Relationship Between Total 25-Hydroxyvitamin D and Parathyroid Hormone Concentrations During Early Gestation in Indian Women
Abstract
1. Introduction
2. Materials and Methods
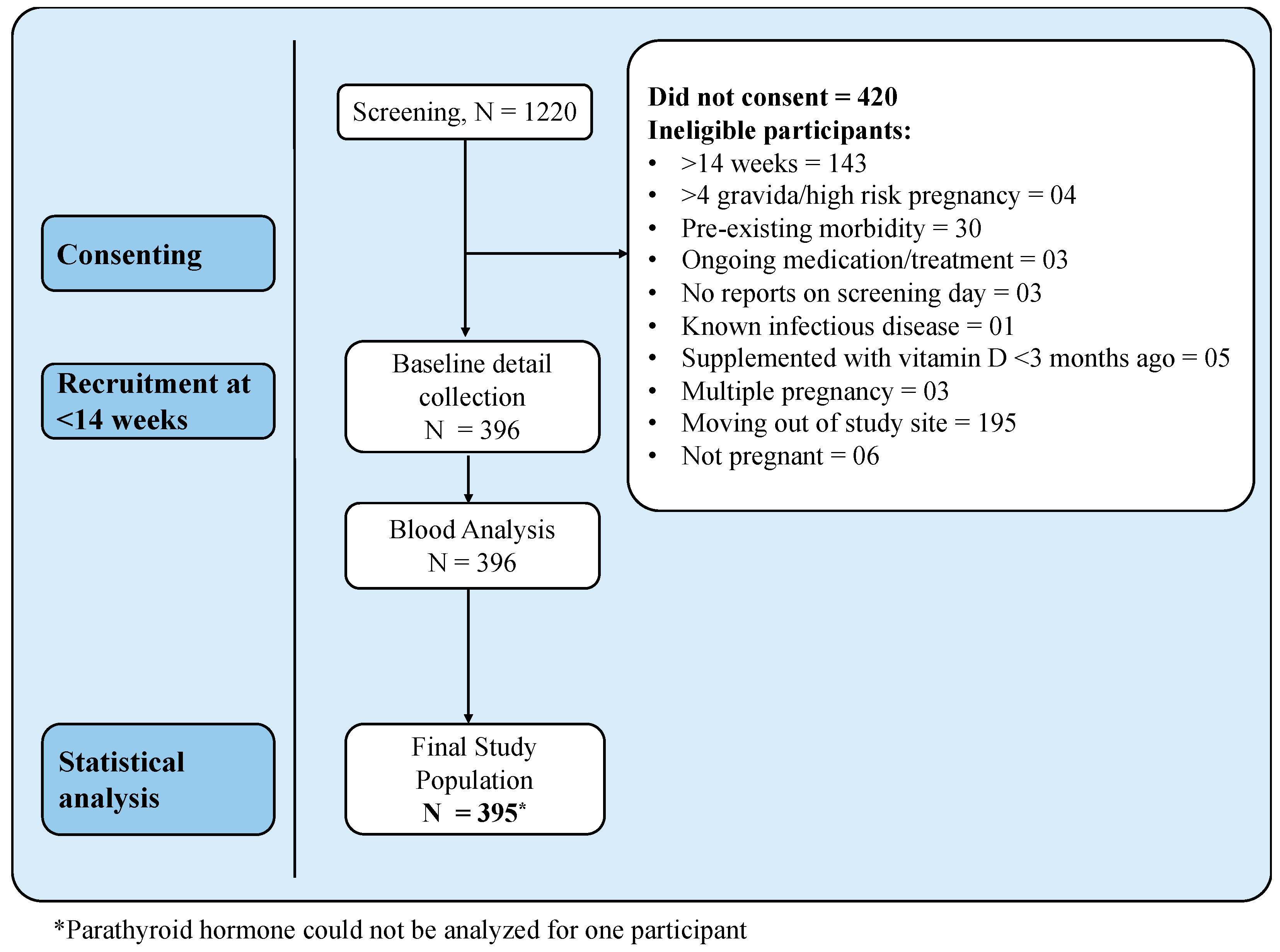
2.1. Study Participants
2.2. Study Measurements
2.3. Laboratory Measurements
2.4. Sample Size and Statistical Analyses
3. Results
3.1. General Characteristics of Study Population
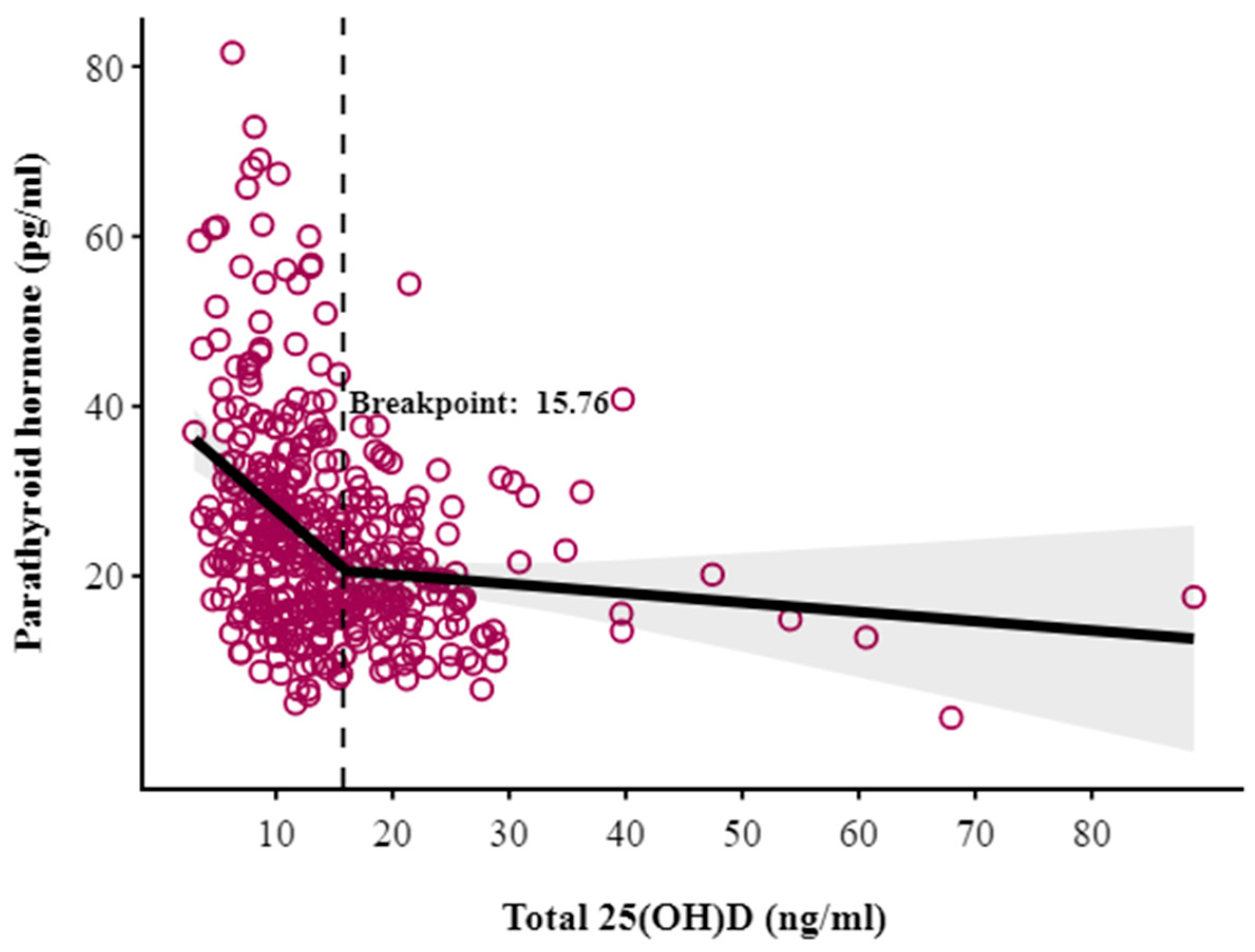
3.2. Breakpoint Analysis of 25-Hydroxyvitamin D and Parathyroid Hormone
3.3. Associations with 25(OH)D Status and Parathyroid Hormone Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VDD | Vitamin D Deficiency |
| PTH | Parathyroid Hormone |
| IEC | Institutional Ethics Committee |
| CTRI | Clinical Trials Registry of India |
| HMSC | Health Ministry’s Screening Committee |
| HIV | Human Immunodeficiency Virus |
| HBsAg | Hepatitis B Surface Antigen |
| EDTA | Ethylenediaminetetraacetic Acid |
References
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and Regional Prevalence of Vitamin D Deficiency in Population-Based Studies from 2000 to 2022: A Pooled Analysis of 7.9 Million Participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef]
- Khadilkar, A.; Kajale, N.; Oza, C.; Oke, R.; Gondhalekar, K.; Patwardhan, V.; Khadilkar, V.; Mughal, Z.; Padidela, R. Vitamin D Status and Determinants in Indian Children and Adolescents: A Multicentre Study. Sci. Rep. 2022, 12, 16790. [Google Scholar] [CrossRef]
- Aggarwal, A.; Pal, R.; Bhadada, S.K.; Ram, S.; Garg, A.; Bhansali, A.; Singh, P.; Thakur, J.S.; Singh, T.; Sachdeva, N.; et al. Bone Mineral Density in Healthy Adult Indian Population: The Chandigarh Urban Bone Epidemiological Study (CUBES). Arch. Osteoporos. 2021, 16, 17. [Google Scholar] [CrossRef]
- Siddiqee, M.H.; Bhattacharjee, B.; Siddiqi, U.R.; MeshbahurRahman, M. High Prevalence of Vitamin D Deficiency among the South Asian Adults: A Systematic Review and Meta-Analysis. BMC Public Health 2021, 21, 1823. [Google Scholar] [CrossRef]
- Mukherjee, A.; Chaudhry, A.; Khatwani, L.; Khanna, T.; Verma, P. Roadmap to Address Vitamin D Deficiency in India; AF Press: New Delhi, India; Indian Council for Research on International Economic Relations (ICRIER): New Delhi, India, 2025. [Google Scholar]
