Adding L-Carnitine and Selenium to Methimazole in Graves’ Disease: A Prospective Randomized Trial on Thyroid Markers and Quality of Life
Abstract
1. Introduction
2. Methods
2.1. Study Design
- First diagnosis of overt GD (TSH < lower limit of normal range (LLN) for the reference laboratory, fT4 > upper limit of normal range (ULN) and/or fT3 > ULN, TRAb titer > ULN) with indication for initiating medical therapy with MMI.
- Pregnancy;
- Age below 18 years;
- GO at enrollment;
- Reported intolerance and/or hypersensitivity reaction to any component of the studied supplement;
- Patients already requiring definitive therapy at enrollment;
- History of cardiovascular disease,
- History of anxiety and/or mood disorders.
2.2. Interventions
- Control Group (C) (30 patients): treated according to standard practice with MMI and beta-blockers only;
- Intervention Group (I) (30 patients): treated with MMI, beta-blockers, and a combined supplement containing selenium (83 mcg) and L-carnitine (500 mg).
- Spontaneous remission: defined as normalization of TSH, fT4, and fT3 levels with concurrent TRAb negativity, confirmed after 2 months off therapy;
- Indication for definitive therapy: when the investigator determined that the patient required thyroidectomy or radioiodine therapy;
- Intolerance to treatments: side effects and/or intolerance were evaluated during each evaluation, allowing patients to withdraw from the study at any time and pursue alternative treatments. If intolerance to the supplement occurred within the first 2 months, the supplement was discontinued, and the patient was subsequently analyzed as part of the Control Group, following an as-treated approach.
More About Treatments
- MMI: MMI was administered in its standard 5 mg tablet formulation. According to international guidelines, an initial dose of 10–30 mg was prescribed, with titration during follow-up to reach euthyroidism, based on fT3 and fT4 levels, aiming for a maintenance dose of typically 5–10 mg. Patients obtained MMI from their local pharmacies. In Italy, only one formulation of MMI is commercially available (Tapazole © tablets), eliminating any source of bias.
- Supplement: The supplement (Tiroxil 0.4 ©) contained 83 µg of elemental selenium in the form of L-selenomethionine, together with 500 mg of L-carnitine, supplied as tablets with a recommended dosage of one tablet daily, irrespective of meals. It is commercially available in Italy and was supplied at no cost by the manufacturing company, Lo.Li. Pharma™ (Rome, Italy). The tablet was self-administered daily at home by the patients; therefore, no adherence monitoring strategies could be implemented. Yet, to enhance adherence, the supplement was periodically provided to patients. Reported side effects were limited to gastrointestinal issues such as diarrhea and nausea.
2.3. Biochemical Evaluations
Methimazole Cumulative Dose
2.4. Symptoms and Quality of Life Assessment
2.5. Clinical and Anamnestic Data Collection
2.6. Endpoints and Outcome Assessment
- Association between the treatment and rates of spontaneous resolution;
- Higher cumulative incidence of TRAb negativity (TRAb < ULN);
- Lower MMI cumulative dose;
- Lower patients reported symptoms over time (lower AUC of overall Symptom Score).
- Variations over time of thyroid function markers and TRAb titer;
- Cumulative incidence of the normalization of thyroid function indices (TSH > LLN, fT4 < ULN, fT3 < ULN);
- Reduction of patient-reported symptoms over time, as single items.
