroxyvitamin D concentrations in wild rabbits (Oryctolagus cuniculus) in southern Finland. Acta Vet. Scand. 2024, 66, 4. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhou, Y.; Liu, Y.; Zhang, H.; Yuan, Z.; Han, Y.; Weng, Q. Seasonal changes of vitamin D3 and ovarian steroidogenesis in the wild ground squirrels (Citellus dauricus Brandt). J. Steroid Biochem. Mol. Biol. 2023, 234, 106385. [Google Scholar] [CrossRef]
- Kiourtzidis, M.; Kühn, J.; Brandsch, C.; Baur, A.C.; Wensch-Dorendorf, M.; Stangl, G.I. Markers Indicating Body Vitamin D Stores and Responses of Liver and Adipose Tissues to Changes in Vitamin D Intake in Male Mice. Nutrients 2020, 12, 1391. [Google Scholar] [CrossRef]
- Lin, E.; Armstrong-Moore, D.; Liang, Z.; Sweeney, J.F.; Torres, W.E.; Ziegler, T.R.; Tangpricha, V.; Gletsu-Miller, N. Contribution of adipose tissue to plasma 25-hydroxyvitamin D concentrations during weight loss following gastric bypass surgery. Obesity 2011, 9, 588–594. [Google Scholar] [CrossRef]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Front. Endocrinol. 2020, 10, 910. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.S.; Rybchyn, M.S.; Abboud, M.; Brennan-Speranza, T.C.; Fraser, D.R. The Role of Skeletal Muscle in Maintaining Vitamin D Status in Winter. Curr. Dev. Nutr. 2019, 3, nzz087. [Google Scholar] [CrossRef]
- Rybchyn, M.S.; Abboud, M.; Puglisi, D.A.; Gordon-Thomson, C.; Brennan-Speranza, T.C.; Mason, R.S.; Fraser, D.R. Skeletal Muscle and the Maintenance of Vitamin D Status. Nutrients 2020, 12, 3270. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.R.; Mason, R.S. Commentary: Cellular functions of vitamin D-binding protein. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2025, 305, 111848. [Google Scholar] [CrossRef]
- Abboud, M.; Rybchyn, M.S.; Liu, J.; Ning, Y.; Gordon-Thomson, C.; Brennan-Speranza, T.C.; Cole, L.; Greenfield, H.; Fraser, D.R.; Mason, R.S. The effect of parathyroid hormone on the uptake and retention of 25-hydroxyvitamin D in skeletal muscle cells. J. Steroid Biochem. Mol. Biol. 2017, 173, 173–179. [Google Scholar] [CrossRef]
- Brock, K.; Cant, R.; Clemson, L.; Mason, R.S.; Fraser, D.R. Effects of diet and exercise on plasma vitamin D (25(OH)D) levels in Vietnamese immigrant elderly in Sydney, Australia. J. Steroid Biochem. Mol. Biol. 2007, 103, 786–792. [Google Scholar] [CrossRef]
- Foo, L.H.; Zhang, Q.; Zhu, K.; Ma, G.; Trube, A.; Greenfield, H.; Fraser, D.R. Relationship between vitamin D status, body composition and physical exercise of adolescent girls in Beijing. Osteoporos. Int. 2009, 20, 417–425. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Z.B. Exercise: A Possibly Effective Way to Improve Vitamin D Nutritional Status. Nutrients 2022, 14, 2652. [Google Scholar] [CrossRef]
- Datta, P.; Philipsen, P.A.; Olsen, P.; Bogh, M.K.; Johansen, P.; Schmedes, A.V.; Morling, N.; Wulfa, H.C. The half-life of 25(OH)D after UVB exposure depends on gender and vitamin D receptor polymorphism but mainly on the start level. Photochem. Photobiol. Sci. 2017, 16, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Rillaerts, K.; Verlinden, L.; Doms, S.; Carmeliet, G.; Verstuyf, A. A comprehensive perspective on the role of vitamin D signaling in maintaining bone homeostasis: Lessons from animal models. J. Steroid Biochem. Mol. Biol. 2025, 250, 106732. [Google Scholar] [CrossRef] [PubMed]
- Lowe, K.E.; Maiyar, A.C.; Norman, A.W. Vitamin D-mediated gene expression. Crit. Rev. Eukaryot. Gene Expr. 1992, 2, 65–109. [Google Scholar]
