Beyond GLP-1 Agonists: An Adaptive Ketogenic–Mediterranean Protocol to Counter Metabolic Adaptation in Obesity Management
Simple Summary
Abstract
1. Introduction
1.1. Contextualizing the Obesity Problem: Historical and Current Trends
1.2. Public Health Consequences
1.3. Main Contributing Factors to the Obesity Epidemic
1.3.1. The Diet–Environment Axis: The Nutrition Transition and the Obesogenic Environment
1.3.2. The Neurobiological Component: Glycemic Load and Compulsive Eating Behavior
1.3.3. Sedentary Behavior and Individual Susceptibility Factors
1.4. International Efforts to Curb the Obesity Trend
- Sugar-sweetened beverage taxes: Multiple systematic reviews confirm their effectiveness, with consumption reductions ranging from 10% to 30% across various European contexts [46]. The case of Mexico—global pioneer since 2014—is emblematic. An observational analysis documented a 7.6% reduction in sugary beverage purchases during the first two years of implementation, with an even greater impact among low-income households, demonstrating the pro-equity potential of the measure [47].
- Front-of-pack labelling (FOPL): Mandatory implementation shows modest but insufficient progress. The latest WHO report for the European Region (2022) indicates that one-third (33%) of countries have already established regulations for interpretive FOPL, an increase from the 27% reported in 2020. This system is critical to guide consumer choices, especially among lower socioeconomic groups [48].
- Child-directed advertising restrictions: Evidence shows that marketing doubles the likelihood of unhealthy food choices in children [23]. A key case study is the province of Quebec, where a ban on child-targeted advertising since 1980 has been associated with more favorable dietary habits and better child health outcomes compared to the rest of Canada [49].
- Comprehensive school-based policies: A meta-analysis of 85 randomized controlled trials demonstrated that school-based lifestyle interventions lead to modest but statistically significant reductions in BMI (approximately 0.22 kg/m2). Parental involvement and extracurricular activities further strengthen these effects [50].
1.5. Surge of Interest in the Ketogenic Diet as a Therapeutic Alternative
1.6. State of the Art: Challenges in Obesity and the Evidence on Ketogenic Mediterranean Diets
1.6.1. Initial Evidence and Pilot Studies
1.6.2. Medium- to Long-Term Interventions
1.6.3. Recent Controlled Trials
1.6.4. Adherence and Feasibility
1.6.5. Emerging Clinical Applications
1.7. The Pharmacological Paradigm: Revolutionary Efficacy and Its Fundamental Limitations
- The first is therapeutic dependence: their effect ceases upon discontinuation, leading to rapid weight regain of most of the lost weight. Data from the STEP-1 Extension trial revealed that after stopping semaglutide, participants regained 11.6 percentage points of the 17.3% initially lost within just 52 weeks, retaining only a 5.6% net weight loss [80].
- The second limitation—more critical from a physiological standpoint—is its inability to address the biological root of weight regain: the metabolic adaptation described in Section 1.6. The weight loss induced by these drugs is accompanied by the expected reduction in resting energy expenditure (REE), without offering any thermogenic advantage to counterbalance it. Van Eyk et al. demonstrated that liraglutide 1.8 mg/day over 26 weeks reduced resting energy expenditure by 52 kcal/day, which is comparable to the adaptive response seen with conventional caloric restriction [81].
- The third limitation—particularly concerning from a body composition perspective—is the suboptimal quality of weight loss. In a DXA substudy of adults receiving semaglutide (STEP 1 trial), 39% of the total weight reduction was lean mass [82], compromising basal metabolism by an additional 90–105 kcal/day. This disproportionate loss of metabolically active tissue not only exacerbates metabolic adaptation but also increases the risk of sarcopenia and functional decline, calling into question the long-term metabolic sustainability of this approach.
1.8. A Proposal for Metabolic Recalibration: The Adaptive Ketogenic–Mediterranean Protocol (AKMP)
1.9. Central Hypothesis and Objectives of the Review
- To formalize and justify the components of the Adaptive Ketogenic–Mediterranean Protocol (AKMP), evaluating the originality of its dynamic biomarker-driven adjustment system as an innovation in the design of personalized dietary interventions.
- To conduct a comprehensive comparative analysis between the dietary-metabolic approach of the AKMP and the pharmacological paradigm of GLP-1/GIP receptor agonists, focusing on their differential effects on energy expenditure, post-treatment sustainability, and the mechanisms of weight regain [79,80,83,89].
2. Materials and Methods
2.1. Search Strategy
- Structured evidence-mapping search: aimed at identifying and analyzing all published Ketogenic Mediterranean Diet (KMD) protocols. The following search terms and combinations were used: “ketogenic Mediterranean diet”, “Mediterranean-style ketogenic diet”, “Mediterranean ketogenic diet”, “modified ketogenic Mediterranean diet”, “ketosis and Mediterranean nutrition”, “Mediterranean diet for ketosis”, “Mediterranean ketogenic protocol”, and “ketogenic low-carbohydrate Mediterranean diet”. The search was restricted to human studies.
- Expert narrative search: To construct the contextual, mechanistic, and clinical framework, a broad and ongoing search was carried out using key terms related to the central themes of the review. Main concepts included, among others: “adaptive thermogenesis”, “metabolic adaptation”, “weight loss plateau”, “appetite regulation”, “satiety hormones”, “ghrelin”, “leptin”, “GLP-1 receptor agonists”, “incretin mimetics”, “semaglutide”, “tirzepatide”, “obesity”, “metabolic syndrome”, “MASLD”, and “PCOS”, often combined with “ketogenic diet” or “low-carbohydrate diet”. For this component, priority was given to the selection of systematic reviews, meta-analyses, high-impact clinical trials, and seminal publications.
2.2. Inclusion and Exclusion Criteria
2.2.1. Criteria for the Focused Systematic Search (KMD Protocols)
- Type of intervention: studies that described a dietary protocol explicitly defined as “ketogenic” AND “Mediterranean.”
- Publication type: peer-reviewed articles—randomised or non-randomised clinical trials, pilot studies, case series, case reports—and systematic or narrative reviews that describe an original KMD protocol in detail.
- Population: human studies with no restrictions on age, sex, or health status
- Language: articles published in English.
- In vitro studies or those using animal models.
- Articles describing non-Mediterranean ketogenic diets, or low-carbohydrate Mediterranean diets that were not explicitly ketogenic.
- Studies that mentioned the Ketogenic Mediterranean Diet but did not provide details regarding macronutrient composition, permitted or restricted foods, or protocol phases.
- Editorials, letters to the editor, conference abstracts, or any publication lacking sufficient data for protocol analysis.
2.2.2. Evidence Selection Criteria for the Narrative Component
- Thematic Relevance: Articles were included if they directly addressed key concepts of the review: mechanisms of appetite and satiety, adaptive thermogenesis, metabolic syndrome and its comorbidities (MASLD, PCOS), and the pharmacology of GLP-1 receptor agonists.
- Hierarchy of Evidence: Priority was given to the inclusion of meta-analyses and systematic reviews to establish general knowledge. For specific issues, high-impact randomized clinical trials and fundamental mechanistic studies were selected.
- Impact and Recency: Both seminal articles that established conceptual foundations and recent publications in high-impact journals were included to ensure the discussion reflects the current state of the art.
- No exclusion criteria were applied based on the direction or statistical significance of reported results, in order to provide a balanced view of the evidence.
2.3. Study Selection Process
- Title and Abstract Screening: All identified references were imported into Mendeley Desktop version 1.19.5 (Elsevier) reference management software for automatic duplicate removal. Titles and abstracts of the remaining articles were then screened to assess their initial relevance against the inclusion criteria.
- Full-Text Review: Articles deemed potentially relevant in the first phase were retrieved for full-text review. At this stage, the inclusion and exclusion criteria were strictly applied to determine the final selection of studies for analysis.
2.4. Analysis and Synthesis of the Evidence