- Gutiérrez, O.M.; Farwell, W.R.; Kermah, D.; Taylor, E.N. Racial Differences in the Relationship between Vitamin D, Bone Mineral Density, and Parathyroid Hormone in the National Health and Nutrition Examination Survey. Osteoporos. Int. 2011, 22, 1745–1753. [Google Scholar] [CrossRef]
- Mendes, M.M.; Hart, K.H.; Lanham-New, S.A.; Botelho, P.B. Suppression of Parathyroid Hormone as a Proxy for Optimal Vitamin D Status: Further Analysis of Two Parallel Studies in Opposite Latitudes. Nutrients 2020, 12, 942. [Google Scholar] [CrossRef]
- Manson, J.E.; Brannon, P.M.; Rosen, C.J.; Taylor, C.L. Vitamin D Deficiency—Is There Really a Pandemic? N. Engl. J. Med. 2016, 375, 1817–1820. [Google Scholar] [CrossRef]
- Kantorovich, V.; Gacad, M.A.; Seeger, L.L.; Adams, J.S. Bone Mineral Density Increases with Vitamin D Repletion in Patients with Coexistent Vitamin D Insufficiency and Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2000, 85, 3541–3543. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garg, M.K.; Tandon, N.; Marwaha, R.K.; Menon, A.S.; Mahalle, N. The Relationship between Serum 25-Hydroxy Vitamin D, Parathormone and Bone Mineral Density in Indian Population. Clin. Endocrinol. 2014, 80, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Olmos, J.M.; Hernández, J.L.; García-Velasco, P.; Martínez, J.; Llorca, J.; González-Macías, J. Serum 25-Hydroxyvitamin D, Parathyroid Hormone, Calcium Intake, and Bone Mineral Density in Spanish Adults. Osteoporos. Int. 2016, 27, 105–113. [Google Scholar] [CrossRef]
- Garabedian, M.; Holick, M.F.; Deluca, H.F.; Boyle, I.T. Control of 25-Hydroxycholecalciferol Metabolism by Parathyroid Glands. Proc. Natl. Acad. Sci. USA 1972, 69, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Crews, B.O.; Moore, J.; Dietzen, D.J. Circulating Intact Parathyroid Hormone Is Suppressed at 25-Hydroxyvitamin D Concentrations >25 Nmol/L in Children. J. Pediatr. Endocrinol. Metab. 2014, 27, 657–660. [Google Scholar] [CrossRef]
- Kazemian, E.; Madreseh, E.; Azizi, F.; Ashrafivand, S.; Gargari, S.S.; Mansournia, M.A.; Wagner, C.L.; Amouzegar, A. The Association of Parathyroid Hormone with Serum 25-Hydroxyvitamin during Pregnancy. J. Nutr. Sci. 2023, 12, e1. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.K.; Ye, C.; Hanley, A.J.; Connelly, P.W.; Sermer, M.; Zinman, B.; Retnakaran, R. The Relationship Between Parathyroid Hormone and 25-Hydroxyvitamin D During and After Pregnancy. J. Clin. Endocrinol. Metab. 2016, 101, 1729–1736. [Google Scholar] [CrossRef]
