Effects of Two Compound Probiotic Formulations on Gastrointestinal Symptoms and Gut Microbiota: A 4-Week Randomized, Double-Blind Intervention Trial
Abstract
1. Introduction
2. Materials and Methods
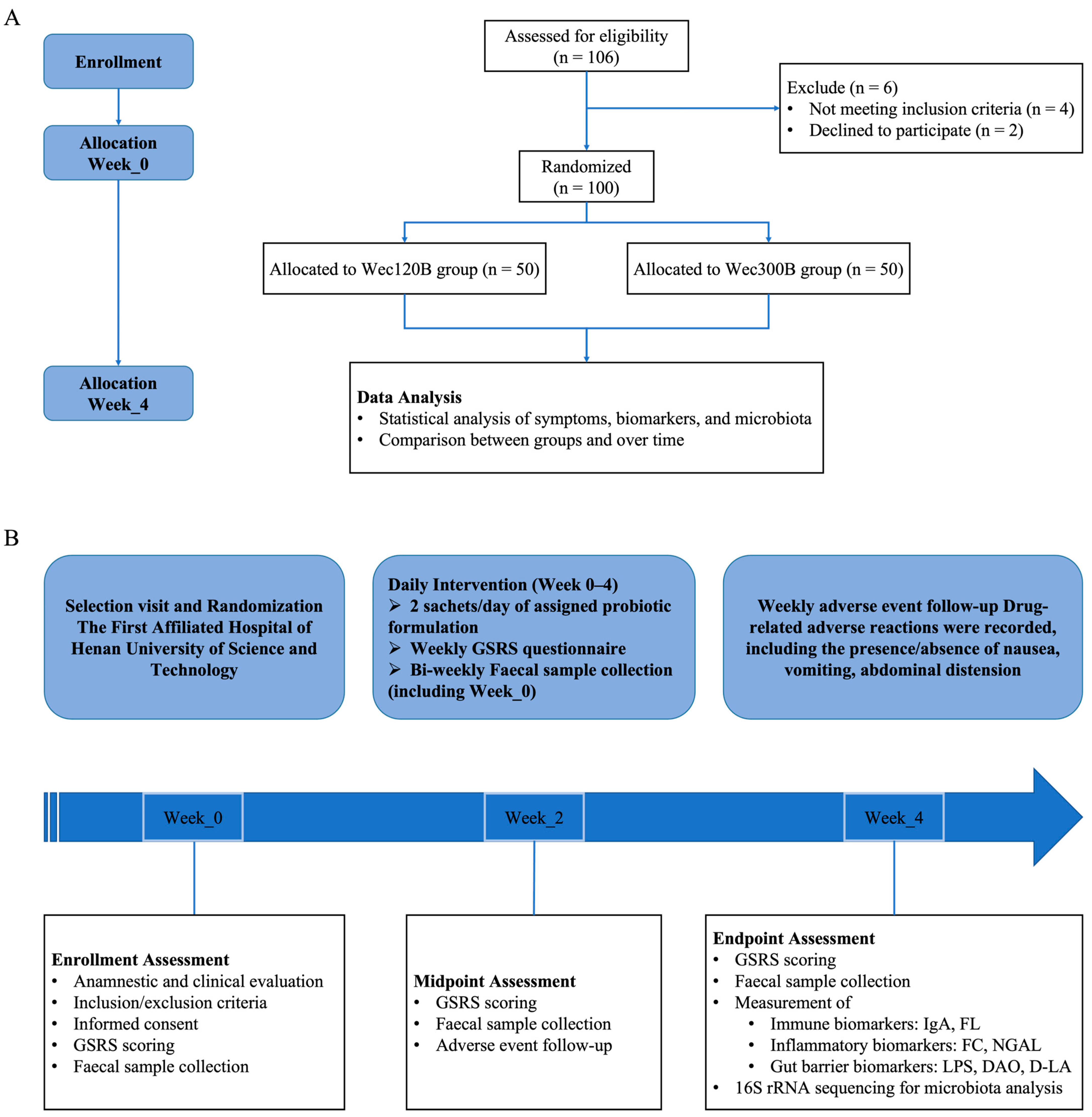
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Sample Size and Randomization
2.4. Participants and Intervention
2.5. Primary and Secondary Outcomes
2.6. Gastrointestinal Symptom Assessment
2.7. Fecal Sample Collection and Biomarker Analysis
2.8. Gut Microbiota Analysis
2.9. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Effects on Immune, Inflammatory, and Gut Barrier Markers
3.3. Comparison of Gastrointestinal Symptoms Before and After Intervention
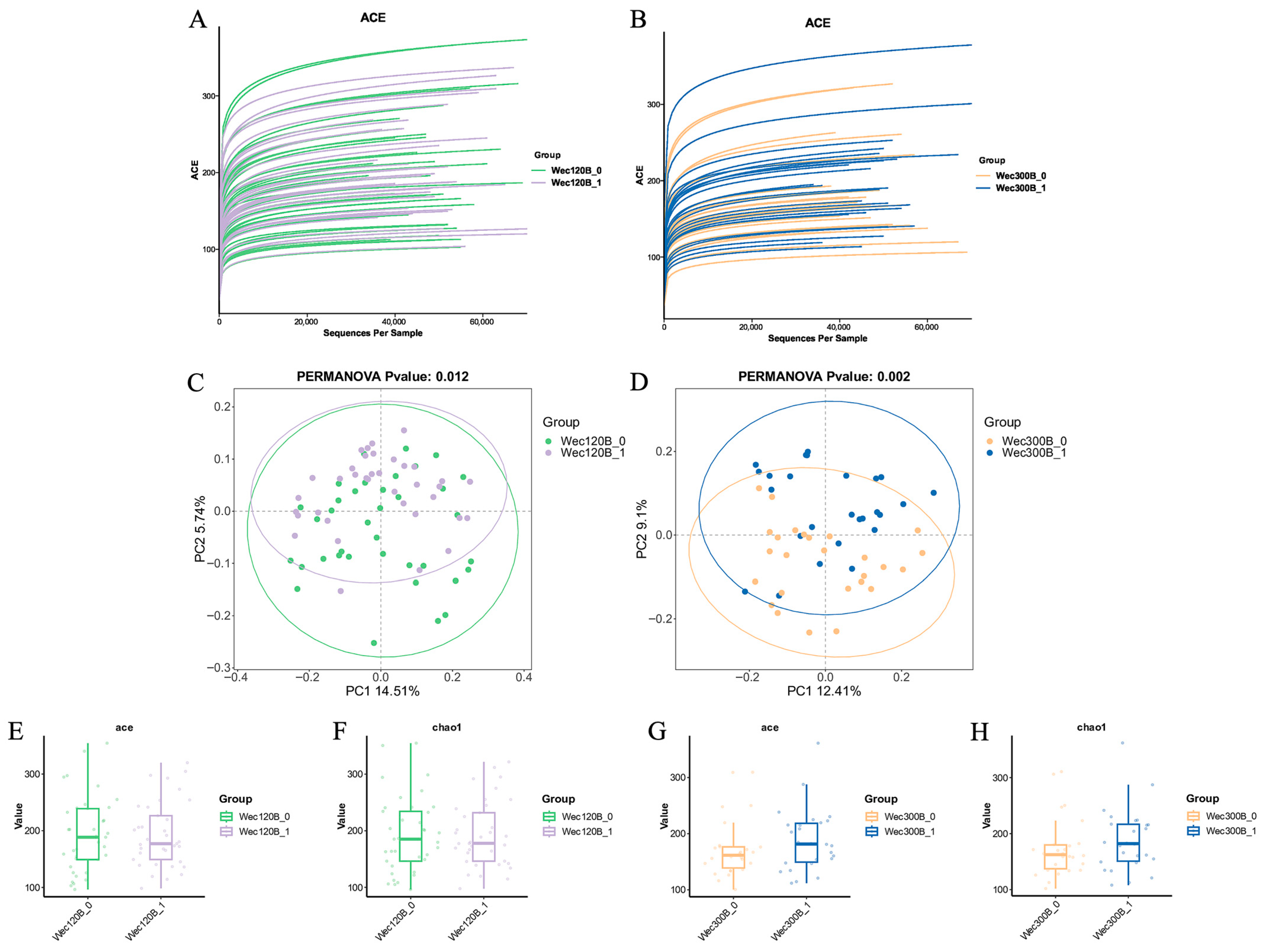
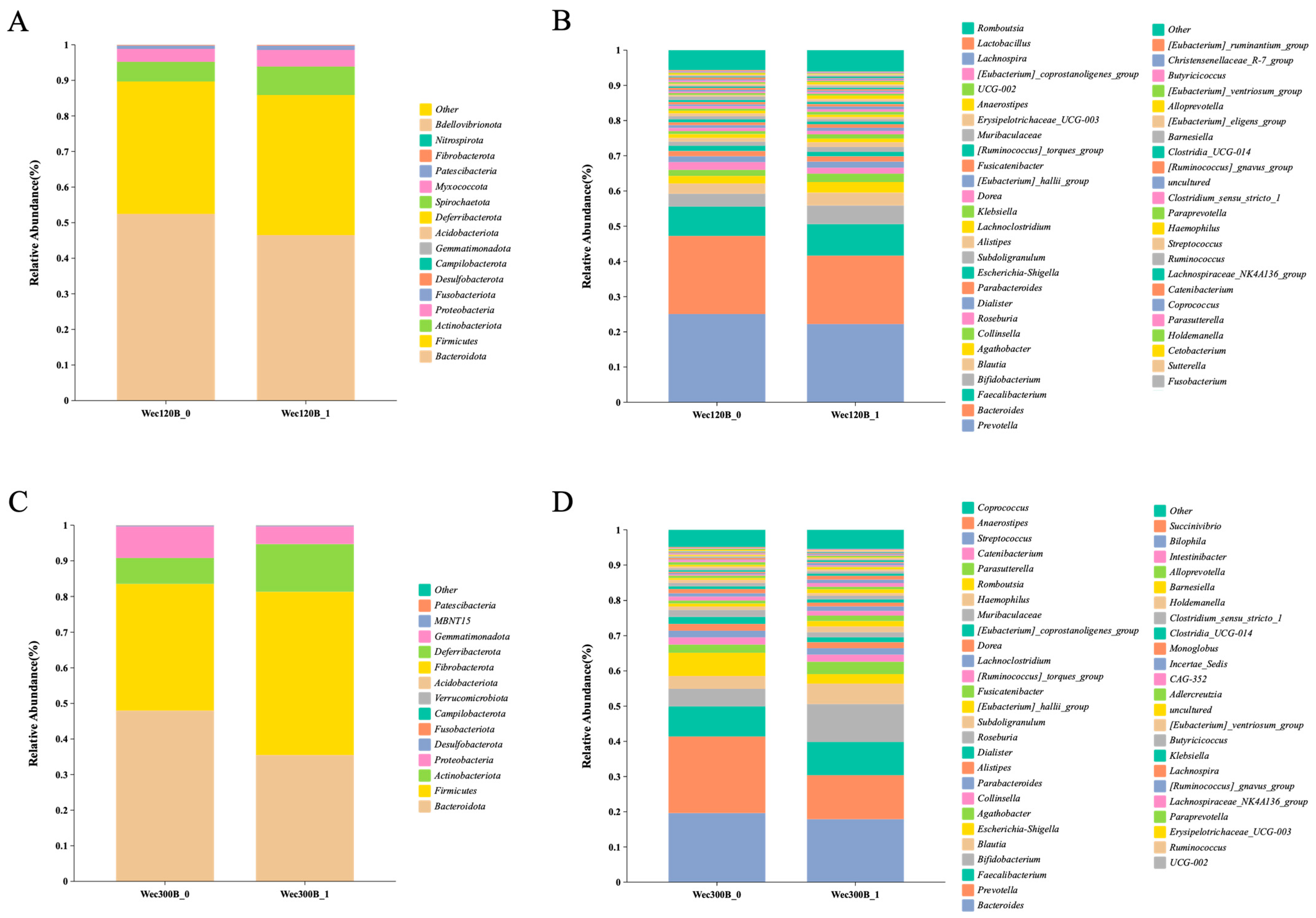
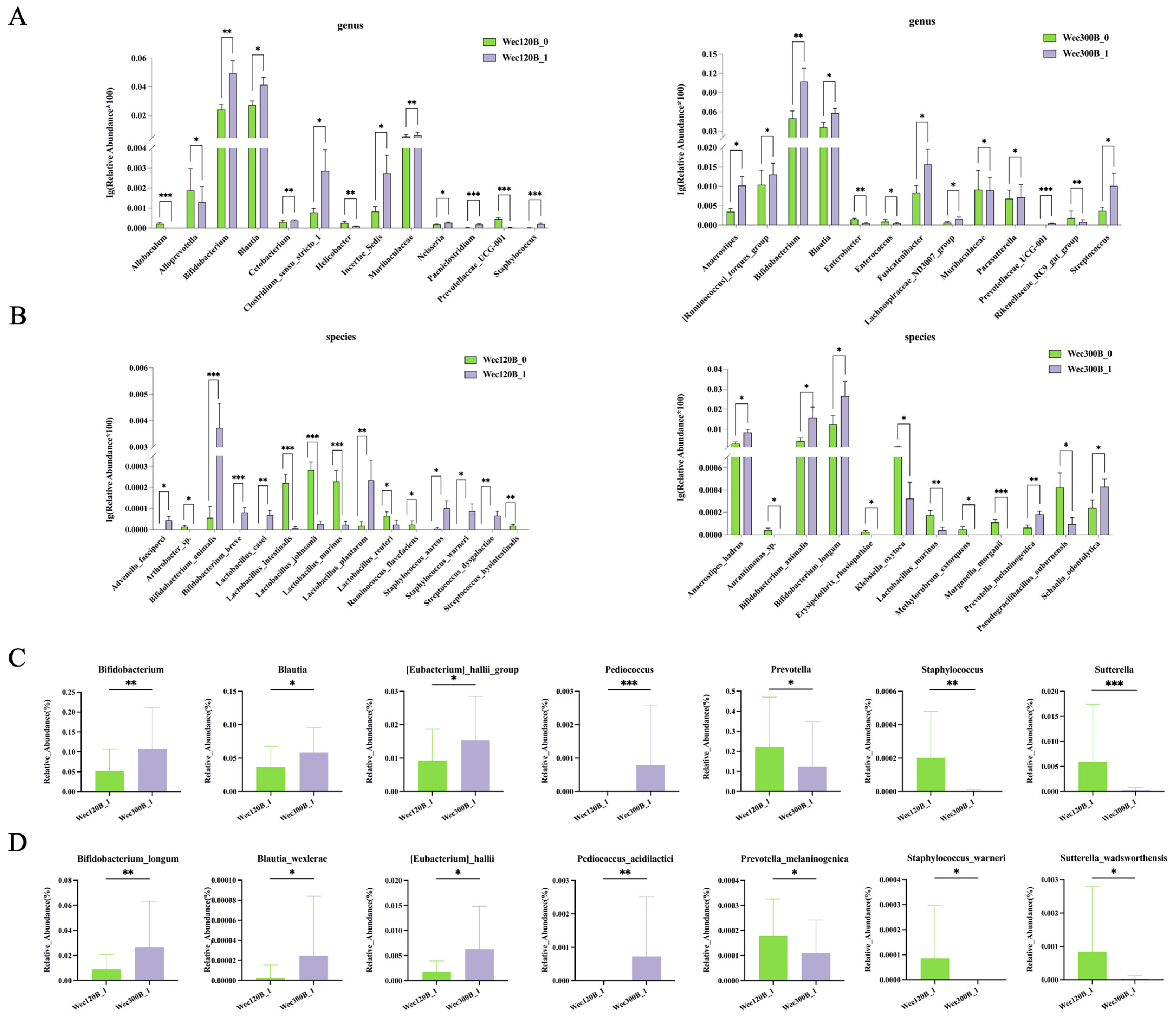
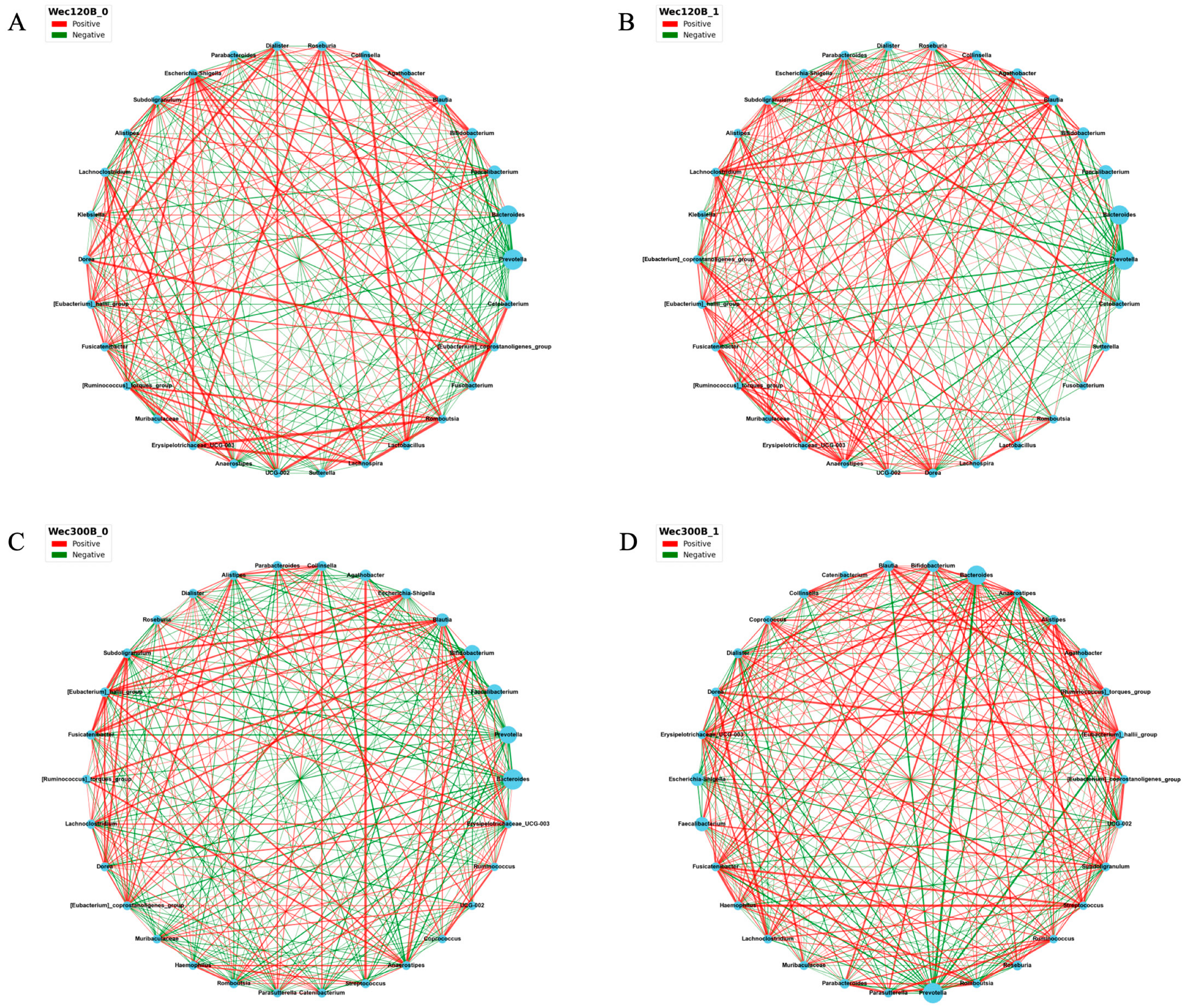
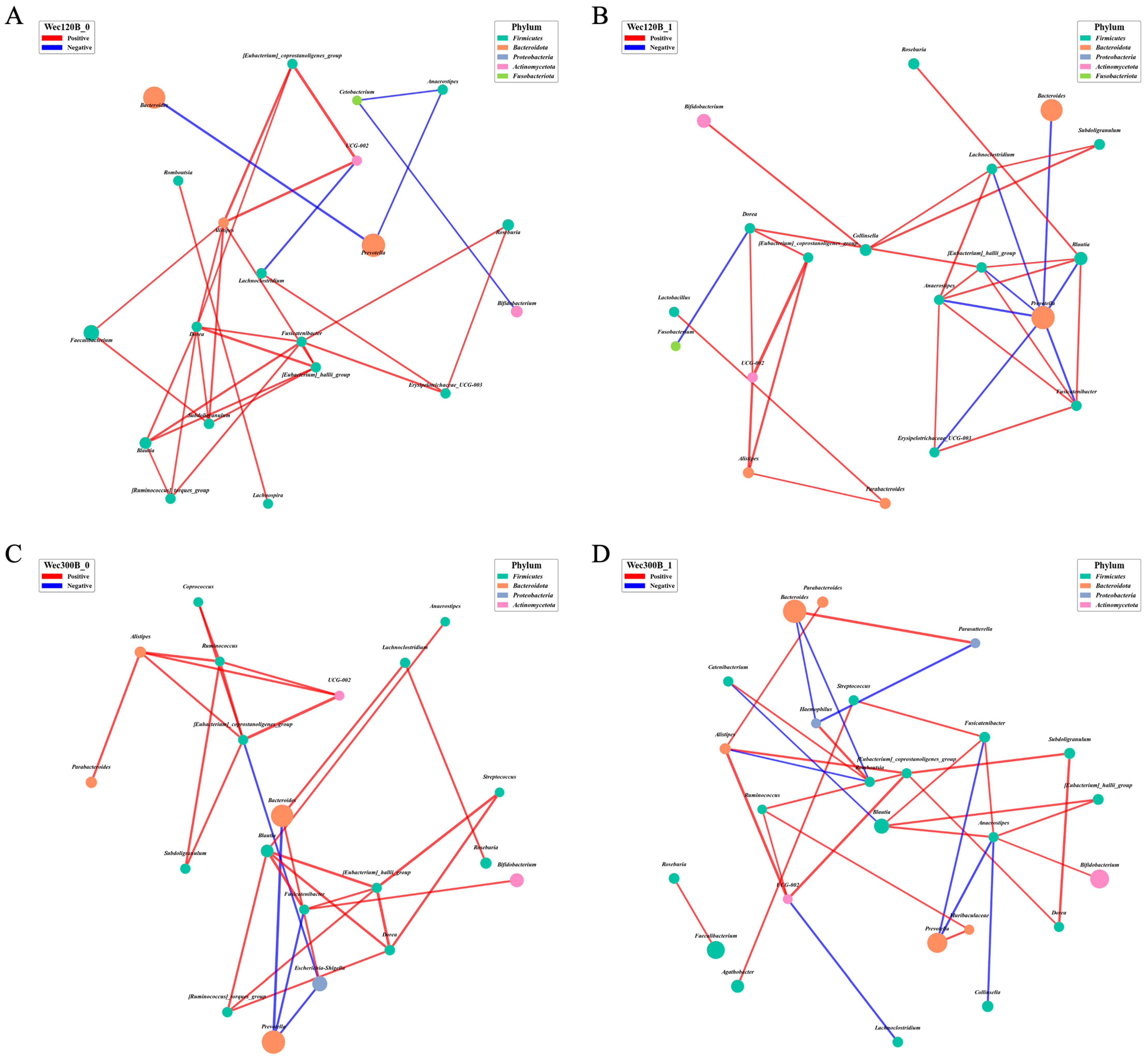