- For studies included in the systematic analysis of (KMD) protocols, qualitative data were extracted—macronutrient ratios, phases, permitted and restricted foods and duration—and summarized in a comparative characteristics table to identify commonalities, variations, and research gaps. Key information from each protocol—including macronutrient ratio, phases, recommended and restricted foods, and duration—was extracted and organized into a characteristics table for comparative analysis, with the goal of identifying common elements, variations, and gaps in literature. Full study-level details of all identified KMD protocols are provided in Supplementary Table S1 (design, population, duration, outcomes, and ketosis-verification methods).
- For the evidence gathered from the expert narrative review, the information was grouped and synthesized thematically. The findings were structured around pathophysiological mechanisms, clinical applications, and comparative therapeutic approaches, to underpin the central argument developed in the Results and Discussion.
2.5. Reporting Framework and Transparency
3. Results and Discussion
3.1. Evidence and Challenges of the Ketogenic Diet as a Baseline Intervention: Ketogenic Variants
- Definitional heterogeneity: lack of consensus on what exactly constitutes a “Ketogenic Mediterranean Diet.”
- Nutritional paradox: tension between carbohydrate restriction and traditional Mediterranean foods.
- Absence of adherence biomarkers: no standardized metrics to evaluate compliance.
- Lack of metabolic-adaptation management: No protocol accounts for adaptive thermogenesis.
- Lack of dynamic personalization based on metabolic response: while some protocols allow flexibility in quantity (ad libitum) or timing (peri-exercise), none implement systematic adjustments based on the detection and management of metabolic adaptation or weight plateaus.
3.2. Safety Profile: Debunking Historical Myths
3.2.1. The Unequivocal Physiological Distinction: Ketosis vs. Ketoacidosis
3.2.2. The Cardiovascular Paradigm: Beyond LDL Cholesterol
3.2.3. Renal Function: Evidence Against the “High-Protein” Fallacy
3.2.4. Body Composition and Bone Health: Preservation of Lean Tissue
3.2.5. Essential Considerations and Contraindications
3.3. Barriers to Long-Term Adherence
3.3.1. Early Physiological Barriers
3.3.2. Psychological and Behavioral Barriers
3.3.3. Sociocultural Barriers
3.3.4. Knowledge and Support Barriers
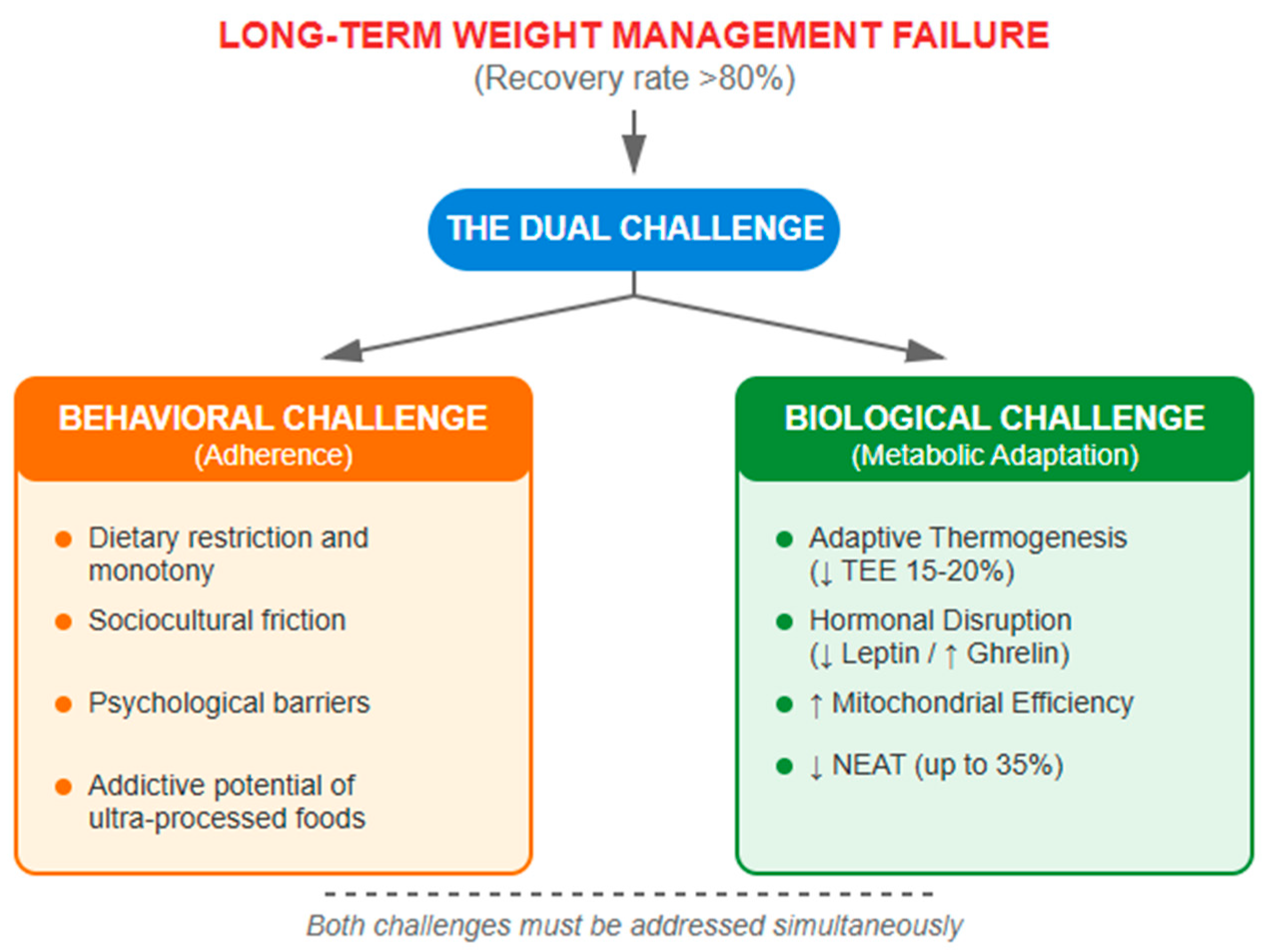
4. Metabolic Adaptation: The Central Challenge in Weight Management
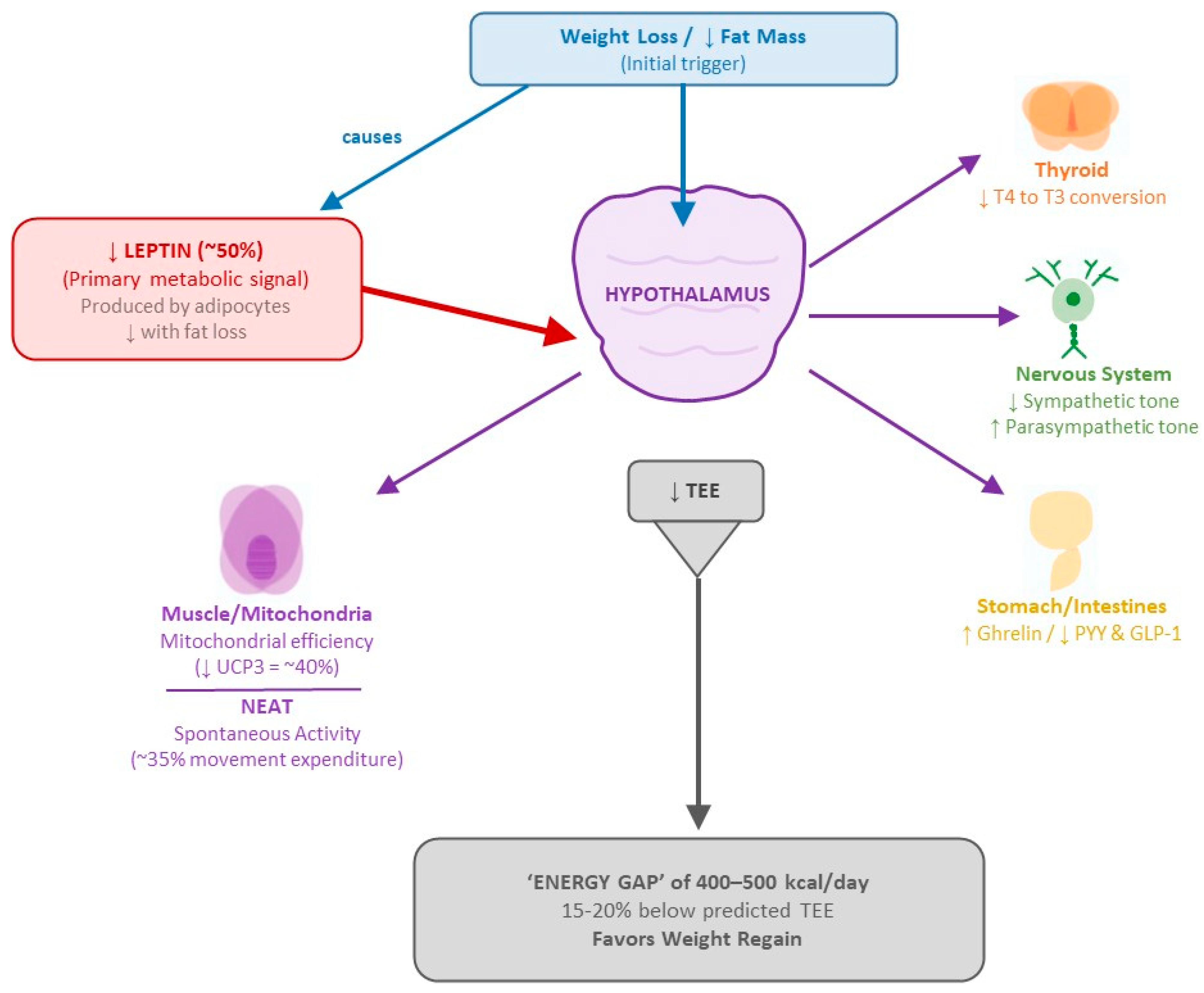
4.1. Mechanisms of Post-Weight Loss Metabolic Adaptation
4.2. Adaptive Thermogenesis: Magnitude and Persistence
- Leptin System: Leptin acts as the primary “metabolic conductor,” signaling the central nervous system about the status of energy stores. During weight loss, leptin levels drop disproportionately—a 10% reduction in fat mass can result in a 50% decrease in circulating leptin [64]. This relative hypoleptinemia triggers a systemic response that includes reduced energy expenditure, increased appetite, and neuroendocrine changes that favor weight regain.
- Thyroid Axis: Weight loss induces significant changes in thyroid hormones. T3 levels drop from 112.7 ± 3.1 to 101.8 ± 2.6 ng/dL (p < 0.001) with no significant changes in TSH or free T4 [138]. Moreover, reverse T3 increases, while peripheral conversion of T4 to active T3 decreases, creating a state of “functional hypothyroidism” that may persist for up to 12 months post-weight loss [139].
- Autonomic Nervous System: Studies using sequential pharmacological blockade have shown a 20–40% reduction in sympathetic activity and a concomitant increase in parasympathetic tone following weight loss [140]. These shifts in autonomic balance contribute to the decrease in basal energy expenditure and lipolysis suppression.
- Gut–Brain Axis: The gastrointestinal hormone profile is profoundly altered. Ghrelin, the orexigenic hormone mainly predominantly secreted in the stomach, increases significantly following diet-induced weight loss and remains elevated for at least 12 months, and independently predicts subsequent weight regain [65]. Conversely, anorexigenic peptides such as PYY and GLP-1 decrease, creating a hormonal environment that promotes persistent hunger and reduces satiety [64].
- Basal Metabolism: Reduced by 15–20% beyond expectations based on body composition changes [141].
- Mitochondrial Efficiency: Increased by 15–25% in ATP production per unit of oxygen consumed, mediated by up to a 40% reduction in uncoupling proteins (especially UCP3) [66].
- Activity-Related Thermogenesis: Non-exercise activity thermogenesis (NEAT) may decreases by up to 35%, while muscular efficiency during exercise increases permanently, requiring less energy to perform the same physical work [68].
5. Current Therapeutic Paradigms: Critical Analysis
5.1. GLP-1/GIP Agonists: A Revolution Centered on Appetite Suppression
5.2. Limitations: Therapeutic Dependence and Metabolic Neutrality
5.2.1. Therapeutic Dependence
5.2.2. The Metabolic Cost: Loss of Lean Mass
5.2.3. Metabolic Neutrality: No Activation of Energy Expenditure
5.3. The Need for Metabolically Active Approaches
6. The Metabolic Advantage of Ketosis: Mechanistic Evidence
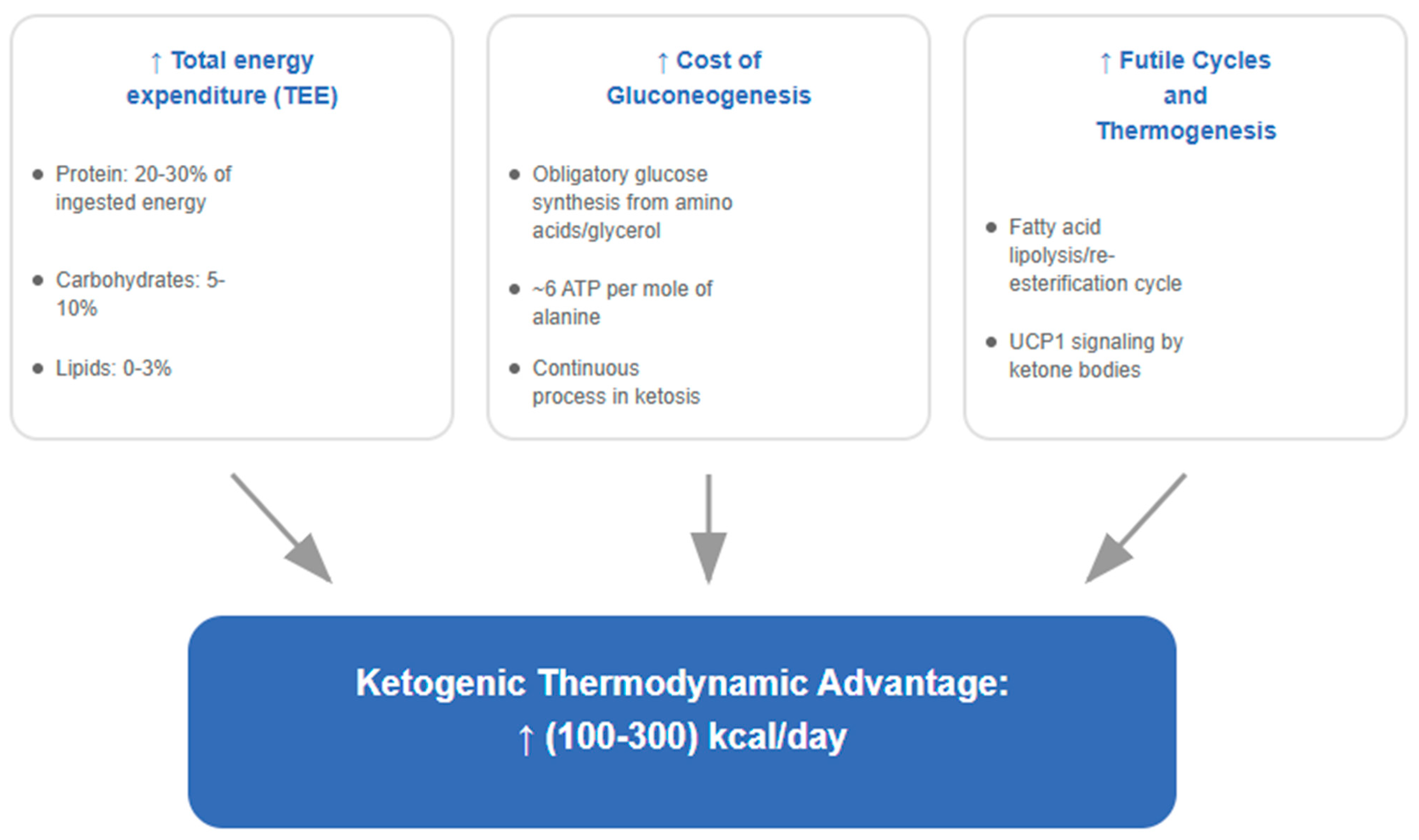
6.1. Increased Energy Expenditure: Quantitative Analysis
6.2. Biochemical Mechanisms: Futile Cycles and Thermogenesis
- Increased Thermic Effect of Food (TEF): Well-formulated ketogenic diets, being adequate in protein, exhibit a higher TEF. Protein metabolism consumes 20–30% of its own energy content, compared to 5–10% for carbohydrates and 0–3% for fats [157].
- Energetic Cost of Gluconeogenesis (GNG): The obligatory synthesis of glucose from precursors such as amino acids and glycerol is energy-intensive, requiring approximately 6 moles of ATP per mole of glucose synthesized from alanine [158], contributing continuously to daily energy expenditure.
- Futile Cycles and Ketogenic Thermogenesis: Ketosis enhances several futile cycles that dissipate energy as heat. The most relevant is the lipolysis/re-esterification cycle, in which fatty acids are released from adipose tissue and a significant portion is re-esterified back into triglycerides—a process with a net ATP cost [159]. Moreover, ketone bodies, beyond serving as fuels, act as signaling molecules through HDAC inhibition [160], which may modulate the expression of thermogenic genes such as UCP1 in brown adipose tissue (BAT), as demonstrated in murine models [161]; translation to functional brown adipose tissue in adult humans has yet to be established.
7. Toward an Integrated Solution: The Adaptative Ketogenic–Mediterranean Protocol (AKMP)
7.1. Evidence-Mapping of Ketogenic Mediterranean Diet Protocols (KMDs)
7.2. Gaps Identified in the Literature: Opportunities for a New Generation of Protocols
- the individual’s life context (including dietary preferences, schedules, and culture), which is key to long-term sustainability; and
- the enormous interindividual variability in metabolic response to ketosis, as reflected in weight loss trajectories and lipid changes—a well-documented phenomenon [162].
7.3. Design of the Adaptive Ketogenic–Mediterranean Protocol (AKMP): Principles and Phases
7.3.1. Foundational Principles
- Principle 1: Optimized Ketogenic–Mediterranean Synergy.The protocol uses the metabolic potency of nutritional ketosis with the cardioprotective profile of the Mediterranean pattern. This integration includes extra virgin olive oil (30–50 mL/day) as the main lipid source, oily fish (≥4 servings/week) to optimize the omega-6:omega-3 ratio (~4:1)—a proportion shown to yield significant benefits in secondary cardiovascular prevention [165]—and the maximum allowable quantity of vegetables, limited to a daily cap of 20 g of net carbohydrates.Paoli et al. have demonstrated that converging the ketogenic and Mediterranean patterns improves both adherence and cardiometabolic risk profile [166]. In an independent study, Paoli’s biphasic protocol achieved sustained weight loss of 16.9 ± 7.1 kg with no regain during 6 months of Mediterranean maintenance, and an adherence rate of 88.2% [59].
- Principle 2: Systematic Exploitation of the Metabolic Advantage.The AKMP is designed to maximize the increase in energy expenditure documented under nutritional ketosis. Fine and Feinman proposed that the “metabolic advantage” of low-carbohydrate diets reflects real differences in metabolic pathway efficiency, without violating the laws of thermodynamics [85,158].This advantage—typically in the ≈100–300 kcal/day range and context-dependent, as detailed in Section 6—derives from multiple mechanisms, including the high thermic effect of protein, ATP-dependent futile cycles, and the energetic cost of gluconeogenesis. The Framingham State Food Study confirmed that participants on a low-carbohydrate diet experienced a ~210 kcal/day increase in total energy expenditure compared to a high-carbohydrate diet [83]. The meta-analysis by Ludwig et al. showed an increase of 50.4 kcal/day for every 10% carbohydrate reduction in studies lasting more than 2.5 weeks [84].
