BMI as a Mediator in the Relationship Between Dietary Trace Elements and Type 2 Diabetes Mellitus: Findings from a Rural Cohort
Highlights
- This study focuses on the association between dietary trace elements and T2DM in rural areas.
- Dietary trace elements have a protective effect on T2DM.
- BMI mediates the association between dietary trace elements and T2DM.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Assessment of Dietary Iron, Copper, and Zinc Intakes
2.3. Assessment of Covariates
2.4. Definition of T2DM
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Participants
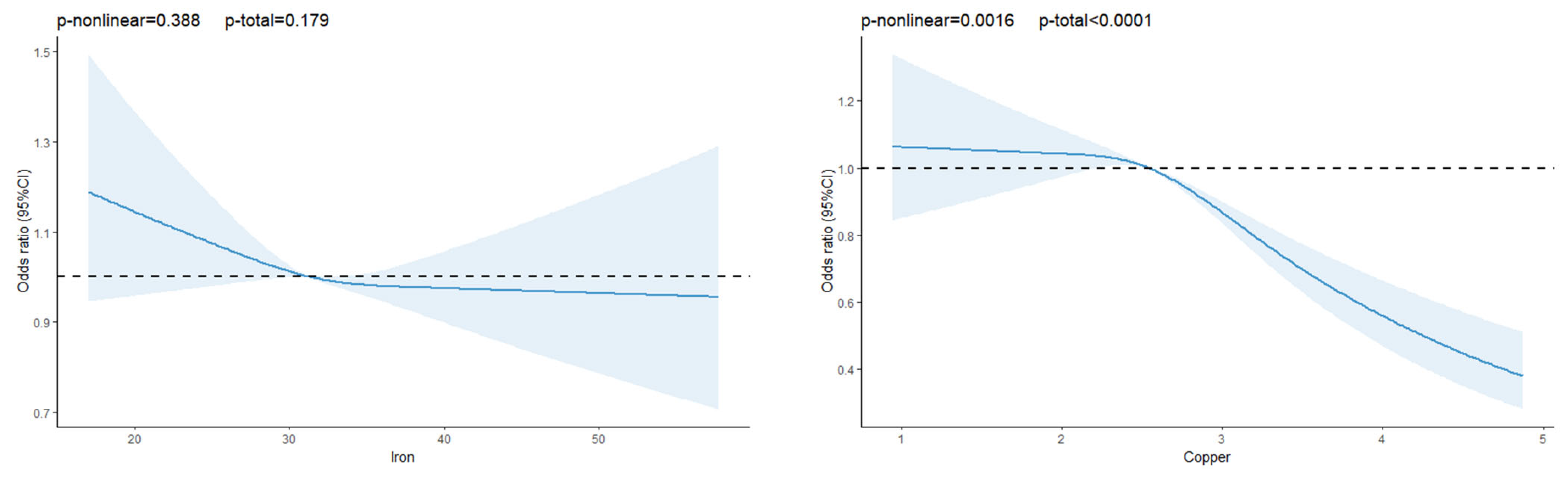
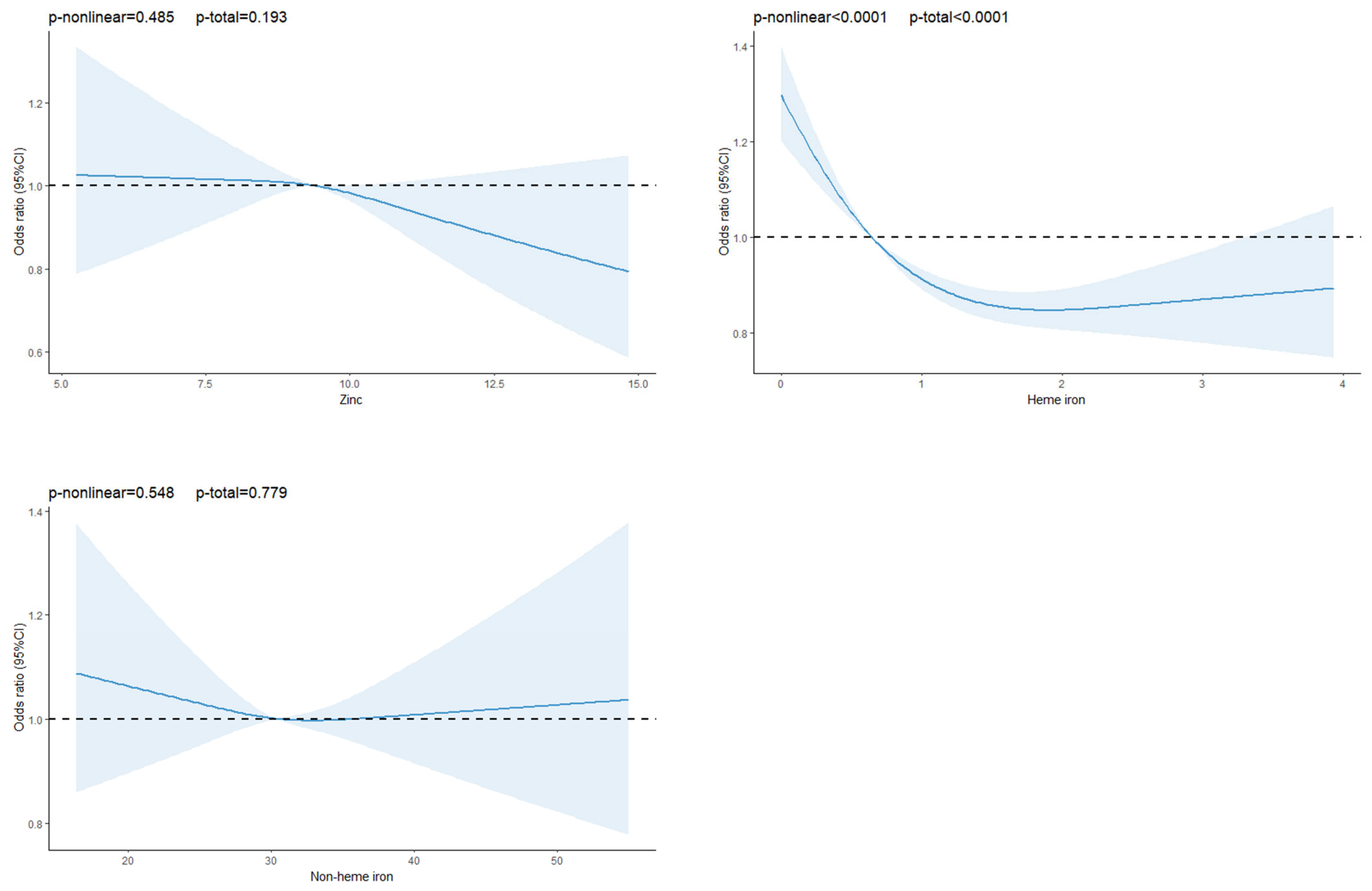
3.2. Association Between Dietary Trace Elements and T2DM
3.3. Stratification Analysis
3.4. Mediation Effects of BMI on Dietary Trace Elements and T2DM Association
3.5. Interaction Analysis and Combined Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al, K.J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045, Diabetes. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Bommer, C.; Heesemann, E.; Sagalova, V.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Vollmer, S. The global economic burden of diabetes in adults aged 20-79 years: A cost-of-illness study. Lancet Diabetes Endocrinol. 2017, 5, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Bao, Y.; Bai, B.; Liu, X.; Shi, B.; Tian, L. An update on the economic burden of type 2 diabetes mellitus in China. Expert Rev. Pharmacoecon. Outcomes Res. 2022, 22, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, P.; Zhang, M.; Huang, Z.; Zhang, D.; Deng, Q.; Li, Y.; Zhao, Z.; Qin, X.; Jin, D.; et al. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA 2017, 317, 2515–2523. [Google Scholar] [CrossRef]
- Schnurr, T.M.; Jakupović, H.; Carrasquilla, G.D.; Ängquist, L.; Grarup, N.; Sørensen, T.I.A.; Tjønneland, A.; Overvad, K.; Pedersen, O.; Hansen, T.; et al. Obesity, unfavourable lifestyle and genetic risk of type 2 diabetes: A case-cohort study. Diabetologia 2020, 63, 1324–1332. [Google Scholar] [CrossRef]
- Tan, W.X.; Sim, X.; Khoo, C.M.; Teo, A.K.K. Prioritization of genes associated with type 2 diabetes mellitus for functional studies. Nat. Rev. Endocrinol. 2023, 19, 477–486. [Google Scholar] [CrossRef]