3.4. Modulation of Gut Microbiota Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Zhang, D.-Y.; Chen, S.; Huang, S.; Zeng, F.; Li, D.; Lv, Y.-T.; Xiang, X.; Chen, R.-X.; Zhang, X.; et al. Prevalence, Types, and Risk Factors of Functional Gastrointestinal Diseases in Hainan Province, China. Sci. Rep. 2024, 14, 4553. [Google Scholar] [CrossRef] [PubMed]
- Sasso, J.M.; Ammar, R.M.; Tenchov, R.; Lemmel, S.; Kelber, O.; Grieswelle, M.; Zhou, Q.A. Gut Microbiome-Brain Alliance: A Landscape View into Mental and Gastrointestinal Health and Disorders. ACS Chem. Neurosci. 2023, 14, 1717–1763. [Google Scholar] [CrossRef] [PubMed]
- Volarić, M.; Šojat, D.; Majnarić, L.T.; Vučić, D. The Association between Functional Dyspepsia and Metabolic Syndrome-The State of the Art. Int. J. Environ. Res. Public Health 2024, 21, 237. [Google Scholar] [CrossRef]
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Jensen, E.T.; Kim, H.P.; Egberg, M.D.; Lund, J.L.; Moon, A.M.; Pate, V.; Barnes, E.L.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology 2022, 162, 621–644. [Google Scholar] [CrossRef]