- Principle 3: Dynamic Anti-Stall Personalization.Unlike all previously published protocols, the AKMP implements a system of continuous monitoring and proactive adjustments. Weight plateaus, operationally defined here as a variation < 1% over 14 days with confirmed ketosis—based on the work of Heymsfield et al. [167], which demonstrated weight stability with a coefficient of variation < 0.5% over 10 days, extended to 14 days for greater clinical certainty—trigger personalized interventions:strategic protein increase (up to 1.6 g/kg ideal body weight) or modest caloric reduction (100–200 kcal/day), guided by clinical dialogue concerning satiety and individual patient preferences.
7.3.2. Temporal Architecture and Nutritional Principles of the Protocol
- Carbohydrates: ≤20 g net/day;
- Protein: 1.2–1.6 g/kg ideal body weight (20–30% of total energy intake);
- Fats: 60–70% of total energy intake;
- Hydration: ≥2 L/day.
- Extra virgin olive oil: 30–40 mL/day as the primary lipid source;
- Mediterranean oily fish: ≥4 servings/week;
- Non-starchy vegetables, customized per individual plan;
- Mediterranean nuts, within carbohydrate limits.
- Increased protein intake if persistent hunger is reported (up to 1.6 g/kg);
- Modest caloric reduction (100–200 kcal/day) if satiety is adequate (as detailed in Section 7.4).
- Priority given to non-starchy vegetables rich in phytochemicals:
- ○
- Cruciferous vegetables (sulforaphane);
- ○
- Leafy greens (lutein, zeaxanthin);
- ○
- Colorful vegetables, such as red and yellow peppers (β-carotene) and tomatoes (lycopene).
- Gradual introduction of berries: offering maximum antioxidant density with minimal glycaemic impact.
- Incorporation of legumes in controlled portions: a source of complex carbohydrates, plant-based protein, and bioactive compounds (e.g., saponins, phytates with antioxidant properties).
- Enhancement with Mediterranean spices:
- ○
- Turmeric (curcumin);
- ○
- Oregano (rosmarinic acid);
- ○
- Rosemary (carnosol).
- Maintenance of extra virgin olive oil intake: 40–50 mL/day.
- Monitoring of weight stabilization, taking into account the recovery of hepatic and muscle glycogen stores.
- Establishment of a modified Mediterranean dietary pattern, integrating the metabolic insights gained during the ketogenic phase.
7.3.3. Methodology for Energy and Macronutrient Personalization
- Men: REE = (10 × weight in kg) + (6.25 × height in cm) − (5 × age in years) + 5
- Women: REE = (10 × weight in kg) + (6.25 × height in cm) − (5 × age in years) − 161
- For participants with BMI < 40 kg/m2: actual body weight is used in the equation.
- For participants with BMI ≥ 40 kg/m2: an adjusted body weight is used to avoid REE overestimation due to excess fat mass. The formula applied is a standard in clinical dietetics [179]:
- ○
- Adjusted Weight = Ideal Weight + 0.25 × (Actual Weight − Ideal Weight)
- PAF 1.2: Sedentary lifestyle (corresponding to PAL 1.0–1.39);
- PAF 1.375: Light activity (corresponding to PAL 1.4–1.59);
- PAF 1.55: Moderate activity (corresponding to PAL 1.6–1.89);
- PAF 1.725: Intense or very active lifestyle (corresponding to PAL 1.9–2.5).
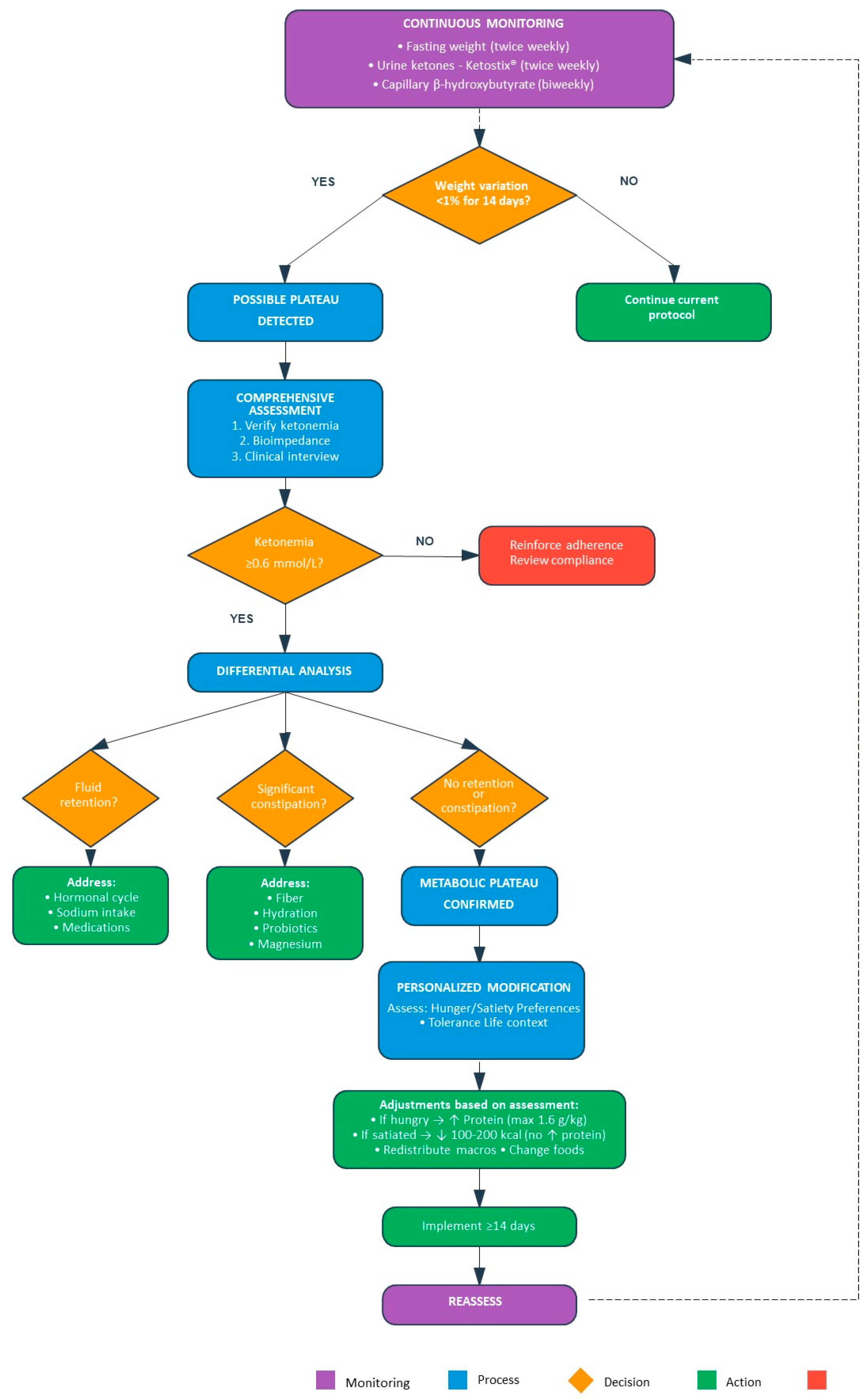
7.4. Dynamic Adjustment System to Optimize Weight Loss
- Systematic Monitoring:A stratified surveillance system is implemented. For high-frequency tracking, twice-weekly measurements of fasted body weight are combined with urine ketone testing using Ketostix® strips—a well-documented, practical, low-cost tool for adherence monitoring [181].
- In the AKMP, ketone measurements are obtained from the first morning urine sample to ensure standardization with fasted weight measurements and maximize patient convenience. Every two weeks, capillary ketonemia (β-hydroxybutyrate) is quantified, with nutritional ketosis defined as 0.6–3.0 mmol/L [166] (In formal research pilots (Section 8.3.2), biomarker frequency is temporarily intensified as per the study schedule.). Bioimpedance analysis (InBody 270) is used when hydration-related changes are suspected of masking actual fat loss [182]. Clinical-practice (pragmatic) versus research-grade monitoring. In routine care, we prioritize low-burden tools—fasted body weight (≥2×/week), weekly urine acetoacetate strips for adherence checks (with explicit acknowledgment of their limitations due to hydration and diurnal variation), and capillary β-hydroxybutyrate at least every two weeks or whenever an adjustment is considered. As cost-efficient “triggers,” consecutive negative urine strips or an abrupt weekly weight uptick (compatible with glycogen/water repletion) prompt an earlier in-clinic capillary β-OHB check. In research settings, gold-standard assessments include body composition by DXA (with operational BIA validated against DXA in a pilot), indirect calorimetry for REE, accelerometry for activity, and DLW for TEE in validation subsets.
- Plateau Detection:A weight plateau is operationally defined as <1% body weight variation over 14 consecutive days with confirmed nutritional ketosis. This 14-day period allows differentiation between normal daily fluctuations in body weight—typically 1–2 kg due to hydration status, glycogen stores, and gastrointestinal contents—and true stagnation in fat loss [183]. When a plateau is detected, an evaluation visit is scheduled to:
- ○
- Confirm adherence through ketonemia ≥ 0.6 mmol/L;
- ○
- Analyze body composition;
- ○
- Clinically assess the patient’s general status.
The 14-day plateau window is a pragmatic choice that balances day-to-day weight variability (fluid/glycogen/intestinal contents) with clinical feasibility; future testing will examine sensitivity to 10- versus 14- versus 21-day windows. - Adjustment Strategies:Upon confirmed plateau, one of two strategies is implemented based on clinical evaluation:
- ○
- Protein Adjustment:If the patient reports persistent hunger despite confirmed ketosis, protein intake is increased incrementally up to 1.6 g/kg ideal body weight. This strategy leverages protein’s higher thermic effect (20–30% of energy content versus 5–10% for carbohydrates and 0–3% for fats) [157], potentially helping to maintain total energy expenditure while enhancing satiety [184].
- ○
- Caloric Adjustment:If the patient reports adequate satiety, a modest caloric reduction of 100–200 kcal/day is introduced, primarily by reducing fat intake while preserving protein.
- Early plateau detection enables timely intervention before motivation and adherence deteriorate.
- Individualized response strategies are tailored to each patient’s specific metabolic response and subjective experience, rather than applying generic modifications.
7.5. Comparative Analysis: Positioning the AKMP Within Current Therapeutic Paradigms
- Adherence compromised by neurobiological factors, addressed through its culturally acceptable Mediterranean foundation;
- Inevitable metabolic adaptation, countered through the systematic exploitation of the ketogenic metabolic advantage (100–300 kcal/day).
7.6. Ethical and Safety Considerations for AKMP
8. General Discussion, Limitations, and Future Directions
8.1. Synthesis of the Central Argument: From Metabolic Adaptation to Adaptive Solution
- Integration of the Mediterranean patterns to enhance adherence and palatability;
- Exploitation of the metabolic advantage of ketosis;
- Personalized adjustments based on individual response to counteract metabolic adaptation.
8.2. The AKMP as a Proposal for Personalized Nutritional Medicine
8.3. Integration with Pharmacotherapy: Acknowledging Patient Diversity
- For patients with mild-to-moderate obesity and strong motivation: A well-calibrated, balanced diet supervised by a professional, combined with increased physical activity, may suffice as first-line therapy.
- For patients with capacity and motivation for exercise: Beginning with a structured physical activity program may be ideal, with dietary modifications added as needed.
- For motivated individuals without severe carbohydrate addiction: They may start directly with the AKMP, without requiring pharmacological support, benefiting from the natural appetite suppression induced by ketosis.
- For severe cases or patients with strong addiction to refined carbohydrates: Recognizing the neurobiological basis of this phenomenon [25], GLP-1 receptor agonists may constitute an essential first-line therapeutic tool, always combined with nutritional supervision to minimize metabolic adaptation and the loss of muscle mass characteristic of conventional hypocaloric diets.
8.3.1. Sequential Therapeutic Algorithms: AKMP-First (Algorithm 1) vs. Incretin-First Pathways (Algorithm 2)
| Algorithm 1. AKMP-first, step-up to incretin therapy if adherence threatens to fail |
| Indication: motivated patients willing to adopt structured menus and monitoring. Steps: (i) Initiate ketogenic–Mediterranean induction per AKMP Phase 1; (ii) maintain dynamic, biomarker-guided adjustments (protein up to 1.6 g/kg IBW if hunger; or −100–200 kcal/day if satiety adequate); (iii) Add a GLP-1/GIP agonist if any of the following triggers occur:
|
| Algorithm 2. Incretin-first, tapered carbohydrate reduction to AKMP |
| Indication: patients unable to “on-ramp” into AKMP due to reward-driven eating, severe cravings, chaotic schedules, or prior failed ketogenic attempts. Steps: (i) Start a professionally supervised Mediterranean diet; (ii) initiate a GLP-1RA or dual GLP-1/GIP agonist; (iii) taper carbohydrates by 25–50 g every 2 weeks while monitoring satiety and biomarkers (target β-hydroxybutyrate ≥ 0.6 mmol/L for ≥2 consecutive weeks); (iv) when hunger/food preoccupation is blunted and BHB threshold is met, transition to AKMP Phase 2 (adaptive metabolic optimization). Mechanistic aim: use incretin therapy to reduce appetite and food-reward responsivity [145,196], then consolidate the behavioral and metabolic gains under AKMP’s biomarker-guided framework. |
8.3.2. Pragmatic Pilot Trial to Validate the AKMP–Incretin Sequencing
8.4. Limitations and Future Directions
- Requires specialized personnel in ketogenic nutrition and metabolic monitoring;
- Necessitates infrastructure for biomarker tracking;
- Demands a high level of patient commitment;
- Its complexity limits scalability in primary care settings.