- Dusso, A.; Brown, A.; Slatopolsky, E. Extrarenal production of calcitriol. Semin. Nephrol. 1994, 14, 144–155. [Google Scholar]
- Ryan, Z.C.; Craig, T.A.; Folmes, C.D.; Wang, X.; Lanza, I.R.; Schaible, N.S.; Salisbury, J.L.; Nair, K.S.; Terzic, A.; Sieck, G.C.; et al. 1α,25-Dihydroxyvitamin D3 Regulates Mitochondrial Oxygen Consumption and Dynamics in Human Skeletal Muscle Cells. J. Biol. Chem. 2016, 291, 514–528. [Google Scholar] [CrossRef]
- Olszewska, A.M.; Zmijewski, M.A. Genomic and non-genomic action of vitamin D on ion channels—Targeting mitochondria. Mitochondrion 2024, 77, 101891. [Google Scholar] [CrossRef]
- Carlberg, C. Genomic signaling of vitamin D. Steroids 2023, 198, 109271. [Google Scholar] [CrossRef] [PubMed]
- Henry, H.L. Vitamin D hydroxylases. J. Cell Biochem. 1992, 49, 4–9. [Google Scholar] [CrossRef]
- Armbrecht, H.J.; Okuda, K.; Wongsurawat, N.; Nemani, R.K.; Chen, M.L.; Boltz, M.A. Characterization and regulation of the vitamin D hydroxylases. J. Steroid Biochem. Mol. Biol. 1992, 43, 1073–1081. [Google Scholar] [CrossRef]
- Adams, J.S.; Ren, S.Y.; Arbelle, J.E.; Horiuchi, N.; Gray, R.W.; Clemens, T.L.; Shany, S. Regulated production and intracrine action of 1,25-dihydroxyvitamin D3 in the chick myelomonocytic cell line HD-11. Endocrinology 1994, 134, 2567–2573. [Google Scholar] [CrossRef]
- Chun, R.F.; Shieh, A.; Gottlieb, C.; Yacoubian, V.; Wang, J.; Hewison, M.; Adams, J.S. Vitamin D Binding Protein and the Biological Activity of Vitamin D. Front. Endocrinol. 2019, 10, 718. [Google Scholar] [CrossRef]
- Mendel, C.M. The free hormone hypothesis: A physiologically based mathematical model. Endocr. Rev. 1989, 10, 232–274. [Google Scholar] [CrossRef]
- Leheste, J.R.; Melsen, F.; Wellner, M.; Jansen, P.; Schlichting, U.; Renner-Müller, I.; Andreassen, T.T.; Wolf, E.; Bachmann, S.; Nykjaer, A.; et al. Hypocalcemia and osteopathy in mice with kidney-specific megalin gene defect. FASEB J. 2003, 17, 247–249. [Google Scholar] [CrossRef]
- Gupta, D.; Vashi, P.G.; Trukova, K.; Lis, C.G.; Lammersfeld, C.A. Prevalence of serum vitamin D deficiency and insufficiency in cancer: Review of the epidemiological literature. Exp. Ther. Med. 2011, 2, 181–193. [Google Scholar] [CrossRef]
- Fekete, M.; Lehoczki, A.; Szappanos, Á.; Zábó, V.; Kaposvári, C.; Horváth, A.; Farkas, Á.; Fazekas-Pongor, V.; Major, D.; Lipécz, Á.; et al. Vitamin D and Colorectal Cancer Prevention: Immunological Mechanisms, Inflammatory Pathways, and Nutritional Implications. Nutrients 2025, 17, 1351. [Google Scholar] [CrossRef]
- Islam, H.; Hassaan, S.M.; Islam, R.; Islam, T.; Zaidi, F.; Rehman, H.U.; Haque, M.M.U.; Turabee, Z.; Asim, M.; Ahmad, I.; et al. Vitamin D’s Role in Cardiovascular Diseases. Discov. Med. 2024, 36, 1973–1986. [Google Scholar] [CrossRef]
- Savran, Z.; Baltaci, S.B.; Aladag, T.; Mogulkoc, R.; Baltaci, A.K. Vitamin D and Neurodegenerative Diseases Such as Multiple Sclerosis (MS), Parkinson’s Disease (PD), Alzheimer’s Disease (AD), and Amyotrophic Lateral Sclerosis (ALS): A Review of Current Literature. Curr. Nutr. Rep. 2025, 14, 77. [Google Scholar] [CrossRef]
- Durá-Travé, T.; Gallinas-Victoriano, F. Type 1 Diabetes Mellitus and Vitamin D. Int. J. Mol. Sci. 2025, 26, 4593. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. COVID-19 and our understanding of vitamin D and immune function. J. Steroid Biochem. Mol. Biol. 2025, 249, 106710. [Google Scholar] [CrossRef] [PubMed]