- Thorpe, L.E.; Adhikari, S.; Lopez, P.; Kanchi, R.; McClure, L.A.; Hirsch, A.G.; Howell, C.R.; Zhu, A.; Alemi, F.; Rummo, P.; et al. Neighborhood Socioeconomic Environment and Risk of Type 2 Diabetes: Associations and Mediation through Food Environment Pathways in Three Independent Study Samples. Diabetes Care 2022, 45, 798–810. [Google Scholar] [CrossRef]
- Altamura, S.; Müdder, K.; Schlotterer, A.; Fleming, T.; Heidenreich, E.; Qiu, R.; Hammes, H.-P.; Nawroth, P.; Muckenthaler, M.U. Iron aggravates hepatic insulin resistance in the absence of inflammation in a novel db/db mouse model with iron overload. Mol. Metab. 2021, 51, 101235. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Ruscica, M.; Rametta, R.; Recalcati, S.; Steffani, L.; Gatti, S.; Girelli, D.; Cairo, G.; Magni, P.; Fargion, S.; et al. Dietary iron overload induces visceral adipose tissue insulin resistance. Am. J. Pathol. 2013, 182, 2254–2263. [Google Scholar] [CrossRef]
- Eshak, E.S.; Iso, H.; Maruyama, K.; Muraki, I.; Tamakoshi, A. Associations between dietary intakes of iron, copper and zinc with risk of type 2 diabetes mellitus: A large population-based prospective cohort study. Clin. Nutr. 2018, 37, 667–674. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Hernell, O. Iron, zinc, copper and selenium status of breast-fed infants and infants fed trace element fortified milk-based infant formula. Acta Paediatr. 1994, 83, 367–373. [Google Scholar] [CrossRef]
- Sandström, B.; Davidsson, L.; Cederblad, Å.; Lönnerdal, B. Oral iron, dietary ligands and zinc absorption. J. Nutr. 1985, 115, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Rong, Y.; Rong, S.; Liu, L. Dietary iron intake, body iron stores, and the risk of type 2 diabetes: A systematic review and meta-analysis. BMC Med. 2012, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Wang, F.; Lu, X.-T.; Zhong, R.-H.; Long, J.-A.; Fang, A.-P.; Zhu, H.-L. Dietary iron intake and the risk of type 2 diabetes mellitus in middle-aged and older adults in urban China: A prospective cohort study. Br. J. Nutr. 2021, 126, 1091–1099. [Google Scholar] [CrossRef]
- Costacou, T.; Mayer-Davis, E. Nutrition and prevention of type 2 diabetes. Annu. Rev. Nutr. 2003, 23, 147–170. [Google Scholar] [CrossRef]