- Pilot studies (3–6 months) to assess feasibility and refine the protocol;
- Randomized trial (12 months) comparing AKMP vs. static Ketogenic Mediterranean Diet, evaluating weight loss, body composition, and metabolic markers;
- Comparative trial (24 months) AKMP vs. semaglutide, measuring not only weight loss but also its quality (lean mass/fat ratio), energy expenditure, and post-intervention maintenance;
- Combination studies assessing the proposed synergy between pharmacotherapy and the AKMP.
8.5. Final Reflection: Personalizing the Approach to Obesity
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Murray, C.J.L.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global Burden of 87 Risk Factors in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Kyle, T.K.; Dhurandhar, E.J.; Allison, D.B. Regarding Obesity as a Disease. Endocrinol. Metab. Clin. N. Am. 2016, 45, 511–520. [Google Scholar] [CrossRef]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. GBD 2015 Obesity Collaborators. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- Department of Agriculture and, U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Department of Agriculture and U.S. Department of Health and Human Services: Washington, DC, USA, 2020. [Google Scholar]
- Okunogbe, A.; Nugent, R.; Spencer, G.; Powis, J.; Ralston, J.; Wilding, J. Economic Impacts of Overweight and Obesity: Current and Future Estimates for 161 Countries. BMJ Glob. Health 2022, 7, e009773. [Google Scholar] [CrossRef] [PubMed]
- Obesity Prevention—PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/topics/obesity-prevention (accessed on 6 June 2025).
- Controlling the Global Obesity Epidemic. Available online: https://www.who.int/activities/controlling-the-global-obesity-epidemic (accessed on 6 June 2025).
- Boutari, C.; Mantzoros, C.S. A 2022 Update on the Epidemiology of Obesity and a Call to Action: As Its Twin COVID-19 Pandemic Appears to Be Receding, the Obesity and Dysmetabolism Pandemic Continues to Rage On. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef]
- Phelps, N.H.; Singleton, R.K.; Zhou, B.; Heap, R.A.; Mishra, A.; Bennett, J.E.; Paciorek, C.J.; Lhoste, V.P.; Carrillo-Larco, R.M.; Stevens, G.A.; et al. Worldwide Trends in Underweight and Obesity from 1990 to 2022: A Pooled Analysis of 3663 Population-Representative Studies with 222 Million Children, Adolescents, and Adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Gakidou, E.; Lo, J.; Abate, Y.H.; Abbafati, C.; Abbas, N.; Abbasian, M.; Abd ElHafeez, S.; Abdel-Rahman, W.M.; Abd-Elsalam, S.; et al. Global, Regional, and National Prevalence of Adult Overweight and Obesity, 1990–2021, with Forecasts to 2050: A Forecasting Study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Lingvay, I.; Sumithran, P.; Cohen, R.V.; le Roux, C.W. Obesity Management as a Primary Treatment Goal for Type 2 Diabetes: Time to Reframe the Conversation. Lancet 2022, 399, 394–405. [Google Scholar] [CrossRef]
- Petrelli, F.; Cortellini, A.; Indini, A.; Tomasello, G.; Ghidini, M.; Nigro, O.; Salati, M.; Dottorini, L.; Iaculli, A.; Varricchio, A.; et al. Association of Obesity with Survival Outcomes in Patients with Cancer. JAMA Netw. Open 2021, 4, e213520. [Google Scholar] [CrossRef]
- Mahamat-saleh, Y.; Aune, D.; Freisling, H.; Hardikar, S.; Jaafar, R.; Rinaldi, S.; Gunter, M.J.; Dossus, L. Association of Metabolic Obesity Phenotypes with Risk of Overall and Site-Specific Cancers: A Systematic Review and Meta-Analysis of Cohort Studies. Br. J. Cancer 2024, 131, 1480–1495. [Google Scholar] [CrossRef]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-Induced Hypertension. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.; Cabezas, M. Dyslipidemia in Obesity: Mechanisms and Potential Targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, R.; Pierce, N.; Koppe, S. Obesity and Nonalcoholic Fatty Liver Disease: Current Perspectives. Diabetes Metab. Syndr. Obes. 2018, 11, 533–542. [Google Scholar] [CrossRef]
- Wearing, S.C.; Hennig, E.M.; Byrne, N.M.; Steele, J.R.; Hills, A.P. Musculoskeletal Disorders Associated with Obesity: A Biomechanical Perspective. Obes. Rev. 2006, 7, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Herrero, M.T.; Capdevila García, L.; Bellido Cambrón, M.d.C.; Ramírez Iñiguez de la Torre, M.V.; Lladosa Marco, S. Riesgo Cardiovascular y Obesidad En El Síndrome de Apnea Del Sueño Valorado Con El Cuestionario Stop-Bang. Endocrinol. Diabetes Nutr. 2017, 64, 544–551. [Google Scholar] [CrossRef]
- Lane, M.M.; Davis, J.A.; Beattie, S.; Gómez-Donoso, C.; Loughman, A.; O’Neil, A.; Jacka, F.; Berk, M.; Page, R.; Marx, W.; et al. Ultraprocessed Food and Chronic Noncommunicable Diseases: A Systematic Review and Meta-analysis of 43 Observational Studies. Obes. Rev. 2021, 22, e13146. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 67–77.e3. [Google Scholar] [CrossRef]
- Nguyen, M.; Jarvis, S.E.; Tinajero, M.G.; Yu, J.; Chiavaroli, L.; Mejia, S.B.; Khan, T.A.; Tobias, D.K.; Willett, W.C.; Hu, F.B.; et al. Sugar-Sweetened Beverage Consumption and Weight Gain in Children and Adults: A Systematic Review and Meta-Analysis of Prospective Cohort Studies and Randomized Controlled Trials. Am. J. Clin. Nutr. 2023, 117, 160–174. [Google Scholar] [CrossRef]
- Boyland, E.; McGale, L.; Maden, M.; Hounsome, J.; Boland, A.; Angus, K.; Jones, A. Association of Food and Nonalcoholic Beverage Marketing with Children and Adolescents’ Eating Behaviors and Health. JAMA Pediatr. 2022, 176, e221037. [Google Scholar] [CrossRef]
- Eskandari, F.; Lake, A.A.; Rose, K.; Butler, M.; O’Malley, C. A Mixed-method Systematic Review and Meta-analysis of the Influences of Food Environments and Food Insecurity on Obesity in High-income Countries. Food Sci. Nutr. 2022, 10, 3689–3723. [Google Scholar] [CrossRef]
- Lennerz, B.; Lennerz, J.K. Food Addiction, High-Glycemic-Index Carbohydrates, and Obesity. Clin. Chem. 2018, 64, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Lennerz, B.S.; Alsop, D.C.; Holsen, L.M.; Stern, E.; Rojas, R.; Ebbeling, C.B.; Goldstein, J.M.; Ludwig, D.S. Effects of Dietary Glycemic Index on Brain Regions Related to Reward and Craving in Men. Am. J. Clin. Nutr. 2013, 98, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-J.; Volkow, N.D.; Logan, J.; Pappas, N.R.; Wong, C.T.; Zhu, W.; Netusll, N.; Fowler, J.S. Brain Dopamine and Obesity. Lancet 2001, 357, 354–357. [Google Scholar] [CrossRef]
- Schulte, E.M.; Avena, N.M.; Gearhardt, A.N. Which Foods May Be Addictive? The Roles of Processing, Fat Content, and Glycemic Load. PLoS ONE 2015, 10, e0117959. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; White, M.A.; Potenza, M.N. Binge Eating Disorder and Food Addiction. Curr. Drug Abus. Rev. 2011, 4, 201–207. [Google Scholar] [CrossRef]
- di Giacomo, E.; Aliberti, F.; Pescatore, F.; Santorelli, M.; Pessina, R.; Placenti, V.; Colmegna, F.; Clerici, M. Disentangling Binge Eating Disorder and Food Addiction: A Systematic Review and Meta-Analysis. Eat. Weight. Disord. Stud. Anorex. Bulim. Obes. 2022, 27, 1963–1970. [Google Scholar] [CrossRef]
- World Health Organization Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight#:~:text=Worldwide%20adult%20obesity%20has%20more,16%25%20were%20living%20with%20obesity (accessed on 14 August 2025).
- van der Valk, E.S.; Savas, M.; van Rossum, E.F.C. Stress and Obesity: Are There More Susceptible Individuals? Curr. Obes. Rep. 2018, 7, 193–203. [Google Scholar] [CrossRef]
- Wu, Y.; Zhai, L.; Zhang, D. Sleep Duration and Obesity among Adults: A Meta-Analysis of Prospective Studies. Sleep. Med. 2014, 15, 1456–1462. [Google Scholar] [CrossRef]
- Yengo, L.; Sidorenko, J.; Kemper, K.E.; Zheng, Z.; Wood, A.R.; Weedon, M.N.; Frayling, T.M.; Hirschhorn, J.; Yang, J.; Visscher, P.M. Meta-Analysis of Genome-Wide Association Studies for Height and Body Mass Index in ∼700,000 Individuals of European Ancestry. Hum. Mol. Genet. 2018, 27, 3641–3649. [Google Scholar] [CrossRef] [PubMed]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic Studies of Body Mass Index Yield New Insights for Obesity Biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Kilpeläinen, T.O.; Qi, L.; Brage, S.; Sharp, S.J.; Sonestedt, E.; Demerath, E.; Ahmad, T.; Mora, S.; Kaakinen, M.; Sandholt, C.H.; et al. Physical Activity Attenuates the Influence of FTO Variants on Obesity Risk: A Meta-Analysis of 218,166 Adults and 19,268 Children. PLoS Med. 2011, 8, e1001116. [Google Scholar] [CrossRef]
- Voerman, E.; Santos, S.; Patro Golab, B.; Amiano, P.; Ballester, F.; Barros, H.; Bergström, A.; Charles, M.-A.; Chatzi, L.; Chevrier, C.; et al. Maternal Body Mass Index, Gestational Weight Gain, and the Risk of Overweight and Obesity across Childhood: An Individual Participant Data Meta-Analysis. PLoS Med. 2019, 16, e1002744. [Google Scholar] [CrossRef]
- Sanyal, D.; Raychaudhuri, M. Hypothyroidism and Obesity: An Intriguing Link. Indian J. Endocrinol. Metab. 2016, 20, 554. [Google Scholar] [CrossRef]
- NCD Countdown 2030 Collaborators. NCD Countdown 2030: Pathways to Achieving Sustainable Development Goal Target 3.4. Lancet 2020, 396, 918–934. [Google Scholar] [CrossRef]
- Transforming Our World: The 2030 Agenda for Sustainable Development | Department of Economic and Social Affairs. Available online: https://sdgs.un.org/2030agenda?utm_source=chatgpt.com (accessed on 1 June 2025).
- World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Noncommunicable Diseases Progress Monitor 2022. Available online: https://www.who.int/publications/i/item/9789240047761 (accessed on 14 August 2025).
- Nugent, R.; Bertram, M.Y.; Jan, S.; Niessen, L.W.; Sassi, F.; Jamison, D.T.; Pier, E.G.; Beaglehole, R. Investing in Non-Communicable Disease Prevention and Management to Advance the Sustainable Development Goals. Lancet 2018, 391, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Acceleration Plan to Stop Obesity; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- World Health Organization. Saving Lives, Spending Less: The Case for Investing in Noncommunicable Diseases; WHO Press: Geneva, Switzerland, 2021; pp. 1–20. [Google Scholar]
- Thow, A.M.; Rippin, H.L.; Mulcahy, G.; Duffey, K.; Wickramasinghe, K. Sugar-Sweetened Beverage Taxes in Europe: Learning for the Future. Eur. J. Public. Health 2022, 32, 273–280. [Google Scholar] [CrossRef]
- Colchero, M.A.; Rivera-Dommarco, J.; Popkin, B.M.; Ng, S.W. In Mexico, Evidence of Sustained Consumer Response Two Years After Implementing A Sugar-Sweetened Beverage Tax. Health Aff. 2017, 36, 564–571. [Google Scholar] [CrossRef] [PubMed]
- WHO. European Regional Obesity Report 2022. Available online: https://www.who.int/europe/publications/i/item/9789289057738 (accessed on 14 August 2025).
- Carroll, J.E.; Emond, J.A.; Griffin, L.L.; Bertone-Johnson, E.R.; VanKim, N.A.; Sturgeon, S.R. Children’s Perception of Food Marketing Across Digital Media Platforms. AJPM Focus. 2024, 3, 100205. [Google Scholar] [CrossRef] [PubMed]
- Oosterhoff, M.; Joore, M.; Ferreira, I. The Effects of School-based Lifestyle Interventions on Body Mass Index and Blood Pressure: A Multivariate Multilevel Meta-analysis of Randomized Controlled Trials. Obes. Rev. 2016, 17, 1131–1153. [Google Scholar] [CrossRef]
- Breda, J.; Castro, L.S.N.; Whiting, S.; Williams, J.; Jewell, J.; Engesveen, K.; Wickramasinghe, K. Towards Better Nutrition in Europe: Evaluating Progress and Defining Future Directions. Food Policy 2020, 96, 101887. [Google Scholar] [CrossRef]
- World Obesity Atlas 2023|World Obesity Federation. Available online: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2023 (accessed on 7 June 2025).