- Hamilton, A.; Poleon, S.; Cherian, J.; Cosgrove, S.; Laxminarayan, R.; Klein, E. COVID-19 and Outpatient Antibiotic Prescriptions in the United States: A County-Level Analysis. Open Forum Infect. Dis. 2023, 10, ofad096. [Google Scholar] [CrossRef]
- Abdul Manan, M. Progress in Probiotic Science: Prospects of Functional Probiotic-Based Foods and Beverages. Int. J. Food Sci. 2025, 2025, 5567567. [Google Scholar] [CrossRef]
- Tremblay, A.; Fatani, A.; Ford, A.L.; Piano, A.; Nagulesapillai, V.; Auger, J.; MacPherson, C.W.; Christman, M.C.; Tompkins, T.A.; Dahl, W.J. Safety and Effect of a Low- and High-Dose Multi-Strain Probiotic Supplement on Microbiota in a General Adult Population: A Randomized, Double-Blind, Placebo-Controlled Study. J. Diet. Suppl. 2021, 18, 227–247. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Hul, M.V.; Cani, P.D. Gut Microbiome and Health: Mechanistic Insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Ma, J.; Piao, X.; Mahfuz, S.; Long, S.; Wang, J. The Interaction among Gut Microbes, the Intestinal Barrier and Short Chain Fatty Acids. Anim. Nutr. 2022, 9, 159–174. [Google Scholar] [CrossRef]
- Tyagi, A.; Kumar, V. The Gut Microbiota-Bile Acid Axis: A Crucial Regulator of Immune Function and Metabolic Health. World J. Microbiol. Biotechnol. 2025, 41, 215. [Google Scholar] [CrossRef]
- Masse, K.E.; Lu, V.B. Short-Chain Fatty Acids, Secondary Bile Acids and Indoles: Gut Microbial Metabolites with Effects on Enteroendocrine Cell Function and Their Potential as Therapies for Metabolic Disease. Front. Endocrinol. 2023, 14, 1169624. [Google Scholar] [CrossRef]
- Carco, C.; Young, W.; Gearry, R.B.; Talley, N.J.; McNabb, W.C.; Roy, N.C. Increasing Evidence That Irritable Bowel Syndrome and Functional Gastrointestinal Disorders Have a Microbial Pathogenesis. Front. Cell Infect. Microbiol. 2020, 10, 468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, C.; Zhang, J.; Sun, F.; Duan, L. Efficacy of Probiotics for Irritable Bowel Syndrome: A Systematic Review and Network Meta-Analysis. Front. Cell Infect. Microbiol. 2022, 12, 859967. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, Y.; Wan, Y.; Hu, T.; Liu, L.; Yang, S.; Gong, Z.; Zeng, Q.; Wei, Y.; Yang, W.; et al. A Novel Postbiotic from Lactobacillus Rhamnosus GG with a Beneficial Effect on Intestinal Barrier Function. Front. Microbiol. 2019, 10, 477. [Google Scholar] [CrossRef]
- Segui-Perez, C.; Huang, L.Z.X.; Paganelli, F.L.; Lievens, E.; Strijbis, K. Probiotic Bifidobacterium bifidum Strains Desialylate MUC13 and Increase Intestinal Epithelial Barrier Function. Sci. Rep. 2025, 15, 8778. [Google Scholar] [CrossRef]
- World Medical Association (WMA). WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Participants. World Medical Association. 2022. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki/ (accessed on 31 October 2024).