- Hu, T.; Jacobs, D.R.; Sinaiko, A.R.; Bazzano, L.A.; Burns, T.L.; Daniels, S.R.; Dwyer, T.; Hutri-Kähönen, N.; Juonala, M.; Murdy, K.A.; et al. Childhood BMI and Fasting Glucose and Insulin Predict Adult Type 2 Diabetes: The International Childhood Cardiovascular Cohort (i3C) Consortium. Diabetes Care 2020, 43, 2821–2829. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.; Bi, S.; Zhou, L.; Li, L.; He, Z. Association between minerals intake and childhood obesity: A cross-sectional study of the NHANES database in 2007-2014. PLoS ONE 2023, 18, e0295765. [Google Scholar] [CrossRef]
- González-Domínguez, Á.; Visiedo-García, F.M.; Domínguez-Riscart, J.; González-Domínguez, R.; Mateos, R.M.; Lechuga-Sancho, A.M. Iron Metabolism in Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 5529. [Google Scholar] [CrossRef]
- Yarizadeh, H.; Setayesh, L.; Majidi, N.; Rasaei, N.; Mehranfar, S.; Ebrahimi, R.; Casazzza, K.; Mirzaei, K. Nutrient patterns and their relation to obesity and metabolic syndrome in Iranian overweight and obese adult women. Eat. Weight Disord. Stud. Anorex. Bulim. Obes. 2022, 27, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mao, Z.; Li, Y.; Wu, W.; Zhang, X.; Huo, W.; Yu, S.; Shen, L.; Li, L.; Tu, R.; et al. Cohort Profile: The Henan Rural Cohort: A prospective study of chronic non-communicable diseases. Leuk. Res. 2019, 48, 1756–1756j. [Google Scholar] [CrossRef] [PubMed]
- Monsen, E.R.; Hallberg, L.; Layrisse, M.; Hegsted, D.M.; Cook, J.D.; Mertz, W.; Finch, C. Estimation of available dietary iron. Am. J. Clin. Nutr. 1978, 31, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustmentfor total energyintakein epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef]
- Xue, Y.; Yang, K.; Wang, B.; Liu, C.; Mao, Z.; Yu, S.; Li, X.; Wang, Y.; Sun, H.; Wang, C.; et al. Reproducibility and validity of an FFQ in the Henan Rural Cohort Study. Public Health Nutr. 2020, 23, 34–40. [Google Scholar] [CrossRef]
- Hou, J.; Liu, X.; Tu, R.; Dong, X.; Zhai, Z.; Mao, Z.; Huo, W.; Chen, G.; Xiang, H.; Guo, Y.; et al. Long-term exposure to ambient air pollution attenuated the association of physical activity with metabolic syndrome in rural Chinese adults: A cross-sectional study. Environ. Int. 2020, 136, 105459. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2013, 36 (Suppl. S1), S67–S74. [Google Scholar] [CrossRef]
- Liu, J.; Shang, X.; Chen, Y.; Tang, W.; Yusufu, M.; Chen, Z.; Chen, R.; Hu, W.; Jan, C.; Li, L.; et al. Diet-Wide Association Study for the Incidence of Type 2 Diabetes Mellitus in Community-Dwelling Adults Using the UK Biobank Data. Nutrients 2023, 16, 103. [Google Scholar] [CrossRef]