- Swinburn, B.A.; Kraak, V.I.; Allender, S.; Atkins, V.J.; Baker, P.I.; Bogard, J.R.; Brinsden, H.; Calvillo, A.; De Schutter, O.; Devarajan, R.; et al. The Global Syndemic of Obesity, Undernutrition, and Climate Change: The Lancet Commission Report. Lancet 2019, 393, 791–846. [Google Scholar] [CrossRef]
- Wing, R.R.; Phelan, S. Long-Term Weight Loss Maintenance. Am. J. Clin. Nutr. 2005, 82, 222S–225S. [Google Scholar] [CrossRef]
- Dyńka, D.; Kowalcze, K.; Paziewska, A. The Role of Ketogenic Diet in the Treatment of Neurological Diseases. Nutrients 2022, 14, 5003. [Google Scholar] [CrossRef]
- McGaugh, E.; Barthel, B. A Review of Ketogenic Diet and Lifestyle. Mo. Med. 2022, 119, 84–88. [Google Scholar]
- Astrup, A.; Larsen, T.M.; Harper, A. Atkins and Other Low-Carbohydrate Diets: Hoax or an Effective Tool for Weight Loss? Lancet 2004, 364, 897–899. [Google Scholar] [CrossRef] [PubMed]
- Dowis, K.; Banga, S. The Potential Health Benefits of the Ketogenic Diet: A Narrative Review. Nutrients 2021, 13, 1654. [Google Scholar] [CrossRef]
- Paoli, A.; Bianco, A.; Grimaldi, K.; Lodi, A.; Bosco, G. Long Term Successful Weight Loss with a Combination Biphasic Ketogenic Mediterranean Diet and Mediterranean Diet Maintenance Protocol. Nutrients 2013, 5, 5205–5217. [Google Scholar] [CrossRef] [PubMed]
- Sheffler, J.L.; Kiosses, D.N.; He, Z.; Arjmandi, B.H.; Akhavan, N.S.; Klejc, K.; Naar, S. Improving Adherence to a Mediterranean Ketogenic Nutrition Program for High-Risk Older Adults: A Pilot Randomized Trial. Nutrients 2023, 15, 2329. [Google Scholar] [CrossRef]
- MacLean, P.S.; Bergouignan, A.; Cornier, M.-A.; Jackman, M.R. Biology’s Response to Dieting: The Impetus for Weight Regain. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 301, R581–R600. [Google Scholar] [CrossRef] [PubMed]
- Leibel, R.L.; Rosenbaum, M.; Hirsch, J. Changes in Energy Expenditure Resulting from Altered Body Weight. N. Engl. J. Med. 1995, 332, 621–628. [Google Scholar] [CrossRef]
- Fothergill, E.; Guo, J.; Howard, L.; Kerns, J.C.; Knuth, N.D.; Brychta, R.; Chen, K.Y.; Skarulis, M.C.; Walter, M.; Walter, P.J.; et al. Persistent Metabolic Adaptation 6 Years after “The Biggest Loser” Competition. Obesity 2016, 24, 1612–1619. [Google Scholar] [CrossRef]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Long-Term Persistence of Hormonal Adaptations to Weight Loss. N. Engl. J. Med. 2011, 365, 1597–1604. [Google Scholar] [CrossRef]
- Cummings, D.E.; Weigle, D.S.; Frayo, R.S.; Breen, P.A.; Ma, M.K.; Dellinger, E.P.; Purnell, J.Q. Plasma Ghrelin Levels after Diet-Induced Weight Loss or Gastric Bypass Surgery. N. Engl. J. Med. 2002, 346, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Vandenborne, K.; Goldsmith, R.; Simoneau, J.-A.; Heymsfield, S.; Joanisse, D.R.; Hirsch, J.; Murphy, E.; Matthews, D.; Segal, K.R.; et al. Effects of Experimental Weight Perturbation on Skeletal Muscle Work Efficiency in Human Subjects. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2003, 285, R183–R192. [Google Scholar] [CrossRef]
- Baldwin, K.M.; Joanisse, D.R.; Haddad, F.; Goldsmith, R.L.; Gallagher, D.; Pavlovich, K.H.; Shamoon, E.L.; Leibel, R.L.; Rosenbaum, M. Effects of Weight Loss and Leptin on Skeletal Muscle in Human Subjects. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 301, R1259–R1266. [Google Scholar] [CrossRef]
- Levine, J.A. Non-Exercise Activity Thermogenesis (NEAT). Best. Pract. Res. Clin. Endocrinol. Metab. 2002, 16, 679–702. [Google Scholar] [CrossRef]
- Pérez-Guisado, J.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Spanish Ketogenic Mediterranean Diet: A Healthy Cardiovascular Diet for Weight Loss. Nutr. J. 2008, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Cenci, L.; Grimaldi, K.A. Effect of Ketogenic Mediterranean Diet with Phytoextracts and Low Carbohydrates/High-Protein Meals on Weight, Cardiovascular Risk Factors, Body Composition and Diet Compliance in Italian Council Employees. Nutr. J. 2011, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Ivan, C.R.; Messina, A.; Cibelli, G.; Messina, G.; Polito, R.; Losavio, F.; Torre, E.L.; Monda, V.; Monda, M.; Quiete, S.; et al. Italian Ketogenic Mediterranean Diet in Overweight and Obese Patients with Prediabetes or Type 2 Diabetes. Nutrients 2022, 14, 4361. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.D.; Landry, M.J.; Perelman, D.; Petlura, C.; Durand, L.R.; Aronica, L.; Crimarco, A.; Cunanan, K.M.; Chang, A.; Dant, C.C.; et al. Effect of a Ketogenic Diet versus Mediterranean Diet on Glycated Hemoglobin in Individuals with Prediabetes and Type 2 Diabetes Mellitus: The Interventional Keto-Med Randomized Crossover Trial. Am. J. Clin. Nutr. 2022, 116, 640–652. [Google Scholar] [CrossRef]
- Zinn, C.; Wood, M.; Williden, M.; Chatterton, S.; Maunder, E. Ketogenic Diet Benefits Body Composition and Well-Being but Not Performance in a Pilot Case Study of New Zealand Endurance Athletes. J. Int. Soc. Sports Nutr. 2017, 14, 22. [Google Scholar] [CrossRef]
- Bostock, E.C.S.; Kirkby, K.C.; Taylor, B.V.; Hawrelak, J.A. Consumer Reports of “Keto Flu” Associated with the Ketogenic Diet. Front. Nutr. 2020, 7, 20. [Google Scholar] [CrossRef]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-Ketogenic Diet Modulates Gut Microbiome and Short-Chain Fatty Acids in Association with Alzheimer’s Disease Markers in Subjects with Mild Cognitive Impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Olivito, I.; Simona, F.; Tarsitano, A.; Pagliuso, M.; Tarantino, C.; De Lorenzo, A.; Alò, R.; Avolio, E.; Facciolo, R.M. Mediterranean Ketogenic Diet Accounts for Reduced Pain Frequency and Intensity in Patients with Chronic Migraine: A Pilot Study. Clinical Nutrition 2024, 43, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D. The Ketone Metabolite β-Hydroxybutyrate Blocks NLRP3 Inflammasome–Mediated Inflammatory Disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Davies, M.; Van Gaal, L.F.; Kandler, K.; Konakli, K.; Lingvay, I.; McGowan, B.M.; Oral, T.K.; Rosenstock, J.; et al. Weight Regain and Cardiometabolic Effects after Withdrawal of Semaglutide: The STEP 1 Trial Extension. Diabetes Obes. Metab. 2022, 24, 1553–1564. [Google Scholar] [CrossRef]
- van Eyk, H.J.; Paiman, E.H.M.; Bizino, M.B.; IJzermans, S.L.; Kleiburg, F.; Boers, T.G.W.; Rappel, E.J.; Burakiewicz, J.; Kan, H.E.; Smit, J.W.A.; et al. Liraglutide Decreases Energy Expenditure and Does Not Affect the Fat Fraction of Supraclavicular Brown Adipose Tissue in Patients with Type 2 Diabetes. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 616–624. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Van Gaal, L.F.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wharton, S.; Yokote, K.; Zeuthen, N.; et al. Impact of Semaglutide on Body Composition in Adults with Overweight or Obesity: Exploratory Analysis of the STEP 1 Study. J. Endocr. Soc. 2021, 5, A16–A17. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Feldman, H.A.; Klein, G.L.; Wong, J.M.W.; Bielak, L.; Steltz, S.K.; Luoto, P.K.; Wolfe, R.R.; Wong, W.W.; Ludwig, D.S. Effects of a Low Carbohydrate Diet on Energy Expenditure during Weight Loss Maintenance: Randomized Trial. BMJ 2018, 371, k4583. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Dickinson, S.L.; Henschel, B.; Ebbeling, C.B.; Allison, D.B. Do Lower-Carbohydrate Diets Increase Total Energy Expenditure? An Updated and Reanalyzed Meta-Analysis of 29 Controlled-Feeding Studies. J. Nutr. 2021, 151, 482–490. [Google Scholar] [CrossRef]
- Fine, E.J.; Feinman, R.D. Thermodynamics of Weight Loss Diets. Nutr. Metab. 2004, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Feinman, R.D.; Fine, E.J. Nonequilibrium Thermodynamics and Energy Efficiency in Weight Loss Diets. Theor. Biol. Med. Model. 2007, 4, 27. [Google Scholar] [CrossRef]
- Sukkar, S.G.; Muscaritoli, M. A Clinical Perspective of Low Carbohydrate Ketogenic Diets: A Narrative Review. Front. Nutr. 2021, 8, 642628. [Google Scholar] [CrossRef]
- Watanabe, M.; Tuccinardi, D.; Ernesti, I.; Basciani, S.; Mariani, S.; Genco, A.; Manfrini, S.; Lubrano, C.; Gnessi, L. Scientific Evidence Underlying Contraindications to the Ketogenic Diet: An Update. Obes. Rev. 2020, 21, e13053. [Google Scholar] [CrossRef]
- Van Eyk, H.J.; Paiman, E.H.M.; Bizino, M.B.; De Heer, P.; Geelhoed-Duijvestijn, P.H.; Kharagjitsingh, A.V.; Smit, J.W.A.; Lamb, H.J.; Rensen, P.C.N.; Jazet, I.M. A Double-Blind, Placebo-Controlled, Randomised Trial to Assess the Effect of Liraglutide on Ectopic Fat Accumulation in South Asian Type 2 Diabetes Patients. Cardiovasc. Diabetol. 2019, 18, 87. [Google Scholar] [CrossRef]
- Wilder, R.M. The effect on ketonemia on the course of epilepsy. Mayo Clin. Bull. 1921, 2, 307–308. [Google Scholar]
- Roehl, K.; Sewak, S.L. Practice Paper of the Academy of Nutrition and Dietetics: Classic and Modified Ketogenic Diets for Treatment of Epilepsy. J. Acad. Nutr. Diet. 2017, 117, 1279–1292. [Google Scholar] [CrossRef]
- Huttenlocher, P.R.; Wilbourn, A.J.; Signore, J.M. Medium-chain Triglycerides as a Therapy for Intractable Childhood Epilepsy. Neurology 1971, 21, 1097. [Google Scholar] [CrossRef]
- Liu, Y.-M.C.; Lowe, H.; Zak, M.M.; Kobayashi, J.; Chan, V.W.; Donner, E.J. Can Children with Hyperlipidemia Receive Ketogenic Diet for Medication-Resistant Epilepsy? J. Child. Neurol. 2013, 28, 479–483. [Google Scholar] [CrossRef]
- van der Louw, E.; van den Hurk, D.; Neal, E.; Leiendecker, B.; Fitzsimmon, G.; Dority, L.; Thompson, L.; Marchió, M.; Dudzińska, M.; Dressler, A.; et al. Ketogenic Diet Guidelines for Infants with Refractory Epilepsy. Eur. J. Paediatr. Neurol. 2016, 20, 798–809. [Google Scholar] [CrossRef]
- Ferraris, C.; Guglielmetti, M.; Neri, L.; Allehdan, S.; Mohsin Albasara, J.; Fareed Alawadhi, H.; Trentani, C.; Perna, S.; Tagliabue, A. A Review of Ketogenic Dietary Therapies for Epilepsy and Neurological Diseases: A Proposal to Implement an Adapted Model to Include Healthy Mediterranean Products. Foods 2023, 12, 1743. [Google Scholar] [CrossRef]
- Kossoff, E.H.; Krauss, G.L.; McGrogan, J.R.; Freeman, J.M. Efficacy of the Atkins Diet as Therapy for Intractable Epilepsy. Neurology 2003, 61, 1789–1791. [Google Scholar] [CrossRef]
- Pfeifer, H.H.; Thiele, E.A. Low-Glycemic-Index Treatment: A Liberalized Ketogenic Diet for Treatment of Intractable Epilepsy. Neurology 2005, 65, 1810–1812. [Google Scholar] [CrossRef] [PubMed]
- Castellana, M.; Conte, E.; Cignarelli, A.; Perrini, S.; Giustina, A.; Giovanella, L.; Giorgino, F.; Trimboli, P. Efficacy and Safety of Very Low Calorie Ketogenic Diet (VLCKD) in Patients with Overweight and Obesity: A Systematic Review and Meta-Analysis. Rev. Endocr. Metab. Disord. 2020, 21, 5–16. [Google Scholar] [CrossRef]
- Noakes, T.D.; Windt, J. Evidence That Supports the Prescription of Low-Carbohydrate High-Fat Diets: A Narrative Review. Br. J. Sports Med. 2017, 51, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Kysel, P.; Haluzíková, D.; Doležalová, R.P.; Laňková, I.; Lacinová, Z.; Kasperová, B.J.; Trnovská, J.; Hrádková, V.; Mráz, M.; Vilikus, Z.; et al. The Influence of Cyclical Ketogenic Reduction Diet vs. Nutritionally Balanced Reduction Diet on Body Composition, Strength, and Endurance Performance in Healthy Young Males: A Randomized Controlled Trial. Nutrients 2020, 12, 2832. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, A.M.; Horgan, G.W.; Murison, S.D.; Bremner, D.M.; Lobley, G.E. Effects of a High-Protein Ketogenic Diet on Hunger, Appetite, and Weight Loss in Obese Men Feeding Ad Libitum. Am. J. Clin. Nutr. 2008, 87, 44–55. [Google Scholar] [CrossRef]