2.7. Randomization and Blinding
2.8. Statistical Analysis
3. Results
3.1. Population
3.2. Follow–up, Resolution, and Definitive Therapies
3.3. Thyroid-Stimulating Hormone (TSH)
3.4. Free Thyroid Hormones (fT3 and fT4)
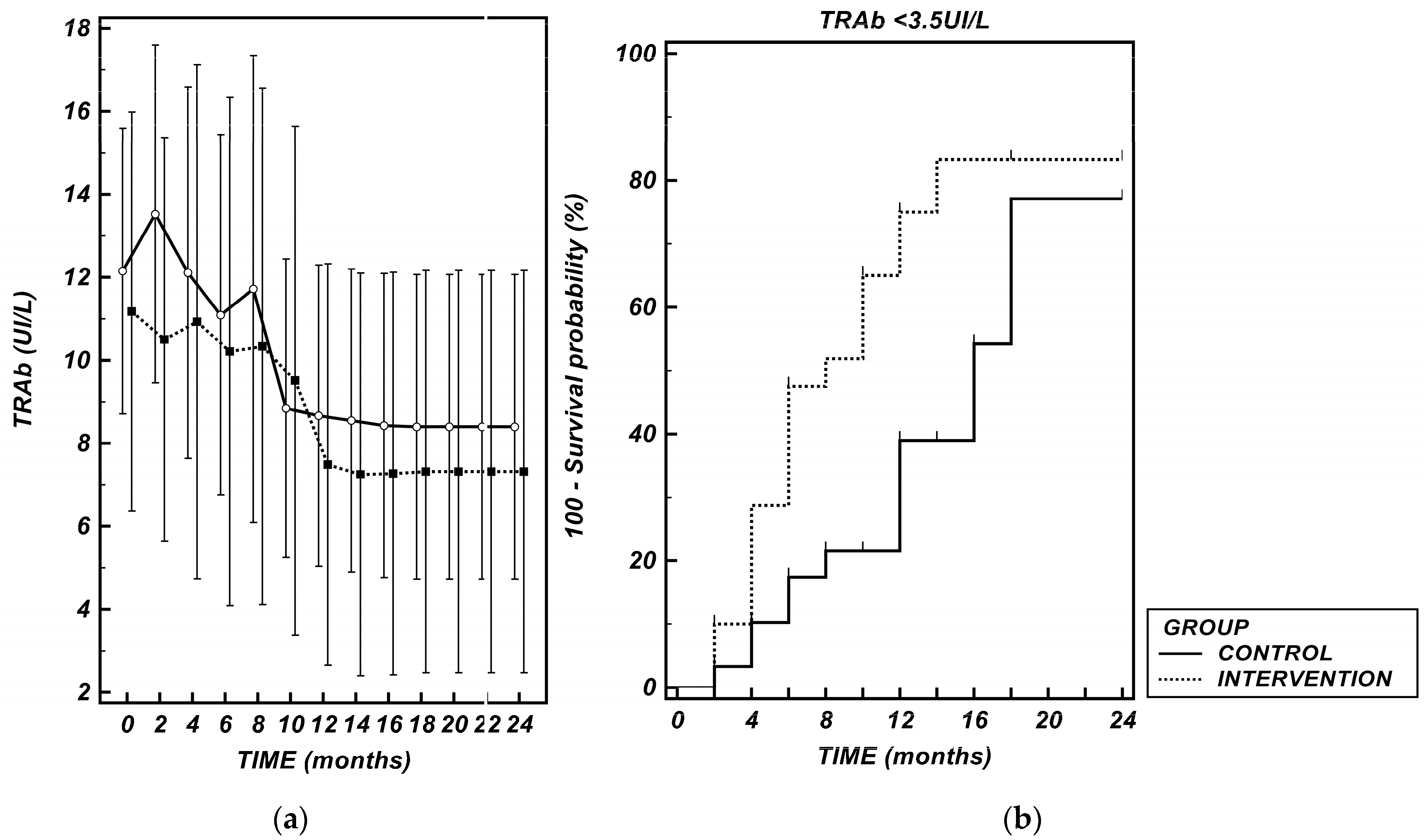
3.5. TSH Receptor Antibodies (TRAb)
- MMI dosage, as an independent variable (β = −0.270, p < 0.001);
- Treatment duration, as an independent variable (β = −0.231, p < 0.001);
- The interaction between Intervention Group and MMI dosage, (β = −0.246, p = 0.005).