- Shahinfar, H.; Jayedi, A.; Shab-Bidar, S. Dietary iron intake and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur. J. Nutr. 2022, 61, 2279–2296. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, S.; Liu, G.; Yan, F.; Ma, X.; Huang, Z.; Tian, H. Body iron stores and heme-iron intake in relation to risk of type 2 diabetes: A systematic review and meta-analysis. PLoS ONE 2012, 7, e41641. [Google Scholar] [CrossRef]
- Cui, Z.; Zhou, H.; Liu, K.; Wu, M.; Li, S.; Meng, S.; Meng, H. Dietary Copper and Selenium Intakes and the Risk of Type 2 Diabetes Mellitus: Findings from the China Health and Nutrition Survey. Nutrients 2022, 14, 2055. [Google Scholar] [CrossRef]
- Drake, I.; Hindy, G.; Ericson, U.; Orho-Melander, M. A prospective study of dietary and supplemental zinc intake and risk of type 2 diabetes depending on genetic variation in SLC30a8. Genes Nutr. 2017, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Yuan, B.; Qi, L.; Dai, Y.; Zuo, H.; Zhou, M. Zinc intake and the risk of hyperglycemia among Chinese adults: The prospective Jiangsu Nutrition Study (JIN). J. Nutr. Health Aging 2010, 14, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; van Dam, R.M.; Willett, W.C.; Hu, F.B. Prospective study of zinc intake and risk of type 2 diabetes in women. Diabetes Care 2009, 32, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Vashum, K.P.; McEvoy, M.; Shi, Z.; Milton, A.H.; Islam, R.; Sibbritt, D.; Patterson, A.; Byles, J.; Loxton, D.; Attia, J. Is dietary zinc protective for type 2 diabetes? Results from the Australian longitudinal study on women’s health. BMC Endocr. Disord. 2013, 13, 40. [Google Scholar] [CrossRef]
- de Oliveira Otto, M.C.; Alonso, A.; Lee, D.-H.; Delclos, G.L.; Bertoni, A.G.; Jiang, R.; Lima, J.A.; Symanski, E.; Jacobs, D.R.; Nettleton, J.A. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. J. Nutr. 2012, 142, 526–533. [Google Scholar] [CrossRef]
- Al-Shaar, L.; Satija, A.; Wang, D.D.; Rimm, E.B.; A Smith-Warner, S.; Stampfer, M.J.; Hu, F.B.; Willett, W.C. Red meat intake and risk of coronary heart disease among US men: Prospective cohort study. BMJ 2020, 371, m4141. [Google Scholar] [CrossRef]
- Hu, B.; He, X.; Sun, H.; Hu, Y.; Li, F.; Sun, Y.; Sun, J.; Feng, L. Red and processed meat intake and risk of cardiovascular disease: A two-sample Mendelian randomization study. Clin. Nutr. ESPEN 2024, 60, 289–297. [Google Scholar] [CrossRef]