- Westman, E.C.; Yancy, W.S.; Mavropoulos, J.C.; Marquart, M.; McDuffie, J.R. The Effect of a Low-Carbohydrate, Ketogenic Diet versus a Low-Glycemic Index Diet on Glycemic Control in Type 2 Diabetes Mellitus. Nutr. Metab. 2008, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Wong, J.M.W.; Kendall, C.W.C.; Esfahani, A.; Ng, V.W.Y.; Leong, T.C.K.; Faulkner, D.A.; Vidgen, E.; Greaves, K.A.; Paul, G.; et al. The Effect of a Plant-Based Low-Carbohydrate (“Eco-Atkins”) Diet on Body Weight and Blood Lipid Concentrations in Hyperlipidemic Subjects. Arch. Intern. Med. 2009, 169, 1046. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Wong, J.M.W.; Kendall, C.W.C.; Esfahani, A.; Ng, V.W.Y.; Leong, T.C.K.; Faulkner, D.A.; Vidgen, E.; Paul, G.; Mukherjea, R.; et al. Effect of a 6-Month Vegan Low-Carbohydrate (Eco-Atkins) Diet on Cardiovascular Risk Factors and Body Weight in Hyperlipidaemic Adults: A Randomised Controlled Trial. BMJ Open 2014, 4, e003505. [Google Scholar] [CrossRef] [PubMed]
- Clemens, Z.; Kelemen, A.; Fogarasi, A.; Tóth, C. Childhood Absence Epilepsy Successfully Treated with the Paleolithic Ketogenic Diet. Neurol. Ther. 2013, 2, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Neal, E.G.; Cross, J.H. Efficacy of Dietary Treatments for Epilepsy. J. Hum. Nutr. Diet. 2010, 23, 113–119. [Google Scholar] [CrossRef]
- Paoli, A. Ketogenic Diet for Obesity: Friend or Foe? Int. J. Environ. Res. Public Health 2014, 11, 2092–2107. [Google Scholar] [CrossRef]
- Family, A.; Westerberg, D.P. Diabetic Ketoacidosis: Evaluation and Treatment. Am. Fam. Physician 2013, 87, 337–346. [Google Scholar]
- Arora, S.; Henderson, S.O.; Long, T.; Menchine, M. Diagnostic Accuracy of Point-of-Care Testing for Diabetic Ketoacidosis at Emergency-Department Triage. Diabetes Care 2011, 34, 852–854. [Google Scholar] [CrossRef]
- Bueno, N.B.; de Melo, I.S.V.; de Oliveira, S.L.; da Rocha Ataide, T. Very-Low-Carbohydrate Ketogenic Diet vs Low-Fat Diet for Long-Term Weight Loss: A Meta-Analysis of Randomised Controlled Trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef]
- Volek, J.S.; Phinney, S.D.; Forsythe, C.E.; Quann, E.E.; Wood, R.J.; Puglisi, M.J.; Kraemer, W.J.; Bibus, D.M.; Fernandez, M.L.; Feinman, R.D. Carbohydrate Restriction Has a More Favorable Impact on the Metabolic Syndrome than a Low Fat Diet. Lipids 2009, 44, 297–309. [Google Scholar] [CrossRef]
- Bruci, A.; Tuccinardi, D.; Tozzi, R.; Balena, A.; Santucci, S.; Frontani, R.; Mariani, S.; Basciani, S.; Spera, G.; Gnessi, L.; et al. Very Low-Calorie Ketogenic Diet: A Safe and Effective Tool for Weight Loss in Patients with Obesity and Mild Kidney Failure. Nutrients 2020, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Grimaldi, K.; D’Agostino, D.; Cenci, L.; Moro, T.; Bianco, A.; Palma, A. Ketogenic Diet Does Not Affect Strength Performance in Elite Artistic Gymnasts. J. Int. Soc. Sports Nutr. 2012, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Kosinski, C.; Jornayvaz, F. Effects of Ketogenic Diets on Cardiovascular Risk Factors: Evidence from Animal and Human Studies. Nutrients 2017, 9, 517. [Google Scholar] [CrossRef]
- Kossoff, E.H.; Zupec-Kania, B.A.; Auvin, S.; Ballaban-Gil, K.R.; Christina Bergqvist, A.G.; Blackford, R.; Buchhalter, J.R.; Caraballo, R.H.; Cross, J.H.; Dahlin, M.G.; et al. Optimal Clinical Management of Children Receiving Dietary Therapies for Epilepsy: Updated Recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 2018, 3, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Wieser, T. Carnitine Palmitoyltransferase II Deficiency. In GeneReviews®; University of Washington, Seattle: Seattle, WA, USA, 2019. [Google Scholar]
- Olpin, S.E.; Murphy, E.; Kirk, R.J.; Taylor, R.W.; Quinlivan, R. The Investigation and Management of Metabolic Myopathies. J. Clin. Pathol. 2015, 68, 410–417. [Google Scholar] [CrossRef]
- Ahmad, A.; Kahler, S.G.; Kishnani, P.S.; Artigas-Lopez, M.; Pappu, A.S.; Steiner, R.; Millington, D.S.; Van Hove, J.L.K. Treatment of Pyruvate Carboxylase Deficiency with High Doses of Citrate and Aspartate. Am. J. Med. Genet. 1999, 87, 331–338. [Google Scholar] [CrossRef]
- Marin-Valencia, I.; Roe, C.R.; Pascual, J.M. Pyruvate Carboxylase Deficiency: Mechanisms, Mimics and Anaplerosis. Mol. Genet. Metab. 2010, 101, 9–17. [Google Scholar] [CrossRef]
- Anderson, K.E.; Bloomer, J.R.; Bonkovsky, H.L.; Kushner, J.P.; Pierach, C.A.; Pimstone, N.R.; Desnick, R.J. Recommendations for the Diagnosis and Treatment of the Acute Porphyrias. Ann. Intern. Med. 2005, 142, 439–450. [Google Scholar] [CrossRef]
- Balwani, M.; Desnick, R.J. The Porphyrias: Advances in Diagnosis and Treatment. Blood 2012, 120, 4496–4504. [Google Scholar] [CrossRef]
- Blau, J.E.; Tella, S.H.; Taylor, S.I.; Rother, K.I. Ketoacidosis Associated with SGLT2 Inhibitor Treatment: Analysis of FAERS Data. Diabetes Metab. Res. Rev. 2017, 33, e2924. [Google Scholar] [CrossRef]
- Peters, A.L.; Buschur, E.O.; Buse, J.B.; Cohan, P.; Diner, J.C.; Hirsch, I.B. Euglycemic Diabetic Ketoacidosis: A Potential Complication of Treatment with Sodium–Glucose Cotransporter 2 Inhibition. Diabetes Care 2015, 38, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- Leow, Z.Z.X.; Guelfi, K.J.; Davis, E.A.; Jones, T.W.; Fournier, P.A. The Glycaemic Benefits of a Very-low-carbohydrate Ketogenic Diet in Adults with Type 1 Diabetes Mellitus May Be Opposed by Increased Hypoglycaemia Risk and Dyslipidaemia. Diabet. Med. 2018, 35, 1258–1263. [Google Scholar] [CrossRef]
- McClean, A.-M.; Montorio, L.; McLaughlin, D.; McGovern, S.; Flanagan, N. Can a Ketogenic Diet Be Safely Used to Improve Glycaemic Control in a Child with Type 1 Diabetes? Arch. Dis. Child. 2019, 104, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Buehler, L.A.; Noe, D.; Knapp, S.; Isaacs, D.; Pantalone, K.M. Ketogenic Diets in the Management of Type 1 Diabetes: Safe or Safety Concern? Cleve Clin. J. Med. 2021, 88, 547–555. [Google Scholar] [CrossRef]
- Patikorn, C.; Saidoung, P.; Pham, T.; Phisalprapa, P.; Lee, Y.Y.; Varady, K.A.; Veettil, S.K.; Chaiyakunapruk, N. Effects of Ketogenic Diet on Health Outcomes: An Umbrella Review of Meta-Analyses of Randomized Clinical Trials. BMC Med. 2023, 21, 196. [Google Scholar] [CrossRef]
- Kumar, N.K.; Merrill, J.D.; Carlson, S.; German, J.; Yancy, W.S., Jr. Adherence to Low-Carbohydrate Diets in Patients with Diabetes: A Narrative Review. Diabetes Metab. Syndr. Obes. 2022, 15, 477–498. [Google Scholar] [CrossRef]
- O’Neill, B.; Raggi, P. The Ketogenic Diet: Pros and Cons. Atherosclerosis 2020, 292, 119–126. [Google Scholar] [CrossRef]
- Westenhoefer, J.; Stunkard, A.J.; Pudel, V. Validation of the Flexible and Rigid Control Dimensions of Dietary Restraint. Int. J. Eat. Disord. 1999, 26, 53–64. [Google Scholar] [CrossRef]
- Harvey, C.J.d.C.; Schofield, G.M.; Zinn, C.; Thornley, S. Effects of Differing Levels of Carbohydrate Restriction on Mood Achievement of Nutritional Ketosis, and Symptoms of Carbohydrate Withdrawal in Healthy Adults: A Randomized Clinical Trial. Nutrition 2019, 67–68, 100005. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.; Schofield, G.; Williden, M. The Lived Experience of Healthy Adults Following a Ketogenic Diet: A Qualitative Study. 2018. Available online: https://www.semanticscholar.org/paper/The-lived-experience-of-healthy-adults-following-a-Harvey-Schofield/b63b40d8da66e2f84e8f14e12f40303b521c8736 (accessed on 8 May 2025).
- Bellamy, E.L.; Hadjiefthyvoulou, F.; Walsh, J.; Brown, J.; Turner, J. Understanding the Experiences of Ketogenic Metabolic Therapy for People Living with Varying Levels of Depressive Symptoms: A Thematic Analysis. Front. Nutr. 2024, 11, 1397546. [Google Scholar] [CrossRef]
- Protogerou, C.; Leroy, F.; Hagger, M.S. Beliefs and Experiences of Individuals Following a Zero-Carb Diet. Behav. Sci. 2021, 11, 161. [Google Scholar] [CrossRef]
- Rana, A.; Arora, M. Ketogenic Diet: Assessing YouTube Video Information Using Quality, Reliability, and Text Analytics Methods. Nutr. Health 2023, 31, 509–516. [Google Scholar] [CrossRef]
- Rogers, M.; Lemstra, M.; Bird, Y.; Nwankwo, C.; Moraros, J. Weight-Loss Intervention Adherence and Factors Promoting Adherence: A Meta-Analysis. Patient Prefer. Adherence 2016, 10, 1547–1559. [Google Scholar] [CrossRef]
- Müller, M.J.; Bosy-Westphal, A. Adaptive Thermogenesis with Weight Loss in Humans. Obesity 2013, 21, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Agnihothri, R.V.; Courville, A.B.; Linderman, J.D.; Smith, S.; Brychta, R.; Remaley, A.; Chen, K.Y.; Simchowitz, L.; Celi, F.S. Moderate Weight Loss Is Sufficient to Affect Thyroid Hormone Homeostasis and Inhibit Its Peripheral Conversion. Thyroid 2014, 24, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Hirsch, J.; Murphy, E.; Leibel, R.L. Effects of Changes in Body Weight on Carbohydrate Metabolism, Catecholamine Excretion, and Thyroid Function. Am. J. Clin. Nutr. 2000, 71, 1421–1432. [Google Scholar] [CrossRef]
- Arone, L.J.; Mackintosh, R.; Rosenbaum, M.; Leibel, R.L.; Hirsch, J. Autonomic Nervous System Activity in Weight Gain and Weight Loss. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1995, 269, R222–R225. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Leibel, R.L. Adaptive Thermogenesis in Humans. Int. J. Obes. 2010, 34, S47–S55. [Google Scholar] [CrossRef]
- Martins, C.; Gower, B.A.; Hill, J.O.; Hunter, G.R. Metabolic Adaptation Is Not a Major Barrier to Weight-Loss Maintenance. Am. J. Clin. Nutr. 2020, 112, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Ebbeling, C.B.; Swain, J.F.; Feldman, H.A.; Wong, W.W.; Hachey, D.L.; Garcia-Lago, E.; Ludwig, D.S. Effects of Dietary Composition on Energy Expenditure During Weight-Loss Maintenance. JAMA 2012, 307, 2627–2634. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Trapp, S.; Brierley, D.I. Brain GLP-1 and the Regulation of Food Intake: GLP-1 Action in the Brain and Its Implications for GLP-1 Receptor Agonists in Obesity Treatment. Br. J. Pharmacol. 2022, 179, 557–570. [Google Scholar] [CrossRef]
- Brierley, D.I.; de Lartigue, G. Reappraising the Role of the Vagus Nerve in GLP-1-mediated Regulation of Eating. Br. J. Pharmacol. 2022, 179, 584–599. [Google Scholar] [CrossRef]
- Samms, R.J.; Coghlan, M.P.; Sloop, K.W. How May GIP Enhance the Therapeutic Efficacy of GLP-1? Trends Endocrinol. Metab. 2020, 31, 410–421. [Google Scholar] [CrossRef]
- Rubino, D.; Abrahamsson, N.; Davies, M.; Hesse, D.; Greenway, F.L.; Jensen, C.; Lingvay, I.; Mosenzon, O.; Rosenstock, J.; Rubio, M.A.; et al. Effect of Continued Weekly Subcutaneous Semaglutide vs. Placebo on Weight Loss Maintenance in Adults with Overweight or Obesity. JAMA 2021, 325, 1414. [Google Scholar] [CrossRef]
- Sjöström, L. Review of the Key Results from the Swedish Obese Subjects (SOS) Trial—A Prospective Controlled Intervention Study of Bariatric Surgery. J. Intern. Med. 2013, 273, 219–234. [Google Scholar] [CrossRef]
- Arterburn, D.E.; Telem, D.A.; Kushner, R.F.; Courcoulas, A.P. Benefits and Risks of Bariatric Surgery in Adults. JAMA 2020, 324, 879. [Google Scholar] [CrossRef] [PubMed]
- Nuijten, M.A.H.; Eijsvogels, T.M.H.; Monpellier, V.M.; Janssen, I.M.C.; Hazebroek, E.J.; Hopman, M.T.E. The Magnitude and Progress of Lean Body Mass, Fat-free Mass, and Skeletal Muscle Mass Loss Following Bariatric Surgery: A Systematic Review and Meta-analysis. Obes. Rev. 2022, 23, e13370. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.J.; Bosy-Westphal, A.; Later, W.; Haas, V.; Heller, M. Functional Body Composition: Insights into the Regulation of Energy Metabolism and Some Clinical Applications. Eur. J. Clin. Nutr. 2009, 63, 1045–1056. [Google Scholar] [CrossRef]