- IBM Corp. IBM SPSS Statistics for Windows, Version 25.0; Released 2017; IBM Corp.: Armonk, NY, USA, 2017. [Google Scholar]
- Bunker, J.J.; Bendelac, A. IgA Responses to Microbiota. Immunity 2018, 49, 211–224. [Google Scholar] [CrossRef]
- Abraham, B.P.; Kane, S. Fecal Markers: Calprotectin and Lactoferrin. Gastroenterol. Clin. 2012, 41, 483–495. [Google Scholar] [CrossRef]
- Ikhtaire, S.; Shajib, M.S.; Reinisch, W.; Khan, W.I. Fecal calprotectin: Its scope and utility in the management of inflammatory bowel disease. J. Gastroenterol. 2016, 51, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Inoue, K.; Kato, J.; Minamishima, S.; Morisaki, H. Functions and Regulation of Lipocalin-2 in Gut-Origin Sepsis: A Narrative Review. Crit. Care 2019, 23, 269. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, X.; Wu, J.; Chen, M. The Correlation between Endotoxin, D-Lactate, and Diamine Oxidase with Endoscopic Activity in Inflammatory Bowel Disease. Dis. Markers 2022, 2022, 9171436. [Google Scholar] [CrossRef]
- Svedlund, J.; Sjödin, I.; Dotevall, G. GSRS—A Clinical Rating Scale for Gastrointestinal Symptoms in Patients with Irritable Bowel Syndrome and Peptic Ulcer Disease. Dig. Dis. Sci. 1988, 33, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Kulich, K.R.; Madisch, A.; Pacini, F.; Piqué, J.M.; Regula, J.; Van Rensburg, C.J.; Újszászy, L.; Carlsson, J.; Halling, K.; Wiklund, I.K. Reliability and Validity of the Gastrointestinal Symptom Rating Scale (GSRS) and Quality of Life in Reflux and Dyspepsia (QOLRAD) Questionnaire in Dyspepsia: A Six-Country Study. Health Qual. Life Outcomes 2008, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Du, T.; Yi, S.; Zhang, C.; Yu, L.; Tian, F.; Chen, W.; Zhai, Q. Response Differences of Gut Microbiota in Oligofructose and Inulin Are Determined by the Initial Gut Bacteroides/Bifidobact. Ratios. Food Res. Int. 2023, 174, 113598. [Google Scholar] [CrossRef] [PubMed]
- Lordan, C.; Roche, A.K.; Delsing, D.; Nauta, A.; Groeneveld, A.; MacSharry, J.; Cotter, P.D.; van Sinderen, D. Linking Human Milk Oligosaccharide Metabolism and Early Life Gut Microbiota: Bifidobacteria and Beyond. Microbiol. Mol. Biol. Rev. 2024, 88, e00094-23. [Google Scholar] [CrossRef]
- Hiraku, A.; Nakata, S.; Murata, M.; Xu, C.; Mutoh, N.; Arai, S.; Odamaki, T.; Iwabuchi, N.; Tanaka, M.; Tsuno, T.; et al. Early Probiotic Supplementation of Healthy Term Infants with Bifidobacterium longum Subsp. infantis M-63 Is Safe and Leads to the Development of Bifidobacterium-Predominant Gut Microbiota: A Double-Blind, Placebo-Controlled Trial. Nutrients 2023, 15, 1402. [Google Scholar] [CrossRef]
- Alatawi, H.; Mosli, M.; Saadah, O.I.; Annese, V.; Al-Hindi, R.; Alatawy, M.; Al-Amrah, H.; Alshehri, D.; Bahieldin, A.; Edris, S. Attributes of Intestinal Microbiota Composition and Their Correlation with Clinical Primary Non-Response to Anti-TNF-α Agents in Inflammatory Bowel Disease Patients. Biomol. Biomed. 2022, 22, 412–426. [Google Scholar] [CrossRef]
- Lin, J.; Li, E.; Li, C. Increasing Degree of Substitution Inhibits Acetate While Promotes Butyrate Production during in Vitro Fermentation of Citric Acid-Modified Rice Starch. Int. J. Biol. Macromol. 2024, 281, 136385. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, X.; Zhu, Z.; Pang, L.; Ding, S.; Li, X.; Kang, Y.; Hei, G.; Zhang, L.; Zhang, X.; et al. Multiomics Analyses Reveal Microbiome–Gut–Brain Crosstalk Centered on Aberrant Gamma-Aminobutyric Acid and Tryptophan Metabolism in Drug-Naïve Patients with First-Episode Schizophrenia. Schizophr. Bull. 2024, 50, 187–198. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Q.; Yang, Y.; Sun, S.; Qi, H.; He, J.; Jin, T.; Yao, P.; Wang, J.; Lin, F. Integrated Analysis of Gut Microbiota, Fecal and Serum Metabolites in Type 2 Diabetes Mellitus with Peripheral Neuropathy. J. Endocrinol. Investig. 2025. [Google Scholar] [CrossRef]
- Gaifem, J.; Mendes-Frias, A.; Wolter, M.; Steimle, A.; Garzón, M.J.; Ubeda, C.; Nobre, C.; González, A.; Pinho, S.S.; Cunha, C.; et al. Akkermansia muciniphila and Parabacteroides distasonis synergistically protect from colitis by promoting ILC3 in the gut. mBio 2024, 15, e00078-24. [Google Scholar] [CrossRef]