- Boucher, B.J. Why do so many trials of vitamin D supplementation fail? Endocr. Connect. 2020, 9, R195–R206. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Boucher, B.J.; Al Anouti, F.; Pilz, S. Comparing the Evidence from Observational Studies and Randomized Controlled Trials for Nonskeletal Health Effects of Vitamin D. Nutrients 2022, 14, 3811. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J. Regarding: Low vitamin D is a marker for poor health and increased risk for disease: But causality is still unclear in most cases. J. Intern. Med. 2023, 293, 791–792. [Google Scholar] [CrossRef]
- Singh, S.; Meena, R.K.; Maharshi, V.; Sinha, N.; Agarwal, N.; Payra, S.; Harsha, D. Vitamin D supplementation trials: Navigating the maze of unpredictable results. Perspect. Clin. Res. 2025, 16, 69–74. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. Brit. Med. J. 2017, 356, i6583. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Vitamin D in the Context of Evolution. Nutrients 2022, 14, 3018. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, G.; Dang, H.T.; Schluter, S.F.; Bernstein, R.M.; Bunag, T.; Manzon, L.A.; Hsieh, G.; Dominguez, C.E.; Youson, J.H.; Haussler, M.R.; et al. Cloning of a functional vitamin D receptor from the lamprey (Petromyzon marinus), an ancient vertebrate lacking a calcified skeleton and teeth. Endocrinology 2003, 144, 2704–2716. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Hewison, M.; Studzinski, G.P.; Li, Y.C.; Kalia, V. Role of vitamin D in cytotoxic T lymphocyte immunity to pathogens and cancer. Crit. Rev. Clin. Lab. Sci. 2016, 53, 132–145. [Google Scholar] [CrossRef]
- Chirumbolo, S.; Bjørklund, G.; Sboarina, A.; Vella, A. The Role of Vitamin D in the Immune System as a Pro-survival Molecule. Clin. Ther. 2017, 39, 894–916. [Google Scholar] [CrossRef]
- Sîrbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 9784. [Google Scholar] [CrossRef]
- Fenercioglu, A.K. The Anti-Inflammatory Roles of Vitamin D for Improving Human Health. Curr. Issues Mol. Biol. 2024, 46, 13514–13525. [Google Scholar] [CrossRef]
- Muñoz, A.; Grant, W.B. Vitamin D and Cancer: An Historical Overview of the Epidemiology and Mechanisms. Nutrients 2022, 14, 1448. [Google Scholar] [CrossRef] [PubMed]
- Kift, R.C.; Webb, A.R. Globally Estimated UVB Exposure Times Required to Maintain Sufficiency in Vitamin D Levels. Nutrients 2024, 16, 1489. [Google Scholar] [CrossRef]
- Voskarides, K.; Philippou, S.; Hamam, M.; Parperis, K. Prevalence of autoimmune diseases is strongly associated with average annual temperatures: Systematic review and linear regression analysis. BMC Rheumatol. 2025, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Feron, F.; Cui, X.; Kesby, J.P.; Harms, L.H.; Ko, P.; McGrath, J.J.; Burne, T.H. Developmental vitamin D deficiency causes abnormal brain development. Psychoneuroendocrinology 2009, 34 (Suppl. S1), S247–S257. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraser, D.R. Perspective: Vitamin D Deficiency Relationship to Initiation of Diseases. Nutrients 2025, 17, 2900. https://doi.org/10.3390/nu17172900
Fraser DR. Perspective: Vitamin D Deficiency Relationship to Initiation of Diseases. Nutrients. 2025; 17(17):2900. https://doi.org/10.3390/nu17172900
Chicago/Turabian StyleFraser, David R. 2025. "Perspective: Vitamin D Deficiency Relationship to Initiation of Diseases" Nutrients 17, no. 17: 2900. https://doi.org/10.3390/nu17172900
APA StyleFraser, D. R. (2025). Perspective: Vitamin D Deficiency Relationship to Initiation of Diseases. Nutrients, 17(17), 2900. https://doi.org/10.3390/nu17172900