3.6. Methimazole (MMI) Dosage
3.7. Symptoms and Quality of Life: Symptom Score (SS)
3.8. Symptoms and Quality of Life: Individual Items
- -
- For irritability, the intervention proved both an independent effect (p < 0.001) on reducing the symptom and a synergistic effect with MMI dosage and time (MMI dose × Group [I]: p < 0.001; Time × Group [I]: p < 0.001; MMI dose × Time × Group [I]: p = 0.006) (Supplementary Table S4);
- -
- For tremor, mood lability, and heat intolerance, the effect was only dependent on time (Time × Group [I]: p = 0.009, p < 0.001, p = 0.024, respectively) (Supplementary Tables S5, S6 and S9);
- -
- For exertional dyspnea, again, the effect was dependent on both time and MMI dosage (Time × Group [I]: p = 0.006; MMI dose × Time × Group [I]: p < 0.001) (Supplementary Table S11).
4. Discussion
Limitations of Our Study
- -
- The absence of a validated QoL questionnaire is one limitation, as it may have exposed the results to several potential biases, including measurement bias due to reduced psychometric robustness and increased susceptibility to interpretation variability by respondents. Furthermore, the absence of prior validation may reduce the comparability of our findings with those obtained in other settings using standardized tools. Nevertheless, the choice of a non-validated questionnaire allowed us to design questions specifically tailored to the clinical features and symptomatic spectrum of hyperthyroidism. At the main follow-up time points, the questionnaire demonstrated good internal consistency (Supplementary Materials—Internal Consistency of QoL Questionnaire (Cronbach’s alpha)), supporting its reliability within the context of our study. In addition, since the same instrument was employed in the study that primarily inspired our protocol, its use facilitated a direct and meaningful comparison with the available literature.
- -
- The lack of placebo and blinded control group is another limitation that could have exposed the study to expectation bias, especially impacting subjective outcomes on symptoms and quality of life. However, it is precisely in these domains that our results have already proven to be of limited significance, thereby minimizing the potential impact of this limitation on the overall study conclusions.
- -
- The absence of baseline and longitudinal serum measurements of LCT and selenium levels is another limitation. These measurements are frequently included in similar studies to correlate clinical outcomes with effective biochemical availability of the supplements.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahaly, G.J.; Bartalena, L.; Hegedüs, L.; Leenhardt, L.; Poppe, K.; Pearce, S.H. 2018 European Thyroid Association Guideline for the Management of Graves’ Hyperthyroidism. Eur. Thyroid. J. 2018, 7, 167–186. [Google Scholar] [CrossRef]
- Trzepacz, P.T.; McCue, M.; Klein, I.; Levey, G.S.; Greenhouse, J. A psychiatric and neuropsychological study of patients with untreated Graves’ disease. Gen. Hosp. Psychiatry 1988, 10, 49–55. [Google Scholar] [CrossRef]