- Tian, C.; Yang, Q.; Lv, H.; Yue, F. Integrative Analysis of Gut Microbiota and Fecal Metabolites in Cynomolgus Monkeys with Spontaneous Type 2 Diabetes Mellitus. Microb. Pathog. 2025, 199, 107228. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Peng, Z.; Jiang, L.; Yan, Y.; Xia, Y.; Wang, Y.; Guo, L.; Miao, J.; Bian, Y. Causal Associations of Gut Microbiota Species with Lymphoma: A Two-Sample Mendelian Randomization Study. Hematol. Oncol. 2025, 43, e70046. [Google Scholar] [CrossRef]
- Luo, C.-H.; Lai, A.C.-Y.; Chang, Y.-J. Butyrate Inhibits Staphylococcus Aureus-Aggravated Dermal IL-33 Expression and Skin Inflammation through Histone Deacetylase Inhibition. Front. Immunol. 2023, 14, 1114699. [Google Scholar] [CrossRef]
- Sebastián Domingo, J.J. Irritable Bowel Syndrome. Med. Clin. (Barc.) 2022, 158, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, Z.; Rostami, M.; Whiting, S.J.; Vatanparast, H. Fast-Food Dietary Pattern Is Linked to Higher Prevalence of Metabolic Syndrome in Older Canadian Adults. J. Nutr. Metab. 2021, 2021, 5712844. [Google Scholar] [CrossRef] [PubMed]
- Castellana, C.; Pecere, S.; Furnari, M.; Telese, A.; Matteo, M.V.; Haidry, R.; Eusebi, L.H. Side effects of long-term use of proton pump inhibitors: Practical considerations. Pol. Arch. Intern. Med. 2021, 131, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Prokinetics in the Management of Functional Gastrointestinal Disorders. Curr. Gastroenterol. Rep. 2017, 19, 53. [Google Scholar] [CrossRef]
- Maideen, N.M.P. Adverse Effects Associated with Long-Term Use of Proton Pump Inhibitors. Chonnam Med. J. 2023, 59, 115–127. [Google Scholar] [CrossRef]
- San Mauro Martín, I.; López Oliva, S.; Garicano Vilar, E.; Sánchez Niño, G.M.; Penadés, B.F.; Terrén Lora, A.; Sanz Rojo, S.; Collado Yurrita, L. Effects of Gluten on Gut Microbiota in Patients with Gastrointestinal Disorders, Migraine, and Dermatitis. Nutrients 2024, 16, 1228. [Google Scholar] [CrossRef]
- Zhao, L.-P.; Wu, J.; Quan, W.; Zhou, Y.; Hong, H.; Niu, G.-Y.; Li, T.; Huang, S.-B.; Qiao, C.-M.; Zhao, W.-J.; et al. DSS-Induced Acute Colitis Causes Dysregulated Tryptophan Metabolism in Brain: An Involvement of Gut Microbiota. J. Nutr. Biochem. 2023, 115, 109282. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, Y.; Ma, Y.; Chen, P.; Tang, J.; Yang, B.; Li, H.; Liang, M.; Xue, Y.; Liu, Y.; et al. Gut Microbiota Dysbiosis Correlates with Long COVID-19 at One-Year After Discharge. J. Korean Med. Sci. 2023, 38, e120. [Google Scholar] [CrossRef]
- Gavzy, S.J.; Kensiski, A.; Lee, Z.L.; Mongodin, E.F.; Ma, B.; Bromberg, J.S. Bifidobacterium Mechanisms of Immune Modulation and Tolerance. Gut Microbes 2023, 15, 2291164. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, H.; Feng, X.; Ma, X.; Zhai, Z.; Hao, Y. Bifidobacterium longum BL300 Alleviates DSS-Induced Colitis by Promoting IgA-Mediated Gut Microbiota Homeostasis. Food Funct. 2025, 16, 4303–4314. [Google Scholar] [CrossRef]
- Benítez-Páez, A.; Gómez del Pugar, E.M.; López-Almela, I.; Moya-Pérez, Á.; Codoñer-Franch, P.; Sanz, Y. Depletion of Blautia Species in the Microbiota of Obese Children Relates to Intestinal Inflammation and Metabolic Phenotype Worsening. mSystems 2020, 5, e00857-19. [Google Scholar] [CrossRef] [PubMed]
- Waclawiková, B.; Codutti, A.; Alim, K.; El Aidy, S. Gut Microbiota-Motility Interregulation: Insights from in Vivo, Ex Vivo and in Silico Studies. Gut Microbes 2022, 14, 1997296. [Google Scholar] [CrossRef]
- Staley, C.; Weingarden, A.R.; Khoruts, A.; Sadowsky, M.J. Interaction of Gut Microbiota with Bile Acid Metabolism and Its Influence on Disease States. Appl. Microbiol. Biotechnol. 2017, 101, 47–64. [Google Scholar] [CrossRef]
- Cai, J.; Chen, H.; Weng, M.; Jiang, S.; Gao, J. Diagnostic and Clinical Significance of Serum Levels of D-Lactate and Diamine Oxidase in Patients with Crohn’s Disease. Gastroenterol. Res. Pract. 2019, 2019, 8536952. [Google Scholar] [CrossRef]
- Wang, Y.; Pei, F.; Wang, X.; Sun, Z.; Hu, C.; Dou, H. Diagnostic Accuracy of Fecal Lactoferrin for Inflammatory Bowel Disease: A Meta-Analysis. Int. J. Clin. Exp. Pathol. 2015, 8, 12319–12332. [Google Scholar] [PubMed]
- Sutton, K.A.; He, M.; Ma, C.; Liu, T.-C.; Faubion, W.A.; Hoffmann, J.; Linneman, L.; Rodriguez, C.; Holtz, L.R. Noninvasive Markers of Inflammation and Protein Loss Augment Diagnosis of Pediatric Celiac Disease. Clin. Transl. Gastroenterol. 2024, 15, e00695. [Google Scholar] [CrossRef] [PubMed]