- Hysaj, O.; Marqués-Gallego, P.; Richard, A.; Elgizouli, M.; Nieters, A.; Quack Lötscher, K.C.; Rohrmann, S. Parathyroid Hormone in Pregnancy: Vitamin D and Other Determinants. Nutrients 2021, 13, 360. [Google Scholar] [CrossRef]
- Mansur, J.L.; Oliveri, B.; Giacoia, E.; Fusaro, D.; Costanzo, P.R. Vitamin D: Before, during and after Pregnancy: Effect on Neonates and Children. Nutrients 2022, 14, 1900. [Google Scholar] [CrossRef]
- Várbíró, S.; Takács, I.; Tűű, L.; Nas, K.; Sziva, R.E.; Hetthéssy, J.R.; Török, M. Effects of Vitamin D on Fertility, Pregnancy and Polycystic Ovary Syndrome—A Review. Nutrients 2022, 14, 1649. [Google Scholar] [CrossRef]
- You, Z.; Mei, H.; Zhang, Y.; Song, D.; Zhang, Y.; Liu, C. The Effect of Vitamin D Deficiency during Pregnancy on Adverse Birth Outcomes in Neonates: A Systematic Review and Meta-Analysis. Front. Pediatr. 2024, 12, 1399615. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, F.R.; Fernández-Alonso, A.M.; Ferrando-Marco, P.; González-Salmerón, M.D.; Dionis-Sánchez, E.C.; Fiol-Ruiz, G.; Chedraui, P. First Trimester Serum 25-Hydroxyvitamin D Status and Factors Related to Lower Levels in Gravids Living in the Spanish Mediterranean Coast. Reprod. Sci. 2011, 18, 730–736. [Google Scholar] [CrossRef]
- Ahmed, F.; Khosravi-Boroujeni, H.; Khan, M.R.; Roy, A.K.; Raqib, R. Prevalence and Predictors of Vitamin D Deficiency and Insufficiency among Pregnant Rural Women in Bangladesh. Nutrients 2021, 13, 449. [Google Scholar] [CrossRef]
- Pratumvinit, B.; Wongkrajang, P.; Wataganara, T.; Hanyongyuth, S.; Nimmannit, A.; Chatsiricharoenkul, S.; Manonukul, K.; Reesukumal, K. Maternal Vitamin D Status and Its Related Factors in Pregnant Women in Bangkok, Thailand. PLoS ONE 2015, 10, e0131126. [Google Scholar] [CrossRef]
- Khan, A.H.; Islam, F.; Rehman, A. Assessment of Serum Vitamin D Level in Third Trimester of Multiparous Women of Sargodha Region with Relation to Sun Exposure and Age Group. Prof. Med. J. 2020, 27, 2483–2487. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Kumar, A.; Jain, A.; Kim, R.; Subramanian, S.V. Trends in the Quality of Antenatal Care in India: Patterns of Change across 36 States and Union Territories, 1999–2021. J. Glob. Health 2024, 14, 04188. [Google Scholar] [CrossRef]