- Shi, W.; Huang, X.; Schooling, C.M.; Zhao, J.V. Red meat consumption, cardiovascular diseases, and diabetes: A systematic review and meta-analysis. Eur. Heart J. 2023, 44, 2626–2635. [Google Scholar] [CrossRef]
- Zhong, V.W.; Van Horn, L.; Greenland, P.; Carnethon, M.R.; Ning, H.; Wilkins, J.T.; Lloyd-Jones, D.M.; Allen, N.B. Associations of Processed Meat, Unprocessed Red Meat, Poultry, or Fish Intake with Incident Cardiovascular Disease and All-Cause Mortality. JAMA Intern. Med. 2020, 180, 503–512. [Google Scholar] [CrossRef]
- Masad, A.; Hayes, L.; Tabner, B.J.; Turnbull, S.; Cooper, L.J.; Fullwood, N.J.; German, M.J.; Kametani, F.; El-Agnaf, O.M.; Allsop, D. Copper-mediated formation of hydrogen peroxide from the amylin peptide: A novel mechanism for degeneration of islet cells in type-2 diabetes mellitus? FEBS Lett. 2007, 581, 3489–3493. [Google Scholar] [CrossRef] [PubMed]
- Ihara, Y.; Toyokuni, S.; Uchida, K.; Odaka, H.; Tanaka, T.; Ikeda, H.; Hiai, H.; Seino, Y.; Yamada, Y. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes 1999, 48, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Li, X.; Zhang, X.; Shi, H.; Vos, M.B.; Wei, X.; Wang, Y.; Gao, H.; Rouchka, E.C.; Yin, X.; et al. Dietary copper-fructose interactions alter gut microbial activity in male rats. Am. J. Physiol. Liver Physiol. 2018, 314, G119–G130. [Google Scholar] [CrossRef] [PubMed]
- Puig, S.; Thiele, D.J. Molecular mechanisms of copper uptake and distribution. Curr. Opin. Chem. Biol. 2002, 6, 171–180. [Google Scholar] [CrossRef]
- Domellöf, M.; Dewey, K.G.; Cohen, R.J.; Lönnerdal, B.; Hernell, O. Iron supplements reduce erythrocyte copper-zinc superoxide dismutase activity in term, breastfed infants. Acta Paediatr. 2005, 94, 1578–1582. [Google Scholar] [CrossRef]

| Characteristics | Healthy Participants (N = 34,768) | Diabetic Subjects (N = 3616) | Total (N = 38,384) | p |
|---|---|---|---|---|
| Mean ± SD or n (%) | Mean ± SD or n (%) | Mean ± SD or n (%) | ||
| Gender (%) | 0.047 | |||
| Male | 13,776 (39.6) | 1371 (37.9) | 15,147 (39.5) | |
| Female | 20,992 (60.4) | 2245 (62.1) | 23,237 (60.5) | |
| Culture (%) | <0.01 | |||
| Primary school or below | 15,189 (43.7) | 2000 (55.3) | 17,189 (44.8) | |
| Junior high school | 14,107 (40.6) | 1196 (33.1) | 15,303 (39.9) | |
| Senior high school or below | 5472 (15.7) | 420 (11.6) | 5892 (15.3) | |
| Marital status (%) | 0.009 | |||