| Group | Age () | Gender | |
|---|---|---|---|
| Male | Female | ||
| Wec120B group (n = 50) | 21.1 ± 5.18 | 29 (58%) | 21 (42%) |
| Wec300B group (n = 50) | 20.42 ± 2.7 | 24 (48%) | 26 (52%) |
| Domain | Evaluation | Score | p-Value (vs. Baseline) | 95% CI (vs. Baseline) |
|---|---|---|---|---|
| Indigestion | Baseline | 7.54 ± 2.56 | - | - |
| W1 | 6.64 ± 2.10 | 0.057 | −0.02815, 1.82815 | |
| W2 | 6.44 ± 1.79 | 0.014 | 0.22461, 1.97539 | |
| W3 | 6.52 ± 1.58 | 0.018 | 0.17625, 1.86375 | |
| W4 | 6.16 ± 1.54 | 0.0008 | 0.57955, 2.18025 | |
| Reflux | Baseline | 2.74 ± 1.38 | - | - |
| W1 | 2.44 ± 0.88 | 0.199 | −0.16052, 0.76052 | |
| W2 | 2.62 ± 1.05 | 0.626 | −0.36675, 0.60675 | |
| W3 | 2.26 ± 0.60 | 0.027 | 0.05715, 0.90285 | |
| W4 | 2.22 ± 0.55 | 0.015 | 0.10296, 0.93704 | |
| Bellyache | Baseline | 5.10 ± 2.38 | - | - |
| W1 | 4.24 ± 1.52 | 0.033 | 0.06863, 1.65137 | |
| W2 | 4.10 ± 1.40 | 0.012 | 0.22569, 1.77431 | |
| W3 | 3.58 ± 1.00 | 0.000 | 0.79758, 2.24242 | |
| W4 | 3.48 ± 0.76 | <0.0001 | 0.91984, 2.32016 | |
| Constipation | Baseline | 0.52 ± 0.58 | - | - |
| W1 | 0.38 ± 0.60 | 0.239 | −0.09467, 0.37467 | |
| W2 | 0.36 ± 0.48 | 0.138 | −0.05215, 0.37215 | |
| W3 | 0.32 ± 0.59 | 0.090 | −0.03157, 0.43157 | |
| W4 | 0.26 ± 0.44 | 0.013 | 0.05518, 0.46482 | |
| Diarrhea | Baseline | 0.54 ± 0.73 | - | - |
| W1 | 0.38 ± 0.64 | 0.247 | −0.11251, 0.43251 | |
| W2 | 0.34 ± 0.66 | 0.155 | −0.07672, 0.47672 | |
| W3 | 0.30 ± 0.54 | 0.066 | −0.01647, 0.49647 | |
| W4 | 0.18 ± 0.39 | 0.003 | 0.12691, 0.59309 |
| Domain | Evaluation | Score | p-Value (vs. Baseline) | 95% CI (vs. Baseline) |
|---|---|---|---|---|
| Indigestion | Baseline | 7.63 ± 2.18 | - | - |
| W1 | 6.73 ± 2.05 | 0.034 | 0.07031, 1.73361 | |
| W2 | 6.73 ± 2.15 | 0.038 | 0.05014, 1.75379 | |
| W3 | 6.53 ± 2.00 | 0.009 | 0.27520, 1.92088 | |
| W4 | 6.10 ± 1.68 | 0.0002 | 0.73682, 2.32270 | |
| Reflux | Baseline | 2.94 ± 1.33 | - | - |
| W1 | 2.57 ± 0.92 | 0.104 | −0.07770, 0.82280 | |
| W2 | 2.61 ± 1.08 | 0.168 | −0.14298, 0.80965 | |
| W3 | 2.27 ± 0.57 | 0.001 | 0.26412, 1.06922 | |
| W4 | 2.29 ± 0.67 | 0.003 | 0.23236, 1.06176 | |
| Bellyache | Baseline | 4.92 ± 2.35 | - | - |
| W1 | 4.20 ± 2.00 | 0.096 | −0.13144, 1.58242 | |
| W2 | 4.27 ± 1.67 | 0.112 | −0.15412, 1.44824 | |
| W3 | 3.98 ± 1.61 | 0.020 | 0.15084, 1.73152 | |
| W4 | 3.75 ± 1.40 | 0.003 | 0.41730, 1.93564 | |
| Diarrhea | Baseline | 0.51 ± 0.61 | - | - |
| W1 | 0.49 ± 0.67 | 0.878 | −0.23346, 0.27268 | |
| W2 | 0.38 ± 0.60 | 0.255 | −0.10065, 0.37516 | |
| W3 | 0.31 ± 0.58 | 0.101 | −0.03875, 0.43091 | |
| W4 | 0.35 ± 0.63 | 0.039 | 0.01334, 0.49647 | |
| Constipation | Baseline | 0.51 ± 0.58 | - | - |
| W1 | 0.55 ± 0.73 | 0.764 | −0.29796, 0.21953 | |
| W2 | 0.32 ± 0.65 | 0.110 | −0.04524, 0.43739 | |
| W3 | 0.22 ± 0.54 | 0.009 | 0.07406, 0.51418 | |
| W4 | 0.14 ± 0.35 | 0.000 | 0.18501, 0.56008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, Z.; Wu, Y.; Jiang, Y.; Fan, J.; Cao, L.; Dong, Y.; Fang, S.; Gu, S. Effects of Two Compound Probiotic Formulations on Gastrointestinal Symptoms and Gut Microbiota: A 4-Week Randomized, Double-Blind Intervention Trial. Nutrients 2025, 17, 2886. https://doi.org/10.3390/nu17172886
Qu Z, Wu Y, Jiang Y, Fan J, Cao L, Dong Y, Fang S, Gu S. Effects of Two Compound Probiotic Formulations on Gastrointestinal Symptoms and Gut Microbiota: A 4-Week Randomized, Double-Blind Intervention Trial. Nutrients. 2025; 17(17):2886. https://doi.org/10.3390/nu17172886
Chicago/Turabian StyleQu, Zhen, Ying Wu, Yiru Jiang, Jiajia Fan, Li Cao, Yao Dong, Shuguang Fang, and Shaobin Gu. 2025. "Effects of Two Compound Probiotic Formulations on Gastrointestinal Symptoms and Gut Microbiota: A 4-Week Randomized, Double-Blind Intervention Trial" Nutrients 17, no. 17: 2886. https://doi.org/10.3390/nu17172886
APA StyleQu, Z., Wu, Y., Jiang, Y., Fan, J., Cao, L., Dong, Y., Fang, S., & Gu, S. (2025). Effects of Two Compound Probiotic Formulations on Gastrointestinal Symptoms and Gut Microbiota: A 4-Week Randomized, Double-Blind Intervention Trial. Nutrients, 17(17), 2886. https://doi.org/10.3390/nu17172886