- Brandt, F.; Thvilum, M.; Almind, D.; Christensen, K.; Green, A.; Hegedüs, L.; Brix, T.H. Graves’ disease and toxic nodular goiter are both associated with increased mortality but differ with respect to the cause of death: A Danish population-based register study. Thyroid 2013, 23, 408–413. [Google Scholar] [CrossRef]
- Yazkan Akgül, G.; Köprülü, Ö. Examination of quality of life and psychiatric symptoms in childhood Graves’ disease. J. Pediatr. Endocrinol. Metab. 2024, 37, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Lane, L.C.; Rankin, J.; Cheetham, T. A survey of the young person’s experience of Graves’ disease and its management. Clin. Endocrinol. 2021, 94, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.N.; Braverman, L.E. Hyperthyroidism: Advantages and disadvantages of medical therapy. Surg. Clin. N. Am. 2004, 84, 833–847. [Google Scholar] [CrossRef]
- Abraham, P.; Avenell, A.; McGeoch, S.C.; Clark, L.F.; Bevan, J.S. Antithyroid drug regimen for treating Graves’ hyperthyroidism. Cochrane Database Syst. Rev. 2010, 2010, CD003420. [Google Scholar] [CrossRef] [PubMed]
- Abraham, P.; Avenell, A.; Park, C.M.; A Watson, W.; Bevan, J.S. A systematic review of drug therapy for Graves’ hyperthyroidism. Eur. J. Endocrinol. 2005, 153, 489–498. [Google Scholar] [CrossRef]
- Konishi, T.; Okamoto, Y.; Ueda, M.; Fukuda, Y.; Harusato, I.; Tsukamoto, Y.; Hamada, N. Drug discontinuation after treatment with minimum maintenance dose of an antithyroid drug in Graves’ disease: A retrospective study on effects of treatment duration with minimum maintenance dose on lasting remission. Endocr. J. 2011, 58, 95–100. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Liu, Y.-Y.; Liu, G.-H.; Lu, H.-B.; Mao, C.-Y. l-Carnitine and heart disease. Life Sci. 2018, 194, 88–97. [Google Scholar] [CrossRef]
- Benvenga, S. Effects of L-carnitine on thyroid hormone metabolism and on physical exercise tolerance. Horm. Metab. Res. 2005, 37, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Strack, E.; Woratz, G.; Rotzsch, W. Effects of carnitine in hyperfunction of the thyroid gland. Endokrinologie 1959, 38, 218–225. [Google Scholar]
- Gilgore, S.G.; DeFelice, S.L. Evaluation of carnitine--an antagonist of thyroid hormone. Clinical pharmacology report. J. New Drugs 1966, 6, 349–350. [Google Scholar] [CrossRef]
- DeFelice, S.L.; Gilgore, S.G. The antagonistic effect of carnitine in hyperthyroidism. Preliminary report. J. New Drugs 1966, 6, 351–353. [Google Scholar] [CrossRef]
- Benvenga, S.; Ruggeri, R.M.; Russo, A.; Lapa, D.; Campenni, A.; Trimarchi, F. Usefulness of L-carnitine, a naturally occurring peripheral antagonist of thyroid hormone action, in iatrogenic hyperthyroidism: A randomized, double-blind, placebo-controlled clinical trial. J. Clin. Endocrinol. Metab. 2001, 86, 3579–3594. [Google Scholar] [CrossRef]
- Benvenga, S.; Lakshmanan, M.; Trimarchi, F. Carnitine is a naturally occurring inhibitor of thyroid hormone nuclear uptake. Thyroid 2000, 10, 1043–1050. [Google Scholar] [CrossRef]
- Benvenga, S.; Lapa, D.; Cannavò, S.; Trimarchi, F. Successive thyroid storms treated with L-carnitine and low doses of methimazole. Am. J. Med. 2003, 115, 417–418. [Google Scholar] [CrossRef]