- Ravinder, S.S.; Padmavathi, R.; Maheshkumar, K.; Mohankumar, M.; Maruthy, K.N.; Sankar, S. Prevalence of Vitamin D Deficiency among South Indian Pregnant Women. J. Fam. Med. Prim. Care 2022, 11, 2884–2889. [Google Scholar] [CrossRef] [PubMed]
- Dwarkanath, P.; Vinotha, P.; Thomas, T.; Joseph, S.; Thomas, A.; Shirley, G.; Sheela, C.N.; Mehta, S.; Kurpad, A.V. Relationship of Early Vitamin D Concentrations and Gestational Diabetes Mellitus in Indian Pregnant Women. Front. Nutr. 2019, 6, 116. [Google Scholar] [CrossRef]
- Sharma, N.; Nath, C.; Mohammad, J. Vitamin D Status in Pregnant Women Visiting a Tertiary Care Center of North Eastern India. J. Fam. Med. Prim. Care 2019, 8, 356. [Google Scholar] [CrossRef]
- Mani, I.; Dwarkanath, P.; Thomas, T.; Thomas, A.; Kurpad, A.V. Maternal Fat and Fatty Acid Intake and Birth Outcomes in a South Indian Population. Int. J. Epidemiol. 2016, 45, 523–531. [Google Scholar] [CrossRef]
- Dwarkanath, P.; Muthayya, S.; Vaz, M.; Thomas, T.; Mhaskar, A.; Mhaskar, R.; Thomas, A.; Bhat, S.; Kurpad, A. The Relationship between Maternal Physical Activity during Pregnancy and Birth Weight. Asia Pac. J. Clin. Nutr. 2007, 16, 704–710. [Google Scholar] [PubMed]
- Betts, M.G.; Forbes, G.J.; Diamond, A.W. Thresholds in Songbird Occurrence in Relation to Landscape Structure. Conserv. Biol. 2007, 21, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.B. Hypothesis Testing When a Nuisance Parameter Is Present Only Under the Alternatives. Biometrika 1987, 74, 33–43. [Google Scholar] [CrossRef]
- Davies, R.B. Hypothesis Testing When a Nuisance Parameter Is Present Only under the Alternative: Linear Model Case. Biometrika 2002, 89, 484–489. [Google Scholar] [CrossRef]
- Guideline on Haemoglobin Cutoffs to Define Anaemia in Individuals and Populations. Available online: https://www.who.int/publications/i/item/9789240088542 (accessed on 19 March 2025).
- de Haas, S.; Ghossein-Doha, C.; van Kuijk, S.M.J.; van Drongelen, J.; Spaanderman, M.E.A. Physiological Adaptation of Maternal Plasma Volume during Pregnancy: A Systematic Review and Meta-Analysis. Ultrasound Obstet. Gynecol. 2017, 49, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Bukhary, N.B.I.; Isa, Z.M.; Shamsuddin, K.; Lin, K.G.; Mahdy, Z.A.; Hassan, H.; Yeop, N.S.H. Risk Factors for Antenatal Hypovitaminosis D in an Urban District in Malaysia. BMC Pregnancy Childbirth 2016, 16, 156. [Google Scholar] [CrossRef]