| Married/cohabiting | 31,271 (89.9) | 3202 (88.6) | 34,473 (89.8) | |
| Single/divorced/widowed | 3497 (10.1) | 414 (11.4) | 3911 (10.2) | |
| Per capita monthly income (%) | <0.01 | |||
| <500 RMB | 12,234 (35.2) | 1424 (39.4) | 13,658 (35.6) | |
| 500–1000 RMB | 11,494 (33.0) | 1160 (32.1) | 12,654 (33.0) | |
| >1000 RMB | 11,040 (31.8) | 1032 (28.5) | 12,072 (31.4) | |
| Exercise (%) | <0.01 | |||
| Low | 10,946 (31.5) | 1411 (39.1) | 12,357 (32.2) | |
| Moderate | 13,235 (38.0) | 1285 (35.5) | 14,520 (37.8) | |
| High | 10,587 (30.5) | 920 (25.4) | 11,507 (30.0) | |
| Smoking (%) | <0.01 | |||
| Never | 25,218 (72.5) | 2730 (75.5) | 27,948 (72.8) | |
| Former or current smoking | 9550 (27.5) | 886 (24.5) | 10,436 (27.2) | |
| Drinking (%) | 0.009 | |||
| Never | 26,809 (77.1) | 2858 (79.0) | 29,667 (77.3) | |
| Former or current drinking | 7959 (22.9) | 758 (21.0) | 8717 (22.7) | |
| Hypertension (%) | <0.01 | |||
| No | 24,063 (69.2) | 1765 (48.8) | 25,828 (67.3) | |
| Yes | 10,674 (30.7) | 1849 (51.2) | 12,523 (32.6) | |
| Family history of T2DM (%) | <0.01 | |||
| No | 33,530 (96.4) | 3259 (90.1) | 36,789 (95.8) | |
| Yes | 1238 (3.6) | 357 (9.9) | 1595 (4.2) | |
| Age (years) | 55.06 ± 12.35 | 60.36 ± 9.27 | 55.56 ± 12.19 | <0.01 |
| Iron (mg/day) | 32.05 ± 5.02 | 31.91 ± 5.05 | 32.04 ± 5.02 | 0.104 |
| Copper (mg/day) | 2.62 ± 0.53 | 2.55 ± 0.49 | 2.61 ± 0.53 | <0.01 |
| Zinc (mg/day) | 9.50 ± 1.14 | 9.46 ± 1.13 | 9.49 ± 1.14 | 0.090 |
| Heme iron (mg/day) | 0.99 ± 0.96 | 0.88 ± 0.92 | 0.98 ± 0.95 | <0.01 |
| Non-heme iron (mg/day) | 31.06 ± 4.79 | 31.03 ± 4.84 | 31.06 ± 4.79 | 0.713 |
| BMI (kg/m2) | 24.70 ± 3.52 | 26.19 ± 3.70 | 24.84 ± 3.57 | <0.01 |
| Total energy (kcal/day) | 1802.80 ± 540.56 | 1744.63 ± 547.89 | 1797.32 ± 541.51 | <0.01 |
| Carbohydrate (g/day) | 317.58 ± 30.76 | 317.43 ± 30.13 | 317.56 ± 30.70 | 0.783 |
| Cereal fiber (g/day) | 6.42 ± 2.02 | 6.57 ± 2.11 | 6.43 ± 2.03 | <0.01 |
| Cholesterol (mg/day) | 436.25 ± 289.77 | 422.98 ± 295.04 | 435.00 ± 290.29 | 0.009 |
| Protein (g/day) | 69.49 ± 7.77 | 69.54 ± 3.70 | 69.50 ± 7.77 | 0.727 |
| Iron | Q1 | Q2 | Q3 | Q4 | p-Trend |
|---|---|---|---|---|---|
| Model 1 | 1.00 (ref) | 1.01 (0.92–1.11) | 0.96 (0.87–1.06) | 0.99 (0.90–1.09) | 0.694 |
| Model 2 | 1.00 (ref) | 1.03 (0.93–1.14) | 1.02 (0.92–1.13) | 1.09 (0.99–1.21) | 0.111 |