- Chee, R.; Agah, R.; Vita, R.; Benvenga, S. L-carnitine treatment in a seriously ill cancer patient with severe hyperthyroidism. Hormones 2014, 13, 407–412. [Google Scholar] [CrossRef]
- Kimmoun, A.; Munagamage, G.; Dessalles, N.; Gerard, A.; Feillet, F.; Levy, B. Unexpected awakening from comatose thyroid storm after a single intravenous injection of L-carnitine. Intensive Care Med. 2011, 37, 1716–1717. [Google Scholar] [CrossRef] [PubMed]
- Asayama, K.; Dobashi, K.; Hayashibe, H.; Megata, Y.; Kato, K. Lipid Peroxidation and Free Radical Scavengers in Thyroid Dysfunction in the Rat: A Possible Mechanism of Injury to Heart and Skeletal Muscle in Hyperthyroidism*. Endocrinology 1987, 121, 2112–2118. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Driessens, N.; Costa, M.; De Deken, X.; Detours, V.; Corvilain, B.; Maenhaut, C.; Miot, F.; Van Sande, J.; Many, M.-C.; et al. Roles of Hydrogen Peroxide in Thyroid Physiology and Disease. J. Clin. Endocrinol. Metab. 2007, 92, 3764–3773. [Google Scholar] [CrossRef]
- Yamada, T.; Mishima, T.; Sakamoto, M.; Sugiyama, M.; Matsunaga, S.; Wada, M. Oxidation of myosin heavy chain and reduction in force production in hyperthyroid rat soleus. J. Appl. Physiol. 2006, 100, 1520–1526. [Google Scholar] [CrossRef]
- Venditti, P.; Balestrieri, M.; Di Meo, S.; De Leo, T. Effect of thyroid state on lipid peroxidation, antioxidant defences, and susceptibility to oxidative stress in rat tissues. J. Endocrinol. 1997, 155, 151–157. [Google Scholar] [CrossRef]
- Leo, M.; Bartalena, L.; Dottore, G.R.; Piantanida, E.; Premoli, P.; Ionni, I.; Di Cera, M.; Masiello, E.; Sassi, L.; Tanda, M.L.; et al. Effects of selenium on short-term control of hyperthyroidism due to Graves’ disease treated with methimazole: Results of a randomized clinical trial. J. Endocrinol. Investig. 2017, 40, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Marcocci, C.; Kahaly, G.J.; Krassas, G.E.; Bartalena, L.; Prummel, M.; Stahl, M.; Altea, M.A.; Nardi, M.; Pitz, S.; Boboridis, K.; et al. Selenium and the Course of Mild Graves’ Orbitopathy. N. Engl. J. Med. 2011, 364, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Köhrle, J. Selenium and the thyroid. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 392–401. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Drutel, A.; Archambeaud, F.; Caron, P. Selenium and the thyroid gland: More good news for clinicians. Clin. Endocrinol. 2013, 78, 155–164. [Google Scholar] [CrossRef]
- Vrca, V.; Mayer, L.; Škreb, F.; Rahelić, D.; Marušić, S. Antioxidant supplementation and serum lipids in patients with Graves’ disease: Effect on LDL-cholesterol. Acta Pharm. 2012, 62, 115–122. [Google Scholar] [CrossRef]
- Kahaly, G.J.; Riedl, M.; König, J.; Diana, T.; Schomburg, L. Double-Blind, Placebo-Controlled, Randomized Trial of Selenium in Graves Hyperthyroidism. J. Clin. Endocrinol. Metab. 2017, 102, 4333–4341. [Google Scholar] [CrossRef]