- Hemmingway, A.; Kenny, L.C.; Malvisi, L.; Kiely, M.E. Exploring the Concept of Functional Vitamin D Deficiency in Pregnancy: Impact of the Interaction between 25-Hydroxyvitamin D and Parathyroid Hormone on Perinatal Outcomes. Am. J. Clin. Nutr. 2018, 108, 821–829. [Google Scholar] [CrossRef]
- Fraser, D.; Kooh, S.W.; Scriver, C.R. Hyperparathyroidism as the Cause of Hyperaminoaciduria and Phosphaturia in Human Vitamin D Deficiency. Pediatr. Res. 1967, 1, 425–435. [Google Scholar] [CrossRef]
- Takaoka, N.; Nishida, K.; Sairenchi, T.; Umesawa, M.; Noguchi, R.; Someya, K.; Kobashi, G. Changes in Vitamin D Status Considering Hemodilution Factors in Japanese Pregnant Women According to Trimester: A Longitudinal Survey. PLoS ONE 2020, 15, e0239954. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Aji, A.S.; Erwinda, E.; Yusrawati, Y.; Malik, S.G.; Lipoeto, N.I. Vitamin D Deficiency Status and Its Related Risk Factors during Early Pregnancy: A Cross-Sectional Study of Pregnant Minangkabau Women, Indonesia. BMC Pregnancy Childbirth 2019, 19, 183. [Google Scholar] [CrossRef]
- Reverzani, C.; Zaake, D.; Nansubuga, F.; Ssempewo, H.; Manirakiza, L.; Kayiira, A.; Tumwine, G. Prevalence of Vitamin D Deficiency and Its Association with Adverse Obstetric Outcomes among Pregnant Women in Uganda: A Cross-Sectional Study. BMJ Open 2025, 15, e089504. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Kim, S.; Yoo, H.; Cho, Y.Y.; Kim, S.W.; Chung, J.H.; Oh, S.; Lee, S.-Y. High Prevalence of Vitamin D Deficiency in Pregnant Korean Women: The First Trimester and the Winter Season as Risk Factors for Vitamin D Deficiency. Nutrients 2015, 7, 3427–3448. [Google Scholar] [CrossRef]
- Pikuleva, I.A.; Waterman, M.R. Cytochromes P450: Roles in Diseases*. J. Biol. Chem. 2013, 288, 17091–17098. [Google Scholar] [CrossRef] [PubMed]
- Toxqui, L.; Vaquero, M.P. Chronic Iron Deficiency as an Emerging Risk Factor for Osteoporosis: A Hypothesis. Nutrients 2015, 7, 2324–2344. [Google Scholar] [CrossRef]