- Maciel, M.G.; Beserra, B.T.S.; Oliveira, F.C.B.; Ribeiro, C.M.; Coelho, M.S.; Neves, F.d.A.R.; Amato, A.A. The Effect of Glucagon-like Peptide 1 and Glucagon-like Peptide 1 Receptor Agonists on Energy Expenditure: A Systematic Review and Meta-Analysis. Diabetes Res. Clin. Pract. 2018, 142, 222–235. [Google Scholar] [CrossRef]
- Sacks, F.M.; Bray, G.A.; Carey, V.J.; Smith, S.R.; Ryan, D.H.; Anton, S.D.; McManus, K.; Champagne, C.M.; Bishop, L.M.; Laranjo, N.; et al. Comparison of Weight-Loss Diets with Different Compositions of Fat, Protein, and Carbohydrates. N. Engl. J. Med. 2009, 360, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.D.; Trepanowski, J.F.; Del Gobbo, L.C.; Hauser, M.E.; Rigdon, J.; Ioannidis, J.P.A.; Desai, M.; King, A.C. Effect of Low-Fat vs Low-Carbohydrate Diet on 12-Month Weight Loss in Overweight Adults and the Association with Genotype Pattern or Insulin Secretion. JAMA 2018, 319, 667. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Bemis, T.; Brychta, R.; Chen, K.Y.; Courville, A.; Crayner, E.J.; Goodwin, S.; Guo, J.; Howard, L.; Knuth, N.D.; et al. Calorie for Calorie, Dietary Fat Restriction Results in More Body Fat Loss than Carbohydrate Restriction in People with Obesity. Cell Metab. 2015, 22, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R. Diet Induced Thermogenesis. Nutr. Metab. 2004, 1, 5. [Google Scholar] [CrossRef]
- Feinman, R.D.; Fine, E.J. “A Calorie Is a Calorie” Violates the Second Law of Thermodynamics. Nutr. J. 2004, 3, 9. [Google Scholar] [CrossRef]
- Reshef, L.; Olswang, Y.; Cassuto, H.; Blum, B.; Croniger, C.M.; Kalhan, S.C.; Tilghman, S.M.; Hanson, R.W. Glyceroneogenesis and the Triglyceride/Fatty Acid Cycle. J. Biol. Chem. 2003, 278, 30413–30416. [Google Scholar] [CrossRef]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of Oxidative Stress by β-Hydroxybutyrate, an Endogenous Histone Deacetylase Inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef]
- Srivastava, S.; Kashiwaya, Y.; King, M.T.; Baxa, U.; Tam, J.; Niu, G.; Chen, X.; Clarke, K.; Veech, R.L. Mitochondrial Biogenesis and Increased Uncoupling Protein 1 in Brown Adipose Tissue of Mice Fed a Ketone Ester Diet. FASEB J. 2012, 26, 2351–2362. [Google Scholar] [CrossRef]
- Yancy, W.S.; Olsen, M.K.; Guyton, J.R.; Bakst, R.P.; Westman, E.C. A Low-Carbohydrate, Ketogenic Diet versus a Low-Fat Diet To Treat Obesity and Hyperlipidemia. Ann. Intern. Med. 2004, 140, 769–777. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Hirsch, J.; Gallagher, D.A.; Leibel, R.L. Long-Term Persistence of Adaptive Thermogenesis in Subjects Who Have Maintained a Reduced Body Weight. Am. J. Clin. Nutr. 2008, 88, 906–912. [Google Scholar] [CrossRef]
- Heinitz, S.; Hollstein, T.; Ando, T.; Walter, M.; Basolo, A.; Krakoff, J.; Votruba, S.B.; Piaggi, P. Early Adaptive Thermogenesis Is a Determinant of Weight Loss after Six Weeks of Caloric Restriction in Overweight Subjects. Metabolism 2020, 110, 154303. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond Weight Loss: A Review of the Therapeutic Uses of Very-Low-Carbohydrate (Ketogenic) Diets. Eur. J. Clin. Nutr. 2013, 67, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Peterson, C.M.; Thomas, D.M.; Hirezi, M.; Zhang, B.; Smith, S.; Bray, G.; Redman, L. Establishing Energy Requirements for Body Weight Maintenance: Validation of an Intake-Balance Method. BMC Res. Notes 2017, 10, 220. [Google Scholar] [CrossRef] [PubMed]
- Roekenes, J.; Martins, C. Ketogenic Diets and Appetite Regulation. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 359–363. [Google Scholar] [CrossRef]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Ketosis and Appetite-Mediating Nutrients and Hormones after Weight Loss. Eur. J. Clin. Nutr. 2013, 67, 759–764. [Google Scholar] [CrossRef]
- Deemer, S.E.; Plaisance, E.P.; Martins, C. Impact of Ketosis on Appetite Regulation—A Review. Nutr. Res. 2020, 77, 1–11. [Google Scholar] [CrossRef]
- Hasan-Olive, M.M.; Lauritzen, K.H.; Ali, M.; Rasmussen, L.J.; Storm-Mathisen, J.; Bergersen, L.H. A Ketogenic Diet Improves Mitochondrial Biogenesis and Bioenergetics via the PGC1α-SIRT3-UCP2 Axis. Neurochem. Res. 2019, 44, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.J.; LaFountain, R.A.; Barnhart, E.; Sapper, T.S.; Short, J.; Arnold, W.D.; Hyde, P.N.; Crabtree, C.D.; Kackley, M.L.; Kraemer, W.J.; et al. A Ketogenic Diet Combined with Exercise Alters Mitochondrial Function in Human Skeletal Muscle While Improving Metabolic Health. Am. J. Physiol.-Endocrinol. Metab. 2020, 319, E995–E1007. [Google Scholar] [CrossRef]
- Hyatt, H.W.; Kephart, W.C.; Holland, A.M.; Mumford, P.; Mobley, C.B.; Lowery, R.P.; Roberts, M.D.; Wilson, J.M.; Kavazis, A.N. A Ketogenic Diet in Rodents Elicits Improved Mitochondrial Adaptations in Response to Resistance Exercise Training Compared to an Isocaloric Western Diet. Front. Physiol. 2016, 7, 533. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.-L.; Kensler, T.W.; et al. Therapeutic Targeting of the NRF2 and KEAP1 Partnership in Chronic Diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Mukamal, K.J.; Liu, L.; Franz, M.; Eliassen, A.H.; Rimm, E.B. High Anthocyanin Intake Is Associated with a Reduced Risk of Myocardial Infarction in Young and Middle-Aged Women. Circulation 2013, 127, 188–196. [Google Scholar] [CrossRef]
- Mifflin, M.; St Jeor, S.; Hill, L.; Scott, B.; Daugherty, S.; Koh, Y. A New Predictive Equation for Resting Energy Expenditure in Healthy Individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef]
- Raymond, J.L.; Morrow, K. Medical Nutrition Therapy for Overweight and Obesity in Adults. In Krause and Mahan’s Food & the Nutrition Care Process; Elsevier: St. Louis, MO, USA, 2021; pp. 396–419. [Google Scholar]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Urbain, P.; Bertz, H. Monitoring for Compliance with a Ketogenic Diet: What Is the Best Time of Day to Test for Urinary Ketosis? Nutr. Metab. 2016, 13, 77. [Google Scholar] [CrossRef]
- Ling, C.H.Y.; de Craen, A.J.M.; Slagboom, P.E.; Gunn, D.A.; Stokkel, M.P.M.; Westendorp, R.G.J.; Maier, A.B. Accuracy of Direct Segmental Multi-Frequency Bioimpedance Analysis in the Assessment of Total Body and Segmental Body Composition in Middle-Aged Adult Population. Clin. Nutr. 2011, 30, 610–615. [Google Scholar] [CrossRef]
- Bhutani, S.; Kahn, E.; Tasali, E.; Schoeller, D.A. Composition of Two-Week Change in Body Weight under Unrestricted Free-Living Conditions. Physiol. Rep. 2017, 5, e13336. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Westman, E.; Mattes, R.D.; Wolfe, R.R.; Astrup, A.; Westerterp-Plantenga, M. Protein, Weight Management, and Satiety. Am. J. Clin. Nutr. 2008, 87, 1558S–1561S. [Google Scholar] [CrossRef]
- Krieger, J.W.; Sitren, H.S.; Daniels, M.J.; Langkamp-Henken, B. Effects of Variation in Protein and Carbohydrate Intake on Body Mass and Composition during Energy Restriction: A Meta-Regression. Am. J. Clin. Nutr. 2006, 83, 260–274. [Google Scholar] [CrossRef]
- McCrimmon, R.J.; Catarig, A.-M.; Frias, J.P.; Lausvig, N.L.; le Roux, C.W.; Thielke, D.; Lingvay, I. Effects of Once-Weekly Semaglutide vs Once-Daily Canagliflozin on Body Composition in Type 2 Diabetes: A Substudy of the SUSTAIN 8 Randomised Controlled Clinical Trial. Diabetologia 2020, 63, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, L.; De Stefano, G.; Persico, F.; Gargiulo, S.; Di Spirito, F.; Griguolo, G.; Petrucciani, N.; Fontas, E.; Iannelli, A.; Pilone, V. A Randomized, Controlled Trial Comparing the Impact of a Low-Calorie Ketogenic vs a Standard Low-Calorie Diet on Fat-Free Mass in Patients Receiving an ElipseTM Intragastric Balloon Treatment. Obes. Surg. 2021, 31, 1514–1523. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Gasparri, C.; Peroni, G.; Spadaccini, D.; Maugeri, R.; Nichetti, M.; Infantino, V.; Perna, S. Current Opinion on Dietary Advice in Order to Preserve Fat-Free Mass during a Low-Calorie Diet. Nutrition 2020, 72, 110667. [Google Scholar] [CrossRef]
- Wycherley, T.P.; Moran, L.J.; Clifton, P.M.; Noakes, M.; Brinkworth, G.D. Effects of Energy-Restricted High-Protein, Low-Fat Compared with Standard-Protein, Low-Fat Diets: A Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2012, 96, 1281–1298. [Google Scholar] [CrossRef] [PubMed]
- Tronieri, J.S.; Wadden, T.A.; Walsh, O.; Berkowitz, R.I.; Alamuddin, N.; Gruber, K.; Leonard, S.; Bakizada, Z.M.; Chao, A.M. Effects of Liraglutide on Appetite, Food Preoccupation, and Food Liking: Results of a Randomized Controlled Trial. Int. J. Obes. 2020, 44, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, I.; Stampfer, M.J.; Schwarzfuchs, D.; Shai, I. Adherence and Success in Long-Term Weight Loss Diets: The Dietary Intervention Randomized Controlled Trial (DIRECT). J. Am. Coll. Nutr. 2009, 28, 159–168. [Google Scholar] [CrossRef]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.W.; le Roux, C.W.; Violante Ortiz, R.; Jensen, C.B.; et al. A Randomized, Controlled Trial of 3.0 Mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef]
- Ma, L.; Liu, G.; Ding, M.; Zong, G.; Hu, F.B.; Willett, W.C.; Rimm, E.B.; Manson, J.E.; Sun, Q. Isoflavone Intake and the Risk of Coronary Heart Disease in US Men and Women. Circulation 2020, 141, 1127–1137. [Google Scholar] [CrossRef]
- Marini, H.R. Mediterranean Diet and Soy Isoflavones for Integrated Management of the Menopausal Metabolic Syndrome. Nutrients 2022, 14, 1550. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.J.; Cox, P.J.; Evans, R.D.; Cyranka, M.; Clarke, K.; de Wet, H. A Ketone Ester Drink Lowers Human Ghrelin and Appetite. Obesity 2018, 26, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Eren-Yazicioglu, C.Y.; Yigit, A.; Dogruoz, R.E.; Yapici-Eser, H. Can GLP-1 Be a Target for Reward System Related Disorders? A Qualitative Synthesis and Systematic Review Analysis of Studies on Palatable Food, Drugs of Abuse, and Alcohol. Front. Behav. Neurosci. 2021, 14, 614884. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; D‘Alessio, D.A. Tirzepatide, a Dual GIP/GLP-1 Receptor Co-Agonist for the Treatment of Type 2 Diabetes with Unmatched Effectiveness Regrading Glycaemic Control and Body Weight Reduction. Cardiovasc. Diabetol. 2022, 21, 169. [Google Scholar] [CrossRef] [PubMed]


| Variant and Acronym | Year/Key Author | Typical Macros (% C/P/F) | Main Application | Key Advantage (Mechanism/Benefit) | Main Limitation (Challenge) | Documented Results | Reference(s) |
|---|---|---|---|---|---|---|---|
| Classic Ketogenic Diet (CKD) | 1921/Wilder | ~4/~8/~90 | Refractory Epilepsy | Maximum ketosis induction through strict 4:1 ratio | Highly restrictive, low adherence, requires strict medical supervision | >50% seizure reduction in drug-resistant epilepsy | [90] |
| Modified Ratios | Clinical evolution/1920s–1930s | ~10/~10–15/~75–80 | Pediatric Epilepsy | Greater nutritional flexibility while maintaining therapeutic efficacy (3:1, 2:1 ratios) | Still requires precise calculation and close monitoring | Efficacy comparable to 4:1 with better tolerance | [91] |
| MCT Diet (MCT) | 1971/Huttenlocher | ~15–20/~10/~70 | Pediatric Epilepsy | Greater flexibility (more CHO/PRO) thanks to direct MCT metabolism | MCT oil cost, GI effects (diarrhea, nausea) | Similar efficacy to CKD with greater dietary variety | [92,93,94,95] |
| Original Atkins | 1972/Atkins | Variable (20→100 g CHO) | Weight Loss | No caloric restriction, gradual CHO increase allows adaptation | Designed for weight loss, not for therapeutic ketosis | Rapid initial loss, variable long-term maintenance | [59] |