- Guerra, L.N.; de Molina, M.d.C.R.; Miler, E.A.; Moiguer, S.; Karner, M.; Burdman, J.A. Antioxidants and methimazole in the treatment of Graves’ disease: Effect on urinary malondialdehyde levels. Clin. Chim. Acta 2005, 352, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Calissendorff, J.; Mikulski, E.; Larsen, E.H.; Möller, M. A Prospective Investigation of Graves’ Disease and Selenium: Thyroid Hormones, Auto-Antibodies and Self-Rated Symptoms. Eur. Thyroid. J. 2015, 4, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Wichman, J.; Winther, K.H.; Bonnema, S.J.; Hegedüs, L. Selenium Supplementation Significantly Reduces Thyroid Autoantibody Levels in Patients with Chronic Autoimmune Thyroiditis: A Systematic Review and Meta-Analysis. Thyroid 2016, 26, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wu, D.; Ying, H.; Zhang, Y. A pilot study on the beneficial effects of additional selenium supplementation to methimazole for treating patients with Graves’ disease. Turk. J. Med. Sci. 2019, 49, 715–722. [Google Scholar] [CrossRef]
- Zheng, H.; Wei, J.; Wang, L.; Wang, Q.; Zhao, J.; Chen, S.; Wei, F.; Kennedy, D.A. Effects of Selenium Supplementation on Graves’ Disease: A Systematic Review and Meta-Analysis. Evid. Based Complement. Alternat. Med. 2018, 2018, 3763565. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Chen, S.R.; Hou, X.; Wang, X.F.; Zhao, S.H.; Song, J.Q.; Wang, Y.G. Effect of Selenium Supplementation on Recurrent Hyperthyroidism Caused by Graves’ Disease: A Prospective Pilot Study. Horm. Metab. Res. 2016, 48, 559–564. [Google Scholar] [CrossRef]
- Bartalena, L.; Kahaly, G.J.; Baldeschi, L.; Dayan, C.M.; Eckstein, A.; Marcocci, C.; Marinò, M.; Vaidya, B.; Wiersinga, W.M.; Ayvaz, G.; et al. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur. J. Endocrinol. 2021, 185, G43–G67. [Google Scholar] [CrossRef]
- Nordio, M. A novel treatment for subclinical hyperthyroidism: A pilot study on the beneficial effects of l-carnitine and selenium. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2268–2273. [Google Scholar]
- Bednarczuk, T.; Schomburg, L. Challenges and perspectives of selenium supplementation in Graves’ disease and orbitopathy. Hormones 2020, 19, 31–39. [Google Scholar] [CrossRef]
- Lanzolla, G.; Marinò, M.; Marcocci, C. Selenium in the Treatment of Graves’ Hyperthyroidism and Eye Disease. Front. Endocrinol. 2020, 11, 608428. [Google Scholar] [CrossRef] [PubMed]


| Population (n= 60) | Control Group (n = 30) | Intervention Group (n = 30) | p | |
|---|---|---|---|---|
| Age (years) | 48.51 (44.66–52.38) | 48.03 (41.95–51.12) | 49.00 (43.87–54.13) | 0.804 |
| Gender (female) | 46 (76.67%) | 23 (76.67%) | 23 (76.67%) | 0.760 |
| Body Mass Index | 23.15 (22.21–24.14) | 23.47 (20.36–25.00) | 22.84 (21.56–24.20) | 0.517 |
| Smoking habit | 0.585 | |||
| Active smokers | 21 (35%) | 9 (25%) | 12 (30%) | 0.588 |
| Never smoked | 32 (53.33%) | 18 (60%) | 14 (50%) | 0.438 |
| Stopped smoking | 7 (11.67%) | 3 (15%) | 4 (20%) | 1.000 |
| TSH * (mUI/L) | 0.008 (0.008–0.010) | 0.006 (0.004–0.009) | 0.008 (0.007–0.010) | 0.184 |
| fT3 * (ng/L) | 9.39 (6.20–19.20) | 12.10 (6.12–20.00) | 8.80 (6.15–14.43) | 0.282 |
| fT4 (ng/L) | 26.82 (23.30–30.33) | 29.03 (23.53–34.52) | 24.53 (19.99–29.07) | 0.203 |
| TRAb (UI/L) | 11.89 (8.94–14.84) | 12.15 (8.71–15.79) | 11.87 (6.80–16.93) | 0.748 |
| Population (n = 60) | Control Group (n = 30) | Intervention Group (n = 30) | p | |