- Ghaleb, A.; Abdi, S.; Yakout, S.; Danish Hussain, S.; Wani, K.; Masoud, M.; Alnaami, A.; Al-Daghri, N.M. Serum Iron Deficiency and 25-Hydroxyvitamin D Deficiency as an Independent Risk Factor for Osteoporosis in Postmenopausal Arab Women. J. King Saud. Univ. Sci. 2021, 33, 101217. [Google Scholar] [CrossRef]
- Hollis, B.W. Circulating 25-Hydroxyvitamin D Levels Indicative of Vitamin D Sufficiency: Implications for Establishing a New Effective Dietary Intake Recommendation for Vitamin D1. J. Nutr. 2005, 135, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.; Simbar, M.; Amiri, M.; Bidhendi-Yarandi, R.; Hosseinpanah, F.; Ramezani Tehrani, F. The Optimal Cut-off Point of Vitamin D for Pregnancy Outcomes Using a Generalized Additive Model. Clin. Nutr. 2021, 40, 2145–2153. [Google Scholar] [CrossRef] [PubMed]
- Merewood, A.; Mehta, S.D.; Chen, T.C.; Bauchner, H.; Holick, M.F. Association between Vitamin D Deficiency and Primary Cesarean Section. J. Clin. Endocrinol. Metab. 2009, 94, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.-C.; Huang, C.-Y.; Wang, J.-H.; Shih, C.-L.; Wu, P. Effects of Vitamin D in Pregnancy on Maternal and Offspring Health-Related Outcomes: An Umbrella Review of Systematic Review and Meta-Analyses. Nutr. Diabetes 2024, 14, 35. [Google Scholar] [CrossRef]
- Gupta, N.; Agarwal, A.; Jindal, R.; Sr, S. Estimating Vitamin D Threshold for the Indian Population: Delving into the Actual Disease Burden. Med. J. Armed Forces India 2023, 79, S224–S229. [Google Scholar] [CrossRef]
| Variables (Mean ± SD) | N = 396 |
|---|---|
| Age (years) | 26.3 ± 4.0 |
| Gestational age | 11.6 ± 2.3 |
| Education, n (%) | |
| Up to high school | 50 (12.6%) |
| PUC and diploma | 98 (24.7%) |
| University and above | 248 (62.6%) |
| Parity, n (%) | |
| Nulliparous | 250 (63.1%) |
| Multiparous | 146 (36.9%) |
| Participant employment, n (%) | |
| Yes | 127 (32.1%) |
| No | 269 (67.9%) |
| Anthropometry | |
| Weight (kg) | 57.5 ± 11.0 |
| Height (cm) | 155.7 ± 5.4 |
| Body Mass Index (kg/m2) | 23.7 ± 4.2 |
| Body Composition | |
| Fat percent | 29.3 ± 3.2 |
| Fat mass (kg) | 17.1 ± 4.7 |
| Fat-free mass (kg) | 40.2 ± 6.3 |
| Blood pressure (mmHg) | |
| Systolic | 97.9 ± 9.5 |
| Diastolic | 68.4 ± 17.2 |
| Dietary intakes | |
| Energy (kcal/d) | 1860 ± 471 |
| Protein (g/d) | 57.4 ± 16.3 |
| Total fat (g/d) | 54.3 ± 17.6 |
| Carbohydrates (g/d) | 285.5 ± 75.4 |