| Model 3 | 1.00 (ref) | 0.91 (0.82–1.01) | 0.85 (0.76–0.95) | 0.81 (0.70–0.92) | 0.002 |
| Copper | |||||
| Model 1 | 1.00 (ref) | 0.98 (0.89–1.08) | 1.00 (0.90–1.10) | 0.80 (0.72–0.89) | <0.01 |
| Model 2 | 1.00 (ref) | 0.97 (0.88–1.07) | 1.00 (0.91–1.10) | 0.81 (0.73–0.90) | <0.01 |
| Model 3 | 1.00 (ref) | 0.85 (0.77–0.94) | 0.83 (0.75–0.93) | 0.64 (0.57–0.72) | <0.01 |
| Zinc | |||||
| Model 1 | 1.00 (ref) | 1.05 (0.95–1.16) | 1.08 (0.98–1.19) | 1.11 (1.01–1.23) | 0.036 |
| Model 2 | 1.00 (ref) | 1.07 (0.97–1.18) | 1.10 (1.00–1.22) | 1.15 (1.04–1.27) | 0.008 |
| Model 3 | 1.00 (ref) | 0.89 (0.80–1.00) | 0.81 (0.71–0.92) | 0.65 (0.55–0.78) | <0.01 |
| Heme iron | |||||
| Model 1 | 1.00 (ref) | 0.92 (0.83–1.01) | 0.97 (0.88–1.07) | 0.94 (0.85–1.04) | 0.499 |
| Model 2 | 1.00 (ref) | 0.94 (0.86–1.04) | 1.01 (0.91–1.11) | 0.99 (0.89–1.10) | 0.760 |
| Model 3 | 1.00 (ref) | 0.90 (0.82–1.00) | 0.88 (0.79–0.98) | 0.69 (0.60–0.80) | <0.01 |
| Non-heme iron | |||||
| Model 1 | 1.00 (ref) | 0.98 (0.89–1.08) | 0.95 (0.86–1.05) | 0.99 (0.90–1.09) | 0.764 |
| Model 2 | 1.00 (ref) | 1.01 (0.91–1.11) | 1.01 (0.92–1.12) | 1.08 (0.98–1.20) | 0.114 |
| Model 3 | 1.00 (ref) | 0.89 (0.80–0.99) | 0.84 (0.75–0.94) | 0.79 (0.70–0.90) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Shi, B.; Li, H.; Li, Y.; Mao, Z.; Wang, C.; Hou, J.; Tian, Y.; Li, L. BMI as a Mediator in the Relationship Between Dietary Trace Elements and Type 2 Diabetes Mellitus: Findings from a Rural Cohort. Nutrients 2025, 17, 2875. https://doi.org/10.3390/nu17172875
Wang J, Shi B, Li H, Li Y, Mao Z, Wang C, Hou J, Tian Y, Li L. BMI as a Mediator in the Relationship Between Dietary Trace Elements and Type 2 Diabetes Mellitus: Findings from a Rural Cohort. Nutrients. 2025; 17(17):2875. https://doi.org/10.3390/nu17172875
Chicago/Turabian StyleWang, Jianwei, Biwen Shi, Haiyang Li, Yuqian Li, Zhenxing Mao, Chongjian Wang, Jian Hou, Yuan Tian, and Linlin Li. 2025. "BMI as a Mediator in the Relationship Between Dietary Trace Elements and Type 2 Diabetes Mellitus: Findings from a Rural Cohort" Nutrients 17, no. 17: 2875. https://doi.org/10.3390/nu17172875
APA StyleWang, J., Shi, B., Li, H., Li, Y., Mao, Z., Wang, C., Hou, J., Tian, Y., & Li, L. (2025). BMI as a Mediator in the Relationship Between Dietary Trace Elements and Type 2 Diabetes Mellitus: Findings from a Rural Cohort. Nutrients, 17(17), 2875. https://doi.org/10.3390/nu17172875