|---|---|---|---|---|
| Spontaneous resolution | 25 (41.67%) | 4 (13.33%) | 19 (63.33%) | <0.001 |
| Time to spontaneous resolution | 11.30 (9.73–12.88) | 11.50 (7.50–15.51) | 11.26 (9.38–13.14) | 0.909 |
| Definitive treatment | 13 (21.67%) | 7 (23.33%) | 6 (20%) | 0.185 |
| Type of definitive treatment | 0.559 | |||
| Radioiodine | 4 (6.67%) | 3 (10%) | 1 (3.33%) | 0.606 |
| Thyroidectomy | 9 (15%) | 4 (13.33%) | 5 (16.67%) | 0.709 |
| Time to definitive treatment | 15.92 (12.00–19.84) | 16.29 (11.46–21.11) | 15.50 (6.85–24.57) | 0.838 |
| Generalized Linear Mixed Model Fit by Maximum Likelihood (Laplace Approximation) | ||||
|---|---|---|---|---|
| Formula: TRAb ~ GROUP × TIME. × MMI.dose. + (1|ID) | ||||
| Family: Gaussian (Identity); | ||||
| Fixed Effects | Estimate | S.E. | t-Value | p-Value |
| GROUP [INTERVENTION] | 0.882 | 3.034 | 0.291 | 0.771 |
| TIME. | −0.231 | 0.043 | −5.321 | <0.001 |
| MMI.dose. | −0.270 | 0.062 | 4.364 | <0.001 |
| GROUP [INTERVENTION]:TIME. | 0.037 | 0.057 | 0.648 | 0.517 |
| GROUP [INTERVENTION]:MMI.dose. | −0.246 | 0.087 | −2.836 | 0.005 |
| TIME.:MMI.dose. | 0.005 | 0.005 | 1.028 | 0.304 |
| GROUP [INTERVENTION]:TIME.:MMI.dose | −0.004 | 0.007 | −0.514 | 0.607 |
| Generalized Linear Model | ||||
|---|---|---|---|---|
| Formula: RESOLUTION ~ AGE + FT4 + SMOKE + SEX + TRAB + GROUP. | ||||
| Family = binomial(logit) | ||||
| Overall Model Fit | ||||
| Null model Log Likelihood 0.025; Full model Log Likelihood 51.257 | ||||
| Chi-squared 23.767; Degrees of Freedom 6; Significance level p = 0.0006 | ||||
| Estimate | S.E. | z-Value | p-Value | |
| AGE | −0.047 | 0.030 | −1.544 | 0.123 |
| FT4 | −0.004 | 0.029 | −0.146 | 0.884 |
| SMOKE [Yes] | −1.312 | 0.825 | −1.590 | 0.112 |
| SEX [Male] | 1.152 | 0.881 | 1.308 | 0.191 |
| TRAB | −0.031 | 0.038 | −0.807 | 0.420 |
| GROUP [INTERVENTION] | 3.015 | 0.824 | 3.658 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, M.; Meomartino, L.; Zavattaro, M.; Selvatico, G.; Rossetto Giaccherino, R.; Pagano, L. Adding L-Carnitine and Selenium to Methimazole in Graves’ Disease: A Prospective Randomized Trial on Thyroid Markers and Quality of Life. Nutrients 2025, 17, 2693. https://doi.org/10.3390/nu17162693
Rossi M, Meomartino L, Zavattaro M, Selvatico G, Rossetto Giaccherino R, Pagano L. Adding L-Carnitine and Selenium to Methimazole in Graves’ Disease: A Prospective Randomized Trial on Thyroid Markers and Quality of Life. Nutrients. 2025; 17(16):2693. https://doi.org/10.3390/nu17162693
Chicago/Turabian StyleRossi, Mattia, Letizia Meomartino, Marco Zavattaro, Gloria Selvatico, Ruth Rossetto Giaccherino, and Loredana Pagano. 2025. "Adding L-Carnitine and Selenium to Methimazole in Graves’ Disease: A Prospective Randomized Trial on Thyroid Markers and Quality of Life" Nutrients 17, no. 16: 2693. https://doi.org/10.3390/nu17162693
APA StyleRossi, M., Meomartino, L., Zavattaro, M., Selvatico, G., Rossetto Giaccherino, R., & Pagano, L. (2025). Adding L-Carnitine and Selenium to Methimazole in Graves’ Disease: A Prospective Randomized Trial on Thyroid Markers and Quality of Life. Nutrients, 17(16), 2693. https://doi.org/10.3390/nu17162693