| Fortified Vitamin D2 (IU/d) | 174.9 (87.2) |
| Calcium intake (mg/d) | 894.5 ± 319.4 |
| Iron (mg/d) | 14.8 ± 4.9 |
| Physical Activity Level (PAL) | 1.5 ± 0.2 |
| Variable (N = 395) | Category | Mean ± SD |
|---|---|---|
| Hemoglobin (g/dL) | – | 11.8 ± 1.3 |
| Anemia Status, n (%) | Normal | 326 (83.0) |
| Mild | 37 (9.3) | |
| Moderate | 30 (7.6) | |
| Severe | 3 (0.8) | |
| Parathyroid Hormone (pg/mL) | – | 24.7 ± 12.4 |
| Total 25(OH)D (ng/mL) | – | 14.6 ± 8.7 |
| Covariates | Unadjusted Odds Ratio (95% CI) | p-Value |
|---|---|---|
| Season | ||
| Monsoon | Ref | |
| Post-Monsoon | 3.00 (1.62–5.55) | <0.001 |
| Winter | 2.43 (1.41–4.19) | 0.001 |
| Parity | ||
| Multiparous | Ref | |
| Nulliparous | 1.74 (1.14–2.67) | 0.011 |
| Anemia status | ||
| Normal | Ref | |
| Moderate/Severe Anemia | 2.76 (1.03–7.41) | 0.044 |
| Physical Activity | 0.24 (0.06–0.97) | 0.045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chopra, N.; Ayoob, F.; C, R.; Karanth, S.; Harish, M.; Thomas, A.; Adiga, V.; Vyakarnam, A.; Hawrylowicz, C.; Kurpad, A.V.; et al. Relationship Between Total 25-Hydroxyvitamin D and Parathyroid Hormone Concentrations During Early Gestation in Indian Women. Nutrients 2025, 17, 2626. https://doi.org/10.3390/nu17162626
Chopra N, Ayoob F, C R, Karanth S, Harish M, Thomas A, Adiga V, Vyakarnam A, Hawrylowicz C, Kurpad AV, et al. Relationship Between Total 25-Hydroxyvitamin D and Parathyroid Hormone Concentrations During Early Gestation in Indian Women. Nutrients. 2025; 17(16):2626. https://doi.org/10.3390/nu17162626
Chicago/Turabian StyleChopra, Nandini, Fathima Ayoob, Roopashree C, Shashikala Karanth, Manjula Harish, Annamma Thomas, Vasista Adiga, Annapurna Vyakarnam, Catherine Hawrylowicz, Anura V. Kurpad, and et al. 2025. "Relationship Between Total 25-Hydroxyvitamin D and Parathyroid Hormone Concentrations During Early Gestation in Indian Women" Nutrients 17, no. 16: 2626. https://doi.org/10.3390/nu17162626
APA StyleChopra, N., Ayoob, F., C, R., Karanth, S., Harish, M., Thomas, A., Adiga, V., Vyakarnam, A., Hawrylowicz, C., Kurpad, A. V., & Dwarkanath, P. (2025). Relationship Between Total 25-Hydroxyvitamin D and Parathyroid Hormone Concentrations During Early Gestation in Indian Women. Nutrients, 17(16), 2626. https://doi.org/10.3390/nu17162626