| Modified Atkins (MAD) | 2003/Kossoff | ~5/Ad libitum/Ad libitum | Epilepsy (Adolescents/Adults) | Easy implementation (no weighing/measuring, no caloric restriction), high adherence | Less control over body composition and macro quality | 40–50% achieve >50% seizure reduction | [91,96] |
| Low Glycemic Index (LGIT) | 2005/Pfeifer & Thiele | ~10–20/~25/~60 | Epilepsy | Allows more carbohydrates (40–60 g) if low GI, improving variety | Requires GI knowledge; less deep ketosis | >90% seizure reduction in 50% of patients | [97,106] |
| Very Low Calorie Ketogenic (VLCKD) | 2000s/Castellana | ~5/~20/~75 (<800 kcal) | Severe obesity | Very rapid weight loss with significant cardiometabolic improvements | Not sustainable long-term; requires strict medical supervision | ↓ 15.6 kg, ↓ HbA1c 0.7%, ↓ TG 60 mg/dL, ↓ SBP 8 mmHg | [98] |
| Targeted (TKD) | 1990s/Various | Standard + 15–30 g CHO peri-exercise | Endurance Athletes | Improves high-intensity performance without compromising keto-adaptation | Requires precise periodization; risk of exiting ketosis | ↑ 2.8% time trial performance | [99] |
| Cyclical (cKD) | 1990s/Various | 5–6 d keto/1–2 d 60–70% CHO | Strength/Bodybuilding | Weekly muscle glycogen replenishment; maintains lean mass during deficit | Planning complexity; repeated keto/glycolysis adaptation | Preserves strength and muscle mass, ↓ body fat | [100] |
| High Protein (HPKD) | 2000s/Westman | ~5/~35/~60 | Weight Loss/Type 2 Diabetes | Maximizes satiety and thermic effect; lean mass preservation; glycemic control | Risk of excessive gluconeogenesis if uncontrolled | Significantly greater weight loss vs. control diets (11.1 vs. 6.9 kg at 24 weeks); 95% ↓ DM2 medication | [101,102] |
| Eco-Atkins (Plant-based) | 2009/Jenkins | ~31/~26/~43 | Dyslipidemia/Vegans | Enables ketosis in vegetarians/vegans; superior cardioprotective profile | Difficulty obtaining complete protein; dependence on processed foods | ↓ CRP 28.2%, ↓ LDL 8.1%, ↓ 10.7 kg in 6 months | [103,104] |
| Paleolithic (PKD) | 2010s/Clemens | ~5–10/~20–25/~70–80 | Autoimmunity/Clinical cases | Eliminates inflammatory foods; emphasis on organs and grass-fed meat | Very socially restrictive; evidence limited to cases | Anecdotal improvements in autoimmune conditions | [105] |
| Spanish Ketogenic Mediterranean (SKMD) | 2008/Pérez-Guisado | ~5/~20/~75 | Obesity/CV Health | Combines keto + Mediterranean benefits; virgin olive oil as primary fat | Cost of quality Mediterranean ingredients | ↓ 14.47 ± 6.88 kg (12 wk), ↓ TG 47.9%, ↑ HDL 10% | [69] |
| KEMEPHY | 2011/Paoli | Variable (<50 g CHO) | Corporate weight loss | Prepared meals + phytoextracts; maximum convenience | Dependence on specific commercial products | 92% adherence, ↓ 10 cm waist (6 wk) | [70] |
| Biphasic Ketogenic Mediterranean Diet | 2013/Paoli | Alternates keto/Mediterranean | Long-term maintenance | Combines initial keto loss with Mediterranean sustainability | Not adaptive to individual response; fixed transitions | ↓ 16.9 ± 7.1 kg/year, 88.2% adherence | [59] |
| Area of Concern | Prevalent Myth | Scientific Evidence | Protective Mechanism | References |
|---|---|---|---|---|
| Ketoacidosis | “Ketosis is dangerous and can lead to ketoacidosis” | Nutritional ketosis (0.5–5 mmol/L) is physiological and safe. DKA (≥3 mmol/L) only occurs with absolute insulin deficiency | Intact insulin feedback prevents uncontrolled ketogenesis | [107,108,109] |
| Cardiovascular Health | “High fat intake increases cardiovascular risk” | Triglyceride reduction (−51%), HDL-C increase (+13%), blood pressure reduction, shift from sdLDL to lbLDL | Improved insulin sensitivity (−55%), reduced systemic inflammation | [110,111,114] |
| Renal Function | “KD damages kidneys due to high protein” | No deterioration in renal function; 27.7% normalization of GFR in mild CKD | KD is adequate, not high in protein; improves glycemic control and BP | [112] |
| Muscle Mass | “Ketosis causes muscle loss” | Complete preservation of strength in elite athletes; maintenance of explosive performance | Ketones reduce protein gluconeogenesis; increased growth hormone | [107,113] |
| Bone Density | “Ketosis causes bone deterioration” | No evidence of bone loss with well-formulated KD | Alkalinizing vegetables; reduced inflammation | [107] |
| Category | Specific Conditions | Clinical Considerations | References |
|---|---|---|---|
| Absolute Contraindications | • CPT I/II deficiency | Inability to metabolize fatty acids | [115,116,117] |
| • Carnitine–acylcarnitine translocase deficiency | Mitochondrial transport impairment | [115,116] | |
| • Mitochondrial β-oxidation defects | Toxic metabolite accumulation | [115,117] | |
| • Acute intermittent porphyria | Risk of porphyric crisis | [115,120,121] | |
| • Pyruvate carboxylase deficiency | Gluconeogenesis impairment | [115,118,119] | |
| • Acute pancreatitis | Exacerbation by fat content | [115] | |
| Special Precautions | • Type 1 Diabetes Mellitus | Mandatory basal/bolus insulin adjustment | [124,125,126] |
| • SGLT-2i | Risk of euglycemic ketoacidosis | [122,123] | |
| • Antihypertensives | Possible need for dose reduction | [107] | |
| • Oral hypoglycemic agents | Adjustment according to blood glucose | [107,126] | |
| Conditions Requiring Modification | • Pregnancy and lactation | Special nutritional requirements | [107] |
| • CKD stage ≥ 4 | Strict renal function monitoring | [112,115] | |
| • Hepatic insufficiency | Ketone metabolism impairment | [115] | |
| • Children < 2 years | Only under refractory epilepsy protocol | [115] | |
| Recommended Monitoring | • Weeks 1–2 | Daily capillary ketones | [115] |
| • Monthly | Complete metabolic panel | [115] | |
| • Quarterly | Renal function, liver function, lipid profile | [112,115] |
| Drug/Class or Condition | Initial Action at AKMP Start | Ongoing Monitoring | Notes/Contingencies |
|---|---|---|---|
| Type 2 diabetes—SGLT-2 inhibitors | Discontinue prior to ketogenic induction to reduce euglycemic DKA risk; consider resumption in non-ketogenic phases if clinically indicated. | SMBG/CGM during Weeks 1–2; assess ketones if symptomatic. | Prefer alternatives during induction; re-evaluate if persistent hyperglycemia. |
| Type 2 diabetes—Insulin | Reduce basal by ~20–30%; withhold/reduce prandial insulin initially. | SMBG/CGM to titrate and avoid hypoglycemia. | Reinstate/titrate prandial doses as carb intake increases in later phases. |
| Type 2 diabetes—Sulfonylureas | Reduce dose by ~50% or discontinue. | SMBG; weekly review during Weeks 1–4. | Prioritize de-escalation if hypoglycemia or rapid weight loss. |
| Metformin/GLP-1 RA | Continue unless intolerance. | GI tolerance; weight trajectory. | Consider GLP-1 RA as adjunct in severe hyperphagia; reassess need over time. |
| Type 1 diabetes | Ketogenic phases only under specialist supervision; proactive basal/bolus adjustments. | Strict ketone-glucose monitoring and sick-day rules. | Abort ketogenic phase if persistent ketosis with hyperglycemia or intercurrent illness. |
| Hypertension/Diuretics | Expect early natriuresis; reassess thiazides/loops and ACEi/ARB doses. | Seated BP daily during Weeks 1–2; electrolytes if symptoms. | Adjust fluids/electrolytes per clinical status. |
| Lipid safety (atherogenic risk) | Baseline apoB (or LDL-P), LDL-C, non-HDL-C. | Repeat at 6–12 weeks. | If apoB rises substantially, shift fats toward MUFA/PUFA, add low-carb fiber/plant sterols; consider pharmacotherapy per guidelines. |
| Drug | Clinical Trial | n | Duration (weeks) | Weight Loss (%) | Weight Loss (kg) | Reference |
|---|---|---|---|---|---|---|
| Semaglutide | STEP 1 | 1961 | 68 | 14.9% | −15.3 kg | Wilding et al., 2021 [78] |
| Semaglutide | STEP 4 | 803 | 68 | 17.4% | −18.2 kg | Rubino et al., 2021 [148] |
| Tirzepatide | SURMOUNT-1 | 2539 | 72 | 20.9% (15 mg) | −22.2 kg | Jastreboff et al., 2022 [79] |
| Study | N | Duration | Protocol/Macros | Weight Loss | Adherence/Completion (%) | Adverse Events | Addresses Metabolic Adaptation? |
|---|---|---|---|---|---|---|---|
| Pérez-Guisado 2008 [69] | 40 | 12 wk | SKMD: <30 g CHO, EVOO ≥ 30 mL/d | −14.1 kg (−13.0%) | 77.5% | Mild headache, constipation | No |
| Paoli 2011 [70] | 106 | 6 wk | KEMEPHY: <20 g CHO + phytoextracts | −7.0 kg | 93.4% | Minimal | No |
| Paoli 2013 [59] | 89 | 12 months | Biphasic: MKD/Med alternating | −16.1 kg (−16.0%) | 88.8% | Transient initial phase | No |
| Cincione 2022 [71] | 80 | 30 days | VLCKD-Med vs. VLCD-Med in T2DM | −7.2 kg | 100% | Not significant | No |
| Gardner 2022 [72] | 40 | 12 wk x2 | Keto vs. Med-Plus crossover | −7–8% weight | 90.0% | LDL elevation with keto | No |
| Sheffler 2023 [60] | 58 | 6 wk | MKD + motivational support | NR | 79–82% | None reported | No |
| Nagpal 2019 [75] | 17 | 6 wk | Modified MKD in MCI | −3.1 kg | 88.2% | Minimal | No |
| Evaluation Domain | Standard Hypocaloric Diet | GLP-1 Agonists | Standard KD | AKMP (Proposed Protocol) |
|---|---|---|---|---|
| Weight Quality (Lean Mass) | Lean mass loss of ~25% of total weight. No specific protective mechanisms [185]. | Induces substantial weight loss composed of both fat mass and significant lean mass reduction, without active muscle protection mechanisms [82,186]. | VLCKD preserves lean mass better than conventional hypocaloric diets [187,188,189]. | Designed to preserve lean mass through protein adequacy (1.6 g/kg) and the anticatabolic effect of ketones. |
| Metabolic Adaptation (Expenditure) | Does not address TEE decline (see Section 4). | Potent appetite suppression, with reduced hunger and food preoccupation [190], without increased energy expenditure; in fact, REE decreases in line with expected metabolic adaptation [81]. | Leverages metabolic advantage (150–300 kcal/day) but without dynamic adjustments [83,84]. | Designed to actively counteract through: (1) Metabolic advantage, and (2) Dynamic adjustment system. |
| Sustainability and Adherence | Average long-term adherence of 60.5%. Improves with supervision (RR 1.65) [136]. | Requires chronic treatment. Recovery of ~2/3 of weight lost upon discontinuation [80]. | High adjusted adherence (93.4%) demonstrated in a 6-week supervised study [70]. Limited long-term sustainability. | Designed to maximize adherence through: (1) Mediterranean pattern, (2) Personalization, and (3) Intensive support. |
| Patient Autonomy | Moderate. Initial success predicts maintenance [191]. | Minimal. Effect depends on continuous drug presence [80]. | Moderate. Requires macro self-management. | Maximum. Central goal of empowering for self-management through Transition Phase and education. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Gorrita, C.; San Onofre, N.; Merino-Torres, J.F.; Soriano, J.M. Beyond GLP-1 Agonists: An Adaptive Ketogenic–Mediterranean Protocol to Counter Metabolic Adaptation in Obesity Management. Nutrients 2025, 17, 2699. https://doi.org/10.3390/nu17162699
García-Gorrita C, San Onofre N, Merino-Torres JF, Soriano JM. Beyond GLP-1 Agonists: An Adaptive Ketogenic–Mediterranean Protocol to Counter Metabolic Adaptation in Obesity Management. Nutrients. 2025; 17(16):2699. https://doi.org/10.3390/nu17162699
Chicago/Turabian StyleGarcía-Gorrita, Cayetano, Nadia San Onofre, Juan F. Merino-Torres, and Jose M. Soriano. 2025. "Beyond GLP-1 Agonists: An Adaptive Ketogenic–Mediterranean Protocol to Counter Metabolic Adaptation in Obesity Management" Nutrients 17, no. 16: 2699. https://doi.org/10.3390/nu17162699
APA StyleGarcía-Gorrita, C., San Onofre, N., Merino-Torres, J. F., & Soriano, J. M. (2025). Beyond GLP-1 Agonists: An Adaptive Ketogenic–Mediterranean Protocol to Counter Metabolic Adaptation in Obesity Management. Nutrients, 17(16), 2699. https://doi.org/10.3390/nu17162699







